ABSTRACT
During the last decades significant progress has been made in the field of cancer immunotherapy. However, cancer vaccines have not been successful in clinical trials due to poor immunogenicity of antigen, limitations of safety associated with traditional systemic delivery as well as the complex regulation of the immune system in tumor microenvironment. In recent years, nanotechnology-based delivery systems have attracted great interest in the field of immunotherapy since they provide new opportunities to fight the cancer. In particular, for delivery of cancer vaccines, multifunctional nanoparticles present many advantages such as targeted delivery to immune cells, co-delivery of therapeutic agents, reduced adverse outcomes, blocked immune checkpoint molecules, and amplify immune activation via the use of stimuli-responsive or immunostimulatory materials. In this review article, we highlight recent progress and future promise of multifunctional nanoparticles that have been applied to enhance the efficiency of cancer vaccines.
KEYWORDS: cancer vaccine, delivery system, immunotherapy, multifunctional nanoparticle, tumor microenvironment
Introduction
Cancer is a severe health threat and includes malignant diseases that are characterized by the unregulated cell proliferation. Although there have been significant advances over the last few decades in the prevention, screening, and treatment of cancer, the risk of recurrence remains a major drawback to the successful treatment of various types of cancer.1 Most of the time, the primary tumor can efficiently be removed by surgery and after that chemotherapy is the first line approach for the treatment of the remaining cancer calls. However, there are some hurdles associated with conventional chemotherapeutic agents include limited accessibility of drug to tumor tissues, which requires a higher dose, leading to intolerable cytotoxicity and nonspecific targeting, and consequently repeated treatment with these chemotherapeutic agents can result in resistance to the chemotherapies or development of multi-drug resistance (MDR).1,2 As a result, in many patients, chemotherapy becomes ineffective in preventing the metastatic spread of the disease through disseminated tumor cells and does not improve life expectancy.3 In recent years, cancer therapy has evolved to strategically develop new therapeutic approaches such as immunotherapy in order to optimize the chance of cure.4 To this end, there has been a growing focus on therapeutic strategies based on nanotechnology to enhance the potency of chemo-immunotherapy approaches by overcoming many biological barriers and efficiently deliver the therapeutic payload to a particular tissue destination.5,6
Nowadays growing and compelling evidence suggests that immune cells play an important role in the control of malignancy.7 In this regard, cancer vaccine and tumor immunotherapy is a promising therapeutic strategy based on the stimulation or activation of the patient's own immune system to recognize and destroy cancer cells. Cancer immunity consists of several key steps, including release of antigens from tumor beds, presentation of tumor antigens by antigen-presenting cells (APCs), priming and activation of T cells by activated APCs, migration and infiltration of effector T cells back to the tumor, and finally the recognition and killing of tumor cells by effector T cells (Fig. 1).8,9 Cancer vaccines are active immunization approaches to induce tumor-specific T cells in patients harnessing the power of the immune system against cancer, which may be developed as a prophylactic tool to prevent future development of cancer or as a therapeutic approach to boost the elimination of tumor by the immune system.10 Significant advantages of these approaches over standard therapies are their ability to 1) target and kill tumor cells in a specific manner, with minimal damage to healthy, non-tumor cells, 2) systemically stimulate anti-tumor immune responses that can prevent the metastatic spread of the disease, and 3) result in immunological memory that would alert the immune system, and provide long-term protection against possible future tumor recurrences.11
Figure 1.
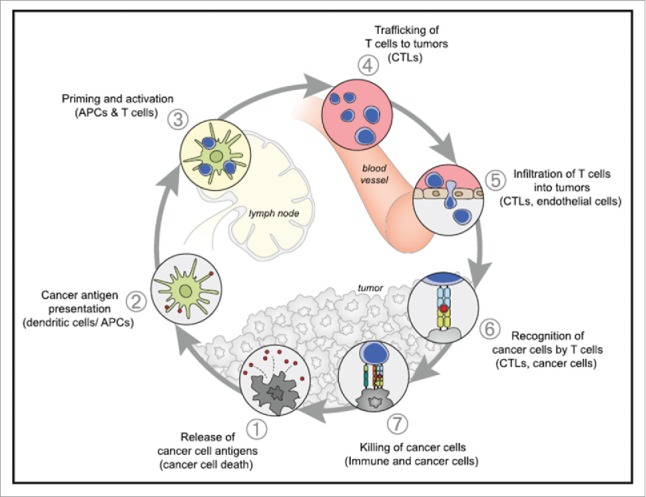
The Cancer-Immunity Cycle The generation of immunity to cancer is a cyclic process that can be self propagating, leading to an accumulation of immune-stimulatory factors that in principle should amplify and broaden T cell responses. The cycle is also characterized by inhibitory factors that lead to immune regulatory feedback mechanisms, which can halt the development or limit the immunity. This cycle can be divided into seven major steps, starting with the release of antigens from the cancer cell and ending with the killing of cancer cells. Each step is described above, with the primary cell types involved and the anatomic location of the activity listed. Abbreviations are as follows: APCs, antigen presenting cells; CTLs, cytotoxic T lymphocytes. Reprinted with permission from ref. 9.
Recent strategies for developing preventative and therapeutic vaccines have focused on the ability to deliver antigen to dendritic cells (DCs) in a targeted and prolonged manner.12 DCs are the most effective antigen-presenting cells (APCs), and have a crucial role in initiating T-cell mediated immunity. DCs can control a substantial part of the adaptive immune response by internalizing and processing antigen through MHC class I and class II pathways and, finally, presenting antigenic peptides to CD8+ and CD4+ T lymphocytes.6,13 The recognition of the crucial role of DCs in initiating anti-tumor immunity has led to the development of several strategies that target vaccine antigens to DCs as an attempt for developing potent, specific and lasting anti-tumor T cell responses.14 In this regard, there are three main categories of vaccines targeting DCs against cancer. First category includes cell-based vaccination by ex vivo strategies. This approach consists of isolating DCs from the blood of patients, exposing them to antigen and other maturation stimuli or genetically modifying them to express immunostimulatory cytokines, chemokines or growth factors, and, finally, re-injecting them into the patient.15 While these strategies show promise, the techniques used are laborious, time consuming and very expensive to carry out in large clinical trials. Second category employs the use of viral vectors to deliver vaccine antigens. Most of these are powerful activators of immune responses; however, safety concerns and problems with viral vectors include immunologic priming to the vector itself, oncogenicity due to insertional mutagenesis, difficult manufacturing, and limited cargo capacity have hindered their human application. Third category is based on non viral delivery systems. The target antigen molecule such as purified protein, peptide, and plasmid DNA will be delivered by a synthetic non viral delivery system. While these new approaches bypass many of the production difficulties associated with cellular vaccines, and there is no concern about viral vectors, a number of significant challenges need to be overcome for design of an efficient cancer vaccine. First critical feature of a therapeutic cancer vaccine is the choice of an appropriate tumor antigen. Most cancer vaccines have used tumor-associated antigens which are expressed in some normal tissues at low levels but are over-expressed in malignant cells. Expression of these antigens in normal cells can trigger tolerance mechanisms that lead to the selection of T cells with low-affinity T cell receptors (TCR).16 Therefore, a fundamental challenge with such approaches is that they require overcoming both central tolerance (whereby autoreactive T cells are deleted in the thymus during development) and peripheral tolerance (whereby mature Tcells are suppressed by regulatory mechanisms).17 Another class of tumor antigens is tumor-specific neoantigens, derived from mutated proteins. Tumor neoantigens may be ideal targets for a therapeutic vaccine because they are present in tumor cells but not normal cells, and therefore neoantigen-specific T cells are not subject to central and peripheral tolerance, and also lack the ability to induce normal tissue destruction. However, many challenges remain in producing and testing neoantigens -based vaccines customized for each patient.16,17 Tumor antigens alone are poorly immunogenic and often result in less efficient vaccines. Therefore, there is a critical need for a specific delivery system and/or adjuvant to deliver antigen to DCs more specifically and induce the subsequent activation of T-cell immunity to enhance immunogenicity. Second, the presence of the immunosuppressive factors in the tumor microenvironment may defeat or disable antitumor immune responses before clinically relevant tumor kill can occur.9 Therefore, cancer vaccine should be able to overcome this tumor- mediated immune suppression and to shift the balance back from immune suppression toward immune stimulation.18 Nanoparticle-based immunotherapy which is the focus of current review paper is a new promising approach that can satisfy these requirements for developing the next generation of cancer vaccine. In this review article, we describe several parameters that should be considered in the design of smart nanoparticle for vaccine delivery in terms of composition, the size and charge, surface modification, molecular recognition for targeting to DCs, sense biologic environment and responsive to alterations in pH, and bypass intracellular biological barriers. In addition, we have shown some examples that highlight the potential of multifunctional nanovaccines for the development of improved immunotherapies to overcome immunosuppressive network of tumor microenvironment and generate strong and long-lasting antitumor immunity.
