Abstract
Purpose
To evaluate the association between menstrual cycle characteristics in early life and adulthood and fecundability.
Methods
Pregnancy Study Online (PRESTO) is an internet-based preconception cohort study of pregnancy planners from the United States and Canada. During the preconception period, we enrolled 2,189 female pregnancy planners age 21–45 years who had been attempting conception for ≤6 cycles. Women self-reported menstrual cycle characteristics via an online baseline questionnaire, and pregnancy status was ascertained through bi-monthly follow-up questionnaires. Proportional probabilities models were used to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs), adjusting for potential confounders.
Results
Compared with usual menstrual cycle lengths of 27–29 days, cycle lengths of <25 (FR=0.81, 95% CI: 0.54–1.22) and 25–26 days (FR=0.92, 95% CI: 0.75–1.14) were associated with reduced fecundability. Compared with women who reached menarche at age 12–13 years, those who reached menarche at <12 years had reduced fecundability (FR=0.87, 95% CI: 0.76–0.99). Women whose cycles never regularized after menarche (FR=0.93, 95% CI: 0.81, 1.06) had slightly reduced fecundability compared with women whose cycles regularized within 2 years of menarche. Bleed length and heaviness of bleeding were not appreciably associated with fecundability.
Conclusions
Menstrual cycle characteristics, specifically cycle length and age at menarche, may act as markers of fertility potential among pregnancy planners.
Keywords: fecundability, menstrual cycle, preconception cohort, time-to-pregnancy
INTRODUCTION
The menstrual cycle is characterized by a series of feedback responsive processes in the hypothalamic-pituitary-ovarian axis. These changes allow for the release of a mature egg from the dominant ovarian follicle and the development of a receptive endometrial lining that can support a pregnancy (1). Menstrual patterns are a marker of ovarian and hormonal function and may be related to fecundity, the biologic capacity for reproduction (2). Women with irregular cycles may have longer time-to-pregnancy due to higher risk of anovulation (2), an underlying disorder of the hypothalamic-pituitary-ovarian axis or the uterus (3), and/or difficulty timing intercourse to the fertile window (4).
Several studies support an association between cycle length and fecundity, even after controlling for age. Short cycles may reflect ovarian aging (5) or a narrow fertile window and are associated with higher risk of anovulation (2) and lower fecundability compared with normal length cycles (6–8). However, evidence assessing the association between long menstrual cycles and fecundability is inconsistent. In an IVF cohort, egg donors with regular menstrual cycles of 34–35 days had lower gonadotropin medication requirements, improved oocyte quality, and better cycle success compared with donors with menstrual cycles of 27–28 days (9). Cycle length has been positively associated with pregnancy rates in women undergoing IVF (10) and with improved fecundability among pregnancy planners (6), but also with increased risk of anovulation (2) and reduced fecundability (7, 8). Long irregular cycles may reflect underlying gynecologic disease, and inconsistencies in prior studies may relate to varying exclusion criteria (e.g., women with irregular cycles or women whose menstrual characteristics are obscured by recent hormone use). Differing study designs and small study sizes may also account for inconsistencies in the literature.
Bleed length and heaviness of bleeding may act as markers of endometrial development. In a study of regularly menstruating, healthy females in the U.S., anovulatory cycles were followed by lighter blood loss and shorter bleed length compared with ovulatory cycles (12). These findings are supported by other prospective cohort studies that have found an association between short cycle length and lower fertility (7, 11). However, a Danish preconception cohort study found only slightly lower fecundability among women with short bleeds or light menstrual flow (6).
In a cohort of North American pregnancy planners, we examined early life menstrual cycle characteristics (age at menarche and time until cycle regularity) and current menstrual cycle characteristics (irregular cycles, cycle length, bleed length, and heaviness of bleed) in relation to fecundability.
MATERIALS AND METHODS
Study population
Pregnancy Study Online (PRESTO) is an internet-based preconception cohort study of pregnancy planners in the U.S. and Canada. The study methodology has been described in detail elsewhere (13). Recruitment began in June 2013 and was conducted primarily through banner advertisements on social media and health-related websites. Eligible women were aged 21–45 years, in a stable relationship with a male partner, and not using contraception or fertility treatments. The institutional review board of Boston University Medical Center approved the study protocol and all participants provided informed consent.
Study procedures
Participants completed an online baseline questionnaire on demographics, medical history, and lifestyle habits, followed by shorter online questionnaires every 8 weeks for up to 12 months or until reported conception. Follow-up questionnaires collected updated exposure information and ascertained pregnancy status. Over 80% of women completed at least one follow-up questionnaire (13).
