abstract
Background. Improving HPV vaccination coverage in the US will require healthcare providers to recommend the vaccine more effectively. To inform quality improvement efforts, we systematically reviewed studies of provider communication about HPV vaccination. Methods. We searched MEDLINE, CINAHL, EMBASE, and POPLINE in August 2015 to identify studies of provider communication about HPV vaccination. Results. We identified 101 qualitative and quantitative studies. Providers less often recommended HPV vaccine if they were uncomfortable discussing sex, perceived parents as hesitant, or believed patients to be low risk. Patients less often received recommendations if they were younger, male, or from racial/ethnic minorities. Despite parents' preference for unambiguous recommendations, providers often sent mixed messages by failing to endorse HPV vaccine strongly, differentiating it from other vaccines, and presenting it as an “optional” vaccine that could be delayed. Conclusion. Interventions are needed to help providers deliver effective recommendations in the complex communication environment surrounding HPV vaccination.
Keywords: adolescent health, health communication, papillomavirus vaccines, physician-patient relations
Introduction
Improving the delivery of human papillomavirus (HPV) vaccine is a public health priority in the United States.1,2 Despite national guidelines for the routine administration of HPV vaccine to 11- and 12-year-old adolescents, only 40% of girls and 22% of boys completed the 3-dose series in 2014.3 These persistently low levels of coverage have prompted a rapid growth in the research literature on determinants of HPV vaccination, and this work has consistently highlighted the powerful influence of healthcare providers' communication. Adolescents who receive a provider's recommendation have substantially higher odds of initiating HPV vaccination compared to those who do not.4-7 However, around half of age-eligible adolescents do not receive recommendations.8-10 Furthermore, a growing body of research suggests that those recommendation adolescents do receive are weaker than recommendations for other adolescent vaccines, such as tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal vaccines.11,12 Understanding and improving provider communication, particularly with regard to recommendations, is imperative to increasing HPV vaccination coverage.
To inform quality improvement efforts in this area, we systematically reviewed the literature on provider communication about HPV vaccination. We organized our review using the classic communication constructs of source (where a message originates), audience (the receiver), message (what is communicated), channel (the medium used to convey the message), and context (where and when communication occurs).13,14 With regard to source and audience, our aims were to understand which providers are preferred sources of information, identify factors associated with the delivery and receipt of recommendations, and characterize the communication roles played by patients and parents in clinical decision making. We additionally sought to assess provider communication about HPV vaccination in terms of message content and style, as well as channels used to educate about HPV vaccination in clinical settings. Finally, we aimed to describe the context of HPV vaccine communication to understand how policy environments, clinical systems, and health communication interventions facilitate or constrain communication.
Methods
We searched four databases (MEDLINE, CINAHL, EMBASE, and POPLINE) in August 2015 to identify studies related to provider communication about HPV vaccination. Searches varied by database, but all used combinations of terms related to HPV, vaccination, and communication. For example, we conducted a search of MEDLINE using the following terms: (“Papillomavirus Vaccines”[mesh] OR ((hpv[tiab] OR papilloma[tiab] OR papillomavirus[tiab]) AND (vaccin*[tiab] OR immuniz*[tiab])) OR gardasil[tiab]) AND (“Health Communication”[mesh] OR “Physician-Patient Relations”[Mesh] OR communicat*[tiab] OR recommend*[tiab] OR deci*[tiab]). We identified additional studies by soliciting in-press and unpublished papers from research groups publishing in this area and by checking reference lists of papers included in our review.
We reviewed studies for inclusion using a two-step process. First, one author (MBG) reviewed paper titles and abstracts to identify relevant studies, and another (ALM) checked these determinations. Second, we conducted full-text reviews of identified studies. In this step, one author (MBG or ALM) further assessed each study's eligibility and coded eligible studies using an abstraction form. The other author checked the data. For each step, authors resolved questions and disagreements in coding through discussion.
Eligible studies were those that used data from a US sample to report quantitative or qualitative findings related to provider communication about HPV vaccination. We defined “provider communication” to include in-person dialog among patients, parents, and providers as well as the use of educational materials or other communication interventions in a clinical setting. For the purposes of this study, “clinical settings” included primary care practices or clinics, school-based health clinics, public health department clinics that deliver vaccines, and pharmacies. We limited our review to studies with US samples to account for the unique practice and policy environments that influence HPV vaccination in this country. Excluded studies were those that reported on: only the association between receiving a provider recommendation and HPV vaccine uptake; message framing without an explicitly stated clinical context; communication in settings, such as dental offices, that do not stock HPV vaccine; reminder/recall interventions; or quality improvement interventions that did not evaluate changes in provider communication.
Results
The search yielded 2,175 unduplicated articles, 101 of which met eligibility criteria and were included in our review (Fig. 1, Table 1). Most studies (67%) were quantitative, primarily using cross-sectional survey designs. About one-third (33%) of included studies used qualitative or mixed methods. Only one study examined recorded dialog among providers, patients, and parents. A majority of studies were conducted with either health care providers (53%) or with parents (42%); about one-quarter included adolescents or young adults (25%). Most studies focused on communication about HPV vaccination for only adolescent girls (53%) with fewer focusing on either boys specifically (11%) or on both boys and girls (36%).
Figure 1.
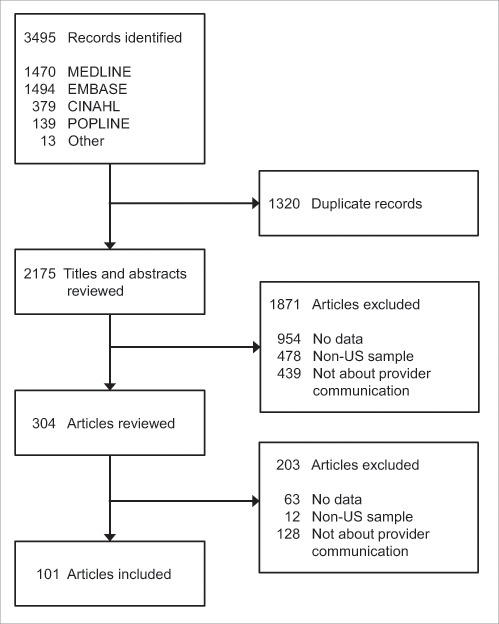
Flow diagram of included and excluded articles.
Table 1.
Characteristics of included studies.
| Guidelines during data collectiona |
Communication constructs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Study design | Females | Males | Sexb | N | Sample population | Source | Audience | Message | Channel | Context |
| Alexander, 201276 | Qualitative, in-person interviews | rout | rout | M | 42 | Unvaccinated adolescent males (ages 13–17) and their parents (21 dyads) recruited from clinics in a mid-western city | • | • | |||
| Alexander, 201421 | Qualitative, in-person interviews | rout | rout | M | 42 | Unvaccinated adolescent males (ages 13–17) and their parents (21 dyads) recruited from clinics in a mid-western city | • | • | |||
| Alexander, 201527 | Qualitative, in-person interviews | rout | rout | M | 20 | Pediatricians serving low income families in a mid-western city | • | • | • | • | |
| Allen, 201271 | Qualitative, focus groups | rout | pre | F | 64 | Parents of girls (ages 9–17) recruited though health and social service agencies in Boston, MA | • | • | |||
| Allison, 201344 | Cross-sectional, mail and online survey | rout | perm | M | 609 | Nationally-representative sample of pediatricians and family physicians | • | • | • | ||
| Aragones, 201598 | Qualitative, focus groups | rout | rout | F, M | 36 | Latino immigrant parents of unvaccinated children (ages 9–17) | • | ||||
| Barnack, 201048 | Cross-sectional, online survey | rout | pre | F, M | 200 | Physicians (pediatricians, primary care physicians and gynecologists; n = 100) and parents of children (ages 7–17; n = 100) | • | ||||
| Bednarczyk, 201172 | Cross-sectional, in-person survey | rout | perm | F | 795 | Women recruited from a university health clinic and classroom settings | • | ||||
| Bhatta, 201555 | Cross-sectional, in-person survey | rout | rout | F, M | 1299 | Middle and high school students (ages 11–18) in a rural Appalachian Ohio county | • | • | |||
| Bruno, 201449 | Cross-sectional, mail survey | rout | perm, rout | F, M | 121 | Primary care providers (pediatricians, family physicians, and internists) serving minority populations in Brooklyn, NY | • | ||||
| Bynum, 201432 | Cross-sectional, mail survey | rout | pre | F | 433 | Florida-based physicians serving Medicaid-enrolled females (ages 9–17) | • | ||||
| Cates, 2011100 | Cross-sectional, mail and email survey | rout | pre. perm | F | 260 | Mothers of adolescent daughters (ages 9–18) (n = 225) and healthcare providers (n = 35) in 4 NC counties | • | • | |||
| Clark, 201584 | Cross-sectional, online survey | rout | rout | M | 809 | Nationally-representative sample of parents of adolescent sons (ages 9–17) | • | ||||
| Daley, 200629 | Cross sectional mail and online survey | pre | pre | F | 294 | National sample of pediatricians | • | • | • | ||
| Daley, 201035 | Cross-sectional, online or mail survey | rout | pre | F | 780 | Nationally-representative sample of pediatricians and family physicians | • | • | • | • | |
| Dempsey, 200980 | Qualitative analysis of cross-sectional, telephone survey | rout | pre | F | 52 | Mothers of adolescent daughters (ages 11–17) seen within the University of Michigan health care system | • | • | |||
| Dempsey, 201515 | Cross sectional, online survey | rout | rout | F | 175 | Women (ages 15–26) attending one of 9 OB/GYN offices in CO | • | ||||
| Dempsey, 201687 | Cross-sectional, mail survey | rout | rout | F, M | 356 | Parents of children (ages 9–14) in Denver, CO metro area | • | • | |||
| Dorrell, 20124 | Cross-sectional telephone survey | rout | perm | F, M | 8652 | National sample of parents of adolescents (ages 13–17) | • | • | |||
| Fontenot, 2013105 | Cross-sectional, online survey | rout | rout | F, M | 519 | Forensic nurses who were members of the International Association of Forensic Nurses (95% from the US) | • | ||||
| Ford, 2014108 | Cross-sectional telephone survey | rout | pre | F, M | 263 | Adolescent health professionals (medical providers and public health practitioners) in 43 states | • | ||||
| Getrich, 201473 | Mixed methods: Cross-sectional survey; qualitative interviews | rout | pre | F | 30 | Clinicians (n = 8), Hispanic mothers (n = 10) and adolescent females (ages 12–17) (n = 12) in New Mexico | • | • | • | ||
| Gilkey, 201633 | Cross-sectional, online survey | rout | rout | F, M | 776 | National sample of pediatricians and family physicians | • | • | • | ||
| Gilkey, 201511 | Cross-sectional, online survey | rout | rout | F, M | 776 | National sample of pediatricians and family physicians | • | • | • | ||
| Gilkey, 201660 | Cross-sectional, online survey | rout | rout | F, M | 1495 | National sample of parents of adolescents (ages 11–17) | • | • | |||
| Gilkey, under review16 | Cross-sectional, online survey | rout | rout | F, M | 1484 | National sample of parents of adolescents (ages 11–17) | • | • | • | • | |
| Goff, 201179 | Mixed-methods analysis of audio-recorded clinical encounters | rout | pre | F | 184 | Clinical encounters between physicians and their female patients (ages 11–26) | • | • | |||