Nanoparticle-based vaccine delivery
The use of nanoparticle to enhance the efficacy of therapeutic agents is being increasingly investigated, and many such carriers have been successfully developed.19 In tumor immunotherapy, these systems comprise three main components; first, an antigen against which the immune responses are induced. It will be peptides from, or DNA encoding tumor associated antigens (TAAs). Second, an adjuvant that acts as danger signals to alert the immune system and activate early as well as long-lasting immune responses. The third component is the delivery system that delivers vaccine antigens and adjuvants to DCs in a targeted and prolonged manner.14 The use of nanoparticle offers several advantages over conventional administration of the antigen for cancer immunotherapy include: 1) protection of the drug/antigen/adjuvant from enzymatic degradation, 2) enhanced absorption of the drug/antigen/adjuvant into targeted tumor tissue either by the enhanced permeation and retention (EPR) effect or via active targeting with the use of ligands, and 3) ability to control the pharmacokinetic and drug/antigen/adjuvant tissue distribution profile and enhance cellular uptake by DCs to trigger a strong immunostimulatory cascade.11 4) delivery systems designed to initiate immunogenic cell death or target immune checkpoint molecules can drive anti-tumoral immune responses and reverse immune suppression.8 Furthermore, these nanoscale carriers offer the unique advantage of multi-component loading, which is of considerable significance, particularly in immunotherapy where simultaneous delivery of antigens, immunoadjuvants and targeting ligands is optimal. Additionally, due to their large surface area, these nanocarriers can be surface functionalized. The fabrication of such multifunctional nanocarriers with controlled properties often requires the conjugation of proteins, peptides, polymers, cell penetrating moieties, reporter groups and other functional and targeting ligands to the carrier surface. Thus, the simplicity of design and use, coupled with multifunctionality makes nanoparticulates a versatile and attractive carrier system for tumor vaccines and immunotherapy.11 Some of these nanoparticle-based cancer vaccines are summarized in Table 1.
Table 1.
A summary of multifunctional nanoparticles for cancer immunotherapy.
| Material | Delivery agents | Size | Targeting ligand | Function | In vitro/in vivo | Ref. |
|---|---|---|---|---|---|---|
| Liposome | B16 melanoma antigens/IFN-γ or lipopolysaccharide | Not reported | CD11c-ScFv or DEC-205-ScFv | Targeted vaccine/Co-delivery of antigen and danger signal | In vitro and in vivo | 20 |
| PLGA | Melanoma antigen tyrosinase-related protein 2(TRP2); toll-like receptor (TLR) ligand(7-acyl lipid A) | 350–410 nm | No | Co-delivery of antigen and TLR4 ligand | In vitro and in vivo | 21 |
| Polybutyl cyanoacrylate (PBCA) | CMV-β-gal plasmid/TGF-β siRNA | Not reported | No | gene delivery | In vivo | 22 |
| PLGA | Tumor antigenic peptide | 150–500 nm | No | Delivery of tumor antigenic peptides | In vitro and in vivo | 23 |
| Chitosan | Interleukin-12-encoded plasmid | 103–170 nm | Mannose | Targeted gene vaccine delivery | In vitro and in vivo | 24 |
| P(MDS-co-CES) | PTX, Interleukin-12-encoded plasmid PTX, Bcl-2 siRNA | 180 nm | No | Co-delivery of gene and drug | In vitro and in vivo | 25,26 |
| PEG-AuNPs | anti-VEGF siRNA (labeled with Alexa Fluor 488) | 20–26 nm | M2 peptide (TAMs-targeting peptide) | Dual Targeted Immunotherapy | In vitro and in vivo | 27 |
Important characteristics of nanoparticle-based vaccine delivery
For decades, nanoparticles (NPs) based on biodegradable and biocompatible polymers have potential applications in cancer therapy and as sustained drug delivery vehicles.28,29 Additionally, these carriers can also be designed as low toxicity systems with suitable physical and chemical structures and specific targeting properties for delivery of cancer vaccines.11,30 To this end, understanding the interactions between nanomaterials and DCs is important in the field of immunotherapy. It is necessary to consider the properties of NPs in terms of composition, adjuvant activity, size, surface properties to fabricate vaccine delivery carriers. As aforementioned, APCs, among which DCs have been considered as the most efficient APC population, are essential for initiating and regulating vaccine-induced immune responses. Indeed, DCs are modified to present TAAs to T cells resulting in TAA-specific CTL activation. The production of such cellular immune responses firstly requires loading of the DCs with TAAs, after which the cells need to be matured, in order to become potent APCs.18 Generally, in contrast to viruses and pathogenic bacteria, tumor cells don't have any danger signal to stimulate DCs maturation. Thus, an in vivo applicable nanoparticulate system for cancer vaccination should not only deliver antigen, but also exhibit immune adjuvant effects and induce complete maturation of the antigen-loaded DCs.18 In line with this requirement, it has been demonstrated that in some cases NPs composed of biomaterials have intrinsic adjuvant activity.13 In contrast to traditional adjuvants such as alum, nanoparticle-based vaccines increase expression of MHC-I and MHC-II molecules on DCs and enhance antigen cross-presentation.31 For example, certain cationic lipids such as the DOTAP used to prepare liposomes are immunostimulatory.32 It was shown that treatment of DCs with these cationic liposomes induced both CD80/CD86 expression on DCs surface and the release of proinflammatory cytokines such as TNF-a by DCs.32 However, the adjuvant activity of NPs is depended on the physicochemical properties (e.g. the material's composition, particle size, surface charge, production methods, and additional surface modifications) and should be explored further.
Influence of particle size and charge
NPs can meet the ultimate goal of cancer vaccines by facilitating antigen presentation and T-cell activation. This is achieved by tuning the size of nanocarriers to target delivery of tumor antigens and adjuvants to APCs and lymphoid tissues. It was reported that particle size is an important factor when designing delivery systems for trafficking into the body as well as uptake by APCs such as DCs. Large particles (>500 nm in diameter) can be physically trapped at the injection site by interaction with extracellular matrix proteins, whereas ultra-small NPs (<10 nm in diameter) or soluble antigen molecules can rapidly diffuse into and out of lymph nodes, thus minimizing the chance of APCs phagocytizing sufficient amount of vaccine particles.8 For uptake into cells, particles of 500 nm or smaller were optimal for uptake by DCs and macrophages.33 However, the influence of size on the induction of immunity is not clear and may depend on the route of administration. For example, particles administered either orally or intranasally, the increased size of the particles (>500 nm) may facilitate their trapping in gut-associated lymphoid tissue or nasal-associated lymphoid tissue, thus inducing efficient mucosal responses.34 In contrast, for transport through the lymphatic vasculature, particles of an intermediate size (10–100 nm in diameter) can both efficiently drain to regional draining lymph nodes and become retained there, thereby increasing the chance of antigen uptake and presentation by APCs, provide enhanced immunogenicity compared with larger systems.8,35 It is also known that in addition to influencing the cellular uptake, particle size can also affect on the type of immune responses induced.34 There are data showing that microparticles promote humoral immune responses, whereas NPs may favor the induction of cellular immune responses. The influence of particle size on the type, level and quality of the immune response may be attributed to differences in pathways and mechanisms for cell uptake, and antigen presentation and processing. It has been reported that particles with a diameter of 500 nm or less are optimal for uptake by DCs or macrophages. Particles of 20–200 nm are generally taken up via endocytosis with subsequent inducement of CD4+ and CD8+, and Th1-type immune responses. In contrast, for particles with a dimension greater than 500 nm, uptake is via phagocytosis or micropinocytosis, leading to a humoral immune response.34 In our recent study nanovaccine have been used in two size ranges of 200 nm (N/P ratio of 10:1 MPG/DNA nanoparticle) and 700 nm (N/P ratio of 5:1 MPG/DNA nanoparticle) to immunization of mice. The results showed that the anti-tumor activity induced by the larger NPs at an N/P ratio of 5:1 was weaker than that induced by the smaller NPs at an N/P ratio of 10:1. In fact, immunization with the 200 nm NPs favored Th1 type immune responses denoted by production of IFN-γ, whereas immunization with the 700 nm particles induced a higher antibody titer.36