Women who completed the baseline questionnaire were randomized with 50% probability to receive a complimentary premium subscription to Fertility Friend (FF), a menstrual cycle charting and fertility information software program. FF users record daily information on the presence and heaviness of menstrual bleeding. They receive email tutorials from FF on monitoring their fertility and using different features of the software program, but were not provided with additional encouragement or incentives to use FF.
Assessment of menstrual cycle characteristics
Participants reported the age when they experienced their first menstrual period on the baseline questionnaire. To assess time from menarche until cycle regularity, we asked, “Did your period become regular on its own without the use of hormonal contraceptives…?” Women who responded “no” were classified as “never regular”. Women who responded “cannot say because I was taking hormones most of the time” were classified as “hormone-obscured.” Women who responded “yes” were asked to report the age when their periods became regular. We calculated time until cycle regularity as the difference between age at menarche and age when periods became regular.
On the baseline questionnaire we asked participants if their menstrual periods were regular in the past couple of years when not using hormonal contraceptives (“regular so you can usually predict about when your next period will begin”). If a woman reported regular cycles, she was asked to report her typical menstrual cycle length when not using contraception, defined as the number of days from the first day of one menstrual period to the first day of the next menstrual period. For women with missing or implausible responses to this question (3.4% of regularly-cycling women who were not long-term hormone users), we used data from follow-up questionnaires (self-reported cycle length or difference in last menstrual period (LMP) dates) to calculate cycle length. We also asked participants about their typical bleed length (defined as the number of days of bleeding, not spotting) and total amount of menstrual flow (light: ≤10 pads/tampons per menses, moderate: 11–20 pads/tampons per menses, moderate/heavy: 21–30 pads/tampons per menses, and heavy: >30 pads/tampons per menses).
Validation of menstrual cycle characteristics
We used the subset of women who provided prospective daily FF data to validate cycle length and bleed length reported on the baseline questionnaire. To calculate cycle length from FF data, we identified the first day of bleeding (not including spotting) that was immediately preceded by a day of spotting or no bleeding for each cycle and took the difference in the first dates of each pair of consecutive cycles. We averaged cycle length across all prospectively reported cycles in FF for each woman and compared it with cycle length reported at baseline.
We identified the first day of each menstrual cycle, as defined above, and the last day of each bleed, defined as a day of bleeding followed by a day of non-bleeding, and took the difference in these days to calculate bleed length. We averaged bleed length across all prospectively-reported cycles in FF for each woman and compared it with bleed length reported at baseline.
Assessment of covariates
Women reported data on age, race/ethnicity, education, income, height, weight, physical activity, parity, perceived stress scale (PSS-10) (14), multivitamin or folic acid intake, smoking, alcohol and caffeine intake, intercourse frequency, last method of contraception at baseline, and history of polycystic ovarian syndrome (PCOS), endometriosis, and uterine leiomyomata (fibroids) diagnoses. We updated information on frequency of intercourse over time using data from the follow-up questionnaires.
Assessment of pregnancy and cycles at risk
On each follow-up questionnaire, participants reported the date of their LMP and whether they had conceived since their last follow-up. We calculated total cycles at risk from the number of cycles attempting conception at study entry, date of LMP before enrollment, usual cycle length, and LMP date on each follow-up questionnaire. Participants contributed cycles to the analysis from enrollment until conception, initiation of fertility treatment, loss to follow-up, or 12 months, whichever came first.
Exclusions
Over 34 months of recruitment, 3,144 women enrolled in PRESTO. We excluded women from this analysis who had been trying to conceive for >6 months before study entry (n=345), did not complete any follow-up questionnaires (n=520), or had insufficient or implausible information about the date of baseline LMP or first pregnancy attempt (n=90). For the analysis of cycle length, bleed length, and heaviness of bleed, we excluded women who reported irregular menstrual cycles (n=331) or recent regular hormone use (n=598).
Data analysis
We categorized menstrual cycle characteristics based on the distribution in the cohort. We used a proportional probabilities model to estimate fecundability ratios (FR) and 95% confidence intervals (CI) for each exposure category relative to the reference category (15). The FR models the per-cycle probability of conception, and an FR<1.0 corresponds to reduced fecundability among exposed women compared with unexposed women. The proportional probabilities model uses discrete time-to-event data and incorporates the decline in baseline fecundability over time by controlling for binary indicators of cycle number at risk (16). It allows for left truncation due to delayed entry into the risk set, which occurs when women enter the study after attempting conception for at least 1 cycle (17, 18).
