Abstract
Purpose of review
Although restless legs syndrome (RLS) is a disorder recognized in the medical literature since the 17th century, there have only recently been significant clinical and scientific advances in diagnosis, epidemiology and understanding the disorder, mainly due to the advent of dopaminergic treatment.
Recent findings
Recent discoveries have uncovered the iron–dopamine connection in RLS and the basic dopaminergic pathology related to the RLS symptoms. These have led to new understanding of the morbidity of RLS and the many conditions associated with RLS, which have also supported new approaches to treatment. These developments are each briefly described here.
Summary
Although there has been progress in understanding, diagnosing and treating RLS, it remains an underdiagnosed and undertreated condition severely impairing functioning of patients with moderate-to-severe disease. Much work is needed to improve on current, as well as other novel therapies.
Keywords: dopamine, iron, pathology, restless legs syndrome, RLS
Introduction
Restless legs syndrome (RLS), also known as Ekbom's syndrome, is a condition consisting of an urge to move that is focused in the legs usually occurring with other abnormal leg sensations such as burning, creeping, tugging, or ‘like insects crawling inside the legs’. The RLS sensations are relieved by movement and have a strong circadian presentation worse in the evening and night with significant relief in the morning independent of the amount of movement. RLS is categorized as either primary (idiopathic) or secondary RLS depending on clinical features. Treatment of RLS includes iron supplementation, dopaminergic agents, opioids, benzodiazepines, and antiepileptic drugs. Increased recognition and management of RLS, an often debilitating condition, can lead to an improved quality of life and potentially improved overall health for the many who suffer from it. This review will focus primarily on the new diagnostic standards and methods, epidemiology, improved treatments and enhanced understanding of the neurobiology and genetics of RLS.
Diagnosis
Restless legs syndrome remains a clinical diagnosis based on confirming the four essential diagnostic features of RLS, which are as follows:
-
(1)
An urge to move the legs, usually but not always, accompanied by or felt to be caused by uncomfortable and unpleasant sensations in the legs.
-
(2)
The urge to move and any accompanying unpleasant sensation begins or worsens during periods of rest or inactivity such as lying down or sitting.
-
(3)
The urge to move and any accompanying unpleasant sensation is partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues.
-
(4)
The urge to move and any accompanying unpleasant sensation during rest or inactivity only occurs or is worse in the evening or night compared to during the day.
Recently experts in the field have been investigating the utility of new tools and questionnaires to enhance diagnostic power [1]. Helpful tools to make an accurate RLS diagnosis include:
-
(1)
Johns Hopkins Telephone Diagnostic Interview.
-
(2)
Medical history (evaluating for four essential diagnostic features of RLS and iron deficiency).
-
(3)
Positive family history of RLS.
-
(4)
Improvement with dopaminergic therapy.
-
(5)
Evaluating and ruling out mimics.
-
(6)
Presence of periodic limb movements of sleep (PLMS) on the sleep study.
In one recent diagnostic questionnaire, attention has focused not only on better identification of the defining diagnostic features of RLS, but also identifying and ruling out RLS mimics [2••]. A variety of conditions, including cramps, positional discomfort, and local leg pathology, can superficially satisfy all four diagnostic criteria for RLS and thereby ‘mimic’ it. Definitive diagnosis of RLS, therefore, requires exclusion of these specific conditions, which may be more common in the population than RLS. Short of an extended clinical interview and work-up, certain features of the presentation help differentiate mimics from RLS [2••] and make for a more certain RLS diagnosis. Moreover, one study of a series of RLS cases has also characterized a potential RLS variant called quiescegenic nocturnal dyskinesia (QND) that shares considerable overlap with RLS and should be considered when evaluating for RLS. Individuals with QND present with three of the diagnostic criteria of RLS except that there are excessive involuntary leg movements when resting in the evening or before sleep onset without any related uncomfortable sensations or urge to move the legs [3•]. Furthermore, many chronic disorders can be associated with RLS and may either cause RLS secondarily or cause exacerbation of RLS symptoms. For this reason it is important to be familiar with these as quality of life can be improved for these patients with proper management of primary or secondary RLS.
