ABSTRACT
The antibody responses of a reduced-dose intradermal seasonal influenza vaccination have never been studied in COPD patients soon after a pandemic. A total of 149 COPD patients (60 y of age or older) were randomized to receive trivalent influenza vaccine (Sanofi-Pasteur, France) either 9 µg of hemagglutinin (HA) per strain split into 2-site intradermal (ID) injections via the Mantoux technique or one intramuscular (IM) injection of 15 µg of HA per strain. The geometric mean titers, seroconversion factors, seroconversion rates and seroprotection rates for influenza A(H3N2) and B administered through the ID injection (n = 75) were similar to those obtained with the IM injection (n = 74) 4 weeks post-vaccination. The antibody responses for influenza A(H1N1)pdm09 administered through the ID injection were lower than those obtained with the IM injection, but all of these responses met the 3 criteria proposed by the Committee for Proprietary Medicinal Products (CPMP) for annual re-licensure. The seroprotection rates 4 weeks post-vaccination for influenza A(H1N1)pdm09 were 64.0% (95%CI 52.7-74.0%) in the ID group vs. 78.4% (95% CI 67.6-86.3%) in the IM group (p = 0.053). Influenza-related acute respiratory illness (ARI), diagnosed as a 4-fold rise in HI titers with a convalescent titer > 1:40, and/or the RT-PCR between the ID group (5.3%) and the IM group (8.1%) were not significantly different. The reduced-dose intradermal influenza vaccine may expand vaccine coverage in cases of vaccine shortage.
KEYWORDS: acute respiratory illness, COPD, immunogenicity, intradermal, influenza vaccine, influenza A(H1N1)pdm09
Introduction
Yearly influenza vaccination is recommended for chronic obstructive pulmonary disease (COPD) patients to reduce serious illness and death.1,2 The conventional dose of the vaccine is an intramuscular injection of 15 µg of hemagglutinin (HA) per strain of each influenza virus but a reduced dose intradermal injection may increase vaccine coverage in cases of vaccine shortage, especially in low-income countries. Our previous study3 demonstrated that the antibody responses of a reduced dose of 6 µg of HA per strain split into 2-site intradermal injections in COPD patients, most of whom were aged 60 y or older, met all of the Committee for Proprietary Medicinal Products (CPMP) criteria for annual re-licensure of influenza vaccine.4 However, the 6 μg of HA per strain intradermal vaccination elicited lower antibody responses than the 15 μg of HA per strain intramuscular vaccination. Previous studies of a new intradermal microinjection system (BD soluviaTM) in healthy adults aged 18 -59 y showed that a reduced doses 9 μg of HA per strain elicited comparable antibody responses to a conventional 15 μg of HA per strain intramuscular vaccination,5 induced non-inferior antibody responses against all 3 strains and superior responses against influenza A(H1N1 and H3N2) compared with the conventional intramuscular injection.6 Another study of the reduced dose intradermal influenza vaccination in the elderly7 demonstrated that 9 µg of HA per strain intradermal injection via the Mantoux technique, the same technique as our study, elicited an immunogenicity that was similar to the 15 µg of HA per strain intramuscular injection in adults 65 y and older. Arnou R et al.8 demonstrated that a 15 μg of HA per strain administered ID using the BD SoluviaTM microinjection system elicited superior immune response as compared to the standard 15 μg of HA per strain IM dose in subjects over 60 y. The 9 µg of HA per strain may be the optimal dose-sparing strategy for intradermal influenza vaccination in the elderly.
The World Health Organization (WHO) declared a pandemic of the novel influenza, A(H1N1)pdm09 in June 20099 and over in August 2010.10 The influenza A(H1N1)pdm09 virus was expected to be the predominant seasonal influenza A(H1N1) during the post-pandemic period. Therefore, the WHO recommended the inclusion of the influenza A(H1N1)pdm09 virus as the H1N1 component in the 2010-2011 seasonal trivalent influenza vaccine. We hypothesized that the immunogenicity of a reduced dose 9 µg of HA per strain of seasonal trivalent influenza vaccine containing influenza A(H1N1)pdm09 virus intradermal injection would elicit similar responses as a 15 µg of HA per strain intramuscular injection in COPD patients, even soon after these pandemics.
Results
Demographic data
Demographic characteristics were not different between the ID group (n = 75) and IM group (n = 74), including age, sex, BMI, severity of COPD, co-morbidities, inhaled corticosteroid use, percentage of previous trivalent influenza vaccinations and duration of last trivalent influenza vaccination. Mean ages of the ID and IM groups were 72 ± 8 (range 60 – 88) and 73 ± 7 (range 60–94) years old, respectively (Table 1).
Table 1.
Demographic and clinical characteristics of COPD patients in the intradermal (ID) group (n = 75) and intramuscular (IM) group (n = 74).