Along with size, charge and the nanoparticle's surface properties are also important. In general, cationic particles are taken up into DCs and macrophages much more readily than those with an overall negative surface charge due to the ionic attraction between the positively charged particles and the negatively charged cell membrane initiates efficient binding and facilitate particle internalization.14 It should be noted that uptake of cationic nanoparticle by DCs is feasible when these targeting cells are localized at the site of injection such as skin, whereas positive surface charge for lymphatic transport and nanoparticle-trafficking in vessels is problematic. Indeed, without additional surface modifications, cationic particles tend to quickly aggregate upon contact with serum proteins. This can result in premature antigen release and a change in particle size, which leads to different cellular uptake and antigen transfer kinetics.18 PEGylation, which is grafting polyethylene glycol (PEG) chains on the outer particle surface, creates a hydrophilic protective layer that can prevent nonspecific absorption of serum protein and avoid the clearance by reticuloendothelial system (RES), thereby effectively accelerating the drainage of NPs into the lymphatic system and increases the chance of nanoparticle encounters with APCs.35,37 Zhuang et al. investigated the effect of PEGylation on lymph node (LN) targeting and the immunogenicity of cationic liposome-formulated vaccines.37 In this study, particle size and zeta analysis showed that the presence of 1 or 5 mol% of DSPE-PEG2000 remarkably decreased the surface charge density of liposome without significantly changing their particle size. Moreover, in vivo results showed that PEGylation not only enhanced passive LN targeting of cationic liposomes, but also regulated the biodistribution, both of which contributed to enhanced immune responses.37
Targeted delivery to peripheral DCs
It has been shown that as long as antigens remain outside the lymphatic tissues, they will be ignored by the immune systems.38-40 Therefore, in order to induce cell mediated anti-tumor immunity via an antigen delivery system, it must find its way to the organized lymph organs such as the lymph nodes. This may be achieved by either effective delivery of the antigen to the lymph organ or by effective targeting of antigen to APCs in the periphery, along with delivery of appropriate “danger” signals to induce APCs maturation and migration to local draining lymph nodes for presentation.40,41
Most delivery systems target peripheral immature DCs in the skin, where the materials are taken up to induce DC maturation and migration to lymph nodes, where the DCs activate T cells. However, the challenge is that in the skin immature peripheral DCs are present in extremely low numbers compared with other phagocytic cells (e.g., macrophages); therefore, the ability to enhance DC-targeting specificity becomes crucial to generating a sufficient immune response.13 According to this fact, several targeting approaches are being explored to enhance the delivery of antigens to DCs. These approaches use the cell surface receptors expressed by DCs such as mannose receptor, DEC-205, CD11c, CD40 and DC-SIGN, which facilitate binding and endocytosis of targeting ligands.42 It was reported that immunization with fused protein vaccine to human mAb specific to DEC-205 has led to Ag-specific immune responses in mice.43 However, the options to link a single antibody to multiple vaccine components, such as antigens and immune modulators, are limited. Therefore, to increase the efficiency of vaccine it is desirable to encapsulate all of vaccine component within NPs and the surface of NPs can be conjugated with targeting moieties (e.g. mannose, anti CD11c and anti-DEC205) to achieve DC-specific delivery. For example, Kwon et al. conjugated anti-DEC205 on microparticles and showed that these particles are taken up by DCs three times higher than non-targeted counterparts in vivo.44 It has been shown that human and murine DCs and macrophages express mannose receptor (MR) on their surface. Several studies have confirmed the feasibility of using mannose or mannan to target protein antigens, liposomes, and other micro and NPs to APCs.41 For example, Cui et al. coated the surface of liposome-protamine-DNA (LPD) nanoparticle with mannan, significantly enhanced both preventive and therapeutic activities when mice were immunized with mannan-coated LPD/E7 than non-targeted particles.41 It is noticeable that mannosyl glycoconjugates are present on the surface of some bacteria, fungi, virus infected cells, and parasites. Thus, it also could be possible that the host immune system considered mannan as a “danger” signal and started a stronger innate immune response against the mannan-coated than the mannan-free LPD/E7. In a similar study Kim et al. employed mannosylated chitosan (MC) for IL-12 gene delivery to the DCs as a potent nanoparticle gene carrier for cancer immunotherapy. In fact, the MC/DNA complex was more efficient for transferring IL-12 gene into DCs than the chitosan/DNA complex, resulting in better induction of IFN-g and mIL-12 p70 from DCs.24
Targeting lymph node–residing DCs
A major challenge in the development of subunit vaccines is the efficient delivery of antigen/adjuvant to secondary lymphoid organs, where immune responses are organized. Antigen delivery to LNs might provide an attractive alternative to the common approach of targeting DCs in peripheral tissues such as skin. DCs are present in much higher concentration in LNs in contrast to the peripheral tissues such as skin or muscle, where DCs reside in much lower numbers and must travel to the LN after antigen uptake.45 In addition, one beneficial advantage of targeting lymph-node DCs is the prevention premature antigen presentation and avoiding antigen tolerance.
Particle size has a crucial role to passively target LNs and the DCs residing in these tissues. Reddy et al. compared the delivery of 20, 45, and 100 nm diameter PEGylated poly(propylene sulfide) (PPS) NPs to DCs in the lymph nodes.46 After intradermal injection, 20 and 45 nm particles drained effectively through lymphatic vessels to the LNs via interstitial flow and could be retained there for at least 120 h after injection, targeting half of the lymph node–residing DCs, while 100 nm particles largely remained at the injection site and were found within only 6% of DCs and could not be visualized within the draining lymph node after 24 h. These authors demonstrated that half of the lymph node–residing DCs had taken up nanopartilces without using any targeting ligands.47
In a recent work Liu et al. used albumin as a shuttle to direct tumor-targeting vaccines to LNs. Human serum albumin (HSA; 66.5 kD) is the most abundant protein with a concentration of ˜35–50 mg/mL that serves to transport fatty acids from the blood into lymphatics and to LNs. Exploiting this role of albumin, lipids containing an albumin binding domain made from a diacyl tail were conjugated to peptide antigens and CpG (a TLR9 agonist that activates TLR pathways). In this approach amphiphiles cancer vaccine (amph-vaccine) was composed of either peptide antigens or adjuvants conjugated to fatty acid tails that would bind albumin. Peptides specific to HPV-derived cervical cancer or melanoma were added to these structures and used to immunize mice after tumor inoculation. Administration of CpG-DNA/peptide amph-vaccines in mice resulted in marked increases in LN accumulation and decreased systemic dissemination relative to their parent compounds, leading to 30-fold increases in T-cell priming and enhanced anti-tumor efficacy while greatly reducing systemic toxicity.45 Of note, already HSA NPs has been used increasingly as delivery system because of their ability to bind to various drug molecules, great stability during storage, no toxicity and antigenicity, and biodegradability.48-51 Bunschoten and co-workers also used HSA-based NPs formed non-covalent self assembled complexes with indocyanine green (ICG) dyes toward tumor draining lymph nodes for imaging.52 Inspired by this strategy, through the synthesis of albumin nanoparticle via novel approaches such as self assembly, it could be possible to control the size of the nanoparticle in a way that can targeted TAAs directly toward the LN and improve the potency of cancer vaccines.