Restless legs syndrome epidemiology: latest findings
The prevalence of RLS has been somewhat controversial. Most of the studies use questionnaires asking for the four defining diagnostic features of RLS, never taking into account the differential diagnosis or RLS mimics noted above. Moreover these questionnaires rarely attend to the problem of the wide range of severity of RLS, from a minor occasionally annoying condition to one that significantly disrupts sleep. The problem here is that the minor annoying conditions are very likely to be mimics, whereas the ones that are more distressing and occur more than weekly are more likely to reflect actual RLS. Large population studies using the full diagnostic criteria previously reported on the prevalence of ‘clinically significant’ RLS (defined as occurring at least twice a week and being at least moderately distressing) being just over 2% in American and European populations, but even these studies failed to exclude mimics [4,5].
A more recent and better designed study used a questionnaire to screen for clinically significant RLS using the standard diagnostic criteria given to all patients appearing in general medical practices in Western European countries [6]. Those identified with RLS by the questionnaire were then medically evaluated by their primary care doctor for diagnosis of RLS and for the degree to which the RLS was a significant medical problem for the patient. The questionnaire had, as expected, only a 58% positive predictive value [6]. It is almost certain that questionnaires not using a validated diagnostic interview or a severity restriction will have at least 50% patients who do not in fact have RLS in their RLS sample. This casts serious doubts on the validity of population-based surveys using only un-validated questionnaires without inclusion of interviews or severity criteria. There has been recent development of a validated diagnostic questionnaire for RLS that has reasonable positive predictive values and should be considered for use in future questionnaire studies [7••].
Genetic advances
Sixty per cent of RLS patients report a positive family history for RLS. Genetic association studies have now identified 5 genes and 10 different risk alleles for RLS [8–10]. These are all on introns probably reflecting effects on expression of proteins associated with these genes. One of the allelic variations associated with increased risk of RLS is also associated with decreased serum ferritin, indicating relative reduction in body iron stores. Otherwise, there has been little progress in relating the genetic findings to RLS pathology.
Pathology: iron–dopamine connection
Most research on the disease mechanism of RLS has focused on the dopamine and iron systems. The relation of iron to RLS was first recognized in the early work by both Ekbom and Nordlander in the middle of the 20th century. The serendipitous finding by Dr Akpinar of the dramatic benefits of dopaminergic treatment of RLS then led to a general sense that there was a dopaminergic abnormality producing the RLS symptoms. Modern studies have sought to find the iron and dopamine pathologies and their relationship in RLS. More specifically, it has been hypothesized by the Johns Hopkins RLS group that brain iron deficiency produces a dopaminergic pathology producing the RLS symptoms. Initial cerebral spinal fluid (CSF), autopsy, and brain imaging studies clearly showed the expected brain iron deficiency particularly affecting the dopamine-producing cells in the substantia nigra and their terminal fields in the striatum. The low brain iron is a well established pathology of RLS.
The dopamine pathology was, however, elusive. Only recently has it been more clearly identified. Animal and cellular iron deficiency studies have provided a surprising result of increased tyrosine hydroxylase activity in the substantia nigra [11••] and decreased D2 receptors in the striatum [12], decreased dopamine transporter (DAT) functioning on the cell surface [13], and increased extracellular dopamine with a much larger (four times) increase in the amplitude of the circadian variation of extracellular dopamine (night–day difference) [14]. These same findings have now been largely replicated in RLS patients. A recent autopsy study of RLS patients found both increased tyrosine hydroxylase in the substantia nigra and decreased D2R in the putamen [11••]. CSF from RLS patients has significantly more 3-O-methyldopa (3-OMD) that correlates with the CSF homovanillic acid and RLS severity, indicating that increased dopamine production is proportional to the severity of RLS symptoms [15•]. CSF tetrahydrobiopterin is increased significantly more in the morning than night for RLS patients compared to normal controls [16] consistent with the larger circadian extracellular dopa-mine pattern in the iron-deprived rat.
