ABSTRACT
The rapid occurrence of emerging infectious diseases demonstrates an urgent need for a new preclinical experimental model that reliably replicates human immune responses. Here, a new homozygous humanized human leukocyte antigen (HLA)-A11/DR1 transgenic mouse (HLA-A11+/+/DR01+/+/H-2-β2m−/−/IAβ−/−) was generated by crossing HLA-A11 transgenic (Tg) mice with HLA-A2+/+/DR01+/+/H-2-β2m−/−/IAβ−/− mice. The HLA-A11-restricted immune response of this mouse model was then examined. HLA-A11 Tg mice expressing a chimeric major histocompatibility complex (MHC) molecule comprising the α1, α2, and β2m domains of human HLA-A11 and the α3 transmembrane and cytoplasmic domains of murine H-2Db were generated. The correct integration of HLA-A11 and HLA-DR1 into the genome of the HLA-A11/DR1 Tg mice (which lacked the expression of endogenous H-2-I/II molecules) was then confirmed. Immunizing mice with a recombinant HBV vaccine or a recombinant HIV-1 protein resulted in the generation of IFN-γ-producing cytotoxic T lymphocyte (CTL) and antigen-specific antibodies. The HLA-A11-restricted CTL response was directed at HLA immunodominant epitopes. These mice represent a versatile animal model for studying the immunogenicity of HLA CTL epitopes in the absence of a murine MHC response. The established animal model will also be useful for evaluating and optimizing T cell-based vaccines and for studying differences in antigen processing between mice and humans.
Keywords: MHC, HLA, HLA-A11/DR1 transgenic mice, MHC-restricted epitopes, Vaccine
Introduction
Major histocompatibility complex (MHC) molecules play a vital role in activating the adaptive immune system. They do this by presenting antigens (usually peptides) to immune cells in a specific context and by participating in the differentiation and maturation of T lymphocytes in the thymus.1,2 The incidence of disease caused by newly emerged viruses is increasing worldwide; therefore, candidate epitope-based vaccines are vital for providing immunoprotection from pathogens via the elicitation of humoral and cellular responses.3–8 Specific cytotoxic T lymphocyte (CTL) responses would lead to a marked reduction in the viral load, and may even clear the virus and cure some autoimmune diseases.9 A protective adaptive immune response is based on the effective activation and mobilization of B cells, cytotoxic T cells, and helper T cells.10 Synergism between MHC class I and II molecules is a key component of an effective host immune response.
Human MHC molecules (known as HLA (human leukocyte antigen) molecules) are the most polymorphic genes in the human genome. MHC restriction differs markedly according to geographical region and ethnicity. Several studies used MHC-I or MHC-II transgenic mice to examine the molecular mechanism(s) underlying disease, evaluate candidate vaccines, and screen HLA-restricted epitopes.11,12 For example, Ishioka et al.13 used HLA-A2 and HLA-A11 transgenic (Tg) mice to evaluate a minigene DNA vaccine encoding multiple HLA-restricted CTL epitopes derived from HIV and HBV, whereas Pajot et al.14 used HLA-DR1 Tg mice to identify novel HLA-DR1-restricted epitopes derived from the HBV envelope protein. T helper cells play a vital role in boosting CTL responses and humoral immune responses against pathogens; indeed, the collaboration between T helper and cytotoxic CD8+ T cells is critical for an effective CTL response.15,16 We previously generated HLA-Tg mice expressing both HLA class I and II molecules (e.g., HLA-A2/DR1 Tg mice17 and HLA-A2/DP4 Tg mice18) and used them to develop and evaluate vaccines.19–22
HLA-A11 is one of the most common HLA class I genotypes in the world, with a phenotypic frequency of about 20–30% in the Chinese population and 10–15% in European and American populations.23 The first HLA-A11/Kb mouse was generated by Alexander et al.24 and was used to develop HLA-A11-restricted epitope-based vaccines.25 However, the relevance of this model to the human immune system was overshadowed by the presence of murine H-2 class I or II molecules, which were used preferentially as restricting elements in response to antigens.26,27 Furthermore, HLA-A11/Kb mice did not accurately reflect human T helper cell responses because they lacked the HLA-II molecules. Thus, a transgenic mouse model expressing both HLA-A11 and HLA-DR1 molecules, but lacking H-2 class I and II molecules, is needed.
Here, we constructed an HLA-A11/DR1 (A11+/+/DR01+/+/β2m−/−/IAβ−/−) Tg mouse model based on the A*1101/DR01 genotypes, which represented 10–15% of the Chinese population, and used it to evaluate novel candidate vaccines.
Results
Generation of homozygous HLA-A11/DR1 (HLA-A11+/+/DR01+/+/β2m−/−/IAβ−/−) Tg mice
The construction of the recombinant gene encoding the chimeric HLA-A11 monochains illustrated in Figure 1A and B. Transgenic mice expressing the integrated HLA-A11 fragment were identified by PCR (data not shown). HLA-A11/DR1 (HLA-A11+/+/DR01+/+/β2m−/−/IAβ−/−) Tg mice were obtained by crossing the parental HLA-A11+/+ (HLA-A11) Tg mice with HLA-A2+/+/DR01+/+/β2m−/−/IAβ−/− (HLA-A2/DR1) Tg mice. The genotype of the HLA-A11+/+/DR1+/+/β2m−/−/IAβ−/−Tg mice was confirmed by PCR, with offspring-derived genomic DNA as a template. The exogenous HLA-A11 and HLA-DR1 fragments were integrated into the mouse genome (Fig. 2A), and the endogenous β2m and H-2 IAβ (Fig. 2B) genes were knocked out.
Figure 1.