We selected potential confounders a priori from the literature. Final models were adjusted for age, race/ethnicity, education, body mass index (BMI), smoking, intercourse frequency, last method of contraception, and history of PCOS, endometriosis, or fibroids. We considered alcohol and caffeine intake, PSS-10 score, daily multivitamin intake, physical activity, and parity, but inclusion of these variables did not appreciably alter the FRs, so we omitted them from the final models.
We used PROC MI to impute missing values for exposures and covariates by creating five imputed datasets (19). We used PROC MIANALYZE to combine coefficient and standard error estimates from the five datasets (19). For menstrual cycle variables, 0.2% were missing for age at menarche, 35.0% for time until cycle regularity, <0.1% for heaviness of bleed and 0% for cycle length and bleed length.
RESULTS
The analysis of early life menstrual cycle characteristics and fecundability includes 2,189 women contributing 9,832 cycles and 1,355 pregnancies. Over 23% of participants reported menarche at <12 years of age. The majority of women (51.4%) had cycles that regularized in <2 years, whereas 4.7% of women had cycles that took ≥4 years to regularize and 21.8% never regularized.
The analysis of current menstrual cycle characteristics and fecundability includes 1,260 regularly-cycling women contributing 5,719 cycles and 777 pregnancies. Fifteen percent of women reported current irregular cycles. The average cycle length in the cohort was 29 days; 3% of women had cycles of <25 days and 6% had cycles of ≥34 days. Ninety-one percent of women had an average bleed length of 3–6 days, and 3% of women reported heavy bleeds.
Compared with women who reached a study endpoint or were followed for 12 cycles, the 227 women who were lost to follow-up had slightly longer menstrual cycles (30.3 vs. 29.8 days) and higher BMI (27.1 vs. 26.3 kg/m2), were more likely to have ≤high school education (4.9 vs. 1.9%) and less likely to identify as white/non-Hispanic (81.9 vs. 86.2%) but were similar with respect to baseline age, age at menarche, current smoking, and last method of contraception.
Table 1 presents baseline characteristics by cycle length. Average cycle length was longer for women who were younger, nulliparous and reached menarche at age <12 years. Women with the shortest and longest cycles were most likely to have a history of gynecologic disease. No other demographic or lifestyle variables were appreciably associated with usual cycle length.
Table 1.
Baseline characteristics of 1,260 pregnancy planners with regular menstrual cycles according to usual menstrual cycle length at enrollment, PRESTO, 2013–15.
| Characteristic* | Menstrual cycle length, days
|
|||||
|---|---|---|---|---|---|---|
| <25 | 25–26 | 27–29 | 30–31 | 32–33 | ≥34 | |
| Number of women | 38 | 147 | 654 | 265 | 75 | 81 |
| Age, years | 30.5 | 30.7 | 30.6 | 30.1 | 30.6 | 30.0 |
| Partner’s age, years | 32.1 | 32.2 | 32.1 | 32.4 | 32.7 | 32.0 |
| White, non-Hispanic (%) | 79.2 | 83.5 | 84.2 | 87.8 | 86.8 | 79.1 |
| <College degree (%) | 30.6 | 18.7 | 23.5 | 20.3 | 11.1 | 24.1 |
| Income <$50,000/year (%) | 27.6 | 15.4 | 18.6 | 15.6 | 5.6 | 17.8 |
| Parous (%) | 39.8 | 34.8 | 34.0 | 36.3 | 29.2 | 37.1 |
| History of infertility (%) | 10.8 | 9.9 | 8.6 | 9.5 | 12.4 | 12.4 |
| First time trying to conceive (%) | 26.9 | 32.5 | 33.0 | 27.9 | 34.4 | 28.2 |
| Body mass index, kg/m2 | 27.8 | 26.3 | 26.3 | 26.6 | 25.5 | 26.7 |
| Physical activity, MET hours/week | 36.6 | 37.5 | 35.3 | 34.8 | 33.0 | 33.0 |
| Current regular smoker (%) | 14.7 | 3.9 | 5.6 | 3.5 | 4.0 | 5.2 |
| Smoking history, pack-years | 1.2 | 0.6 | 1.0 | 0.8 | 0.9 | 0.7 |
| Current alcohol intake, drinks/week | 2.9 | 3.7 | 3.5 | 3.4 | 3.0 | 3.0 |
| Current caffeine intake, mg/day | 131.6 | 112.0 | 123.2 | 122.8 | 102.7 | 109.3 |
| Perceived stress scale score | 15.0 | 14.9 | 15.2 | 15.4 | 15.6 | 15.1 |
| Daily multivitamin intake (%) | 77.9 | 80.4 | 83.8 | 82.5 | 92.7 | 88.7 |
| Intercourse frequency <1 time/week (%) | 23.0 | 29.6 | 22.4 | 19.4 | 20.9 | 17.4 |
| Hormonal last method of contraception (%) | 39.6 | 19.8 | 29.1 | 20.5 | 23.3 | 20.4 |
| History of gynecologic disease (%)† | 8.1 | 3.7 | 7.2 | 6.6 | 7.8 | 15.9 |
| Age at menarche <12 years (%) | 33.4 | 28.2 | 24.4 | 21.2 | 20.5 | 25.5 |
| Bleed length ≥7 days (%) | 7.2 | 0.7 | 3.2 | 5.8 | 3.6 | 11.6 |
| Self-reported heavy bleeds (%) | 4.6 | 0.8 | 3.5 | 2.2 | 4.3 | 0.0 |
All characteristics except for age are age-standardized to the cohort at baseline.
