Abstract
Objectives: To define the learning curve associated with adopting ultrasound guidance for prone percutaneous nephrolithotomy (PCNL) for the experienced surgeon.
Methods: A prospective cohort study of consecutive patients undergoing PCNL with ultrasound guidance for renal tract access and dilation was performed. Clinical data reviewed included success in gaining renal access with ultrasound guidance, total fluoroscopic screening time, and radiation exposure dose. PCNL cases performed with fluoroscopic guidance matched for stone size served as control cases.
Results: One hundred consecutive ultrasound-guided procedures performed by a single experienced endourologist were divided into five experience groups. Significant improvement in renal access success rate with ultrasound was seen after 20 cases (p < 0.05). Total fluoroscopic screening time, radiation exposure dose, and operative time were also statistically significantly improved over the study period. When compared with fluoroscopy-guided PCNL, significant decreases in total fluoroscopic screening time (33.4 ± 35.3 seconds vs 157.5 ± 84.9 seconds, p < 0.05) and radiation exposure (7.0 ± 8.7 mGy vs 47.8 ± 45.9 mGy, p < 0.05) were seen. No differences in complication rates were found.
Conclusions: Ultrasound-guided renal access for PCNL can be performed effectively after 20 cases. Transition to the use of ultrasound will quickly reduce radiation exposure for patients and intraoperative personnel.
Introduction
Percutaneous nephrolithotomy (PCNL) is a procedure commonly performed by urologists worldwide.1,2 Important steps in this procedure are collecting system imaging and stone localization, renal access, tract dilation, and evaluation of possible residual fragments. Fluoroscopy is the imaging modality most commonly used for these purposes.3 However, a major drawback of fluoroscopy is the associated ionizing radiation exposure to the patient and intraoperative personnel.4
Ultrasonography (US) can be a reliable modality for localizing renal stones.5 It facilitates identification of posterior renal calices while avoiding vasculature and surrounding organs and is free of ionizing radiation compared with fluoroscopy.6 These features facilitate safe percutaneous renal access into a posterior calix while reducing radiation exposure to the patient, surgeon, and staff. Other benefits may also be derived, including reduced surgeon and staff fatigue by obviating the need to wear lead aprons.
Urologists who adopt ultrasound guidance for PCNL could gain these benefits, although a learning curve is involved.7 We transitioned from fluoroscopic to ultrasonographic guidance for PCNL, and the goal of this study is to report our experience and describe the associated learning curve.
Patients and Methods
This was a prospective cohort study of 120 PCNL procedures performed at two academic medical centers between July 2013 and February 2016. Institutional review board (IRB) approval for prospective collection of human clinical information for all nephrolithiasis patients was obtained (CHR #14-4533). All procedures were performed by a single endourology fellowship-trained surgeon (T.C.). Before May 2014, the study surgeon performed all PCNL procedures entirely under fluoroscopic guidance and had minimal experience with performing diagnostic renal ultrasound imaging. In May 2014, after participating in six mentored hands on hands cases with a surgeon experienced in using ultrasound guidance for PCNL (J.L.), the operative surgeon adopted ultrasound guidance for PCNL.
During these mentored cases, the study surgeon performed the procedure with the mentor surgeon present and scrubbed in, providing hands on feedback as needed. After adopting ultrasound guidance, ultrasound was used initially for renal imaging and percutaneous access only, and eventually for all steps of renal access and dilation. Clinical data for 100 consecutively performed PCNL procedures were collected between May 2014 and February 2016. Twenty fluoroscopy-guided PCNL cases performed between July 2013 and May 2014 by the same operative surgeon were used as a control comparison group. The study surgery performed all steps (including access, tract dilation, and lithotripsy) for these control fluoroscopy-guided cases. The complexity for fluoroscopy guided vs ultrasound guided cases was relatively similar, matched for stone size, patient body mass index (BMI), and the presence of abnormal anatomy or staghorn stones.
Preoperative patient demographic data were obtained. Stone burden was determined by measuring the maximal stone length, stone location, and degree of hydronephrosis on the preoperative CT scan. Skin to stone distance (SSD) was calculated on CT scan by three measured distances from the center of the stone to the skin (0°, 45°, and 90°) at the widest dimension of the stone on cross-sectional images.8,9 Kidney depth (KD) was also collected, determined by average the anterior and posterior depths at the renal hilum on axial images.10
The operative surgeon was a fellowship-trained attending surgeon whose prior experience in PCNL had been exclusively with fluoroscopic guidance for renal tract access and dilation. We have previously published details regarding surgical technique used for this study.11 Briefly, all PCNLs were performed under general anesthesia in the prone position. Collecting system puncture was done under real-time ultrasonographic monitoring freehand without the aid of a needle guide. We used a 3.5-MHz convex abdominal transducer (Hitachi Aloka Alpha 7; Hitachi Aloka Medical) to localize the stone position as well as an ideally suited posterior calix for puncture. A high-pressure 24F balloon (BARD X-Force; Bard Medical) was used for tract dilation and insertion of the working sheath.