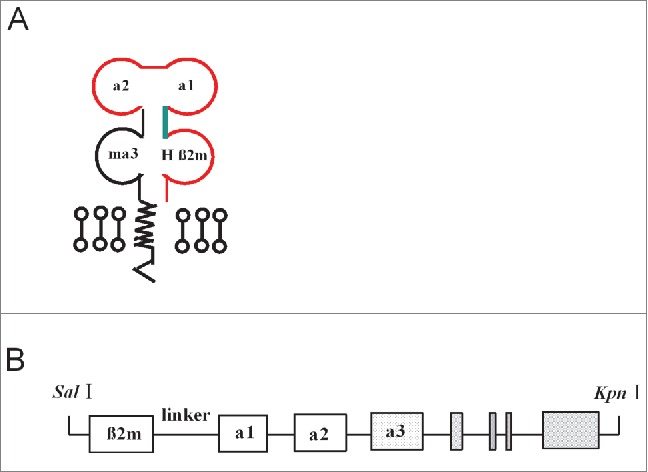
Schematic diagram of the chimeric human/mouse MHC class I gene. (A) Schematic diagram of the monochain chimeric human/mouse MHC class I gene showing the HHD structure of the chimeric human α1, α2, and β2m HLA-A11/murine H-2 α3 molecule. The murine H2 α3 domain is covalently linked to human β2m via a 15 amino acid linker. (B) Liner representation of the final construct.
Figure 2.
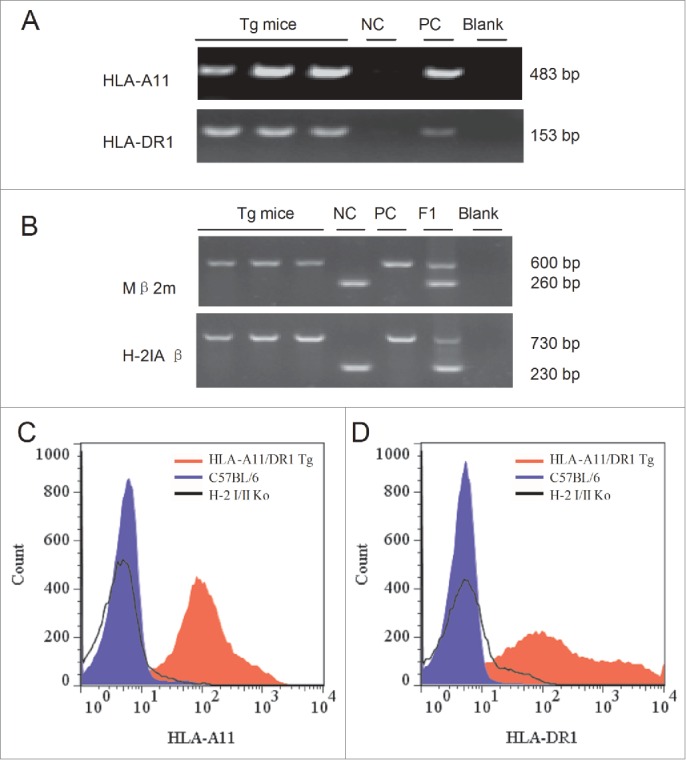
Genotype of HLA-A11/DR1 Tg mice and flow cytometric analysis of transgenic HLA molecules expressed on the surface of mouse immune cells. Genomic DNA purified from HLA-A11/DR1 Tg mice was analyzed by PCR using primers specific for HLA-A11, HLA-DR1, mouse β2m, and H-2 IAβ. (A) Identification of HLA-A11 (upper panel) and HLA-DR1 (lower panel). Tg mice, HLA-A11/DR1 Tg mice; NC, Negative control; PC, Positive control; Blank, dH2O. (B) Identification of mouse β2m(upper panel) and H-2 IAβ (lower panel).Tg mice,HLA-A11/DR1 Tg mice; NC, Negative control; PC, Positive control; F1, Heterozygote control; Blank, dH2O.(C and D) Splenocytes from HLA-A11/DR1 (red histogram), H-2-I/II knockout mice (black histogram), and wild-type C57BL/6 (blue histogram) mice were isolated and stained with a PE-anti-human-HLA-ABC antibody (C) or an FITC-anti-human-HLA-DR antibody (D) to detect HLA-A11 and HLA-DR1 expression, respectively.
Expression of HLA-A11 and HLA-DR1 in splenocytes isolated from HLA-A11/DR1 Tg mice was detected by flow cytometry analysis. HLA-A11/DR1 Tg mice expressed both HLA-A11 (Fig. 2C) and HLA-DR1 (Fig. 2D), whereas no HLA transgenes were expressed in wild-type C57BL/6 mice or H-2-I/II knockout mice.
Taken together, these results demonstrated that the HLA-A11 and HLA-DR1 transgenes had integrated into the mouse genome and were expressed by splenocytes from HLA-A11/DR1 Tg mice, while competitive inhibition by mouse endogenous H-2-I/II molecules had been eliminated. Thus, the mouse model could be used to examine cooperation between human HLA-I/II molecules without interference from mouse H-2 molecules.
Proportions of CD8+ and CD4+ T lymphocytes in the peripheral blood of HLA-A11/DR1 Tg mice
To test whether the expression of exogenous HLA transgenes would still allow positive and negative selection of CD4+ and CD8+ T lymphocytes in HLA-A11/DR1 Tg mice, we next examined the populations of HLA-regulated T lymphocytes in the periphery. The numbers of CD3+CD4+ and CD3+CD8+ T lymphocytes were counted by flow cytometry.
The isolated splenocytes were labeled with a PE-anti-mouse CD3 antibody, followed by a PEcy5-anti-mouse CD8 antibody. Figure 3A-D shows that 1.48% of the lymphocytes in HLA-A11/DR1 Tg mice, 3.32% of the lymphocytes in HLA-A11 Tg mice, and 1.16% of lymphocytes in HLA-DR1 Tg mice were CD8+ T cells. By contrast, 11.7% of lymphocytes in wild-type C57BL/6 mice were CD8+ T cells. Peripheral CD4+ T lymphocytes were labeled with a PE-anti-mouse CD3 antibody, followed by an APC-anti-mouse CD4 antibody. Figure 3E-H shows that 92.2% of the lymphocytes in HLA-A11/DR1 Tg mice, 62.6% in HLA-A11 Tg mice, and 94.8% in HLA-DR1 Tg mice were CD4+ T cells, compared with 64.6% in wild-type C57BL/6 mice.
Figure 3.