Cytosolic antigen delivery and endosomal escape
In general, non-viral delivery systems have been developed to mimic the receptor-mediated cell entry mechanism of viruses and the main mechanism of internalization is endocytosis. This pathway is composed of vesicles known as endosomes with an internal pH around 5 that mature in a unidirectional manner from early endosomes to late endosomes before fusing with intracellular organelles called lysosomes which contain certain digestive enzymes. Thus, particles entering the cells via the endocytic pathway become entrapped in endosomes and eventually end up in the lysosome, where active enzymatic degradation processes take place.53 Therefore, the entrapment of internalized peptide antigen and DNA in endocytic compartments prevents further intracellular transport toward the cytoplasm and nucleus respectively, and will often result in degradation and end up in MHC II presentation pathway, leading to the activation of Th cells. As a result, Protein and peptide based vaccines as exogenous proteins in the lysosomal compartments are cleaved to immunogenic peptides and loaded onto MHC class II molecules and presented to CD4+ T-cell result in antibody responses. For this reason, vaccination with peptide-based vaccines derived from the sequence of tumor-associated antigens typically generates only antibody-mediated (“humoral”) immune responses. However, effective cancer vaccinations require cell-mediated responses for generation of cytotoxic CD8+ T lymphocytes (CTL) cells that kill tumor cells.54 Moreover, this approach is far from optimal because recognition of these peptide epitopes alone, in the absence of co-stimulatory molecules, can lead to immunological tolerance.55 Synthetic long peptides (SLP) have been developed as a solution for these problems that have surfaced with short peptide vaccines. In this approach the potency of peptide vaccines has been improved by the conjugation of minimal TH and TC peptide to form a single linear peptide or by the conjugation of the Toll-like receptor (TLR) ligand to peptides. Indeed, an increase in the length of the peptide used for vaccination strongly affects the magnitude of the induced CD8+ cytotoxic T-cell responces.55 CTL responses will occur when antigen is presented through MHC-I pathway. Indeed, the main difference between these two pathways lies in the intracellular location for processing and loading of antigens to MHC molecules: the vacuolar pathway utilizes endosomes while the cytosolic pathway utilizes endoplasmic reticulum for formation of MHC-II/antigen peptide and MHC-I/antigen peptide complexes, respectively. Through a process known as cross presentation, exogenous antigens can escape endocytic vesicles and enter the cytoplasm where they are cleaved into peptides by the proteasome, imported into the endoplasmic reticulum and loaded onto MHC class I which present antigen to CD8+ T cells. However, APCs are not efficient in the uptake and processing of exogenous antigens via the MHC class I pathway because the lack of potency in endosomal escape.56 Thus, particular emphasis should be given to the design of nanoparticle-based delivery systems that promote antigen escape from endosomes into the cytosol to enhance MHC class I presentation. To this end, comprehensive efforts have been focused on design of smart NPs based on pH-sensitive delivery systems that can retain their cargo under the physiological pH condition while triggering release of antigens and disruption of endocytic vacuoles at the acidic (˜pH 6) endosomal microenvironment.8 For example, liposomes, which are the most used for bimolecular delivery, have been equipped with pH-responsive moieties, such as phosphatidylethanolamine (PE) or unsaturated DOPE.57 In such pH sensitive delivery systems, pH-responsive moieties trigger liposome destabilization after endocytosis and promote lipid membrane fusion under acidic conditions, releasing its content into the cytoplasm and lead to effective MHC class I presentation pathway and therefore improved CTL responses.58 An alternative approach is surface modification of liposomes with pH-sensitive fusogenic molecules such as pH-sensitive polymers or fusogenic peptides either encapsulated or incorporated in lipid bilayers. This approach might be beneficial for producing functional liposomes having both high stability and strong fusion properties. Yuba et al. developed pH-sensitive polymer-lipids that consists of pH-sensitive fusogenic polymer moieties such as 3-methyl glutarylated poly(glycidol) and 2-carboxycyclohexane-1-carboxylated poly(glycidol), connected to a phosphatidylethanolamine head group. Incorporation of these pH-sensitive polymer-lipids into egg yolk phosphatidylcholine liposomes produced highly pH-sensitive liposomes. Immunization of mice with these OVA-loaded pH-sensitive polymer-lipid-incorporated liposomes induced strong OVA specific immunity, which achieved complete rejection of OVA-expressing E.G7-OVA cells and marked regression of E.G7-OVA tumors.59
Viruses and some pathogenic bacteria have pH-sensitive surface proteins that change conformation in mildly acidic environments such as in endosomes, and exhibit membrane-disruptive (fusogenic or endosomolytic) properties. Synthetic fusogenic peptides that mimic the sequences of these natural proteins have been confirmed to increase cytoplasmic gene delivery.60 Examples of these endosome-disruptive peptides are the influenza HA2 peptide, melittin, the T-domain of the diphtheria toxin or the GALA peptide.61 In an effort for achieving both active cellular entry and endosomal escape, a packaging concept referred to as “Programmed Packaging,” in which various types of devices are incorporated into NPs was proposed. Based on this concept, multifunctional envelope-type nano-devices (MEND) were originally established for use as a plasmid DNA (pDNA) carrier. For the application of this system to siRNA delivery, nano-sized complexed cores were similarly formed with siRNA using an amphiphatic polycation (i.e. stearylated octaarginine; STR-R8), which leads to its loading in the lipid envelope by hydration methods (MENDhydo). octaarginine (R8) and the lipid composition may synergistically function in membrane fusion, which induces cellular uptake of the particle by macropinocytosis, a useful pathway that avoids lysosomal degradation. This novel delivery system was improved for intracellular trafficking of siRNA by using a pH-dependent fusogenic peptide (GALA) permits an enhanced endosomal escape in response to the low pH in endosomes. In this study it was demonstrated siRNA loaded in R8/GALA-MENDSUV efficiently suppresses endogenous gene expression and consequently enhances the potency of dendritic cell-based cancer vaccine.62
Cellular and nuclear localization of DNA vaccines
Application of gene therapy and nucleic acids in medicine holds great potential for the treatment of many different diseases such as cancer. DNA vaccination has been identified as a promising treatment strategy and may provide a solution to many technical challenges that hinder traditional vaccine systems including rapid development, production and induction of robust cell mediated immune responses. Intracellular production of antigens from DNA can result in coordinated activation of both humoral and cell-mediated responses, hence DNA vaccines potentially allow for both prophylactic and therapeutic vaccination strategies.54,63 Despite their successful applications in some preclinical models, their potency in clinical trials has been insufficient to generate effective immunity. The reasons for low immunogenicity may be related to poor delivery of DNA to APCs. These therapeutic molecules are usually unable to cross cellular barriers efficiently by passive diffusion, due to their strong negative charge, high molecular weight (MW) and hydrophilicity which make cellular membrane impermeable to them.64 Therefore, an appropriate delivery system must be able to transport plasmid DNA molecule across cellular barriers including the cellular and endosomal membrane into the nucleus for expression of protein antigen to occur. Among the different available non-viral delivery systems, cell-penetrating peptides (CPPs) represent an interesting alternative to bypass the problem of poor membrane permeability to nucleic acids. These peptides consist of less than 30 amino acids; mostly, possess cationic and hydrophobic residues that help them to establish interactions with the cell-surface negative charges.64 In addition these peptides are able to aid in the release of DNA from the endosome and target the DNA to the nucleus and allow entry through the nuclear pore complexes (NPCs).65 In our recent study, MPG peptide which is a short amphipathic peptide carrier was used for in vitro and in vivo delivery of HPV16 E7 DNA as a model antigen.36 The results of this study demonstrated several properties of MPG that propose it as an ideal vector for use in DNA vaccine delivery. As it is shown in Figure 2, MPG was able to interact and form stable non-covalent NPs with DNA through electrostatic interactions, which take place between the negative charges of the nucleic acids (phosphate groups) and the positively charged moiety of MPG. Furthermore, the condensation of DNA with MPG peptide protects DNA during formulation and preserves its structure in serum. Additionally, one of the unique features of MPG is the presence of a nuclear localization signal (NLS) which plays a crucial role in both electrostatic interactions with DNA and nuclear uptake.66 The result of this study indicated that the MPG/DNA NPs were stable in transfection media containing serum and overcame the intracellular barriers and target nucleus, which led to significant E7 antigen expression in transfected cells. In addition, in vitro experiments indicated that internalization of MPG based NPs was carried out through non-endosomal pathway, which confirmed previous reports.64 In our study, C57BL/6 mice were vaccinated twice (2 weeks interval) with the aforementioned NPs after TC-1 challenge, and the result of cytokine assay indicated that the immune response elicited by MPG based NPs was a dominant Th1 response denoted by the production of IFN-γ.36
Figure 2.
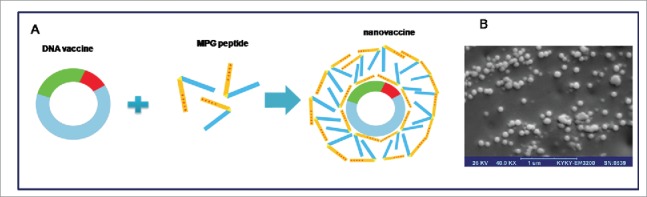
Schematic illustration of MPG-based nanovaccine (A), and The SEM micrograph of the spherical nanoparticles formed at N/P 10:1 at 20,000× magnification (B).
Multifunctional nanoparticle for targeting immune suppressive players within the tumor microenvironment
Increasing evidence indicates that during tumor development a growth-supporting microenvironment is created, which is characterized by the prevalence of many immune suppressive cell types and immunoinhibitory pathways which allow the tumor to escape from immune recognition and will support rather than suppress tumor growth.18,31 Indeed, via different processes, tumors stimulate their own growth and deeper tissue invasion. In this regard, to provide nutritional supplements, rapidly growing tumors stimulate angiogenesis by secretion of the vascular endothelial growth factor (VEGF). In addition, tumor cells down-regulate expression of surface antigens and co-stimulatory molecules, thus reducing T cell recognition and stimulation. Tumor cells also secrete immunosuppressive cytokines such as IL-10 and TGFβ creating an environment that inhibits DC maturation, thus abrogating their capacity to efficiently present antigens and induce T cell activation.67,68 Finally, cancerous tissue can also attract a number of immune suppressive cell types to the tumor microenvironment. These cells include tumor associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid derived suppressor cells (MDSCs). TAMs have been shown to promote cancer progression through the release of cytokines that induce angiogenesis, metastasis, and cell growth and can produce anti-inflammatory signals that suppress immune effectors such as natural killer (NK) cells and T cells.69,70 Suppressive DCs will even stimulate Tregs that suppress various immune cells, including cytotoxic T cells and DCs; this suppression is induced by Tregs using multiple paths of action including release of immunomodulatory factors like TGFβ, IL-10, cell-cell contact dependent molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4).71 MDSCs are aberrantly differentiated myeloid cells which secrete suppressive cytokines, and the main actions of MDSCs to promote tumor progression are impairing CTL migrationin to the tumor, reducing NK cell function, supporting TAM activity and expanding Treg populations.18,72,73 Therefore, cancer immunotherapeutic strategies demand new approaches for targeting these immune suppressive factors to control tumor microenvironment and overcome the immune suppressive condition. There are many of the immune activating factors that can be used to promote the efficiency of cancer vaccines and could improve the immunity, but systemic application of these factors generally leads to severe side effects due to unspecific immune activation. Therefore, more localized and targeted approaches are reasonable. As a result, it seems that designing multifunctional intelligent delivery systems to locally co-deliver antigens together with adjuvants can induce the effective proliferation of antitumor CTLs, their recruitment to the tumor while reducing the immune-resistant nature of the tumor microenvironment. In the next sections our aim is to describe new approaches that are being developed to target the suppressive tumor microenvironment and how NPs can be used to combine antigen and adjuvant to target and counteract tumor- mediated immune suppression and improve the immunotherapeutic outcome.
