This Letter to the Editor comments upon a recent publication on the impact of Cervarix vaccination on Human Papillomavirus (HPV) infection and cervical cancer in the United Kingdom in which estimates of long-term, cross-protection against non-vaccine HPV types of 20 y were provided. However, the article fails to cite several studies regarding the durability of cross-protection. Overall, studies have shown that while virus-like particle (VLP) HPV vaccines have demonstrated consistently high and long-lasting type-specific protection against the included HPV types, cross-protection efficacy against non-vaccine types is lower and wanes over time. It is also noted that cross-protection antigens are not specifically manufactured, quality-controlled or potency tested as are the vaccine HPV VLPs that undergo such testing before release of product.
The report by Van Effelterre, et al. (2016) presented various ‘vaccine scenarios’ for the efficacy of the bivalent Human Papillomavirus (HPV) vaccine in order to inform the health economics model presented in the paper.1 Estimates of efficacy included impact of vaccination on lifelong, 20-year cross-protection versus no protection against cervical intraepithelial neoplasia (CIN2+) and cancer due to HPV 31/33/45 and 9 other HPV types. Estimates of efficacy were drawn from the end of study results of the phase III study, PATRICIA, which lasted 4 y.2
Unfortunately, the authors failed to cite some important findings regarding the likely durability of cross-protection, including reports from study-extension data on the long term effectiveness of the bivalent HPV vaccine. Although long-term studies have demonstrated continued protection against those specifically manufactured HPV types present in a quadrivalent HPV vaccine (6/11/16/18) through 8 y of follow-up3 and the bivalent HPV vaccine (16/18) for up to 9 years,4 long-term follow-up studies that have evaluated continued effectiveness due to cross-protection post-vaccination are more limited, and have shown at best, partial protection that markedly wanes over time.
For example, Malagon, et al., performed a meta-analysis of HPV vaccine clinical trials including the 4-year PATRICIA trial and longer-term trials of the bivalent vaccine.5 The analysis reported that while the bivalent vaccine conferred cross-protection for HPV types 31, 33, and 45 at varying degrees, estimates of efficacy were lost by 6 y of follow-up. An evaluation of a long-term extension of a Phase II study of the bivalent vaccine by Naud, et al. showed no cross-protection against persistent infection and high-grade disease for any non-vaccine type, including 31, 33, and 45 at 9 y of follow-up.4 Another analysis of the efficacy across these HPV types for the bivalent vaccine using extension study and registry data by Saah et al., similarly showed a waning of response in non-vaccine types (see Table 1).6 Estimates of efficacy based on persistent infection were assessed, which obviated complications of HPV attribution in tissue specimens tested by PCR. Efficacy against HPV types 16 and 18 ranged from 92 to 100% for all time points; whereas efficacy for HPV types 31, 33, and 45 ranged from 43% to 79% at 4 years, then declined by 6.4 y to −16% to 52%, and to −52% to 10% at 8 y. At year 8, the case distribution for HPV 16 was 12 in the placebo group vs. none in the vaccine group, whereas the 12 cases due to HPV 31 were evenly split between vaccine (6 cases) and placebo groups (6 cases). A similar unfavorable case split was seen for HPV 33 and also for HPV 45. In an analysis of 2 phase III studies of the bivalent vaccine cross-protection efficacy for persistent HPV 31/33/45 infection was not found in women vaccinated with a 2-dose regimen of the bivalent vaccine,7 adding evidence of the relatively weak nature of the immune response to cross-protective antigens in the vaccines.
Table 1.
Bivalent HPV vaccine efficacy against individual vaccine and non-vaccine HPV types: Comparison of 6-month persistent infection.
| Phase III (Study 008-PATRICIA).9,10 Follow-up: 4 y TVC-Na |
Phase II (Study 007).11 Follow-up: up to 6.4 y ATP-Eb |
Phase II (Study 023).12 Follow-up: up to 8 y ATP-Eb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV Type | Vaccine N = 5427 | Control N = 5399 | VE % (95% CI) | VaccinecN = 560 | ControlcN = 553 | VE % (95% CI) | Vaccine N = 223 | Control N = 213 | VE % (95% CI) |
| HPV16/18 | 35 | 521 | 94 (91.1, 95.6)* | 0 | 34 | 100 (90, 100)* | 0 | 17 | 100 (80, 100)* |
| HPV16 | 22 | 395 | 95 (91.8, 96.7)* | 0 | 27 | 100 (87, 100)* | 0 | 12 | 100 (69, 100)* |
| HPV18 | 13 | 166 | 92 (86.5, 96)* | 0 | 10 | 100 (60, 100)* | 0 | 8 | 100 (50, 100)* |
| HPV31 | 38 | 163 | 77 (67.2, 84.4)* | 5 | 9 | 48 (<0, 86) | 6 | 6 | 10 (<0, 76) |
| HPV33 | 53 | 92 | 43 (19.3, 60.2)* | 6 | 5 | −16 (<0, 71) | 6 | 4 | −37 (<0, 68) |
| HPV45 | 13 | 61 | 79 (61.3, 89.4)* | 2 | 4 | 52 (<0, 96) | 5 | 3 | −52 (<0, 70) |
*= statistical significance; ATP-E = according-to-protocol for efficacy; TVC-N = total vaccinated cohort-naive; VE = vaccine effectiveness
TVC-N: included subjects who were given at least 1 vaccine dose, were evaluable for efficacy and at baseline had normal cytology, were DNA (−) for all 14 oncogenic HPV types investigated, and were sero (−) for HPV 16 and 18; cases were counted after Day 1; in Protocol 008, the efficacy analyses for HPV 16 and/or 18 were performed in the ATP-E population.