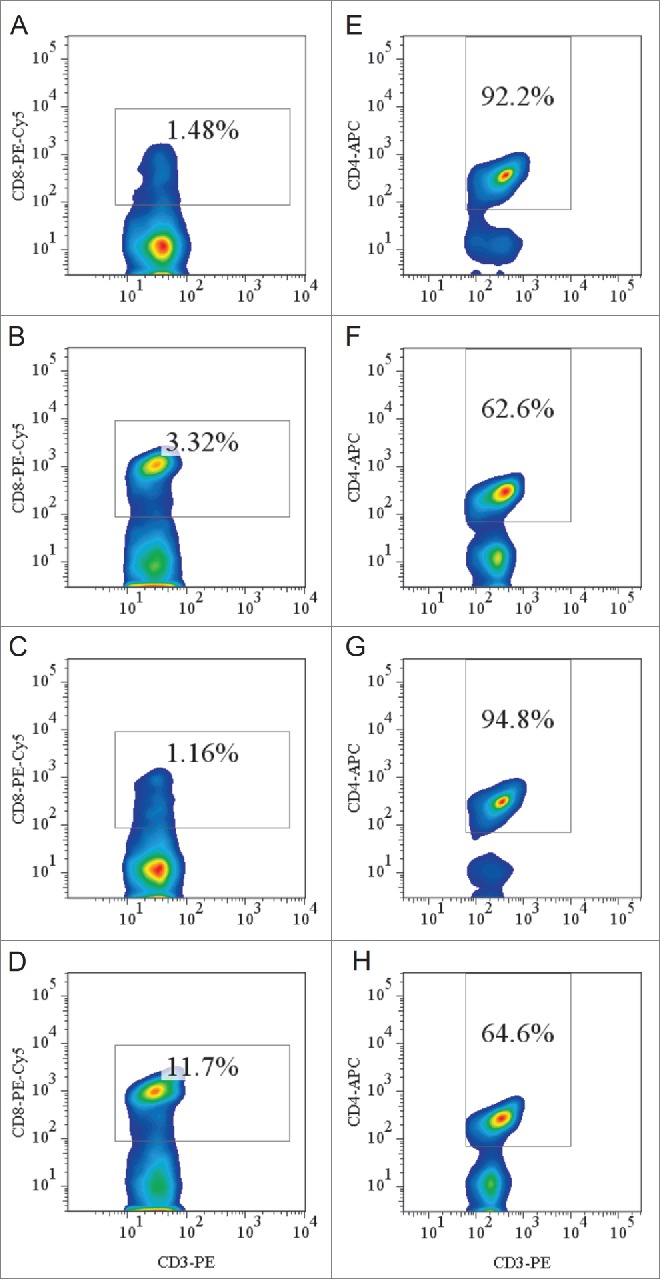
Flow cytometric analysis of peripheral CD8+ and CD4+ (T)lymphocytes. (A-D) Splenocytes from HLA-A11/DR1 mice (A), HLA-A11 mice (B), HLA-DR1 mice (C), and wild-type C57BL/6 mice (D) were isolated. CD3+ T lymphocytes were gated by staining with an FITC-anti-CD3 mAb and CD8+ T lymphocytes were gated by staining with a PEcy5-anti-CD8 mAb. (E-H) Splenocytes from HLA-A11/DR1 mice (E), HLA-A11 mice (F), HLA-DR1 mice (G), and wild-type C57BL/6 mice (H) were isolated. CD3+ T lymphocytes were gated by staining with a PE-anti-CD3 mAb, and CD4+ T lymphocytes were gated by staining with an APC-anti-CD4 mAb.
These results showed that the percentage of CD4+ T cells in HLA-A11/DR1 Tg mice were similar to that in wild-type C57BL/6 mice, while the number of CD8+ T cells in HLA-A11/DR1 Tg mice was lower than that in wild-type C57BL/6 mice. However, the lower percentage of CD8+ T cell was in accordance with that in HLA-A2/DR1 Tg mice or HLA-A2/DP4 Tg mice in which efficient humoral and cellular immune responses could be developed.17,18 Thus, HLA transgenes enabled the differentiation and maturation of CD4+ and CD8+ T lymphocytes in transgenic mice.
HLA-A11/DR1 Tg mice mount effective humoral and cellular responses
To examine the humoral and cellular responses to vaccination in HLA-A11/DR1 Tg mice, the mice were immunized with a recombinant HBV vaccine (derived from the HBV envelope protein28) or with a multi-epitope HIV-1-based candidate vaccine.29
The amount of HBsAg-specific IgG antibodies in the serum of HLA-A11/DR1 Tg mice was similar to that in the serum of wild-type C57BL/6 mice (Fig. 4A). It is worth noting that HLA-A11/DR1 Tg mice generated 4–5-fold more IFN-γ than wild-type mice, indicating a satisfactory cellular immune response and an ability to mimic the human cytotoxic response (Fig. 4B).
Figure 4.
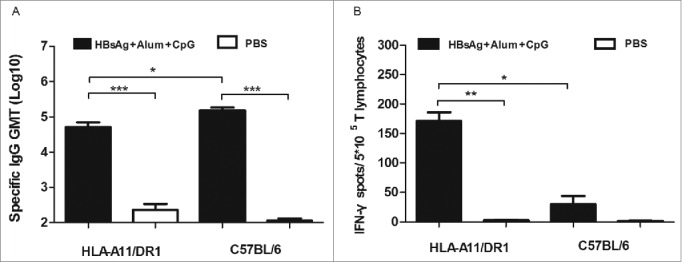
HBs-specific antibody- and cell-mediated responses after immunization with a recombinant HBsAg vaccine. (A) Sera were collected from immunized HLA-A11/DR1 Tg mice and wild-type C57BL/6 mice, and the titers of anti-HBs (IgG) antibodies were compared with those of PBS-immunized (hollow bar) mice in an ELISA test. (B) HBs epitope-specific IFN-γ production by cytotoxic T lymphocytes was examined by measuring the response of both HLA-A11/DR1 Tg mice and wild-type C57BL/6 mice to a recombinant HBs vaccine or PBS.