| Characteristics | ID group | IM group | P-value |
|---|---|---|---|
| Age, years (mean ± SD, range) | 72 ± 8, 60 - 88 | 73 ± 7, 60 - 94 | 0.765 |
| Male sex, no. (%) | 68 (90.7) | 68 (91.9) | 0.791 |
| BMI (kg/m2)a, no. (%) | |||
| < 18.5 (underweight) | 15 (20.0) | 16 (21.6) | 0.253 |
| 18.5-24.9 (normal) | 44 (58.7) | 47 (63.5) | 0.273 |
| 25-29.9 (overweight) | 13 (17.3) | 10 (13.5) | 0.691 |
| ≥ 30 (obese) | 3 (4.0) | 1 (1.4) | 0.558 |
| Severity of COPDb, no. (%) | |||
| Mild | 18 (24.0) | 18 (24.3) | 0.963 |
| Moderate | 36 (48.0) | 29 (39.2) | 0.278 |
| Severe | 18 (24.0) | 25 (33.8) | 0.188 |
| Very severe | 3 (4.0) | 2 (2.7) | 0.660 |
| Co-morbidity, no. (%) | |||
| Hypertension | 50 (66.7) | 38 (51.4) | 0 .057 |
| Dyslipidemia | 34 (45.3) | 32 (43.2) | 0.797 |
| Diabetes mellitus | 11 (14.7) | 9 (12.2) | 0.654 |
| Coronary artery disease | 13 (17.3) | 10 (13.5) | 0.519 |
| Cerebrovascular accident | 3 (4.0) | 7 (9.5) | 0.183 |
| Inhaled corticosteroid use, no. (%) | 36 (48.0) | 41 (55.4) | 0.366 |
| Previous TIV, no. (%) | 63 (84.0) | 57 (77.0) | 0.282 |
| Previous TIV, months (mean + SD) | 16.2 ± 8.9 | 14.7 ± 7.0 | 0.303 |
BMI = body mass index,
WHO BMI Classification30,
a spirometric classification of COPD severity1, TIV = trivalent influenza vaccination
Immunogenicity
A total of 147 of the 149 patients (98.7%) were older than 60 y old, and only one patient in each group was 60 y old. Therefore, we used the CPMP criteria for aged older than 60 y to evaluate the antibody responses for all patients.4 Pre-vaccination GMTs and seroprotection rates (the percentage of patients with HI titer ≥ 1:40) for each influenza strain in the ID group (n=75) and IM group (n=74) were similar (Table 2). Each of the influenza strains of the ID and IM vaccinations met CPMP criteria for annual re-licensure at 4 weeks post-vaccination. GMTs, seroconversion factors, seroconversion rates and seroprotection rates for each influenza strain were not statistically significant different between the ID and IM groups 4 weeks post-vaccination. However, the antibody responses for influenza A(H1N1)pdm09 in the ID group tended to be lower than the IM group. The seroprotection rates for influenza A(H1N1)pdm09 were 64.0% (95%CI 52.7-74.0%) in the ID group and 78.4% (95% CI 67.6-86.3%) in the IM group 4 weeks post-vaccination (p = 0.053), and the seroconversion rates were 50.7 (39.6-61.7) in the ID group versus 66.2 (54.8-76.0) in the IM group (p = 0.054). The antibody responses for influenza B in the ID and IM groups were quite low. Seroprotection rates for influenza B 4 weeks post-vaccination were 41.3% (95% CI 30.9–52.6%) in the ID group and 36.5% (95% CI 26.4–47.9%) in the IM group. Only seroconversion factors of influenza B met the CPMP criteria (> 2.0), but with low values: 4.6 (95% CI 3.1 – 6.1) in the ID group and 5.7 (95% CI 3.5 – 7.8) in the IM group.
Table 2.
Hemagglutination inhibition (HI) antibody titers in the intradermal (ID) group (n = 75) compared with intramuscular (IM) group (n = 74) pre-vaccination and 4 weeks post-vaccination.
| H1N1pdm09 |
H3N2 |
B |
|||||
|---|---|---|---|---|---|---|---|
| CPMP Age > 60 yrs | ID | IM | ID | IM | ID | IM | |
| Pre-vaccination GMT (95% CI) | 13.8(10.2 – 18.7) | 13.0(10.0-17.0) | 30.3(21.9-41.9) | 26.0(18.7-36.2) | 9.6(7.5-12.4) | 8.9(7.1-11.3) | |
| P-value | 0.984 | 0.479 | 0.812 | ||||
| Post-vaccination GMT(95% CI) | 70.3(49.5– 99.8) | 97.4(69.8-135.8) | 160.0(116.8-219.2) | 141.7(102.0-196.7) | 23.2(17.2-31.3) | 20.2(14.8-27.5) | |
| P-value | 0.192 | 0.601 | 0.449 | ||||
| Seroconversion factor (95% CI) | > 2.0 | 13.1(9.0-17.2) | 19.9(12.7-27.1) | 16.4(10.1-22.7) | 17.2(10.7-23.8) | 4.6(3.1-6.1) | 5.7(3.5-7.8) |
| P-value | 0.113 | 0.971 | 0.371 | ||||
| Seroconversion rate %, (95% CI) | > 30 | 50.7(39.6-61.7) | 66.2(54.8-76.0) | 60.0(48.7-70.3) | 55.4(44.1-66.2) | 26.7(18.0-37.7) | 24.3(15.9-35.3) |
| P-value | 0.054 | 0.570 | 0.743 | ||||
| Pre-vaccination seroprotection rate%, (95% CI) | 24.0(15.7-34.9) | 28.4(19.3-39.6) | 50.7(39.6-61.7) | 40.5(30.1-52.0) | 17.3(10.3-27.6) | 14.9(8.3-24.9) | |
| P-value | 0.543 | 0.215 | 0.682 | ||||
| Post-vaccination seroprotection rate%, (95% CI) | > 60 | 64.0(52.7-74.0) | 78.4(67.6-86.3) | 81.3(71.0-88.7) | 79.7(69.1-87.4) | 41.3(30.9-52.6) | 36.5(26.4-47.9) |
| P-value | 0.053 | 0.805 | 0.544 | ||||
CPMP = Committee for Proprietary Medicinal Products.4
GMT = Geometric mean titers.
Seroconversion factor = the ratio of the HI titer after vaccination to the HI titer before vaccination.
Seroconversion rate = the percentage of post-vaccination HI titer ≥ 1:40 in patients with pre-vaccination titer < 1:10 or ≥ 4-fold increase in post-vaccination HI titer in patients with a pre-vaccination titer ≥ 1:10.
Seroprotection rate = the percentage of patients with post-vaccination HI titer ≥ 1:40.
Four of the 149 patients (2.7%) received monovalent inactivated influenza A(H1N1)pdm09 vaccine during the pandemic 3, 4, 6 and 8 months before enrollment, and 2 other patients had influenza A(H1N1)pdm09 pneumonia that was confirmed using RT-PCR before enrollment. All of these patients were randomly allocated to the IM group. These factors may have affected the antibody responses to influenza A(H1N1)pdm09 in the IM group. Therefore, we excluded these patients from the analysis, including 11 patients in the ID group and 6 patients in the IM group who previously received trivalent influenza vaccine containing influenza A(H1N1)pdm09 at least 12 months prior to enrollment. We found that the antibody responses in these patients were similar to those observed in the patients included in the analysis. The antibody responses for influenza A(H1N1)pdm09 in the ID group remained lower than the IM group after the exclusion of these patients, but this difference was not statistically significant (Table 3). The seroprotection rates at 4 weeks post-vaccination in the ID group and IM groups were 67.2% (95% CI 55.0-77.5%, n = 64) and 79.0% (95% CI 67.2-87.5%, n = 62), respectively (p = 0.134). The antibody responses for influenza A(H1N1)pdm09 in the ID group still met all 3 criteria of the CPMP.
Table 3.