Includes polycystic ovarian syndrome, endometriosis, and uterine fibroids.
Among the 1,260 women included in the analysis of current menstrual cycle characteristics and fecundability, 491 women who did not already have an FF account were randomized to receive a premium subscription to FF and 247 used at least one FF feature (50.3% of those randomized). Seventy-three and 94 women reported sufficient data to calculate cycle length and bleed length, respectively. We compared cycle length and bleed length reported at baseline with prospectively-collected FF data, and categorized each variable as in the fecundability analyses (Figure 1). On average, cycle length derived from FF was 2.0 days longer than cycle length reported at baseline (standard deviation (SD)=4.2). Bleed length from FF was, on average, 0.4 days longer than report at baseline (SD=1.1).
Figure 1.
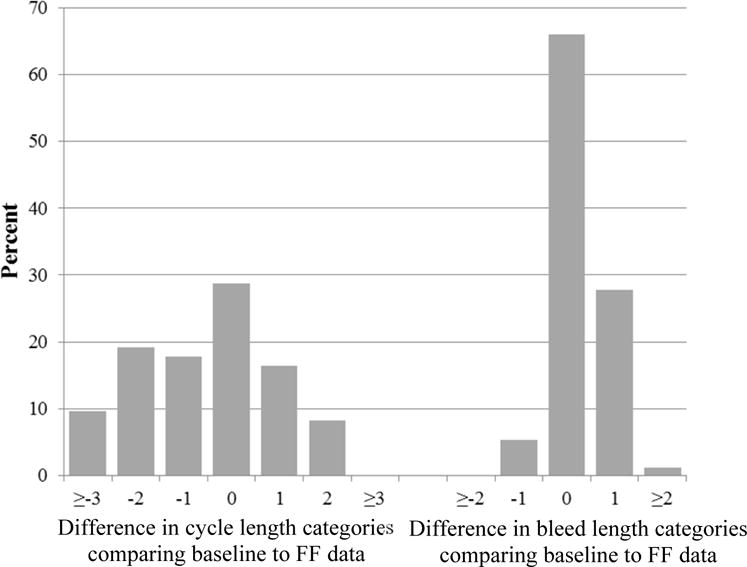
Comparison of menstrual cycle length (n=73) and bleed length (n=94) reported at baseline with prospective Fertility Friend (FF) results in PRESTO participants. The bars display the percentage of women whose baseline data are the specified number of categories different from the FF data (e.g., the −1 category indicates that the baseline data are 1 categories shorter than the FF data).
Compared with women who reached menarche at age 12–13 years, the adjusted FRs for those with age at menarche <12 or ≥15 years were 0.87 (95% CI: 0.76–0.99) and 0.92 (95% CI: 0.77–1.10), respectively (Table 2). In addition, women whose cycles took ≥4 years to regularize had similar fecundability compared with women whose cycles regularized within 2 years (adjusted FR=1.01, 95% CI: 0.79–1.27), whereas women whose cycles never regularized had slightly reduced fecundability (adjusted FR=0.93, 95% CI: 0.81–1.06).
Table 2.
