Abstract
Nanomedicine involves the use of nanoparticles for therapeutic and diagnostic purposes. During the past two decades, a growing number of nanomedicines have received regulatory approval and many more show promise for future clinical translation. In this context, it is important to evaluate the safety of nanoparticles in order to achieve biocompatibility and desired activity. However, it is unwarranted to make generalized statements regarding the safety of nanoparticles, since the field of nanomedicine comprises a multitude of different manufactured nanoparticles made from various materials. Indeed, several nanotherapeutics that are currently approved, such as Doxil and Abraxane, exhibit fewer side effects than their small molecule counterparts, while other nanoparticles (e.g. metallic and carbon-based particles) tend to display toxicity. However, the hazardous nature of certain nanomedicines could be exploited for the ablation of diseased tissue, if selective targeting can be achieved. This review discusses the mechanisms for molecular, cellular, organ, and immune system toxicity, which can be observed with a subset of nanoparticles. Strategies for improving the safety of nanoparticles by surface modification and pretreatment with immunomodulators are also discussed. Additionally, important considerations for nanoparticle safety assessment are reviewed. In regards to clinical application, stricter regulations for the approval of nanomedicines might not be required. Rather, safety evaluation assays should be adjusted to be more appropriate for engineered nanoparticles.
Keywords: nanomedicine, nanoparticle, nanosafety, nanotoxicity, safety, toxicity
Graphical Abstract
This mini-review addresses the safety considerations for nanoparticles in medicine.
1. Introduction
The field of nanomedicine encompasses the utilization of nanoparticles for diagnostic and therapeutic purposes. In general, nanoparticles are used as delivery vehicles for imaging and therapeutic agents, e.g. small molecules, proteins, peptides, and nucleic acids. Numerous materials have been employed to construct such nanoparticles, including lipids [1, 2], metal [3, 4], silicon and silica [5–7], polymers [8], proteins [9], and carbon [10–12]. To date, a plethora of nano-based drugs has been designed to treat various diseases such as neurological disorders, diabetes, cancer, infectious diseases, and allergy [13, 14]. Accordingly, many nanotherapeutics have made it to clinical trials and several have gained regulatory approval [15, 16]. The major categories of nanomedicines that are clinically approved are lipid, polymer, and protein-based particles. For instance, 14 liposomal drugs are available on the USA, European or Chinese markets, while over 20 are enrolled in clinical trials [17–19].
Nanocarriers can protect the payload from degradation and enable sustained and controlled drug release. Furthermore, nanoparticles have the potential to decrease clearance and improve accumulation of drugs in diseased tissue, thereby increasing therapeutic efficacy and reducing side effects. The body contains several barriers that must be overcome for a drug to reach an intended location [20]. These barriers include, immunological clearance, renal clearance, enzymatic and mechanical degradation, vascular endothelium, the extracellular matrix, the cell membrane, the lysosome, and membrane pumps. In essence, the properties of nanoparticles can be optimized to overcome such obstacles. In particular, nanoplatforms enable a multifaceted approach, where various materials and compartments can be combined to efficiently combat the challenges of targeted delivery. For example, the multistage vector, which sequentially releases cargo, is well equipped to handle the various in vivo conditions and compartments that are encountered upon systemic injection [21]. Furthermore, certain nanoparticles have unique electrical and optical properties that can be employed for therapeutic purposes. For instance, metal nanoparticles combined with external energy can be used to thermally ablate diseased tissue. As an illustration, gold nanoparticles can be heated with infrared light [22] and radio waves [23], while iron oxide particles can generate heat when placed in a magnetic field [24].
Taken together, these advantages suggest that nanoparticles could be effectively used to combat several diseases. Since the field of nanomedicince displays great promise, it is imperative to also develop safety tests that can accurately predict the potential toxicity of nanotherapeutics. Especially since nanoparticles exhibit distinct and unique properties that cannot be predicted from analyzing the bulk material, suitable assays for evaluation of nanoparticle toxicity should be taken into practice. Nevertheless, it may not be necessary to establish stricter guidelines for the approval of nanoparticles, as compared to small molecule drugs. Rather, the methods for assessing safety may in certain cases be different. Moreover, as nanoparticles are solely defined by size criteria and encompass a large quantity of particles with different composition and morphology, general statements regarding the safety or toxicity of nano-sized objects are impossible to make. This review will examine how nanoparticles can be used to lower drug toxicity and the major mechanisms by which certain nanoparticles exert toxicity. Furthermore, the safety assessment of nanoparticles will be discussed.
2. Reduction of drug toxicity through nanomedicine
The earliest nanotherapeutics were approved based on similar efficacy, but lower toxicity than their free-drug counterparts. The first nanomedicine to gain clinical approval was Doxil, which is a liposomal formulation of doxorubicin. Doxil was approved by the US Food and Drug Administration (FDA) in 1995 for AIDS-related Kaposi's sarcoma, and has since then been approved for other cancers, e.g. multiple myeloma [25]. The main advantage of Doxil in comparison to free-doxorubicin is reduced cardiotoxicity [25]. In essence, nanoparticles can cause fewer side effects by improving the accumulation of drugs in diseased tissue, thereby reducing the dose required to achieve therapeutic efficacy. As an illustration, less than 0.01% of the injected dose of agents in the angstrom size range (e.g. antibodies) typically accumulates in the target region [26], while the same value is approximately 1–5% for nanoparticles [27]. The major mechanism for increased deposition of nanoparticles in tumor tissue is the enhanced permeability and retention (EPR) effect. Whereas small molecules can freely pass through the vasculature of any tissue, the movement of nanoparticles is more restrictive. The EPR effect arises primarily due to differences between the vasculature of tumors and normal tissue [28, 29]. Namely, cancer blood vessels have larger fenestrations, thereby permitting improved access of nanoparticles to tumor tissue. However, it is important to note that the EPR effect may not be present in all human tumors, and large heterogeneity is likely to exist between patients and cancer types [30].
Another mechanism by which nanoparticles can reduce drug toxicity is associated with the administration of hydrophobic therapeutics. Namely, nanoparticles can serve as an alternative to toxic solubilizing agents [31–33]. Indeed, harmful solvents have been used extensively in vitro and in vivo to permit the delivery of non-water-soluble drugs. For instance, dimethyl sulfoxide (DMSO) is usually the first agent of choice for cell culture [34, 35]. However, DMSO has displayed toxicity in multiple studies [36–39]. Moreover, several clinically approved hydrophobic drugs are formulated with toxic agents. Examples include paclitaxel and docetaxel, which are administered with a polyethoxylated castor oil (Cremophor EL) and dehydrated ethanol mixture [40], and polysorbate 80 (Tween 80) [41], respectively. Patients may require treatment with antihistamines and corticosteroids to alleviate side effects arising form these solubilizing agents [42]. Consequently, nanoparticles represent a better alternative for the administration of poorly water-soluble drugs. For instance, the nanotherapeutic Abraxane, which consists of protein-bound paclitaxel, was approved by the FDA in 2005 for pretreated metastatic breast cancer, and has since then also been approved for the treatment of other cancers [43]. Accordingly, higher doses of Abraxane in comparison to Chremophor El-based paclitaxel can be tolerated by patients [44]. In conclusion, nanoparticles can decrease the toxicity of drugs by improving the biodistribution profile or by eliminating the need for harmful solubilizing agents.
3. Toxicity of nanoparticles in medicine
There are certain categories of nanoparticles that have frequently been reported to have cytotoxic effects. For example, carbon-based nanoparticles have displayed toxicity in multiple in vitro and in vivo assays, although conflicting results exist [45–47]. In particular, carbon nanotubes have been shown to induce mesothelioma, thereby mimicking the toxicity of asbestos, a naturally occurring carcinogenic mineral fiber [48, 49]. Accordingly, the harmful effects of carbon nanotubes may not be a consequence of the actual material, but rather the shape [50], demonstrating that the safety of nanoparticles is highly dependent on particle morphology. Likewise, the size of nanoparticles may also be a determining factor for biocompatibility. For instance, gold nanoparticles with a diameter of 1.4 nm where found to be toxic, while the same particles with a diameter of 15 nm did not display toxicity [51]. In addition to gold nanoparticles, other metal-based particles have also demonstrated cytotoxic effects. For instance several studies have revealed cytotoxic effects of silver nanoparticles [52, 53]. Moreover, iron oxide particles have also been found to exhibit harmful characteristics both in vitro and in vivo [54–56], mainly due to the generation of reactive oxygen species (ROS) [57].