Restless legs syndrome, unlike Parkinson's disease, is a hyperdopaminergic condition with an apparent postsynaptic desensitization that overcompensates during the circadian low point of dopaminergic activity in the evening and night. This overcompensation leads to the RLS symptoms that can be easily corrected by adding dopamine stimulation at that time. But this is adding fuel to the fire and often leads to increasing postsynaptic desensitization and augmentation of the RLS. Thus, for some, long-term use of dopamine agonists results in much worse RLS than before treatment, a condition referred to as RLS augmentation. These patients often need RLS treatment with nondopaminergic medications.
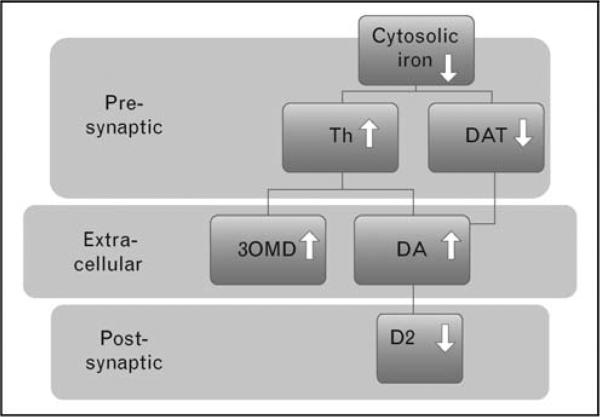
Brain iron deficiency has a profound effect on the brain neurotransmitters adenosine and dopamine as well as on the spinal systems. The primary finding from multiple studies indicates that the iron deficiency affects dopaminergic function by increasing tyrosine hydroxylase which then increases extracellular dopamine, resulting in a decrease in DAT on the cell surface and, in extreme cases, also causes a decrease in the number of D2 receptors (see Fig. 1). Even the spinal sensory system appears to be altered in mice, producing increased pain response accompanied by an elevated expression of c-Fos immunoreactive cells at the ipsilateral dorsal horn. Increased pain sensitivity has also been reported in RLS, although it is not clear how much this results from RLS per se or from the effects of the sleep loss caused by RLS [17].
Figure 1. Model of iron–dopamine relation in restless legs syndrome.
Iron decrease also increases circadian amplitude of dopamine (DA) changes from day to night. Postsynaptic adjustment suffices for the increased dopamine in the day, but fails to adjust for the relatively lower dopamine levels at night. DAT, dopamine transporter; 3OMD, 3-O-methyldopa; Th, tyrosine hydroxylase.
Restless legs syndrome and cortical activity
Tyvaert et al. [18] studied cortical function via movement-related beta and mu rhythm reactivity in patients with idiopathic, primary RLS relative to healthy individuals. On the basis of a rhythm reactivity study, authors concluded that the symptoms of RLS are related to cortical sensorimotor dysfunction. This would be consistent with the disruptions in the adenosine and dopaminergic systems regulating sensorimotor responses that have been reported for iron deficiency [18].
Restless legs syndrome and morbidity
Health-related quality of life (HRQoL) is substantially affected by RLS, which is comparable to other chronic neurological disorders such as Parkinson's disease and stroke. Severity of RLS and depressive symptoms have the most significant impact on HRQoL, but further studies are needed to evaluate the effect of disease symptoms on HRQoL and their change due to medication [19]. Sleep disturbance is the most common subjective complaint expressed by the RLS sufferer. A community-based cross-sectional observational study confirmed these subjective complaints objectively by evaluating HRQoL and polysomnography in the home of RLS patients. In this study, RLS patients had longer adjusted mean sleep latencies and higher arousal index than controls. However, there were no differences in sleep stage percentages between participants with and without RLS. Patients with RLS also reported poorer HRQoL in all physical domains as well as in the mental health and vitality domains [20••].
