Abstract
The influence of nutrition on offspring metabolism has become a hot topic in recent years owing to the growing prevalence of maternal and childhood obesity. Studies in mammals have identified several factors correlating with parental and early offspring dietary influences on progeny health; however, molecular mechanisms that underlie these factors remain undiscovered. Mammalian metabolic tissues and pathways are heavily conserved in Drosophila melanogaster, making the fly an invaluable genetic model organism for studying metabolism. In this review, we discuss the metabolic similarities between mammals and Drosophila and present evidence supporting its use as an emerging model of metabolic programming.
Introduction
The environmental impact during in utero and childhood development on adult health has recently emerged as a hot topic due to the increased prevalence of obesity in childhood and adolescence and the fact that 25–30% of pregnant women in the United States are obese, both of which place offspring at an increased risk for developing major health complications (O’Brien et al., 2003; Freedman et al., 2005, 2009; Dokras et al., 2006; Metwally et al., 2008; Catalano et al., 2009; Nohr et al., 2009; Stothard et al., 2009; Vahratian, 2009; Clausen et al., 2009; Biro and Wien, 2010; Lowe et al., 2011; Forno et al., 2014; Diesel et al., 2015). David Barker introduced the concept that maternal malnourishment as well as altered infant nutrition can permanently influence offspring metabolism and pre-dispose progeny to cardiovascular and metabolic diseases (Barker, 1990; Hales and Barker, 1992). Additional studies have also demonstrated strong correlations between the malnourished parent and compromised offspring health (Painter et al.; Stanner et al., 1997; Ravelli et al., 1998; Kaati et al., 2002; Pembrey et al., 2006). Substantial evidence from both human and animal models has implicated several factors including altered epigenetic gene regulation, ER stress, and mitochondrial disruption as vehicles through which parental diet impacts offspring development and health (Gemma et al., 2006, 2009; Bruce et al., 2009; Ng et al., 2010; Vucetic et al., 2010; Wu et al., 2010, 2015; Carone et al., 2010; Igosheva et al., 2010; Borengasser et al., 2011; Luzzo et al., 2012; Soubry et al., 2013; Herbstman et al., 2013; Malti et al., 2014; Melo et al., 2014; Radford et al., 2014; Sharp et al., 2015; Casas-Agustench et al., 2015; Gallardo et al., 2015). However, to date, the molecular mechanisms by which altered nutrition influences these factors to impact offspring health are not entirely clear.
Recently, Drosophila melanogaster has emerged as a promising tool to uncover the molecular mechanisms of metabolic programming. In the fly, the impact of parental diet on offspring health can be determined by altering the maternal or paternal diet, or both. Because fly embryogenesis occurs outside of the maternal female, scientists can also investigate the influence of the pre-gestational maternal diet on offspring nutritional programming without the highly invasive procedures of in vitro fertilization and intracytoplasmic sperm injection required of mammalian studies (Ceelen et al., 2007, 2008; Giritharan et al., 2007; Scott et al., 2010; Wu et al., 2015). Moreover, understanding the impact of nutrition during early development on adult health within a single generation can be modeled in the holometabolous fly by manipulating the larval diet and assessing changes in adulthood. Use of the fly in nutritional programming is attractive for several additional reasons including the ease by which laboratories can economically design and produce customized diets, the high conservation of metabolic pathways in Drosophila, and the already extensive use of the fly in metabolic and developmental research. Also, because one of the main questions regarding metabolic programming is the impact it has on subsequent generations, the rapid fly life cycle, 10 days from embryo to adult, provides a further benefit for using Drosophila to understand the molecular mechanisms of the transgenerational impact of the parental diet. This review will focus on the benefits of using Drosophila to understand human metabolism and the emergence of the fly as a tool to model the impact of parental and early offspring nutrition on metabolic programming.
Benefits of the Drosophila model in metabolic studies
Genetics and dietary manipulation
Mammalian models have been successful in identifying several factors that may contribute to metabolic programming; however, many of these observations remain correlative. Recently, Drosophila melanogaster has proven a powerful tool in uncovering the molecular mechanisms involved in several human metabolic diseases – in fact an average of 75% of the known human disease-related genes are conserved in the fly (Reiter et al., 2001; Sanchez-Martinez et al., 2006; Baker and Thummel, 2007; Fernández-Moreno et al., 2007; Birse et al., 2010; Musselman et al., 2011; Na et al., 2013). Methods exist in the fly for the analysis of metabolic profiles including measurement of circulating and stored lipids and carbohydrates and ATP levels as well as tools for metabolomics (Tennessen et al., 2014).
Much of the interest in Drosophila is due to the exceptionally well-developed genetic tools allowing for the rapid generation of whole-body, tissue-specific, and/or developmental stage-specific gene manipulated strains as well as the development of sophisticated genetic screens (del Valle Rodríguez et al., 2012). Drosophila offer enormous transgenic libraries, some covering up to 91% of the fly protein-coding genome and include over 26,000 RNAi lines and a plethora of overexpression strains, enabling researchers to investigate various mutant versions of a single gene (Dietzl et al., 2007; Center, 2015). Specific gene mutants that are not commercially available can be quickly and relatively easily generated using either the classic method of transposon-mediated mutagenesis or the new approach of CRISPR genome editing (Bassett et al., 2013). Several groups have taken advantage of the sophistication and ease of fly genetics to identify novel metabolic regulators (Beller et al., 2008; Guo et al., 2008; Jumbo-Lucioni et al., 2010; Pospisilik et al., 2010; Baumbach et al., 2014). One such study used large-scale RNAi to knockdown 49% of protein-coding Drosophila genes in the fat body and portions of the midgut and identified 77 genes that altered organismal fat content, 83% of which have a human ortholog (Baumbach et al., 2014). One of the conserved gene, store-operated calcium entry, was identified as a novel regulator of adiposity (Baumbach et al., 2014). Another group performed a Drosophila genome-wide obesity screen, targeting 10,489 open reading frames with 11,594 transgenic RNAi fly lines to identify genes involved in fly adiposity (Pospisilik et al., 2010). From their screen Pospisilik et al. identified 500 candidate regulators of fly triglyceride levels, including genes involved in feeding behavior and key lipid regulators such as fatty-acid synthetase and Drosophila homologs to PI3K and the insulin receptor. Interestingly, this screen revealed a previously unknown role for hedgehog signaling in fat body adiposity and identified the hedgehog pathway as a determinate of mammalian brown versus white adipocyte cell fate (Pospisilik et al., 2010).
Dietary manipulations in the fly are relatively easy, economical, and the variations in diet are limitless primarily due to the fact that most diets can be produced in the laboratory. Moreover, several methods exist to quantify food ingestion (Deshpande et al., 2014; Tennessen et al., 2014). The concentrations and types of lipids, carbohydrates, amino acids, and other ingredients can be easily and quickly adjusted or omitted from fly diets to test the contribution of specific nutrients to metabolic programming. Specific compounds and pharmaceuticals can also be added to fly diets to either assess their therapeutic potential or as a method of inhibiting or activating a particular signaling pathway (Kang et al., 2002; Agrawal et al., 2005). Taking advantage of these benefits, many studies have used Drosophila to investigate the mechanisms involved in dietary restriction and overnutrition (previously reviewed by Tatar et al., 2014).