STAT3 silencing in dendritic cells
An important pathway that mediates immune suppression at the tumor microenvironment, is STAT3 (signal transducer and activator of transcription3) signaling. In tumor cells, many tumor-derived factors (TDFs) such as vascular endothelial growth factor (VEGF), IL-6, and IL-10 induce the transcriptional activity of STAT3.74 The secretion of these TDFs in tumor milieu leads to further induction of STAT3 in DCs and forces them to remain immature and suppresses their antitumor activity. STAT3 mediates tumor growth by promoting angiogenesis and hypoxia, increasing the expression of MMPs and by inducing the secretion of suppressive cytokines (e.g.,IL-10,IL6, TGF β) while reducing the production of proinflammatory cytokines (e.g.IL-12, IFN γ, TNF).18 Therefore, it seems that silencing STAT3 in DCs is beneficial for cancer immunotherapy. It was shown that STAT3 knockdown in B16 murine melanoma by siRNA polyplexes of polyethylenimine (PEI) encapsulated in PLGA NPs induces B16 cell death in vitro and in vivo. In this study, STAT3 silencing by PLGA NPs restored DC maturation and functionality as evidenced by the upregulation of CD86 expression, high secretion of TNF-α and significant allogenic T cell proliferation. Moreover, encapsulation of STAT3 siRNA in PLGA NPs significantly reduced PEI-associated toxicity on DCs.75
Plasma high-density lipoprotein (HDL) particles are taken up through scavenger receptor class B type 1 (SR-B1) that is primarily expressed in the liver and most malignant cells.76 However, SR-B1 expression in malignant cells is quite prominent. In fact, to maintain a high level of growth, tumor cells scavenge high-density lipoprotein (HDL) particles by overexpressing of this receptor.77 For example, breast cancer cells increase uptake of cholesterol esters by increasing SR-B1 expression.78 Thanks to the role of SR-B1 in HDL homing to tumor cells, Shahzad and coworkers established a novel formulation of reconstituted HDL (rHDL) NPs for selective delivery of therapeutic payloads for gene silencing of STAT3 and focal adhesion kinase (FAK), which is a critical factor for tumor cell survival, migration, and invasion.77 Over expression of FAK has been reported in colorectal, breast, ovarian, thyroid, and prostate carcinoma. In ovarian cancer patients, FAK overexpression is associated with aggressive tumor features resulting to poor overall survival.79 In this regard, systemic targeting of FAK with liposomal NPs or small molecule inhibitors has shown reduction in tumor growth and metastasis,77,80 but such approaches are not tumor-specific and could result in undesired side effects. This highlights the need for targeted delivery of these therapeutic molecules. Hence, in Shahzad and co-workers' study STAT3 or FAK siRNA targeted delivery was achieved using rHDL NPs in mouse models of ovarian and colorectal cancer and resulted in significant reduction in tumor growth and metastasis, reduction in angiogenesis, and decreased tumor cell survival without any obvious effects on other organs.77
Combination delivery of TGF-β inhibitor and IL-2 by nano liposomal polymeric gels
Transforming growth factor β (TGF-β) is one of the major negative regulatory signals produced in tumors. Secretion of inflammatory mediators such as TGF-β by the tumor cells will cause the inactivation of DCs, thus abolish their capacity to present antigens and induce Tcell activation. Besides, TGF-β decreases the number and activity of NK cells, and that reduces the activity of CTLs while increasing the number of Tregs. This cytokine has been found at high levels in a large number of different tumors. It is believed that TGF-β is essential for tumor cell growth and differentiation, as well as for maintaining an immunosuppressive environment to protect an established tumor from the host immune response, rendering it an ideal target for cancer therapies. In one study, TGF-β receptor-I inhibitor in combination with IL-2 as an immunostimulant has been used to induce tumor immunity against immunosuppressive tumor microenvironment.81 In this work Park et al. designed a multifunctional core−shell delivery system comprising nanoscale liposome encapsulated polymeric gels (nanolipogels) for co-delivery of TGF-β inhibitor (a hydrophobic small drug molecule) and IL-2 (a hydrophilic protein). To achieve sustained release of the hydrophobic drug in conjunction with encapsulated proteins, β-cyclodextrins as solubilization agent was incorporated into the interior of the liposomes.81 TGF-β inhibitor and IL-2 released from liposomal polymeric gels significantly delayed tumor growth, improved survival of tumor-bearing mice, and increased CD8+ T-cell and NK cell expansion while blocking a key immunosuppressive pathway. In this study it was demonstrated that a new biodegradable nanoparticle (120 nm) could facilitate sustained co-delivery of hydrophilic and hydrophobic immunomodulators to enhance anti-tumor activity against subcutaneous and metastatic melanomas.81
Intelligent multifunctional nanoparticle targeted to tumor-associated macrophages (TAMs)
Monocytes that are attracted into the tumor microenvironment can differentiate into macrophages which either promote or counteract tumor growth, depending on the local environment. M1 polarized macrophages (stimulated by INF γ) are tumoricidal and produce large amounts of pro-inflammatory cytokines, whereas M2 polarized macrophages, also known as tumor-associated macrophages (TAMs, differentiation upon encounter of TGF-β, IL-10,IL-4 and IL-13) will stimulate tumor progression by producing high amounts of IL-10 but not IL-12 and exhibit anti-inflammatory and tissue-repair functions.18 TAMs have been proven to be a driving force in the initiation, proliferation, metastasis and angiogenesis of various tumors. Therefore, it might be beneficial to develop a cancer immunotherapy that targets TAMs. The principle functions of IL-10 are to limit and ultimately terminate inflammatory responses. The IL-10 receptor is composed of at least 2 subunits, namely, IL-10RA and IL-10RB, and blocking IL-10RA with monoclonal antibodies could abolish all IL-10 activity. It has already been proven that CpG in addition to blocking the IL-10 pathway redirected TAMs polarization and had a potent anti-tumor effect.82 However, prolonged and high-dose systemic administration of CpG oligodeoxynucleotide (ODN) and an antibody blocking the IL-10 receptor may seriously interfere with the body's immune homeostasis and not only the immune cells in the tumor microenvironment. To solve these problems, Huang et al. developed an intelligent multifunctional delivery system responsive to the tumor microenvironment which targeted TAMs. This multifunctional nanocarrier consists three components (Fig. 3): first, CpG ODN, anti-IL-10 ODN and anti-IL-10RA ODN were used in combination as therapeutic agent to alter the phenotype of TAMs and stimulate their potential tumoricidal activity. Second, galactosylated cationic dextran (gal-C-dextran), which can associate with ODN to form stable nano-complex (GDO, gal-C-dextran+ODN), to target TAMs. Third, the pH sensitive material PEG-histidine-modified alginate (PHA) was used to combine the GDO to form PDO (PHA+gal-C-dextran+ODN), which specifically releases the GDO in the acidic microenvironment of the tumor. In the circulatory system, PHA could prolong the circulation time of PDO as a result of PEG modification. Next, due to an EPR effect, long lasting PDO particles primarily accumulated at the tumor sites. As illustrated in Figure 3, after entering the tumor site, the acidic microenvironment triggered an alteration in the charge of PHA from negative to positive, which finally led to the dissociation of PHA from the complex and exposed the galactose-labeled GDO complex. TAMs express high levels of macrophage galactose-type lectin (Mgl), which is responsible for receptor mediated endocytosis and could facilitate the uptake of nanoparticle with TAMs. The result of this study showed that this smart nanocarrier was significantly efficient in suppressing the pro-tumor functions and stimulating the anti-tumor activities of TAMs by inducing IL-12 production and inhibiting the IL-10 pathway.82 This nucleic acid drug-based immuneregulation was restricted to the tumor microenvironment and did not cause an upregulation of serum inflammatory cytokines, represents a potential therapeutic approach for current cancer immunotherapy.
Figure 3.
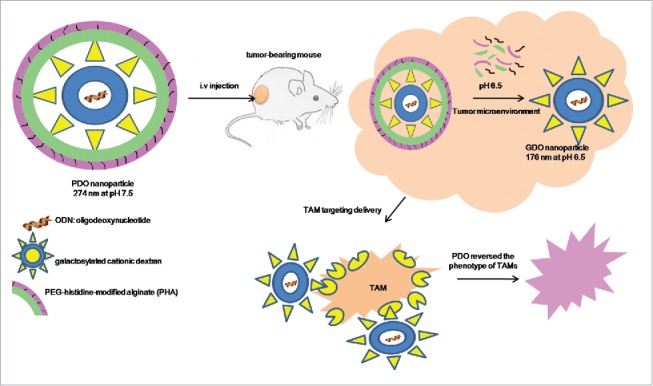
Schematic representation of the intelligent multifunctional nanoparticle targeted to tumor-associated macrophages (TAMs).