At the end of the procedure, a nephrostomy tube was placed in all patients. Initially after adopting ultrasound guidance, only percutaneous renal access was performed utilizing US imaging, and the remainder of the procedure with fluoroscopy. Over the course of the study period, the operative surgeon utilized ultrasound imaging for additional steps, including tract dilation, and determination of the presence of residual stone fragments. By the end of the study period, nephrostomy tube placement, performed under fluoroscopic guidance in the vast majority of cases, was also done under ultrasound guidance to confirm final positioning.
Fluoroscopic screening (GE OEC 9800 Plus Mobile C-arm; GE Healthcare) was utilized by the same operative surgeon when ultrasound imaging was inadequate to achieve effective guidance for that step in the renal access and dilation process. During cases in which renal access could not be obtained by ultrasound guidance, fluoroscopy was utilized and the ultrasound success was scored as a failure. For each procedure, fluoroscopic screening time, total radiation exposure dose measured in milliGray (mGy), and total operative time were recorded.
Fluoroscopic screening time and estimated total radiation exposure values were taken directly from recordings made on the fluoroscopy unit at the end of each case. Serum creatinine and hematocrit levels were checked on the first postoperative day. A combination of plain radiograph kidney, ureter, and bladder (KUB) and renal ultrasound was used to evaluate stone-free status 30 days after surgery, which was divided into three categories: stone free, insignificant residual fragment (<4 mm), and significant residual fragment. All perioperative complications occurring within 30 days postoperatively were recorded according to the Clavien–Dindo classification system.12
To evaluate the learning curve, cases consecutively performed with US guidance were divided into five temporally collected groups for which intraoperative parameters and perioperative complications were compared. US cases were then compared with fluoroscopy cases matched for stone size and BMI.
ANOVA, chi-square test, and Student's t-test were used to compare intergroup differences, and statistical analyses were performed using Stata/IC version 13.1 (StataCorp). Data are expressed as mean ± standard deviation or percentage with a significance level of p < 0.05.
Results
This study cohort comprised 120 patients who underwent PCNL for stone removal, 100 of whom underwent consecutive ultrasound-guided renal access and 20 nonconsecutive fluoroscopy-guided renal access. The ultrasound cohort was split into groups 1 through 5 temporally, with 20 cases in each group. Mean age of patients was 52.3 ± 15.6 years, with female patients slightly predominant. Half of the patients had an American Society of Anesthesiologists (ASA) physical status classification of 2 with an average BMI of 28.1 ± 7.3 kg/m2. SSD and KD were comparable across the five ultrasound groups. Renal pelvis, caliceal, and staghorn stones were found in 28.0%, 26.0%, and 20% of patients, respectively, with 55% of stones seen on the left side. Mean stone size was 28.8 ± 16.9 mm and, in the majority of cases, no or mild hydronephrosis was found on preoperative imaging (Table 1).