Overnutrition in the form of excess fat and sucrose in Drosophila mimics the pathophysiology of mammalian obesity including insulin resistance, hyperglycemia, non-adipose lipid accumulation, cardiomyopathy, shortened lifespan, and elevated expression of lipogenic and gluconeogenic genes (Birse et al., 2010; Musselman et al., 2011, 2013; Pasco and Leopold, 2012; Na et al., 2013). Using these models of overnutrition, several studies identified new roles for classic metabolic pathways in obesity-related human disease including insulin-TOR pathway, SREBP, PGRC-1, Retinol-Binding Protein 4, and the hexosamine biosynthetic pathway (Birse et al., 2010; Pasco and Leopold, 2012; Na et al., 2013; Diop et al., 2015). Such studies underscore the many benefits of using the fly to uncover new functions for known genes as well as to identify novel genes related to human metabolic disease.
Metabolic tissue and pathway conservation
Midgut – Nutrient absorption
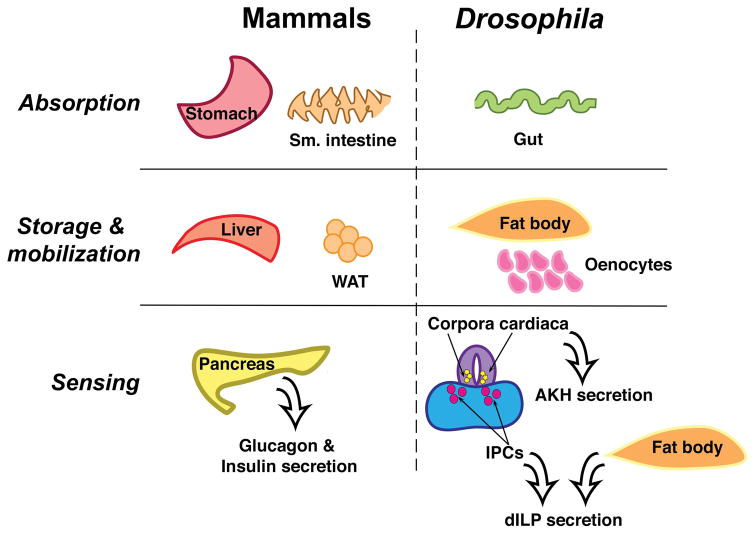
There exist many similarities between the mammalian and fly digestive systems (Apidianakis and Rahme, 2011) (Figure 1). The pharynx, esophagus, and crop (analogous to the stomach) comprise the fly foregut, one of three sections of the Drosophila gut. The hindgut follows the midgut and is the site of water absorption. While nutrient absorption and digestion occur in the mammalian stomach and small intestines, in the fly these activities are primarily restricted to the midgut. The gut is also the site of lipid absorption whereby dietary TAG is metabolized most notably by a homolog of mammalian gastric lipase, Magro, into monoacylglycerides and fatty acids which can then be absorbed by enterocytes, converted to diacylglycerides, and transported in the hemolymph as lipoproteins (Sieber and Thummel, 2009). Malpighian tubules are at the junction of the midgut and hindgut and perform functions analogous to mammalian kidneys.
Figure 1. Conservation of metabolic tissues in Drosophila.
Nutrient absorption and digestion in mammals primarily occurs in the stomach and small intestine. Drosophila contain a three-sectioned gut (foregut, midgut, and hindgut) in which the midgut performs the bulk of these functions. The fly malpighian tubules at the junction of the midgut and hindgut perform functions similar to the mammalian kidney. Storage and mobilization of carbohydrates and lipids occurs in the fat body and is analogous to mammalian white adipose tissue (WAT) and the liver. Oenocytes also play a key role in lipid storage and mobilization. The action of glucagon in response to decreased glucose levels mirrors that of the fly adipokinetic hormone (AKH), while, like insulin, Drosophila insulin-like peptides (dILPs) respond to increased nutrient availability. AKH is also known to act similarly to mammalian β3 agonist and induce mobilization of TAG from lipid droplets.
Fat body – Nutrient storage, mobilization, and sensing
Nutrients absorbed by the midgut are circulated through the hemolymph, the Drosophila equivalent of mammalian blood, and delivered to the fat body. The fat body is analogous to the liver and white adipose tissue and is the site of lipid and carbohydrate storage and mobilization and de novo lipogenesis (Dobrosotskaya et al., 2002; Seegmiller et al., 2002; Liu and Huang, 2013). Lipids reach the fat body in the form of lipoproteins, with lipophorin being the most abundant type (Kutty et al., 1996; Arrese et al., 2001; Palm et al., 2012). Lipophorins are taken up by the fat body via the lipophorin receptor, a member of the LDL receptor family, and converted to TAG and stored in lipid droplets (Arrese and Soulages, 2010). Dietary sugars are metabolized in the midgut and transferred to the fat body for storage in the form of glycogen. A fairly recent discovery identified larval oenocytes as Drosophila hepatocyte counterparts, expressing many genes homologous to mammalian lipid metabolic genes, as well as serving a key role in lipid droplet storage and fatty acid utilization (Gutierrez et al., 2007).
In addition to nutrient storage, the fat body is also a site of lipid and carbohydrate mobilization. During times of nutrient deprivation or increased energy expenditure, the fat body synthesizes trehalose from glycogen stores for release into the hemolymph for subsequent use by other tissues. Mobilization of fat body lipid droplets requires the hydrolysis of TAG to DAG by the specialized lipase Brummer, a homolog of human adipose triglyceride lipase (Grönke et al., 2005). As in mammals, specific signaling events regulate nutrient mobilization in Drosophila and are discussed in further detail.
The fat body also functions as a nutrient sensor in the control of organismal growth. Studies have shown that modulating components specifically in the fat body of the insulin/insulin-like growth factor and TSC/TOR pathways, both highly conserved in the fly, alter organismal size. Inhibiting activation of the insulin receptor (InR)/PI3K pathway through fat body-specific expression of a dominant negative p60 produces developmentally arrested larvae with proportionately small organs; on the other hand, promotion of insulin signaling either by inducing fat body-specific expression of the insulin-like peptide dILP6 or by targeting the PI3K inhibitor u-shaped (USH) through fat body-expressed microRNA mir-8 increases growth of both fat body cells and the organism (Britton et al., 2002; Hyun et al., 2009; Okamoto et al., 2009; Slaidina et al., 2009). As in mammals, Drosophila TOR (dTOR) can be activated by both the InR/PI3K pathway and extracellular nutrient availability to control cellular growth (Oldham et al., 2000; Zhang et al., 2000). Depletion of the amino acid transporter slimfast in the fat body produces developmentally delayed and smaller larvae relative to control animals that die during the pupal stage (Colombani et al., 2003). When a milder RNAi knockdown condition was used, slimfast-depleted larvae developed beyond the pupal stage, producing adults that were over 50% smaller than control flies (Colombani et al., 2003). Interestingly, the growth defect in the slimfast mutant was partially rescued by expression of the dTOR downstream target S6 Kinase and larvae expressing dominant negative dTOR in the fat body phenotypically resembled slimfast knockdown animals (Colombani et al., 2003). Depletion of slimfast in the fat body also inhibited dILP2 release from IPCs and systemically suppressed the InR/PI3K pathway (Colombani et al., 2003; Géminard et al., 2009). This failure to secrete dILP2 in response to suppressing amino acid import in the fat body was also observed in dietary restricted animals when dTOR signaling was disrupted, indicating that notification of amino acid availability is relayed from the fat body to the brain by dTOR (Géminard et al., 2009).