Hemagglutination inhibition (HI) antibody titers in the intradermal (ID) group (n = 64) compared with intramuscular (IM) group (n = 62) pre-vaccination and 4 weeks post-vaccination in patients who had no previous vaccination containing influenza A(H1N1)pdm09 virus or no history of influenza A(H1N1)pdm09 infection.
| H1N1pdm09 |
H3N2 |
B |
||||
|---|---|---|---|---|---|---|
| ID | IM | ID | IM | ID | IM | |
| Pre-vaccination GMT (95% CI) | 14.0(10.1-19.4) | 12.9(9.6-17.4) | 32.6(22.8-46.6) | 25.0(17.6-35.5) | 9.2(7.1-11.9) | 8.5(6.7-10.7) |
| P-value | 0.789 | 0.307 | 0.835 | |||
| Post-vaccination GMT(95% CI) | 74.2 (51.6-106.6) | 95.7 (66.7-137.2) | 165.3 (117.0-233.5) | 127.9 (88.9-184.2) | 21.3(15.5-29.4) | 19.1(13.6 -26.8) |
| P-value | 0.316 | 0.296 | 0.517 | |||
| Seroconversion factor* (95% CI) | 12.4(8.3-16.5) | 19.9(12.1-27.7) | 17.0(9.6-24.2) | 15.6(9.0-22.2) | 4.3(2.9-5.7) | 5.8(3.4-8.2) |
| P-value | 0.168 | 0.935 | 0.490 | |||
| Seroconversion rate* %, (95% CI) | 53.1(41.1-64.8) | 66.1(53.7-76.7) | 60.9(48.7-72.0) | 56.5(44.1-68.1) | 17.2(9.7-28.4) | 17.7(10.0-29.2) |
| P-value | 0.137 | 0.609 | 0.935 | |||
| Pre-vaccination seroprotection rate%, (95% CI) | 23.4(14.6-35.2) | 27.4(17.8-39.7) | 54.7(42.6-66.3) | 40.3(29.0-52.8) | 15.6(8.5-26.6) | 12.9(6.4-23.7) |
| P-value | 0.608 | 0.107 | 0.662 | |||
| Post-vaccination seroprotection rate*%, (95% CI) | 67.2(55.0-77.5) | 79.0(67.2-87.5) | 82.8(71.6-90.3) | 77.4(67.2-87.5) | 37.5(26.7-49.8) | 33.9(23.3-46.3) |
| P-value | 0.134 | 0.448 | 0.671 | |||
Committee for Proprietary Medicinal Products (CPMP) criteria4: Seroconversion factor > 2.0, Seroconversion rate > 30, seroprotection rate > 60.
Antibody responses 4 weeks post-vaccination in baseline seronegative and seropositive patients were also analyzed. GMTs and seroprotection rates in seronegative patients were lower than the seropositive patients 4 weeks post-vaccination (Table 4). GMTs and seroprotection rates in seronegative and seropositive patients for influenza A(H3N2) and influenza B in the ID and IM groups were similar. However, GMTs and seroprotection rates in seronegative and seropositive patients for influenza A(H1N1)pdm09 were lower in the ID group than the IM group. Seroprotection rates 4 weeks post-vaccination for influenza A(H1N1)pdm09 in the seronegative ID and IM groups were 47.2% (95%CI 32.0-63.0%, n = 36) and 61.1% (95%CI 44.8-75.2%, n = 36), respectively (p = 0.237), and seroprotection rates in the seropositive ID and IM groups were 79.5% (95%CI 64.2-89.5%, n = 39) and 94.7% ( 95%CI 81.8-99.5%, n = 38), respectively (p = 0.047).
Table 4.
Hemagglutination inhibition (HI) antibody titers 4 weeks post-vaccination by baseline seronegative and seropositive status in the intradermal (ID) group (total n = 75) compared to intramuscular (IM) group (total n = 74).
| Seronegative (HI titers < 1:10) |
Seropositive (HI titers > 1:10) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1N1pdm09 |
H3N2 |
B |
H1N1pdm09 |
H3N2 |
B |
||||||||||
| CPMPAge> 60 yrs | ID n = 36 | IM n = 36 | ID n = 14 | IM n = 18 | ID n = 51 | IM n = 51 | ID n = 39 | IM n = 38 | ID n = 61 | IM n = 56 | ID n = 24 | IM n = 23 | |||
| Pre-vaccination GMT (95% CI) | 5 | 5 | 5 | 5 | 5 | 5 | 35.3(23.8-52.5) | 32.1(23.7-43.6) | 45.8(33.5-62.8) | 44.2(31.7-61.6) | 38.9(25.6-59.0) | 32.4(22.0-47.7) | |||
| P-value | 0.892 | 0.881 | 0.566 | ||||||||||||
| Post-vaccination GMT(95% CI) | 37.0 (23.0-59.7) | 52.4 (31.4-87.4) | 97.5 (40.4-235.1) | 74.1 (34.5-159.2) | 15.9 (11.8-21.3) | 14.6 (10.4-20.6) | 127.0(80.9-199.2) | 175.3(122.8-250.2) | 179.3(128.0-251.1) | 174.5(122.5-248.6) | 51.9(27.9-96.6) | 41.2(23.0-73.8) | |||
| P-value | 0.325 | 0.604 | 0.400 | 0.410 | 0.900 | 0.493 | |||||||||
| Seroconversion factor (95% CI) | > 2.0 | 17.6(10.3-24.8) | 29.2 (15.3-43.1) | 44.9 (16.3-73.4) | 37.3 (14.4-60.2) | 5.7(3.7-7.8) | 6.8(3.9-9.6) | 9.0(4.9-13.1) | 11.0(6.9-15.1) | 9.8(6.6-13.1) | 10.6 (6.7-14.8) | 2.2(1.2-3.1) | 3.3(0.3-6.4) | ||
| P-value | 0.325 | 0.604 | 0.400 | 0.162 | 0.886 | 0.630 | |||||||||
| Seroconversion rate %, (95% CI) | > 30 | 47.2 (32.0-63.0) | 61.1 (44.8-75.2) | 71.4 (45.0-88.7) | 66.7 (43.5-83.9) | 27.5 (17.0-41.0) | 27.5 (17.0-41.0) | 53.8 (38.6-68.4) | 71.1 (55.1-83.1) | 57.4 (44.9-69.0) | 51.8 (39.0-64.3) | 25.0 (11.7-45.2) | 17.4 (6.3-37.7) | ||
| P-value | 0.237 | 0.773 | 1.000 | 0.119 | 0.544 | 0.524 | |||||||||
| Pre-vaccination seroprotection rate%, (95% CI) | 0 | 0 | 0 | 0 | 0 | 0 | 46.2 (31.6-61.4) | 55.3 (39.7-69.9) | 62.3(49.7-73.4) | 53.6(40.7-66.0) | 54.2(35.1-72.1) | 47.8 (29.2-67.0) | |||
| P-value | 0.424 | 0.339 | 0.664 | ||||||||||||
| Post-vaccination seroprotection rate%, (95% CI) | > 60 | 47.2 (32.0-63.0) | 61.1 (44.8-75.2) | 71.4 (45.0-88.7) | 66.7 (43.6-83.9) | 27.5 (17.0-41.0) | 27.5 (17.0-41.0) | 79.5 (64.2-89.5) | 94.7 (81.8-99.5) | 83.6 (72.2-91.0) | 83.9 (72.0-91.5) | 70.8 (50.6-85.3) | 56.5 (36.8-74.4) | ||
| P-value | 0.237 | 0.773 | 1.000 | 0.047* | 0.962 | 0.307 | |||||||||
The level of critical significance was assigned at p < 0.05.