The hazardous properties of certain nanoparticles do not necessarily hinder them from being used for medical purposes. Although a nanoparticle that simultaneously displays biocompatibility and ability to deliver drugs to a tissue of interest is usually considered optimal, harmful nanoparticles could also be utilized. Namely, the toxic properties of nanoparticles could be directly harnessed to ablate diseased tissue, thereby eliminating the need for a drug component. Nevertheless, this approach would require selective targeting of nanoparticles, in order to spare healthy tissue from damage. Additionally, the harmful effects of pristine nanoparticles can be reduced using several approaches, such as surface modification [58]. As an illustration, the addition of hydroxyl groups to gadolinium fullerene particles prevented the generation of ROS, thereby reducing toxicity [11, 59, 60]. Similarly, a polymer coating on the surface of iron oxide nanoparticles dramatically improved cell viability [61].
4. Mechanisms of nanoparticle toxicity
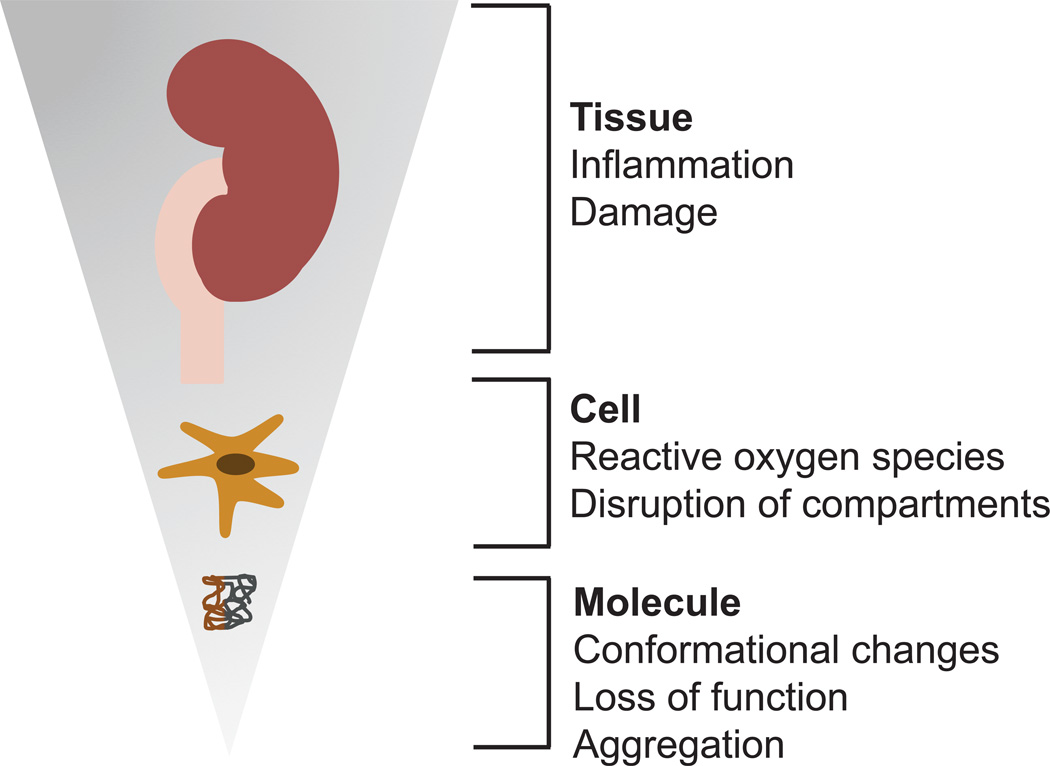
The toxicity of certain nanoparticles can be manifested at the molecular, cellular, and tissue level (Figure 1). Indeed, as nanoparticles move through the body they can be exposed to different biological microenvironments, including the blood, the extracellular matrix, the cytoplasm, and cellular organelles. Consequently, the interactions that occur at the nano-bio interface may impact the function of biomolecules, cellular components and tissue structures. As described in the previous section, certain nanoparticles are prone to elicit a toxic response. The mechanisms by which such nanoparticles cause toxicity will be outlined in the next sections.
Figure 1.
Schematic representation of some of the major toxic effects that can be induced by a subset of nanoparticles. These effects can manifest at the tissue, cellular and molecular level.
4.1. Molecular toxicity of nanomedicines
Upon entering the body, nanoparticles instantaneously interact with the biological environment. Namely, biomolecules that are present in biological fluids form a coat around the particle surface, due to the high surface free energy of nanoparticles [62]. This coating, termed the protein corona, is believed to consist of a soft and hard layer. (Figure 2) [63]. The hard layer is tightly associated with the nanoparticle, while the soft layer is more dynamic. The characteristics of nanoparticles can influence the composition of the hard corona [64, 65], which has been shown to contain more than 100 different proteins [66, 67]. Notably, the presence of a protein corona can drastically change nanoparticle properties, such as shape, size, and charge [62]. For example, protein interactions can increase [67–70] or decrease [71] the size of nanoparticles, and typically cause the zeta potential to become more anionic [67].
Figure 2.
Schematic representation of the current protein corona hypothesis. The hard corona consists of a layer of tightly associated biomolecules, while the soft corona consists of loosely associated biomolecules.
In addition to changes in nanoparticle characteristics, endogenous biomolecules that are exposed to the nanoparticle interface may also undergo structural and functional alterations. Such changes can have important implications for the safety of nanoparticles [62, 72, 73]. Previously, it has been shown that certain nanoparticles induce structural alterations in proteins, such as albumin [74], cytochrome c [75], and ribonuclease A [76]. In general, the ability of nanoparticles to cause protein unfolding is correlated with increased particle size [77–79]. Accordingly, the lower surface curvature of larger nanoparticles increases the protein interaction surface, subsequently causing more alterations in protein structure. Inevitably, conformational changes may compromise protein function. For example, binding of transferrin to iron oxide nanoparticles was shown to irreversibly change the structure and function of this protein, resulting in the premature release of iron [80]. Therefore, it is possible that intravenously injected iron oxide nanoparticles could permanently damage iron transport. Nanoparticles have also been found to cause unfolding of fibrinogen, consequently stimulating inflammatory signaling pathways [81]. Another safety concern is nanoparticle-induced protein aggregation, which can take place as proteins are tightly clustered together on the nanoparticle surface. As an illustration, the rate of β2-microglobulin fibril formation in an acidic environment was increased in the presence of nanoparticles [82]. Such fibrillar aggregates could potentially give rise to pathological conditions, as amyloid formation has been linked to several diseases [83]. However, it is unclear whether β2-microglobulin fibrillation would take place in an in vivo environment, since multiple proteins compete for binding to the nanoparticle surface, thereby decreasing the likelihood that identical proteins come in close contact with each other.
In this context, it is worth noting that while the protein corona may give rise to abnormal protein structures, nanoparticle toxicity is usually decreased in the presence of a protein layer [84, 85]. Correspondingly, as the density of the protein coat increases, the toxicity decreases [84]. One explanation for this protective effect is that coated nanoparticles have less affinity for membrane proteins, thereby preserving cell membrane integrity [84, 86, 87]. Moreover, the protein corona has also been found to prevent nanoparticle-induced platelet activation and hemolysis [67]. Taken together, the initial contact between nanoparticles and proteins in biological fluids reduces further interactions with biomolecules that are part of tissue structures. Therefore, while the formation of a protein corona may cause damage to some circulating proteins, it generally mitigates the overall toxicity of nanomedicines.
4.2. Cellular toxicity of nanomedicines
Nanoparticles may also elicit toxicity by disrupting various membranes within the cell. When membrane integrity is compromised the content inside the membrane-surrounded compartments can leak out. Such leakage is likely to trigger cell stress and interfere with cell and organelle function. For instance, several nanoparticles have been shown to cause disruption of the lysosome membrane, e.g. titanium dioxide [88], zinc oxide [89], polystyrene [90], and polycation particles [8]. A major consequence of lysosomal damage is the release of protons, iron, and hydrolytic enzymes, which can result in oxidative stress, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and protein aggregation [91]. Previously it was thought that the proton sponge effect was the major mechanism by which polycations escape from the lysosome [92]. The proton sponge hypothesis postulates that polycations can cause osmotic swelling and subsequent lysosome rupture, due to their capability to buffer protons and trigger an influx of chloride anions and water. However, recent evidence suggests that osmotic pressure cannot be solely responsible for lysosomal escape. Indeed, even when polycations have maximum proton sequestering capability, the generated osmotic pressure would only cause the area of the membrane to expand by ~2.3%, which is not enough to cause lysosomal rupture [93]. It is possible that the expansion of the cell membrane could make it easier for polycation chains to protrude the lipid bilayer, consequently generating holes in the membrane. Another mechanism that may cause nanoparticle-induced lysosome disruption is the generation of ROS, as oxidative stress has previously been linked to lysosomal membrane permeabilization [94]. Nevertheless, as most nanoparticles become entrapped in the lysosome following endocytosis [95], it is usually necessary that they escape from the lysosome in order to exert therapeutic activity in the correct cellular location. Therefore, it is critical to find a balance between endosomal escape and toxicity. Alternatively, nanoparticles may impair lysosomal function without compromising the membrane. For example, gold nanoparticles were found to alkalize the lysosome compartment, thereby causing accumulation of autophagosomes [96].
