Abstract
Background:
Noncontiguous cervical spondylotic myelopathy (CSM) is a special degenerative disease because of the intermediate normal level or levels between supra and infraabnormal levels. Some controversy exists over the optimal procedure for two noncontiguous levels of CSM. The study was to evaluate the outcomes of the anterior cervical discectomy and fusion (ACDF) with zero-profile devices for two noncontiguous levels of CSM.
Materials and Methods:
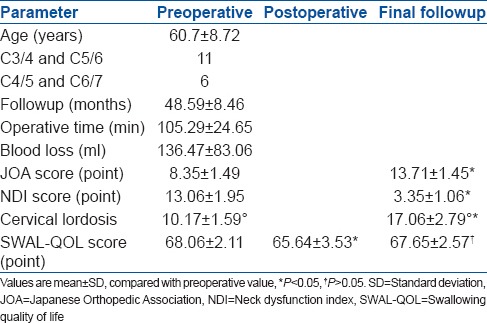
17 consecutive patients with two noncontiguous levels of CSM operated between December 2009 and August 2012 were included in the study. There were 12 men and 5 women with a mean age of 60.7 years (range 45–75 years). Involved disc levels were C3/4 and C5/6 in 11 patients and C4/5 and C6/7 in six patients. Preoperative plain radiographs, computed tomography (CT) with 3-D reconstruction and magnetic resonance imaging (MRI) of the cervical spine were taken in all patients. All radiographs were independently evaluated by 2 spine surgeons and 1 radiologist. The outcomes were assessed by the average operative time, blood loss, Japanese Orthopedic Association (JOA) score, improvement rate, neck dysfunction index (NDI), swallowing quality of life (SWAL-QOL) score, the cervical lordosis and complications.
Results:
The mean followup was 48.59 months (range 24-56 months). The average operative time and blood loss was 105.29 min and 136.47 ml, respectively. The preoperative JOA score was 8.35, which significantly increased to 13.7 at the final followup (P < 0.01). The NDI score was significantly decreased from preoperative 13.06 to postoperative 3.35 (P < 0.01). The operation also provided a significant increase in the cervical lordosis (P < 0.01) from preoperative 10.17° to postoperative 17.06°. The fusion rate was 94.1% at 6 months postoperatively, and 100% at 12 months after surgery. The mean SWAL-QOL score decreased from preoperative 68.06 to immediate postoperatively 65.65 and then increased to 67.65 at final followup. There was a statistically significant difference between preoperative and immediate postoperatively values (P < 0.05), but none between preoperative and at final followup (P > 0.05). Cerebrospinal fluid leak, dysphagia and radiological adjacent segment degeneration occurred in one patient, respectively.
Conclusion:
The ACDF with zero-profile devices is generally effective and safe in treating two noncontiguous levels of CSM.
Keywords: Cervical spondylotic myelopathy, noncontiguous levels, zero-profile devices, anterior cervical decompression, anterior cervical fusion
MeSh terms: Spine, cervical vertebral, myelopathy, spinal cord disease, spinal fusion
INTRODUCTION
Noncontiguous cervical spondylotic myelopathy (CSM) is a special degenerative disease because of the intermediate normal level or levels between supra and infraabnormal levels. When it cannot respond to conservative treatment, surgical treatment is usually chosen. Surgical management consists of two capital steps: Decompressing the involved nerve root(s) or spinal cord and stabilizing the involved vertebral bodies.1 Anterior cervical discectomy and fusion (ACDF) has historically been the gold standard procedure for CSM using anterior plate and cervical cage. However, ACDF with plate and cage in treating two noncontiguous levels of CSM, two plates have to be used. Moreover, ACDF inevitably costs the loss of range of motion at involved levels. The loss of range of motion at the operated level is commonly compensated for at the adjacent levels and the compensatory stress within adjacent intervertebral discs also increases after fusion. When ACDF with two anterior plates and cervical cages was used in treating two noncontiguous levels of CSM, the intermediate segment will bear more stress, thereby the potential for accelerated the intermediate level disc degeneration will be raised. Worrying about this, some surgeons advocate including the normal level in the fusion construct in patients with noncontiguous CSM. However, it is not sensible to sacrifice the motion of normal level for preventing the possible intermediate level disc degeneration. Besides the adjacent segment degeneration (ASD), dysphagia is also a common complication of ACDF. It was reported that the adhesion formation around the plate was the reason for dysphagia.2
For reducing the potential for the intermediate level disc degeneration, preserving the kinematics of the intermediate level and decreasing the incidence of dysphagia, ACDF with zero-profile devices (Zero-P, Synthes GmbH Switzerland, Oberdorf, Switzerland) has been used for many years. The zero-profile device combines an interbody spacer with an anterior plate [Figure 1]. It has been shown to provide similar biomechanical stability to that of fusion using an anterior plate and cage.3 To evaluate its results and feasibility, a case series study was done.