| Greenfield, 201517 | Cross-sectional, in-person survey; qualitative focus groups | rout | rout | F, M | 222 | WA-based samples of Hispanic, Somalai, and Ethiopian/Eritrian adolescents (survey, n = 45) and parents (survey, n = 157); mothers of children (ages 11–18; focus groups, n = 27); providers (n = 20) | • | • | • | ||
| Griffioen, 201218 | Qualitative, in-person interviews | rout | – | F | 65 | Females (ages 11–12; n = 33) and their mothers (n = 32) recruited from an FQHC in Chicago, IL | • | • | • | ||
| Hamlish, 201277 | Qualitative, in-person interviews | rout | – | F | 38 | African-American females (ages 9–18) attending school in Chicago, IL or northern IN and mothers (19 dyads) | • | • | |||
| Head, 201394 | Qualitative, in-person interviews | rout | perm | F | 8 | Nurses and a physician in rural Appalachian KY | • | • | • | ||
| Hughes, 201174 | Qualitative, in-person interviews | rout | pre | F | 60 | Mother-daughter-clinician triads recruited from practices in a multi-state, primary care practice-based research network | • | • | • | ||
| Ishibashi, 200847 | Cross-sectional, online survey | rout | pre | F | 373 | Random, national sample of pediatricians | • | • | • | ||
| Jensen, 200940 | Cross-sectional mail survey | rout | pre | F, M | 204 | Physicians, nurse practitioners, and physician's assistants in Dane County, WI | • | • | • | ||
| Jim, 2012101 | Cross-sectional, online survey; qualitative in-person interviews | rout | pre, perm | F | 319 | Health care providers in all 12 Indian Health Service (HIS) areas (survey, n = 269; interviews, n = 51) | • | ||||
| Kahn, 200923 | Cross-sectional, online survey | rout | pre | F, M | 1122 | Statewide sample of physicians (pediatricians, family medicine, OB/GYN, internal medicine) in TX | • | • | |||
| Kahn, 200524 | Cross-sectional, mail survey | pre | pre | F, M | 513 | Random, national sample of pediatricians | • | • | |||
| Kahn, 200737 | Qualitative in-person interviews | pre | pre | F, M | 31 | Pediatricians practicing in OH, KY, and IN | • | • | |||
| Katz, 2009102 | Cross-sectional telephone interviews; immunization database review | rout | pre | F | 234 | Health department personnel in 7 Appalachian states | • | ||||
| Kepka, 201252 | Cross-sectional survey | rout | pre | F | 421 | National sample of primary care providers (including OB/GYBN, family practice physicians, internists, mid-level providers) | • | • | |||
| Kester, 201388 | Cross-sectional, online survey | rout | perm | F | 501 | National sample of mothers of adolescent females (ages 14–17) | • | ||||
| Klosky, 201556 | Cross-sectional, in-person written survey | rout | perm | F | 344 | Young adult, female cancer survivors (ages 18–26, n = 114) and maternal caregivers of adolescent female survivors (ages 9–17; n = 230) | • | ||||
| Kramer, 201266 | Cross-sectional, telephone survey | rout | pre | F | 17,264 | National, population-based sample of parents of adolescent females (ages 12–17) | • | ||||
| Krieger, 201246 | Cross-sectional, mail survey | rout | pre | F | 334 | Pediatricians with practices in Appalachia or in non-Appalachia KY and WV | • | ||||
| Kulczycki, 201534 | Cross-sectional mail and online survey | rout | perm | F | 301 | Pediatricians and family physicians from Alabama, Mississippi, and 10 randomly selected states | • | • | |||
| Lau, 20127 | Cross-sectional telephone survey | rout | pre | F | 16462 | National sample of parents of adolescent daughters (ages 12–17) | • | ||||
| Luque, 2012104 | Qualitative in-person interviews | rout | pre | F | 63 | Mexican and Honduran female farmworkers (n = 46) and health care workers (n = 17) recruited from FQHCs | • | ||||
| Luque, 201457 | Cross-sectional mail and online surveys | rout | perm | F, M | 217 | Physicians in GA (pediatricians, family medicine, OB/GYN, and other clinical specialties) | • | ||||
| Malo, 201430 | Cross-sectional mail survey | rout | perm | M | 728 | Nationally-representative samples of pediatricians and family physicians | • | ||||
| Malo, 201622 | Cross-sectional mail survey | rout | perm, rout | M | 355 | Nationally-representative samples of pediatricians and family physicians | • | • | • | • | |
| Mayne, 2012109 | Cross-sectional, telephone survey | rout | perm | F | 162 | Parents of adolescent girls (ages 11–17) who were due for HPV vaccine and had a recent primary care visit | • | ||||
| McRee, 201412 | Cross-sectional, online survey | rout | rout | F, M | 615 | Statewide sample of physicians (pediatricians and family physicians) and nurse practitioners in MN | • | • | • | • | • |
| Mehta, 201262 | Cross-sectional, telephone interviews; medical record reviews | rout | pre | F | 269 | Women diagnosed with precancerous cervical lesions, identified through pathology lab in New Haven County, CT | • | ||||
| Morales-Campos, 201378 | Qualitative focus groups | rout | pre | F | 52 | Hispanic adolescent girls (ages 14–18; n = 28) and mothers (n = 24) at an urban school district in southeast TX | • | • | |||
| Moss, under review92 | Cross-sectional, phone survey | rout | pre, perm | F, M | 9021 | Nationally-representative sample of parents of adolescents (ages 13–17) | • | ||||
| Moss, 201693 | Cross-sectional, phone survey | rout | pre | F | 4124 | Nationally-representative sample of parents of daughters (ages 13–17) | • | ||||
| Mullins, 201382 | Cross-sectional (baseline), qualitative interviews | rout | pre | F | 84 | Girls (ages11–12) who had initiated HPV vaccination (n = 33), their mothers (n = 32), and their clinicians (n = 19) (33 triads) | • | ||||
| Niccolai, 201483 | Qualitative, in-person interviews | rout | rout | F, M | 38 | Clinic-based sample of parents of children (female or male; ages 10–18) in northeastern US | • | ||||
| Niccolai, 201591 | Qualitative, in-person interviews | rout | rout | F, M | 38 | Clinic-based sample of parents of children (female or male; ages 10–18) in northeastern US | • | ||||
| Perkins, 201241 | Qualitative, in-person interviews | rout | – | F | 34 | Pediatric and family medicine providers (physicians and nurse practitioners) | • | • | • | ||
| Perkins, 201228 | Qualitative, in-person interviews | rout | pre, perm | F, M | 31 | Physicians and nurse practitioners at 4 community health centers in Boston, MA | • | • | |||
| Perkins, 201353 | Cross-sectional, mail survey | rout | perm, rout | F | 366 | Random, national sample of OB/GYNs | • | • | • | ||
| Perkins, 201397 | Qualitative, in-person interviews | rout | perm, rout | F | 120 | Clinic-based sample of parents of adolescent sons (ages 11–17) | • | ||||
| Perkins, 201438 | Qualitative, in-person interviews | rout | rout | F, M | 161 | Adolescent males and females ages 11–21 (n = 124) and health care providers (n = 36) at 8 neighborhood health centers in Boston, MA | • | • | • | • | |
| Perkins, 201685 | Qualitative interviews | rout | rout | F | 65 | Clinic-based sample of parents of daughters (ages 11–17) who received at least one dose of HPV vaccine | • | ||||
| Polonijo, 201367 | Yearly cross-sectional telephone survey | rout | pre, perm | F | 41,358 | Nationally-representative samples of parents of adolescents (ages 13–17) | • | ||||
| Quinn, 201263 | Qualitative analysis of open-ended items on a cross-sectional, mail survey | rout | pre | F | 112 | National sample of pediatricians, family physicians and OB/GYNs | • | ||||
| Rahman, 201565 | Cross-sectional, telephone survey | rout | perm, rout | F, M | 23,564 | National sample of adolescents (ages 13–17) | • | ||||
| Raley, 200442 | Cross-sectional survey | pre | pre | F | 181 | Random sample of American College of Gynecology fellows | • | • | • | ||
| Rand, 201181 | Cross-sectional telephone survey | rout | pre | F, M | 638 | Parents with children (ages 11–17; n = 430) and the older daughters/sons (ages 15–17; n = 208) recruited at 9 primary care practices in Monroe County, New York | • | ||||
| Reimer, 201464 | Cross-sectional in-person, self-administered survey | rout | perm | F, M | 507 | Adolescents and young adults (ages 15–30) recruited from waiting rooms of 3 health care clinics in a Midwestern city | • | ||||
| Reiter, 2012107 | Cross-sectional, online survey | rout | rout | M | 404 | National sample of parents of adolescent males (ages 11–17) | • | ||||
| Riedesel, 200525 | Cross-sectional, mail survey | pre | pre | F, M | 145 | National sample of family physicians (AAFP members) | • | • | |||
| Roberto, 201154 | Cross-sectional, mail survey | rout | pre | F | 406 | Pediatricians in a mid-western state | • | ||||
| Roland, 201458 | Cross-sectional, mail survey | rout | pre | F | 98 | Physicians, nurse practitioners, nurse-midwives, and physician assistants in FQHCs in IL | • | ||||
| Rosen, 201526 | Cross-sectional, written survey | rout | rout | F, M | 137 | School nurses attending the Ohio Association of School Nurses conference | • | ||||
| Rosenthal, 201189 | Cross-sectional mail survey | rout | pre | F | 530 | Women (ages 19–26) identified via claims data from a large managed care plan | • | ||||
| Same, 201495 | Cross-sectional, in-person, written survey | rout | – | M | 346 | Clinic-based sample of men (ages 16–35) in New York, NY and Baltimore, MD | • | ||||
| Sanders-Thompson, 201219 | Qualitative, in-person interviews | rout | pre | F | 30 | African American parents of daughters (ages 9–17) in St. Louis, MO area | • | • | • | ||
| Savas, 201268 | Cross-sectional telephone survey | rout | pre, perm | F | 99 | Parents of daughters (ages 9–17) in Houston, TX who called the 2–1–1 Texas/United Way Helpline | • | ||||
| Schnatz, 201031 | Cross-sectional mail survey | rout | pre | F | 345 | Statewide sample of pediatricians in CT | • | ||||
| Shah, 2014103 | Cross-sectional online survey | rout | rout | M | 524 | National sample of parents and their sons (ages 11–19) | • | ||||
| Soon, 201536 | Cross-sectional online survey | rout | rout | F, M | 71 | Pediatricians and family medicine physicians in HI | • | ||||
| Staras, 201486 | Cross-sectional telephone and mail survey | rout | perm | F | 2127 | Stratified-random sample of parents of non-privately insured adolescent females (ages 9–17) in FL | • | ||||
| Stephens, 201320 | Qualitative in-person interviews | rout | perm | F | 31 | Immigrant Haitian mothers of unvaccinated daughters (ages 11–18) | • | • | |||
| Stokley, 201410 | Yearly, cross-sectional telephone surveys | rout | perm, rout | F, M | 89,915 | Nationally-representative sample of parents of females (2007–2013) and males (2011–2013) (ages 13–17) | • | ||||
| Sussman, 200739 | Qualitative in-person interviews | pre | pre | F | 37 | Primary care clinicians in New Mexico | • | ||||
| Sussman, 201559 | Mixed methods: cross-sectional, in-person and telephone interviews; mail and online survey | rout | perm | F, M | 123 | Primary care clinicians, health policy makers, and immunization experts (interviews, n = 25) and clinician members of a primary care research network in New Mexico (survey, n = 98) | • | • | • | • | |
| Taylor, 201296 | Cross-sectional, in-person survey | rout | perm | F | 96 | Cambodian mothers of adolescent daughters (ages 9–18) | • | ||||
| Tissot, 200743 | Qualitative, in-person interviews | pre | pre | F, M | 31 | Pediatricians in a 3-state region surrounding Cincinnati, OH | • | • | • | • | |
| Vadaparampil, 20119 | Cross-sectional, mail survey | rout | pre | F | 1013 | Nationally-representative sample of physicians (family physicians, pediatricians, and OB/GYNs) | • | • | • | ||
| Vadaparampil, 2013106 | Cross-sectional, mail survey | rout | perm | F, M | 134 | Nationally-representative sample of physicians (family physicians, pediatricians, and OB/GYNs) | • | ||||
| Vadaparampil, 201450 | Two cross-sectional mail surveys | rout | pre, perm | F | 1941 | Nationally-representative sample of physicians (family physicians, pediatricians, and OB/GYNs) (n = 1013 in 2009, n = 928 in 2011) | • | ||||
| Warner, 201599 | Qualitative focus groups | pre | pre | F, M | 52 | Latino parents of adolescents (ages 11–17) recruited from 2 community organizations | • | • | |||