In addition, polyethylenimine (PEI), which is frequently incorporated into polymeric nanoparticles, was shown to damage the mitochondrial membrane, causing leakage of protons and inhibition of cytochrome c oxidase activity [97]. Consequently, the activity of the electron transport system and the synthesis of adenosine triphosphate (ATP) were suppressed. The proposed mechanism for mitochondrial protrusion is the formation of pores in the mitochondrial membrane [97]. It is also possible that other organelles, such as the ER, could be disrupted as a result of nanoparticle exposure.
In summary, a major cause of nanoparticle cytotoxicity is damage to lipid membranes, which serve to compartmentalize cellular components. Loss of endomembrane integrity is likely to disrupt homeostasis within a cell. Another proposed mechanism for nanoparticle-induced toxicity is the generation of ROS, which can damage DNA, proteins, and lipids. Nanoparticles can cause formation of ROS directly through catalyzing free radical reactions or indirectly by interfering with cellular homeostasis [98]. The presence of ROS can further cause damage to several organelles, e.g. the ER, as has been demonstrated with zinc oxide nanoparticles [99]. In addition, nanoparticle-induced ROS can sensitize cells to other forms of stress [100]. As a result of acquired cellular damage, nanoparticles can induce different pathways of programmed cell death, including apoptosis, regulated necrosis, and autophagic cell death [101]. Factors such as the nanoparticle size [51, 52], dose [102], incubation period [102], and charge [103] can impact which of these pathways become activated. Nevertheless, the different cell death pathways are tightly associated with each other, making it difficult to precisely pinpoint the mode of death. Inevitably, different cell lines also respond in varying ways to nanoparticle-induced cell death [52, 104].
4.3. Tissue toxicity of nanomedicines
It is foreseeable that nanoparticle-induced damage elicited upon cells and endogenous biomolecules could affect the function of entire organs. There are some examples in the literature of incidents where nanoparticles have caused harm to tissues. Such damage can be traced back to molecular and cellular defects triggered by nanoparticle exposure. In general, the organs that are affected the most are the ones which have the highest levels of nanoparticle accumulation, e.g. the lungs following intratracheal installation and the liver following intravenous injection. For instance, the deposition of carbon nanotubes in the lungs resulted in lung inflammation [105, 106]. Moreover, positively charged lipid nanoparticles injected into the blood were found to cause hepatotoxicity, which was evident from the increased presence of liver enzymes in the blood [107]. The suggested mechanism for cytotoxicity was nanoparticle-mediated activation of the immune system trough toll-like receptor (TLR) signaling. Additional ways in which nanoparticles elicit immune reactions will be discussed in the next section.
5. Immunological responses to nanomedicines
A major challenge in nanomedicine is unwanted immunological recognition. Following intravenous injection, nanoparticles accumulate primarily in the spleen and liver [108, 109]. Indeed, macrophages in these organs recognize and engulf nanoparticles as they pass through the circulation. These phagocytic cells make up the reticuloendothelial system, which is responsible for eliminating foreign bodies. The process of recognition and clearance of nanoparticles relies on the interplay between opsonins and macrophages. Opsonins are immunostimulatory plasma proteins that bind to the surface of foreign particles, thereby marking them for phagocytosis. The most common opsonins are immunoglobulins and complement proteins. Immunoglobulins can trigger nanoparticle engulfment by binding directly to macrophages or by activating the complement system [110]. In essence, the purpose of the complement system is to recognize foreign microparticles and nanoparticles. This recognition relies heavily on identifying surface patterns, since bacteria and viruses usually express repetitive surface units [111, 112]. Therefore, nanoparticle-induced complement activation is also dependent on the same principle, suggesting that a uniform nanoparticle surface could be unbeneficial [111]. Along with surface morphology, the size, composition, charge, and shape of nanoparticles also influence immunological recognition [111]. For example, larger size, increased cholesterol content, and cationic or anionic charge have been related to liposome-induced complement activation [113]. Furthermore, there also exists a correlation between nanoparticle hydrophobicity and immunological activation [114], which likely stems from the recognition of hydrophobic molecules as damage associated-patterns [115]. In fact, the disruption of cells causes hydrophobic cellular components to become revealed, thereby alarming the immune system of the occurred damage. In addition to foreign materials, endogenous plasma proteins that have undergone nanoparticle-induced conformational changes can also activate the complement system [62]. As previously discussed, proteins that bind to the surface of nanoparticles may become misfolded, causing the immune system to mistake them for foreign bodies. For instance, one study speculated that polymeric nanoparticles where activating the complement system, due to conformational changes in albumin [116]. Once nanoparticles have been covered by opsonins, they are internalized by immune cells through non-specific interactions or through binding to Fc receptors [110, 117]. Moreover, certain nanomaterials can directly stimulate the immune system through interactions with TLRs [118, 119]. Although TLRs and the complement system can function independently to induce immune reactions, they may also act synergistically to enhance inflammatory responses [120].
Macrophage-mediated clearance results in decreased accumulation of nanoparticles in the target tissue, consequently lowering therapeutic efficacy. However, complement fragments can also stimulate other immune cells and vascular endothelial cells, causing the production of proinflammatory and vasoactive agents [121]. Therefore, nanoparticle-induced complement activation could also have more serious consequences, such as adverse inflammatory reactions [122]. Indeed, hypersensitivity to the nanotherapeutic Doxil, manifested by e.g. skin reactions, hypotension, hypertension, respiratory issues or pain, has been reported in humans [123] and pigs [124]. These reactions were believed to be caused by activation of the complement system. Another clinically approved nanotherapeutic that has displayed complement-induced adverse reactions in patients is Taxol, which is administered as a micellar formulation [113]. It is worth stressing that several small molecule drugs also cause immunological hypersensitivity reactions through non-complement mediated pathways [125]. Therefore, these observations do not necessarily indicate that nanotherapeutics are more immunogenic than conventional drugs, rather they suggest that the main mechanisms for immune activation could be different. Furthermore, stimulation of TLRs may also cause adverse immune reactions, as certain TLR agonists have been found to trigger excessive production of proinflammatory cytokines, thereby giving rise to a cytokine storm [126]. Correspondingly, cationic liposomes where found to initiate the release of proinflammatory cytokines in a TLR4-dependent manner [107].
Going forward, it is imperative to develop in vitro assays that can accurately predict nanoparticle-induced immunotoxicity. In an attempt to understand how nanoparticles interact with an in vivo environment, numerous studies have identified the major proteins that bind to the surface of nanoparticles upon exposure to serum or plasma [66, 67, 127–129]. In these studies, complement proteins have consistently been reported as one of the main compositional elements of the protein corona. Nevertheless, the mere presence of complement proteins on the nanoparticle surface offers little information about immunological activity. Accordingly, activated complement proteins that initially bind to the nanoparticle surface may be released in the fluid phase [111]. Hence, a complete analysis of complement activation should encompass examination of the nanoparticle surface and the fluid phase. Furthermore, in several protein corona studies, blood coagulation has been prevented through the use of ethylenediaminetetraacetic acid (EDTA) [66, 70, 129], which is also an inhibitor of complement activation [130]. Consequently, the unintentional suppression of the complement system causes disparity between experimental and in vivo conditions. An example of a more informative way of predicting immune activation is the use of an enzyme-linked immunosorbent assay (ELISA) to measure the production of complement activation products in the fluid phase following incubation of nanoparticles with serum or heparin-treated plasma samples. For instance, studies using this method have shown that pegylated liposomes [124], carbon nanotubes [131], and polystyrene particles [132] elicit complement activation in vitro. Another way to predict nanoparticle-induced immune reactions is the measurement of cytokine production in human peripheral blood mononucleated cells (PBMCs) [133]. In particular, cytokines such as interferon gamma (IFN-γ), tumor necrosis factor (TNF-α), and interleukin 12 (IL-12) are indicators of inflammatory responses. Moreover, immunological adverse drug reactions have previously been linked to distinct individual differences on the genome level [134]. The ability to identify individuals who are sensitive to the infusion of nanoparticles would be useful for avoiding adverse reactions in the clinic [135].