Figure 1.

Photograph showing the zero-profile device (Synthes, GmbH Switzerland, Oberdorf, Switzerland)
MATERIALS AND METHODS
17 patients with two noncontiguous levels of CSM who underwent ACDF with zero-profile devices between December 2009 and August 2012 were included in the study. There were 12 men and 5 women with a mean age of 60.7 years (range 45–75 years). Involved disc levels were C3/4 and C5/6 in 11 patients and C4/5 and C6/7 in six patients [Table 1]. The inclusion criteria were: (1) Patients with two noncontiguous levels of CSM (2) Atleast one level intermediate segment was not involved e.g. C3/C4 and C5/C6 or C4/C5 and C6/C7 and (3) All those patients who had not responded to conservative treatment for at least 6 weeks. The exclusion criteria were the contiguous multilevel or single level CSM, the development stenosis due to thickened yellow ligament, osteoporosis, infection or tumor.
Table 1.
Clinical details of patients
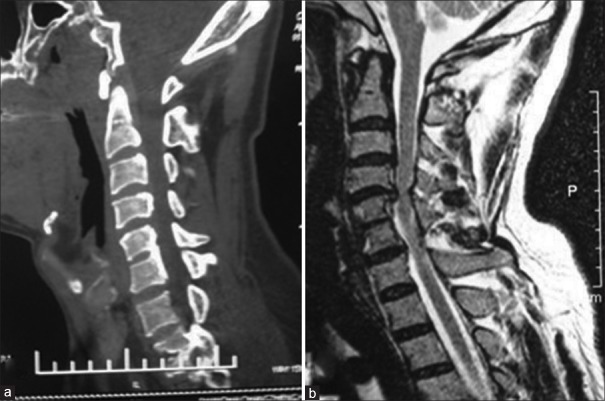
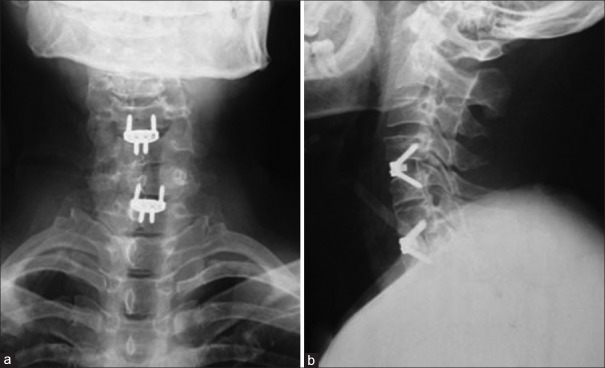
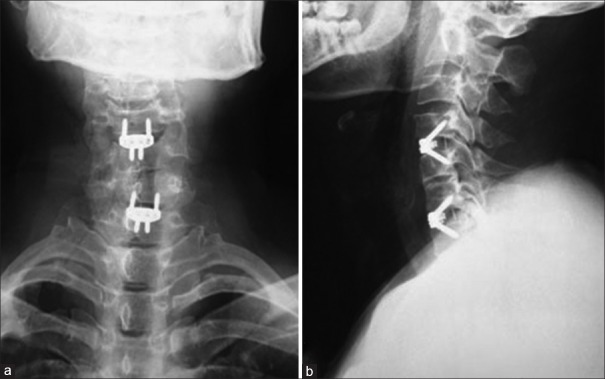
The study was approved by the Local Ethical Committee and Institutional Review Board. Preoperative plain radiographs, computed tomography (CT) with 3-D reconstruction and magnetic resonance imaging (MRI) of the cervical spine were taken in all patients [Figures 2 and 3]. Radiographs were taken immediate postoperatively, at 3, 6, and 12 months postoperatively and yearly thereafter in neutral lateral and lateral view in extension flexion position [Figures 4 and 5]. All radiographs were independently evaluated by 2 spine surgeons and 1 radiologist. When a difference about the imaging diagnosis existed between the 2 spine surgeons, the decision was made by the radiologist. If dural sac was shown being compressed on CT and MRI because of the cervical disc herniation, ossification of the posterior longitudinal ligament or osteophyte formation and associated with the neurological symptoms, the patient was diagnosed with CSM [Figure 2].
Figure 2.
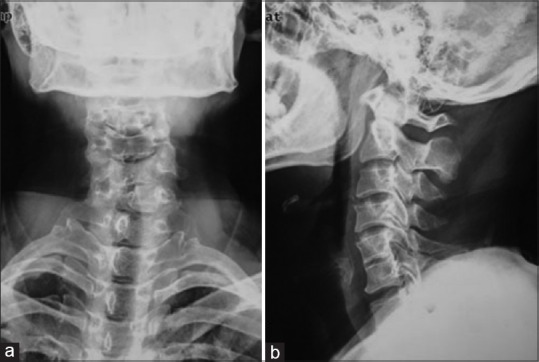
The preoperative anteroposterior (a) and lateral (b) radiograph showing the degeneration of the cervical spine
Figure 3.