The nutrient sensing capabilities of the fat body are also modulated by the steroid hormone ecdysone. Suppressing ecdysone signaling in the fat body by targeting the ecdysone receptor (EcR) produced larger larvae and pupae compared to control animals (Colombani et al., 2005). Two distinct mechanisms by which ecdysone may control organismal size have been reported. One study demonstrated that fat body knockdown of EcR increases dMyc expression causing both a decrease in fat cell ribosomal number and overall animal size (Delanoue et al., 2010). Additionally, it was also shown that overexpressing dMyc in the fat body increases larval size (Delanoue et al., 2010; Parisi et al., 2013). Another report showed, using fat body EcR knockdown and a dominant negative EcR, that ecdysone controls organismal size by inhibiting expression miR-8 in the fat body, which leads to increased USH activity and blunted organismal growth (Jin et al., 2012).
Conservation of nutrient pathways
Several signaling pathways that control the sensing and utilization of carbohydrates, lipids, and amino acids are highly conserved in the fly. In the pancreas, α- and β-cells control blood glucose levels through the balanced secretion of glucagon and insulin, respectively, and are secreted at low levels in the basal non-fasting state (Campbell and Drucker, 2015). In the fly, there are three tissues that regulate glucose levels, the corpora cardiaca, the IPCs in the brain, and the fat body. The ring gland houses the neurosecretory cells of the corpora cardiaca, which, like pancreatic α-cells, secrete the glucagon-like protein adipokinetic hormone (AKH) (Kim and Rulifson, 2004). Like glucagon, AKH is derived from the processing of a preprohormone and in its mature form binds to the AKH G-protein-coupled transmembrane receptor located on the plasma membrane of fat body cells (Rayne and O’Shea, 1994; Noyes et al., 1995; Staubli et al., 2002). AKH receptor binding promotes glycogenolysis, the synthesis and subsequent release of trehalose, the major circulating sugar in the fly, and lipolysis (Staubli et al., 2002; Van der Horst, 2003; Rhea et al., 2010).
Another highly conserved gene in the regulation of carbohydrate metabolism is the Drosophila ChREBP homologue Mlx interactor (Mio/Mondo), which is activated in response to increased glucose and induces expression of genes involved in lipid biosynthesis and glycolysis, and as such, it’s promotion of lipogenesis is required for survival on an obesogenic high-sucrose diet (Postic et al., 2007; Musselman et al., 2013). Recently it has been reported that, in the brain, Mio can also regulate nutrient storage and feeding and that control of food consumption occurs in the IPCs likely via the Mio-driven down regulation of dILP3 mRNA (Docherty et al., 2015).
Insulin in the fly exists as eight (1–8) distinct homologs denoted as Drosophila insulin-like-peptides (dILPs). In larvae, 5 of the 8 dILPs (dILPs 1–5) are secreted from specialized neurosecretory cells known as IPCs found in the brain. IPC ablation decreases adult and larval size and increases circulating glucose and trehalose levels, which can be rescued by restoring dILP2 expression (Rulifson et al., 2002). In addition to the brain, several dILPs are expressed in multiple tissues, including the fat body, depending on fly developmental stage (Brogiolo et al., 2001).
Similar to mammalian insulin, dILP secretion is controlled by nutrient availability. Under starvation and amino acid-poor conditions, transcript levels of many IPC originating dILPs are altered with some dILP proteins being sequestered to IPCs as well (Ikeya et al., 2002; Géminard et al., 2009). Contrary to IPC produced dILPs, dILP6, which originates in the fat body, is increased in response to starvation (Slaidina et al., 2009). The known physiological functions of dILPs are varied and, depending on the developmental stage of the organisms, include regulation of organismal growth, cell size, lifespan, lipid storage, and carbohydrate metabolism (Brogiolo et al., 2001; Broughton et al., 2008; Slaidina et al., 2009; Grönke et al., 2010).
Activation and regulation of the insulin signaling pathway is highly conserved in the fly and is a key component of lipid and carbohydrate mobilization, uptake, and storage. Recent studies have demonstrated that release of dILPs from the IPCs is controlled by changes in amino acid and trehalose levels in a TOR-dependent fashion as well as lipids involving the leptin homolog Unpaired 2 (Géminard et al., 2009; Rajan and Perrimon, 2012; Kim and Neufeld, 2015). dILP secretion is also regulated by the Drosophila short neuropeptide F (sNPF), an ortholog of mammalian neuropeptide Y (Lee et al., 2008). Overexpression of either sNPF or its receptor, sNPFR1, results in increased food intake and overall organismal size and, at the molecular level, down regulation of fat body AKT signaling (Lee et al., 2004, 2008). sNPF orchestrates these effects by controlling dILP secretion via ERK signaling in IPCs (Lee et al., 2008). Secreted dILPs circulate and bind to Drosophila InR causing the receptor to oligomerize and activating a highly conserved set of molecular events that lead to AKT activation (Garofalo, 2002; Oldham and Hafen, 2003). Active Drosophila AKT inhibits many of the same metabolic targets as mammalian AKT including dFOXO, dTSC2, and GSK-3β (Garofalo, 2002).
Additional lipid regulatory components conserved in the fly include the Drosophila sterol regulatory element-binding protein (dSREBP) transcription factor. Unlike in mammals where sterols regulate SREBP activation, in the cholesterol auxotrophic fly, dSREBP is controlled by intracellular levels of phosphatidylethanolamine (PE), the most abundant phospholipid in flies, and undergoes proteolytic cleavage in the absence of phospholipids leading to transcription of genes for fatty acid and phospholipid biosynthesis (Dobrosotskaya et al., 2002; Seegmiller et al., 2002). Inducing dSREBP activity by decreasing PE not only impacts whole body lipid homeostasis, but leads to cardiac hyperlipidemia and dysfunction (Lim et al., 2011). Using a Drosophila model of obesity-associated heart dysfunction, Diop et al. demonstrated that dSREBP contributed to cardiac lipotoxicity in flies fed a high-fat diet, highlighting a potential role for SREBP in the control of human obesity-associated cardiomyopathies (Diop et al., 2015). Drosophila also controls lipid levels by modulation of lipid storage via the fly perilipin homologue Lsd2 that resides on the surface of lipid droplets (Grönke et al., 2003; Teixeira et al., 2003).
Lipid homeostasis is also regulated by AKH-dependent lipid mobilization, although the precise mechanisms by which AKH promotes lipid release from the fat body is not fully understood (Trinh and Boulianne, 2013). Binding of AKH to its receptor activates glycogen phosphorylase leading to breakdown of glycogen stores and subsequent synthesis of trehalose (Leopold and Perrimon, 2007). Control of lipid stores by AKH is less clear and is thought to involve a mechanism similar to fat mobilization in mammalian adipose tissue. AKH binding to the AKH receptor stimulates Brummer lipase activity, a homolog of human adipose triglyceride lipase, leading to breakdown of TAG lipid droplet stores and release of DAG into hemolymph (Trinh and Boulianne, 2013). Secretion of AKH relies on the activity of AMPK, whose function as an energy sensor is conserved in Drosophila (Pan and Hardie, 2002; Braco et al., 2012). In the fly, depleting AMPK activity produces small larvae with depleted TAG stores that die in the pupal stage (Bland et al., 2010). This phenotype was shown to be due to a requirement for AMPK in the visceral musculature to promote normal gut function and subsequent uptake of dietary nutrients (Bland et al., 2010). Several other important studies on AMPK in the fly have described a conserved role for the energy sensor in autophagy and organismal longevity, starvation, and maintenance of cell structure, further highlighting Drosophila as a model tool in understanding the physiological impact and molecular mechanisms of nutrient sensing and energy expenditure (Lee et al., 2007; Johnson et al., 2010; Stenesen et al., 2013; Ulgherait et al., 2014). However, with regards to lifespan, treatment of flies with the AMPK agonist metformin failed to increase fly longevity – resulting in a dose-response increase in animal mortality; yet, metformin did activate AMPK and decrease TAG stores (Jafari et al., 2007; Slack et al., 2012). Interestingly, metformin also activates the TOR pathway, independently of AMPK, suggesting that off-target effects of the drug may be a contributing factor and that the function of metformin with regards to lifespan may prove more complex (Kalender et al., 2010).