Table 1.
Ultrasound Cohort Patient Characteristics
| Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Total | p |
|---|---|---|---|---|---|---|---|
| Mean (SD) age (years) | 55.6 ± 9.2 | 50.1 ± 13.5 | 49.9 ± 16.7 | 53.6 ± 17.8 | 52.5 ± 14.9 | 52.3 ± 15.6 | 0.77 |
| Gender, n (%) | |||||||
| Male | 12 (60) | 6 (30) | 9 (45.0) | 9 (45.0) | 10 (50.0) | 46 (46.0) | 0.44 |
| Female | 8 (40) | 14 (70) | 11 (55.0) | 11 (55.0) | 10 (50.0) | 54 (54.0) | |
| Mean (SD) BMI (kg/m2) | 30.9 ± 9.4 | 27.7 ± 6.6 | 26.2 ± 5.7 | 25.7 ± 6.0 | 30.1 ± 7.3 | 28.1 ± 7.3 | 0.10 |
| Mean (SD) skin to stone distance (mm) | 120.8 ± 40.2 | 101.3 ± 23.8 | 104.2 ± 29.5 | 98.5 ± 25.8 | 97.6 ± 21.0 | 105.0 ± 29.9 | 0.10 |
| Mean (SD) kidney depth (mm) | 98.7 ± 30.4 | 83.1 ± 18.7 | 90.8 ± 24.2 | 83.7 ± 21.7 | 85.3 ± 21.5 | 88.5 ± 24.1 | 0.22 |
| Mean (SD) preoperative serum creatinine (mg/dL) | 0.96 ± 0.30 | 1.04 ± 0.72 | 0.90 ± 0.35 | 0.92 ± 0.29 | 0.95 ± 0.36 | 0.95 ± 0.42 | 0.38 |
| Mean (SD) preoperative hematocrit (%) | 42.3 ± 6.2 | 38.7 ± 4.5 | 38.6 ± 5.8 | 39.8 ± 6.8 | 40.7 ± 4.5 | 40.0 ± 5.7 | 0.23 |
| ASA physical status, n (%) | |||||||
| Class 1 | 0 | 5 (25.0) | 3 (15.0) | 3 (15.0) | 1 (5.0) | 12 (12.0) | 0.50 |
| Class 2 | 13 (65.0) | 10 (50.0) | 10 (50.0) | 11 (55.0) | 14 (70.0) | 58 (58.0) | |
| Class 3 | 6 (30.0) | 5 (25.0) | 7 (35.0) | 6 (30.0) | 4 (20.0) | 28 (28.0) | |
| Class 4 | 1 (5.0) | 0 | 0 | 0 | 1 (5.0) | 2 (2.0) | |
| Stone laterality, n (%) | |||||||
| Right | 11 (55.0) | 9 (45.0) | 9 (45.0) | 6 (30.0) | 10 (50.0) | 45 (45.0) | 0.59 |
| Left | 9 (45.0) | 11 (55.0) | 11 (55.0) | 14 (70.0) | 10 (50.0) | 55 (55.0) | |
| Stone type and position, n (%) | |||||||
| Caliceal stone | 6 (30.0) | 4 (20.0) | 5 (25.0) | 6 (30.0) | 5 (25.0) | 26 (26.0) | 0.66 |
| Staghorn stone | 5 (25.0) | 9 (45.0) | 7 (35.0) | 4 (20.0) | 3 (15.0) | 28 (28.0) | |
| Pelvic stone | 4 (20.0) | 3 (15.0) | 2 (10.0) | 4 (20.0) | 7 (35.0) | 20 (20.0) | |
| Upper ureteral stone | 2 (10.0) | 1 (5.0) | 1 (5.0) | 3 (15.0) | 0 | 7 (7.0) | |
| Multiple stones | 3 (15.0) | 3 (15.0) | 5 (25.0) | 3 (15.0) | 5 (25.0) | 19 (19.0) | |
| Mean (SD) stone size (mm) | 27.6 ± 15.0 | 25.6 ± 16.4 | 24.3 ± 13.3 | 28.1 ± 19.7 | 38.6 ± 17.2 | 28.8 ± 16.9 | 0.06 |
| Degree of hydronephrosis, n (%) | |||||||
| None | 6 (30.0) | 7 (35.0) | 12 (60.0) | 9 (45.0) | 14 (70.0) | 48 (48.0) | 0.09 |
| Mild | 5 (25.0) | 10 (50.0) | 3 (15.0) | 6 (30.0) | 3 (15.0) | 27 (27.0) | |
| Moderate | 5 (25.0) | 3 (15.0) | 4 (20.0) | 4 (20.0) | 2 (10.0) | 18 (18.0) | |
| Severe | 4 (20.0) | 0 | 1 (5.0) | 1 (5.0) | 1 (5.0) | 7 (7.0) | |
ASA = American Society of Anesthesiologists; BMI = body mass index; SD = standard deviation.
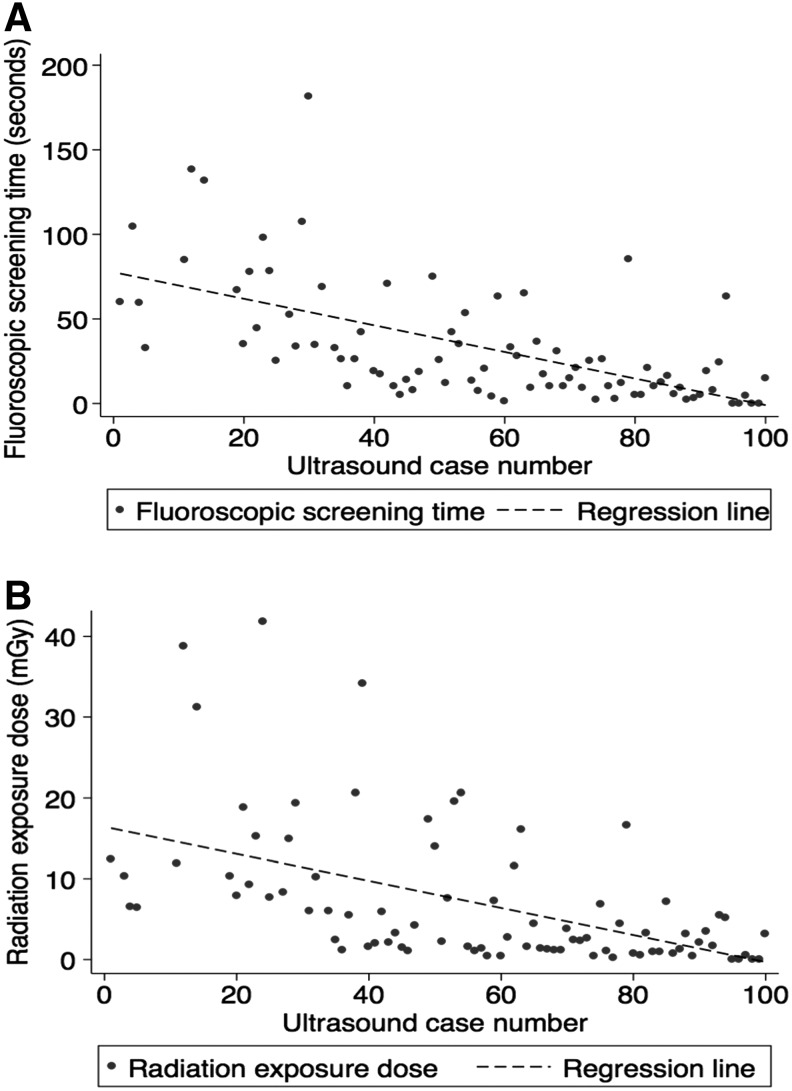
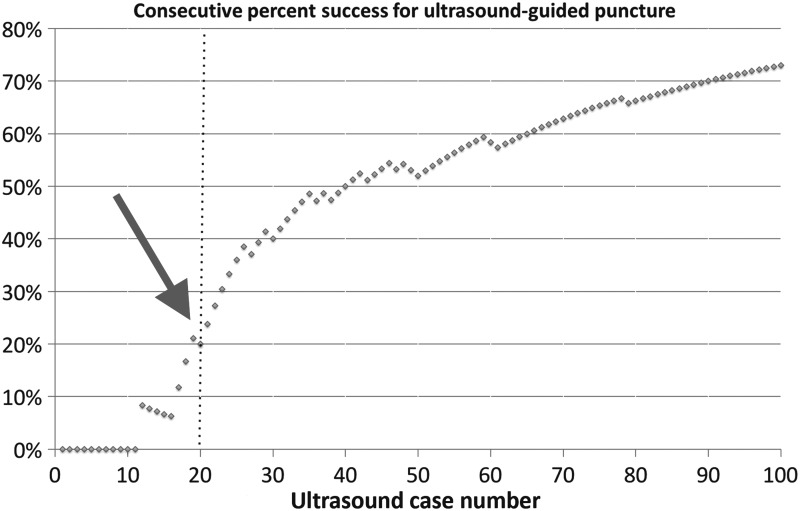