HIV-1 protein vaccine, and the amount of HIV-specific IgG antibodies and the amount of IFN-γ secreted by CD8+ T lym HIV-1 protein vaccine, and the amount of HIV-specific IgG antibodies and the amount of IFN-γ secreted by CD8+ T lymphocytes was measured respectively. The results revealed an increase (p<0.05) in the levels of specific IgG antibodies in HLA-A11/DR1 Tg mice (Fig. 5A). HLA-A11/DR1 Tg mice also secreted more IFN-γ than wild-type mice (Fig. 5B). These results suggest that HLA-A11/DR1 Tg mice show stronger cellular immune responses than C57BL/6 mice and are therefore a suitable model for evaluating vaccines.
Figure 5.
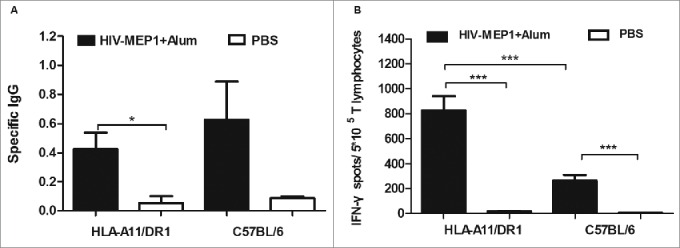
HIV-specific antibody and CD8+ (T)cell responses after immunization with a recombinant HIV-1 protein. (A) HIV-specific antibody titers in HLA-A11/DR1 Tg and C57BL/6 mice immunized with a recombinant HIV-1 protein or PBS. (B) HIV-specific IFN-γ production by cytotoxic T lymphocytes was examined by measuring responses to the recombinant HIV-1 protein in immunized mice (PBS-immunized mice were used as control).
HLA-A11/DR1 Tg mice show HLA-A11-restricted cytotoxic responses
To verify that HLA-A11/DR1 Tg mice mount a fully functional immune response, 2 known immunodominant HLA-A11-restricted epitopes, one derived from HIV-Pol177–188 (QMAVFIHNFKRK)30 and the other derived from HIV-GP16032–45 (KLWVTVYYGVPVWR),30 were used to measure IFN-γ production by CD8+ T lymphocytes from HLA-A11/DR1 Tg mice immunized with a recombinant HIV-1 protein vaccine.
Compared with those from wild-type C57BL/6 mice, CD8+T lymphocytes from HLA-A11/DR1 Tg mice were functionally restricted by the Tg human MHC class I molecules (HLA-A11). The HLA-A11-restricted HIV-specific CTL immune responses were specific for the HLA-A11-restricted-epitopes HIV-Pol177–188(Fig. 6A) and HIV-GP16032–45(Fig. 6B). Collectively, these data show that HLA-A11/DR1 Tg mice mount a functional HLA-A11 restricted immune response.
Figure 6.
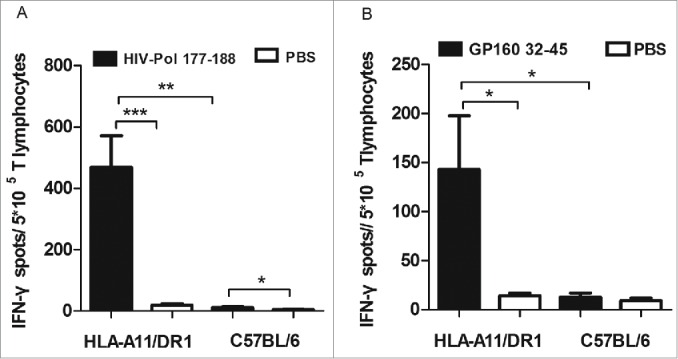
HLA-A11 restricted cytotoxic responses in HLA-A11 transgenic mice. Responses to (A) the HLA-A11-restricted epitope, HIV-Pol177–188, and (B) the HLA-A11-restricted epitope, HIV-GP32–45, were assessed in immunized HLA-A11 transgenic and wild-type C57BL/6 mice by counting the number of IFN-γ-secreting spots in ELISPOT assays.
Discussion
Vaccines based on multiple epitopes can be used to target well-defined ethnic populations and prime robust immune responses.30,31 To facilitate the evaluation and development of novel candidate epitope-based vaccines, we constructed a novel humanized HLA-A11/DR1 Tg mouse model expressing both HLA-A11 and HLA-DR1 molecules in the absence of H-2 class I/II molecules. Different from previous HLA-A2/DR117 and HLA-A2/DP4 Tg mouse models,18 HLA-A11/DR1 Tg mice showed combined expression of HLA-I allele A11 and HLA-II allele DR1, both of which are prevalent in the Chinese population. Because a quarter of the Chinese population express the HLA-A11 and HLA-DR1 genotypes,23 this novel animal model could be used to predict and evaluate the cellular responses of this Chinese population more accurately than previous transgenic mouse models.
The HLA system is the most polymorphic gene cluster in human beings. HLA class I and II alleles are expressed in a co-dominant manner in any particular individual. Competition among different HLA molecules results in the selection of different immunodominant epitopes that recognize and present processed antigens to immune cells. Examining the function of a single HLA molecule in the absence of competitive inhibition is a real challenge. Humanized mice have allowed the investigation of individual HLA molecules due to the integration of specific HLA alleles into the mouse genome. Indeed, double-Tg mice (expressing human HLA class I and class II molecules) in the absence of murine H-2 molecules have been developed.17,18
The phenotypic frequency of HLA-A11 in the Chinese population is about 20–30% compared with 10–15% in European, American, and Middle Eastern populations.23 Thus, much research focused on HLA-A11 Tg mice for evaluating HLA-A11-restricted epitope-based vaccines.32,33
To ensure that the transgenes functioned within the mouse genome, we designed a HLA-A11 HHD chimeric monochain expressing the α1/α2/β2m domains of HLA-A11 and the α3 domain of endogenous H-2. Replacing the human α3 domain with the mouse α3 domain preserved the species-specific binding affinity between the HLA-A11 chimeric monochain and the mouse CD8 molecule. Inactivating important components of the endogenous H-2 molecule prevented H-2 molecules from competing with the transgenic HLA molecules. Therefore, transgenic mice could only mount HLA-restricted immune responses.