Metabolic programming in the offspring
Larval influence on the adult
In addition to the influences of parental diet, the contributions of gestational and early childhood nutrition on the progeny are key for understanding the mechanisms of metabolic programming. Many studies have used Drosophila to successfully model the effects of caloric restriction during early development (i.e, at the larval stage) on adult lifespan and reproductive capacity (Min et al., 2006; Aguila et al., 2007, 2013; Kolss et al., 2009; Andersen et al., 2010; May et al., 2015). Several reports have also demonstrated the metabolic influences of larval overnutrition on adulthood. Feeding larvae a high-sucrose diet significantly prolonged pupation time and produced adults with heightened levels of whole-body lipids and protein (Musselman et al., 2011; Rovenko et al., 2015a). High-sucrose fed larvae exhibited increased circulating dILPs in hemolymph, whether this continues into adulthood has not been reported; however, these adults did show increased amounts of brain dILP mRNAs (Musselman et al., 2011; Rovenko et al., 2015a). Interestingly, adults raised on high-sucrose diet as larvae also exhibited decreased lipid peroxides and reduced superoxide dismutase mRNA and activity levels, but increased catalase mRNA and activity compared to controls on a low-sucrose high-protein diet (Rovenko et al., 2015a). When comparing the impact of rearing larvae on glucose versus fructose, while both sugars produced an obese-like phenotype, fructose-fed larvae consumed more food, and as adults had increased stores of carbohydrates and lipids and decreased dILP mRNAs compared to glucose-fed larvae (Rovenko et al., 2015b). However, glucose-fed larvae had prolonged pupation rates and increased mortality (Rovenko et al., 2015b). Conversely access to excess dietary protein early in life proved beneficial for survival of physical stress including exposure to extreme temperatures and generated females with increased fecundity (Andersen et al., 2010).
Parental influence
In light of the global obesity epidemic and the increased prevalence of maternal obesity, a greater understanding of how parental diet influences offspring health as well as its impact on subsequent generations is of paramount importance. The benefits afforded by the fly, including ease of genetic and dietary manipulation, a rapid life-cycle, conserved metabolic tissues and signaling pathways, make it an ideal tool for elucidating molecular mechanisms of parental metabolic programming. Several Drosophila studies have focused on the contribution of parental diet on offspring health focusing on both under- and overnutrition (Vijendravarma et al., 2010; Valtonen et al., 2012a; Matzkin et al., 2013; Colines et al., 2015; Hardy et al., 2015).
When male and female Drosophila were exposed to isocaloric diets that varied in protein and sucrose concentrations, flies fed a high-sucrose, low-protein diet had elevated levels of whole-body glycogen, decreased protein amounts, and females laid fewer eggs than flies raised on a low-sucrose, high-protein diet (Matzkin et al., 2013) (Table 1). Although progeny from both groups were reared on standard diets, offspring of the high-sucrose, low-protein parents underwent a longer metamorphosis than offspring from high-protein, low-sucrose parents (Matzkin et al., 2013). However, while developmental timing was effected, altering the parental diet did not impact offspring survival, but it did alter offspring reproduction (Matzkin et al., 2013). Female offspring of high-sucrose, low-protein parents produced fewer eggs and exhibited increased body weight and overall glycogen content compared to females from low-sucrose, high-protein parents (Matzkin et al., 2013). Decreasing both dietary sucrose and protein to one quarter of the amounts of a general laboratory fly diet resulted in a less profound result than the high-sucrose diet – when both parents were reared on the malnourished diet, females produced heavier eggs than those fed a standard diet (Vijendravarma et al., 2010). When eggs of malnourished parents were laid on nutrient poor food they pupated at a faster rate than eggs from standard diet-fed parents (Vijendravarma et al., 2010). Studies in the fly have also demonstrated that a carbohydrate enriched parental diet can ameliorate the influence of mature parental age on offspring asymmetry relative to old parents reared solely on a protein-rich diet (Colines et al., 2015).
Table 1.
Effect of high sucrose diet on metabolic programming in Drosophila
| Paternal High Sucrose Ost et al. 2014 |
Maternal High Sucrose Buescher et al. 2013 |
Parental High Sucrose - Low Protein Matzkin et al. 2013 |
|||
|---|---|---|---|---|---|
| F0 |
 TAG Adult stage TAG Adult stage
|
 TAG, trehalose, & glycogen TAG, trehalose, & glycogen |
Adult stage |
 Glycogen; Glycogen;
 Protein Protein |
Adult stage |
 Glucose & body weight Glucose & body weight |
Fewer eggs laid | ||||
|
| |||||
| F1 |
 Body weight (adult males & females) Body weight (adult males & females)Obesogenic Challenge (adult males & females):  Body weight, TAG, & lipid droplet size Body weight, TAG, & lipid droplet size Development time & body size Development time & body sizeDesilenced peri-centric X chromosome (adult females) |
Larval stage (males): Glucose & trehalose Glucose & trehalose Glycogen & cholesterol; Glycogen & cholesterol;
 TAG TAGAltered lipid & cholesterol gene expression |
 Development time (males & females) Development time (males & females)Fewer eggs laid (adult females) |
||
Adult stage (males): Glucose & body weight* Glucose & body weight* Trehalose*, TAG*, & glycogen Trehalose*, TAG*, & glycogenAltered lipid & cholesterol gene expression | |||||
|
| |||||
| F2 | No effect observed (adult males; females unreported) |
Larval males: Glucose & trehalose Glucose & trehalose |
Not reported | ||
Larval females: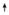 Trehalose; Trehalose;
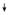 TAG TAG | |||||
Observed impact is exacerbated by obesogenic challenge
These data demonstrate that altering parental diet in Drosophila, as in mammals, not only produces reproductive repercussions in the parents, but also impacts offspring health. However, whether the observed impact of diet on parental and offspring health is a result of excess sugar, decreased protein, or both remains to be understood. One study has specifically focused on the contribution of a low protein parental diet on developmental programming and demonstrated that offspring from low protein-fed parents had longer developmental times relative to progeny from standard diet-fed parents and when only a single parent was malnourished (Valtonen et al., 2012b). It is interesting to note that the offspring developmental time when only a single parent was malnourished was shorter than the standard diet timing, but progeny from two malnourished parents was not faster than the single-parent result, but rather slower than both single malnourished parent progeny and the control standard parent progeny. With regards to severe malnourishment, studies have shown that selecting for starvation resistant flies produces cohorts with altered metabolic features (Schwasinger-Schmidt et al., 2012; Hardy et al., 2015). One particular group demonstrated that selecting for starvation resistant flies for over 65 generations produced organisms with anatomically mislocalized hearts that had decreased contractility and were dilated (Hardy et al., 2015). These cardiac dysfunctions correlated with an accumulation of lipids in the dorsal cuticle, since prolonged fasting of these animals was able to rescue the observed dilation and impaired contractility (Hardy et al., 2015). Interestingly, in another study, selecting for flies exposed to a high-protein diet over 17 generations produced progeny with increased total body mass and lipids and significantly increased mortality rates relative to standard fed controls (Kristensen et al., 2011). Taken together these studies demonstrate that the dietary inclination of each parent has a complex impact on the offspring and that the combined influence of both parent’s diets leads to a further complexity in offspring health.
