ABSTRACT
Concern over the release of variola virus as an agent of bioterrorism remains high and a rapid vaccination regimen is desirable for use in the event of a confirmed release of virus. A single, high-dose (5×108 TCID50) of Bavarian Nordic's IMVAMUNE was tested in a Phase-II clinical trial, in humans, as a substitute for the standard (1×108 TCID50), using a 2-dose, 28-days apart regimen. Prior to this clinical trial taking place a Good Laboratory Practice, repeated high-dose, toxicology study was performed using IMVAMUNE, in New Zealand white rabbits and the results are reported here. Male and female rabbits were dosed twice, subcutaneously, with 5×108 TCID50 of IMVAMUNE (test) or saline (control), 7-days apart. The clinical condition, body-weight, food consumption, haematology, blood chemistry, immunogenicity, organ-weight, and macroscopic and microscopic pathology were investigated. Haematological investigations indicated changes within the white blood cell profile that were attributed to treatment with IMVAMUNE; these comprised slight increases in neutrophil and monocyte numbers, on study days 1-3 and a marginal increase in lymphocyte numbers on day 10. Macroscopic pathology revealed reddening at the sites of administration and thickened skin in IMVAMUNE, treated animals. After the second dose of IMVAMUNE 9/10 rabbits seroconverted, as detected by antibody ELISA on day 10, by day 21, 10/10 rabbits seroconverted. Treatment-related changes were not detected in other parameters. In conclusion, the subcutaneous injection of 2 high-doses of IMVAMUNE, to rabbits, was well tolerated producing only minor changes at the site of administration. Vaccinia-specific antibodies were raised in IMVAMUNE-vaccinated rabbits only.
KEYWORDS: IMVAMUNE, MVA, rabbits, smallpox, toxicology, vaccine
Introduction
Routine smallpox vaccination declined throughout the world after the causative agent, variola virus, was declared eradicated by the WHO in 1980.1 Only 20% of the global population has some immunity to smallpox, due to previous vaccination,2,3 hence a deliberate release of this virus would have catastrophic consequences. Stockpiles of first and second generation vaccines based on replicating-vaccinia virus (eg. Dryvax and ACAM2000) are maintained in some countries, for instance, the United States, to help counter the threat of re-emerging smallpox following a bioterrorist attack.4,5 First and second generation (conventional) smallpox vaccines have been shown to be highly efficacious, however, they have also been associated with rare but severe adverse events, especially in populations with compromised immune systems.6,7 These adverse events include progressive vaccinia eczema vaccinatum, myo/pericarditis, Stevens-Johnson syndrome, fetal vaccinia encephalititis and occasionally death.5 In 2002, Kemper et al estimated that 25% of the US population would be excluded from vaccination with conventional smallpox vaccines because they are, or have close contact with, individuals who have eczema or are immunocompromised.8 Safer alternative smallpox vaccines are thus desirable and interest in attenuated viruses, such as modified-vaccinia Ankara virus (MVA), has led to the development of new third generation smallpox vaccines.
MVA was generated by passaging vaccinia virus more than 500 times through chicken embryo fibroblasts. During this time the virus acquired multiple deletions and mutations and lost its capacity to replicate efficiently in people and most mammalian cell lines.9 MVA was used during the smallpox eradication campaign during the 1970s, in Germany, as a priming vaccine prior to the administration of conventional smallpox vaccine, to mitigate potential reactogenicity.10,11 More than 120,000 people took part in this program. Several high-risk groups were vaccinated, including young children with skin conditions.10-12 There were no reports of serious adverse events using this 2-step inoculation process.12
IMVAMUNE, a vaccine based on a strain of the modified-vaccinia Ankara (MVA) virus, is currently being developed as a safe and effective vaccine, by Bavarian-Nordic (BN), Denmark.12 Early studies determined whether IMVAMUNE was safe, prior to the initiation of human trials. These studies included repeat administrations (subcutaneous and intramuscular) in animal models and the results showed reversible non-dosing-limiting injection site reactions and lymphoid changes.12 Tetraology studies in rats and rabbits did not demonstrate teratogenic or intrauterine toxicity, and peri- and postnatal studies did not reveal toxicity to embryos or developing offspring at doses up to 1 × 108 TCID50.4 IMVAMUNE was tested in humans for safety and immunogenicity using subcutaneous and intramuscular, administration of a range of doses (106−108 TCID50) and different vaccination regimes; 1 or 2 doses at various intervals sometimes followed by a conventional smallpox vaccine.4,12-15 The protective efficacy of IMVAMUNE was also demonstrated in animal models16-20 with promising results against rabbitpox and monkeypox viruses (surrogates for variola virus).