Safety
There were no serious side effects associated with vaccination. The two-site intradermal injection was more painful than the intramuscular injection (visual analog scales 1.6 ± 1.5 vs. 1.0 ± 1.1, p = 0.006) that overall pain scores were low (out of 10 maximum) in both groups and patients tolerated this pain. Sixty-seven patients in each group (ID 89.3% and IM 90.5%) returned their diary records to the investigators. The incidence of local reactions of erythema, itching, induration and ecchymosis in the ID injection group was significantly higher than the IM injection group. Systemic reactions (fever and headache) were significantly more frequently found in the IM injection group than the ID injection group (Table 5). Nevertheless fever and headache were low incidence (less than 5% in the IM group), transient and mild intensity.
Table 5.
Side effects of influenza vaccination in the intradermal (ID) group (n = 67) and the intramuscular (IM) group (n = 67).
| Side effects | ID n (%) | IM n (%) | P-value | |
|---|---|---|---|---|
| Local | Erythema | 67 (100.0) | 34 (50.7) | < 0.001* |
| Itching | 18 (26.9) | 1 (1.5) | < 0.001* | |
| Swelling | 66 (98.5) | 11 (16.4) | < 0.001* | |
| Ecchymosis | 16 (23.9) | 2 (3.0) | 0.001* | |
| Systemic | Fever | 0 (0) | 2 (3.0) | < 0.001* |
| Myalgia | 1 (1.5) | 4 (6.0) | 0.43 | |
| Headache | 0 (0) | 3 (4.5) | < 0.001* | |
Statistically significant at P < 0.05
Influenza-related acute respiratory illness
No patients were lost to follow up during the 1-year post-vaccination period. One patient in the ID group died from pulmonary tuberculosis with hemoptysis and respiratory failure. Four patients in the IM group died, but none of these deaths were related to influenza: 2 patients died from bacterial pneumonia with respiratory failure, and 2 patients died from COPD exacerbation with respiratory failure. A total of 70 ARI episodes (38 patients) were reported in the ID group, and 76 episodes (34 patients) were reported in the IM group. The incidence of each ARI type was not significantly different between the ID and IM groups. The most common ARI was COPD exacerbation, which was reported in 57 of 70 (81.4%) ARI episodes in the ID group and 56 of 76 (73.7%) episodes in the IM group. Pneumonia occurred in 17 of 70 (24.3%) ARI episodes in the ID group and 14 of 76 (18.4%) episodes in the IM group (Fig. 1.). Paired-serum HI titers were performed in ARI patients: 57 of 70 (81.4 %) ARI episodes in the ID group and 54 of 76 (71.0%) episodes in the IM group. RT-PCR was performed in patients who had an onset of the ARI for 7 d or less. Twenty-nine specimens in the ID group and 26 specimens in the IM group were evaluated using RT-PCR.
Figure 1.
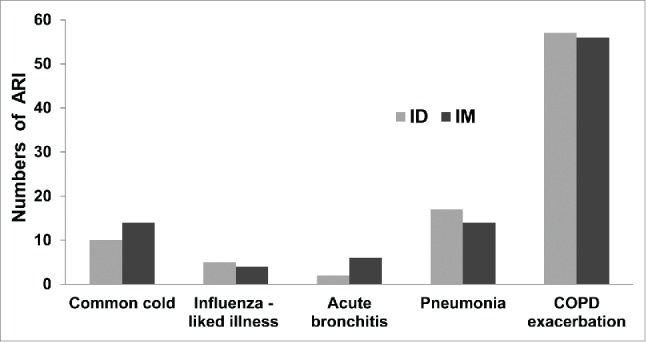
Numbers and types of acute respiratory illness (ARI) in the intradermal injection (ID, total events = 70) and intramuscular injection (IM, total events = 76) groups. The numbers of patients in each ARI type was not significantly different between the ID and IM groups.
Ten of 149 COPD patients were diagnosed with influenza-related acute respiratory illness by HI titers and/or RT-PCR: 4 patients (5.3%, 4 of 75) in the ID group and 6 patients (8.1%, 6 of 74) in the IM group. The most common clinical presentation of influenza-related ARI (7 of 10 patients) was COPD exacerbation. All influenza-related ARI were diagnosed 6 months post-vaccination, except one case in the IM group developed influenza-related ARI 34 d post-vaccination (Table 6). Most influenza related-ARI were influenza A. Four patients (67.7%) in the IM group and one patient (25%) in the ID group were diagnosed as influenza A(H1N1)pdm09. One patient in the IM and ID groups was diagnosed as influenza-related ARI using RT-PCR (Table 6), and both of these patients had pneumonia and required hospitalization. Influenza A(H1N1)pdm09 pneumonia in the IM group was diagnosed by RT-PCR and HI titers, whereas influenza A(H3N2) pneumonia in the ID group was diagnosed by RT-PCR only.
Table 6.
Influenza-related acute respiratory illness diagnosed by hemagglutination inhibition (HI) titers or RT-PCR in the intradermal (ID) injection and intramuscular (IM) injection.