Paternal influence
Teasing out the contribution to metabolic programming of each parent is imperative in order to elucidate the molecular mechanisms at play in controlling offspring health. Work in Drosophila has demonstrated a paternal contribution to metabolic programming (Valtonen et al., 2012b; Ost et al., 2014; Aldrich and Maggert, 2015). Exposure of male flies to a low-protein diet from embryo through adulthood resulted in progeny with shortened developmental times and larger male offspring, but no change in female offspring size, relative to paternal males raised on standard food (Valtonen et al., 2012b). When dietary sucrose rather than protein amounts were altered in paternal flies, it caused paternal TAG levels to increase in correlation with sugar concentrations and also resulted in a concentration dependent increase in offspring body weight (Ost et al., 2014). Offspring of high sucrose fed males appeared to be pre-sensitized to an obesogenic diet since, upon exposure to the diet, body weight, TAG and lipid droplet size all increased (Ost et al., 2014). Interestingly, this obese phenotype was evident in progeny whose fathers had only been on a high sucrose diet for two days (Ost et al., 2014). Developmental times and overall offspring size remained unchanged regardless of the paternal diet (Ost et al., 2014). Moreover, there was no apparent transgenerational impact of the high sucrose paternal diet to generations after F1 (Ost et al., 2014).
Taking full advantage of the benefits of fly genetics and dietary manipulation, two groups have uncovered profound insight into the mechanistic underpinnings of metabolic programming, highlighting a key role for genomic alteration (Ost et al., 2014; Aldrich and Maggert, 2015). Male flies fed a protein-rich diet exhibit decreased rDNA copy numbers resulting in rDNA instability in both somatic and germ cells which was InR-dependent (Aldrich and Maggert, 2015). To investigate the impact on germline transmission, Aldrich et al. crossed these male flies with females carrying an rDNA-deficient compound X chromosome to generate female progeny whose only source of rDNA originated from the Y-linked rDNA gene. This genetic strategy revealed the rDNA phenotype was transferred to female offspring, resulting in progeny with reduced rDNA copy numbers and persisted for up to two generations even though progeny were fed a standard diet (Aldrich and Maggert, 2015). The observation that the generational impact on rRNA copy number was blocked by inhibition of the TOR pathway coupled with the fact that the impact of protein-rich diet on fathers is InR-dependent implicate the insulin/TOR signaling pathway as a mechanism of germline rDNA instability (Aldrich and Maggert, 2015). How paternal-diet-induced rDNA instability impacts offspring gene expression and subsequent health is not known; however, alterations to rDNA integrity have been linked to changes in the global chromatin state and organismal longevity (Paredes and Maggert, 2009; Kobayashi, 2011; Kwan et al., 2013).
Changes in fly paternal diet have also been associated with an altered offspring chromatin state (Ost et al., 2014). Male Drosophila fed a high-sucrose diet produced offspring with desilenced peri-centric heterochromatin on the X chromosome and an increase in gene expression including genes involved in energy metabolism as well as several unknown genes (Ost et al., 2014). Gene desilencing was also observed in the sperm of the high sucrose-fed fathers, suggesting that diet-induced changes to sperm gene expression is a vehicle by which progeny gene expression is influenced (Ost et al., 2014).
Maternal influence
Several studies in Drosophila have focused on understanding the contribution of maternal diet to metabolic programming (Buescher et al., 2013; Matzkin et al., 2013; Prasad et al., 2003; Valtonen et al., 2012; Vijendravarma et al., 2010). Most maternal programming studies have focused on the influence of undernourishment on progeny health and have demonstrated that females exposed to a protein- and sucrose- poor diet produce heavier eggs (Vijendravarma et al., 2010). However, decreased nutrition in the form of sucrose and protein also results in decreased egg production and fewer mating events for females (Chapman and Partridge, 1996). When maternal dietary protein levels were decreased, females produced larger progeny with shorter developmental times compared to offspring of protein-rich females (Valtonen et al., 2012b). Additionally, protein-poor females produced progeny with increased survivorship at the larval stage compared to offspring of protein-rich females; however, by the pupal stage, survivorship between both groups was comparable (Prasad et al., 2003). It is interesting to note that, although protein deficient females generated larger offspring, actual maternal egg production and ovary size correlate negatively with dietary protein concentrations (Drummond-Barbosa and Spradling, 2001). While the molecular mechanisms by which maternal diet controls reproduction and offspring health are not completely understood, there is evidence demonstrating a role for Hedgehog and insulin signaling pathways in regulating the diet-induced proliferation of ovarian stem cells (Drummond-Barbosa and Spradling, 2001; Hsu et al., 2008; Hsu and Drummond-Barbosa, 2009; Hartman et al., 2013).
Most maternal diet studies in Drosophila focus on undernourished conditions; however, to understand the molecular mechanisms of maternal overnutrition (i.e., maternal obesity) we developed a fly model that incorporated comparing the effects of a high versus low sucrose diet on offspring health (Buescher et al., 2013). Rearing female flies on a high sucrose diet produced an obese-like phenotype, marked by increased whole-body TAG, glycogen, and trehalose, insulin resistance, and elevated dILP expression compared to females on a low sucrose diet (Buescher et al., 2013; J G Duncan, unpublished observations). High sucrose-fed females produced male offspring with increased whole-body glucose and trehalose levels, while female progeny only had decreased levels of whole-body cholesterol (Buescher et al., 2013). Changes in gene expression levels of male offspring were assessed by RNA sequencing, which revealed several differentially expressed genes involved in lipid and carbohydrate metabolism, including lipases Lip3 and CG17191, fatty acid synthase, acetyl-CoA-carboxylase, pyruvate kinase, enolase, and a putative sugar transporter CG4797 (Buescher et al., 2013). Offspring of high sucrose-fed females were pre-sensitized to an obesogenic diet, exhibiting increased whole-body TAG, glycogen, and trehalose levels as well as altered expression of several carbohydrate and lipid metabolic genes (Buescher et al., 2013). A transgenerational effect was also observed whereby F2 male progeny of high sucrose-fed F0 females showed increased glycogen and trehalose levels while F2 females displayed increased trehalose and decreased TAG levels (Buescher et al., 2013). The factors contributing to these transgenerational observations are currently being investigated by the lab and are hypothesized to involve inheritance of altered maternal mitochondria – a phenomena that has been reported in mammalian metabolic programming studies; however, these reports only investigated the F1 generation (Grindler and Moley, 2013). The multigenerational reach of the maternal obesogenic diet as well as the transmission of maternal mitochondria makes the organelle a strong candidate for influencing metabolic programming across generations.