| Weiss, 201051 | Cross-sectional mail survey | rout | pre | M | 1094 | Random samples of pediatricians and family physicians who vaccinated females | • | • | |||
| Wilson, 201361 | Qualitative focus groups | rout | perm | F, M | 44 | African American, Caribbean, Haitian and African women recruited from Federally Qualified Health Centers | • | • | |||
| Wong, 201270 | Cross-sectional telephone survey | rout | pre | F | 1631 | National sample of women (ages 18 and older) | • | ||||
| Ylitalo, 201369 | Cross-sectional, phone survey | rout | pre | F | 9274 | Nationally-representative sample of female adolescents (ages 13–17) | • | ||||
| Young, 201145 | Cross-sectional, mail survey | rout | pre | F | 385 | Statewide sample of OB/GYNS and family physicians in VA | • | • | |||
| Zimet, 201090 | Cross-sectional, mail survey | rout | pre | F | 185 | National sample of insured women (ages 19–26) | • | ||||
| Zimet, 201175 | Cross-sectional, mail and fax survey | rout | pre | F | 271 | National sample of physicians who deliver HPV vaccine to adolescent or young adult patients (ages 9–26) | • | ||||
ACIP recommendations for HPV vaccination at the time of data collection. Pre = pre-recommendation (May 2006 or earlier for females; September 2009 or earlier for males). Perm = permissive recommendation (October 2009 to September 2011 for males). Rout = routine recommendation (June 2006 and later for females; October 2011 and later for males). Dash (–) = date for data collection not reported.
Sex of patients the study examined communication about HPV vaccination for; F = females; M = males
Source
Preferences in communication source by provider type
Studies that compared sources of HPV vaccine communication found a preference for talking with physicians versus other providers.15-17 For example, far more parents viewed physicians than nurses as helpful for making decisions about HPV vaccination.16 Similarly, only about one-third of young adults were comfortable getting HPV vaccine from a nurse or medical assistant without first talking with a doctor.15 Qualitative studies suggested that these communication preferences were based on the perception that physicians were most knowledgeable about vaccines as well as patients' social and medical histories.17-20 However, two studies indicated that preferences for talking with a physician about HPV vaccination lessened after initial discussions; respondents were more open to communicating with other providers or office staff for follow-up.15,21 The extent to which parent and patient preferences are currently being met is unknown; however, one recent study found that about half of physicians in a national sample did not make the initial HPV vaccine recommendation for patients in their practice, but rather relied on a nurse practitioner, medical assistant, or other provider to do so.22
Variation in recommendations by provider factors
Studies of variation in HPV vaccine recommendations by provider factors focused on providers' knowledge, perceptions, and professional characteristics. Evidence on whether HPV-related knowledge influenced providers' communication about HPV vaccination was mixed, but suggested a modest association overall. Several quantitative studies found that knowledge was associated with recommendation intention and behavior.23-26 Qualitative studies further supported the hypothesis that knowledge informed provider communication, with incomplete knowledge of HPV-attributable cancers in males identified as a key barrier to HPV vaccine recommendations for boys.27,28 However, other studies found no association between providers' knowledge and their HPV vaccine recommendations.29-31
Studies assessed a wide range of provider perceptions and their relationship to HPV vaccine recommendations, with the most important perceptions being those related to discomfort talking about sex, parental hesitancy, and the role of professional organizations. Discomfort talking about sex or sexually transmitted infections was associated with providing less frequent and lower-quality HPV vaccine recommendations,27,32-34 as was believing that talking about sex was a prerequisite to HPV vaccination.29,35,36 Perceptions of parental hesitancy toward HPV vaccination were also negatively associated with communication; providers who perceived parents as unsupportive less often recommended or intended to recommend HPV vaccine.12,29,33,35,37-39 Conversely, providers who perceived professional organizations as influential more often recommended or intended to recommend the vaccine.23-25,37,40-43
Other frequently studied perceptions included those related to providers' confidence in HPV vaccine and their own abilities to discuss it. For example, providers who perceived high HPV vaccine efficacy consistently reported more positive recommendation intentions and behaviors.29,32,37,44,45 In contrast, no studies found a correlation between providers' perceptions of HPV vaccine safety and their recommendation behaviors, perhaps because concerns about safety were relatively uncommon.27,32,36,38,44,45 In terms of providers' perceptions of themselves, self-efficacy to communicate about HPV vaccine was associated with recommending and intending to recommend the vaccine.12,26,46 Interestingly, providers who viewed themselves as “early adopters” were also more likely to recommend HPV vaccine.30,45,47
Studies assessing the relationship between provider characteristics and HPV vaccine recommendations most often focused on clinical specialty and demographics. Most studies found that pediatricians reported more positive HPV vaccine recommendation practices than family physicians9,11,12,30,33,48-51 or other types of providers.12,32 Other studies failed to find variation in recommendations by specialty,23,34-36,40,52,53 but several of these studies did not include pediatricians.34,52,53 Most studies did not find variation in HPV vaccine recommendations by provider sex,9,23,30,36,39,44,47-49,54 but those that did most often favored female providers.24,25,29,33,53 Similarly, most studies did not find variation in HPV vaccine recommendations by provider age or years in practice,30,33,36,47,49 although a few did with mixed results.9,24,50 Studies assessing provider race/ethnicity suggested that minority status was associated with recommending HPV vaccine.9,30,32,34
Studies of clinic or practice-level characteristics focused on the composition of patient populations in terms of race/ethnicity, insurance type, and geographic location. Providers who served a higher proportion of Hispanic patients reported more often recommending or intending to recommend HPV vaccine for boys,23,30 but not girls.23,32 In contrast, one study reported a negative association between seeing a higher proportion of non-Hispanic Black patients and recommending HPV vaccine.32 Studies of providers with higher participation in Medicaid or the Vaccines for Children program found that this participation was associated with more positive HPV vaccine recommendation practices9,23,30,33-35 or no effect.29,44,48 Provider recommendations did not vary substantially by national region,30,33-35,39,44 but providers practicing in urban areas more often reported recommending HPV vaccine than those in rural or suburban ones.34,37,44,46
Audience
Variation in recommendations by patient factors
Studies assessing patient factors associated with receiving an HPV vaccine recommendation most often examined patient demographics and providers' perceptions of patients' risk of HPV infection. A consistent finding for adolescent patients was that providers' recommendation intentions and behavior improved with patient age, such that recommendations were stronger and more frequent for older adolescents than for those in the target age range of 11–12 years old.9,22-25,27,29,33,35,37,38,40,42,44,51-53,55-60 Relatively few providers recommended or intended to recommend HPV vaccination for younger patients, ages 9 and 10.22,23,27,35,40,51,59 These recommendation practices corresponded with the preferences of parents, who tended to favor communicating with providers about HPV vaccination in their adolescents' teenage vs. preteen years.16,19,61 Among studies focusing on young adult patients, provider recommendations declined slightly in frequency for patients in their twenties.35,41,56,62 Two studies suggested that a minority of providers continued to recommend HPV vaccine for older adults, ages 27 and over, who fell outside of the recommended age range for catch up vaccination.52,63
Provider communication was consistently associated with patients' sex such that providers' recommendation intentions and behavior were more supportive of HPV vaccination for girls versus boys.10,12,24,25,29,33,37,44,55,57,58,60,64,65 For example, the 2014 NIS-Teen found that 64% of age-eligible girls had received a provider recommendation compared to just 42% of boys.10 All but one study examining patients' race/ethnicity suggested disparities in provider communication with parents of African American and Hispanic adolescents less often discussing HPV vaccine with a provider or receiving HPV recommendations than parents of non-Hispanic White adolescents.7,64,66-70 This pattern of findings is concerning given that both quantitative and qualitative studies suggested that provider recommendations were especially influential among parents from racial/ethnic minority backgrounds.19,69,71-73
Providers more often recommended HPV vaccination for patients they perceived to be at higher risk for HPV infection. Studies found that providers prioritized HPV vaccination for subpopulations including sexually active adolescents, males who might have same sex partners, and adolescents of lower socioeconomic status.19,33,38,44,74 Similarly, some providers based their HPV vaccine recommendations on risk-related factors such as the results of a Pap or HPV test, number of sexual partners, or relationship status.52,58,75 Ironically, providers in one qualitative study acknowledged that their ability to accurately assess whether adolescents were sexually active was limited.38
Communication roles
Studies of parents' and adolescents' communication roles in clinical settings consistently found that a parent, most often the mother, was responsible for making the ultimate decision about HPV vaccination.4,18-20,71,74,76-78 However, the extent to which parents communicated about HPV vaccination beyond giving consent varied, with qualitative studies suggesting that parents from racial/ethnic minorities or with lower socioeconomic status were less likely to be engaged by providers and more likely than parents from more socially privileged backgrounds to defer to providers' advice.19,71,73,74,77 Parents were also more likely to follow providers' advice without question when they received a strong, unambiguous recommendation.38,74,77
Findings on adolescents' role in clinical communication about HPV vaccination were also mixed. Some studies emphasized their lack of participation, particularly in the case of younger adolescents.4,19,74,78,79 Indeed, some parents indicated a preference for vaccinating in the preteen years specifically because they viewed adolescents as having little say during this time.18,80 In contrast, other studies found that adolescents did play a role in communication and decision-making in clinical settings, one which increased with age, maturity, and social privilege.18,27,71,73,76,80 For some parents, the desire to maximize adolescents' role was even a reason for delaying HPV vaccination.80 Studies suggested that parent-adolescent decisions were largely concordant and that most dyads ultimately reached agreement about the vaccination decision, but they sometimes looked to providers for guidance in the case of initial disagreement.76,81 Interestingly, one qualitative study noted that adolescents' participation in medical dialog was often subtle and included non-verbal forms of communication such as nods, suggesting that adolescents' communication role could be easy to overlook.76