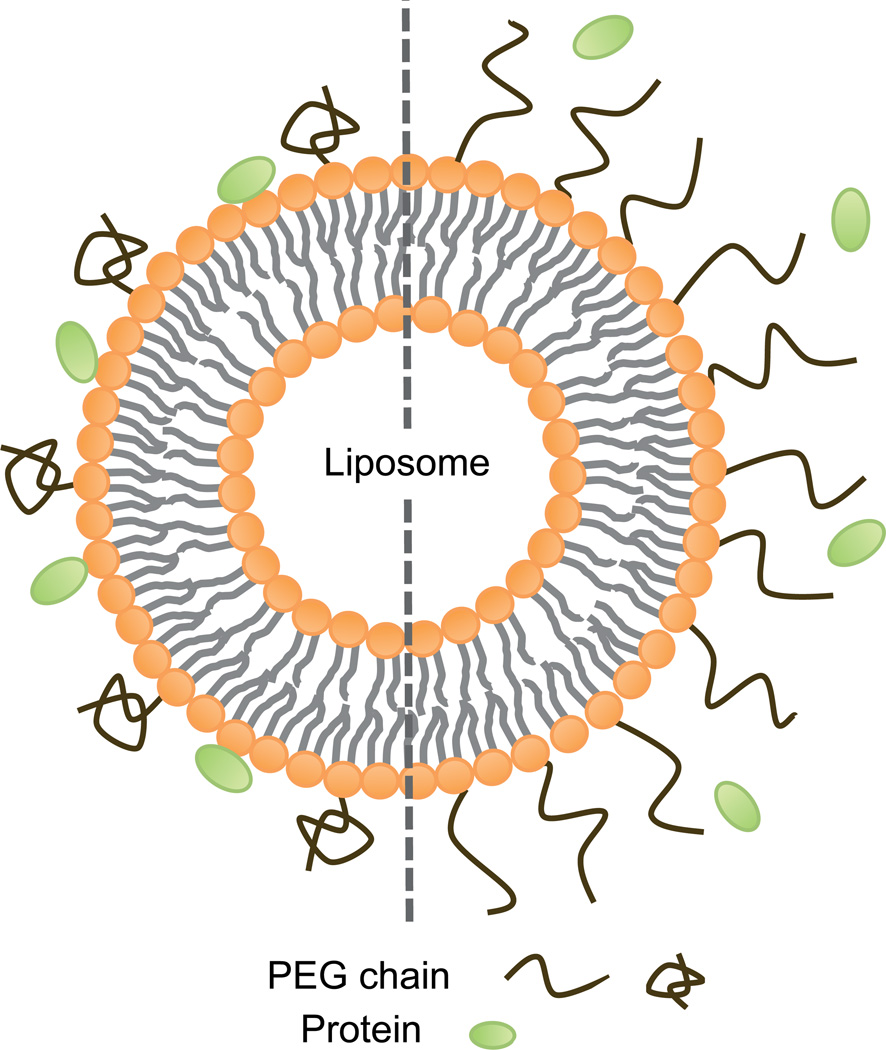
Fortunately, there are several ways in which immunological activation can be partially prevented. The most common way of decreasing immunological recognition is the use of antifouling agents, which reduce protein binding. For example, polyethylene glycol (PEG) is a stealth polymer, which has been widely used in various nanodelivery systems [8, 31, 32, 136]. Nevertheless, the coating of nanoparticles with PEG does not provide complete protection from protein binding [132, 137]. In some respects, the stealth effect can be optimized by regulating the density of PEG chains. For instance, by increasing the density of PEG on a liposome surface the conformation changes from a mushroom to a brush structure, thereby exhibiting greater protection from protein binding (Figure 3) [138]. Despite the benefits of pegylation, PEG may also cause immunological reactions. In certain cases, PEG has paradoxically been found to activate the complement system when incubated with human serum [139]. Moreover, repeated injections of pegylated nanoparticles can cause the formation of anti-PEG antibodies, which results in the accelerated blood clearance (ABC) phenomenon that causes rapid removal of particles from the circulation [140].
Figure 3.
Schematic representation of a pegylated liposome. Sparsely positioned polyethylene glycol (PEG) chains display a mushroom confirmation (left side), which provides poor protection against protein interactions. Densely placed PEG chains display a brush confirmation (right side), which can reduce protein binding.
Besides the use of antifouling agents, other strategies have also been developed to prevent immunological activation. For example, in vitro studies indicate that lipoproteins, such as high-density lipoprotein (HDL) and low-density lipoprotein (LDL), are able to prevent complement activation caused by polymeric nanostructures, presumably through their interactions with these nanoparticles [141]. Likewise, these lipoproteins suppress complement activation by cholesterol-rich liposomes in vitro, and mitigate adverse reactions following intravenous injection of these nanoparticles in vivo [142]. Along with lipoproteins, it has been suggested that inhibitors of the complement system could be used to reduce immunological recognition of nanoparticles [122]. These inhibitors could be conjugated to the nanoparticle surface or administered prior to nanoparticle injection. One study demonstrated that Doxil-induced hypersensitivity reactions in pigs could be reduced through pretreatment with complement inhibitors [124]. Although these strategies are in the early stages of development, they provide promising opportunities for improving the safety of nanotherapeutics. Furthermore, biomimetic approaches for immunological disguise may prove useful in designing non-immunogenic nanoparticles. Indeed, pathogenic organisms can evade complement activation through multiple mechanisms, such as inhibiting or enzymatically degrading complement proteins and interfering with complement regulation [143].
In this context, it is worth emphasizing that the immunological recognition of nanoparticles can also be utilized for therapeutic purposes. Numerous nanoparticles have been shown to stimulate the immune system, thereby providing tools for implementing immunotherapy [144–147]. For instance, poly(methyl vinyl ether-co-maleic anhydride) nanoparticles were shown to improve the survival of mice exposed to a bacterial infection, through binding to various TLRs, causing subsequent stimulation of CD8+ T-cells [119]. Additionally, polyethylenimine nanoparticles with untargeted small interfering RNA (siRNA), triggered therapeutic antitumor activity through stimulation of TLR5, resulting in increased survival of tumor-bearing mice [148]. Besides TLR activation, complement fragments may also serve as immune adjuvants [149]. This phenomenon was exploited in a study, where nanoparticle-induced complement activation conferred immunity to a model antigen conjugated to the nanoparticle [150].
6. Methodological considerations for safety assessment of nanomedicines
When evaluating the cytotoxicity of nanoparticles in vitro, it is important to select assays that are suitable for such applications. The majority of available safety tests have been developed for conventional therapeutic agents. However, nanoparticles behave differently than small molecules, due to their distinct size and surface properties. In particular, nano-sized objects can aggregate, sediment, and display different diffusion dynamics. Therefore, nanoparticles may be unsuitable for test conditions or they may interfere with test results. For example, the Ames test that uses bacteria to measure the mutagenicity of compounds is not particularly fitting for nanoparticles. One study demonstrated that nanofibers that displayed genotoxicity in a mammalian assay, failed to do so in the Ames test [151]. Previously, the suitability of the Ames test for nano-objects has been questioned, due to the presumed inability of nanoparticles to penetrate the bacterial membrane [152]. However, in the aforementioned case, the nanofibers were detected inside the bacteria, suggesting that other factors are responsible for decreased sensitivity of the Ames test to nanoparticles. In addition, carbon nanotubes have been found to interact with the tetrazolium salt in the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, thereby interfering with accurate viability measurements [153]. Namely, while four other viability tests revealed that carbon nanotubes do not elicit toxicity under the reported conditions, the MTT assay showed a 60% reduction in viability. The proposed mechanism for this disparity is that carbon nanotubes clump together with formazan crystals produced in the MTT assay, thus preventing their solubilization and accurate colorimetric readings. Given that nanoparticles may in certain cases interfere with in vitro assays, a good practice is to conduct multiple toxicity tests in parallel, to confirm safety assessment results.