The preoperative computed tomography (a) and mid sagittal T2W magnetic resonance imaging (b) showing the degeneration of the cervical spine and C4-5, C6-7 disc herniation with significant compression on dural sac
Figure 4.
The immediately postoperative anteroposterior (a) and lateral (b) radiograph showing the zero-profile devices in patients with two noncontiguous levels of cervical spondylotic myelopathy
Figure 5.
The anteroposterior (a) and lateral (b) radiograph at 1-year followup showing fusion between C4-C5 and C6-C7
Solid fusion of the involved segments was achieved <2° on extension-flexion radiographs, and ≤50% of the area of the outer surfaces of the implants were radiolucent.4 The cervical lordosis was determined by the Cobb angle preoperatively and at final followup. The angle formed by lines along the inferior endplates of C2 to the inferior endplate of C7 in the neutral position. Instrument dislodgement was defined as instrument beyond the leading edge of the upper and lower vertebral connecting 2–4 mm on lateral radiographs. The instrument subsidence was defined as loss of height of the operative segments on lateral radiographs. Radiographic evidence of ASD included the presence of any of the following parameters:5,6 (1) New anterior or enlarging osteophyte formation, (2) Narrowing of the disc space increased by ≥30%, or (3) Calcification of the anterior longitudinal ligament.
Periprocedural parameters, including operative time and operative blood loss were collected. The Japanese Orthopedic Association (JOA) score was used to evaluate the neurological status, and neck dysfunction index (NDI) score was used to assess the neck function. An improvement rate (IR) was calculated as IR = (Postoperative JOA score − preoperative JOA score/17 − preoperative JOA score) ×100%. They were evaluated preoperatively and at the final followup. The complications including cerebrospinal fluid (CSF) leak, hoarseness, dysphagia, hematoma, ASD, instrument dislodgement, breakage, and subsidence were collected. The dysphagia was assessed by the modified swallowing quality of life (SWAL-QOL) questionnaire.7 All the patients completed the SWAL-QOL questionnaire before surgery, immediate postoperatively, and at the last followup.