Menstrual cycle characteristics and time to pregnancy, PRESTO, 2013–15.
| Exposure | No. of Cycles | No. of Pregnancies | Unadjusted FR (95% CI) | Fully adjusted FR (95% CI)* |
|---|---|---|---|---|
| Age at menarche (yrs) | ||||
| <12 | 2542 | 287 | 0.82 (0.72–0.93) | 0.87 (0.76–0.99) |
| 12–13 | 5255 | 770 | Reference | Reference |
| 14 | 1154 | 182 | 1.07 (0.92–1.23) | 1.07 (0.92–1.24) |
| ≥15 | 881 | 116 | 0.91 (0.76–1.09) | 0.92 (0.77–1.10) |
| Time until cycle regularity (yrs)† | ||||
| <2 | 5041 | 695 | Reference | Reference |
| 2–3 | 1035 | 149 | 1.03 (0.86–1.24) | 1.03 (0.86–1.24) |
| ≥4 | 466 | 66 | 1.01 (0.79–1.28) | 1.01 (0.79–1.27) |
| Never regular | 2223 | 275 | 0.89 (0.78–1.01) | 0.93 (0.81–1.06) |
| Cycle regularity† | ||||
| Regular cycles | 5719 | 777 | Reference | Reference |
| Irregular cycles | 1494 | 184 | 0.92 (0.79––1.06) | 0.96 (0.82–1.12) |
| Menstrual cycle length (days)‡ | ||||
| <25 | 190 | 21 | 0.82 (0.55–1.23) | 0.81 (0.54–1.22) |
| 25–26 | 679 | 87 | 0.97 (0.79–1.20) | 0.92 (0.75–1.14) |
| 27–29 | 3040 | 415 | Reference | Reference |
| 30–31 | 1159 | 157 | 0.98 (0.83–1.16) | 0.98 (0.83–1.16) |
| 32–33 | 337 | 45 | 0.97 (0.73–1.29) | 0.91 (0.68–1.20) |
| ≥34 | 314 | 52 | 1.18 (0.91–1.53) | 1.25 (0.96–1.62) |
| Bleed length (days)‡ | ||||
| <3 | 266 | 38 | 1.06 (0.78–1.43) | 1.13 (0.83–1.53) |
| 3–4 | 3101 | 420 | Reference | Reference |
| 5–6 | 2089 | 286 | 1.05 (0.92–1.21) | 1.08 (0.94–1.24) |
| ≥7 | 263 | 33 | 0.94 (0.68–1.30) | 0.98 (0.70–1.35) |
| Heaviness of bleed‡ | ||||
| Light | 1045 | 158 | 1.05 (0.89–1.23) | 1.02 (0.86–1.20) |
| Moderate | 3142 | 450 | Reference | Reference |
| Moderate/heavy | 1331 | 145 | 0.82 (0.69–0.98) | 0.88 (0.74–1.05) |
| Heavy | 201 | 24 | 0.84 (0.58–1.23) | 0.85 (0.58–1.24) |
Adjusted for age (<25, 25–29, 30–34, ≥35 years), race (white, non-white), education (<college degree, college degree, graduate school), BMI (<25, 25–29, 30–34, ≥35 kg/m2), smoking (never, <5, ≥5 pack-years), intercourse frequency (<1, 1, 2–3, ≥4 times/week), last method of contraception (hormonal, barrier, other methods), and history of gynecologic disease.
Excludes women who reported that they could not say if their cycle became regular on its own because they were taking hormones the whole time.
Excludes women with irregular cycles and women who could not say if their cycles were regular because they were on hormones for the past several years.
Women with current irregular cycles had similar fecundability to women with current regular cycles (adjusted FR=0.96, 95% CI: 0.82–1.12). When we did not control for history of gynecologic disease, the adjusted FR was 0.92 (95% CI: 0.79–1.07). Women who reported regular short cycles (<25 days and 25–26 days) had reduced fecundability compared with women who reported cycles of 27–29 days (adjusted FR=0.81, 95% CI: 0.54–1.22 and FR=0.92, 95% CI: 0.75–1.14, respectively), even after controlling for history of gynecologic disease (Table 2). Women with regular longer cycles had slightly increased fecundability compared with women who had average length cycles (adjusted FR=1.25, 95% CI: 0.96–1.62). Figure 2 presents the results from a restricted cubic spline model predicting fecundability from usual cycle length. The FR increased monotonically from across the range of cycle lengths, although the slope was slightly smaller after28 days.
Figure 2.