Restless legs syndrome and related conditions
Restless legs syndrome frequently occurs in patients with kidney disease. The prevalence of RLS, which is high in dialysis patients and which has been associated with increased risk for cardiovascular disease in the general population, could also play a role in the pathogenesis of hypertension during sleep in renal patients. In renal failure, attention to sleep quality and related perturbations of the sleep/wake cycle may help prevent the occurrence and progression of cardiovascular disease [21]. The prevalence of both insomnia and RLS is reduced in kidney-transplanted patients compared to dialysis patients, and it is similar to the prevalence observed in the general population [22]. It should be noted that i.v. iron treatment reduces the RLS symptoms in patients with end-stage renal disease [23]. The use of i.v. iron and erythropoietin has appeared to significantly reduce the severity of RLS [24]. It seems likely that the compromised iron status in these patients drives the RLS symptoms. RLS is common in rheumatologic disorders such as rheumatoid arthritis (RA) or Sjögren's syndrome. Since RLS symptoms can be similar to, and mistaken for, symptoms of rheumatologic diseases, patients may be referred to rheumatologists [25]. It deserves note that RA, but not osteoarthritis, is associated with increased risk of RLS [26], and also that RA often occurs with reduced iron status that appears to largely predict the co-occurrence of RLS [27]. The reduced iron status in these patients may be related to the systemic inflammatory process. There is a more than five times higher chance of having RLS in migraine patients who report dopaminergic premonitory symptoms (such as yawning, nausea, somnolence or food craving), supporting a dopaminergic imbalance in RLS and migraine [28]. Interestingly, the substantia nigra has been reported to have decreased iron stores in patients with migraine that may contribute to increased risk of RLS [29]. Multiple sclerosis is not known to be associated with an increased risk of RLS; however, in a recent study authors showed a relationship of higher disability and cervical cord damage that are associated with a significant risk factor for RLS in multiple sclerosis (MS) patients. Recent studies have indicated that the reduced iron status in RLS may produce a mild compromise in myelination and may thereby interact with demyelinating diseases such as MS [29,30]. Some recent studies indicate an increased prevalence of RLS among patients with Parkinson's disease, but most of these studies report data after these patients have started dopaminergic treatment and after the diagnosis of RLS. They may also be associated with lower serum ferritin [29,31]. The dopaminergic treatment can produce RLS symptoms, particularly in patients with some compromise in the dopamine system (e.g. PLMS patients) [29,32]. This is generally referred to as RLS augmentation and appears more likely to occur in patients with reduced serum ferritin [33]. Thus the higher rate of occurrence of RLS with Parkinson's disease seems related to the augmenting effects of the dopaminergic treatment. Whereas peripheral neuropathy is believed to be a potential cause of RLS, this remains controversial. In a recent study, Hattan et al. [34] report that symptoms suggestive of RLS occur more frequently among patients with peripheral neuropathy but the percentage of patients who ultimately met the diagnostic criteria for RLS is not significantly different from controls. Interestingly, the prevalence of RLS was higher among patients with hereditary neuropathies [34]. RLS is common during pregnancy, especially during the last trimester, and iron deficiency may be a major cause. Symptoms of RLS usually disappear soon after childbirth.
Restless legs syndrome treatment: new observations and potential discoveries
Dopaminergic drugs are the first-line treatment for severe RLS. Dopamine agonists provide the effects of dopamine by acting on dopamine receptors in the brain and are generally preferred to L-dopa due to a better side effect profile. Dopamine agonists have been shown to relieve symptoms in 70–90% of patients. The newer nonergota-mine derivatives such as ropinirole and pramipexole may induce fewer side effects than ergot-derived drugs. These dopamine agonists stimulate most D2 and D3 receptors. Rotigotine, unlike the other drugs, is administered via transdermal patches, which provide continuous release of the drug over 24 h. Rotigotine has been approved for RLS treatment in Europe, but it has not been approved for use by the US Food and Drug Administration (FDA) [35,36]. Other dopamine agonists that have shown some promise in small studies includes alpha-dihydroergocryptine (Almirid) and piribedil (Trivastal).