Overall, the safety and efficacy data generated for IMVAMUNE, so far, have been very promising. Currently, the optimal vaccination schedule is a prime-boost regimen with 2 doses (1 × 108 TCID50) administered with a 28-day interval.13 If, however, this vaccine were to be used to protect the population following a bioterrorist attack or response scenario, the use of a single dose would be highly advantageous as this could potentially help to limit casualties quickly.13 An investigation into the suitability of a single high-dose of IMVAMUNE (5 × 108 TCID50) was thus initiated. Since early safety studies utilised different doses up to and including 1 × 108 TCID50 it was necessary to perform a repeat-dose toxicology experiment using the higher proposed dose of 5 × 108 TCID50 to ensure this vaccine dose was safe prior to it being given to humans. Vaccination doses were given to rabbits on day 0 and day 7, this allowed sufficient time for recovery from local reactogenicity to the first vaccination before the second vaccination was given. This work was conducted according to Good Laboratory Practice (GLP). After this toxicology study was performed this high-dose of virus was used in the randomized Phase II clinical trial (http://clinicaltrials.gov/show/NCT00879762) reported by Frey et al.21
Results
Clinical signs: Neither abnormal systemic nor local clinical signs were observed, and no deaths were recorded prior to the pre-determined necropsy times. There were changes in the skin at the vaccination sites, observed at necropsy on days 10 and 21 (Table 2). Changes were not observed at day 35. On day 10, when compared with controls, an increased number of dark red areas were noted after injection of IMVAMUNE at site 1 and site 2. Thickened areas were seen in one male and one female animal 3 d after the second treatment with IMVAMUNE. On day 21, when compared with controls, an increased incidence of dark red areas at the vaccination sites with IMVAMUNE (site 1) were seen; a similar response was seen (14 d after the second vaccination) at site 2. Thickened areas were seen in one male and one female animal after treatment with IMVAMUNE at site 2. At the end of the study (day 35) macroscopic, treatment related findings were not apparent.
Table 2.
Summary of treatment-related changes in the average number of Leucocytes.
| Treatment group and sex |
|||||
|---|---|---|---|---|---|
| Male |
Female |
||||
| Cell Types (mean) | Saline Control | IMVAMUNE | Saline Control | IMVAMUNE | |
| Total white | Pretreatment | 8.71 | 7.94 | 7.77 | 8.37 |
| blood cells (x109/L) | Day 1 | 8.95 | 8.49 | 9.05 | 9.03 |
| Day 2 | 8.22 | 8.56 | 8.18 | 8.92 | |
| Day 3 | 8.56 | 8.60 | 8.08 | 8.58 | |
| Day 10 | 7.96 | 8.89 | 7.71 | 9.25 | |
| Day 21 | 8.69 | 8.14 | 7.11 | 8.02 | |
| Day 35 | 6.95 | 6.49 | 6.30 | 8.45* | |
| Mean Neutrophils | Pretreatment | 1.62 | 1.81 | 1.72 | 1.62 |
| (x109/L) | Day 1 | 1.83 | 2.37* | 1.86 | 1.74 |
| Day 2 | 1.54 | 2.03 | 1.46 | 1.56 | |
| Day 3 | 1.78 | 1.87 | 1.64 | 1.61 | |
| Day 10 | 1.44 | 1.87* | 1.51 | 1.91 | |
| Day 21 | 1.62 | 1.74 | 1.32 | 1.33 | |
| Day 35 | 0.79 | 0.85 | 1.13 | 1.22 | |
| Monocytes | Pretreatment | 0.12 | 0.17* | 0.10 | 0.08 |
| (x109/L) | Day 1 | 0.08 | 0.09 | 0.08 | 0.13** |
| Day 2 | 0.07 | 0.16** | 0.11 | 0.26* | |
| Day 3 | 0.08 | 0.12 | 0.13 | 0.17 | |
| Day 10 | 0.17 | 0.17 | 0.06 | 0.08* | |
| Day 21 | 0.10 | 0.10 | 0.11 | 0.09 | |
| Day 35 | 0.10 | 0.17 | 0.04 | 0.09 | |
| Lymphocytes | Pretreatment | 6.30 | 5.31** | 5.42 | 5.93 |
| (x109/L) | Day 1 | 6.34 | 5.42* | 6.61 | 6.46 |
| Day 2 | 5.98 | 5.78 | 6.07 | 6.41 | |
| Day 3 | 6.04 | 6.02 | 5.76 | 6.10 | |
| Day 10 | 5.75 | 6.27 | 5.56 | 6.83 | |
| Day 21 | 6.31 | 5.75 | 5.22 | 6.08 | |
| Day 35 | 5.62 | 5.12 | 4.67 | 6.55 | |
Statistically significant when compared with the Control (Group 1): *-p<0.05; **-p<0.01.
Body weight and food consumption: Body weights and food consumption were unaffected by treatment (data not shown).