| Influenza-related ARI diagnosed by |
HI titers at baseline and 4 wks post-vaccination |
HI titers of influenza-related ARI |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1N1 |
H3N2 |
B |
H1N1 |
H3N2 |
B |
|||||||||||
| Case | HI titers | RT-PCR | ARI after vaccination (days) | 0 wk | 4 wks | 0 wk | 4 wks | 0 wk | 4 wks | 1st serum | 2nd serum | 1st serum | 2nd serum | 1st serum | 2nd serum | |
| IM1 | Flu A undefined subtype | NA | 34 | 5 | 5 | 5 | 10 | 5 | 5 | 5 | 640 | 10 | 320 | 5 | 5 | |
| IM2 | Flu Undefined type | NA | 182 | 40 | 640 | 10 | 320 | 5 | 80 | 20 | 160 | 20 | 80 | 10 | 40 | |
| IM3 | H1N1 | NA | 173 | 5 | 10 | 80 | 640 | 5 | 10 | 40 | 160 | 160 | 40 | 5 | 5 | |
| IM4 | H1N1 | NA | 189 | 20 | 40 | 80 | 640 | 20 | 80 | 5 | 80 | 640 | 320 | 5 | 5 | |
| IM5 | H1N1 | Neg. | 329 | 5 | 10 | 5 | 80 | 5 | 80 | 80 | 640 | 40 | 80 | 10 | 40 | |
| IM6 | H1N1 | H1N1 | 337 | 5 | 40 | 40 | 640 | 5 | 10 | 20 | 640 | 5 | 5 | 10 | 5 | |
| ID1 | FluUndefined type | Neg. | 334 | 10 | 20 | 10 | 320 | 5 | 160 | 5 | 80 | 5 | 80 | 5 | 40 | |
| ID2 | H1N1 | NA | 171 | 40 | 640 | 40 | 80 | 5 | 10 | 10 | 320 | 20 | 5 | 20 | 10 | |
| ID3 | H3N2 | Neg. | 362 | 20 | 640 | 40 | 640 | 5 | 40 | 80 | 80 | 40 | 640 | 5 | 5 | |
| ID4 | Neg. | H3N2 | 345 | 640 | 640 | 10 | 20 | 5 | 5 | 20 | 40 | 5 | 5 | 5 | 5 | |
IM = intramuscular group, ID = intradermal group, ARI = acute respiratory illness, RT-PCR = reverse transcription-polymerase chain reaction
NA = not available, Neg. = negative, H1N1 = influenza A(H1N1)pdm09, H3N2 = influenza A(H3N2), B = influenza B
Discussion
Antibody responses against each influenza strain of the reduced-dose (total dose 9 µg of HA per strain) trivalent seasonal influenza vaccine via 2-site ID injections using the Mantoux technique met the CPMP criteria4 for annual re-licensure in COPD patients 4 weeks post-vaccination (Table 2 and Table 3). Reduced-dose intradermal influenza vaccinations were studied in healthy and diseased subjects at various doses of the vaccine (3 μg to 9 μg HA per strain).3,5-8,11-14 Our previous study3 demonstrated that 6 µg of HA per strain ID injection elicited lower antibody responses than the 15 µg of HA per strain IM injection in COPD patients, but the responses met CPMP criteria. We expected that antibody responses of the reduced dose ID injection of seasonal influenza vaccine containing the pandemic virus soon after the pandemic would elicit lower antibody responses than regular seasonal influenza vaccines. We designed to increase the vaccine dose from 6 µg to 9 µg of HA per strain based on the studies of Chi et al.7 and Leroux-Roels I. et al,6 which were performed before the pandemic. Our study was conducted soon after the pandemic and demonstrated that the antibody responses of the reduced dose intradermal injection were similar to the intramuscular injection for influenza A(H3N2) and influenza B, but not influenza A(H1N1)pdm09. The antibody responses for influenza A(H1N1)pdm09 in the ID injection were lower than the IM injection, but this difference was not statistically significant. All antibody responses met all 3 criteria of the CPMP.15 Another study of the low dose intradermal vaccination after the pandemic was studied by Hung IF et al.16 The study compared 3μg and 9 μg of HA per strain delivered by the MicronJet600TM (NanoPass Technologies, Israel) with 9 μg of HA per strain ID, Intanza®9 (Sanofi-Pasteur, France) delivered by the BD SoluviaTM microinjection system and 15 μg of HA per strain intramuscular injection in the elderly and the chronically ill adults. They found that the antibody responses for all 3 strains of the low dose ID groups were non-inferior to the IM group. Direct comparison among the 3 ID groups was not significantly different. They demonstrated that the antibody responses of the H1N1 strain were significantly higher in the all ID groups compared with the IM group, which the results were contradictory to our study. The Mantoux technique and the microinjection system were different at least in terms of administration techniques and sizes of needles. The Mantoux technique needs well trained personnel. Our study performed by the experience personnel who can deliver the vaccine into the dermis to form the bleb in most subjects (71 of 75 patients (94.7%) on both sites and 3 of 75 patients (4.0%) on one site of the injections) and no leakage was observed by naked eyes. However we found that the bled formation was varied in sizes, which may contribute to the somewhat-reduced immunogenicity of the ID injection. We did not measure the bleb size immediately after the injection, but we measured it within 30 minutes after vaccination to teach the patients and their relatives to measure the diameters of erythema and induration. We did not know the association between bleb formation and the antibody responses. We found that one patient who had no bleb formation on both sites of the injections produced the antibody responses for influenza A(H1N1)pdm09 with HI titers 1:10 at baseline to 1:320 at 4 weeks post vaccination.
This study was performed in 2010-2011. We found that pre-vaccination seroprotective rates for influenza A(H1N1)pdm09 in COPD patients were 24.0% (95%CI 15.7-34.9) and 28.4% (19.3-39.6%) in the ID and IM groups, respectively, which were lower than the values in Thai elderly subjects in the study of Prachayangprecha et al. in 2009 (seroprotection rate 35.7 % in aged 61 y old or older) and higher than pre-pandemic sera collected in 2008 (seroprotection rates only 2 of 100 (2%) stored sera of pre-pandemic subjects in aged range of 51-60 y and over 70 years).17 These differences may be due to the different times of the study, sample groups, and geography. However, these data suggest that the seroprotection rate against influenza A(H1N1)pdm09 was low in the pre-pandemic era. Seronegativity and seropositivity may affect the antibody responses. Therefore, further analyses of antibody responses 4 weeks post-vaccination in pre-vaccination seronegative (HI titers < 1:10) and seropositive (HI titers ≥ 1:10) were performed.