Adverse effects of dopaminergic treatment have been increasingly recognized as a problem. Compulsive behaviors have been reported and are now considered to be due to drug-related response complications [37–39]. In contrast to the RLS patients without compulsive behaviors, those with compulsive habits reported experiencing more stress, depression and sleep problems. Patients with RLS with mood and stress states may be at greater risk of developing compulsive behaviors while receiving standard dosage treatment. These behaviors are clearly linked to short-term satisfaction and underscore the role of dopaminergic mesolimbic stimulation in the reinforcement process of rewarding behavioral sequences [40]. RLS augmentation with symptoms worse then before starting the medication appears to occur with longer-term treatment and at higher dose of L-dopa and dopamine agonists [32,41]. This limits the usefulness of these dopamine medications.
A recent double-blinded, placebo-controlled trial showed that oral iron treatment significantly reduced RLS severity [42•]. To that end, consideration of oral iron therapy for ferritin levels below 75 μg/l may be useful [43]. Moreover, large doses of i.v. iron dextran produced complete resolution of RLS symptoms lasting for several weeks to months in some patients [44]. This has now been confirmed in a double-blinded, placebo-controlled trial using large doses of ferric carboxymaltose [7••, 45•]. This needs to be further studied but offers hope as a treatment alternative.
Opioids are a good alternative therapy in place of dopamine agonists and may be considered first-line therapy for patients presenting with neuropathy or painful dysthesias [46]. Short-acting agents such as hydrocodone, oxycodone, and codeine may be used for intermittent symptoms or symptoms occurring only at night [46]. Long-acting opiates such as oxycodone, methadone, or the fentanyl patch can be considered for more severe disease [46]. Opioids have been shown to be effective in double-blind, placebo-controlled studies in terms of relieving RLS symptom severity, sleep quality, and night-time leg activity [47]. Side effects of long-term opioid use include development or exacerbation of sleep apnea, decreased rapid eye movement (REM) and slow wave sleep, sedation, fatigue, constipation, addiction, and Q-T interval prolongation and torsades de pointes (with the use of methadone).
Potential new treatment options for RLS include the alpha-2-delta anticonvulsants. As such, pregabalin, a modulator of the alpha-2 delta receptor is approved for the treatment of epilepsy, neuropathic pain, generalized anxiety, and fibromyalgia. RLS symptoms were assessed using the international RLS (IRLS) scale and sleep architecture using polysomnography. Of the patients taking pregabalin, 63.3% achieved symptom remission, defined as a final IRLS score of less than 7, compared with 28.6% of patients taking placebo [48•]. This is exciting news for RLS patients since pregabalin is a different class of medication and represents another option for RLS patients that may also improve sleep quality.
Conclusion
Several recently reported studies have provided new clues towards uncovering the iron–dopamine connection and the basic dopaminergic pathology related to RLS symptoms, which have led to a better understanding of the morbidity of RLS and the many conditions associated with it. There has also been important progress recently regarding better diagnosis and treatment strategies. The challenge now is to build on the recent progress to improve the quality of life for our patients.
Acknowledgements
Dr Allen has received research support from GlaxoSmithKline and Sepracor; has consulted for GlaxoSmithKline, Boehringer Ingelheim, UCB, Xenoport, Sepracor, Novartis, Orion Pharma, Respironics, IM Systems, Pfizer, Jazz, and Schwarz Pharma; has participated in speaking engagements for Boehringer Ingelheim and GlaxoSmithKline; and has financial interests in IM Systems.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 439–440).
