Abstract
Background:
The quest for ideal bone graft substitutes still haunts orthopedic researchers. The impetus for this search of newer bone substitutes is provided by mismatch between the demand and supply of autogenous bone grafts. Bone banking facilities such as deep frozen and freeze-dried allografts are not so widely available in most of the developing countries. To overcome the problem, we have used partially decalcified, ethanol preserved, and domestic refrigerator stored allografts which are economical and needs simple technology for procurement, preparation, and preservation. The aim of the study was to assess the radiological and functional outcome of the partially decalcified allograft (by weak hydrochloric acid) in patients of benign lytic lesions of bone. Through this study, we have also tried to evolve, establish, and disseminate the concept of the bone bank.
Materials and Methods:
42 cases of lytic lesions of bone who were treated by decalcified (by weak hydrochloric acid), ethanol preserved, allografts were included in this prospective study. The allograft was obtained from freshly amputated limbs or excised femoral heads during hip arthroplasties under strict aseptic conditions. The causes of lytic lesions were unicameral bone cyst (n = 3), aneurysmal bone cyst (n = 3), giant cell tumor (n = 9), fibrous dysplasia (n = 12), chondromyxoid fibroma, chondroma, nonossifying fibroma (n = 1 each), tubercular osteomyelitis (n = 7), and chronic pyogenic osteomyelitis (n = 5). The cavity of the lesion was thoroughly curetted and compactly filled with matchstick sized allografts.
Results:
Quantitative assessment based on the criteria of Sethi et al. (1993) was done. There was complete assimilation in 27 cases, partial healing in 12 cases, and failure in 3 cases. Functional assessment was also done according to which there were 29 excellent results, 6 good, and 7 cases of failure (infection, recurrence, and nonunion of pathological fracture). We observed that after biological incorporation, the graft participates in bone physiology and morphology. We did not observe any adverse host graft antigenic reaction.
Conclusions:
We conclude that decalcified allograft is suitable alloimplant for use in benign lesions of bone, is easy to prepare and store, and is thus well suited for use in developing countries.
Keywords: Curettage, decalcified allograft, graft incorporation, lytic lesions of bone
MeSh terms: Gratfing, bone, curettage, allograft, bone cysts
INTRODUCTION
It is universally accepted that autogenous bone graft is the most effective osseous graft material and is also the most commonly used one.1,2 However, practical difficulties to procure enough amount of autogenous bone graft provide the thrust to find newer bone substitutes. The ideal bone graft substitute should have osteoinductive, osteoconductive, nonantigenic properties and it should be able to remodel to participate in bone morphology and physiology.3,4 Autograft is the best bone graft as it provides high degree of osteogenesis.5 However, allografts have got many advantages over autografts such as unlimited supply, availability in various shape and sizes, and avoidance of the additional incision and operation required for harvesting the graft.6
Urist7 provided guidelines to the development of decalbone and its preservation in ethanol. Hosny et al.8 found no significant difference in the amount of newly formed bone in demineralized bone preserved at −70°, −4°, and 25°C for a period of up to 6 months. Delloye et al.9 confirmed that hydrochloric decalcification alone either at 4°C or at room temperature provide a higher bone yield than antigen extracted autodigested allografts (AAA Bone).
Deep frozen and freeze-dried allografts devised since that of Inclan,10 which require sophisticated technology are next best materials to autogenous bone graft but are impracticable in developing countries because of perpetual electricity failures and lack of sophisticated facilities.11 Moreover, these also have a varying degree of antigenicity.12,13 Above all, bone banking facilities are not so widely available in most of the developing countries. For developing countries to establish a bone bank up to the level of district hospital, it should be economical and should require simple technology for procurement, preparation and preservation and must obviate the need of advanced sophisticated technology. To overcome the problem, we have used decalcified, ethanol-preserved, and domestic refrigerator stored allografts which are economical and needs simple technology for procurement, preparation, and preservation. Through this study, we have tried to evolve, establish, and disseminate the concept of bone bank for third world countries, where deep frozen and freeze-dried allografts are not readily available.