The SPSS for Windows version 13.0 (SPSS, Chicago, IL, USA) was used for the analysis. The Student's t-test was used for comparison of paired data. P < 0.05 was considered as statistically significant.
RESULTS
All the 17 patients were followup for a mean period of 48.59 months (range 24–56 months). No patient received reoperation. Table 1 shows the demographic data of the patients. Bony fusion was achieved in 16 (94.1%) patients at 6 months postoperatively. At 12 months postoperatively, solid fusion was observed in all patients (100%). No instrument dislodgement, breakage, and subsidence occurred during the whole followup period. The mean cervical lordosis was 10.17 ± 1.59° before operation and significantly increased to 17.06 ± 2.79° after operation [Figure 6]. On sagittal MRIs, ASD was found in one patient at 52 months after operation.
Figure 6.
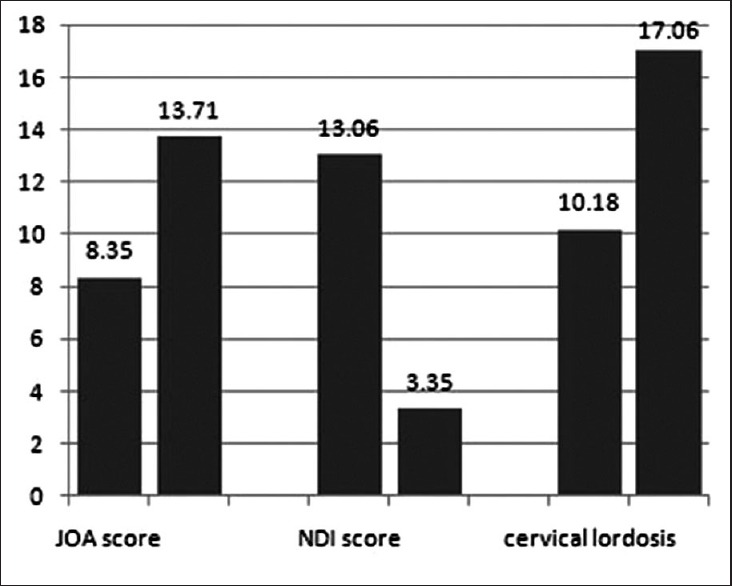
Bar graph showing the comparison of Japanese Orthopedic Association score, neck dysfunction index score and cervical lordosis between before surgery and after surgery
The operative time and blood loss was 105.29 ± 24.65 min and 136.47 ± 83.06 ml, respectively. No patient received blood transfusion. The preoperative JOA score was 8.35 ± 1.49 which significantly increased to 13.71 ± 1.45 at the final followup (P < 0.01) [Figure 6], with the IR of 62.8 ± 15.1%. The NDI score was significantly decreased from preoperative 13.06 ± 1.95 to postoperative 3.35 ± 1.06 (P < 0.01) [Figure 6]. The mean SWAL-QOL score was decreased from preoperative 68.06 ± 2.11 to postoperative 65.64 ± 3.53, and then increased to 67.65 ± 2.57 at final followup [Figure 7]. There was a statistically significant difference between preoperative and immediate postoperative (P < 0.05), but none between preoperative and at final followup (P > 0.05).
Figure 7.