Nanoparticle for delivery of toll-like receptor ligands
Agonists for Toll-like receptors (TLRs) have been widely investigated as adjuvants for cancer vaccines. Although TLRs are mainly involved in innate immunity by sensing pathogenic danger signals, they are crucial for induction of adaptive immune responses as they can promote cross-presentation in APCs to activate CD8+ T cells or prime APCs to release cytokines that can polarize CD4+ TH cells to specific phenotypes. Since the TH1 responses elicited by activation of TLR3, TLR7, or TLR9 contribute to CD8+ T cell responses, agonists of these TLRs have been widely examined for cancer nanovaccines.8 It has been demonstrated that co-delivery of TLR ligand along with TAAs to the same DC population provides the three signals required for optimum CTL activation. DC stimulated with TLR ligand increase the expression of peptide/MHC I complex on the cell surface (signal 1), upregulate costimulatory molecules, e.g., CD40, CD80 and CD86 (signal 2), and secrete various cytokines, e.g., IL-12 (signal 3). The three signals combined lead to enhanced activation and proliferation of specific CD8+ T cell. On the other hand, TLR activated DCs are able to reverse the Treg suppressive effects resulting in breaking self-tolerance. It has been shown that IL-6 secreted by TLR4-activated DCs renders antigen specific T cells resistant to the suppressive activity of Treg.83 Hamdy et al. used PLGA NPs co-encapsulating the poorly immunogenic melanoma antigen, tyrosinase-related protein 2 (TRP2), along with Toll-like receptor 4 (TLR) ligand (7-acyl lipid A). Nanoparticle-vaccinated mice showed TRP2-specific CD8+ T cell responses capable of mediating therapeutic anti-tumor response. More importantly, this vaccine strategy led to the reversal of immune suppressive milieu of the tumor microenvironment, as evidenced by the increase in the level of pro-inflammatory T helper 1 (Th1) related cytokines (IL-2, IL-6, IL-12, TNF-α and IFN-γ) and the decrease in the level of vascular endothelial growth factor (VEGF), an immunosuppressant factor required for tumor growth.21 In a recent study, Silva et al. investigated multiple factors that have an efficient impact on the therapeutic effect of a cancer vaccine.84 These authors demonstrated that the multifunctional properties of NPs in terms of targeting, the synergy between TLR ligands and the relevance of combining multiple TAAs, including MHC class I- and class II-restricted peptides and co-entrapment of antigen/adjuvant have high cancer immunotherapeutic potential. For constructing this delivery system, two TLR ligands, Poly(I:C) and CpG, known to be Th1-immunopotentiators, were co-entrapped along with melanoma-associated antigens in mannose-functionalized aliphatic polyester-based nanoparticles (NPs). High entrapment efficiencies of antigens and immunopotentiators in 150 nm NPs were obtained. The nanoparticulate vaccines decreased the growth rate of murine B16F10 melanoma tumors in therapeutic and prophylatic settings.84 In another study Roy et al. used a TLR4 agonist as an immunostimlunat for enhancement the effect of chemotherapeutic agent. In this study paclitaxel as a cytotoxic drug was co-encapsulated with a TLR4 agonist through a PLGA based nanoparticle and evaluated its anticancer activity.85 The mean diameter of the particles was found to be 255 nm. In vivo tumor regression studies demonstrated that when paclitaxel was co-encapsulated with TLR4 agonist into PLGA NPs resulted in an improved therapeutic outcome compared to the paclitaxel alone. The mean tumor volume of the NPs treated animals was found to be 40% less than that of the Paclitaxel treated animals.85 Indeed, in the tumor microenvironment TLR4 agonist converts TAMs into M1 macrophages. On the other hand, in a synergistic manner, apoptotic bodies produced by cytotoxic activity of paclitaxel giving the immune system new targets to combat, resulting in more activation of antigen presenting cells and T cytotoxic cells.
Blockading the PD-1/PD-L1 pathway
Tumors evade the host immune attack via an immunological phenomenon termed as “tumor immune escape.” A unique feature of this phenomenon is that tumors frequently use physiological immunosuppressive mechanisms to escape from host immunity. For example, programmed cell death 1 (PD-1), an immunoinhibitory receptor belonging to the CD28/CTLA4 family expressed on activated lymphoid cells, has been found to play a critical role in the tumor immune escape. PD-1 ligand 1 and 2 (PD-Ls) expressed on APCs have been shown to induce T cell anergy or apoptosis via PD-1 on T cells. It was shown that PD-L1 is highly expressed on a broad spectrum of carcinomas but minimally expressed on adjacent normal tissue. The aberrant expression of PD-Ls on tumor cells impairs antitumor immunity, resulting in the immune evasion of the tumor cells.86 In fact, antibody blockade of PD-L1 improves T cell–mediated antitumor responses.87 Knowledge on the PD-L1:PD-1 pathway has lead to the production of NPs that interfere with this pathway. For example, linear PEI-based NPs encapsulating siRNA were used to silence PD-L1 expression on mouse tumor-associated DCs.88 In addition, in this study linear PEI was identified as a novel TLR5 agonist and indicates that activation of TLR5 and TLR7 reversed the tolerogenic phenotype of human and mouse ovarian tumor–associated DCs. The results of this study demonstrated that transforming ovarian cancer–associated DCs in vivo from an immunosuppressive to an immunostimulatory and tumoricidal cell type is not only feasible using siRNA-PEI nanocomplexes, but also more effective against aggressive ovarian tumors than what previously reported synergistic effect of standard chemotherapies combined with DC depletion.88
Concluding remarks and future perspectives
The major goal for improvement of immune response to cancer vaccines is to find strategies that can deliver antigen to DCs more specifically and induce the subsequent activation of lasting T cell immune responses against cancer antigens and at the same time be able to reverse immunosuppressive network of tumor microenvironment. In this regard, advances in antigen-delivery techniques resulted in the production of NPs to carry antigenic material toward DCs in the skin or the lymphatics, since the use of particle structures offers benefits over free antigen. One beneficial advantage of nanoparticle is that many immunomodulatory drugs have failed as systemically administered treatments, due to the severe toxicity. Nanoparticle formulations can greatly increase the localization of these drugs in target lymphoid tissues or within immune cells and thereby increase their potency as well as enhance their safety. Of note, antigens are susceptible to digestive enzymes (proteases and nucleases) in blood and interstitial fluid. Thus, encapsulating antigens within NPs protects them from premature degradation. Additionally, some biomaterials that are used as antigen carrier themselves have intrinsic immunomodulatory function, acting as adjuvants or immune potentiators. It should be noticed that potent immune responses can only be induced when antigens are presented to T cells by mature DCs, whereas antigen-presentation by their immature counterparts will rather lead to tolerance and suppression of effector antigen-specific T cells. Thus, an in vivo applicable particulate system for DC vaccination should not only deliver antigen, but also exhibit immune adjuvant effects and induce complete maturation of the antigen-loaded DCs.18 In this review, we have tried to show some examples that highlight the potential of NPs to enhance the efficacy of cancer vaccines by improving delivery, by incorporating targeting approaches and/or stimuli-responsive agents to modulate immune activation. Future studies should focus on designing of novel therapeutic strategies to deal with immunosuppressive cells and breaking immunotolerance in the tumor microenvironment. Research in this field is still in its infancy, but targeting tumor microenvironment is now possible using the nanobiotechnology approaches as number of these reports described in this review. Compared to conventional NPs delivery systems, multifunctional NPs have strong capability to achieve multiple purposes in a simultaneous manner, such as co-delivery of multiple components including TAAs coupled with adjuvant and immune potentiator, and specific targeted delivery by modification of the nanoparticle surface. These NPs are promised to manipulate the immune system through promoting effector immune cells and enhancing immune responses against cancer, and by targeting immunosuppressor components to reverse the ‘immunosuppressive milieu’ of the tumor microenvironment. Overall, it seems that in the coming years, nanobiotechnology through combination strategies could play a critical role for creating novel chemo-immunotherapy approaches to induce more long-lasting immune response and to prevent cancer recurrence and to improve the life of cancer patients.