Decalcified bovine bone chips were first used, with good results, by Senn14 for filling sterile osteomyelitic cavities. Urist7 demonstrated the osteoinductive potential of decalcified allogenic bone experimentally, and subsequently, various reports by Gupta and Tuli,11 Goel et al.,15 Tuli et al.16 have found decalcified allogenic bone to be one of the best replacement/substitute materials for autogenous bone in clinical conditions. Many natural and fabricated complex biomaterials are currently available to replace diseased bone. However, replacement or repair of a bone defect by bone is superior to all other materials, because after biological incorporation of the graft, the bone has the unique ability to repair the bone itself and adopt its mechanical properties and shape according to the external force to which it is subjected. Beyond doubt, grafted bone is the best replacement for diseased bone.4 Hey-Groves17 performed experiments on animals, and he found that grafts are much less likely to favor infective process than metallic plates and screws.
The basis of this study are threefold: The presence of bone morphogenic proteins (BMP) located in bone matrix, a catalyst for osteoinduction in demineralized bone which is uncovered during the demineralization process.18 Allograft behaves in same fashion as autogenous bone graft in the process of bone graft incorporation.7 Defect of human bone must be replaced by human bone because it is superior to all other complex and fabricated materials, the underlying reason being that the porous structure of human bone responsible for osteoinduction, cannot be replicated by any other synthetic material.19
This study assessed the radiological and functional outcome of the partially decalcified allograft in patients with various benign lytic lesions of bone.
MATERIALS AND METHODS
Forty two patients of lytic lesions of bone caused by benign/locally aggressive bone tumors or due to chronic infections (tubercular or pyogenic bacteria), in the age group of 4–50 years (mean age 19.5 years), admitted between 1994 and 2006 were enrolled in the study. The causes of lytic lesions [Table 1] were unicameral bone cyst (n = 3), aneurysmal bone cyst (n = 3), giant cell tumor (n = 9), fibrous dysplasia (n = 12), chondromyxoid fibroma, chondroma, nonossifying fibroma (n = 1 each), tubercular osteomyelitis (n = 7), and chronic pyogenic osteomyelitis (n = 5). The exclusion criteria were (1) malignant tumor (suspected or diagnosed) (2) acute infections (3) traumatic bone loss (4) large tumor size not amenable to curettage and bone grafting. Patients underwent intralesional curettage of tumor followed by decalcified allografting. A cortical window of size larger than the greatest dimension of the tumor was first made through which the cavity was curetted. In no case, we went for any form of internal or external fixation. The limb was protected in appropriate external plaster splint/brace until radiological evidence of healing was noticed.
Table 1.
Histopathology of various lesions in our study

Highest number of patients were in second and third decade of life. Male to female ratio was 5:2, showing more incidence in males. In our series of 42 cases, femur (n = 17) was most commonly involved, followed by tibia (n = 10). Other sites involved were humerus (n = 7), calcaneum (n = 2), metacarpal, phalanx of hand, radius, ulna and metatarsal (1 case each) [Table 2].
Table 2.
Various sites of lesions in our study

All the 42 cases (100%) presented with chief complaints of pain and swelling ranging from few days to surprisingly 8 years. 30 cases had either difficulty in walking or were unable to do so. 13 cases presented clinically with pathological fractures.
Donor selection was a very rigorous process. Medical history included transmissible diseases in the donor, intravenous drug abuse and recent tattooing history, etc. Human bone was obtained from freshly amputated extremities or excised femoral heads during hip arthroplasties under strict aseptic precautions. The cases where the bone was removed due to tumor or infection were not used. Donor patients were submitted for HIV test, test for Australian antigen (hepatitis B surface antigen), and venereal disease research laboratory test and anyone found positive for the same was excluded. In our series, we used hydrochloric acid treated partially decalcified allogenic bone to fill the cystic lesions. After removal of soft tissues and periosteum, the bone was washed with distilled water and immersed in 0.6 N hydrochloric acid. The graft was stored in a domestic refrigerator for 3–5 days, the solution being changed every 24 h. The partially decalcified bone was washed in distilled water to remove any traces of acid and sealed in 50–70% ethanol and stored at 4°C in domestic refrigerator. The stored bone was used within 6 months. The graft samples were sent for culture and sensitivity 3 days prior to use. Before transplantation, the patients were informed about the risks and benefits of allografting and informed consent was taken. At the time of implantation, the decalcified bone was cut into matchstick-shaped grafts after thorough cleaning and rinsing with sterile water. The cavity was packed with snugly fitting grafts.