Intraoperatively, mean fluoroscopic screening time (reflective of the total amount of time the fluoroscopy pedal was depressed and the imaging unit was activated) was reduced respectively from 79.2 ± 38.6 seconds in group 1 to 11.1 ± 14.3 seconds in group 5 (p < 0.01). Similarly, mean radiation exposure dose showed a consecutive downward trend from 15.1 ± 11.7 mGy in group 1 to 2.0 ± 2.1 mGy in group 5 (p < 0.01) (Table 2 and Fig. 1). The largest number of cases was performed through a lower pole caliceal access site (36.0%). The overall success rate for gaining percutaneous renal access with ultrasound guidance alone was 30% for group 1. The later groups showed significantly improved success rates of 75%, 85%, 95%, and 100% for groups 2 through 5, respectively (p < 0.05). Plotting the cumulative success of ultrasound-guided renal access over consecutive cases revealed that the inflection point for success was seen at the 20th case (Fig. 2).
Table 2.
Ultrasound Cohort Intraoperative and Postoperative Outcomes
| Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Total | P |
|---|---|---|---|---|---|---|---|
| Mean (SD) total fluoroscopic screening time (seconds) | 79.2 ± 38.6 | 56.2 ± 42.8 | 26.0 ± 23.6 | 22.6 ± 20.9 | 11.1 ± 14.3 | 33.4 ± 35.3 | <0.01 |
| Mean (SD) total radiation exposure dose (mGy) | 15.1 ± 11.7 | 13.1 ± 11.3 | 6.0 ± 6.8 | 4.1 ± 4.9 | 2.0 ± 2.1 | 7.0 ± 8.7 | <0.01 |
| Mean (SD) total operative time (minutes) | 190.1 ± 74.4 | 150.3 ± 45.3 | 136.6 ± 39.4 | 117.7 ± 41.7 | 124.2 ± 37.7 | 143.8 ± 55.3 | <0.01 |
| Success in puncture with US-guided, n (%) | 6 (30.0) | 15 (75.0) | 17 (85.0) | 19 (95.0) | 20 (100.0) | 77 (77.0) | <0.01 |
| Puncture approach | |||||||
| Lower calix | 12 (60.0) | 5 (25.0) | 7 (35.0) | 7 (35.0) | 5 (25.0) | 36 (36.0) | 0.11 |
| Middle calix | 3 (15.0) | 9 (45.0) | 4 (20.0) | 4 (20.0) | 5 (25.0) | 25 (25.0) | |
| Upper calix | 4 (20.0) | 2 (10.0) | 8 (40.0) | 8 (40.0) | 8 (40.0) | 30 (30.0) | |
| Multiple tracts | 1 (5.0) | 4 (20.0) | 1 (5.0) | 1 (5.0) | 2 (10.0) | 9 (9.0) | |
| Mean (SD) postoperative serum creatinine (mg/dL) | 1.12 ± 0.76 | 1.02 ± 0.57 | 0.88 ± 0.31 | 0.92 ± 0.43 | 0.93 ± 0.30 | 0.97 ± 0.50 | 0.58 |
| Mean (SD) difference in preoperative and postoperative serum hematocrit (%) | 3.1 ± 5.2 | 5.1 ± 4.3 | 3.1 ± 5.4 | 5.0 ± 5.4 | 5.6 ± 3.3 | 4.4 ± 4.8 | 0.33 |
| Mean (SD) hospital stay | 2.8 ± 0.7 | 3.2 ± 1.3 | 3.2 ± 1.4 | 2.8 ± 1.1 | 2.4 ± 0.5 | 2.9 ± 1.1 | 0.16 |
| Postoperative complication, n (%) | |||||||
| None | 18 (90.0) | 16 (80.0) | 19 (95.0) | 18 (90.0) | 18 (90.0) | 89 (89.0) | 0.65 |
| Complication | 2 (10.0) | 4 (20.0) | 1 (5.0) | 2 (10.0) | 2 (10.0) | 11 (11.0) | |
| Grade 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade 2 | 0 | 1 | 5 | 2 | 1 | 9 | |
| Grade 3 | 1 | 1 | 0 | 0 | 1 | 3 | |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade 5 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Stone-free status, n (%) | |||||||
| Stone-free | 17 (85.0) | 18 (90.0) | 20 (100.0) | 17 (85.0) | 16 (80.0) | 88 (88.0) | 0.14 |
| Insignificant residual stone | 0 | 0 | 0 | 2 (10.0) | 3 (15.0) | 5 (5.0) | |
| Significant residual stone | 3 (15.0) | 2 (10.0) | 0 | 1 (5.0) | 1 (5.0) | 7 (7.0) | |
| Secondary procedure, n (%) | 3 (15.0) | 0 | 0 | 0 | 0 | 3 (3.0) | <0.05 |
US = ultrasonography.