Conclusion
The fly is quickly emerging as an important ally for understanding human metabolic diseases, a trend in part owed to the highly conserved series of metabolic tissues and pathways present within the fly and a multitude of genetic tools available (Liu and Huang, 2013; Rajan and Perrimon, 2013). As the prevalence of maternal and paternal obesity increase the threat to offspring health and to subsequent generations will only worsen, making Drosophila an invaluable tool in uncovering the complexity of metabolic programming.
Acknowledgments
Funding
This work was supported by grants from the American Heart Association (IRG5450013 and GRNT12080056 to J.G. Duncan), the Washington University Diabetes Research Center (P30DK020579 to J.G. Duncan) and the National Institutes of Health (K12HD001459 to R.T. Brookheart).
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
- Agrawal N, Pallos J, Slepko N, Apostol BL, Bodai L, Chang L-WW, Chiang A-SS, Thompson LM, Marsh JL. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc Natl Acad Sci U S A. 2005;102:3777–3781. doi: 10.1073/pnas.0500055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK. The role of larval fat cells in adult Drosophila melanogaster. The Journal of Experimental Biology. 2007;210:956–963. doi: 10.1242/jeb.001586. [DOI] [PubMed] [Google Scholar]
- Aguila JR, Hoshizaki DK, Gibbs AG. Contribution of larval nutrition to adult reproduction in Drosophila melanogaster. The Journal of Experimental Biology. 2013;216:399–406. doi: 10.1242/jeb.078311. [DOI] [PubMed] [Google Scholar]
- Aldrich JC, Maggert KA. Transgenerational inheritance of diet-induced genome rearrangements in Drosophila. PLoS Genetics. 2015;11:e1005148. doi: 10.1371/journal.pgen.1005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen LH, Kristensen TN, Loeschcke V, Toft S, Mayntz D. Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. Journal of Insect Physiology. 2010;56:336–340. doi: 10.1016/j.jinsphys.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Disease Models & Mechanisms. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annual Review of Entomology. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA. Lipid storage and mobilization in insects: current status and future directions. Insect Biochemistry and Molecular Biology. 2001;31:7–17. doi: 10.1016/s0965-1748(00)00102-8. [DOI] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Tibbit C, Ponting CP, Liu J-L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Reports. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach J, Hummel P, Bickmeyer I, Kowalczyk KM, Frank M, Knorr K, Hildebrandt A, Riedel D, Jäckle H, Kühnlein RP. A drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metabolism. 2014;19:331–343. doi: 10.1016/j.cmet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Beller M, Sztalryd C, Southall N, Bell M, Jäckle H, Auld DS, Oliver B. COPI complex is a regulator of lipid homeostasis. PLoS Biology. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Wien M. Childhood obesity and adult morbidities. The American Journal of Clinical Nutrition. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metabolism. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, Lee RJ, Magallanes JM, Foskett JK, Birnbaum MJ. AMPK supports growth in Drosophila by regulating muscle activity and nutrient uptake in the gut. Developmental Biology. 2010;344:293–303. doi: 10.1016/j.ydbio.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJJ, Badger TM, Shankar K. Maternal Obesity during Gestation Impairs Fatty Acid Oxidation and Mitochondrial SIRT3 Expression in Rat Offspring at Weaning. PLoS ONE. 2011;6:e24068. doi: 10.1371/journal.pone.0024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braco JT, Gillespie EL, Alberto GE, Brenman JE, Johnson EC. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics. 2012;192:457–466. doi: 10.1534/genetics.112.143610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the drosophila insulin receptor and insulin-like peptides in growth control. Current Biology. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PloS One. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson Ma, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- Buescher JL, Musselman LP, Wilson Ca, Lang T, Keleher M, Baranski TJ, Duncan JG. Evidence for transgenerational metabolic programming in Drosophila. Disease Models & Mechanisms. 2013;6:1123–1132. doi: 10.1242/dmm.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Islet α cells and glucagon—critical regulators of energy homeostasis. Nature Reviews Endocrinology. 2015;11:329–338. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Agustench P, Fernandes FS, Tavares do Carmo MG, Visioli F, Herrera E, Dávalos A. Consumption of distinct dietary lipids during early pregnancy differentially modulates the expression of microRNAs in mothers and offspring. PloS One. 2015;10:e0117858. doi: 10.1371/journal.pone.0117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. The American Journal of Clinical Nutrition. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. The Journal of Clinical Endocrinology and Metabolism. 2007;92:3417–3423. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. The Journal of Clinical Endocrinology and Metabolism. 2008;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- Center VDR. VDRC Transgenic RNAi Libraries. 2015. [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proceedings of the Royal Socieity Biological Sciences. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2009;94:2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- Colines B, Rodríguez NC, Hasson ER, Carreira V, Frankel N. Parental age influences developmental stability of the progeny in Drosophila. Proceedings. Biological Sciences/The Royal Society. 2015;282:20142437. doi: 10.1098/rspb.2014.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carré C, Noselli S, Léopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science (New York, NY ) 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Delanoue R, Slaidina M, Léopold P. The steroid hormone ecdysone controls systemic growth by repressing dMyc function in Drosophila fat cells. Developmental Cell. 2010;18:1012–1021. doi: 10.1016/j.devcel.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, Ja WW. Quantifying Drosophila food intake: comparative analysis of current methodology. Nature Methods. 2014;11:535–540. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesel JC, Eckhardt CL, Day NL, Brooks MM, Arslanian SA, Bodnar LM. Is gestational weight gain associated with offspring obesity at 36 months? Pediatric Obesity. 2015;10:305–310. doi: 10.1111/ijpo.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Diop SBB, Bisharat-Kernizan J, Birse RTT, Oldham S, Ocorr K, Bodmer R. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Reports. 2015;10:1572–1584. doi: 10.1016/j.celrep.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller aC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science (New York, NY ) 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Docherty JEB, Manno JE, McDermott JE, DiAngelo JR. Mio acts in the Drosophila brain to control nutrient storage and feeding. Gene. 2015;568:190–195. doi: 10.1016/j.gene.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstetrics and Gynecology. 2006;108:61–69. doi: 10.1097/01.AOG.0000219768.08249.b6. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Developmental Biology. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno MA, Farr CL, Kaguni LS, Garesse R. Drosophila melanogaster as a model system to study mitochondrial biology. Methods in Molecular Biology (Clifton, NJ ) 2007;372:33–49. doi: 10.1007/978-1-59745-365-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal Obesity in Pregnancy, Gestational Weight Gain, and Risk of Childhood Asthma. PEDIATRICS. 2014;134:e535–e546. doi: 10.1542/peds.2014-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115:22–27. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. Risk factors and adult body mass index among overweight children: the Bogalusa Heart Study. Pediatrics. 2009;123:750–757. doi: 10.1542/peds.2008-1284. [DOI] [PubMed] [Google Scholar]
- Gallardo JM, Gómez-López J, Medina-Bravo P, Juárez-Sánchez F, Contreras-Ramos A, Galicia-Esquivel M, Sánchez-Urbina R, Klünder-Klünder M. Maternal obesity increases oxidative stress in the newborn. Obesity (Silver Spring, Md ) 2015;23:1650–1654. doi: 10.1002/oby.21159. [DOI] [PubMed] [Google Scholar]