Here, we confirmed the cell surface expression of HLA-I and HLA-II molecules by immune cells from HLA-A11 Tg mice. Although the percentage of CD8+ T lymphocytes in HLA-A11/DR1 mice was lower than that in the wild-type mice, the Tg HLA molecules efficiently presented antigens to immune cells to generate functional humoral and cellular immune responses. The low percentage of CD8+ T lymphocytes in HLA-A11/DR1 mice is in accordance with that in HLA-A2/DR1 Tg mice,17 HLA-A2/DP4 Tg mice,18 and other HLA class I transgenic mice models which also could develop efficient humoral and cellular immune responses similar to those in humans.34,35 Furthermore, studies show that the lower percentage of CD8+ T lymphocytes does not preclude the use of HLA-A2/DR1 Tg mice for studies of immune responses to pathogens such as HBV and HIV.34,36 Interesting, it is now well documented by several comparative studies that transgenic HLA class I molecules in chimeric monochain form are superior than traditional transgenic HLA class I molecule mice to induce more efficient T cell responses, even the number of CD8 T cell is lower.37,38
We also confirmed that HLA-A11/DR1 Tg mice mount efficient and functional humoral and cellular immune responses to a recombinant HBV vaccine and a recombinant HIV-1 protein, and especially mount the specific A11-restricted cellular immune response to a recombinant HIV-1 protein. In our HLA-A11/DR1 transgenic mice, the HLA-DR1 genes were derived from the HLA-A2/DR1 transgenic mice in which the HLA-DR1-restricted CD4+T cell responses were described with the HLA-DR1-restricted epitope HBsAg179–19417. Furthermore, by using the HLA-A2/DR1-transgenic, H-2 class I/class II KO mice, Pajot et al.36 identified 2 new HLA-DR1-restricted HIV-1 Gag p24-derived epitopes (Gag321–340 and Gag331–350) and confirmed the immunogenicity of 7 epitopes that had been described previously. In addition, it is well documented that in absence of MHC class II expression, there are no CD4+T cells and more interesting, there are also no humoral responses in the case of vaccination against HBV, as shown in HLA-A2 single transgenic H-2 class I/class II knock-out (KO) mice.36 In the present HLA-A11/DR1 transgenic mice, we confirmed the HLA-DR1-restricted epitope HBsAg179–194responses as reported in HLA-A2/DR1 transgenic mice (data no shown). Furthermore, we show an efficient specific antibodies responses (Fig. 4) which are reported highly protective against HBV viral infection. The results indicated that the novel HLA-A11/DR1 transgenic mice model has potential for evaluating both T cells (CD8 and CD4) and B cells activities as HLA-A2/DR1 transgenic mice, but covering different population. The two models are mutuel important to evaluate available commercial vaccines and novel vaccine candidates development.
In summary, we generated a novel HLA-A11/DR1 Tg mouse strain that faithfully reproduced a human immune response and may be useful for identifying protective epitopes, designing human vaccine and for evaluating vaccination strategies. The humanized HLA-A11/DR1 Tg mouse is a promising and versatile preclinical model that will facilitate the study of human immune responses to a variety of antigens.
Materials and methods
Construction of the HHD chimeric HLA-A11 transgenic plasmid
A 2873bp chimeric HLA-A*1101 (GenBank accession number, D16841.1) monochain fragment was synthesized using GenScript and subcloned into the pET32a plasmid via the SalI and KpnI restriction sites to generate pET32a-A11, which encompassed the human HLA-A11 leader sequence, the α1 and α2 domains, murine H-2Db(from the α3 domain to the COOH terminus), and human β2-microglobulin (β2m), which was covalently linked between the leader sequence and the 5′ end of the HLA-A11 (α1 and α2) domains via a 15 amino acid linker encoding Gly4Ser3.39 The mouse α3 domain within the chimeric HLA-A11 molecule facilitates interaction with mouse CD8 molecules.39
Generation of HHD chimeric HLA-A11 Tg mice
The purified HLA-A11 fragment was released from pET32a-A11 by digestion with SalI and KpnI and microinjected into pronuclei from fertilized (C57BL/6J × C57BL/6J) F1 mouse eggs to generate Tg embryos. The embryos were then re-implanted into pseudopregnant female mice. HLA-A11 Tg mice were identified by PCR of genomic DNA and by fluorescence activated cell sorting of T lymphocytes (see below). HLA-A11 Tg mice showing the highest expression of HLA-A11 were then crossed with HLA-A2/DR1 (HLA-A2+/+/DR01+/+/β2m−/−/IAβ−/−) Tg mice on a C57BL/6 background (originally obtained from Dr. Yuchun Lone17). Offsprings with the HLA-A2−/− A11+/+/DR1+/+/β2m−/−/IAβ−/− genotype were identified by PCR.
Mice were bred in the animal facility at the Laboratory Animal Center, Chinese Academy of Medical Sciences, Beijing, China. All experiments were performed according to the approved protocols and guidelines of the animal facility at the Institutional Animal Care and Use Committees of the Laboratory Animal Center, State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology (permit number, BIME 2014–0017), and the recommendations set out in the guide for the Care and Use of Laboratory Animals.
Genotype identification
HLA-A11 and HLA-DR1 transgenes and β2m/IAβ knockout mice were identified by PCR. Murine genomic DNA was extracted as described previously,17 and PCR was conducted using the following primers: HLA-A11 forward, 5′-CTGGGTTTCATCCATCCG-3′, and reverse, 5′-GATCGGTCTGGCTCTGAGC-3′; HLA-DR1 forward, 5′-GCTTCGAAATGGAAAACCTG-3′, and reverse, 5′-ATGTGCCTTACAGAGGCCCC-3′; mouse β2m forward, 5′-CTGAGCTCTGTTTTCGTCTG-3′, and reverse, 5′-CTTAACTCTGCAGGCGTATG-3′; H-2 IAβ forward, 5′-TTCGTGTACCAGTTCATGGG-3′, and reverse, 5′-TAGTTGTGTCTGCACACCGT-3′; and Neo55a, 5′-CCTGCCGAGAAAGTATCCA-3′.