FIG. 1.
Correlations between number of cases performed with ultrasound guidance to (A) fluoroscopic screening time (seconds) and (B) radiation exposure dose (mGy). The Pearson coefficient of correlation for fluoroscopic screening time was r2 = 0.37 and for radiation exposure dose was r2 = 0.27 with resultant p-values of p < 0.001 for each.
FIG. 2.
Consecutive percent of success for ultrasound-guided renal access. The inflection point of success (arrow) was seen at the 20th case.
Over the whole ultrasound-guided PCNL cohort, mean operative time decreased from 190.1 ± 74.4 minutes in group 1 to 124.2 ± 37.7 minutes in group 5 (Table 2). Eighty eight percent of patients were rendered stone free after a single procedure, with 3.0% requiring secondary procedures for completion of stone removal (Table 2).
Postoperatively, overall mean hospital stay (2.9 ± 1.1 days), blood transfusion rate (3.0%), and complication rate (11.0%) did not differ over the five ultrasound experience groups. The majority of complications experienced were Clavien–Dindo classification grade 2 and mostly comprised transient postoperative fever necessitating prolonged antibiotic treatment (Table 2).
Comparing these findings to the control fluoroscopy cohort of 20 cases matched for stone size and BMI, total radiation exposure and fluoroscopic screening times were reduced nearly sixfold in the ultrasound group (7.0 ± 8.7 mGy vs 47.8 ± 45.9 mGy and 33.4 ± 35.3 seconds vs 157.5 ± 84.9 seconds, respectively, p < 0.01). Other intraoperative and perioperative parameters were unchanged between the two groups (Table 3).
Table 3.
Comparative Analysis Between Cases Performed with Ultrasound vs Fluoroscopy Guidance
| Parameter | Ultrasound group (n = 100) | Fluoroscopic group (n = 20) | p |
|---|---|---|---|
| Mean (SD) stone size (mm) | 28.8 ± 16.9 | 29.2 ± 14.4 | 0.93 |
| Mean (SD) total fluoroscopic screening time (seconds) | 33.4 ± 35.3 | 157.5 ± 84.9 | <0.01 |
| Mean (SD) total radiation exposure dose (mGy) | 7.0 ± 8.7 | 47.8 ± 45.9 | <0.01 |
| Mean (SD) total operative time (minutes) | 143.8 ± 55.3 | 140.3 ± 53.0 | 0.80 |
| Mean (SD) difference in preoperative and postoperative serum hematocrit (%) | 4.4 ± 4.8 | 2.3 ± 4.2 | 0.08 |
| Mean (SD) hospital stay | 2.9 ± 1.1 | 3.2 ± 2.4 | 0.33 |
| Postoperative complication, n (%) | |||
| None | 89 (89.0) | 18 (90.0) | 0.90 |
| Complication | 11 (11.0) | 2 (10.0) | |
| Stone-free status, n (%) | |||
| Stone free | 88 (88.0) | 18 (90.0) | 0.95 |
| Insignificant residual stone | 5 (5.0) | 1 (5.0) | |
| Significant residual stone | 7 (7.0) | 1 (5.0) | |
| Secondary procedure, n (%) | 3 (3.0) | 1 (5.0) | 0.65 |
Discussion
PCNL is the primary procedure utilized for the management of patients with renal stones who are not candidates for retrograde intrarenal surgery or shockwave lithotripsy.13 Fluoroscopic guidance is the most commonly used imaging modality for obtaining percutaneous renal access, establishing the working tract, and performing stone extraction.3 Given the recurrent nature of nephrolithiasis, cumulative patient lifetime exposure to radiation may be a significant concern.14 For intraoperative personnel, cumulative exposure to ionizing radiation acquired during procedures can also increase the risk of developing cancer, cataracts, or other secondary illnesses.15,16 Therefore, reducing the use of ionizing radiation during procedures that traditionally rely on fluoroscopic imaging can be of benefit to both patients and providers.