Message
Content
Studies of the preferred and actual content of provider communication about HPV vaccination focused on prevention topics, vaccine safety, and vaccination logistics. Of these, content related to prevention featured most prominently, with parents and adolescents most often wanting providers to discuss the specific diseases HPV vaccine prevents and its efficacy.18,21,82,83 In correspondence with preferred content, prevention of cancer, genital warts, and sexually transmitted infections (STIs) constituted the major topics of actual HPV vaccine communication. Almost all providers reported mentioning cancer prevention when they discussed HPV vaccination.33,59 However, studies of girls suggested that providers placed a higher priority on cancer prevention 17,29,35,38,40,81 than studies of boys.22,27,29,40,76 For girls, discussions centered on cervical cancer prevention, and included genital warts and STIs less often.35,38,40,79,81 For boys, prevention topics were more varied with the prevention of genital warts and STIs featuring as or more prominently than the prevention of cancer;22,27,29,76 cancer topics included cervical cancer prevention for future partners and, less frequently, the prevention of male cancers in patients themselves.22,27,29,40,76 Several qualitative studies found that some providers chose to discuss cancer, but not genital warts or STIs, so as to avoid talking about sex;27,59 however, a quantitative study found that, for boys, very few providers discussed one without the other.22 Although two studies sought to assess the association between the prevention content and HPV vaccine acceptance, results were inconclusive.60,84
Content about safety and side effects featured somewhat less prominently in studies of provider communication about HPV vaccination. Qualitative studies suggested that some parents and adolescents prioritized safety and side effects as important topics for discussion,19,21,38,61,82 but studies of actual content suggested that such communication was fairly limited.27,79,82 For example, one qualitative study of parents and their adolescent sons found that most recalled that their providers had told them HPV vaccination was generally safe, but few reported receiving information or asking questions about specific side effects.76
Topics related to provider communication about the logistics of HPV vaccination were varied. Parents reported a preference for being informed of: the number of doses needed to complete the vaccine series; the vaccine's availability for boys; cost; and the recommended timing of administration.21 With regard to timing, some parents expressed a particular interest in the rationale for vaccinating adolescents in their preteen years vs. later.18,21,38 In contrast to these preferences, several studies indicated deficiencies in provider communication about how to complete the 3 dose series.27,76,81,85 Fewer studies assessed actual content in terms of the availability of HPV vaccine for boys, cost, and timing.22,76
Style
Studies of communication style focused on four areas: 1) recommendation strength; 2) recommendation quality; 3) the framing of HPV vaccine in relation to other adolescent vaccines; and 4) collaborative communication. First, with regard to recommendation strength, quantitative studies consistently found an association between the extent to which providers endorsed the importance of HPV vaccine and parents' positive perceptions of HPV vaccine,60,86 their intention to get their children vaccinated,60,87 and adolescents' receipt of HPV vaccine.60,87-89 Qualitative studies also found strong provider endorsement to be uniquely influential on parents' HPV vaccination decisions.27,38,74,77 Unfortunately, these same studies suggested that providers often failed to strongly endorse HPV vaccine.11,27,38,44,47,60,86,87,89,90 For example, one national study found that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of provider endorsement.60
Building on the notion of recommendation strength, a second series of studies found that recommendation quality, or the extent to which providers delivered guideline-consistent recommendations, was also associated with HPV vaccination. In addition to strength of endorsement, these studies assessed the quality indicators of timeliness (i.e., routinely recommending HPV vaccine by age 12 versus later), consistency (using a routine versus risk-based approach to recommendations), and urgency (recommending same-day vaccination). A national study of primary care physicians found that half reported 2 or more lower-quality recommendation practices.33 A corresponding survey of parents found that those who received high- versus low-quality recommendations had more often initiated HPV vaccination for their adolescents and had less often refused or delayed the vaccine.60
A third area of communication style centered on understanding how providers communicated about HPV vaccine in relation to other vaccines; qualitative studies identified two main approaches in this regard. In one, providers framed HPV vaccine as one of several vaccines in the routine schedule, avoided drawing special attention to it, and offered their strong endorsement.38,74 Providers using this approach reported low levels of parental hesitancy and high levels of vaccine uptake.38,74 In the other approach, providers distinguished HPV vaccine from other adolescent vaccines by presenting it as an “optional” vaccine which was not required for school; this approach often involved a more lengthy discussion of risks and benefits as well as obtaining parental consent separately from, not along with, other vaccines.38,74,77,79,91 Providers using the second approach reported higher levels of parental hesitancy and vaccine refusal or delay, but nevertheless felt obligated to mention the absence of school entry requirements for HPV vaccine and offer the option to delay vaccination in order to more closely coincide with sexual debut.38,74 Providers who presented HPV vaccine as optional expressed the hope that this open-ended communication style would foster trust with vaccine hesitant parents, thereby encouraging the acceptance of other adolescent vaccines in the short term as well as HPV vaccine in the long term.38 Unfortunately, some parents who delayed HPV vaccination with the intention of getting it later reported that they never followed-up to do so.38,91
The extent to which providers adhere to these overall approaches of aligning HPV vaccine with or distinguishing it from other vaccines is unknown. However, the existing literature consistently supported the assertion that providers often communicated about HPV vaccine differently from other vaccines in discrete ways. Quantitative and qualitative studies found that providers spent longer talking about HPV vaccine than other vaccines,11,27,35,74,76,84 endorsed HPV vaccine less strongly than Tdap and meningococcal vaccines,11,12,22,74,87 and often presented HPV vaccine as an “optional” vaccine vs. one that was “routine” or “required.”12,22,38,74,79,91 Finally, one study found that, among providers with a preferred order for discussing adolescent vaccines, over two-third preferred to discuss HPV vaccine last.11
A fourth area of communication style considered the extent to which providers engaged parents and patients in discussion about HPV vaccination. For example, one series of quantitative studies assessed “collaborative” communication based on parental reports of whether they discussed HPV vaccination with a provider, whether they received enough time to make a decision, and whether providers played a role in decision making. These studies found that collaborative communication was associated with HPV vaccine uptake.92 However, traditionally underserved groups, such as parents of Hispanic and non-privately insured adolescents, were less likely to report collaborative communication, which adversely affected HPV vaccination coverage for these groups.93 Qualitative research generally supported the finding that providers were less likely to engage non-English speaking parents or parents from disadvantaged backgrounds in HPV vaccine-related communication.73,76 Subgroup differences aside, a quantitative analysis of transcribed medical encounters found that providers were verbally dominant in discussions about HPV vaccination, speaking over three-quarters of the words spoken, taking longer turns than parents, and using on average more than 11 technical terms per visit.79 All studies that assessed who initiated HPV vaccine conversations found in favor of providers; parents and patients brought up the topic only rarely and preferred that providers initiate the discussion.76,77,79,87,91,94-97
Decisional timeframe
Both qualitative and quantitative studies found that parents often preferred not to make an immediate decision about HPV vaccination during discussions with a provider, but rather wished to decide later after thinking more about the issue and getting more information.12,16,18,73,74,79,80 Studies suggested that providers may contribute to extending the timeframe for HPV vaccination decision making, given that many gave parents a choice about when to vaccinate or, even at times, actively suggested delay.33,38,60,74,77,91 For example, a national survey of physicians found that only about half usually recommended same-day vaccination for 11- and 12-year-old patients.33
Satisfaction
No studies examined parents' and patients' satisfaction with provider communication about HPV as a primary focus; however, a few reported on certain aspects of parent satisfaction, such as receiving adequate time and informational support. For example, most parents participating in the 2010 NIS-Teen reported that their daughters' providers did give them enough time to discuss HPV vaccine; however, this perception was less common among parents of unvaccinated versus vaccinated daughters.4 One small study found that most parents who discussed HPV vaccination with a provider reported receiving adequate information on prevention topics.84 In contrast, qualitative studies with parents from racial/ethnic minority backgrounds suggested some degree of dissatisfaction with HPV vaccine communication, with some parents reporting too little information, limited opportunities to ask questions, or ambiguous recommendations.61,71,77,78,98,99 For these parents, the perception that providers were withholding information was a source of confusion that introduced doubt about the value of HPV vaccination, discouraged vaccine acceptance, and in some cases undercut parents' trust in providers.61,71,77,78,98,99 Parents emphasized their desire for clear, unambiguous messages from providers about the importance of HPV vaccination.38,71,77,99
Channel
Relatively few studies assessed modes of clinical communication about HPV vaccination beyond provider dialog, but those that did focused primarily on written materials. Written brochures and fact sheets, such as the CDC's Vaccine Information Sheet,12,87,100-103 and posters87,100 were commonly used in traditional primary care settings to support provider communication about HPV vaccination. In studies examining preferred materials, both parents and providers favored brief written materials,12,16,87,94,100,101,103 with parents additionally expressing interest in websites.87,103 Parents and providers across several studies voiced the need for educational materials that were tailored to parents' cultural background, language preference, and literacy level.12,43,94,99,101,104 Given concerns about literacy, some providers suggested video as a promising educational channel,43,94 but only a minority of parents viewed informational videos as helpful.16 Notably, no studies examined educational materials designed specifically for adolescent patients themselves.