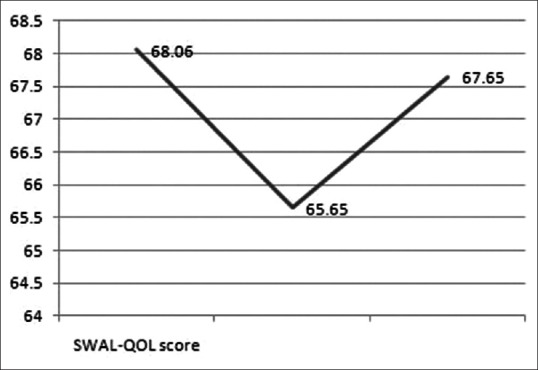
Diagram of curves showing the changes of swallowing quality of life score from preoperative to immediately postoperative until to the final followup
In this series, CSF leak and dysphagia occurred in one patient each. No patients underwent reoperation. CSF leak occurred after an intraoperative dural tear due to tight adhesion with the dura, and stopped after approximately 1-week of conservative treatment of local pressure. Dysphagia was transient and resolved within 1 month after treatment with an aerosol inhalation mixture of 5 mg dexamethasone, chymotrypsin and a humidifier given 3 times daily. One patient had radiological ASD at the intermediate-level at 52 months after the operation but patient was symptomatic.
DISCUSSION
The procedure of ACDF is suitable for noncontiguous CSM. In the current study, 17 patients were diagnosed with CSM on clinical examination, CT and MRI. Because of the existence of osteoporosis, osteophyte, instability or hypermobility, local or global kyphosis, they underwent ACDF instead of artificial disc replacement, posterior laminectomy or laminoplasty. However, the ASD following this procedure is becoming a clinical concern. Matsumoto et al.8 (2010) found a significantly higher incidence of radiographic progression of ASD in the ACDF patients than that of in the healthy control subjects. It was presumed that hypermobility and increased stress in the adjacent segment according to biomechanical study were the reasons for the development of ASD.9,10 The loss of range of motion at the operated level is commonly compensated for at the adjacent levels and the compensatory stress within adjacent intervertebral discs also increases after fusion.9,11 Radiologic but not symptomatic ASD was found in one patient, with the rate of 5.88% in the current study.
It was believed that more segments were fused, more compensatory activity occurred in the adjacent segments and the likelihood of ASD increased. Park et al.12 reported 2-level fusion significantly increased compensatory stress within adjacent intervertebral discs compared with 1 level fusion. For the skip level ACDF, the intermediate segment will theoretically bear more additive forces from fusion masses on both sides of supra and infra segments. Preventing it, some surgeons advocate including the normal level in the fusion construct in patients with noncontiguous CSM. However, a recent biomechanical study challenged the theory of additive stress on an intermediate segment in a cadaveric model. Finn et al.13 evaluated biomechanical forces exerted on intermediate and adjacent segments after 2- or 3-level fusion for the treatment of noncontiguous levels. They found that infra and supra adjacent levels experienced a marked increase in strain in all movements with a three level fusion, whereas supra and infra adjacent segments of a two-level fusion experienced modest strain to intact intermediate level. According to their results, noncontiguous fusions instead of a three level fusion were recommended to treat two noncontiguous levels of CSM. In our series of noncontiguous CSM, we performed the skip-level ACDF. The neurological and clinical outcomes of this procedure are satisfactory. It is not sensible to sacrifice the motion of normal level for preventing the possible ASD.
For a better control of immediate instability and fusion rate, anterior plate was recommend in ACDF. However, a higher incidence of ASD was reported by Ji et al. (2015)14 when using plate along with ACDF. They found that the mean intervertebral disc height change of the adjacent segment was more severe in patients treated with ACDF using cage and plate constructs than that of in patients treated with ACDF using stand-alone cages.14 Moreover, skip-level ACDF needs two anterior plates and cervical cages, which may prolong the operative time, increase the operative blood loss and raise the incidence of soft tissue injury and dysphagia.