Besides selecting suitable assays for nanoparticles, another important point to consider is the use of appropriate doses. A major criticism directed towards in vitro nanoparticle cytotoxicity studies is the use of inappropriately high concentrations that are not achievable in vivo, thereby giving a false impression of potential toxicity [154]. For instance, titanium dioxide nanoparticles were found to damage cultured brain microglia cells at a dose of 25 µg/ml [155]. However, if these particles are inhaled at high concentration for several hours, even the epithelial cells in the lung will not be exposed to this dose [156]. Similarly, several in vivo measurements of nanoparticle toxicity have also employed doses that are excessively high. As an illustration, one study found that carbon nanotubes were carcinogenic when administered intravenously at a dose of ~ 3 mg/mouse (1.46 ml blood volume) [157], corresponding to more than 10 g/human (5 L blood volume). Notably, even this concentration of blood glucose is considered toxic [158]. Furthermore, in regards to nanoparticle dosing, it is also important to take into account that different cell types display varying rates of nanoparticle uptake. It should not be assumed that nanoparticles would be equally distributed in cells covering a given surface area in vivo. Indeed, macrophages will rapidly engulf most of the particles, causing other cell types to be exposed to lower nanoparticle doses than would be expected by measuring the nanoparticle amount per surface area. Therefore, it can be challenging to correlate in vitro nanoparticle exposure doses to in vivo conditions. Yet another point of consideration for nanoparticle safety assessment is the purity of the sample. For instance, some carbon nanotube preparations contain high amounts of hazardous metals, which can impact toxicity measurements [159–161].
One approach to improve in vitro toxicology assays is the use of 3D cultures, which closer mimic tissue architecture and also enable co-culturing of different cell types [162]. In fact, one study revealed that nanoparticles showed similar heterogeneous distribution patterns in 3D culture and in vivo, while conventional 2D models displayed a uniform distribution [163]. Accordingly, several studies have showed that nanoparticles exhibit lower toxicity in 3D models in comparison to monolayer cultures [164–166]. Moreover, dynamic flow models can also be implemented to give a more realistic depiction of nanoparticle interactions with endothelial cells [162]. As can be expected, nanoparticles under flow conditions have less contact time with cells, thereby affecting intracellular uptake kinetics [167, 168]. By virtue of this, one study found that the toxicity of nanoparticles was reduced under flow conditions in comparison to static cultures [169]. For detailed information about various cell-based assays used for safety assessment of nanoparticles please refer to reviews by Joris et al. [162] and Stone et al. [170]. In summary, caution must be taken to identify the appropriate safety assessment assays, nanoparticle doses, and sample purity, to gain valuable insight into the potential toxicity of nanoparticles.
7. Regulatory aspects of nanomedicine
We believe that it may not be necessary to institute stricter guidelines for the approval of nanotherapeutics as compared to conventional drugs. Rather, the appropriate assays for safety assessment should be identified. In particular, it would be time and cost efficient to develop in vitro nanoparticle assays that can accurately predict in vivo toxicity. In some cases, such assay could be identical to those used for conventional drugs, while in other cases, adjustments to safety tests may be required. Notably, prior to drug approval, the characterization process for nanoparticles is likely to be more laborious in comparison to conventional drugs, since nanoparticles display additional physicochemical properties, such as surface area, shape, and drug release. In this regard, the design of safe and effective nanoparticles could be facilitated by the development of high throughput screening platforms and safety assessment tests that would yield predictive information about structure-activity relationships [171]. Nanoparticle safety libraries that link physicochemical characteristics to hazard potential could increase overall understanding of nanoparticle safety and provide guidance for the initial phases of nanoparticle design. Although detailed information about the physical properties of nanoparticles is important for understanding the mechanisms for toxicity and therapeutic efficacy, it is the concrete safety tests that will ultimately determine whether a nanotherapeutic is suitable for clinical applications. In this context, it would be challenging to identify toxic side effects that are only specific for nanotherapeutics or small molecule drugs. For instance, while small molecule drugs can easily penetrate the blood brain barrier, potentially causing neurological effects, certain nanoparticles can also accumulate in brain tissue. Similarly, while immunological activation remains a major problem for nanoparticles, several small molecule drugs can cause adverse immune reactions.
With this in mind, it may not be warranted to adhere to a stricter approval process for nanotherapeutics in comparison to conventional drugs. Given that different therapeutics regardless of size have a huge variety of biological effects, a regulatory framework that can be used for the assessment of small molecule drugs or biologics should be sufficient for nanoparticles. Indeed, the display of nanodimensions does not constitute a safety risk by itself. Notably, the majority of biological components, such as cellular organelles, proteins, and nucleic acids are in the nano size range. However, one area of the regulatory process where nanotherapeutics may require different guidelines is the approval of ‘nanosimilars’. Whereas generic small molecule drugs can relatively easily be reproduced, biologics and nanoparticles display increased complexity that is difficult to replicate, unless the identical materials and manufacturing procedures are used. Since small changes in nanoparticle properties could change the biological impact, it is debatable whether nanoparticles should be permitted to undergo accelerated approval based on bioequivalence [172]. Furthermore, it is anticipated that precision medicine will play an important role in the future for the approval of all therapeutics, regardless of category. Namely, the genome, epigenome, transcriptome, proteome, and metabolome could be used to predict drug responses, in order to avoid hypersensitivity reactions and select patients that are likely to benefit from therapy [173].
8. Conclusions
The field of nanomedicine is equally, if not more, diverse to the field of small molecule drugs. It would be unwarranted to make general statements about the potential toxicity of small molecule drugs, as they are useful for various purposes, ranging from placebo drugs (e.g. sugar molecules) to euthanasia drugs (death-inducing molecules). Therefore, the same notion of avoiding generalization also applies to nanoparticles. Many of the nanotherapeutics that are currently used in the clinic, such as Abraxane and Doxil, actually serve to reduce the toxicity of the encapsulated drugs. On the contrary, a small subset of nanoparticles that are currently undergoing preclinical investigation, e.g. carbon and metal-based nanoparticles, typically display cytotoxic properties. The harmful effects of these particles are mainly based on ROS generation, disruption of cellular compartments, and immune reactions. Nevertheless, the inherent toxic properties of such nanoparticles could be exploited to ablate diseased tissue, as long as healthy organs are protected through selective targeting. Moreover, there are several strategies (e.g. surface modification) that can be utilized to eliminate toxicity.
Acknowledgments
The work was supported by funds from the MOST 973 Program (2011CB933400, 2012CB934001) (Y.Z.), NSFC (B070704, 21320102003) (Y.Z.) and the Houston Methodist Research Institute (M.F.). Partial funds were acquired from the Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), the National Natural Science Foundation of China (31100721) (M.Z.), the US Department of Defense (W81XWH-09-1-0212) (M.F.), the National Institute of Health (U54CA143837, U54CA151668) (M.F.), Nylands nation Finland (J.W.), Victoriastiftelsen Finland (J.W.), Department of Defense grant W81XWH-12-1-0414 (M.F.), and the State of Texas CPRIT grant RP121071 (M.F. and H.S.)