Context
Visit type
Studies assessing visit type found that almost all providers who discussed HPV vaccination used well-child visits to do so, whereas only about three-quarters used school physicals, and about half used camp or sports physicals.17,22 Fewer providers reported discussing HPV vaccine during visits for acute care, even when patients' complaints were mild.11,22,41,63,105 Providers perceived communicating about HPV vaccination to be difficult in the context of acute care, with concerns including the lack of time or fear that parents might blame the vaccine if the child's illness worsened.11,59 Despite avoiding HPV vaccine communication during acute care visits, some providers acknowledged that such visits were sometimes their only contact with adolescents, particularly older adolescents who they perceived as less likely to make well-child visits.59,73,85
Barriers to communication
Studies assessing barriers to provider communication about HPV vaccination considered both policy- and clinic-level factors. Although providers reported being highly motivated to follow practice guidelines, many perceived guidelines for HPV vaccine administration to be complex and unclear; indeed, providers with this perception recommended HPV vaccination less often.27,34,59,106 Providers also perceived the lack of school entry requirements for HPV vaccination as a barrier to making strong recommendations because of the implicit assertion that HPV vaccine was less important than Tdap and other recommended vaccines.9,11,41,63,74 Other studies suggested that policies requiring parental consent prior to HPV vaccine administration to minors may be a barrier to direct communication with adolescents.107,108
In terms of barriers related to the clinical environment, studies most often identified deficiencies in scheduling as limiting provider communication about HPV vaccination. Providers identified patient reminder/recall as critical to their efforts to recommend HPV vaccination, but many reported that they did not use these systems, and instead relied on patients to initiate scheduling.11,43,59,85,94 Finally, providers reported that time constraints in the clinical encounter were also a barrier to HPV vaccine communication.9,11,12,35,41,45,59,63
Interventions to improve communication
Only two studies evaluated the impact of interventions on providers' HPV vaccine communication. One study tested a multi-component, clinic-based intervention that included provider education and provider alerts in patients' electronic medical records; parents of adolescent patients who attended intervention clinics were more likely to discuss HPV vaccination with a provider, but were no more likely to receive a strong recommendation.109 A second study evaluated a social marketing campaign that included public service announcements and the provision of clinic-based educational materials to parents of adolescents; most providers who participated reported that the campaign made them more likely to discuss and recommend HPV vaccination.100
Discussion
Findings from this systematic review of over 100 quantitative and qualitative studies suggest that healthcare providers face a highly complex communication environment when discussing HPV vaccination in clinical settings. First, the audience for clinical communication is not uniform; studies noted that patients could present with or without their parents and that expectations about the communication roles accorded to each of these parties shifted with patients' age and maturity, as well as with parents' preferences.18,27,71,73,76,80 In addition to age, patients' sex added complexity to communication, with providers offering different prevention messages to boys and girls.17,22,27,29,35,38,40,76,81 Second, at the interpersonal level, many providers perceived parents as being unsupportive of HPV vaccination, a view which hindered guideline-consistent delivery of recommendations.12,29,33,35,37-39 Third, studies consistently documented a challenging policy context, including unclear practice guidelines and a lack of school entry requirements for HPV vaccination.11,27,34,59,74 Finally, participants across several studies perceived clinical systems as unconducive to communication due to factors such as time constraints and deficiencies in reminder/recall systems.11,43,59,85,94 In these ways, an audience with dynamic communication needs came together with unsupportive interpersonal, policy, and clinical contexts to create challenges for effective HPV vaccine communication.
Given these complexities, it is perhaps not surprising that the research literature documented shortcomings in provider communication about HPV vaccination. Communication practices such as describing HPV vaccine as “optional,” differentiating it from other adolescent vaccines, failing to endorse it strongly, and recommending delayed vs. same-day vaccination33,38,60,75 were common and likely compromised providers' ability to deliver HPV vaccine according to national guidelines. Although some providers expressed the hope that merely offering, rather than strongly endorsing, HPV vaccine would honor parents' preferences and earn their trust,38 the findings of this review suggest that this view is likely misguided. Rather, qualitative research suggests that parents found open-ended communication ambiguous, frustrating and worrying, leading them to delay HPV vaccination for their adolescents.61,71,77,78,98,99 Instead of encouraging shared decision making, mixed messages appeared to lead to a “default” communication style in which neither providers nor parents engaged in the decision-making process.110
Findings also provide evidence of communication disparities in terms of who receives a provider recommendation for HPV vaccination. More specifically, parents of younger adolescents, males, and adolescents from racial and ethnic minorities less often received recommendations. This pattern of findings is concerning given that HPV vaccination is more effective when administered to preteens versus older adolescents and may be especially beneficial for traditionally underserved populations who experience a disproportionate burden of cervical cancer later in life.111 Although HPV vaccination coverage among adolescents from racial and ethnic minorities tends to be on par with or higher than coverage for non-Hispanic Whites, eliminating communication disparities could raise coverage for these high priority populations even higher. Studies also indicate that many providers used risk-based approaches to recommending HPV vaccine, prioritizing the vaccine for adolescents they perceived as likely to be sexually active.33,52,74,75 Such risk-based strategies have been shown to be ineffective given the extremely high prevalence of HPV infection and the difficulty of accurately predicting adolescents' sexual debut.112
In terms of implications for clinical practice, this review highlights several promising approaches for communicating about HPV vaccination (Table 2). More specifically, quality improvement efforts aimed at strengthening provider communication about HPV vaccination should emphasize the need to say HPV vaccination is important, recommend same-day vaccination, and deliver routine recommendations to all 11- and 12-year-old patients.38,74 Other promising strategies include emphasizing cancer prevention benefits and discussing HPV vaccine at the same time and in the same way as other recommended adolescent vaccines.
Table 2.
Promising practices for recommending HPV vaccination in clinical settings.
| Quality | Communication practice |
|---|---|
| Strength of endorsement | Emphasize the importance of HPV vaccine |
| Urgency | Recommend same-day vaccination |
| Timeliness | Deliver recommendations by age 12 |
| Consistency | Deliver recommendations for all adolescents, not just those perceived to be “at risk” |
| Prevention message | Emphasize cancer prevention |
| Concomitance | Recommend HPV vaccine at the same time and in the same way as other adolescent vaccines |
| Guidelines-based rationale | Focus on the “routine” immunization schedule versus what is “required” for school entry |
This review suggests opportunities for additional research, particularly in the areas of communication source and audience, by raising questions about the relative roles of parents, patients, and providers in HPV vaccine communication. The extent to which parents wish to engage in communication about HPV vaccination—or the impact of their engagement on their HPV vaccination decisions—is largely unknown and warrants further study. In the case of patients, the findings of this review suggests that the communication role of adolescents is under-represented in the research literature, particularly given that several studies found that adolescents can have a positive influence on HPV vaccine decision making.4,76 In the case of providers, this review found that, although parents preferred to communicate with physicians, other providers, including nurse practitioners and medical assistants, were often responsible for delivering recommendations. Given this discordance, more research is needed to understand what impact the source of HPV vaccine recommendations has on uptake and how to delegate communication roles among the care team to maximize efficiency.
It is noteworthy that our search identified only two studies that sought to measure the impact of interventions on provider communication about HPV vaccination.100,109 Additional intervention research is urgently needed to evaluate approaches for supporting effective provider communication and to prospectively evaluate the impact of those interventions on HPV vaccine uptake. Message testing to understand the impact of providers' communication content on parents' HPV vaccine decision making and satisfaction could be especially useful. Although the broader literature on message framing has yield mixed results as to the benefits of gain- versus loss-framed messages,113-115 other areas of potential importance include the relative influence of different prevention messages in terms of disease types and number of diseases,116-118 the length or amount of content delivered, and cognitive versus affective approaches to conveying HPV vaccine-related information.
Strengths of this systematic review include the use of two coders and a standardized form to perform data abstraction across databases in multiple fields of study. Limitations include this review's reliance on studies that most often used relatively weak, cross-sectional designs, convenience samples, and self-reported measures of providers' recommendation behavior and adolescents' vaccination status. Although our narrative analytic approach was well-suited to the aims of this study and the variable quality of the available evidence, future studies should seek to use rigorous study designs to assess the impact of specific communication practices, such as the delivery of provider recommendations, on HPV vaccine uptake. Finally, we acknowledge that this review is limited in its primary focus on provider dialog; related literatures in other areas such as the use of reminder/recall and message faming are also relevant to understanding clinical communication about HPV vaccination.
Conclusion
Improving provider communication is one of the most highly prioritized goals in the national movement to increase HPV vaccination coverage.2 This review identifies promising communication practices, such as strongly endorsing HPV vaccine and using routine approaches to delivering recommendations, that can inform quality improvement efforts and potentially reduce communication disparities that currently limit HPV vaccine recommendations for younger adolescents, males, and racial/ethnic minorities. At the same time, this review suggests that providers face substantial barriers to communicating about HPV vaccination due to challenging interpersonal, clinical, and policy contexts. Interventions are needed to support providers in recommending HPV vaccination effectively and efficiently in the highly complex communication environment surrounding HPV vaccination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Jane Carpenter for providing administrative support.
Funding
This study was supported by a career development award from the National Cancer Institute (K22 CA186979). The funder did not play a role in study design, data analysis, report writing, or the decision to submit the article for publication.