Dysphagia is also a common complication of ACDF. The incidence of dysphagia has been reported to be between 3% and 21% when using anterior plate in ACDF.2,15 Several studies have investigated the etiology of postoperative dysphagia, suggesting prevertebral swelling, esophageal retraction and prominence of the cervical plate as possible causes.16,17,18,19,20,21,22 Prevertebral swelling, as a result of hemorrhage or intraoperative soft injury, may impair esophageal motility.20,22 According to study of Mendoza-Lattes et al. (2008), retraction of the esophagus may produce ischemia and its release may lead to a reperfusion injury, thereby lead to dysphagia.19 In addition, longer duration of surgery was associated with a higher incidence of dysphagia, perhaps owing to longer period of esophageal retraction.23,24 It was reported that the use of thicker plates was significantly associated with dysphagia.16 The use of a nonprotruding zero-profile plate was associated with a lower incidence of dysphagia.21 Moreover, cervical plating requires more esophageal retraction to insert the contralateral drill, tap, and screws that are usually inserted at a convergent angle, which may lead to dysphagia.25 As such, stand-alone cages were introduced for its simple, minimal blood loss and effective procedure resulting in successful fusion.
In the current study, the zero-profile devices were used in ACDF. After cervical discectomy and decompression, the zero-profile device was inserted and then locked with four screws into intervertebral space. ACDF with zero-profile device is relatively simple to manipulate and can gain immediate stability postoperatively. Using the zero-profile devices in treating the two noncontiguous levels of CSM may cut down operative time and operative blood loss and decrease the incidence of soft tissue injury. Decreased operative time and the incidence of soft tissue injury may lead to shorten the period of esophageal retraction and lower incidence of prevertebral swelling and thus lead to lower incidence of dysphagia. In our series of noncontiguous CSM, dysphagia occurred only in one patient and resolved within 1-month. The clinical outcome was also satisfactory with significant increased JOA score and the cervical lordosis, and significant decreased NDI score. However, this study has some limitations such as there was no control group, the number of patients were less and the followup was short. Further studies should be needed in the future.
Based on the results of this study, ACDF with zero-profile devices was generally effective and safe for two noncontiguous levels of CSM if indications were well controlled.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cho SK, Riew KD. Adjacent segment disease following cervical spine surgery. J Am Acad Orthop Surg. 2013;21:3–11. doi: 10.5435/JAAOS-21-01-3. [DOI] [PubMed] [Google Scholar]
- 2.Fountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310–7. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 3.Scholz M, Reyes PM, Schleicher P, Sawa AG, Baek S, Kandziora F, et al. A new stand-alone cervical anterior interbody fusion device: Biomechanical comparison with established anterior cervical fixation devices. Spine (Phila Pa 1976) 2009;34:156–60. doi: 10.1097/BRS.0b013e31818ff9c4. [DOI] [PubMed] [Google Scholar]
- 4.Qizhi S, Xuelei W, Lili Y, Lei L, Linwei C, Yang L, et al. Segmental anterior decompression and fusion for multilevel ossification of the posterior longitudinal ligament. Orthopedics. 2012;35:e403–8. doi: 10.3928/01477447-20120222-38. [DOI] [PubMed] [Google Scholar]
- 5.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: A prospective 2-year study. J Neurosurg Spine. 2005;3:417–23. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 6.Kim SW, Limson MA, Kim SB, Arbatin JJ, Chang KY, Park MS, et al. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur Spine J. 2009;18:218–31. doi: 10.1007/s00586-008-0854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siska PA, Ponnappan RK, Hohl JB, Lee JY, Kang JD, Donaldson WF., 3rd Dysphagia after anterior cervical spine surgery: A prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976) 2011;36:1387–91. doi: 10.1097/BRS.0b013e31822340f2. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: Comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging followup study. Spine (Phila Pa 1976) 2010;35:36–43. doi: 10.1097/BRS.0b013e3181b8a80d. [DOI] [PubMed] [Google Scholar]