Real-time diagnostic US is commonly used by urologists in some areas outside of the United States to obtain percutaneous renal access for PCNL.17 US is totally free of ionizing radiation. It provides imaging information beyond that provided by fluoroscopy, including the location of visceral and vascular structures between the skin and renal calix, easy differentiation between posterior and anterior renal calices, as well as the depth of the access needle.11,18,19 It does not require retrograde contrast injection and can be used for cases marked by ineffective retrograde ureteral catheterization.18 Importantly, it is also suitable for use in pediatric patients where radiation exposure may be of particular concern.20 Moreover, US imaging at the end of the procedure can be used to locate nonopaque and semiopaque residual stones that are not easily visualized by fluoroscopic radiography.21
Applying US to PCNL leads to significant reduction in radiation exposure and provides an alternative method for renal access and instrumentation, however, adopting it is associated with an expected learning curve. Describing this learning curve was the main goal for our study with the hopes of encouraging experienced urologists to adopt US for PCNL.
While a single definition for a PCNL learning curve does not exist, operative time, fluoroscopic screening time, and radiation dose may be used as surrogate markers for how well the procedure has been learned.22 Allen et al. reported a case series of a novice endourologist performing solo PCNL and found that mean operative and fluoroscopic screening times approached those of a senior surgeon after 60 cases.23 Ziaee et al. examined surgical outcomes for an inexperienced endourology fellow performing 105 consecutive cases of solo PCNL.24 Their analysis demonstrated that significant improvement was observed in operative time and complication rate over time and a performance plateau was reached after 45 cases. Similarly, others have examined total operative time as a marker for competency in PCNL.25,26 Schilling et al. retrospectively studied the total operative time of a novice surgeon who performed 35 PCNLs over the course of 1 year and compared their outcomes to an expert surgeon.26 The novice surgeon demonstrated significant improvement over the course of 1 year, and for their last 10 procedures, the mean operative time approached that of the expert surgeon. Song et al. reported a series of 120 ultrasound-guided PCNLs performed by one novice surgeon. They concluded that 60 procedures were needed to achieve a mean operative time, renal access time, and tract dilation time equivalent to those of the senior surgeon.27 All of these studies have implied that for PCNL, the steep learning curve is mainly related to obtaining renal access, which undoubtedly contributes to the fact that as few as 11% of practicing urologists have reported obtaining their own access in a survey based out of the United States.28
To apply ultrasound guidance to achieving renal access for PCNL, two technical skills are required. These include imaging the kidney clearly with accurate interpretation and coordinating the needle hand with the imaging hand to advance the needle within the imaging plane into the chosen target.11 During the early part of our learning curve, when achieving renal access using ultrasound was only 30% effective, failures were attributable to suboptimal imaging, misinterpretation of images (particularly in nondilated kidneys when the collecting system was hard to differentiate from renal pyramids or sinus fat), and inaccurate needle placement.
To improve accuracy of needle placement and, thereby, reduce the number of technical skills required to perform ultrasound-guided PCNL from two down to one, a needle guide can be used.29 Needle guides facilitate accurate needle placement within the plane of imaging; however, they are also accompanied with some limitations. Namely, needle guides only permit a limited number of angles of entry into the kidney relative to the probe. For example, in some instances it might be advantageous to approach a renal calix from a steeper or more shallow trajectory to the skin to avoid bony structures such as the ribs or hips. A needle guide may limit one's ability to adjust these angles in an unlimited manner. We elected to not use a needle guide to learn how to achieve maximal flexibility in angle of entry to the kidney, but use of a needle guide might have improved early success rates by reducing the technical challenge of keeping the needle in the imaging plan.
While the operative surgeon underwent six mentored cases before adopting ultrasound, it is also likely that accuracy in performing and interpreting renal imaging may have improved more quickly if more mentored cases had been possible or if they had had more exposure to ultrasound earlier in their training. While a learning curve of 20 cases may be relatively short, with a different structured environment for learning this technique, the learning curve might be shortened further.
In describing the learning curve in our study, we used effective renal access with ultrasound guidance as one parameter for competency. Arguably, the two most challenging steps encountered during PCNL are accurate collecting system puncture and placement of the access sheath.30 We defined effective US-guided access as using only US to gain access into a posterior calix and the ability to enter the kidney and complete the procedure with this access. For all of our cases, the fluoroscopic C arm was available if the US-gained access was deemed inappropriate for dilation by the surgeon. In those cases, fluoroscopy was used to obtain alternative access into the collecting system, and these cases were not counted as effective.
In our own study, the success rate significantly increased after the first 20 cases, from 30% to 75–100%, which defines our learning curve. This learning curve reflects that which could be applied to an experienced surgeon adopting ultrasound as a new technique. While our 20 cases cannot be directly compared with the previously cited 45 to 60 cases in the fluoroscopy studies that often evaluated a novice urologist learning the entire procedure, the context is certainly valuable. Perhaps even more striking is the dramatic drop that occurred after the first 20 cases in the two main learning curve parameters, radiation exposure and fluoroscopic screening time. An increasing comfort level with ultrasound imaging was likely associated with the decreased reliance on fluoroscopy over time. By the end of the study period, the operative surgeon was routinely attempting to use ultrasound to direct nephrostomy tube placement and confirm the tube position in the collecting system, which resulted in continued decreases in fluoroscopic screening time. This trend continued, and by the 100th case, the radiation exposure during ultrasonographic PCNL for patients and providers is less than one-sixth of that compared with fluoroscopic PCNL.