Clinical chemistry and haematology: The biochemistry of the plasma was unaffected by treatment. Minimal changes in the white blood cell profile were noted following treatment with IMVAMUNE (Table 3). A slight increase in neutrophil numbers was apparent in males on days 1 (P<0.05) and 10 (P<0.05) and a slight increase in monocyte numbers was evident in females on days 1 and 2 (P<0.05) and in males on day 2 (P<0.01). A marginal increase in lymphocyte numbers was evident 3 d after the last dose (day 10) in females but this was not statistically significant.
Table 3.
Summary of treatment-related changes in adrenal and prostate weights (g). Absolute values and difference from control (xn).
| Treatment group and sex |
|||||
|---|---|---|---|---|---|
| Male |
Female |
||||
| OrganDay euthanised | No. of animals | Saline Control | IMVAMUNE | Saline Control | IMVAMUNE |
| Adrenals | |||||
| Day 10 | n = 5 | 0.211 | 0.170 (x0.81) | 0.249 | 0.256 (x1.03) |
| Day 21 | n = 5 | 0.234 | 0.233 (x1.00) | 0.237 | 0.282 (x1.19) |
| Day 35 | n = 3 | 0.167 | 0.285* (x1.71) | 0.287 | 0.370 (x1.29) |
| Prostate | |||||
| Day 10 | n = 5 | 0.797 | 0.603 (x0.76) | – | – |
| Day 21 | n = 5 | 0.683 | 0.503 (x0.74) | – | – |
| Day 35 | n = 3 | 0.597 | 1.115 (x1.87) | – | – |
Statistically significant when compared with the Saline Control (Group 1): *-p<0.05
Immunogenicity (BN Nordic ELISA): Animals treated with saline (control) were negative for vaccinia-specific IgG at all time-points. Animals treated with IMVAMUNE were negative for vaccinia-specific IgG before the first administration (day 0) (Fig. 1) and on day 7 prior to the second administration of IMVAMUNE. Vaccinia-specific IgG could be detected in the majority of IMVAMUNE immunized rabbits 10 d after the first and 3 d after the second administration in both males and females. Out of 5 males, 3 had high titres and 2 were close to the limit of detection. Out of 5 females, one did not have antibodies and 4 were at the limit of detection. Titres increased until day 21, the first day when all animals had seroconverted, and were maintained on day 35. Males tended to have slightly higher titres than females although this was not significantly different (P>0.05) on days 21 and 35.
Figure 1.
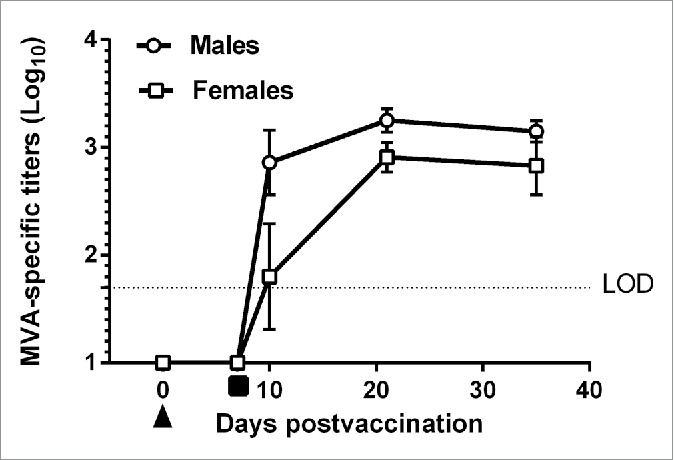
Mean vaccinia-specific IgG titer (Log10 ± 1 SE) of New Zealand white rabbits vaccinated with MVA-BN (IMVAMUNE) vaccine (Group 2). Animals were bled on various days (Prebleed Day 0, Day 7 after the first immunization, 3 d (Day 10), 14 d (Day 21) and 28 d (Day 35) after the second immunisation. Mean values are from 13 (Day 0 and Day 7), 5 (Day 10 and Day 21) or 3 (Day 35) animals each. LOD = Limit of Detection (1.7 log10). First immunization (▴), Second immunisation (▪). For graphical purposes values below the limit of detection (LOD) were assigned a value of 1Log10.
Organ weights: Mean adrenal weights that were higher than control values were apparent among IMVAMUNE treated males on day 35 (P<0.05) (Table 4). No difference in mean adrenal weights in IMVAMUNE treated females were observed (P>0.05) compared with controls. In males, on day 10 and 21, mean prostate weight was lower than controls, however, on day 35, mean prostate weight was higher than controls (none of these observations were significant (P>0.05)). Pathological change was not observed in the adrenal glands and prostate, therefore, the relationship between treatments and organ weight was uncertain and they were considered not to be adverse.
Table 4.