ATP-E: included subjects who met all eligibility criteria, complied with study procedures, and had data available for the efficacy measure considered; TVC-N in Protocol 008 and ATP-E in Protocol 007/023 are equivalent since subjects in 007/023 were only enrolled if they were PCR (−) to the 14 HPV types tested, sero (−) to HPV 16 and 18, and had a normal Pap test at screening.
Number of subjects represent enrollment into the initial study, Protocol 001; of these, 393 and 383 vaccine and placebo recipients, respectively, continued in the follow-up study (Protocol 007); all subjects with follow-up were included in efficacy analyses.
An analysis was undertaken by the authors of this letter to estimate the impact of HPV vaccination on HPV infection as related to vaccine-specific types over time. The effects of the quadrivalent vaccine (types 6/11/16/18) and a nonavalent vaccine (types 6/11/16/18/31/33/45/52/58) on the incidences of cervical cancer, CIN1 and CIN2/3 were compared using a model adapted from that previously reported by Elbasha and Dasbach,8 based on clinical trial data from both vaccines during up to 6 years, similar to the model used by Van Effelterre et al.1 The incidences of cervical cancer, CIN1 and CIN2/3 were reduced for the respective HPV types (16 and 18) for the quadrivalent vaccine and (16/18/31/33/45/52/58) for the nonavalent vaccine over time. Compared with the quadrivalent vaccine, the nonavalent vaccine reduced cervical cancer cases by an additional 20% (Fig. 1A), CIN1 by an additional 38% (Fig. 1B), and CIN2/3 by an additional 33% (Fig. 1C) in >16,000 females, as estimated through 100 y.
Figure 1.
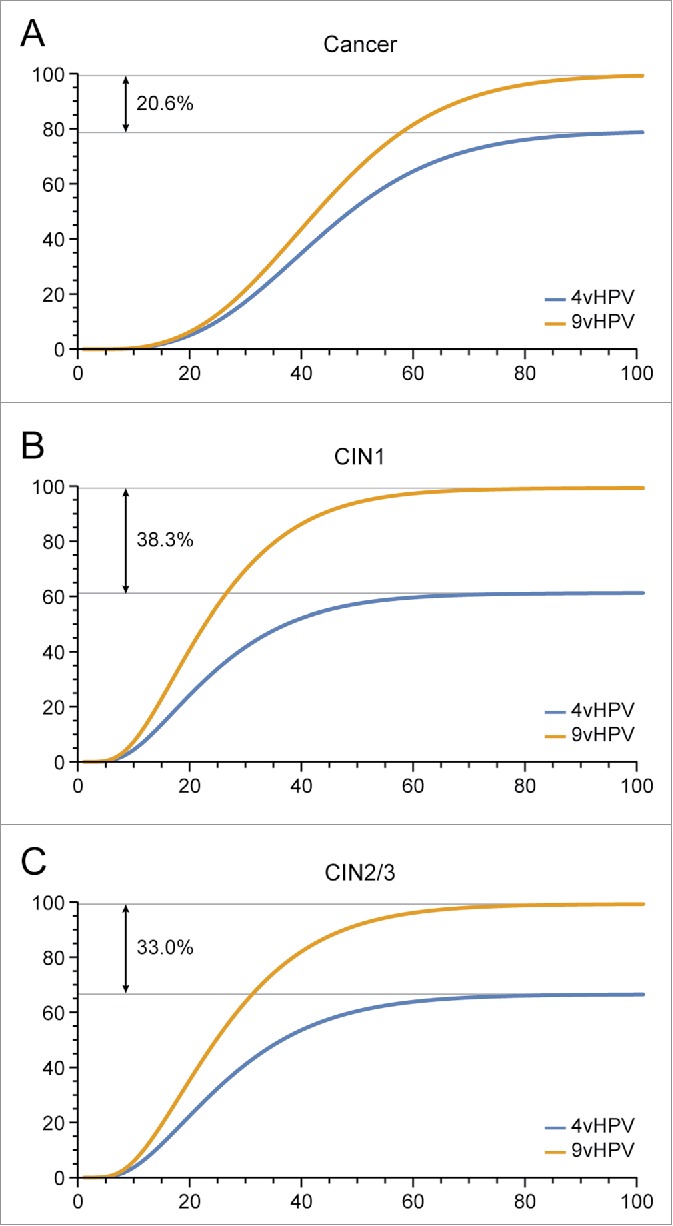
Estimated Human Papillomavirus (HPV) (types 16/18/31/33/45/52/58)-related incidences of cervical cancer (A), cervical intraepithelial neoplasia (CIN)1 (B), CIN2/3 (C) among females over 100 y.