References
- [1].Saraswathy M, Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv 2013; 31:1397-407; PMID:23800690; http://dx.doi.org/ 10.1016/j.biotechadv.2013.06.004 [DOI] [PubMed] [Google Scholar]
- [2].Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 2012; 17:1044-52; PMID:22652342; http://dx.doi.org/ 10.1016/j.drudis.2012.05.010 [DOI] [PubMed] [Google Scholar]
- [3].Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J 2006; 1:138-47; PMID:16892244; http://dx.doi.org/ 10.1002/biot.200500044 [DOI] [PubMed] [Google Scholar]
- [4].Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest 2004; 113:1515-25; PMID:15173875; http://dx.doi.org/ 10.1172/JCI21926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist updates 2011; 14:150-63; http://dx.doi.org/ 10.1016/j.drup.2011.01.003 [DOI] [PubMed] [Google Scholar]
- [6].Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev 2015; 115(19):11109-46; PMID:26154342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science 2004; 305:200-205; PMID:15247469; http://dx.doi.org/ 10.1126/science.1100369 [DOI] [PubMed] [Google Scholar]
- [8].Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines 2015; 3:662-85; PMID:26350600; http://dx.doi.org/ 10.3390/vaccines3030662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1-10; PMID:23890059; http://dx.doi.org/ 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- [10].Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol 2010; 40:2123-30; PMID:20853498; http://dx.doi.org/ 10.1002/eji.201040630 [DOI] [PubMed] [Google Scholar]
- [11].Krishnamachari Y, Geary SM, Lemke CD, Salem AK. Nanoparticle delivery systems in cancer vaccines. Pharmaceut Res 2011; 28:215-36; http://dx.doi.org/ 10.1007/s11095-010-0241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paulis LE, Mandal S, Kreutz M, Figdor CG. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol 2013; 25:389-95; PMID:23571027; http://dx.doi.org/ 10.1016/j.coi.2013.03.001 [DOI] [PubMed] [Google Scholar]
- [13].Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol 2006; 27:573-79; PMID:17049307; http://dx.doi.org/ 10.1016/j.it.2006.10.005 [DOI] [PubMed] [Google Scholar]
- [14].Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev 2011; 63:943-55; PMID:21679733; http://dx.doi.org/ 10.1016/j.addr.2011.05.021 [DOI] [PubMed] [Google Scholar]
- [15].Engleman EG. Dendritic cell-based cancer immunotherapy. Semin Oncol 2003; 30:23-29; PMID:12881809; http://dx.doi.org/ 10.1016/S0093-7754(03)00229-X [DOI] [PubMed] [Google Scholar]
- [16].Lu Y-C, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol 2015; S1044-5323(15):00073-1; PMID:26653770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res 2013: 1:11-5; PMID:24777245; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dewitte H, Verbeke R, Breckpot K, De Smedt SC, Lentacker I. Nanoparticle design to induce tumor immunity and challenge the suppressive tumor microenvironment. Nano Today 2014; 9:743-58; http://dx.doi.org/ 10.1016/j.nantod.2014.10.001 [DOI] [Google Scholar]
- [19].Davis ME, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 2008; 7:771-82; PMID:18758474; http://dx.doi.org/ 10.1038/nrd2614 [DOI] [PubMed] [Google Scholar]
- [20].van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting Dendritic Cells with Antigen-Containing Liposomes A Highly Effective Procedure for Induction of Antitumor Immunity and for Tumor Immunotherapy. Cancer Res 2004; 64:4357-65; PMID:15205352; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0138 [DOI] [PubMed] [Google Scholar]
- [21].Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008; 26:5046-57; PMID:18680779; http://dx.doi.org/ 10.1016/j.vaccine.2008.07.035 [DOI] [PubMed] [Google Scholar]
- [22].Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol 2008; 195:21-27; PMID:18304655; http://dx.doi.org/ 10.1016/j.jneuroim.2007.12.005 [DOI] [PubMed] [Google Scholar]
- [23].Ma W, Chen M, Kaushal S, McElroy M, Zhang Y, Ozkan C, Bouvet M, Kruse C, Grotjahn D, Ichim T. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine 2012; 7:1475-87; PMID:22619507; http://dx.doi.org/ 10.2147/IJN.S29506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim TH, Jin H, Kim HW, Cho M-H, Cho CS. Mannosylated chitosan nanoparticle–based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther 2006; 5:1723-32; PMID:16891458; http://dx.doi.org/ 10.1158/1535-7163.MCT-05-0540 [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Gao S, Ye W-H, Yoon HS, Yang Y-Y. Co-delivery of drugs and DNA from cationic core–shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater 2006; 5:791-96; PMID:16998471; http://dx.doi.org/ 10.1038/nmat1737 [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Wang L-S, Goh S-H, Yang Y-Y. Synthesis and characterization of cationic micelles self-assembled from a biodegradable copolymer for gene delivery. Biomacromolecules 2007; 8:1028-37; PMID:17298094; http://dx.doi.org/ 10.1021/bm061051c [DOI] [PubMed] [Google Scholar]
- [27].Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, Byrne HJ, Artzi N, Tian F. Dual Targeted Immunotherapy via In Vivo Delivery of Biohybrid RNAi‐Peptide Nanoparticles to Tumor‐Associated Macrophages and Cancer Cells. Adv Func. Mater 2015; 25:4183-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces 2010; 75:1-18; http://dx.doi.org/ 10.1016/j.colsurfb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- [29].Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Future Med 2007; 2(5):669-80. [DOI] [PubMed] [Google Scholar]
- [30].Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, Sadat SM. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother 2014; 10:321-32; PMID:24128651; http://dx.doi.org/ 10.4161/hv.26796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Andorko JI, Hess KL, Jewell CM. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J 2014; 17:323-38; PMID:25533221; http://dx.doi.org/ 10.1208/s12248-014-9708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 2013; 3: 13-13; PMID:23532930; http://dx.doi.org/ 10.3389/fcimb.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li X, Sloat BR, Yanasarn N, Cui Z. Relationship between the size of nanoparticles and their adjuvant activity: data from a study with an improved experimental design. Eur J Pharm Biopharm 2011; 78: 107-16; PMID:21182941; http://dx.doi.org/ 10.1016/j.ejpb.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oyewumi M, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines 2010; 9:1095-107; PMID:20822351; http://dx.doi.org/ 10.1586/erv.10.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. Future Med 2008; 3(3):343-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saleh T, Bolhassani A, Shojaosadati SA, Aghasadeghi MR. MPG-based nanoparticle: An efficient delivery system for enhancing the potency of DNA vaccine expressing HPV16E7. Vaccine 2015; 33:3164-70; PMID:26001433; http://dx.doi.org/ 10.1016/j.vaccine.2015.05.015 [DOI] [PubMed] [Google Scholar]
- [37].Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, Liu F, Cai L. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J Control Release 2012; 159:135-42; PMID:22226776; http://dx.doi.org/ 10.1016/j.jconrel.2011.12.017 [DOI] [PubMed] [Google Scholar]
- [38].Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kündig T, Hengartner H. Antigen localisation regulates immune responses in a dose‐and time‐dependent fashion: a geographical view of immune reactivity. Immunol Rev 1997; 156:199-209; PMID:9176709; http://dx.doi.org/ 10.1111/j.1600-065X.1997.tb00969.x [DOI] [PubMed] [Google Scholar]
- [39].Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A 1999; 96:2233-8; PMID:10051624; http://dx.doi.org/ 10.1073/pnas.96.5.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dileo J, Banerjee R, Whitmore M, Nayak JV, Falo LD, Huang L. Lipid–protamine–DNA-mediated antigen delivery to antigen-presenting cells results in enhanced anti-tumor immune responses. Mol Ther 2003; 7:640-8; PMID:12718907; http://dx.doi.org/ 10.1016/S1525-0016(03)00064-9 [DOI] [PubMed] [Google Scholar]
- [41].Cui Z, Han S-J, Huang L. Coating of mannan on LPD particles containing HPV E7 peptide significantly enhances immunity against HPV-positive tumor. Pharmaceut Res 2004; 21:1018-25; http://dx.doi.org/ 10.1023/B:PHAM.0000029292.66792.4f [DOI] [PubMed] [Google Scholar]
- [42].Joshi MD, Unger WJ, Storm G, van Kooyk Y, Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release 2012; 161:25-37; PMID:22580109; http://dx.doi.org/ 10.1016/j.jconrel.2012.05.010 [DOI] [PubMed] [Google Scholar]
- [43].Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, Schuler G, Schaft N, Dörrie J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II–restricted antigen presentation. Blood 2010; 116:2277-85; PMID:20566893; http://dx.doi.org/ 10.1182/blood-2010-02-268425 [DOI] [PubMed] [Google Scholar]