Summary of treatment-related changes in the axillary lymph nodes on days 10, 21 and 35. Number of animals with treatment related changes/Total number of animals.
| Prominent germinal centers | Treatment group and sex |
|||
|---|---|---|---|---|
| Male |
Female |
|||
| |
Saline Control |
IMVAMUNE |
Saline Control |
IMVAMUNE |
| Day 10 | ||||
| Minimal | 0/5 | 0/5 | 0/4 | 3/5 |
| Slight | 0/5 | 2/5 | 0/5 | 1/5 |
| Day 21 | ||||
| Minimal | 0/5 | 4/5 | 0/5 | 4/5 |
| Day 35 | ||||
| Minimal | 0/3 | 3/3 | 0/3 | 3/3 |
Microscopic findings: Changes were observed at the vaccination sites and are summarised in Supplementary Data Tables 1-3. On day 10, the dermis and subcutaneous tissues were infiltrated by mixed inflammatory cells. Dermal and subcutaneous hemorrhage, subcutaneous fibrosis and myofibre necrosis of the panniculus muscle, were noted at both sites. On day 21, at sites 1 and 2, dermal and/or subcutaneous inflammatory cell infiltrates, subcutaneous fibrosis and subcutaneous hemorrhage were evident. On day 35, dermal and/or subcutaneous mixed inflammatory cell infiltrates and/or subcutaneous hemorrhage were apparent.
Table 1.
Vaccination sites examined on rabbits vaccinated with IMVAMUNE or Saline Control. No macroscopic change related to treatment was apparent at necropsy on day 35 (n = 3).
| Treatment Group and Sex |
||||
|---|---|---|---|---|
| Male |
Female |
|||
| Parameters | Saline Control n=5 | IMVAMUNEn = 5 | Saline Control n=5 | IMVAMUNE n=5 |
| Animals euthanised on Day 10 | ||||
| Treated site 1 | ||||
| Dark area(s) | 3 | 5 | 1 | 2 |
| Treated Site 2 | ||||
| Dark area(s) | 2 | 5 | 2 | 4 |
| Thickened / Oedematous | 0 | 1 | 0 | 1 |
| Animals euthanised on Day 21 | ||||
| Treated Site 1 | ||||
| Dark area(s) | 0 | 2 | 1 | 3 |
| Treated Site 2 | ||||
| Dark area(s) | 1 | 2 | 1 | 3 |
| Thickened / Oedematous | 0 | 1 | 0 | 1 |
In the axillary lymph nodes, on days 10, 21 and 35, germinal centers appeared to be increased (minimal or slight) (Table 5) in IMVAMUNE treated animals. Changes were not observed in the controls.
Table 5.
Study Plan: Animals were vaccinated subcutaneously twice (7-days apart) with either MVA-BN (IMVAMUNE) (Group 2), or with saline control (Group 1). Animals were monitored for 27 d (upto day 35) after the last vaccination. Animals were euthanised at days 10, 21 and 35.
| Number of animals |
||||||||
|---|---|---|---|---|---|---|---|---|
| Euthanised Day 10 |
Euthanised Day 21 |
Euthanised Day 35 |
||||||
| Group No. | Treatment | Dose volume(ml) | Male | Female | Male | Female | Male | Female |
| 1 | Saline Control | 2×1ml | 5 | 5 | 5 | 5 | 3 | 3 |
| 2 | IMVAMUNE | 2×1mla | 5 | 5 | 5 | 5 | 3 | 3 |
One dose of IMVAMUNE consisted of 2 × 0.5ml equivalent to 4.9×108 TCID50.
Discussion
The optimal dosing regimen for IMVAMUNE, a third generation smallpox vaccine, is 2 doses of 1 × 108 TCID50 of virus, 28-days apart. However, should a deliberate release of Variola major virus occur, a post-exposure vaccination program with a single dosing schedule would be desirable to limit casualties. To address this issue, a single high-dose (5 × 108 TCID50) of MVA was tested in vaccinia-naïve individuals in a phase II randomized clinical trial21 [21] and, prior to this being carried out, this GLP, repeated-dose, toxicology study was perforrmed, to assess the safety profile of this new dose, before it was given to humans. This is the first time a toxicology study has been reported using a high-dose (5 × 108 TCID50) of IMVAMUNE in rabbits, this contributes information toward the safety assessment for the clinical use of this vaccine in humans.