In conclusion, while HPV virus-like particle (VLP)-vaccines have demonstrated consistently high and long-lasting type-specific protection, cross-protection efficacy against non-vaccine HPV types is lower and declines over time. It should also be noted that cross-protection antigens are not specifically manufactured, quality controlled or potency tested as are the vaccine HPV VLPs that undergo such testing before release of product. Overall, HPV vaccination is expected to provide consistent and long-term protection for the manufactured HPV types present in the vaccine. This includes a high level type-specific protection against the 9 types represented in the nonavalent HPV vaccine. Cross-protection effectiveness is essentially an epiphenomenon that is short-lived and should not be used in calculating favorable outcomes for 20 years, much less lifetime protection.
Disclosure of potential conflicts of interest
Darron Brown has served on an Advisory Board at Merck and Co., Inc. and has lectured on the quadrivalent HPV vaccine (honoraria received from Merck and Co., Inc. are donated to charities). His laboratory has received research funding from Merck and Co., Inc. Indiana University and Merck and Co., Inc. have an agreement that pays the University, based on certain landmarks related to vaccine development. Darron Brown receives a portion of these funds as income. Amit Sharad Kulkarni, Matt Pillsbury, Alain Luxembourg and Alfred Saah are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and may hold stock/stock options in the company.
Acknowledgment
Joanne E Tomassini, PhD., Merck and Co., Inc. for writing support.
Funding
This research was funded by Merck & Co., Inc., Kenilworth, NJ.
References
- [1].Van Effelterre TP, Hogea C, Taylor SM. Projected impact of Cervarix(R) vaccination on oncogenic human papillomavirus infection and cervical cancer in the United Kingdom. Hum Vaccin Immunother 2016; 12:8–19; PMID:25984886; http://dx.doi.org/10.1080/21645515.2015.1054584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al.. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301-14; PMID:19586656; http://dx.doi.org/ 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- [3].Kjaer SK, Nygard M, Dillner J, Munk C, Marshall B, Hansen B, Sigurdardottir L, Hortlund M, Tryggvadottir L, Saah A. Long-Term Effectiveness and Safety af Gardasil™ in the Nordic Countries. European Res Organization Genital Infect Neoplasia February 4–7. 2015. Oc 6–1 Available at http://www.eurogin.com/2015/images/pdf/eurogin-2015_abstracts_part_2.pdf (accessed 21December2015) [Google Scholar]
- [4].Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, Zahaf T, Catteau G, Geeraerts B, Descamps D. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother 2014; 10:2147-62; PMID:25424918; http://dx.doi.org/ 10.4161/hv.29532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781-89; PMID:22920953; http://dx.doi.org/ 10.1016/S1473-3099(12)70187-1 [DOI] [PubMed] [Google Scholar]
- [6].Saah A, Luxembourg A, Brown D. Addressing an unmet medical need using a 9-valent virus-like particle (VLP)-based HPV vaccine. European Res Organization Genital Infect Neoplasia 2015. February 4–7 MSS 7–5 (2015) Available at http://www.eurogin.com/2015/images/pdf/eurogin_2015_abstracts_part_1.pdf (accessed 21December2015) [Google Scholar]
- [7].Kreimer AR, Struyf F, Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David MP, Wheeler CM. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol 2015; 16:775-86; PMID:26071347; http://dx.doi.org/ 10.1016/S1470-2045(15)00047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010; 28:6858-67; PMID:20713101; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.030 [DOI] [PubMed] [Google Scholar]
- [9].Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, et al.. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100-10; PMID:22075170; http://dx.doi.org/ 10.1016/S1470-2045(11)70287-X [DOI] [PubMed] [Google Scholar]
- [10].Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al.. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89-99; PMID:22075171; http://dx.doi.org/ 10.1016/S1470-2045(11)70286-8 [DOI] [PubMed] [Google Scholar]
- [11].GlaxoSmithKline clinical study register A phase IIb, blinded, multi-center, long-term follow-up study of the efficacy of candidate HPV-16/18 VLP vaccine in the prevention of HPV-16 and/or HPV-18 cervical infection in adolescent and young adult women in North America and Brazil vaccinated in primary study 580299/001. HPV-16/18 VLP vaccine: GlaxoSmithKline Biologicals' virus-like particle (VLP) vaccine against human papillomaviruses (HPV)16 and 18. http://download.gsk-clinicalstudyregister.com/files/20401.pdf (accessed 21December2015) [Google Scholar]
- [12].GlaxoSmithKline clinical study register Follow-up study to evaluate the long-term efficacy of a HPV vaccine (580299) in healthy young adult women in Brazil. HPV vaccine (580299) (HPV): GlaxoSmithKline (GSK) Biologicals' vaccine against human papillomaviruses 16 and 18. https://gsk.sylogent.com/files/20207.pdf (accessed 21December2015) [Google Scholar]