GMTs and seroprotection rates were significantly lower in seronegative patients than seropositive patients 4 weeks post-vaccination, which is consistent with Gorse G.J. et al. who investigated the immunogenicity of an ID influenza vaccine (Fluzone®→).18 Antibody responses for influenza A(H1N1)pdm09 in the ID group were lower than the IM group in seronegative and seropositive patients. The seroprotection criterion (HI titers ≥ 1:40) is applied to the immunogenicity assessment of seasonal influenza vaccines, and most of the vaccinated population exhibits some degree of preexisting immunity against the vaccine strains. This criterion may not be valid in an immunologically naïve population.15 However, this study used the same criterion, even in seronegative patients. Seroprotection rates (HI titers ≥ 1:40) for influenza A(H1N1)pdm09 in the seronegative ID and IM groups were 47.2% (95%CI 32.0-63.0%) and 61.1% (95%CI 44.8-75.2%), respectively (P = 0.237), and rates in the seropositive ID and IM groups were 79.5% (95%CI 64.2-89.5) and 94.7% (95%CI 81.8-99.5%), respectively (P = 0.047). Seroprotective rates of influenza A(H1N1)pdm09 in the seronegative ID group were lower than the CPMP criterion for annual re-licensure (> 60%), but rates ranged from 30-50% of the population, which is sufficient to lower and control the infection rate of the influenza A(H1N1)pdm09 in ‘herd immunity’ to protect non-immunized individuals from infection.17
The Thai Ministry of Public Health provided 2 million doses of monovalent influenza A(H1N1)pdm09 to health care personnel and high-risk groups, including COPD patients, during the pandemics. Unfortunately, only 2.7% (4/150) of COPD patients in this study received monovalent influenza, which contrasts the high coverage (78.7%, 6,210 of 7886) in health care personnel in one university hospital.19 A dose-sparing strategy using ID injections via the Mantoux technique may expand vaccine coverage in cases of limited resource of the vaccine, including during pandemics, especially in low-income countries. The Mantoux technique of ID injection is familiar to medical personnel in countries who have experience with the tuberculin test or ID rabies vaccinations. The technique requires trained personnel, but it is not difficult to perform. This technique can be used in the aging skin, as in this study, and local side effects are tolerable. However the Mantoux may give inconsistent results.20 The novel intradermal technique such as the microinjection system is more convenient, easier to use, more reliable 5,6,8,21 and gives better immunogenicity 16 than the Mantoux technique. The Mantoux technique should be limited the use in case the better technique is not available.
We also followed up patients for 1 y after the vaccination to compare the incidences of ARI and laboratory-confirmed influenza-related ARI between the ID and IM groups. The incidences of ARI and laboratory-confirmed influenza illness using the RT-PCR and/or a 4-fold increase in HI titers were not significantly different between the ID and IM groups, but the antibody responses 4 weeks post-vaccination for influenza A(H1N1)pdm09 tended to be lower in the ID group than the IM group. The overall incidence of laboratory confirmed influenza-related ARI in vaccinated COPD patients (ID and IM injection) was 6.7% (10 of 149 patients), which was similar to the IM vaccinations in COPD patients (6.8%) in the study conducted by Wongsurakiat et al.2 Most influenza-related ARI were diagnosed 6 months or more post-vaccination, which is also similar to vaccinated COPD patients (7 months or more post-vaccination) in the Kositanont U et al. study.22 Some cases may have been non-responders to the vaccination, and some cases may have exhibited decreased antibody responses after 6 months.3,22
One patient with influenza-related ARI in each of the ID and IM groups was hospitalized, and both ARI were RT-PCR-positive pneumonia. The patient in the ID group who was RT-PCR positive for influenza A(H3N2) exhibited no increase in HI titers against influenza A(H3N2) (Table 6). This patient may have been a non-responder to the vaccination or infected with a new strain of the influenza A(H3N2). Influenza B-related ARI was not detected in this study, which may due to the low prevalence of influenza B infection during the study period.23
In conclusion antibody responses to the reduced dose 9 µg of HA per strain containing influenza A(H1N1)pdm09 using the 2-site intradermal injection via the Mantoux technique met the CPMP criteria for annual re-licensure to the same degree as the conventional 15 µg HA per strain intramuscular (IM) injection in COPD patients soon after a pandemic. Local side effects of the intradermal injection were tolerable. Influenza-related acute respiratory illnesses were also not different between ID and IM administration. The dose-sparing strategy using intradermal injections via the Mantoux technique may expand vaccine coverage in cases of vaccine shortage, especially in low-income countries.
The limitation of this study was that the Mantoux technique was performed by the well trained personnel who is familiar with the technique. Therefore the results may not apply to general nursing population. Another limitation was that we found the varied sizes of the bleb after the injection, which the size may affect the inconsistent immunogenicity. Further study may compare the Mantoux technique with the microinjection system which is available in the market in terms of the immunogenicity and the cost effectiveness.
Patients and methods
Subjects and study design
A prospective randomized, open-label study was conducted to evaluate the immunogenicity, safety and influenza-related ARI of 2-site intradermal administration of a trivalent, inactivated, split-virion influenza vaccine (Sanofi-Pasteur, France) with total dose of 9 µg of HA per strain compared to a 15 µg of HA per strain intramuscular administration in COPD patients. Patients who were diagnosed as COPD with a ratio of post-bronchodilator forced expiratory volume at one second (FEV1) to forced vital capacity (FVC) less than 0.701 were enrolled at the COPD Clinic, Siriraj Hospital, Bangkok, Thailand. COPD patients who were 60 y or older and had no seasonal influenza vaccination or had a previous seasonal influenza vaccination more than one year prior were included. Patients were excluded if they had ongoing fever (BT > 38 ºC), were immunocompromised hosts or receiving any immunosuppressive drugs, including systemic corticosteroids, had malignancy with an expected survival time of less than a year, or an allergy to vaccine components. The Siriraj Institutional Review Board (SIRB), Faculty of Medicine Siriraj Hospital, Mahidol University reviewed and approved the study protocol. Written informed consent was obtained from each patient prior to enrollment.
Vaccination
The trivalent, inactivated, split-virion seasonal influenza vaccine (15 µg of HA per strain/0.5 ml) was used. The vaccine contained influenza A/California/7/2009(H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus and B/Brisbane/60/2008-like virus, as recommended by the WHO for use in 2010 – 2011. The final bulk vaccine from Sanofi-Pasteur, France was distributed by Government Pharmaceutical Organization – Merieux Biological Products Co., Ltd., was supplied in 5-mL multi-dose vials and supported by Department of Disease Control, Ministry of Public Health, Bangkok, Thailand (Lot numbers 07B1003 and 07B1114).
A sample size at least 50 subjects per group was needed to evaluate the antibody responses using the CPMP criteria.4 A total of 149 COPD patients were enrolled in 2010-2011, and a computerized block randomization was performed with 10 patients in each block. A co-investigator who did not participate in the vaccination randomly assigned patients to receive either an intradermal (ID) injection of 9 µg of HA per strain of influenza vaccine split into 2-site injections or an intramuscular (IM) injection of 15 µg of HA per strain. Seventy-five and 74 patients were enrolled in the ID group and IM group, respectively. Vaccines with Lot number 07B1003 were used in 60 and 61 patients in the ID and IM groups, respectively, and vaccines with Lot number 07B1114 were used in 15 and 13 patients in the ID and IM groups, respectively. Each vaccine vial was shaken before vaccine withdrawal. A 1-ml tuberculin syringe attached to a 25-gauge needle, 5/8 inch (16 mm) in length was used for the injection in the ID group. The vaccine was withdrawn from the vial, and the needle was changed. A final volume of 0.3 ml (9 µg of HA per strain) was used in the syringe. The Mantoux technique intradermal injection was performed as described previously.3 The tip of the needle with the bevel upwards was inserted almost parallel to the stretched skin surface. A half dose (approximately 0.15 ml) of the vaccine was slowly injected into the dermis of the ventral surface of the right forearm and the needle was slowly withdrew to prevent the leakage. The rest of the vaccine (approximately 0.15 ml) was injected into the left forearm using the same technique. A pale orange-peel appearance papule (bleb) immediately appeared which confirmed that the intradermal injection was performed correctly. The bleb formation, leakage and bleeding were observed. A 1-ml tuberculin syringe attached to a 25-gauge needle, 5/8 inch (16 mm) in length was also used in the IM group for convenience. The vaccine (0.5 ml) was withdrawn from the vial and injected perpendicularly into the deltoid muscle of the non-dominant arm.