- [44].Kwon YJ, James E, Shastri N, Fréchet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci U S A 2005; 102:18264-8; PMID:16344458; http://dx.doi.org/ 10.1073/pnas.0509541102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014; 507:519-22; PMID:24531764; http://dx.doi.org/ 10.1038/nature12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly (propylene sulfide) nanoparticles. J Control Release 2006; 112:26-34; PMID:16529839; http://dx.doi.org/ 10.1016/j.jconrel.2006.01.006 [DOI] [PubMed] [Google Scholar]
- [47].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 2007; 25:1159-64; PMID:17873867; http://dx.doi.org/ 10.1038/nbt1332 [DOI] [PubMed] [Google Scholar]
- [48].Kouchakzadeh H, Safavi MS, Shojaosadati SA. Chapter Four-Efficient Delivery of Therapeutic Agents by Using Targeted Albumin Nanoparticles. Adv Protein Chem Struct Biol. 2015; 98:121-43; PMID:25819278; http://dx.doi.org/ 10.1016/bs.apcsb.2014.11.002 [DOI] [PubMed] [Google Scholar]
- [49].Elsadek B, Kratz F. Impact of albumin on drug delivery—New applications on the horizon. J Control Release 2012; 157:4-28; PMID:21959118; http://dx.doi.org/ 10.1016/j.jconrel.2011.09.069 [DOI] [PubMed] [Google Scholar]
- [50].Kouchakzadeh H, Shojaosadati SA, Tahmasebi F, Shokri F. Optimization of an anti-HER2 monoclonal antibody targeted delivery system using PEGylated human serum albumin nanoparticles. Int J Pharm 2013; 447:62-9; PMID:23454849; http://dx.doi.org/ 10.1016/j.ijpharm.2013.02.043 [DOI] [PubMed] [Google Scholar]
- [51].Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 2012; 157:168-82; PMID:21839127; http://dx.doi.org/ 10.1016/j.jconrel.2011.07.031 [DOI] [PubMed] [Google Scholar]
- [52].Bunschoten A, Buckle T, Kuil J, Luker GD, Luker KE, Nieweg OE, van Leeuwen FW. Targeted non-covalent self-assembled nanoparticles based on human serum albumin. Biomaterials 2012; 33:867-75; PMID:22024362; http://dx.doi.org/ 10.1016/j.biomaterials.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bolhassani A, Saleh T. Challenges in Advancing the Field of Cancer Gene Therapy: An Overview of the Multi-Functional Nanocarriers. Intech Open Access Publisher; 2013. [Google Scholar]
- [54].Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv Mater 2009; 21:847-67; http://dx.doi.org/ 10.1002/adma.200801478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Melief CJ, van der Burg SH. Immunotherapy of established (pre) malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-60; PMID:18418403. [DOI] [PubMed] [Google Scholar]
- [56].Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. Fut Med 2008; 343-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliv Rev 2007; 59:718-28; PMID:17683826; http://dx.doi.org/ 10.1016/j.addr.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 2005; 4:145-60; PMID:15688077; http://dx.doi.org/ 10.1038/nrd1632 [DOI] [PubMed] [Google Scholar]
- [59].Yuba E, Kono Y, Harada A, Yokoyama S, Arai M, Kubo K, Kono K. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials 2013; 34:5711-21; PMID:23639528; http://dx.doi.org/ 10.1016/j.biomaterials.2013.04.007 [DOI] [PubMed] [Google Scholar]
- [60].Du FS, Wang Y, Zhang R, Li ZC. Intelligent nucleic acid delivery systems based on stimuli-responsive polymers. Soft Matter 2010; 6:835-48; http://dx.doi.org/ 10.1039/B915020J [DOI] [Google Scholar]
- [61].Vercauteren D, Rejman J, Martens TF, Demeester J, De Smedt SC, Braeckmans K. On the cellular processing of non-viral nanomedicines for nucleic acid delivery: Mechanisms and methods. J Control Release 2012; 161:566-81; PMID:22613879; http://dx.doi.org/ 10.1016/j.jconrel.2012.05.020 [DOI] [PubMed] [Google Scholar]
- [62].Akita H, Kogure K, Moriguchi R, Nakamura Y, Higashi T, Nakamura T, Serada S, Fujimoto M, Naka T, Futaki S. Nanoparticles for ex vivo siRNA delivery to dendritic cells for cancer vaccines: programmed endosomal escape and dissociation. J Control Release 2010; 143:311-7; PMID:20080139; http://dx.doi.org/ 10.1016/j.jconrel.2010.01.012 [DOI] [PubMed] [Google Scholar]
- [63].Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer 2008; 8:108-20; PMID:18219306; http://dx.doi.org/ 10.1038/nrc2326 [DOI] [PubMed] [Google Scholar]
- [64].Saleh T, Bolhassani A, Shojaosadati SA, Hosseinkhani S. Evaluation of Cell Penetrating Peptide Delivery System on HPV16E7 Expression in Three Types of Cell Line. Iran J Biotech 2015; 13:55-62; http://dx.doi.org/ 10.15171/ijb.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J 2007; 9:18-29; http://dx.doi.org/ 10.1208/aapsj0901003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide‐based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res 2003; 31:2717-24; PMID:12771197; http://dx.doi.org/ 10.1093/nar/gkg385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21:137-48; PMID:15308095; http://dx.doi.org/ 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- [68].Bhutia SK, Mallick SK, Maiti TK. Tumour escape mechanisms and their therapeutic implications in combination tumour therapy. Cell Biol Int 2010; 34:553-63; PMID:20384587; http://dx.doi.org/ 10.1042/CBI20090206 [DOI] [PubMed] [Google Scholar]
- [69].Weigert A, Sekar D, Brüne B. Tumor-associated macrophages as targets for tumor immunotherapy. Fut Med 2009; 1(1):83-95. [DOI] [PubMed] [Google Scholar]
- [70].Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86:1065-73; PMID:19741157; http://dx.doi.org/ 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- [71].Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des 2009; 15:1879-92; PMID:19519430; http://dx.doi.org/ 10.2174/138161209788453211 [DOI] [PubMed] [Google Scholar]
- [72].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006; 16(1):53-65; PMID:16168663; http://dx.doi.org/ 10.1016/j.semcancer.2005.07.005 [DOI] [PubMed] [Google Scholar]
- [74].Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7:41-51; PMID:17186030; http://dx.doi.org/ 10.1038/nri1995 [DOI] [PubMed] [Google Scholar]
- [75].Alshamsan A, Haddadi A, Hamdy S, Samuel J, El-Kadi AO, Uludag H, Lavasanifar A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol Pharm 2010; 7:1643-54; PMID:20804176; http://dx.doi.org/ 10.1021/mp100067u [DOI] [PubMed] [Google Scholar]
- [76].Connelly MA, Williams DL. SR‐BI and HDL cholesteryl ester metabolism. Endocr Res 2004; 30:697-703; PMID:15666814; http://dx.doi.org/ 10.1081/ERC-200043979 [DOI] [PubMed] [Google Scholar]
- [77].Shahzad MM, Mangala LS, Han HD, Lu C, Bottsford-Miller J, Nishimura M, Mora EM, Lee J-W, Stone RL, Pecot CV. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia 2011; 13:309-IN8; PMID:21472135; http://dx.doi.org/ 10.1593/neo.101372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pussinen PJ, Lindner H, Glatter O, Reicher H, Kostner GM, Wintersperger A, Malle E, Sattler W. Lipoprotein-associated α-tocopheryl-succinate inhibits cell growth and induces apoptosis in human MCF-7 and HBL-100 breast cancer cells. BBA-Mol Cell Biol L 2000; 1485:129-44 [DOI] [PubMed] [Google Scholar]
- [79].Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol 2004; 165:1087-95; PMID:15466376; http://dx.doi.org/ 10.1016/S0002-9440(10)63370-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Halder J, Kamat AA, Landen CN, Han LY, Lutgendorf SK, Lin YG, Merritt WM, Jennings NB, Chavez-Reyes A, Coleman RL. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res 2006; 12:4916-24; PMID:16914580; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Limon PL. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 2012; 11:895-905; PMID:22797827; http://dx.doi.org/ 10.1038/nmat3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Huang Z, Zhang Z, Jiang Y, Zhang D, Chen J, Dong L, Zhang J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J Control Release 2012; 158:286-92; PMID:22119956; http://dx.doi.org/ 10.1016/j.jconrel.2011.11.013 [DOI] [PubMed] [Google Scholar]
- [83].Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 2003; 299:1033-6; PMID:12532024; http://dx.doi.org/ 10.1126/science.1078231 [DOI] [PubMed] [Google Scholar]
- [84].Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Préat V, Florindo HF. In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model. J Control Release 2015; 198:91-103; PMID:25483429; http://dx.doi.org/ 10.1016/j.jconrel.2014.11.033 [DOI] [PubMed] [Google Scholar]
- [85].Roy A, Singh MS, Upadhyay P, Bhaskar S. Nanoparticle mediated co-delivery of paclitaxel and a TLR-4 agonist results in tumor regression and enhanced immune response in the tumor microenvironment of a mouse model. Int J Pharm 2013; 445:171-80; PMID:23376226; http://dx.doi.org/ 10.1016/j.ijpharm.2013.01.045 [DOI] [PubMed] [Google Scholar]
- [86].Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci 2007; 104:3360-5; PMID:17360651; http://dx.doi.org/ 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brown JA, Dorfman DM, Ma F-R, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170:1257-66; PMID:12538684; http://dx.doi.org/ 10.4049/jimmunol.170.3.1257 [DOI] [PubMed] [Google Scholar]
- [88].Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest 2009; 119:2231; PMID:19620771. [DOI] [PMC free article] [PubMed] [Google Scholar]