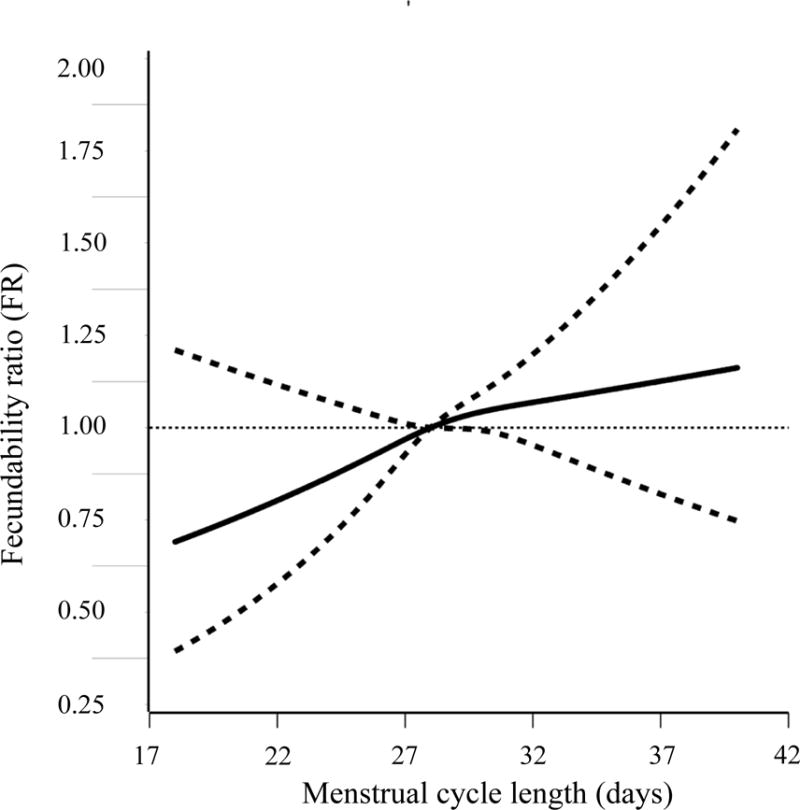
Association between usual menstrual cycle length and fecundability, fitted by restricted cubic splines, PRESTO, 2013–2015. The reference level for the FR is a cycle length of 28 days. The curves are adjusted for age, race, education, body mass index, smoking, intercourse frequency, and last method of contraception and history of gynecologic disease. The spline is trimmed at the 99th percentile and has three knot points located at 26, 28, and 32 days, respectively.
Bleed length was not appreciably associated with fecundability (Table 2). Women who reported heavy bleeds had slightly lower fecundability compared with women who reported moderate bleeds (adjusted FR=0.85, 95% CI: 0.58–1.24), although results were imprecise.
We performed a sensitivity analysis excluding women whose usual cycle length was imputed using information from the follow-up questionnaires (n=81), and results were slightly strengthened. Relative to average cycle lengths of 27–29 days, adjusted FRs for cycle lengths of <25, 25–26, 30–31, 32–33, and ≥34 days were 0.76 (95% CI: 0.49–1.18), 0.88 (95% CI: 0.71–1.10), 0.98 (95% CI: 0.82–1.17), 0.86 (95% CI: 0.64–1.16), and 1.23 (95% CI: 0.94–1.61), respectively.
The finding of reduced fecundability among women with usual cycles of <27 days was stronger among women who were <30 years of age, overweight, parous, and who had been attempting conception for 3–6 cycles at study entry (Supplementary Table 1). However, stratified results were imprecise due to small numbers.
DISCUSSION
In this preconception cohort study, short cycle length, early age at menarche, and heavy menstrual bleeds were associated with reduced fecundability. Bleed length and time until cycle regularity were not appreciably associated with fecundability. Our results were imprecise due to small numbers, but were also reasonably consistent with prior literature.
Previous studies have reported an association between short cycle length and fecundability (6–8, 20). In a Danish preconception cohort study of 2,653 pregnancy planners, cycles of <25 days were associated with reduced fecundability (FR=0.64) compared with cycle lengths of 27–29 days (6). In 6,271 IVF treatment cycles in Sweden, cumulative pregnancy rates were lowest (16.9%) in women with cycles of <26 days and highest (31.3%) in women with cycles of 32–34 days (10). Likewise, in a Spanish IVF cohort, recipients whose donors had cycle lengths of 25–26 and 34–35 days had the lowest (RR=0.74) and highest (RR=1.37) risk of pregnancy, compared with donor cycles of 27–29 days (9, 21), indicating that the relation between cycle length and fertility may be due to oocyte quality rather than endometrial factors.