Assessment of side effects
An investigator who did not perform the injection recorded the pain at the injected site immediately after vaccination using a visual analog scale that ranged from 0 cm (no pain) to 10 cm (the most severe pain). Patients were closely observed for 30 minutes after vaccination to detect acute serious reactions. Patients and their relatives were taught and practiced how to record any side effects, including measuring the temperature and diameter of erythema and induration. Patients were asked to record side effects into a diary for the first 7 d post-vaccination, and the diary was returned on the next visit 4 weeks after vaccination. Local reactions that appeared on at least one site of the forearm in the ID group were recorded as the local reaction. In cases where erythema or induration appeared in both forearms, the largest diameter was chosen for analysis.
Assessment of antibody responses
The hemagglutination inhibition (HI) titer was used to evaluate immunogenicity. Venous blood was drawn from each patient before vaccination and 4 weeks after vaccination. Sera were separated and stored at - 20◦C until analysis. The details of the HI test procedures were described elsewhere.11,22,24-26 Pre- and post-vaccination sera were tested simultaneously in duplicate. Antigens for HI titer testing included influenza antigen A/California/7/2009 (H1N1)v (NYMCX-179A)(Cell Derived) NIBSC code: 09/174, influenza antigen A/Victoria/210/2009 (H3N2)(NYMCX-187) NIBSC code: 10/102, and influenza antigen B/Brisbane/60 /08 (NYMCBX-35) NIBSC code: 10/106 and were supplied by the National Institute for Biological Standards and Control (NIBSC, UK).
HI titers ≥ 1:10 were considered to contain HI antibody. HI titers < 1:10 were considered undetectable and were expressed as 5 for analysis. Protective HI titer was defined at ≥ 1:40 as previously described.2,3,11,22,24-26 Geometric mean titers (GMTs), seroconversion factor (the ratio of the HI titer after vaccination to the HI titer before vaccination), seroconversion rate (the percentage of post-vaccination HI titer ≥ 1:40 in patients with pre-vaccination titers < 1:10 or ≥ 4-fold increase in the post-vaccination HI titers in patients with pre-vaccination titers ≥ 1:10), and seroprotection rates (the percentage of patients with a HI titer of ≥ 1:40) in sera 4 weeks post-vaccination were analyzed and compared with pre-vaccination sera. The CPMP criteria for annual re-licensure of influenza vaccine4 were used for assessments of antibody responses. At least one of the following criteria must be met for each strain approximately 3 weeks after vaccination for adults aged over 60 y old: mean geometric increase > 2.0, seroconversion rate > 30%, or seroprotection rate > 60%.
Assessment of influenza-related acute respiratory illness
Patients were followed up for one year after vaccination to evaluate influenza-related ARI. Patients regularly visited the COPD clinic every 4-8 weeks. Patients and their relatives were informed about symptoms of ARI and received a card that detailed these symptoms. Patients were instructed to visit the COPD clinic earlier than the schedule if they experienced ARI. Patients were also asked about ARI in the past during each regular visit.
The clinical characteristics of ARI were categorized into one of the 5 following groups: common cold, influenza-like illness, acute bronchitis, pneumonia, and COPD exacerbation. Common cold was defined as an upper respiratory tract infection with predominating rhinitis and pharyngitis.27 Influenza-like illness was defined as generalized aches, fever and headache with or without upper respiratory tract symptoms.27 Acute bronchitis was defined as an acute respiratory infection that manifested predominantly by cough with no evidence of pneumonia or common cold.28 Pneumonia was diagnosed using compatible symptoms (fever, cough and/or dyspnea) with new pulmonary infiltrations on chest X-ray. COPD exacerbation was diagnosed in patients who had at least 2 of 3 symptoms (increased dyspnea, increased sputum volume or increased purulent sputum) or at least one of these symptoms with at least one of the following symptoms: (1) upper respiratory tract infection (sore throat, nasal discharge) within the past 5 days, (2) fever without any other cause, (3) increased wheezing, (4) increased cough, or (5) an increase in respiratory rate or heart rate by 20% compared to baseline.29
Laboratory-confirmed influenza infections were measured using HI titers and/or reverse transcription-polymerase chain reaction (RT-PCR). Paired venous blood samples were obtained from patients with ARI for HI titers. The first sample (acute serum) was drawn at the first visit of ARI, and the second sample (convalescent serum) was drawn 2 - 4 weeks later. If patients had symptom onset for 7 d or less, then a nasopharyngeal (NP) wash in non-intubated patients or tracheal suction in intubated patients was obtained for RT-PCR of influenza A and B.
Influenza-related acute respiratory illness was diagnosed if ARI patients exhibited a 4-fold increase in HI titers with a titer of ≥ 1:40 in convalescent serum compared to acute serum and/or positive RT-PCR for influenza A or B from nasopharyngeal (NP) wash or tracheal suction specimen.
Statistical analysis
Data were analyzed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). Quantitative and qualitative data are described as the means and standard deviation (SD) and percentages, respectively. Analysis of variance of log-transformed results was used to compare the GMTS of the ID and IM groups. Student's t- test and Mann-Whitney U test were used in normally and non-normally distributed continuous variables, respectively, to compare the ID and IM groups. The Chi square (χ2) test was used for comparisons of categorical variables between 2 groups. A P-value of < 0.05 or no overlap of 95% CI was considered a statistically significant difference.
Abbreviations
- HA
hemagglutinin
- HI
hemagglutination inhibition
- ID
intradermal
- IM
intramuscular
- GMT
geometric mean titers
- CPMP
Committee for Proprietary Medicinal Products
- ARI
acute respiratory illness
- TIV
Trivalent influenza vaccine.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Department of Disease Control, Ministry of Public Health, Thailand for supplying the vaccine. We also thank the staff in the COPD clinic, Division of Respiratory Disease and Tuberculosis, Department of Medicine, Siriraj Hospital who contributed to this study.