References
- [1].US. Department of Health and Human Services Healthy People 2020 Objectives: Immunization and infectious diseases [cited 2013 April 8]. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=23 [Google Scholar]
- [2].Accelerating HPV vaccine uptake: Urgency for action to prevent cancer Bethesda, MD: National Cancer Institute; 2014. Available from: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm#sthash.vOfexidh.dpbs [Google Scholar]
- [3].Reagan-Steiner S, Yankey D, Jeyarajah D, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years–United States, 2014. Morbidity and Mortality Weekly Report 2015; 64(29):784-92; PMID:26225476; http://dx.doi.org/ 10.15585/mmwr.mm6429a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dorell C, Yankey D, Kennedy A, Stokley S. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2012; 52(2):162-70; PMID:23221308; http://dx.doi.org/ 10.1177/0009922812468208 [DOI] [PubMed] [Google Scholar]
- [5].Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 2012; 30(24):3546-56; PMID:22480928; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.063 [DOI] [PubMed] [Google Scholar]
- [6].Reiter PL, McRee AL, Pepper JK, Gilkey MB, Galbraith KV, Brewer NT. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health 2013; 103(8):1419-27; PMID:23763402; http://dx.doi.org/ 10.2105/AJPH.2012.301189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children's Health. Vaccine 2012; 30(20):3112-8; PMID:22425179; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.034 [DOI] [PubMed] [Google Scholar]
- [8].Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the National Immunization Survey-Teen. Vaccine 2013; 31(26):2816-21; PMID:23602667; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vadaparampil ST, Kahn JA, Salmon D, Lee JH, Quinn GP, Roetzheim R, Bruder K, Malo TL, Proveaux T, Zhao X, et al.. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29(47):8634-41; PMID:21924315; http://dx.doi.org/ 10.1016/j.vaccine.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014–United States. MMWR Morb Mortal Wkly Rep 2014; 63(29):620-4; PMID:25055185 [PMC free article] [PubMed] [Google Scholar]
- [11].Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Prev Med 2015; 77:181-5; PMID:26051197; http://dx.doi.org/ 10.1016/j.ypmed.2015.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McRee AL, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. J Pediatr Health Care 2014; 28(6):541-9; PMID:25017939; http://dx.doi.org/ 10.1016/j.pedhc.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berlo DK. The Process of Communication: An Introduction to Theory and Practice. New York: Holt, Rinehart, and Winston; 1960 [Google Scholar]
- [14].Maibach EW, Parrott RL. Designing health messages: Approaches from communication theory and public health practice: Sage Publications; 1995 [Google Scholar]
- [15].Dempsey AF, Pyrzanowski J, Brewer S, Barnard J, Sevick C, O'Leary ST. Acceptability of using standing orders to deliver human papillomavirus vaccines in the outpatient obstetrician/gynecologist setting. Vaccine. 2015; 33(15):1773-9; PMID:25731788; http://dx.doi.org/ 10.1016/j.vaccine.2015.02.044 [DOI] [PubMed] [Google Scholar]
- [16].Gilkey MB, Calo WA, Marciniak MW, Brewer NT. Counseling parents who refuse or delay HPV vaccine. Under Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Greenfield LS, Page LC, Kay M, Li-Vollmer M, Breuner CC, Duchin JS. Strategies for increasing adolescent immunizations in diverse ethnic communities. J Adolesc Health 2015; 56(5 Suppl):S47-53; PMID:25863555; http://dx.doi.org/ 10.1016/j.jadohealth.2014.10.274 [DOI] [PubMed] [Google Scholar]
- [18].Griffioen AM, Glynn S, Mullins TK, Zimet GD, Rosenthal SL, Fortenberry JD, Kahn JA. Perspectives on decision making about human papillomavirus vaccination among 11- to 12-year-old girls and their mothers. Clin Pediatr (Phila). 2012; 51(6):560-8; PMID:22589477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sanders Thompson VL, Arnold LD, Notaro SR. African American parents' HPV vaccination intent and concerns. J Health Care Poor Underserved 2012; 23(1):290-301; PMID:22643477; http://dx.doi.org/ 10.1353/hpu.2012.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stephens DP, Thomas TL. Cultural Values Influencing Immigrant Haitian Mothers' Attitudes Toward Human Papillomavirus Vaccination for Daughters. J Black Psychol 2013; 39(2):156-68; PMID:25342865; http://dx.doi.org/ 10.1177/0095798412461807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alexander AB, Stupiansky NW, Ott MA, Herbenick D, Reece M, Zimet GD. What parents and their adolescent sons suggest for male HPV vaccine messaging. Health Psychol 2014; 33(5):448-56; PMID:24588632; http://dx.doi.org/ 10.1037/a0033863 [DOI] [PubMed] [Google Scholar]
- [22].Malo TL, Ali KN, Sutton SK, Giulinano AR, Vadaparampil ST. How are physicians communicating about HPV vaccine with male patients? Hum Vaccin Immunother. Forthcoming. [Google Scholar]
- [23].Kahn JA, Cooper HP, Vadaparampil ST, Pence BC, Weinberg AD, LoCoco SJ, Rosenthal SL. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18(8):2325-32; PMID:19661092; http://dx.doi.org/ 10.1158/1055-9965.EPI-09-0184 [DOI] [PubMed] [Google Scholar]
- [24].Kahn JA, Zimet GD, Bernstein DI, Riedesel JM, Lan D, Huang B, Rosenthal SL. Pediatricians' intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health 2005; 37(6):502-10; PMID:16310128; http://dx.doi.org/ 10.1016/j.jadohealth.2005.07.014 [DOI] [PubMed] [Google Scholar]
- [25].Riedesel JM, Rosenthal SL, Zimet GD, Bernstein DI, Huang B, Lan D, Kahn JA. Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol 2005; 18(6):391-8; PMID:16338604; http://dx.doi.org/ 10.1016/j.jpag.2005.09.004 [DOI] [PubMed] [Google Scholar]
- [26].Rosen BL, Ashwood D, Richardson GB. School Nurses' Professional Practice in the HPV Vaccine Decision-Making Process. J Sch Nurs. 2015:1059840515583312; PMID:25962388 [DOI] [PubMed] [Google Scholar]
- [27].Alexander AB, Best C, Stupiansky N, Zimet GD. A model of health care provider decision making about HPV vaccination in adolescent males. Vaccine 2015; 33(33):4081-6; PMID:26143612; http://dx.doi.org/ 10.1016/j.vaccine.2015.06.085 [DOI] [PubMed] [Google Scholar]
- [28].Perkins RB, Clark JA. Providers' attitudes toward human papillomavirus vaccination in young men: challenges for implementation of 2011 recommendations. Am J Mens Health 2012; 6(4):320-3; PMID:22398992; http://dx.doi.org/ 10.1177/1557988312438911 [DOI] [PubMed] [Google Scholar]
- [29].Daley MF, Liddon N, Crane LA, Beaty BL, Barrow J, Babbel C, Markowitz LE, Dunne EF, Stokley S, Dickinson LM, et al.. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics 2006; 118(6):2280-9; PMID:17142510; http://dx.doi.org/ 10.1542/peds.2006-1946 [DOI] [PubMed] [Google Scholar]
- [30].Malo TL, Giuliano AR, Kahn JA, Zimet GD, Lee JH, Zhao X, Vadaparampil ST. Physicians' human papillomavirus vaccine recommendations in the context of permissive guidelines for male patients: a national study. Cancer Epidemiol Biomarkers Prev 2014; 23(10):2126-35; PMID:25028456; http://dx.doi.org/ 10.1158/1055-9965.EPI-14-0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schnatz PF, Humphrey K, O'Sullivan DM. Assessment of the perceptions and administration of the human papillomavirus vaccine. J Low Genit Tract Dis 2010; 14(2):103-7; PMID:20354417; http://dx.doi.org/ 10.1097/LGT.0b013e3181b240ca [DOI] [PubMed] [Google Scholar]
- [32].Bynum SA, Staras SA, Malo TL, Giuliano AR, Shenkman E, Vadaparampil ST. Factors associated With Medicaid providers' recommendation of the HPV vaccine to low-income adolescent girls. J Adolesc Health 2014; 54(2):190-6; PMID:24064282; http://dx.doi.org/ 10.1016/j.jadohealth.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: Findings from a national survey. Cancer Epidemiol Biomarkers Prev 2015; 24(11):1673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kulczycki A, Qu H, Shewchuk R. Primary care physicians' adherence to guidelines and their likelihood to prescribe the human papilomavirus vaccine for 11- and 12-year-old girls. Women's Health Issues 2015; 26(1):34-9; PMID:26344447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Daley MF, Crane LA, Markowitz LE, Black SR, Beaty BL, Barrow J, Babbel C, Gottlieb SL, Liddon N, Stokley S, et al.. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics 2010; 126(3):425-33; PMID:20679306; http://dx.doi.org/ 10.1542/peds.2009-3500 [DOI] [PubMed] [Google Scholar]
- [36].Soon R, Dela Cruz MR, Tsark JU, Chen JJ, Braun KL. A Survey of Physicians' Attitudes and Practices about the Human Papillomavirus (HPV) Vaccine in Hawai'i. Hawaii J Med Public Health 2015; 74(7):234-41; PMID:26225269 [PMC free article] [PubMed] [Google Scholar]
- [37].Kahn JA, Rosenthal SL, Tissot AM, Bernstein DI, Wetzel C, Zimet GD. Factors influencing pediatricians' intention to recommend human papillomavirus vaccines. Ambul Pediatr 2007; 7(5):367-73; PMID:17870645; http://dx.doi.org/ 10.1016/j.ambp.2007.05.010 [DOI] [PubMed] [Google Scholar]
- [38].Perkins RB, Clark JA, Apte G, Vercruysse JL, Sumner JJ, Wall-Haas CL, Rosenquist AW, Pierre-Joseph N. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics 2014; 134(3):e666-74; PMID:25136036; http://dx.doi.org/ 10.1542/peds.2014-0442 [DOI] [PubMed] [Google Scholar]
- [39].Sussman AL, Helitzer D, Sanders M, Urquieta B, Salvador M, Ndiaye K. HPV and cervical cancer prevention counseling with younger adolescents: implications for primary care. Ann Fam Med 2007; 5(4):298-304; PMID:17664495; http://dx.doi.org/ 10.1370/afm.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jensen ME, Hartenbach E, McElroy JA, Faerber A, Havighurst T, Kim KM, Bailey HH. Brief report: Measuring the attitudes of health care professionals in Dane County toward adolescent immunization with HPV vaccine. WMJ 2009; 108(4):203-5; PMID:19753828 [PMC free article] [PubMed] [Google Scholar]
- [41].Perkins RB, Clark JA. What affects human papillomavirus vaccination rates? A qualitative analysis of providers' perceptions. Womens Health Issues 2012; 22(4):e379-86; PMID:22609253; http://dx.doi.org/ 10.1016/j.whi.2012.04.001 [DOI] [PubMed] [Google Scholar]