We did not find any substantial relation of bleed length with fecundability. These findings are consistent with those from the Danish preconception cohort (6) and the study of oocyte donors (9). However, other cohort studies reported that short (7) or long (7, 11) bleed lengths were associated with lower fecundability. We examined usual bleed length, whereas other studies have collected prospective daily diary data and examined the most recent bleed length in relation to fecundability. There is some intra-woman variability in bleed length, which may be due to variation in the development of the endometrial lining from cycle to cycle. This difference in assessment of bleed length could explain our discrepant findings.
To our knowledge, only one other study has investigated the association between early life menstrual cycle characteristics and fecundability, and results are reasonably consistent with the present analysis (6). In this Danish preconception cohort study, women whose cycles took ≥4 years to regularize had reduced fecundability compared with women whose cycles regularized in <2 years (FR=0.89). However, that study did not create a separate category for women whose cycles never regularized; this omission could explain the discrepancy between their results and those presented here. In addition, the Danish study reported no substantial relation between age at menarche and fecundability, whereas we found a 13% reduction in fecundability associated with earlier menarche. Danish children begin puberty later than American children (22), and unmeasured early life environmental, genetic, dietary, or lifestyle factors may explain the relation of early menarche and fecundability in North America.
The main limitation of our study is the potential for exposure misclassification. Studies have found moderate agreement between self-reported usual cycle length and prospective daily diary data, with higher agreement among sexually active (23–25) and less fecund women (24). We found moderate agreement between prospective FF data and self-reported cycle length at baseline. However, FF use was optional and women who used FF consistently may differ from women who did not in terms reporting accuracy. In addition, up to 22% of pregnancies are lost before clinical detection which may result in slightly delayed menses (26). Thus, in our population of women actively attempting to conceive, the slightly longer menstrual cycles observed during follow-up compared with usual cycle lengths reported at baseline might be influenced by early unrecognized early pregnancy losses. Despite these concerns, misclassification of cycle length is likely to be non-differential with respect to detected pregnancy; therefore results are expected to be biased towards the null in the extreme exposure categories.
Bleed length, heaviness of bleed, and age at menarche are also susceptible to non-differential exposure misclassification, which could contribute to our findings. Self-reported amount of menstrual bleeding better distinguishes a woman’s changes in bleeding volume from cycle to cycle than it distinguishes average differences between women (2). Despite using an objective indicator for blood loss (i.e. number of pads/tampons per menses), differences in size and type of pad/tampon and in hygiene patterns between women preclude an accurate assessment of heaviness of bleed using self-reported data. With regard to menstrual history, participants were asked to report their age at menarche and age at cycle regularity up to 30 years later. Prior studies assessing the validity of self-reported age at menarche in adulthood (27), and the presence of over 30% missing data for age at cycle regularity in our cohort indicate that women have difficulty remembering details about their adolescent menstrual history.
It is possible that fertile women with short cycles are less likely to be included in our cohort because they conceive quickly and have more opportunities to conceive in a given amount of calendar time. This selection factor would result in a downward bias and could potentially explain our finding of an association between reduced fecundability among women with short cycles. However, the difference in the number of opportunities to conceive in a given time between women with cycles of <25 and 27–29 days would only be substantial if magnified over many cycles; therefore we expect this type of bias, if present, to be weak in this cohort.
Internet-based recruitment has been criticized because internet users and non-users differ, as do individuals who self-select into epidemiologic studies compared with those who choose not to participate (28). However, there is little reason to believe that self-selection or internet use would be related to both menstrual characteristics and fertility, and internet-based studies can have good internal and external validity (29).
In summary, we found that short menstrual cycles were associated with reduced fecundability among North American pregnancy planners, independent of age, irregular cycles, and history of reproductive illness. We also found that age at menarche and heavy bleeds were inversely associated with fecundability. These results indicate that menstrual cycle characteristics may serve as markers of fertility potential among pregnancy planners.
Supplementary Material
Acknowledgments
We acknowledge the contributions of PRESTO participants and staff. We thank Mr. Michael Bairos for his technical support with developing the web-based infrastructure of PRESTO. We are grateful to Dr. Frederic Montoya and Dr. Heather Bromberg for their generous donation of FertilityFriend.com memberships. We thank Dr. Kristen A. Hahn and Ms. Alina Chaiyasarikul for their technical assistance and instrumental support of the study.
Funding Sources: This study was funded by NICHD Grant R21HD072326 (PI: Wise). Ms. Amelia K. Wesselink was funded in part by the Boston University Reproductive, Perinatal, and Pediatric Epidemiology Training Grant NIH #T32HD052458. Dr. Shruthi Mahalingaiah was funded in part by NICHD grant #HD000849.