Patients were followed at 6 weeks, 3, 6, 9, 12, 18, 24 months and yearly thereafter till final followup. In the followups, the patients were examined clinically to see for any complications which might arise out of the procedure and serial X-rays were done to look for graft incorporation.
Radiological assessment of healing was done on the basis of percentage of osseous replacement of the original cavity after allografting.20 Functional assessment was done on the basis of criteria as mentioned in Table 3.
Table 3.
Functional status according to the rating system

RESULTS
The mean followup was 7.5 years (range 4-20 years). Complete healing (>75% osseous replacement of the cavity) was seen in 27 cases, partial healing (>25–75% replacement) in 12 cases, and failure (<25% replacement) in 3 cases [Table 4]. Among the patients who had complete or partial healing of the lesion (39 cases), the time of incorporation was quite variable ranging from 6 months to 3 years. The duration was lesser in younger patients. The failure cases, in our study, were those in whom recurrence was seen. Recurrence may be a result of inadequate curettage or the nature of the lesion. Due to recurrence, there was a radiological picture of <25% replacement of tumor cavity leading to radiological failure.
Table 4.
Radiological incorporation of the graft after curettage and allografting

We had excellent results in 29 cases, 6 were good, and there were 7 cases of failure due to either infection, recurrence, or nonunion in case of pathological fracture [Table 3].
Local recurrence of the tumor was noted in 3 cases - (2 of fibrous dysplasia and 1 of giant cell tumor). The later one underwent amputation. There were 3 cases of local site infections, wherein pus were subjected for culture and sensitivity. Staphylococcus aureus was the etiological agent in all 3 of them. Systemic dissemination leading to septicemia was not observed in any of them. In one case of pathological fracture, there was nonunion. There was no case of any immunological reaction or graft rejection [Table 5].
Table 5.
Complications noted during the followups

Some of the cases of our study has been shown in Figures 1–3.
Figure 1.
X-ray leg bones with ankle joint anteroposterior and lateral views showing (a) Fibrous dysplasia of left distal tibia in a 14-year-old female. (b) Followup at 3 months. (c) Followup at 10 years. (d) Followup at 18.5 years showing the grafted material to be well-consolidated and incorporated with the host bone
Figure 3.
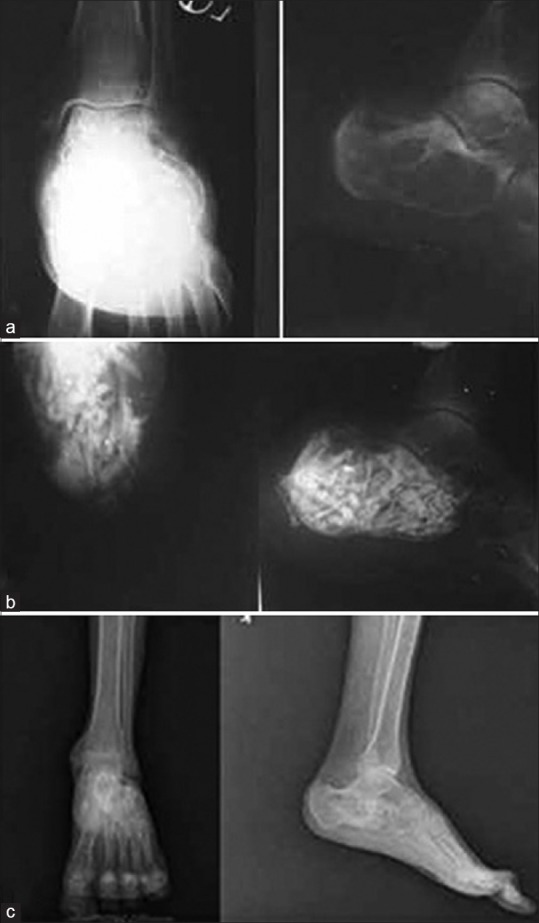
(a) X-ray ankle joint anteroposterior and lateral views showing aneurysmal bone cyst of left calcaneum in an 18 years old hemophilic male. Patient underwent curettage and allografting by decalcified allogenic bone. The patient was operated under cover of Factor VIII. (b) Postoperative X-ray axial and lateral views showing obliteration of cavity (c) Followup (10 years) x-ray anteroposterior and lateral views showing good incorporation of the graft
Figure 2.