- 1.Hening WA, Allen RP, Washburn M, et al. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9:283–289. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 2••.Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (‘mimics’). Sleep Med. 2009;10:976–981. doi: 10.1016/j.sleep.2008.09.015. [An excellent article on differentiating RLS from RLS mimics; another aid in diagnosing RLS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Salas RE, Gamaldo CE, Allen RP, Earley CJ. Quiescegenic nocturnal dyskinesia: a restless legs syndrome (RLS) variant or a new syndrome? Sleep Med. 2009;10:396–397. doi: 10.1016/j.sleep.2008.04.001. [A case report series on a new RLS variant.] [DOI] [PubMed] [Google Scholar]
- 4.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 5.Hening W, Walters AS, Allen RP, et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–246. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–37. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7••.Allen RP, Burchell BJ, MacDonald B, et al. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10:1097–1100. doi: 10.1016/j.sleep.2008.10.007. [A great diagnostic tool for RLS.] [DOI] [PubMed] [Google Scholar]
- 8.Schormair B, Kemlink D, Roeske D, et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 2008;40:946–948. doi: 10.1038/ng.190. [DOI] [PubMed] [Google Scholar]
- 9.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 11••.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403–2412. doi: 10.1093/brain/awp125. [Study showing the dopamine differences in the brain of RLS patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erikson KM, Jones BC, Hess EJ, et al. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 13.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 14.Bianco LE, Unger EL, Earley CJ, Beard JL. Iron deficiency alters the day-night variation in monoamine levels in mice. Chronobiol Int. 2009;26:447–463. doi: 10.1080/07420520902820905. [DOI] [PubMed] [Google Scholar]
- 15•.Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–128. doi: 10.1016/j.sleep.2007.11.012. [Study showing CSF 3-OMD increases in RLS patients related to severity of the disorder.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001;16:144–149. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Dowling P, Klinker F, Amaya F, et al. Iron-deficiency sensitizes mice to acute pain stimuli and formalin-induced nociception. J Nutr. 2009;139:2087–2092. doi: 10.3945/jn.109.112557. [DOI] [PubMed] [Google Scholar]
- 18.Tyvaert L, Houdayer E, Devanne H, et al. Cortical involvement in the sensory and motor symptoms of primary restless legs syndrome. Sleep Med. 2009;10:1090–1096. doi: 10.1016/j.sleep.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10:295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20••.Winkelman JW, Redline S, Baldwin CM, et al. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–778. doi: 10.1093/sleep/32.6.772. [Reports increased risk of cardiovascular health problems for RLS patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portaluppi F, Cortelli P, Buonaura GC, et al. Do restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) play a role in nocturnal hypertension and increased cardiovascular risk of renally impaired patients? Chronobiol Int. 2009;26:1206–1221. doi: 10.3109/07420520903245276. [DOI] [PubMed] [Google Scholar]
- 22.Molnar MZ, Novak M, Mucsi I. Sleep disorders and quality of life in renal transplant recipients. Int Urol Nephrol. 2009;41:373–382. doi: 10.1007/s11255-009-9527-z. [DOI] [PubMed] [Google Scholar]
- 23.Sloand JA, Shelly MA, Feigin A, et al. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004;43:663–670. doi: 10.1053/j.ajkd.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Grote L, Leissner L, Hedner J, Ulfberg J. A Randomized, double-blind, placebo controlled, multicenter study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24:1445–1452. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- 25.Hening WA, Caivano CK. Restless legs syndrome: a common disorder in patients with rheumatologic conditions. Semin Arthritis Rheum. 2008;38:55–62. doi: 10.1016/j.semarthrit.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Salih AM, Gray RE, Mills KR, Webley M. A clinical, serological and neuro-physiological study of restless legs syndrome in rheumatoid arthritis. Br J Rheumatol. 1994;33:60–63. doi: 10.1093/rheumatology/33.1.60. [DOI] [PubMed] [Google Scholar]