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Gentile E, Cilurzo F, Di Marzio L, et al. Liposomal chemotherapeutics. Future Oncology. 2013;9:1849–1859. doi: 10.2217/fon.13.146. [DOI] [PubMed] [Google Scholar]
- 2.Paolino D, Cosco D, Gaspari M, et al. Targeting the thyroid gland with thyroid stimulating hormone (TSH)-nanoliposomes. Biomaterials. 2014;35(25):7101–7109. doi: 10.1016/j.biomaterials.2014.04.088. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Kim HC, Mu C, et al. Multifunctional gold nanorods for siRNA gene silencing and photothermal therapy. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201400103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Liu Y, Chen Z, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–2012. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Xu R, Mai J, et al. High capacity nanoporous silicon carrier for systemic delivery of gene silencing therapeutics. ACS nano. 2013;7(11):9867–9880. doi: 10.1021/nn4035316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J, Kim HC, Su H, et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4(5):487–497. doi: 10.7150/thno.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen J, Wu X, Lee Y, et al. Porous silicon microparticles for delivery of siRNA therapeutics. J Vis Exp. 2014 doi: 10.3791/52075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molinaro R, Wolfram J, Federico C, et al. Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin Drug Deliv. 2013;10(12):1653–1668. doi: 10.1517/17425247.2013.840286. [DOI] [PubMed] [Google Scholar]
- 9.Choi KM, Choi SH, Jeon H, Kim IS, Ahn HJ. Chimeric capsid protein as a nanocarrier for siRNA delivery: stability and cellular uptake of encapsulated siRNA. ACS nano. 2011;5(11):8690–8699. doi: 10.1021/nn202597c. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Liu Y, Chen Z, et al. Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine. Adv Mater. 2013;25(41):5928–5936. doi: 10.1002/adma.201300583. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Xing G, Wang J, et al. Multihydroxylated [Gd@C82(OH)22]n nanoparticles: antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5(10):2050–2057. doi: 10.1021/nl051624b. [DOI] [PubMed] [Google Scholar]
- 12.Meng L, Chen R, Jiang A, et al. Short multiwall carbon nanotubes promote neuronal differentiation of PC12 cells via up-regulation of the neurotrophin signaling pathway. Small. 2013;9(9–10):1786–1798. doi: 10.1002/smll.201201388. [DOI] [PubMed] [Google Scholar]
- 13.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 14.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 16.Cattaneo AG, Gornati R, Sabbioni E, et al. Nanotechnology and human health: risks and benefits. J Appl Toxicol. 2010;30(8):730–744. doi: 10.1002/jat.1609. [DOI] [PubMed] [Google Scholar]
- 17.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koudelka S, Turanek J. Liposomal paclitaxel formulations. J Control Release. 2012;163(3):322–334. doi: 10.1016/j.jconrel.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Silverman JA, Deitcher SR. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28(4):181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godin B, Tasciotti E, Liu X, Serda RE, Ferrari M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res. 2011;44(10):979–989. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, You J, Zhang G, et al. Cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv Healthc Mater. 2012;1(1):84–89. doi: 10.1002/adhm.201100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin Cancer Res. 2010;16(23):5712–5721. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannsen M, Thiesen B, Jordan A, et al. Magnetic fluid hyperthermia (MFH)reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate. 2005;64(3):283–292. doi: 10.1002/pros.20213. [DOI] [PubMed] [Google Scholar]
- 25.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Li KC, Pandit SD, Guccione S, Bednarski MD. Molecular imaging applications in nanomedicine. Biomed Microdevices. 2004;6(2):113–116. doi: 10.1023/b:bmmd.0000031747.05317.81. [DOI] [PubMed] [Google Scholar]
- 27.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 29.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 30.Bogart LK, Pourroy G, Murphy CJ, et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS nano. 2014;8(4):3107–3122. doi: 10.1021/nn500962q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfram J, Suri K, Huang Y, et al. Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells. J Microencaps. 2014;31(5):501–507. doi: 10.3109/02652048.2013.879932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celia C, Trapasso E, Locatelli M, et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf B Biointerfaces. 2013;112:548–553. doi: 10.1016/j.colsurfb.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Norvaisas P, Ziemys A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J Pharm Sci. 2014;103(7):2147–2156. doi: 10.1002/jps.23996. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Wolfram J, Boom K, Fang X, Shen H, Ferrari M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem Funct. 2013;31(5):374–379. doi: 10.1002/cbf.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Wolfram J, Shen H, Fang X, Ferrari M. Hesperetin: an inhibitor of the transforming growth factor-beta (TGF-beta) signaling pathway. Eur J Med Chem. 2012;58:390–395. doi: 10.1016/j.ejmech.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Delgado GJ, Mancias-Guerra C, Tamez-Gomez EL, et al. Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 2009;122(1):1–5. doi: 10.1159/000227267. [DOI] [PubMed] [Google Scholar]
- 37.Zenhausern R, Tobler A, Leoncini L, Hess OM, Ferrari P. Fatal cardiac arrhythmia after infusion of dimethyl sulfoxide-cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol. 2000;79(9):523–526. doi: 10.1007/s002770000186. [DOI] [PubMed] [Google Scholar]
- 38.Montaguti P, Melloni E, Cavalletti E. Acute intravenous toxicity of dimethyl sulfoxide, polyethylene glycol 400, dimethylformamide, absolute ethanol, and benzyl alcohol in inbred mouse strains. Arzneimittelforschung. 1994;44(4):566–570. [PubMed] [Google Scholar]
- 39.Rubin LF. Toxicity of dimethyl sulfoxide, alone and in combination. Ann N Y Acad Sci. 1975;243:98–103. doi: 10.1111/j.1749-6632.1975.tb25348.x. [DOI] [PubMed] [Google Scholar]
- 40.Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 41.Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. International journal of nanomedicine. 2014;9:495–504. doi: 10.2147/IJN.S56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis P, Schneider J, Hann L, et al. Phase II trial of docetaxel in patients with platinum-refractory advanced ovarian cancer. J Clin Oncol. 1994;12(11):2301–2308. doi: 10.1200/JCO.1994.12.11.2301. [DOI] [PubMed] [Google Scholar]
- 43.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. International journal of nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 45.Zhao Y, Xing G, Chai Z. Nanotoxicology: Are carbon nanotubes safe? Nat Nanotechnol. 2008;3(4):191–192. doi: 10.1038/nnano.2008.77. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46(3):702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 47.Jia G, Wang H, Yan L, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39(5):1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 48.Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 50.Johnston HJ, Hutchison GR, Christensen FM, et al. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physicochemical characteristics. Nanotoxicology. 2010;4(2):207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- 51.Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 52.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100(4):1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- 53.Pratsinis A, Hervella P, Leroux JC, Pratsinis SE, Sotiriou GA. Toxicity of silver nanoparticles in macrophages. Small. 2013;9(15):2576–2584. doi: 10.1002/smll.201202120. [DOI] [PubMed] [Google Scholar]
- 54.Zhu MT, Wang Y, Feng WY, et al. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. Journal of nanoscience and nanotechnology. 2010;10(12):8584–8590. doi: 10.1166/jnn.2010.2488. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Feng W, Zhu M, et al. Neurotoxicity of low-dose repeatedly intranasal instillation of nano- and submicron-sized ferric oxide particles in mice. J Nanopart Res. 2009;11(1):41–53. [Google Scholar]
- 56.Zhu MT, Wang B, Wang Y, et al. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol Lett. 2011;203(2):162–171. doi: 10.1016/j.toxlet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Wang B, Yin J-J, Zhou X, et al. Physicochemical Origin for Free Radical Generation of Iron Oxide Nanoparticles in Biomicroenvironment: Catalytic Activities Mediated by Surface Chemical States. The Journal of Physical Chemistry C. 2012;117(1):383–392. [Google Scholar]
- 58.Yan L, Zhao F, Li S, Hu Z, Zhao Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3(2):362–382. doi: 10.1039/c0nr00647e. [DOI] [PubMed] [Google Scholar]
- 59.Meng H, Xing G, Sun B, et al. Potent angiogenesis inhibition by the particulate form of fullerene derivatives. ACS nano. 2010;4(5):2773–2783. doi: 10.1021/nn100448z. [DOI] [PubMed] [Google Scholar]
- 60.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7(10):1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 61.Gupta AK, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobioscience. 2004;3(1):66–73. doi: 10.1109/tnb.2003.820277. [DOI] [PubMed] [Google Scholar]