- 9.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976) 2002;27:2431–4. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Espina CG, Amirouche F, Havalad V. Multilevel cervical fusion and its effect on disc degeneration and osteophyte formation. Spine (Phila Pa 1976) 2006;31:972–8. doi: 10.1097/01.brs.0000215205.66437.c3. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Liu M, Li T, Huang F, Tang T, Xiang Z. A meta-analysis comparing the results of cervical disc arthroplasty with anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical disc disease. J Bone Joint Surg Am. 2013;95:555–61. doi: 10.2106/JBJS.K.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park DH, Ramakrishnan P, Cho TH, Lorenz E, Eck JC, Humphreys SC, et al. Effect of lower two-level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine. 2007;7:336–40. doi: 10.3171/SPI-07/09/336. [DOI] [PubMed] [Google Scholar]
- 13.Finn MA, Samuelson MM, Bishop F, Bachus KN, Brodke DS. Two-level noncontiguous versus three-level anterior cervical discectomy and fusion: A biomechanical comparison. Spine (Phila Pa 1976) 2011;36:448–53. doi: 10.1097/BRS.0b013e3181fd5d7c. [DOI] [PubMed] [Google Scholar]
- 14.Ji GY, Oh CH, Shin DA, Ha Y, Kim KN, Yoon do H, et al. Stand-alone cervical cages versus anterior cervical plates in 2-level cervical anterior interbody fusion patients: Analysis of adjacent segment degeneration. J Spinal Disord Tech. 2015;28:E433–8. doi: 10.1097/BSD.0b013e3182a355ad. [DOI] [PubMed] [Google Scholar]
- 15.Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: A 5 to 11 year followup study. Eur Spine J. 2005;14:677–82. doi: 10.1007/s00586-004-0849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on Dysphagia: A 2-year prospective longitudinal followup study. J Spinal Disord Tech. 2005;18:406–9. doi: 10.1097/01.bsd.0000177211.44960.71. [DOI] [PubMed] [Google Scholar]
- 17.Chin KR, Eiszner JR, Adams SB., Jr Role of plate thickness as a cause of dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2007;32:2585–90. doi: 10.1097/BRS.0b013e318158dec8. [DOI] [PubMed] [Google Scholar]
- 18.Papavero L, Heese O, Klotz-Regener V, Buchalla R, Schröder F, Westphal M. The impact of esophagus retraction on early dysphagia after anterior cervical surgery: Does a correlation exist? Spine (Phila Pa 1976) 2007;32:1089–93. doi: 10.1097/01.brs.0000261627.04944.cf. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza-Lattes S, Clifford K, Bartelt R, Stewart J, Clark CR, Boezaart AP. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am. 2008;90:256–63. doi: 10.2106/JBJS.G.00258. [DOI] [PubMed] [Google Scholar]
- 20.Kang SH, Kim DK, Seo KM, Kim KT, Kim YB. Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery. J Clin Neurosci. 2011;18:1369–73. doi: 10.1016/j.jocn.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Scholz M, Schnake KJ, Pingel A, Hoffmann R, Kandziora F. A new zero-profile implant for stand-alone anterior cervical interbody fusion. Clin Orthop Relat Res. 2011;469:666–73. doi: 10.1007/s11999-010-1597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kepler CK, Rihn JA, Bennett JD, Anderson DG, Vaccaro AR, Albert TJ, et al. Dysphagia and soft-tissue swelling after anterior cervical surgery: A radiographic analysis. Spine J. 2012;12:639–44. doi: 10.1016/j.spinee.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Riley LH, 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: Prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005;30:2564–9. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 24.Rihn JA, Kane J, Albert TJ, Vaccaro AR, Hilibrand AS. What is the incidence and severity of dysphagia after anterior cervical surgery? Clin Orthop Relat Res. 2011;469:658–65. doi: 10.1007/s11999-010-1731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, Regan JJ, et al. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech. 2010;23:1–8. doi: 10.1097/BSD.0b013e31819e2ab8. [DOI] [PubMed] [Google Scholar]