- 27.Györfi MSZKP. Restless legs syndrome and serum transferrin receptor and ferritin levels in patients with rheumatoid arthritis. Sleep. 2003;26 [Google Scholar]
- 28.Cologno D, Cicarelli G, Petretta V, et al. High prevalence of dopaminergic premonitory symptoms in migraine patients with restless legs syndrome: a pathogenetic link? Neurol Sci. 2008;29(Suppl 1):S166–S168. doi: 10.1007/s10072-008-0915-4. [DOI] [PubMed] [Google Scholar]
- 29.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 30.Manconi M, Rocca MA, Ferini-Strambi L, et al. Restless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damage. Mult Scler. 2008;14:86–93. doi: 10.1177/1352458507080734. [DOI] [PubMed] [Google Scholar]
- 31.Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59:421–424. doi: 10.1001/archneur.59.3.421. [DOI] [PubMed] [Google Scholar]
- 32.Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–213. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Trenkwalder C, Hogl B, Benes H, Kohnen R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med. 2008;9:572–574. doi: 10.1016/j.sleep.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Hattan E, Chalk C, Postuma RB. Is there a higher risk of restless legs syndrome in peripheral neuropathy? Neurology. 2009;72:955–960. doi: 10.1212/01.wnl.0000336341.72621.db. [DOI] [PubMed] [Google Scholar]
- 35.Merlino G, Serafini A, Robiony F, et al. Restless legs syndrome: differential diagnosis and management with rotigotine. Neuropsychiatr Dis Treat. 2009;5:67–80. doi: 10.2147/ndt.s3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin CM, Keating GM. Rotigotine transdermal patch: in restless legs syndrome. CNS Drugs. 2008;22:797–806. doi: 10.2165/00023210-200822100-00001. [DOI] [PubMed] [Google Scholar]
- 37.Salas RE, Allen RP, Earley CJ, Gamaldo CE. Drug hoarding: a case of atypical dopamine dysregulation syndrome in a RLS patient. Mov Disord. 2009;24:627–628. doi: 10.1002/mds.22443. [DOI] [PubMed] [Google Scholar]
- 38.Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14:28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Salas RE, Allen RP, Earley CJ, Gamaldo CE. A case of compulsive behaviors observed in a restless legs syndrome patient treated with a dopamine agonist. Sleep. 2009;32:587–588. doi: 10.1093/sleep/32.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pourcher E, Remillard S, Cohen H. Compulsive habits in restless legs syndrome patients under dopaminergic treatment. J Neurol Sci. 2010;290(1–2):52–56. doi: 10.1016/j.jns.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Hogl B, Garcia-Borreguero D, Kohnen R, et al. Progressive development of augmentation during long-term treatment with levodopa in restless legs syndrome: results of a prospective multicenter study. J Neurol. 2010;257:230–237. doi: 10.1007/s00415-009-5299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Wang J, O'Reilly B, Venkataraman R, et al. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: a randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–975. doi: 10.1016/j.sleep.2008.11.003. [Documents the benefits of oral iron treatment for RLS patients with serum ferritin less than 75 mg/1.] [DOI] [PubMed] [Google Scholar]
- 43.Earley CJ. The importance of oral iron therapy in restless legs syndrome. Sleep Med. 2009;10:945–946. doi: 10.1016/j.sleep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–235. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45•.Allen RPBADW. Double-blind, placebo-controlled multicenter evaluation of restless legs syndrome (RLS) treatment with A 1,000 Mg of IV iron (ferric carboxymaltose, FCM). Sleep. in press. [A well controlled study of the long-term benefits of a large dose of i.v. iron for treatment of RLS (even for normal serum ferritin levels).] [Google Scholar]
- 46.Gamaldo CE, Earley CJ. Restless legs syndrome: a clinical update. Chest. 2006;130:1596–1604. doi: 10.1378/chest.130.5.1596. [DOI] [PubMed] [Google Scholar]
- 47.Walters AS. Review of receptor agonist and antagonist studies relevant to the opiate system in restless legs syndrome. Sleep Med. 2002;3:301–304. doi: 10.1016/s1389-9457(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 48•.Goodman A. Pregabalin reported to improve restless legs symptoms and sleep. Neurol Today. 2009;9:25–27. [First study to show the benefit of pregabalin in the treatment of RLS.] [Google Scholar]