Flow cytometry analysis
The mice from experimental and control mice were euthanized and the splenocytes were isolated. The expression of HLA-A11 and HLA-DR1 was examined by flow cytometry analysis with PE-anti-human HLA-ABC and FITC-anti-human HLA-DR antibodies (anti-HLA class I and anti-HLA class II, respectively; BioLegend, San Diego, CA, USA). Splenocytes isolated from wild-type C57BL/6 and H-2-I/II knockout mice were used as controls. To determine the percentage of CD4+ and CD8+cells, splenocytes were first labeled with a PE-anti-mouse CD3 antibody (BioLegend), followed by PE-cy5-anti-mouse CD8 or APC-anti-mouse CD4 (BioLegend). Splenocytes from wild-type C57BL/6 mice were used as the control.
Immunization of HLA-A11/DR1 Tg mice
Female HLA-A11/DR1 Tg mice or wild-type C57BL/6 mice (6–8 weeks old) received 3 intramuscular (i.m.) injections of recombinant HBV S antigen (1 μg) adsorbed to 100 μg of Al(OH)3 (alum adjuvant) in combination with 10 μg of CpG-ODNs (kindly provided by Dr.Honglin Xu28) (total injected volume, 100 μl). Each immunization was separated by an interval of 14 d. Sera were collected both before and after each immunization for serological analysis. Mice were sacrificed 10 d after the final boost, and the cytolytic responses to are combinant HBsAg vaccine were examined in an IFN-γ ELISPOT assay.
Female Tg and wild-type mice (6–8 weeks old) were anesthetized, and the tibialis anterior muscles were injected with 10 μg of recombinant HIV-1 protein in alum adjuvant; mice received 3 injections at 14 day intervals.29 The immunogenicity of 2 known HLA-A11-restricted epitopes derived from the HIV-Pol gene (aa177–188: QMAVFIHNFKRK)30 and the HIV-GP160 gene (aa32–45: KLWVTVYYGVPVWR)30 was examined by measuring the amount of IFN-γ secreted by CD8+ T lymphocytes from HLA-A11/DR1 Tg mice and wild-type C57BL/6 mice. Mice were sacrificed 10 d after the final boost, and the cytolytic responses and HLA-A11-restricted CTL responses to the recombinant HIV-1protein were determined in an ELISPOT assay.
Measurement of serum antibodies by ELISA
The serum levels of antibodies specific for the recombinant HBV vaccine and the recombinant HIV-1 protein in immunized HLA-A11/DR1 Tg mice and wild-type C57BL/6 mice were measured in ELISA. The plates coated with the HBV vaccine antigen or the recombinant HIV-1 protein were blocked with PBS supplemented with 0.1% Tween20 and 10% FCS and then washed 3 times. After the addition of mouse serum for 1 h, the plates were washed again, and bound antibodies were detected with HRP (horseradish peroxidase)-labeled anti-mouse IgG (Santa Cruz Biotechnology, Inc.). Absorbance was then measured at 450nm and 630nm in a plate reader. The antibody titers (the mean of at least 3 determinations) were calculated using the serial end-point dilution method.40
ELISPOT assay
An ELISPOT assay was performed to detect IFN-γ secreted by CD8+ T lymphocytes with BD™ ELISPOT kit (BD Biosciences, CA). Briefly, 96-well ELISPOT plates were coated with an anti-IFN-γ mAb overnight at 4°C and then blocked with blocking solution. Splenocytes (5 × 105) were added to each well and cultured in the presence of recombinant HBV vaccine, synthetic peptides, or recombinant HIV-1 protein (all at 10 μg/ml) and incubated at 37°C/5% CO2 for 48 h. IFN-γ-secreting cells were captured by an anti-IFN-γ mAb and detected by incubation with a biotinylated anti-IFN-γ mAb for 2 h at 37°C, followed by streptavidin-HRP for 1 h. The plates were then developed for 5–10 minutes in an ACE substrate solution (BD Biosciences, CA), washed 3 times, and air-dried at room temperature. Positive spots were counted using an ELISPOT plate reader (Cellular Technology Ltd).
Statistical analysis
Each experiment was repeated at least 3 times, and data were expressed as the mean ± SEM. The means or geometric means of multiple groups were compared using Student's t-test. All statistical analysis was performed using GraphPad Prism version 5.0. A P value <0.05 was considered statistically significant.
Abbreviations
- MHC
major histocompatibility complex
- HLA
human leukocyte antigen
- CTL
cytotoxic T lymphocyte
- HIV
human immunodeficiency virus
- HBV
hepatitis B virus
Funding
This work was supported by the National Program of Infectious Diseases (No. 2012ZX10004–502 and No.2011ZXJ09201–031), the Basic Research 973 Project (2011CB504706).
Disclosure of potential conflicts of interest
All authors agree to submit our manuscript to Human Vaccines & Immunotherapeutics. None of the authors have any conflicts of interest to declare.