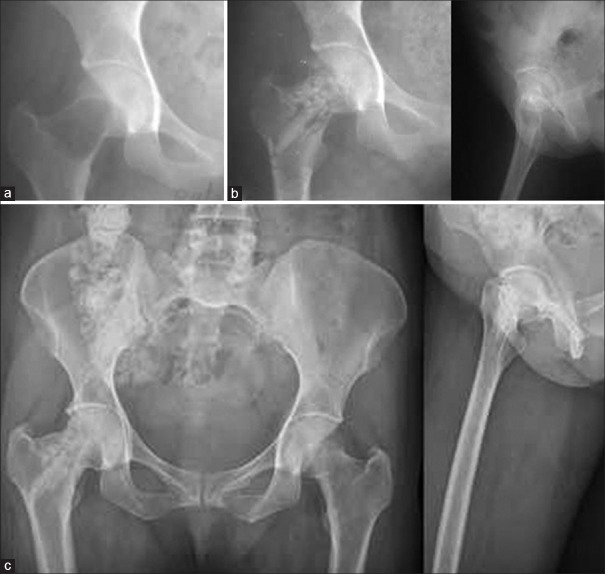
(a) X-ray (R) hip joint anteroposterior view showing tubercular cystic lesion in the right femoral neck of a 24-year-old female. The patient underwent curettage of the cavity with allografting by decalcified allograft along with fibular strut allograft. (b) Postoperative radiograph anteroposterior and lateral views showing the obliteration of cavity at 6 weeks. (c) Followup at 14 years showing complete obliteration of cystic lesion
DISCUSSION
Because of the limitations associated with harvesting autografts, allografts have been applied clinically and experimentally as a common alternative to autografts. The major concern with the use of allograft is immunogenicity and risk of disease transmission.21 Fresh bone allografts are seldom used because of higher risks of both of them. Use of frozen and freeze-dried bone allografts reduces these risks. Furthermore, the biological and mechanical properties which are needed for bone grafting are only mildly affected.22 However, the lack of freeze-drying and bone-banking facilities in the developing world is a matter of concern.15 Regarding immunogenicity, we are of the opinion that serial chemical treatment of allograft with hydrochloric acid reduces the immunogenicity of the cells of the graft. It has been revealed by many series that antigenicity of allogenic bone graft is reduced by cryopreservation, storage, and processing.23 On the other hand, few workers reported that an allograft retains some antigenic potential even if this may not clinically manifest.13 Both Goel et al.15 and Sethi et al.20 did not notice any immune related complications. However, due to the fact that allogenic bone is a foreign tissue, the chances of immune-related complications cannot be ruled out.
However, the use of physical, chemical, and thermal agents may destroy the bone cells and denature proteins present in the graft and alter osteoconductive and osteoinductive characteristics, essentially eliminating the osteogenic properties.21 In the methods that we are using to prepare partially decalcified allograft (decal bone), we have to strike a balance between reducing antigenicity and preserving the strength and trabecular architecture of the allograft. Serial chemical treatment with hydrochloric acid reduces the immunogenicity but doing too much of the same will weaken the graft and lead to loss of trabecular pattern so much that it will not incorporate easily with the host bone due to loss of osteoconductive property. The stored decalcified allograft can be used within 1–6 months.15
We did not evaluate the structural strength of the hydrochloric acid treated partially decalcified allograft with other forms of bone grafts. Various studies comparing the properties of bone grafts show that fresh frozen and freeze-dried allograft have got similar structural strength as that of autograft. However, intrinsic strength of demineralized bone matrix is minimal.24,25,26 Hence, we feel that the strength of allograft used in our study, where partial decalcification has been done to be somewhat between the two extremes. Gupta and Tuli11 conducted a study on rabbits wherein the aim was to evaluate the reparative capacity of partially decalcified preserved diaphyseal allogenic bone used to bridge large osteoperiosteal defects in rabbits, and they found radiological success in 97.2% of the cases.