- Garofalo RS. Genetic analysis of insulin signaling in Drosophila. Trends in Endocrinology and Metabolism: TEM. 2002;13:156–162. doi: 10.1016/s1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- Géminard C, Rulifson EJ, Léopold P. Remote Control of Insulin Secretion by Fat Cells in Drosophila. Cell Metabolism. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Gemma C, Sookoian S, Alvariñas J, García SI, Quintana L, Kanevsky D, González CD, Pirola CJ. Mitochondrial DNA depletion in small- and large-for-gestational-age newborns. Obesity (Silver Spring, Md ) 2006;14:2193–2199. doi: 10.1038/oby.2006.257. [DOI] [PubMed] [Google Scholar]
- Gemma C, Sookoian S, Alvariñas J, García SI, Quintana L, Kanevsky D, González CD, Pirola CJ. Maternal pregestational BMI is associated with methylation of the PPARGC1A promoter in newborns. Obesity (Silver Spring, Md ) 2009;17:1032–1039. doi: 10.1038/oby.2008.605. [DOI] [PubMed] [Google Scholar]
- Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson AT, Rinaudo PF. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction (Cambridge, England) 2007;134:63–72. doi: 10.1530/REP-06-0247. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: Potential mechanisms emerging from mouse model systems. Molecular Human Reproduction. 2013;19:487–494. doi: 10.1093/molehr/gat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Beller M, Fellert S, Ramakrishnan H, Jäckle H, Kühnlein RP. Control of fat storage by a Drosophila PAT domain protein. Current Biology_: CB. 2003;13:603–606. doi: 10.1016/s0960-9822(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metabolism. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular Evolution and Functional Characterization of Drosophila Insulin-Like Peptides. PLoS Genetics. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–280. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hardy CM, Birse RT, Wolf MJ, Yu L, Bodmer R, Gibbs AG. Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2015;309:R658–R667. doi: 10.1152/ajpregu.00160.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TR, Strochlic TI, Ji Y, Zinshteyn D, O’Reilly AM. Diet controls Drosophila follicle stem cell proliferation via Hedgehog sequestration and release. The Journal of Cell Biology. 2013;201:741–757. doi: 10.1083/jcb.201212094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Wang S, Perera FP, Lederman SA, Vishnevetsky J, Rundle AG, Hoepner LA, Qu L, Tang D. Predictors and consequences of global DNA methylation in cord blood and at three years. PLoS One. 2013;8:e72824. doi: 10.1371/journal.pone.0072824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Horst DJ. Insect adipokinetic hormones: release and integration of flight energy metabolism. Comparative Biochemistry and Physiology Part B, Biochemistry & Molecular Biology. 2003;136:217–226. doi: 10.1016/s1096-4959(03)00151-9. [DOI] [PubMed] [Google Scholar]
- Hsu H-J, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-J, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Developmental Biology. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biology. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Jafari M, Khodayari B, Felgner J, Bussel II, Rose MR, Mueller LD. Pioglitazone: an anti-diabetic compound with anti-aging properties. Biogerontology. 2007;8:639–651. doi: 10.1007/s10522-007-9105-7. [DOI] [PubMed] [Google Scholar]
- Jin H, Kim VN, Hyun S. Conserved microRNA miR-8 controls body size in response to steroid signaling in Drosophila. Genes & Development. 2012;26:1427–1432. doi: 10.1101/gad.192872.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Kazgan N, Bretz CA, Forsberg LJ, Hector CE, Worthen RJ, Onyenwoke R, Brenman JE. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PloS One. 2010;5:e12799. doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbo-Lucioni P, Ayroles JF, Chambers MM, Jordan KW, Leips J, Mackay TF, De Luca M. Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics. 2010;11:297. doi: 10.1186/1471-2164-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. European Journal of Human Genetics_: EJHG. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci U S A. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Neufeld TP. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nature Communications. 2015;6:6846. doi: 10.1038/ncomms7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. How does genome instability affect lifespan? Genes to Cells. 2011;16:617–624. doi: 10.1111/j.1365-2443.2011.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolss M, Vijendravarma RK, Schwaller G, Kawecki TJ. Life-history consequences of adaptation to larval nutritional stress in Drosophila. Evolution; International Journal of Organic Evolution. 2009;63:2389–2401. doi: 10.1111/j.1558-5646.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- Kristensen TN, Overgaard J, Loeschcke V, Mayntz D. Dietary protein content affects evolution for body size, body fat and viability in Drosophila melanogaster. Biology Letters. 2011;7:269–272. doi: 10.1098/rsbl.2010.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty RK, Kutty G, Kambadur R, Duncan T, Koonin EV, Rodriguez IR, Odenwald WF, Wiggert B. Molecular Characterization and Developmental Expression of a Retinoid- and Fatty Acid-binding Glycoprotein from Drosophila: A PUTATIVE LIPOPHORIN. Journal of Biological Chemistry. 1996;271:20641–20649. doi: 10.1074/jbc.271.34.20641. [DOI] [PubMed] [Google Scholar]
- Kwan EX, Foss EJ, Tsuchiyama S, Alvino GM, Kruglyak L, Kaeberlein M, Raghuraman MK, Brewer BJ, Kennedy BK, Bedalov A. A natural polymorphism in rDNA replication origins links origin activation with calorie restriction and lifespan. PLoS Genetics. 2013;9:e1003329. doi: 10.1371/journal.pgen.1003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-S, You K-H, Choo J-K, Han Y-M, Yu K. Drosophila short neuropeptide F regulates food intake and body size. The Journal of Biological Chemistry. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee S-H, Shong M, Kim J-M, Kim J, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lee K-S, Kwon O-Y, Lee JH, Kwon K, Min K-J, Jung S-A, Kim A-K, You K-H, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nature Cell Biology. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Lim HY, Wang W, Wessells RJ, Ocorr K, Bodmer R. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes and Development. 2011;25:189–200. doi: 10.1101/gad.1992411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Huang X. Lipid metabolism in Drosophila_: development and disease Using Drosophila System to Study Lipid Metabolism Lipids Function in Drosophila Early Development. Acta Biochim Biophys Sin. 2013a;45:44–50. doi: 10.1093/abbs/gms105. [DOI] [PubMed] [Google Scholar]
- Liu Z, Huang X. Lipid metabolism in Drosophila: development and disease. Acta Biochim Biophys Sin (Shanghai) 2013b;45:44–50. doi: 10.1093/abbs/gms105. [DOI] [PubMed] [Google Scholar]
- Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B. Maternal obesity during pregnancy as a risk for early-life asthma. J Allergy Clin Immunol. 2011;128:1102–1107. doi: 10.1016/j.jaci.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–416. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Matzkin LM, Johnson S, Paight C, Markow Ta. Preadult parental diet affects offspring development and metabolism in Drosophila melanogaster. PLoS One. 2013;8:e59530. doi: 10.1371/journal.pone.0059530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CM, Doroszuk A, Zwaan BJ. The effect of developmental nutrition on life span and fecundity depends on the adult reproductive environment in Drosophila melanogaster. Ecology and Evolution. 2015;5:1156–1168. doi: 10.1002/ece3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AM, Benatti RO, Ignacio-Souza LM, Okino C, Torsoni AS, Milanski M, Velloso LA, Torsoni MA. Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metabolism. 2014;63:682–692. doi: 10.1016/j.metabol.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertility and Sterility. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Min K-J, Hogan MF, Tatar M, O’Brien DM. Resource allocation to reproduction and soma in Drosophila: a stable isotope analysis of carbon from dietary sugar. Journal of Insect Physiology. 2006;52:763–770. doi: 10.1016/j.jinsphys.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Brent MR, Turk J, Baranski TJ. Role of fat body lipogenesis in protection against the effects of caloric overload in drosophila. Journal of Biological Chemistry. 2013;288:8028–8042. doi: 10.1074/jbc.M112.371047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad NG, Mallikarjun Shakarad MR, AJ Interaction between the effects of maternal and larval levels of nutrition on pre-adult survival in Drosophila melanogaster. Evolutionary Ecology Research. 2003;5:903–911. [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013a;9:e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013b;9:e1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S-F, Lin RCY, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Timpson NJ, Andersen CS, Davey Smith G, Olsen J, Sorensen TI. Severe obesity in young women and reproductive health: the Danish National Birth Cohort. PLoS One. 2009;4:e8444. doi: 10.1371/journal.pone.0008444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes BE, Katz FN, Schaffer MH. Identification and expression of the Drosophila adipokinetic hormone gene. Molecular and Cellular Endocrinology. 1995;109:133–141. doi: 10.1016/0303-7207(95)03492-p. [DOI] [PubMed] [Google Scholar]