Differences were not observed between rabbits treated with IMVAMUNE or saline in respect of clinical observations, blood clinical chemistry, food consumption or body weight. However, a local skin reaction to treatment with IMVAMUNE was evident post mortem comprising dark red areas and thickened vaccination sites. Microscopically, inflammatory cell infiltrates, both mononuclear and polymorphonuclear, and hemorrhage were evident in the dermis and subcutis of the administration sites. Subcutaneous fibrosis and inflammation with myofibre necrosis of the panniculus muscle were also apparent at the inoculation sites. A higher incidence and longer persistence of these changes were apparent in animals receiving IMVAMUNE compared with the controls, indicating that IMVAMUNE exerted a local response. Overall, the incidence was low and the severity minimal or slight. This low severity was confirmed by the absence of an overt behavioral change. The incidence was highest at the dose sites 3 d after the second administration (day 10) and diminished subsequently. However, recovery was not complete at the sites 21 and 35 d after treatment. Local skin reactions in humans following the administration of IMVAMUNE have also been reported and this was, therefore, not an unexpected finding.13-15,21 Collectively the data from this study and others suggest that skin reactions at the site of injection should be monitored during clinical trials with IMVAMUNE.
Marginal increases in peripheral neutrophil and monocyte numbers after the first dose were seen and may be related to the role of these cells in the immunological response to IMVAMUNE virus particles following immunisation on day 0. The marginal increase in lymphocyte numbers in females after the second dose may represent an immune response to the second insult of the vaccine. These effects were not considered adverse.
The prominent germinal centers of the axillary lymph nodes, seen following vaccination with IMVAMUNE, were considered to be part of an immune response to the virus. This change decreased in severity on days 21 or 35, when compared to those at day 10, indicating germinal center involution had occurred following the initial immune response. These observations are consistent with reports of lymphoid changes and reversible non-dose-limiting injection site reactions reported in early safety tests on IMVAMUNE in animal models, as reviewed by Kennedy and Greenberg (2009).12
In this study, both male and female test rabbits raised vaccinia-specific antibodies when vaccinated with IMVAMUNE. Initially, titres were higher in males than females although by days 21 and 35 there was no significant difference between groups. Interestingly, Troy et al (2015) report on gender differences in the immune response to vaccination with IMVAMUNE in humans, males tended to have higher levels of antibody than females.22 In this work, none of the rabbits that received saline mounted a detectable vaccinia-specific antibody response, on any of the days tested. Other immune parameters for example, plaque reduction neutralisation assay [PRNT], cell mediated responses and vaccine efficacy (challenge against rabbitpox) could have been analyzed in this study, however, they were excluded because detailed studies addressing these issues had already been performed elsewhere.16-19
Repeated-dose and embryofetal toxicity studies in animals for the standard dose (1 × 108 TCID50) of IMVAMUNE have already been performed and severe adverse events were not reported.12 Similarly, in the present study in rabbits, a repeated-dose of IMVAMUNE at a high concentration (5 × 108 TCID50), was tolerated, producing only minor changes at the site of administration. This good safety profile, in rabbits supported the use of a high-dose of this vaccine in humans and, as a result, a phase-II clinical trial was conducted.21
Materials and methods
Animals: Male (n = 26) and female (n = 26) New Zealand White rabbits were acclimatised for at least 12 d before treatment. At the start of treatment, animals were 12-17 weeks old and males weighed between 2.9-3.3 kg (inclusive) and females between 2.9-3.4 kg (inclusive). Rabbits were housed individually in stainless steel cages and environmental controls were set to maintain the following conditions: temperature range 16-20ºC, relative humidity 40-70%, 12-hr light/12-hr dark cycle. The animals were offered 150 g of a standard laboratory diet each day throughout the study. This diet contained no added antibiotic or other chemotherapeutic or prophylactic agent; water (from the public water supply) was given via water bottle, ad libitum.
Experimental studies were conducted at Envigo CRS Limited (Cambridge, UK). The general procedures were in compliance with the “Code of practice for the housing and care of animals used in scientific procedures,” published by the UK Home Office which forms part of the Animal (Scientific Procedures) Act (1986). This study was also performed in compliance with GLP.
Vaccine formulation and study design: The duration of this study was 35 d. The test vaccine, IMVAMUNE, supplied by Bavarian Nordic (Batch No 0061205), had a viral concentration of 4.9 × 108 TCID50/ml. Animals were weighed and assigned randomly to groups; group 1, (control) (n=26), group 2 (test) (n=26) (Table 1). The dorsum was shorn using electric clippers; 2 injection sites (site 1 and site 2) were identified, on the back of the neck. The animals received 2 doses (2 × 0.5 ml each) of the vaccine (test) or physiological saline (control) by subcutaneous injection on day 0 at site 1 (dose 1) and on day 7 at site 2 (dose 2). Thus, the animals received 4.9 × 108 TCID50 of virus on each day of administration.
Necropsy procedures were undertaken on days 10, 21 and 35 (Table 1).