Successful graft incorporation requires that an appropriate match is made among the biologic effect of bone graft, the condition of the perigraft environment and the mechanical environment. Factors which affect the bone graft incorporation includes - revascularization, new bone formation, and host-graft union. In the case of decalcified allograft, revascularization depends solely on the surrounding tissue. New bone forming tissue is also derived from the host tissue. Host graft union depends on the construct stability and contact between host bone and the graft. A vascularized bed of tissue along with snug filling of the cavity after thorough curettage will result in minimum dead space between host and recipient bed and will facilitate neovascularization and invasion of cells, thereby helping in better and faster incorporation of the graft.27,28
In our study, the time of incorporation varied considerably between patient to patient ranging from 6 months to 3 years, the duration being shorter in younger patients. Though we have treated all the patients by conservative means, we feel that mechanical environment for faster bone incorporation can be improved by internal or external fixation depending on the site and size of the lesion and the presence of pathological fracture.
In cases of simple bone cyst in children and adolescents, curettage alone may lead to healing of the lesion but addition of an osteoconductive material such as allograft leads to better results.29 Goel et al.15 reported 46 patients with benign cystic lesions of bone treated by curettage and bone grafting using allogenic decalcified bone and they obtained healing in 71% cases. Sethi et al.20 studied 17 patients of benign cystic osseous lesions by curettage and grafting by allogenic decalcified bone and they found complete healing in 65% cases, partial healing in 24%, and failure in 6%. In one case, cyst recurred. According to them, the time required for adequate incorporation of the graft varied from 6 to 9 months in children and 9–15 months in adults.
The postoperative infections in our study was in 7% cases which may be considered a bit high as the surgeries were planned and elective. However, one must see that it was conducted at a time when the operation theatre set ups were not as good as it is now. Goel et al.15 reported infections in 5 out of 46 cases and Sethi et al.20 got a figure of 3 out of 17.
Recurrence is more likely dependent on the aggressiveness of the lesion, thoroughness of the curettage, and the effectiveness of any adjuvant agent used before implantation of graft material. The partially decalcified allograft has not got any adjuvant property of its own.
CONCLUSION
We conclude that partially decalcified allograft is suitable alloimplant for use in benign lesions of bone. It is easy to prepare and store and is thus well-suited for use in developing countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Parrish FF. Treatment of bone tumors by total excision and replacement with massive autologous and homologous grafts. J Bone Joint Surg Am. 1966;48:968–90. [PubMed] [Google Scholar]
- 2.Tuli SM. Bridging of bone defects by massive bone grafts in tumorous conditions and in osteomyelitis. Clin Orthop Relat Res. 1972;87:60–73. [PubMed] [Google Scholar]
- 3.Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–43. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- 4.Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J Med Res. 2010;132:15–30. [PubMed] [Google Scholar]
- 5.Athanasiou VT, Papachristou DJ, Panagopoulos A, Saridis A, Scopa CD, Megas P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med Sci Monit. 2010;16:BR24–31. [PubMed] [Google Scholar]
- 6.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urist MR. Surface-decalcified allogeneic bone (SDAB) implants. A preliminary report of 10 cases and 25 comparable operations with undecalcified lyophilized bone implants. Clin Orthop Relat Res. 1968;56:37–50. [PubMed] [Google Scholar]