We contend that the relatively short learning curve associated with adopting ultrasound-guided renal tract access and dilation and significantly reduced radiation exposure, even after only 20 cases, provide a strong rationale for urologists to obtain their own renal access using ultrasound guidance. The remainder of the procedure can still be performed using fluoroscopic guidance, as was done during the early cases of this series, with the immediate benefit of reduced radiation exposure to the patient and intraoperative personnel.
To evaluate the safety and efficacy of ultrasound-guided PCNL, we compared the surgical outcomes to those of fluoroscopy-guided PCNL. No significant differences were seen between the two groups. This held true when looking at the entire ultrasound cohort as a whole and when comparing the early experience cohort only to the fluoroscopic group. We experienced similar stone-free status with the use of fluoroscopy (90%) and ultrasound (88%) for PCNL guidance as well as similar relatively low rates of secondary procedures for patients (5% and 3%, respectively). Our standard practice is to perform ultrasound and KUB 30 days after surgery to look for residual stone fragments, and secondary procedures are reserved for patients with symptomatic fragments. While this practice might differ from surgeon to surgeon, our study demonstrated that using either imaging guidance technique did not cause a shift in rate of residual stone fragments or need for secondary procedure within one surgeon's practice. From these analyses, it appears that if ultrasound guidance for PCNL is adopted in a staged manner, that is, with a focus on just obtaining access at the beginning and over time applying ultrasound to additional steps of the procedure, stable, safe clinical outcomes can be achieved.
For urologists interested in transitioning toward ultrasound-guided PCNL, our learning curve experience may offer some insight on how to initiate that transition. When starting out with renal ultrasound, imaging the lower pole is easier, as it takes some experience to effectively avoid rib shadows to image the upper pole well. This is reflected by the nonstatistically significantly higher rate of lower pole access seen in the earliest experience cohort. In addition, when looking within each group for factors that predicted success in renal access, we are unable to identify any definitive element. This may be because our groups were underpowered to identify these differences. Anecdotally, patients with a lower BMI, dilated collecting systems, and smaller stones may be easier to effectively access with ultrasound guidance. Initially, using the lower pole in patients with these characteristics may be a good setting in which the operative surgeon can build skills and confidence to effectively transition toward performing PCNL using ultrasound guidance.
A primary limitation of this study was that it reflected the experience of a single experienced surgeon. This surgeon was fellowship trained in endourology and had significant prior experience in performing PCNL under fluoroscopic guidance. To adopt ultrasound for renal access and dilation, he performed mentored cases with a surgeon experienced in the use of ultrasound for PCNL. This might contribute to our finding of a relatively short learning curve to adopt US for PCNL compared with earlier learning curve studies. Thus, our learning curve may not be directly applicable to the novice urologist or urologist who is unfamiliar with percutaneous renal access, but it provides a valuable context and the results are encouraging. Importantly, the radiation exposure reduction occurs early in the learning curve period and should be achievable both by urologists who regularly perform their own renal access and those interested in learning this portion of the procedure. Since completing this study, at our institution, residents and trainees have begun formal training in learning to use ultrasound to direct renal access for PCNL. In a structured environment for learning ultrasound skills, trainees appear to be able to adopt these skills with relative efficiency. Studies evaluating the learning curve for novice surgeons and across institutions are currently ongoing.
Conclusions
The learning curve associated with ultrasound-guided PCNL centers around collecting system puncture and working tract establishment. As our study demonstrates, achieving effective ultrasound-guided renal access can occur within 20 cases and is associated with significantly reduced total radiation exposure dose and fluoroscopic screening time. This technique may be of great value to the practicing urologist interested in reducing radiation exposure with PCNL without compromising clinical outcomes.