Observations and clinical signs: During the study periods, each animal was examined twice daily for evidence of ill-health or reaction to treatment. On each day of administration, 5 observations were recorded; 1) immediately before, 2) immediately after dosing, 3) on completion of dosing each group, 4) between 1-2 hours after completion of dosing, and 5) as late as possible in the working day. In addition, a more detailed weekly physical examination was performed on each animal to monitor general health. Injection sites were assessed daily for 3 d after each injection and weekly throughout the study. Injection sites were scored according to the numerical scoring system of Draize.23
Body-weight: The weight of each rabbit was recorded one week before treatment commenced (day -7), on the day treatment commenced (day 0), weekly throughout the study and before necropsy. Group mean weight changes were calculated from the weight changes of individual animals.
Food and water consumption: The weight of food supplied to each animal, food remaining and an estimate of any spilled, was recorded for the week before treatment started (week -1), and each week throughout the study. From these data the estimated weekly consumption per animal (g/rabbit/week) was calculated.
Haematological studies: Before the commencement of treatment (day 0) and on days 1, 2, 3, 10, 21 and 35 of the study, blood samples (0.5 ml) were collected into EDTA anticoagulant (TekLab, County Durham, UK, Catalogue No: K1230) from the central auricular artery. Blood samples were examined using a Bayer Advia 120 haematology analyzer (Siemens, Surrey, UK). The following characteristics were determined; haematocrit (L/L), hemoglobin concentration (g/dL), erythrocyte count (RBC), reticulocyte count (%), mean cell hemoglobin (pg), mean cell hemoglobin concentration (g/dL), mean cell volume (fL), total leucocyte count (cells/L), differential leucocyte count (including neutrophils, lymphocytes, eosinophils, basophils, monocytes, large unstained cells and platelet count) (cells/L).
Additional blood samples were collected into citrate anticoagulant (TekLab, Catalogue No: C1130) and examined for Prothrombin time (sec) using an ACL 3000 Plus analyzer (Instrument Laboratory, Cheshire, UK) with IL PT-Fibrinogen reagent (Instrument Laboratory; Catalogue No: 0008469810). Also, activated partial thromboplastin time (sec) was measured using an ACL 3000 Plus Analyser (Instrument Laboratory) and IL APTT reagent (Instrument Laboratory; Catalogue No: 0020006800).
Clinical biochemistry: When blood was obtained for haematology, additional samples (0.7 ml) were collected into lithium heparin anticoagulant (TekLab, Catalogue No: H2130). Plasma was separated and concentrations of alkaline phosphatase (U/L), alanine amino-transferase (U/L), aspartate amino-transferase (U/L), total bilirubin (µmol/L), urea (mmol/L), creatinine (µmol/L), glucose (mmol/L), total cholesterol (mmol/L), triglycerides (mmol/L), sodium (mmol/L), potassium (mmol/L), chloride (mmol/L), calcium (mmol/L), inorganic phosphorus (mmol/L) and total protein (g/L) were determined, using a Hitachi 917 Clinical Chemistry Analyser (Roche, Sussex, UK).
Electrophoretic protein fractions; albumin (g/L), α1 globulin (g/L), α2 globulin (g/L), β-globulin (g/L) and γ-globulin (g/L) were analyzed with agarose gel and scanning with a densitometer. Albumin/globulin ratio (A/G ratio) was calculated from total protein concentration and analyzed albumin concentration.
Immunogenicity: Before dosing on days 0 and 7 and on days of euthanasia (days 10, 21, and 35), blood samples (1.5 ml) were taken from the central auricular artery. Serum was isolated and frozen (−20ºC). Samples were assayed for immunoglobulin G (IgG) serum antibodies to vaccinia virus using an enzyme-linked immunosorbent assay (ELISA) by Bavarian Nordic (the ELISA was not performed under GLP conditions).
Necropsy: Animals were killed humanely on days 10, 21 and 35 by an intravenous overdose (2.5 ml/animal) of pentobarbitone (Pharmasol, Hampshire, UK; Catalogue No: 80640). All external features and orifices were examined visually, including the parenteral site. The cranial roof was removed to allow observation of the brain, pituitary gland and cranial nerves. After ventral mid-line incision, the neck and associated tissues and the thoracic, abdominal and pelvic cavities and their viscera were exposed and examined in-situ.
Organ weights: Adrenals, brain, heart, kidneys, liver, lungs, pituitary gland, salivary glands, spleen, thyroid with parathyroids and thymus were weighed in all animals. Additionally from males, epididymides, prostate, seminal vesicles and testes were weighed and from females, uterus with cervis and ovaries; bilateral organs were weighed together.