- 62.Wolfram J, Yang Y, Shen J, et al. The nano-plasma interface: Implications of the protein corona. Colloids Surf B Biointerfaces. 2014 doi: 10.1016/j.colsurfb.2014.02.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 64.Zhu M, Perrett S, Nie G. Understanding the particokinetics of engineered nanomaterials for safe and effective therapeutic applications. Small. 2013;9(9–10):1619–1634. doi: 10.1002/smll.201201630. [DOI] [PubMed] [Google Scholar]
- 65.Zhu M, Nie G, Meng H, Xia T, Nel A, Zhao Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc Chem Res. 2013;46(3):622–631. doi: 10.1021/ar300031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tenzer S, Docter D, Rosfa S, et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS nano. 2011;5(9):7155–7167. doi: 10.1021/nn201950e. [DOI] [PubMed] [Google Scholar]
- 67.Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 68.Mortensen NP, Hurst GB, Wang W, Foster CM, Nallathamby PD, Retterer ST. Dynamic development of the protein corona on silica nanoparticles: composition and role in toxicity. Nanoscale. 2013;5(14):6372–6380. doi: 10.1039/c3nr33280b. [DOI] [PubMed] [Google Scholar]
- 69.Goy-Lopez S, Juarez J, Alatorre-Meda M, et al. Physicochemical characteristics of protein-NP bioconjugates: the role of particle curvature and solution conditions on human serum albumin conformation and fibrillogenesis inhibition. Langmuir. 2012;28(24):9113–9126. doi: 10.1021/la300402w. [DOI] [PubMed] [Google Scholar]
- 70.Monopoli MP, Walczyk D, Campbell A, et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133(8):2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 71.Wolfram J, Suri K, Yang Y, et al. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf B Biointerfaces. 2014;114C:294–300. doi: 10.1016/j.colsurfb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelaz B, Charron G, Pfeiffer C, et al. Interfacing engineered nanoparticles with biological systems: anticipating adverse nano-bio interactions. Small. 2013;9(9–10):1573–1584. doi: 10.1002/smll.201201229. [DOI] [PubMed] [Google Scholar]
- 73.Zuo G, Kang SG, Xiu P, Zhao Y, Zhou R. Interactions between proteins and carbon-based nanoparticles: exploring the origin of nanotoxicity at the molecular level. Small. 2013;9(9–10):1546–1556. doi: 10.1002/smll.201201381. [DOI] [PubMed] [Google Scholar]
- 74.Shang L, Wang Y, Jiang J, Dong S. pH-dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: a spectroscopic study. Langmuir. 2007;23(5):2714–2721. doi: 10.1021/la062064e. [DOI] [PubMed] [Google Scholar]
- 75.Shang W, Nuffer JH, Muniz-Papandrea VA, Colon W, Siegel RW, Dordick JS. Cytochrome C on silica nanoparticles: influence of nanoparticle size on protein structure, stability, and activity. Small. 2009;5(4):470–476. doi: 10.1002/smll.200800995. [DOI] [PubMed] [Google Scholar]
- 76.Shang W, Nuffer JH, Dordick JS, Siegel RW. Unfolding of ribonuclease A on silica nanoparticle surfaces. Nano Lett. 2007;7(7):1991–1995. doi: 10.1021/nl070777r. [DOI] [PubMed] [Google Scholar]
- 77.Teichroeb JH, Forrest JA, Jones LW. Size-dependent denaturing kinetics of bovine serum albumin adsorbed onto gold nanospheres. Eur Phys J E Soft Matter. 2008;26(4):411–415. doi: 10.1140/epje/i2007-10342-9. [DOI] [PubMed] [Google Scholar]
- 78.Teichroeb JH, Forrest JA, Ngai V, Jones LW. Anomalous thermal denaturing of proteins adsorbed to nanoparticles. Eur Phys J E Soft Matter. 2006;21(1):19–24. doi: 10.1140/epje/i2006-10040-2. [DOI] [PubMed] [Google Scholar]
- 79.Lundqvist M, Sethson I, Jonsson BH. Protein adsorption onto silica nanoparticles: conformational changes depend on the particles' curvature and the protein stability. Langmuir. 2004;20(24):10639–10647. doi: 10.1021/la0484725. [DOI] [PubMed] [Google Scholar]
- 80.Mahmoudi M, Shokrgozar MA, Sardari S, et al. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale. 2011;3(3):1127–1138. doi: 10.1039/c0nr00733a. [DOI] [PubMed] [Google Scholar]
- 81.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6(1):39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 82.Linse S, Cabaleiro-Lago C, Xue WF, et al. Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci U S A. 2007;104(21):8691–8696. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 84.Ge C, Du J, Zhao L, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci U S A. 2011;108(41):16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Li J, Pan J, et al. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: understanding the reduced damage in cell membranes. J Am Chem Soc. 2013;135(46):17359–17368. doi: 10.1021/ja406924v. [DOI] [PubMed] [Google Scholar]
- 86.Hu W, Peng C, Lv M, et al. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS nano. 2011;5(5):3693–3700. doi: 10.1021/nn200021j. [DOI] [PubMed] [Google Scholar]
- 87.Lesniak A, Fenaroli F, Monopoli MP, Aberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS nano. 2012;6(7):5845–5857. doi: 10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho WS, Duffin R, Howie SE, et al. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lunov O, Syrovets T, Loos C, et al. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS nano. 2011;5(12):9648–9657. doi: 10.1021/nn203596e. [DOI] [PubMed] [Google Scholar]
- 91.Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Won YY, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J Control Release. 2009;139(2):88–93. doi: 10.1016/j.jconrel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berndt C, Kurz T, Selenius M, Fernandes AP, Edgren MR, Brunk UT. Chelation of lysosomal iron protects against ionizing radiation. Biochem J. 2010;432(2):295–301. doi: 10.1042/BJ20100996. [DOI] [PubMed] [Google Scholar]
- 95.Iversen TG, Skotland T, Sandvig K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today. 2011;6(2):176–185. [Google Scholar]
- 96.Ma X, Wu Y, Jin S, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS nano. 2011;5(11):8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 97.Hall A, Larsen AK, Parhamifar L, Meyle KD, Wu LP, Moghimi SM. High resolution respirometry analysis of polyethylenimine-mediated mitochondrial energy crisis and cellular stress: Mitochondrial proton leak and inhibition of the electron transport system. Biochim Biophys Acta. 2013;1827(10):1213–1225. doi: 10.1016/j.bbabio.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Yan L, Gu Z, Zhao Y. Chemical mechanisms of the toxicological properties of nanomaterials: generation of intracellular reactive oxygen species. Chem Asian J. 2013;8(10):2342–2353. doi: 10.1002/asia.201300542. [DOI] [PubMed] [Google Scholar]
- 99.Chen R, Huo L, Shi X, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS nano. 2014;8(3):2562–2574. doi: 10.1021/nn406184r. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Wang C, Li Z, et al. Unraveling stress-induced toxicity properties of graphene oxide and the underlying mechanism. Adv Mater. 2012;24(39):5391–5397. doi: 10.1002/adma.201202678. [DOI] [PubMed] [Google Scholar]
- 101.Andon FT, Fadeel B. Programmed cell death: molecular mechanisms and implications for safety assessment of nanomaterials. Acc Chem Res. 2013;46(3):733–742. doi: 10.1021/ar300020b. [DOI] [PubMed] [Google Scholar]
- 102.Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190(2):156–162. doi: 10.1016/j.toxlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 103.Schaeublin NM, Braydich-Stolle LK, Schrand AM, et al. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale. 2011;3(2):410–420. doi: 10.1039/c0nr00478b. [DOI] [PubMed] [Google Scholar]
- 104.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS nano. 2008;2(1):85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 105.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77(1):117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 106.Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Kedmi R, Ben-Arie N, Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31(26):6867–675. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 108.Kumar R, Roy I, Ohulchanskky TY, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS nano. 2010;4(2):699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee MJ, Veiseh O, Bhattarai N, et al. Rapid pharmacokinetic and biodistribution studies using cholorotoxin-conjugated iron oxide nanoparticles: a novel non-radioactive method. PLoS One. 2010;5(3):e9536. doi: 10.1371/journal.pone.0009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodridge HS, Underhill DM, Touret N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. 2012;13(8):1062–1071. doi: 10.1111/j.1600-0854.2012.01382.x. [DOI] [PubMed] [Google Scholar]
- 111.Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63(12):1000–1007. doi: 10.1016/j.addr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol. 2013;78(2):181–193. doi: 10.1111/sji.12070. [DOI] [PubMed] [Google Scholar]
- 113.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 114.Moyano DF, Goldsmith M, Solfiell DJ, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134(9):3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 116.Vauthier C, Persson B, Lindner P, Cabane B. Protein adsorption and complement activation for diblock copolymer nanoparticles. Biomaterials. 2011;32(6):1646–1656. doi: 10.1016/j.biomaterials.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 117.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Yang Y, Wolfram J, Fang X, Shen H, Ferrari M. Polyarginine Induces an Antitumor Immune Response through Binding to Toll-Like Receptor 4. Small. 2014;10(7):1250–1254. doi: 10.1002/smll.201302887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17(9):1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holst B, Raby AC, Hall JE, Labeta MO. Complement takes its Toll: an inflammatory crosstalk between Toll-like receptors and the receptors for the complement anaphylatoxin C5a. Anaesthesia. 2012;67(1):60–64. doi: 10.1111/j.1365-2044.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 121.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moghimi SM, Farhangrazi ZS. Nanomedicine and the complement paradigm. Nanomedicine. 2013;9(4):458–460. doi: 10.1016/j.nano.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 123.Chanan-Khan A, Szebeni J, Savay S, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14(9):1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 124.Szebeni J, Baranyi L, Savay S, et al. Complement activation-related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function. Am J Physiol Heart Circ Physiol. 2006;290(3):H1050–H1058. doi: 10.1152/ajpheart.00622.2005. [DOI] [PubMed] [Google Scholar]