Abbreviations Used
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CT
computed tomography
- KD
kidney depth
- KUB
kidney, ureter, and bladder
- PCNL
percutaneous nephrolithotomy
- SSD
skin to stone distance
- US
ultrasonography
Acknowledgment
This study was supported by K12-DK-07-006: Multidisciplinary K12 Urologic Research Career Development Program (T.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Patel SR, Nakada SY. The modern history and evolution of percutaneous nephrolithotomy. J Endourol 2015;29:153–157 [DOI] [PubMed] [Google Scholar]
- 2.Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: Diagnosis and treatment recommendations. J Urol 2005;173:1991–2000 [DOI] [PubMed] [Google Scholar]
- 3.Andonian S, Scoffone CM, Louie MK, et al. Does imaging modality used for percutaneous renal access make a difference? A matched case analysis. J Endourol 2013;27:24–28 [DOI] [PubMed] [Google Scholar]
- 4.Lodh B, Gupta S, Singh AK, Sinam RS. Ultrasound guided direct percutaneous nephrostomy (PCN) tube placement: Stepwise report of a new technique with its safety and efficacy evaluation. J Clin Diagn Res 2014;8:84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanno T, Kubota M, Sakamoto H, et al. The efficacy of ultrasonography for the detection of renal stone. Urology 2014;84:285–288 [DOI] [PubMed] [Google Scholar]
- 6.Penbegul N, Hatipoglu NK, Bodakci MN, et al. Role of ultrasonography in percutaneous renal access in patients with renal anatomic abnormalities. Urology 2013;81:938–942 [DOI] [PubMed] [Google Scholar]
- 7.Mishra S, Jagtap J, Sabnis RB, Desai MR. Training in percutaneous nephrolithotomy. Curr Opin Urol 2013;23:147–151 [DOI] [PubMed] [Google Scholar]
- 8.Pareek G, Hedican SP, Lee FT, Jr., Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 2005;66:941–944 [DOI] [PubMed] [Google Scholar]
- 9.Gonulalan U, Akand M, Coban G, et al. Skin-to-stone distance has no impact on outcomes of percutaneous nephrolithotomy. Urol Int 2014;92:444–448 [DOI] [PubMed] [Google Scholar]
- 10.Taylor A, Lewis C, Giacometti A, Hall EC, Barefield KP. Improved formulas for the estimation of renal depth in adults. J Nucl Med 1993;34:1766–1769 [PubMed] [Google Scholar]
- 11.Chu C, Masic S, Usawachintachit M, et al. Ultrasound-guided renal access for percutaneous nephrolithotomy: A description of three novel ultrasound-guided needle techniques. J Endourol 2016;30:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 13.Ziemba JB, Matlaga BR. Guideline of guidelines: Kidney stones. BJU Int 2015;116:184–189 [DOI] [PubMed] [Google Scholar]
- 14.Chen TT, Wang C, Ferrandino MN, et al. Radiation exposure during the evaluation and management of nephrolithiasis. J Urol 2015;194:878–885 [DOI] [PubMed] [Google Scholar]
- 15.Faulkner K, Vano E. Deterministic effects in interventional radiology. Radiat Prot Dosimetry 2001;94:95–98 [DOI] [PubMed] [Google Scholar]
- 16.Majidpour HS. Risk of radiation exposure during PCNL. Urol J 2010;7:87–89 [PubMed] [Google Scholar]
- 17.Li J, Xiao B, Hu W, et al. Complication and safety of ultrasound guided percutaneous nephrolithotomy in 8,025 cases in China. Chin Med J (Engl) 2014;127:4184–4189 [PubMed] [Google Scholar]
- 18.Lojanapiwat B. The ideal puncture approach for PCNL: Fluoroscopy, ultrasound or endoscopy? Indian J Urol 2013;29:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbulut F, Tok A, Penbegul N, et al. Colon perforation related to percutaneous nephrolithotomy: From diagnosis to treatment. Urolithiasis 2015;43:521–526 [DOI] [PubMed] [Google Scholar]
- 20.Penbegul N, Soylemez H, Bozkurt Y, et al. An alternative and inexpensive percutaneous access needle in pediatric patients. Urology 2012;80:938–940 [DOI] [PubMed] [Google Scholar]
- 21.Yan S, Xiang F, Yongsheng S. Percutaneous nephrolithotomy guided solely by ultrasonography: A 5-year study of >700 cases. BJU Int 2013;112:965–971 [DOI] [PubMed] [Google Scholar]
- 22.de la Rosette JJ, Laguna MP, Rassweiler JJ, Conort P. Training in percutaneous nephrolithotomy—A critical review. Eur Urol 2008;54:994–1001 [DOI] [PubMed] [Google Scholar]
- 23.Allen D, O'Brien T, Tiptaft R, Glass J. Defining the learning curve for percutaneous nephrolithotomy. J Endourol 2005;19:279–282 [DOI] [PubMed] [Google Scholar]
- 24.Ziaee SA, Sichani MM, Kashi AH, Samzadeh M. Evaluation of the learning curve for percutaneous nephrolithotomy. Urol J 2010;7:226–231 [PubMed] [Google Scholar]
- 25.Tanriverdi O, Boylu U, Kendirci M, Kadihasanoglu M, Horasanli K, Miroglu C. The learning curve in the training of percutaneous nephrolithotomy. Eur Urol 2007;52:206–211 [DOI] [PubMed] [Google Scholar]
- 26.Schilling D, Gakis G, Walcher U, Stenzl A, Nagele U. The learning curve in minimally invasive percutaneous nephrolitholapaxy: A 1-year retrospective evaluation of a novice and an expert. World J Urol 2011;29:749–753 [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Ma Y, Song Y, Fei X. Evaluating the learning curve for percutaneous nephrolithotomy under total ultrasound guidance. PLoS One 2015;10:e0132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird VG, Fallon B, Winfield HN. Practice patterns in the treatment of large renal stones. J Endourol 2003;17:355–363 [DOI] [PubMed] [Google Scholar]
- 29.Desai M. Ultrasonography-guided punctures-with and without puncture guide. J Endourol 2009;23:1641–1643 [DOI] [PubMed] [Google Scholar]
- 30.Jagtap J, Mishra S, Bhattu A, Ganpule A, Sabnis R, Desai MR. Which is the preferred modality of renal access for a trainee urologist: Ultrasonography or fluoroscopy? Results of a prospective randomized trial. J Endourol 2014;28:1464–1469 [DOI] [PubMed] [Google Scholar]