Histopathological examination: Testes and epididymides were fixed in Bouin's solution prior to transfer to 70% methylated spirit and eyes were fixed in Davidson's fluid prior to transfer to 70% methylated spirit. Other tissues comprising adrenals, aorta-thoracic, brain, caecum, colon, duodenum, femur, gall bladder, Harderian glands, heart, ileum, jejunum, kidneys, lachrymal glands, larynx, liver, lungs, lymph nodes (mandibular, axillary, inguinal, mesenteric, draining and distal nodes), mammary area (caudal), esophagus, optic nerves, ovaries, pancreas, pituitary, prostate, rectum, salivary glands (submandibular, parotid, sublingual), sciatic nerves, seminal vesicles, skeletal muscle, skin (treated site), skin (untreated site), spinal cord, spleen, sternum, stomach, thymus, thyroid with parathyroids, tongue, trachea, ureters, urinary bladder, uterus and cervix and vagina were fixed in 10% neutral buffered formalin. All tissues were processed to paraffin wax, sections were cut at 5 µm, and then stained with haematoxylin and eosin.
Statistical analysis: Startox version 3.2 was used for the statistical analysis of the haematological and blood chemistry data. Quasar version 1.1 was used to analyze the body weight, organ weight and pathological data. All analyses were carried out using the individual animal as the basic experimental unit. The following data types were analyzed separately at each timepoint: bodyweight, using gains over appropriate study periods; blood chemistry and haematology; organ weights, both absolute and adjusted for terminal bodyweight.
The following sequence of statistical tests was used if 75% of the data (across all groups) were the same value, for example c, then a frequency analysis was applied. Groups were compared using pairwise Fisher's Exact tests (FE) both for i) values < c versus values > c, and for ii) values ≤ c vs. values > c, as applicable.
If Bartlett's test for variance homogeneity was not significant at the 1% level, then parametric analysis was applied. Groups were compared using t-tests (Tt). If Bartlett's test was significant at the 1% level, then logarithmic and square-root transformations were tried. If Bartlett's test was still significant, then non-parametric tests were applied. Groups were compared using the Wilcoxon rank sum tests (Wc).
For organ weight data, analysis of covariance was initially performed using terminal bodyweight as covariate. If the within group relationship between organ weight and bodyweight was significant at the 10% level,24 then the treatment comparisons were made on adjusted group means (calculated using analysis of covariance, where the factor is group and the covariate is the terminal bodyweight) in order to allow for differences in bodyweight which might influence the organ weights.
Supplementary Material
Abbreviations
- GLP
Good Laboratory practice
- ELISA
Enzyme-linked-immunoassay
- EDTA
Ethylenediaminetetraacetic acid
- Kg
kilogram
- hr
hour
- g
gram
- UK
United Kingdom
- Pg
picograms
- %
percentage
- sec
second
- cells/L
cells per liter
- No
number
- n
number
- ml
milliliter
- Wc
Wilcoxon
- Tt
t-tests
- FE
fisher's exact
- PHE
Public Health England
- L
liter
- A
α
- mmol
millimolar
- A/G
albumin/globulin
- BN
Bavarian Nordic
- IgG
Immunoglobulin
- ml
milliliter
- PT
Prothrombin Time
- RBC
Red Blood Cells
- NIAID
National Institute of Allergy and Infectious Diseases
- ACL
Automated Coagulation Analyzer
- Sec
second
- U/L
units per liter
- APPT
Activated Partial Thromboplastin Time
- β
beta
- γ
gamma
- MVA
modified vaccinia Ankara
- TCID50
50% Tissue Culture Infective Dose
- ºC
degrees Celsius
- d
day
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr Ariane Volkmann for performing the antibody ELISAs. We thank Professor Geoff Pearson (PHE) and Dr Robert Johnson and Dr Lynda Lanning (NIAID) for reviewing this manuscript.
Funding
This work was funded by the National Institute of Allergy and Infectious Diseases (Contract NO1-AI-30062, Task Order 10). The views in the paper are of the authors and not necessarily those of the funding body.