Funding
This study was supported by the Siriraj Research Development Fund, the Faculty of Medicine Siriraj Hospital, Mahidol University.
References
- [1]. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al.. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532-55; PMID:17507545; http://dx.doi.org/ 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- [2]. Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 2004; 125:2011-20; PMID:15189916; http://dx.doi.org/ 10.1378/chest.125.6.2011 [DOI] [PubMed] [Google Scholar]
- [3]. Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine 2010; 28:4045-51; PMID:20412877; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.006 [DOI] [PubMed] [Google Scholar]
- [4]. Committee for Proprietary Medicinal Products (CPMP) Note for guidance on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96). Euro Agency Eval Med Products 12 March 1997. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf (accessed March 8, 2010). [Google Scholar]
- [5]. Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, Demanet E, Heijmans S, Van Belle P, Weber F, et al.. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med 2009; 7:13; PMID:19341446; http://dx.doi.org/ 10.1186/1741-7015-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, Leroux-Roels G. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine 2008; 26:6614-9; PMID:18930093; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.078 [DOI] [PubMed] [Google Scholar]
- [7]. Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis 2010; 50:1331-8; PMID:20377407; http://dx.doi.org/ 10.1086/652144 [DOI] [PubMed] [Google Scholar]
- [8]. Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine 2009; 27:7304-12; PMID:19849996; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.033 [DOI] [PubMed] [Google Scholar]
- [9]. World Health Organization World now at the start of 2009 influenza pandemic Available from: http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/ (accessed March 8, 2010). [Google Scholar]
- [10]. World Health Organization H1N1 in post-pandemic period. Available at http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/ (accessed August 20, 2010). [Google Scholar]
- [11]. Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 2007; 25:659-63; PMID:17011678; http://dx.doi.org/ 10.1016/j.vaccine.2006.08.026 [DOI] [PubMed] [Google Scholar]
- [12]. Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med 2004; 351:2286-94; PMID:15525713; http://dx.doi.org/ 10.1056/NEJMoa043555 [DOI] [PubMed] [Google Scholar]
- [13]. Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 2007; 25:6755-63; PMID:17692438; http://dx.doi.org/ 10.1016/j.vaccine.2007.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/ 10.1056/NEJMoa043540 [DOI] [PubMed] [Google Scholar]
- [15]. Committee for Proprietary Medicinal Products (CPMP) Guideline on influenza vaccines prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). Euro Agency Eval Med Prod 24 January 2007. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003872.pdf (accessed July 17, 2014). [Google Scholar]
- [16]. Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 2012; 30:6427-35; PMID:22910287; http://dx.doi.org/ 10.1016/j.vaccine.2012.08.014 [DOI] [PubMed] [Google Scholar]
- [17]. Prachayangprecha S, Makkoch J, Payungporn S, Chieochansin T, Vuthitanachot C, Vuthitanachot V, Theamboonlers A, Poovorawan Y. Serological analysis of human pandemic influenza (H1N1) in Thailand. J Health Popul Nutr 2010; 28:537-44; PMID:21261198; http://dx.doi.org/ 10.3329/jhpn.v28i6.6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Gorse GJ, Falsey AR, Fling JA, Poling TL, Strout CB, Tsang PH. Intradermally-administered influenza virus vaccine is safe and immunogenic in healthy adults 18-64 years of age. Vaccine 2013; 31:2358-65; PMID:23499604; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.008 [DOI] [PubMed] [Google Scholar]
- [19]. Kiertiburanakul S, Malathum K, Watcharananan SP, Bunupuradah P, Piebpien P, Rujiraviroj U, Likitsinsipon W, Apivanich S, Kehachindawat P, Burakitcharoen R, et al.. High coverage and safety of influenza A (H1N1) 2009 monovalent vaccination among health care personnel in Thailand. Am J Infect Control 2011; 39:525-8; PMID:21612842; http://dx.doi.org/ 10.1016/j.ajic.2010.09.011 [DOI] [PubMed] [Google Scholar]
- [20]. Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest 1994; 106:1463-5; PMID:7956403; http://dx.doi.org/ 10.1378/chest.106.5.1463 [DOI] [PubMed] [Google Scholar]
- [21]. Arnou R, Frank M, Hagel T, Prebet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther 2011; 28:555-65; PMID:21626269; http://dx.doi.org/ 10.1007/s12325-011-0035-z [DOI] [PubMed] [Google Scholar]
- [22]. Kositanont U, Kanyok R, Wasi C, Wongsurakiat P, Suthamsmai T, Maranetra N. Occurrence and protective level of influenza infections using serology in patients with COPD in vaccination study. J Med Assoc Thai 2004; 87:964-9; PMID:15471303 [PubMed] [Google Scholar]
- [23].Prachayangprecha S, Makkoch J, Suwannakarn K, Vichaiwattana P, Korkong S, Theamboonlers A, Poovorawan Y. Epidemiology of seasonal influenza in Bangkok between 2009 and 2012. J Infect Dev Ctries 2013; 7:734-40. [DOI] [PubMed] [Google Scholar]
- [24]. Kositanont U, Assantachai P, Wasi C, Puthavathana P, Praditsuwan R. Kinetics of the antibody response to seasonal influenza vaccination among the elderly. Viral Immunol 2012; 25:471-6; PMID:23061793; http://dx.doi.org/ 10.1089/vim.2012.0024 [DOI] [PubMed] [Google Scholar]
- [25]. Kositanont U, Wongsurakiat P, Pooruk P, Maranetra N, Puthavathana P. Induction of cross-neutralizing antibody against H5N1 virus after vaccination with seasonal influenza vaccine in COPD patients. Viral Immunol 2010; 23:329-34; PMID:20565296; http://dx.doi.org/ 10.1089/vim.2009.0082 [DOI] [PubMed] [Google Scholar]
- [26]. Praditsuwan R, Assantachai P, Wasi C, Puthavatana P, Kositanont U. The efficacy and effectiveness of influenza vaccination among Thai elderly persons living in the community. J Med Assoc Thai 2005; 88:256-64; PMID:15962680 [PubMed] [Google Scholar]
- [27]. Eadie MB, Stott EJ, Grist NR. Virological studies in chronic bronchitis. Br Med J 1966; 2:671-3; PMID:20791109; http://dx.doi.org/ 10.1136/bmj.2.5515.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, et al.. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129:1S-23S; PMID:16428686; http://dx.doi.org/ 10.1378/chest.129.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196-204; PMID:3492164; http://dx.doi.org/ 10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- [30]. Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157-63; PMID:14726171; http://dx.doi.org/ 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]