References
- [1].Viret C, Janeway CA Jr. MHC and T cell development. Rev Immunogenet 1999; 1:91-104; PMID:11256575 [PubMed] [Google Scholar]
- [2].Sprent J, Gao EK, Webb SR. T cell reactivity to MHC molecules: immunity versus tolerance. Science 1990; 248:1357-63; PMID:1694041; http://dx.doi.org/ 10.1126/science.1694041 [DOI] [PubMed] [Google Scholar]
- [3].Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol 2003; 15:461-70; PMID:12900280; http://dx.doi.org/ 10.1016/S0952-7915(03)00083-9 [DOI] [PubMed] [Google Scholar]
- [4].Sette A, Livingston B, McKinney D, Appella E, Fikes J, Sidney J, Newman M, Chesnut R. The development of multi-epitope vaccines: epitope identification, vaccine design and clinical evaluation. Biologicals 2001; 29:271-6; PMID:11851327; http://dx.doi.org/ 10.1006/biol.2001.0297 [DOI] [PubMed] [Google Scholar]
- [5].Comber JD, Philip R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther Adv Vaccines 2014; 2:77-89; PMID:24790732; http://dx.doi.org/ 10.1177/2051013614525375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011; 12:509-17; PMID:21739679; http://dx.doi.org/ 10.1038/ni.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol 2011; 29:294-306; PMID:21459467; http://dx.doi.org/ 10.1016/j.tibtech.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oyarzun P, Ellis JJ, Gonzalez-Galarza FF, Jones AR, Middleton D, Boden M, Kobe B. A bioinformatics tool for epitope-based vaccine design that accounts for human ethnic diversity: application to emerging infectious diseases. Vaccine 2015; 33:1267-73; PMID:25629524; http://dx.doi.org/ 10.1016/j.vaccine.2015.01.040 [DOI] [PubMed] [Google Scholar]
- [9].Himoudi N, Abraham JD, Fournillier A, Lone YC, Joubert A, Op De Beeck A, Freida D, Lemonnier F, Kieny MP, Inchauspé G. Comparative vaccine studies in HLA-A2.1-transgenic mice reveal a clustered organization of epitopes presented in hepatitis C virus natural infection. J Virol 2002; 76:12735-46; PMID:12438599; http://dx.doi.org/ 10.1128/JVI.76.24.12735-12746.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Healey GD, Elvin SJ, Morton M, Williamson ED. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance postinfection. Infect Immun 2005; 73:5945-51; PMID:16113315; http://dx.doi.org/ 10.1128/IAI.73.9.5945-5951.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raffegerst SH, Hoelzlwimmer G, Kunder S, Mysliwietz J, Quintanilla-Martinez L, Schendel DJ. Diverse hematological malignancies including hodgkin-like lymphomas develop in chimeric MHC class II transgenic mice. PLoS One 2009; 4:e8539; PMID:20046882; http://dx.doi.org/ 10.1371/journal.pone.0008539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boesen A, Sundar K, Coico R. Lassa fever virus peptides predicted by computational analysis induce epitope-specific cytotoxic-T-lymphocyte responses in HLA-A2.1 transgenic mice. Clin Diagn Lab Immunol 2005; 12:1223-30; PMID:16210487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ishioka GY, Fikes J, Hermanson G, Livingston B, Crimi C, Qin M, del Guercio MF, Oseroff C, Dahlberg C, Alexander J, et al.. Utilization of MHC class I transgenic mice for development of minigene DNA vaccines encoding multiple HLA-restricted CTL epitopes. J Immunol 1999; 162:3915-25; PMID:10201910 [PubMed] [Google Scholar]
- [14].Pajot A, Michel ML, Mancini-Bourgine M, Ungeheuer MN, Ojcius DM, Deng Q, Lemonnier FA, Lone YC. Identification of novel HLA-DR1-restricted epitopes from the hepatitis B virus envelope protein in mice expressing HLA-DR1 and vaccinated human subjects. Microbes Infect 2006; 8:2783-90; PMID:17045504; http://dx.doi.org/ 10.1016/j.micinf.2006.08.009 [DOI] [PubMed] [Google Scholar]
- [15].BenMohamed L, Krishnan R, Longmate J, Auge C, Low L, Primus J, Diamond DJ. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum Immunol 2000; 61:764-79; PMID:10980387; http://dx.doi.org/ 10.1016/S0198-8859(00)00139-7 [DOI] [PubMed] [Google Scholar]
- [16].Wasik TJ, Wierzbicki A, Whiteman VE, Trinchieri G, Lischner HW, Kozbor D. Association between HIV-specific T helper responses and CTL activities in pediatric AIDS. Eur J Immunol 2000; 30:117-27; PMID:10602033; http://dx.doi.org/ 10.1002/1521-4141(200001)30:1%3c117::AID-IMMU117%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- [17].Pajot A, Michel ML, Fazilleau N, Pancre V, Auriault C, Ojcius DM, Lemonnier FA, Lone YC. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1-transgenic H-2 class I-/class II-knockout mice. Eur J Immunol 2004; 34:3060-9; PMID:15468058; http://dx.doi.org/ 10.1002/eji.200425463 [DOI] [PubMed] [Google Scholar]
- [18].Ru Z, Xiao W, Pajot A, Kou Z, Sun S, Maillere B, Zhao G, Ojcius DM, Lone YC, Zhou Y. Development of a humanized HLA-A2.1/DP4 transgenic mouse model and the use of this model to map HLA-DP4-restricted epitopes of HBV envelope protein. PLoS One 2012; 7:e32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dion S, Bourgine M, Godon O, Levillayer F, Michel ML. Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules. J Virol 2013; 87:5554-63; PMID:23468504; http://dx.doi.org/ 10.1128/JVI.03134-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reiser M, Wieland A, Plachter B, Mertens T, Greiner J, Schirmbeck R. The immunodominant CD8 T cell response to the human cytomegalovirus tegument phosphoprotein pp65(495-503) epitope critically depends on CD4 T cell help in vaccinated HLA-A*0201 transgenic mice. J Immunol 2011; 187:2172-80; PMID:21810614; http://dx.doi.org/ 10.4049/jimmunol.1002512 [DOI] [PubMed] [Google Scholar]