References
- [1].Artenstein AW, Grabenstein JD. Smallpox vaccines for biodefense: need and feasibility. Expert Rev Vaccines 2008; 7:1225-37; PMID:18844596; http://dx.doi.org/ 10.1586/14760584.7.8.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Russell PK. Vaccines in civilian defense against bioterrorism. Emerg Infect Dis 1999; 5:531-3; PMID:10458959; http://dx.doi.org/ 10.3201/eid0504.990413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, et al.. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama 1999; 281:2127-37; PMID:10367824; http://dx.doi.org/ 10.1001/jama.281.22.2127 [DOI] [PubMed] [Google Scholar]
- [4].Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, Hill H, Yan LK, Chaplin P, Vollmar J, Chaitman BR, et al.. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine 2007; 25:8562-73; PMID:18036708; http://dx.doi.org/ 10.1016/j.vaccine.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Metzger W, Mordmueller BG. Vaccines for preventing smallpox. Cochrane Database Syst Rev 2007 Jul 18; (3):CD004913; PMID:17636779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis 1970; 122:303-9; PMID:4396189; http://dx.doi.org/ 10.1093/infdis/122.4.303 [DOI] [PubMed] [Google Scholar]
- [7].Thomas TN, Reef S, Neff L, Sniadack MM, Mootrey GT. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis 2008; 46 Suppl 3:S212-20; PMID:18284361; http://dx.doi.org/ 10.1086/524742 [DOI] [PubMed] [Google Scholar]
- [8].Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract 2002; 5:84-90; PMID:11990216 [PubMed] [Google Scholar]
- [9].Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, et al.. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004; 428:182-5; PMID:15014500; http://dx.doi.org/ 10.1038/nature02331 [DOI] [PubMed] [Google Scholar]
- [10].Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. [MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl)]. Dtsch Med Wochenschr 1974; 99:2386-92; PMID:4426258; http://dx.doi.org/ 10.1055/s-0028-1108143 [DOI] [PubMed] [Google Scholar]
- [11].Mayr A, Stickl H, Muller HK, Danner K, Singer H. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl)]. Zentralbl Bakteriol B 1978; 167:375-90; PMID:219640 [PubMed] [Google Scholar]
- [12].Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Exp Rev Vaccines 2009; 8:13-24; PMID:19093767; http://dx.doi.org/ 10.1586/14760584.8.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frey SE, Winokur PL, Salata RA, El-Kamary SS, Turley CB, Walter EB Jr., Hay CM, Newman FK, Hill HR, Zhang Y, et al.. Safety and immunogenicity of IMVAMUNE(R) smallpox vaccine using different strategies for a post event scenario. Vaccine 2013; 31:3025-33; PMID:23664987; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, Schlereth B, Handley A, King L, Hulsemann V, et al.. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 2006; 24:2065-70; PMID:16337719; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.022 [DOI] [PubMed] [Google Scholar]
- [15].von Krempelhuber A, Vollmar J, Pokorny R, Rapp P, Wulff N, Petzold B, Handley A, Mateo L, Siersbol H, Kollaritsch H, et al.. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 2010; 28:1209-16; PMID:19944151; http://dx.doi.org/ 10.1016/j.vaccine.2009.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, Funnell SG, Bate SR, Steeds K, Tipton T, et al.. Assessment of the Protective Effect of Imvamune and Acam2000 Vaccines against Aerosolized Monkeypox Virus in Cynomolgus Macaques. J Virol 2013; 87:7805-15; PMID:23658452; http://dx.doi.org/ 10.1128/JVI.03481-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keckler MS, Carroll DS, Gallardo-Romero NF, Lash RR, Salzer JS, Weiss SL, Patel N, Clemmons CJ, Smith SK, Hutson CL, et al.. Establishment of the black-tailed prairie dog (Cynomys ludovicianus) as a novel animal model for comparing smallpox vaccines administered preexposure in both high- and low-dose monkeypox virus challenges. J Virol 2011; 85:7683-98; PMID:21632764; http://dx.doi.org/ 10.1128/JVI.02174-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Garza NL, Hatkin JM, Livingston V, Nichols DK, Chaplin PJ, Volkmann A, Fisher D, Nalca A. Evaluation of the efficacy of modified vaccinia Ankara (MVA)/IMVAMUNE against aerosolized rabbitpox virus in a rabbit model. Vaccine 2009; 27:5496-504; PMID:19632316; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stabenow J, Buller RM, Schriewer J, West C, Sagartz JE, Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against Monkeypox virus. J Virol 2010; 84:3909-20; PMID:20130052; http://dx.doi.org/ 10.1128/JVI.02012-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Townsend MB, Keckler MS, Patel N, Davies DH, Felgner P, Damon IK, Karem KL. Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations. J Virol 2013; 87:900-11; PMID:23135728; http://dx.doi.org/ 10.1128/JVI.02089-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Frey SE, Winokur PL, Hill H, Goll JB, Chaplin P, Belshe RB. Phase II randomized, double-blinded comparison of a single high dose (5×10(8) TCID50) of modified vaccinia Ankara compared to a standard dose (1×10(8) TCID50) in healthy vaccinia-naive individuals. Vaccine 2014; 32:2732-9; PMID:24607004; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Troy JD, Hill HR, Ewell MG, Frey SE. Sex difference in immune response to vaccination: A participant-level meta-analysis of randomized trials of IMVAMUNE((R)) smallpox vaccine. Vaccine 2015; 33:5425-31; PMID:26319063; http://dx.doi.org/ 10.1016/j.vaccine.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Therapies 1944; 82:377-90 [Google Scholar]
- [24].Angervall L, Carlstrom E. Theoretical criteria for the use of relative organ weights and similar ratios in biology. J Theoret Biol 1963; 4:254-259; PMID:5875198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