Abbreviations
- BMI
Body mass index
- CI
confidence interval
- FF
Fertility Friend
- FR
fecundability ratio
- IVF
in vitro fertilization
- LMP
last menstrual period
- PRESTO
Pregnancy Study Online
- RR
risk ratio
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heffner LJ, Schust DJ. The menstrual cycle The Reproductive System at a Glance. UK: Wiley-Blackwell; 2010. pp. 38–9. [Google Scholar]
- 2.Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev. 1995;17(2):265–86. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- 3.Heffner LJ, Schust DJ. Secondary amenorrhoea The Reproductive System at a Glance. UK: Wiley-Blackwell; 2010. pp. 70–1. [Google Scholar]
- 4.Wilcox AJ, Dunson D, Baird DD. The timing of the “fertile window” in the menstrual cycle: day specific estimates from a prospective study. BMJ. 2000;321(7271):1259–62. doi: 10.1136/bmj.321.7271.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gizzo S, Andrisani A, Noventa M, Quaranta M, Esposito F, Armanini D, et al. Menstrual cycle length: a surrogate measure of reproductive health capable of improving the accuracy of biochemical/sonographical ovarian reserve test in estimating the reproductive chances of women referred to ART. Reprod Biol Endocrinol. 2015;13:28. doi: 10.1186/s12958-015-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174(6):701–9. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small CM, Manatunga AK, Klein M, Feigelson HS, Dominguez CE, McChesney R, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17(1):52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422–8. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Vassena R, Vidal R, Coll O, Vernaeve V. Menstrual cycle length in reproductive age women is an indicator of oocyte quality and a candidate marker of ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2014;177:130–4. doi: 10.1016/j.ejogrb.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Brodin T, Bergh T, Berglund L, Hadziosmanovic N, Holte J. Menstrual cycle length is an age-independent marker of female fertility: results from 6271 treatment cycles of in vitro fertilization. Fertil Steril. 2008;90(5):1656–61. doi: 10.1016/j.fertnstert.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Vitzthum VJ, Spielvogel H, Caceres E, Miller A. Vaginal bleeding patterns among rural highland Bolivian women: relationship to fecundity and fetal loss. Contraception. 2001;64(5):319–25. doi: 10.1016/s0010-7824(01)00260-8. [DOI] [PubMed] [Google Scholar]
- 12.Dasharathy SS, Mumford SL, Pollack AZ, Perkins NJ, Mattison DR, Wactawski-Wende J, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175(6):536–45. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–71. doi: 10.1111/ppe.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 15.Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129(5):1072–8. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg CR, Wilcox AJ. Methdologic issues in reproductive epidemiology. In: Rothman KJ, Greenland S, Lash T, editors. Modern Epidemiology. Third. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 620–40. [Google Scholar]
- 17.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165(4):444–52. doi: 10.1093/aje/kwk027. [DOI] [PubMed] [Google Scholar]
- 18.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27(5):491–502. doi: 10.1111/ppe.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SAS Institute. SAS/Stat 9.3 user’s guide. Cary, NC: SAS Institute; 2008. [Google Scholar]
- 20.Kolstad HA, Bonde JP, Hjollund NH, Jensen TK, Henriksen TB, Ernst E, et al. Menstrual cycle pattern and fertility: a prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertil Steril. 1999;71(3):490–6. doi: 10.1016/s0015-0282(98)00474-9. [DOI] [PubMed] [Google Scholar]
- 21.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 22.Juul A, Teilmann G, Scheike T, Hertel NT, Holm K, Laursen EM, et al. Pubertal development in Danish children: comparison of recent European and US data. Int J Androl. 2006;29(1):247–55. doi: 10.1111/j.1365-2605.2005.00556.x. discussion 86–90. [DOI] [PubMed] [Google Scholar]
- 23.Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007;17(3):163–70. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167(1):25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachand AM, Cragin LA, Reif JS. Reliability of retrospectively assessed categorical menstrual cycle length data. Ann Epidemiol. 2009;19(7):501–3. doi: 10.1016/j.annepidem.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 27.Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006;60(11):993–7. doi: 10.1136/jech.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keiding N, Slama R. Commentary: time-to-pregnancy in the Real World. Epidemiology. 2015;26(1):119–21. doi: 10.1097/EDE.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 29.Hatch EE, Hahn KA, Wise LA, Mikkelsen EM, Kumar R, Fox MP, et al. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