- 8.Hosny M, Arcidi C, Sharawy M. Effects of preservation on the osteoinductive capacity of demineralized bone powder allografts. J Oral Maxillofac Surg. 1987;45:1051–4. doi: 10.1016/0278-2391(87)90162-5. [DOI] [PubMed] [Google Scholar]
- 9.Delloye C, Hebrant A, Munting E, Piret L, Coutelier L. The osteoinductive capacity of differently HCl-decalcified bone alloimplants. Acta Orthop Scand. 1985;56:318–22. doi: 10.3109/17453678508993024. [DOI] [PubMed] [Google Scholar]
- 10.Inclan A. The use of preserved bone in orthopaedic surgery. J Bone Joint Surg. 1942;24:81. [Google Scholar]
- 11.Gupta D, Tuli SM. Osteoinductivity of partially decalcified alloimplants in healing of large osteoperiosteal defects. Acta Orthop Scand. 1982;53:857–65. doi: 10.3109/17453678208992839. [DOI] [PubMed] [Google Scholar]
- 12.Brooks DB, Heiple KG, Herndon CH, Powell AE. Immunological factors in homogenous bone transplantation. IV. The effect of various methods of preparation and irradiation on antigenicity. J Bone Joint Surg Am. 1963;45:1617–26. [PubMed] [Google Scholar]
- 13.Friedlaender GE, Strong DM, Sell KW. Studies on the antigenicity of bone. I. Freeze-dried and deep-frozen bone allografts in rabbits. J Bone Joint Surg Am. 1976;58:854–8. [PubMed] [Google Scholar]
- 14.Senn N. On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Am J Med Sci. 1889;98:219–43. doi: 10.1097/00000658-188907000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel SC, Tuli SM, Singh HP, Sharma SV, Saraf SK, Srivastava TP. Allogenic decalbone in the repair of benign cystic lesions of bone. Int Orthop. 1992;16:176–9. doi: 10.1007/BF00180212. [DOI] [PubMed] [Google Scholar]
- 16.Tuli SM, Srivastava TP, Sharma SV, Goel SC, Gupta D, Khanna S. The bridging of large osteoperiosteal gaps using ‘Decalbone’. Int Orthop. 1988;12:119–24. doi: 10.1007/BF00266976. [DOI] [PubMed] [Google Scholar]
- 17.Hey-Groves EW. 3rd ed. Vol. 1. Bristol: John wright and Sons Ltd; 1921. On Modern Methods of Treating Fractures Including the Jacksonian Prize Essay on bone Grafting; p. 248. [Google Scholar]
- 18.Nazirkar G, Singh S, Dole V, Nikam A. Effortless effort in bone regeneration: A review. J Int Oral Health. 2014;6:120–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: An update. Injury. 2005;36(Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Sethi A, Agarwal K, Sethi S, Kumar S, Marya SK, Tuli SM. Allograft in the treatment of benign cystic lesions of bone. Arch Orthop Trauma Surg. 1993;112:167–70. doi: 10.1007/BF00662282. [DOI] [PubMed] [Google Scholar]
- 21.Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br. 2001;83:3–8. doi: 10.1302/0301-620x.83b1.11952. [DOI] [PubMed] [Google Scholar]
- 22.Malinin T, Temple HT. Comparison of frozen and freeze-dried particulate bone allografts. Cryobiology. 2007;55:167–70. doi: 10.1016/j.cryobiol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Burwell RG. The fate of bone grafts. In: Apley AG, editor. Recent Advances in Orthopaedics. London: J & A Churchill; 1969. pp. 115–207. [Google Scholar]
- 24.Cornell CN. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop Clin North Am. 1999;30:591–8. doi: 10.1016/s0030-5898(05)70112-7. [DOI] [PubMed] [Google Scholar]
- 25.Perry CR. Bone repair techniques, bone graft, and bone graft substitutes. Clin Orthop Relat Res. 1999;360:71–86. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–24. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res. 2000;371:38–45. doi: 10.1097/00003086-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Kokavec M, Fristakova M, Polan P, Bialik GM. Surgical options for the treatment of simple bone cyst in children and adolescents. Isr Med Assoc J. 2010;12:87–90. [PubMed] [Google Scholar]