- 125.Pichler WJ, Adam J, Daubner B, Gentinetta T, Keller M, Yerly D. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. Med Clin North Am. 2010;94(4):645–664. doi: 10.1016/j.mcna.2010.04.003. xv. [DOI] [PubMed] [Google Scholar]
- 126.Wen H, Lei Y, Eun SY, Ting JP. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J Exp Med. 2010;207(13):2943–2957. doi: 10.1084/jem.20101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5(2):106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martel J, Young D, Young A, et al. Comprehensive proteomic analysis of mineral nanoparticles derived from human body fluids and analyzed by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418(1):111–125. doi: 10.1016/j.ab.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 129.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zapf S, Loos M. Effect of EDTA and citrate on the functional activity of the first component of complement, C1, and the C1q subcomponent. Immunobiology. 1985;170(3):123–132. doi: 10.1016/S0171-2985(85)80085-1. [DOI] [PubMed] [Google Scholar]
- 131.Andersen AJ, Robinson JT, Dai H, Hunter AC, Andresen TL, Moghimi SM. Single-walled carbon nanotube surface control of complement recognition and activation. ACS nano. 2013;7(2):1108–1119. doi: 10.1021/nn3055175. [DOI] [PubMed] [Google Scholar]
- 132.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering. ACS nano. 2010;4(11):6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 133.Hanley C, Thurber A, Hanna C, Punnoose A, Zhang J, Wingett DG. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale research letters. 2009;4(12):1409–1420. doi: 10.1007/s11671-009-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Daly AK. Using genome-wide association studies to identify genes important in serious adverse drug reactions. Annu Rev Pharmacol Toxicol. 2012;52:21–35. doi: 10.1146/annurev-pharmtox-010611-134743. [DOI] [PubMed] [Google Scholar]
- 135.Moghimi SM, Wibroe PP, Helvig SY, Farhangrazi ZS, Hunter AC. Genomic perspectives in inter-individual adverse responses following nanomedicine administration: The way forward. Adv Drug Deliv Rev. 2012;64(13):1385–1393. doi: 10.1016/j.addr.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 136.Schipper ML, Iyer G, Koh AL, et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5(1):126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim HR, Andrieux K, Delomenie C, et al. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip system. Electrophoresis. 2007;28(13):2252–2261. doi: 10.1002/elps.200600694. [DOI] [PubMed] [Google Scholar]
- 138.Bartucci R, Pantusa M, Marsh D, Sportelli L. Interaction of human serum albumin with membranes containing polymer-grafted lipids: spin-label ESR studies in the mushroom and brush regimes. Biochim Biophys Acta. 2002;1564(1):237–242. doi: 10.1016/s0005-2736(02)00458-3. [DOI] [PubMed] [Google Scholar]
- 139.Hamad I, Hunter AC, Szebeni J, Moghimi SM. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol. 2008;46(2):225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 140.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 141.Hamad I, Hunter AC, Moghimi SM. Complement monitoring of Pluronic 127 gel and micelles: suppression of copolymer-mediated complement activation by elevated serum levels of HDL, LDL, and apolipoproteins AI and B-100. J Control Release. 2013;170(2):167–174. doi: 10.1016/j.jconrel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 142.Moein Moghimi S, Hamad I, Bunger R, et al. Activation of the human complement system by cholesterol-rich and PEGylated liposomes-modulation of cholesterol-rich liposome-mediated complement activation by elevated serum LDL and HDL levels. J Liposome Res. 2006;16(3):167–174. doi: 10.1080/08982100600848801. [DOI] [PubMed] [Google Scholar]
- 143.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6(2):132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tian X, Zhu M, Nie G. How can nanotechnology help membrane vesicle-based cancer immunotherapy development? Hum Vaccin Immunother. 2013;9(1):222–225. doi: 10.4161/hv.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu M, Tian X, Song X, et al. Nanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activation. Small. 2012;8(18):2841–2848. doi: 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- 146.Liu Y, Jiao F, Qiu Y, et al. Immunostimulatory properties and enhanced TNF- alpha mediated cellular immunity for tumor therapy by C60(OH)20 nanoparticles. Nanotechnology. 2009;20(41):415102. doi: 10.1088/0957-4484/20/41/415102. [DOI] [PubMed] [Google Scholar]
- 147.Yang D, Zhao Y, Guo H, et al. [Gd@C(82)(OH)(22)](n) nanoparticles induce dendritic cell maturation and activate Th1 immune responses. ACS nano. 2010;4(2):1178–1186. doi: 10.1021/nn901478z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cubillos-Ruiz JR, Engle X, Scarlett UK, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119(8):2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 150.Reddy ST, van der Vlies AJ, Simeoni E, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 151.Clift MJ, Raemy DO, Endes C, et al. Can the Ames test provide an insight into nano-object mutagenicity? Investigating the interaction between nano-objects and bacteria. Nanotoxicology. 2013;7(8):1373–1385. doi: 10.3109/17435390.2012.741725. [DOI] [PubMed] [Google Scholar]
- 152.Ng CT, Li JJ, Bay BH, Yung LY. Current studies into the genotoxic effects of nanomaterials. J Nucleic Acids. 2010;2010:ID947859. doi: 10.4061/2010/947859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 154.Oberdorster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 155.Long TC, Tajuba J, Sama P, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oberdörster G, Yu CP. The carcinogenic potential of inhaled diesel exhaust: a particle effect? J Aerosol Sci. 1990;21(Supplement 1(0)):S397–S401. [Google Scholar]
- 157.Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 158.Corathers SD, Falciglia M. The role of hyperglycemia in acute illness: supporting evidence and its limitations. Nutrition. 2011;27(3):276–281. doi: 10.1016/j.nut.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 159.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77(1):126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 160.Ge C, Li Y, Yin J-J, et al. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012;4:e32. [Google Scholar]
- 161.Ge C, Meng L, Xu L, et al. Acute pulmonary and moderate cardiovascular responses of spontaneously hypertensive rats after exposure to single-wall carbon nanotubes. Nanotoxicology. 2012;6(5):526–542. doi: 10.3109/17435390.2011.587905. [DOI] [PubMed] [Google Scholar]
- 162.Joris F, Manshian BB, Peynshaert K, De Smedt SC, Braeckmans K, Soenen SJ. Assessing nanoparticle toxicity in cell-based assays: influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem Soc Rev. 2013;42(21):8339–8359. doi: 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- 163.Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS nano. 2012;6(5):4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Godugu C, Patel AR, Desai U, Andey T, Sams A, Singh M. AlgiMatrix based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One. 2013;8(1):e53708. doi: 10.1371/journal.pone.0053708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee J, Lilly GD, Doty RC, Podsiadlo P, Kotov NA. In vitro toxicity testing of nanoparticles in 3D cell culture. Small. 2009;5(10):1213–1221. doi: 10.1002/smll.200801788. [DOI] [PubMed] [Google Scholar]
- 166.Movia D, Prina-Mello A, Bazou D, Volkov Y, Giordani S. Screening the cytotoxicity of singlewalled carbon nanotubes using novel 3D tissue-mimetic models. ACS nano. 2011;5(11):9278–9290. doi: 10.1021/nn203659m. [DOI] [PubMed] [Google Scholar]
- 167.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Control Release. 2012;157(3):485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin A, Sabnis A, Kona S, et al. Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. J Biomed Mater Res A. 2010;93(3):833–842. doi: 10.1002/jbm.a.32592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mahto SK, Yoon TH, Rhee SW. A new perspective on in vitro assessment method for evaluating quantum dot toxicity by using microfluidics technology. Biomicrofluidics. 2010;4(3) doi: 10.1063/1.3486610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39(7):613–626. doi: 10.1080/10408440903120975. [DOI] [PubMed] [Google Scholar]
- 171.Nel A, Zhao Y, Madler L. Environmental health and safety considerations for nanotechnology. Acc Chem Res. 2013;46(3):605–606. doi: 10.1021/ar400005v. [DOI] [PubMed] [Google Scholar]
- 172.Moein Moghimi S, Shadi Farhangrazi Z. Defining and characterizing nonbiological complex drugs (NBCDs) – Is size enough? The case for liposomal doxorubicin generics (‘liposomal nanosimilars’) for injection. GaBI J. 2014;3(2):56–62. [Google Scholar]
- 173.Rosenblum D, Peer D. Omics-based nanomedicine: The future of personalized oncology. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.07.029. in press. [DOI] [PubMed] [Google Scholar]