- [42].Raley JC, Followwill KA, Zimet GD, Ault KA. Gynecologists' attitudes regarding human papilloma virus vaccination: a survey of Fellows of the American College of Obstetricians and Gynecologists. Infect Dis Obstet Gynecol 2004; 12(3–4):127-33; PMID:15763912; http://dx.doi.org/ 10.1080/10647440400020661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tissot AM, Zimet GD, Rosenthal SL, Bernstein DI, Wetzel C, Kahn JA. Effective strategies for HPV vaccine delivery: the views of pediatricians. J Adolesc Health 2007; 41(2):119-25; PMID:17659214; http://dx.doi.org/ 10.1016/j.jadohealth.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Allison MA, Dunne EF, Markowitz LE, O'Leary ST, Crane LA, Hurley LP, Stokley S, Babbel CI, Brtnikova M, Beaty BL, et al.. HPV vaccination of boys in primary care practices. Acad Pediatr 2013; 13(5):466-74; PMID:24011749; http://dx.doi.org/ 10.1016/j.acap.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Young JL, Bernheim RG, Korte JE, Stoler MH, Guterbock TM, Rice LW. Human papillomavirus vaccination recommendation may be linked to reimbursement: a survey of Virginia family practitioners and gynecologists. J Pediatr Adolesc Gynecol 2011; 24(6):380-5; PMID:21906978; http://dx.doi.org/ 10.1016/j.jpag.2011.06.016 [DOI] [PubMed] [Google Scholar]
- [46].Krieger JL, Katz ML, Kam JA, Roberto A. Appalachian and non-Appalachian pediatricians' encouragement of the human papillomavirus vaccine: implications for health disparities. Womens Health Issues 2012; 22(1):e19-26; PMID:21907591; http://dx.doi.org/ 10.1016/j.whi.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ishibashi KL, Koopmans J, Curlin FA, Alexander KA, Ross LF. Paediatricians' attitudes and practices towards HPV vaccination. Acta Paediatr 2008; 97(11):1550-6; PMID:18671696; http://dx.doi.org/ 10.1111/j.1651-2227.2008.00958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barnack JL, Reddy DM, Swain C. Predictors of parents' willingness to vaccinate for human papillomavirus and physicians' intentions to recommend the vaccine. Womens Health Issues 2010; 20(1):28-34; PMID:20123174; http://dx.doi.org/ 10.1016/j.whi.2009.08.007 [DOI] [PubMed] [Google Scholar]
- [49].Bruno DM, Wilson TE, Gany F, Aragones A. Identifying human papillomavirus vaccination practices among primary care providers of minority, low-income and immigrant patient populations. Vaccine 2014; 32(33):4149-54; PMID:24886959; http://dx.doi.org/ 10.1016/j.vaccine.2014.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vadaparampil ST, Malo TL, Kahn JA, Salmon DA, Lee JH, Quinn GP, Roetzheim RG, Bruder KL, Proveaux TM, Zhao X, et al.. Physicians' human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med 2014; 46(1):80-4; PMID:24355675; http://dx.doi.org/ 10.1016/j.amepre.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weiss TW, Zimet GD, Rosenthal SL, Brenneman SK, Klein JD. Human papillomavirus vaccination of males: attitudes and perceptions of physicians who vaccinate females. J Adolesc Health 2010; 47(1):3-11; PMID:20547286; http://dx.doi.org/ 10.1016/j.jadohealth.2010.03.003 [DOI] [PubMed] [Google Scholar]
- [52].Kepka D, Berkowitz Z, Yabroff KR, Roland K, Saraiya M. Human papillomavirus vaccine practices in the USA: do primary care providers use sexual history and cervical cancer screening results to make HPV vaccine recommendations? Sex Transm Infect 2012; 88(6):433-5; PMID:22522751; http://dx.doi.org/ 10.1136/sextrans-2011-050437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Perkins RB, Anderson BL, Gorin SS, Schulkin JA. Challenges in cervical cancer prevention: a survey of US obstetrician-gynecologists. Am J Prev Med 2013; 45(2):175-81; PMID:23867024; http://dx.doi.org/ 10.1016/j.amepre.2013.03.019 [DOI] [PubMed] [Google Scholar]
- [54].Roberto AJ, Krieger JL, Katz ML, Goei R, Jain P. Predicting pediatricians' communication with parents about the human papillomavirus (hpv) vaccine: an application of the theory of reasoned action. Health Commun 2011; 26(4):303-12; PMID:21424964; http://dx.doi.org/ 10.1080/10410236.2010.550021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bhatta MP, Phillips L. Human papillomavirus vaccine awareness, uptake, and parental and health care provider communication among 11- to 18-year-old adolescents in a rural Appalachian Ohio county in the United States. J Rural Health 2015; 31(1):67-75; PMID:25040612; http://dx.doi.org/ 10.1111/jrh.12079 [DOI] [PubMed] [Google Scholar]
- [56].Klosky JL, Russell KM, Simmons JL, Foster RH, Peck K, Green DM, Hudson MM. Medical and sociodemographic factors associated with human papillomavirus (HPV) vaccination adherence among female survivors of childhood cancer. Pediatr Blood Cancer 2015; 62(9):1630-6; PMID:25900433; http://dx.doi.org/ 10.1002/pbc.25539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Luque JS, Tarasenko YN, Dixon BT, Vogel RL, Tedders SH. Recommendations and administration of the HPV vaccine to 11- to 12-year-old girls and boys: a statewide survey of Georgia vaccines for children provider practices. J Low Genit Tract Dis 2014; 18(4):298-303; PMID:24633170; http://dx.doi.org/ 10.1097/LGT.0000000000000011 [DOI] [PubMed] [Google Scholar]
- [58].Roland KB, Benard VB, Greek A, Hawkins NA, Saraiya M. Primary care providers human papillomavirus vaccine recommendations for the medically underserved: a pilot study in US. Federally Qualified Health Centers Vaccine 2014; 32(42):5432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sussman AL, Helitzer D, Bennett A, Solares A, Lanoue M, Getrich CM. Catching Up With the HPV Vaccine: Challenges and Opportunities in Primary Care. Ann Fam Med 2015; 13(4):354-60; PMID:26195681; http://dx.doi.org/ 10.1370/afm.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016. Jan 23. pii: S0264–410X(16):00058-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wilson R, Brown DR, Boothe MA, Harris CE. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health 2013; 15(4):747-57; PMID:23197180; http://dx.doi.org/ 10.1007/s10903-012-9749-5 [DOI] [PubMed] [Google Scholar]
- [62].Mehta NR, Julian PJ, Meek JI, Sosa LE, Bilinski A, Hariri S, Markowitz LE, Hadler JL, Niccolai LM. Human papillomavirus vaccination history among women with precancerous cervical lesions: disparities and barriers. Obstet Gynecol 2012; 119(3):575-81; PMID:22353956; http://dx.doi.org/ 10.1097/AOG.0b013e3182460d9f [DOI] [PubMed] [Google Scholar]
- [63].Quinn GP, Murphy D, Malo TL, Christie J, Vadaparampil ST. A national survey about human papillomavirus vaccination: what we didn't ask, but physicians wanted us to know. J Pediatr Adolesc Gynecol 2012; 25(4):254-8; PMID:22516792; http://dx.doi.org/ 10.1016/j.jpag.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Reimer RA, Schommer JA, Houlihan AE, Gerrard M. Ethnic and gender differences in HPV knowledge, awareness, and vaccine acceptability among White and Hispanic men and women. J Community Health 2014; 39(2):274-84; PMID:24150246; http://dx.doi.org/ 10.1007/s10900-013-9773-y [DOI] [PubMed] [Google Scholar]
- [65].Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr (Phila) 2015; 54(4):371-5; PMID:25238779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kramer MR, Dunlop AL. Inter-state variation in human papilloma virus vaccine coverage among adolescent girls in the 50 US states, 2007. Matern Child Health J 2012; 16 Suppl 1:S102-10; PMID:22453332; http://dx.doi.org/ 10.1007/s10995-012-0999-6 [DOI] [PubMed] [Google Scholar]
- [67].Polonijo AN, Carpiano RM. Social inequalities in adolescent human papillomavirus (HPV) vaccination: a test of fundamental cause theory. Soc Sci Med 2013; 82:115-25; PMID:23337830; http://dx.doi.org/ 10.1016/j.socscimed.2012.12.020 [DOI] [PubMed] [Google Scholar]
- [68].Savas LS, Fernandez ME, Jobe D, Carmack CC. Human papillomavirus vaccine: 2-1-1 helplines and minority parent decision-making. Am J Prev Med 2012; 43(6 Suppl 5):S490-6; PMID:23157770; http://dx.doi.org/ 10.1016/j.amepre.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health 2013; 103(1):164-9; PMID:22698055; http://dx.doi.org/ 10.2105/AJPH.2011.300600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wong KY, Do YK. Are there socioeconomic disparities in women having discussions on human papillomavirus vaccine with health care providers? BMC Womens Health 2012; 12:33; PMID:23033931; http://dx.doi.org/ 10.1186/1472-6874-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Allen JD, de Jesus M, Mars D, Tom L, Cloutier L, Shelton RC. Decision-Making about the HPV Vaccine among Ethnically Diverse Parents: Implications for Health Communications. J Oncol 2012; 2012:401979; PMID:22174715; http://dx.doi.org/ 10.1155/2012/401979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bednarczyk RA, Birkhead GS, Morse DL, Doleyres H, McNutt LA. Human papillomavirus vaccine uptake and barriers: association with perceived risk, actual risk and race/ethnicity among female students at a New York State university, 2010. Vaccine 2011; 29(17):3138-43; PMID:21376797; http://dx.doi.org/ 10.1016/j.vaccine.2011.02.045 [DOI] [PubMed] [Google Scholar]
- [73].Getrich CM, Broidy LM, Kleymann E, Helitzer DL, Kong AS, Sussman AL. Different models of HPV vaccine decision-making among adolescent girls, parents, and health-care clinicians in New Mexico. Ethn Health 2014; 19(1):47-63; PMID:24261842; http://dx.doi.org/ 10.1080/13557858.2013.857767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr 2011; 11:74; PMID:21878128; http://dx.doi.org/ 10.1186/1471-2431-11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zimet GD, Stupiansky NW, Weiss TW, Rosenthal SL, Good MB, Vichnin MD. Influence of patient's relationship status and HPV history on physicians' decisions to recommend HPV vaccination. Vaccine 2011; 29(3):378-81; PMID:21111781; http://dx.doi.org/ 10.1016/j.vaccine.2010.11.027 [DOI] [PubMed] [Google Scholar]
- [76].Alexander AB, Stupiansky NW, Ott MA, Herbenick D, Reece M, Zimet GD. Parent-son decision-making about human papillomavirus vaccination: a qualitative analysis. BMC Pediatr 2012; 12:192; PMID:23241217; http://dx.doi.org/ 10.1186/1471-2431-12-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hamlish T, Clarke L, Alexander KA. Barriers to HPV immunization for African American adolescent females. Vaccine 2012; 30(45):6472-6; PMID:22910288; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.085 [DOI] [PubMed] [Google Scholar]
- [78].Morales-Campos DY, Markham CM, Peskin MF, Fernandez ME. Hispanic mothers' and high school girls' perceptions of cervical cancer, human papilloma virus, and the human papilloma virus vaccine. J Adolesc Health 2013; 52(5 Suppl):S69-75; PMID:23601613; http://dx.doi.org/ 10.1016/j.jadohealth.2012.09.020 [DOI] [PubMed] [Google Scholar]