- O’Brien TE, Ray JG, Chan W-S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology (Cambridge, Mass ) 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, O’Connor MB, Mizoguchi A. A Fat Body-Derived IGF-like Peptide Regulates Postfeeding Growth in Drosophila. Developmental Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends in Cell Biology. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes & Development. 2000;14:2689–2694. doi: 10.1101/gad.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–1364. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reproductive Toxicology (Elmsford, NY) 20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S. Lipoproteins in Drosophila melanogaster-assembly, function, and influence on tissue lipid composition. PLoS Genetics. 2012:8. doi: 10.1371/journal.pgen.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. The Biochemical Journal. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes S, Maggert KA. Ribosomal DNA contributes to global chromatin regulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17829–17834. doi: 10.1073/pnas.0906811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi F, Riccardo S, Zola S, Lora C, Grifoni D, Brown LM, Bellosta P. dMyc expression in the fat body affects DILP2 release and increases the expression of the fat desaturase Desat1 resulting in organismal growth. Developmental Biology. 2013;379:64–75. doi: 10.1016/j.ydbio.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco MY, Leopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One. 2012;7:e36583. doi: 10.1371/journal.pone.0036583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, Golding J. Sex-specific, male-line transgenerational responses in humans. European Journal of Human Genetics_: EJHG. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Schramek D, Schnidar H, Cronin SJF, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Postic C, Dentin R, Denechaud P-D, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annual Review of Nutrition. 2007;27:179–192. doi: 10.1146/annurev.nutr.27.061406.093618. [DOI] [PubMed] [Google Scholar]
- Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, Seisenberger S, Hore TA, Reik W, Erkek S, et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science (New York, NY ) 2014;345:1255903. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biology. 2013;11:38. doi: 10.1186/1741-7007-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet (London, England) 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Rayne RC, O’Shea M. Reconstitution of adipokinetic hormone biosynthesis in vitro indicates steps in prohormone processing. European Journal of Biochemistry/FEBS. 1994;219:781–789. doi: 10.1111/j.1432-1033.1994.tb18558.x. [DOI] [PubMed] [Google Scholar]
- Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Research. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea JM, Wegener C, Bender M. The proprotein convertase encoded by amontillado (amon) is required in Drosophila corpora cardiaca endocrine cells producing the glucose regulatory hormone AKH. PLoS Genetics. 2010;6:e1000967. doi: 10.1371/journal.pgen.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovenko BM, Kubrak OI, Gospodaryov DV, Perkhulyn NV, Yurkevych IS, Sanz A, Lushchak OV, Lushchak VI. High sucrose consumption promotes obesity whereas its low consumption induces oxidative stress in Drosophila melanogaster. Journal of Insect Physiology. 2015a;79:42–54. doi: 10.1016/j.jinsphys.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Rovenko BM, Perkhulyn NV, Gospodaryov DV, Sanz A, Lushchak OV, Lushchak VI. High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology. 2015b;180:75–85. doi: 10.1016/j.cbpa.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martinez A, Luo N, Clemente P, Adan C, Hernandez-Sierra R, Ochoa P, Fernandez-Moreno MA, Kaguni LS, Garesse R. Modeling human mitochondrial diseases in flies. Biochim Biophys Acta. 2006;1757:1190–1198. doi: 10.1016/j.bbabio.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwasinger-Schmidt TE, Kachman SD, Harshman LG. Evolution of starvation resistance in Drosophila melanogaster: measurement of direct and correlated responses to artificial selection. Journal of Evolutionary Biology. 2012;25:378–387. doi: 10.1111/j.1420-9101.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KA, Yamazaki Y, Yamamoto M, Lin Y, Melhorn SJ, Krause EG, Woods SC, Yanagimachi R, Sakai RR, Tamashiro KLK. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biology of Reproduction. 2010;83:220–227. doi: 10.1095/biolreprod.109.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB. The SREBP pathway in Drosophila: Regulation by palmitate, not sterols. Developmental Cell. 2002;2:229–238. doi: 10.1016/s1534-5807(01)00119-8. [DOI] [PubMed] [Google Scholar]
- Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, Suderman M, Shihab HA, Lyttleton O, McArdle W, Ring SM, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thummel CS. The DHR96 Nuclear Receptor Controls Triacylglycerol Homeostasis in Drosophila. Cell Metabolism. 2009;10:481–490. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PloS One. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P. A Drosophila Insulin-like Peptide Promotes Growth during Nonfeeding States. Developmental Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013;11:29. doi: 10.1186/1741-7015-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanner SA, Bulmer K, Andrès C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. BMJ (Clinical Research Ed ) 1997;315:1342–1348. doi: 10.1136/bmj.315.7119.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli F, Jorgensen TJD, Cazzamali G, Williamson M, Lenz C, Sondergaard L, Roepstorff P, Grimmelikhuijzen CJP. Molecular identification of the insect adipokinetic hormone receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3446–3451. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesen D, Suh JM, Seo J, Yu K, Lee K-S, Kim J-S, Min K-J, Graff JM. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metabolism. 2013;17:101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends in Endocrinology and Metabolism: TEM. 2014;25:509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Rabouille C, Rørth P, Ephrussi A, Vanzo NF. Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mechanisms of Development. 2003;120:1071–1081. doi: 10.1016/s0925-4773(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Barry WE, Cox J, Thummel CS. Methods for studying metabolism in Drosophila. Methods. 2014;68:105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh I, Boulianne GL. Modeling obesity and its associated disorders in Drosophila. Physiology (Bethesda, Md ) 2013;28:117–124. doi: 10.1152/physiol.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Reports. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–273. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle Rodríguez A, Didiano D, Desplan C. Power tools for gene expression and clonal analysis in Drosophila. Nature Methods. 2012;9:47–55. doi: 10.1038/nmeth.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen TM, Kangassalo K, Polkki M, Rantala MJ. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS One. 2012a;7:e31611. doi: 10.1371/journal.pone.0031611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen TM, Kangassalo K, Pölkki M, Rantala MJ, Polkki M, Rantala MJ. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS One. 2012b;7:e31611. doi: 10.1371/journal.pone.0031611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijendravarma RK, Narasimha S, Kawecki TJ. Effects of parental larval diet on egg size and offspring traits in Drosophila. Biology Letters. 2010;6:238–241. doi: 10.1098/rsbl.2009.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai T-SS, St John JC, Norman RJ, Febbraio Ma, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–691. doi: 10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes & Development. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]