- [21].Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother 2011; 34:569-80; PMID:21904219; http://dx.doi.org/ 10.1097/CJI.0b013e31822b5b1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Smith HA, Cronk RJ, Lang JM, McNeel DG. Expression and immunotherapeutic targeting of the SSX family of cancer-testis antigens in prostate cancer. Cancer Res 2011; 71:6785-95; PMID:21880588; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2127 [DOI] [PubMed] [Google Scholar]
- [23].Lee TD, Zhao TM, Mickey R, Sun YP, Lee G, Song CX, Cheng DZ, Zhou S, Ding SQ, Cheng DX, et al.. The polymorphism of HLA antigens in the Chinese. Tissue Antigens 1988; 32:188-208; PMID:3217935; http://dx.doi.org/ 10.1111/j.1399-0039.1988.tb01656.x [DOI] [PubMed] [Google Scholar]
- [24].Alexander J, Oseroff C, Sidney J, Wentworth P, Keogh E, Hermanson G, Chisari FV, Kubo RT, Grey HM, Sette A. Derivation of HLA-A11/Kb transgenic mice: functional CTL repertoire and recognition of human A11-restricted CTL epitopes. J Immunol 1997; 159:4753-61; PMID:9366399 [PubMed] [Google Scholar]
- [25].Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, et al.. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J Immunol 2011; 187:4268-79; PMID:21918184; http://dx.doi.org/ 10.4049/jimmunol.1101970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ureta-Vidal A, Firat H, Perarnau B, Lemonnier FA. Phenotypical and functional characterization of the CD8+ T cell repertoire of HLA-A2.1 transgenic, H-2Kb null Db null double knockout mice. J Immunol 1999; 163:2555-60; PMID:10452993 [PubMed] [Google Scholar]
- [27].Firat H, Cochet M, Rohrlich PS, Garcia-Pons F, Darche S, Danos O, Lemonnier FA, Langlade-Demoyen P. Comparative analysis of the CD8(+) T cell repertoires of H-2 class I wild-type/HLA-A2.1 and H-2 class I knockout/HLA-A2.1 transgenic mice. Int Immunol 2002; 14:925-34; PMID:12147629; http://dx.doi.org/ 10.1093/intimm/dxf056 [DOI] [PubMed] [Google Scholar]
- [28].Liu Y, Luo X, Yang C, Yu S, Xu H. Three CpG oligodeoxynucleotide classes differentially enhance antigen-specific humoral and cellular immune responses in mice. Vaccine 2011; 29:5778-84; PMID:21664398; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.087 [DOI] [PubMed] [Google Scholar]
- [29].Yang Y, Sun W, Guo J, Zhao G, Sun S, Yu H, Guo Y, Li J, Jin X, Du L, et al.. In silico design of a DNA-based HIV-1 multi-epitope vaccine for Chinese populations. Hum Vaccin Immunother 2015; 11:795-805; PMID:25839222; http://dx.doi.org/ 10.1080/21645515.2015.1012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Groot AS, Jesdale B, Martin W, Saint Aubin C, Sbai H, Bosma A, Lieberman J, Skowron G, Mansourati F, Mayer KH. Mapping cross-clade HIV-1 vaccine epitopes using a bioinformatics approach. Vaccine 2003; 21:4486-504; PMID:14505932; http://dx.doi.org/ 10.1016/S0264-410X(03)00390-6 [DOI] [PubMed] [Google Scholar]
- [31].Zhao L, Zhang M, Cong H. Advances in the study of HLA-restricted epitope vaccines. Hum Vaccin Immunother 2013; 9:2566-77; PMID:23955319; http://dx.doi.org/ 10.4161/hv.26088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen HW, Leng CH, Liu HY, Cheng WF, Chang YW, Wu PY, Lien SP, Huang TY, Chiang SK, Lin MH, et al.. Identification of HLA-A11-restricted CTL epitopes derived from HPV type 18 using DNA immunization. Cancer Biol Ther 2009; 8:2025-32; PMID:19738415; http://dx.doi.org/ 10.4161/cbt.8.21.9732 [DOI] [PubMed] [Google Scholar]
- [33].Gavioli R, Kurilla MG, de Campos-Lima PO, Wallace LE, Dolcetti R, Murray RJ, Rickinson AB, Masucci MG. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol 1993; 67:1572-8; PMID:7679748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Deng Q, Mancini-Bourgine M, Zhang X, Cumont MC, Zhu R, Lone YC, Michel ML. Hepatitis B virus as a gene delivery vector activating foreign antigenic T cell response that abrogates viral expression in mouse models. Hepatology 2009; 50:1380-91; PMID:19821533; http://dx.doi.org/ 10.1002/hep.23150 [DOI] [PubMed] [Google Scholar]
- [35].Boucherma R, Kridane-Miledi H, Bouziat R, Rasmussen M, Gatard T, Langa-Vives F, Lemercier B, Lim A, Bérard M, Benmohamed L, et al.. HLA-A*01:03, HLA-A*24:02, HLA-B*08:01, HLA-B*27:05, HLA-B*35:01, HLA-B*44:02, and HLA-C*07:01 monochain transgenic/H-2 class I null mice: novel versatile preclinical models of human T cell responses. J Immunol 2013; 191:583-93; PMID:23776170; http://dx.doi.org/ 10.4049/jimmunol.1300483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pajot A, Schnuriger A, Moris A, Rodallec A, Ojcius DM, Autran B, Lemonnier FA, Lone YC. The Th1 immune response against HIV-1 Gag p24-derived peptides in mice expressing HLA-A02.01 and HLA-DR1. Eur J Immunol 2007; 37:2635-44; PMID:17668896; http://dx.doi.org/ 10.1002/eji.200636819 [DOI] [PubMed] [Google Scholar]
- [37].Ramage JM, Metheringham R, Moss R, Spendlove I, Rees R, Durrant LG. Comparison of the immune response to a self antigen after DNA immunisation of HLA*A201/H-2Kb and HHD transgenic mice. Vaccine 2004;22:1728-31; PMID:15068856; http://dx.doi.org/ 10.1016/j.vaccine.2004.01.034 [DOI] [PubMed] [Google Scholar]
- [38].Firat H, Cochet M, Rohrlich PS, Garcia-Pons F, Darche S, Danos O, Lemonnier FA, Langlade-Demoyen P. Comparative analysis of the CD8(+) T cell repertoires of H-2 class I wild-type/HLA-A2.1 and H-2 class I knockout/HLA-A2.1 transgenic mice. Int Immunol. 2002;14:925-34; PMID:12147629; http://dx.doi.org/ 10.1093/intimm/dxf056 [DOI] [PubMed] [Google Scholar]
- [39].Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 1997; 185:2043-51; PMID:9182675; http://dx.doi.org/ 10.1084/jem.185.12.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang C, Shi H, Zhou J, Liang Y, Xu H. CpG oligodeoxynucleotides are a potent adjuvant for an inactivated polio vaccine produced from Sabin strains of poliovirus. Vaccine 2009; 27:6558-63; PMID:19729087; http://dx.doi.org/ 10.1016/j.vaccine.2009.08.047 [DOI] [PubMed] [Google Scholar]