- [79].Goff SL, Mazor KM, Gagne SJ, Corey KC, Blake DR. Vaccine counseling: a content analysis of patient-physician discussions regarding human papilloma virus vaccine. Vaccine 2011; 29(43):7343-9; PMID:21839136; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.082 [DOI] [PubMed] [Google Scholar]
- [80].Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol 2009; 19(8):531-8; PMID:19394865; http://dx.doi.org/ 10.1016/j.annepidem.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rand CM, Schaffer SJ, Humiston SG, Albertin CS, Shone LP, Heintz EV, Blumkin AK, Stokley S, Szilagyi PG. Patient-provider communication and human papillomavirus vaccine acceptance. Clin Pediatr (Phila) 2011; 50(2):106-13; PMID:20837607 [DOI] [PubMed] [Google Scholar]
- [82].Mullins TL, Griffioen AM, Glynn S, Zimet GD, Rosenthal SL, Fortenberry JD, Kahn JA. Human papillomavirus vaccine communication: perspectives of 11–12 year-old girls, mothers, and clinicians. Vaccine 2013; 31(42):4894-901; PMID:23916986; http://dx.doi.org/ 10.1016/j.vaccine.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Niccolai LM, Hansen CE, Credle M, Ryan SA, Shapiro ED. Parents' views on human papillomavirus vaccination for sexually transmissible infection prevention: a qualitative study. Sex Health 2014; 11(3):274-9; PMID:24990400; http://dx.doi.org/ 10.1071/SH14047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Clark SJ, Cowan AE, Filipp SL, Fisher AM, Stokley S. Parent HPV vaccine perspectives and the likelihood of HPV vaccination of adolescent males. Hum Vaccin Immunother 2015:0:1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Perkins RB, Chigurupati NL, Apte G, Vercruysse J, Wall-Haaf C, Rosenquist A, Lee L, Clark JA, Pierre-Joseph N. Why don't adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother 2016 Jan 25:0 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Staras SA, Vadaparampil ST, Patel RP, Shenkman EA. Parent perceptions important for HPV vaccine initiation among low income adolescent girls. Vaccine 2014; 32(46):6163-9; PMID:25180815; http://dx.doi.org/ 10.1016/j.vaccine.2014.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dempsey AF, Pyrzanowski J, Lockhart S, Campagna E, Barnard J, O'Leary ST. Parents' perceptions of provider communication regarding adolescent vaccines. Hum Vaccin Immunother. 2016. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J 2013; 17(5):879-85; PMID:22729660; http://dx.doi.org/ 10.1007/s10995-012-1066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician's recommendation. Vaccine 2011; 29(5):890-5; PMID:20056186; http://dx.doi.org/ 10.1016/j.vaccine.2009.12.063 [DOI] [PubMed] [Google Scholar]
- [90].Zimet GD, Weiss TW, Rosenthal SL, Good MB, Vichnin MD. Reasons for non-vaccination against HPV and future vaccination intentions among 19–26 year-old women. BMC Womens Health 2010; 10:27; PMID:20809965; http://dx.doi.org/ 10.1186/1472-6874-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Niccolai LM, Hansen CE, Credle M, Shapiro ED. Parents' Recall and Reflections on Experiences Related to HPV Vaccination for Their Children. Qual Health Res 2015:1049732315575712; PMID:25779984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Moss JL, Reiter PL, Reimer BK, Brewer NT. Collaborative patient-provider communication and uptake of adolescent vaccines. Hum Vaccin Immunother 2016 Jan 19:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Moss JL, Gilkey MB, Reimer BK, Brewer NT. Disparities in collaborative patiernt-provider communication about human papillomavirus vaccination. Hum Vaccin Immunother. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Head KJ, Vanderpool RC, Mills LA. Health care providers' perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs 2013; 30(4):351-60; PMID:23808860; http://dx.doi.org/ 10.1111/phn.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Same RV, Bell DL, Rosenthal SL, Marcell AV. Sexual and reproductive health care: adolescent and adult men's willingness to talk and preferred approach. Am J Prev Med 2014; 47(2):175-81; PMID:24951042; http://dx.doi.org/ 10.1016/j.amepre.2014.03.009 [DOI] [PubMed] [Google Scholar]
- [96].Taylor VM, Burke N, Do H, Liu Q, Yasui Y, Bastani R. HPV vaccination uptake among Cambodian mothers. J Cancer Educ 2012; 27(1):145-8; PMID:21861237; http://dx.doi.org/ 10.1007/s13187-011-0269-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Perkins RB, Apte G, Marquez C, Porter C, Belizaire M, Clark JA, Pierre-Joseph N. Factors affecting human papillomavirus vaccine use among White, Black and Latino parents of sons. Pediatr Infect Dis J 2013; 32(1):e38-44; PMID:22914585; http://dx.doi.org/ 10.1097/INF.0b013e31826f53e3 [DOI] [PubMed] [Google Scholar]
- [98].Aragones A, Genoff M, Gonzalez C, Shuk E, Gany F. HPV Vaccine and Latino Immigrant Parents: If They Offer It, We Will Get It. J Immigr Minor Health 2015; PMID:26001843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Warner EL, Lai D, Carbajal-Salisbury S, Garza L, Bodson J, Mooney K, Kepka D. Latino Parents' Perceptions of the HPV Vaccine for Sons and Daughters. J Community Health 2015; 40(3):387-94; PMID:25269400; http://dx.doi.org/ 10.1007/s10900-014-9949-0 [DOI] [PubMed] [Google Scholar]
- [100].Cates JR, Shafer A, Diehl SJ, Deal AM. Evaluating a County-Sponsored Social Marketing Campaign to Increase Mothers' Initiation of HPV Vaccine for their Pre-teen Daughters in a Primarily Rural Area. Soc Mar Q 2011; 17(1):4-26; PMID:21804767; http://dx.doi.org/ 10.1080/15245004.2010.546943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jim CC, Lee JW, Groom AV, Espey DK, Saraiya M, Holve S, Bullock A, Howe J, Thierry J. Human papillomavirus vaccination practices among providers in Indian health service, tribal and urban Indian healthcare facilities. J Womens Health (Larchmt). 2012; 21(4):372-8; PMID:22309210 [DOI] [PubMed] [Google Scholar]
- [102].Katz ML, Reiter PL, Kluhsman BC, Kennedy S, Dwyer S, Schoenberg N, Johnson A, Ely G, Roberto KA, Lengerich EJ, et al.. Human papillomavirus (HPV) vaccine availability, recommendations, cost, and policies among health departments in seven Appalachian states. Vaccine 2009; 27(24):3195-200; PMID:19446191; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shah PD, McRee AL, Reiter PL, Brewer NT. What parents and adolescent boys want in school vaccination programs in the United States. J Adolesc Health 2014; 54(4):421-7; PMID:24287015; http://dx.doi.org/ 10.1016/j.jadohealth.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Luque JS, Castaneda H, Tyson DM, Vargas N, Meade CD. Formative research on HPV vaccine acceptability among Latina farmworkers. Health Promot Pract 2012; 13(5):617-25; PMID:21881079; http://dx.doi.org/ 10.1177/1524839911414413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fontenot HB. Intersection of HPV and sexual assault: an opportunity for practice change. J Forensic Nurs 2013; 9(3):146-54; PMID:24158152; http://dx.doi.org/ 10.1097/JFN.0b013e318291b276 [DOI] [PubMed] [Google Scholar]
- [106].Vadaparampil ST, Murphy D, Rodriguez M, Malo TL, Quinn GP. Qualitative responses to a national physician survey on HPV vaccination. Vaccine 2013; 31(18):2267-72; PMID:23499608; http://dx.doi.org/ 10.1016/j.vaccine.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Reiter PL, McRee AL, Pepper JK, Brewer NT. Default policies and parents' consent for school-located HPV vaccination. J Behav Med 2012; 35(6):651-7; PMID:22271328; http://dx.doi.org/ 10.1007/s10865-012-9397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ford CA, Skiles MP, English A, Cai J, Agans RP, Stokley S. Minor consent and delivery of adolescent vaccines. J Adolesc Health 2014; 54:183-9; PMID:24074605; http://dx.doi.org/ 10.1016/j.jadohealth.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mayne S, Karavite D, Grundmeier RW, Localio R, Feemster K, DeBartolo E, Hughes CC, Fiks AG. The implementation and acceptability of an HPV vaccination decision support system directed at both clinicians and families. AMIA Annu Symp Proc 2012; 2012:616-24; PMID:23304334 [PMC free article] [PubMed] [Google Scholar]
- [110].Roter D, Hall JA. Doctors talking with patients/patients talking with doctors: improving communication in medical visits: Praeger; Westport, CT; 2006 [Google Scholar]
- [111].Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004; 54(2):78-93; PMID:15061598; http://dx.doi.org/ 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- [112].Dempsey AF, Gebremariam A, Koutsky LA, Manhart L. Using risk factors to predict human papillomavirus infection: implications for targeted vaccination strategies in young adult women. Vaccine 2008; 26(8):1111-7; PMID:18242793; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.088 [DOI] [PubMed] [Google Scholar]
- [113].Gainforth HL, Cao W, Latimer‐Cheung AE. Message framing and parents' intentions to have their children vaccinated against HPV. Public Health Nurs 2012; 29(6):542-52; PMID:23078425; http://dx.doi.org/ 10.1111/j.1525-1446.2012.01038.x [DOI] [PubMed] [Google Scholar]
- [114].Gainforth HL, Latimer AE. Risky business: risk information and the moderating effect of message frame and past behaviour on women's perceptions of the human papillomavirus vaccine. J Health Psychol 2012; 17(6):896-906; PMID:22147061; http://dx.doi.org/ 10.1177/1359105311431173 [DOI] [PubMed] [Google Scholar]
- [115].Gerend MA, Shepherd JE. Using message framing to promote acceptance of the human papillomavirus vaccine. Health Psychol 2007; 26(6):745; PMID:18020847; http://dx.doi.org/ 10.1037/0278-6133.26.6.745 [DOI] [PubMed] [Google Scholar]
- [116].Leader AE, Weiner JL, Kelly BJ, Hornik RC, Cappella JN. Effects of information framing on human papillomavirus vaccination. J Women's Health 2009; 18(2):225-33; PMID:19183094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].McRee AL, Reiter PL, Chantala K, Brewer NT. Does framing human papillomavirus vaccine as preventing cancer in men increase vaccine acceptability? Cancer Epidemiol Biomarkers Prev 2010; 19(8):1937-44; PMID:20647398; http://dx.doi.org/ 10.1158/1055-9965.EPI-09-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Juraskova I, O'Brien M, Mullan B, Bari R, Laidsaar-Powell R, McCaffery K. HPV vaccination and the effect of information framing on intentions and behaviour: An application of the theory of planned behaviour and moral norm. Int J Behav Med 2012; 19(4):518-25; PMID:21879340; http://dx.doi.org/ 10.1007/s12529-011-9182-5 [DOI] [PubMed] [Google Scholar]


