ABSTRACT
Chronic wasting disease (CWD) is a geographically expanding prion disease of wild and captive cervids in North America. Disease can be transmitted directly, animal to animal, or indirectly via the environment. CWD contamination can occur residually in the environment via soil, water, and forage following deposition of bodily fluids such as urine, saliva, and feces, or by the decomposition of carcasses. Recent work has indicated that plants may even take up prions into the stems and leaves. When a carcass or gut pile is present in the environment, a large number of avian and mammalian species visit and consume the carrion. Additionally, predators like coyotes, likely select for disease-compromised cervids. Natural cross-species CWD transmission has not been documented, however, passage of infectious prion material has been observed in the feces of crows. In this study we evaluated the ability of CWD-infected brain material to pass through the gastrointestinal tract of coyotes (Canis latrans) following oral ingestion, and be infectious in a cervidized transgenic mouse model. Results from this study indicate that coyotes can pass infectious prions via their feces for at least 3 days post ingestion, demonstrating that mammalian scavengers could contribute to the translocation and contamination of CWD in the environment.
Keywords: chronic wasting disease, coyotes, environmental contamination, feces, prions, scavengers, transmission
Introduction
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy, or prion disease, of deer (Odocoileus virginianus and O. hemionus), elk (Cervus elaphus) and moose (Alces alces). First identified in 1967 in a wildlife research center in Colorado, CWD is the only prion disease enzootic to wild animals.1 Transmission of CWD can occur directly, animal to animal,2 or indirectly through the environment.3 Contamination of the environment can occur by deposition of bodily fluids4-6 or by decay of infected carcasses.3 Ingestion or inhalation of contaminated soil particles can also lead to disease transmission.7,8
Each year the number of states reporting incidences of CWD in captive or wild cervid populations increases. Currently, 21 states have been affected (http://www.cwd-info.org/index.php/fuseaction/about.map). In some regions the spread has been contiguous, such as that seen in Colorado, Wyoming, and Nebraska. Other incidences are far removed from known CWD-positive foci. The mechanisms for this expansion are unclear, and likely vary by circumstance. Several human behaviors, such as movement of captive cervids9 and the dumping of CWD-positive carcasses from hunter kills in CWD-negative regions,10 have likely contributed to the expansion, but may not explain all incidences.
The role scavengers play in the spread of the disease has been evaluated primarily from a cross-species transmission aspect.11,12 A wide variety of avian and mammalian scavenger species have been documented to feed upon deer carcasses and gut piles.12 The array of tissues that contain CWD include brain, eyes, lymph nodes, neural tissue, heart, spleen, and muscle,13-17 and are all readily accessible in both carcasses and gut piles. Common scavengers from CWD-enzootic areas (raccoons (Procyon lotor), opossums (Didelphis virginiana) and coyotes (Canis latrans)) have been evaluated for the presence of CWD in their tissues, but no evidence of CWD was detected, suggesting that they do not play a direct role in transmission or become infected.11 They may, however, play a more indirect role. Recent work demonstrated that infectious mouse-adapted scrapie prions could be viably passed in the feces of crows (Corvus brachyrrhynchos) after ingestion.10 Deposition of infectious feces from scavengers could then be another unexplored mode for environmental contamination. Mammalian scavengers, such as coyotes, are of particular interest in western states such as Colorado and Wyoming, where there are both a high number of CWD-infected deer and elk, and coyotes.
Coyotes are opportunistic and widespread carnivores found throughout much of North America and everywhere CWD is enzootic in the wild.18 Their diet is composed primarily of rodents and lagomorphs, however, diet composition can vary seasonally and by geographical location and include ungulates.19,20 For example, coyotes in the Black Hills of South Dakota feed preferentially on white-tailed deer (Odocoileus virginianus) throughout the year, with the highest consumption occurring (72%) in the winter.21 In addition to predation, coyotes will opportunistically forage on carcasses and entrails left by human hunters.22-24 Both predation and scavenging exposes coyotes to CWD in affected regions.
Little is known about the degradation of CWD-infected tissue and infectivity after passage through the gastrointestinal tract of mammalian scavengers. In this study we investigated the potential for coyotes to translocate infectious CWD prions, via feces, after oral consumption of CWD-infected elk brain, utilizing a cervidized transgenic mouse bioassay.
Methods and Materials
Coyotes
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the USDA National Wildlife Research Center in accordance with the USDA Animal Welfare Act Regulation. CFR, title 9, chapter 1, subchapter A, parts 1-4. Six coyotes, 2 males and 4 females, was transported from the National Wildlife Research Center (NWRC) field station in Logan, Utah to the NWRC headquarters in Fort Collins, Colorado. Upon arrival, coyotes were individually housed in outdoor kennels with den boxes for 3 weeks to allow time for acclimation. During this time, feces was collected from each coyote for pre-exposure controls and frozen at −80°C. Coyotes were given water ad libitum and were fed dry dog food once a day. Prior to initiation of the study, coyotes were given a small amount of CWD-negative elk brain homogenate to test for palatability. The coyotes readily ate the homogenate when housed in their outdoor enclosures.
Two coyotes were placed in the control group and remained in their outdoor kennels, while the remaining 4 were transported to indoor wire runs (Table 1). After DPI 5 fecal collection, coyotes were sedated intramuscularly with ketamine and xylazine, then euthanized intravenously with euthanasia solution. Complete necropsies were conducted, and the brain and lymph nodes placed in 10% buffered formalin.
TABLE 1.
Coyote number, sex, age and treatment group
| Coyote Number | Sex | Age (yrs) | Treatment Group |
|---|---|---|---|
| 132 | Male | 6 | CWD Negative Brain |
| 134 | Female | 10 | CWD Negative Brain |
| 133 | Male | 10 | CWD Positive Brain |
| 135 | Female | 2 | CWD Positive Brain |
| 136 | Female | 6 | CWD Positive Brain |
| 137 | Female | 2 | CWD Positive Brain |
Inoculum Preparation
CWD-negative and positive 10% elk brain homogenates (wt/vol) were prepared using 1X phosphate buffered saline (PBS) (Invitrogen) in a blender, then aliquoted into 50 ml volumes and stored at −80°C until needed. The CWD-negative elk brain was an archived sample from a captive cull and the CWD-positive elk brain was an archived sample from a terminally ill captive elk. The CWD status of the elk brains was verified by Western blot as previously described.8 CWD-negative brain was prepared first in a new blender, then the CWD-positive inoculum was prepared.
Oral Inoculation
After being moved indoors for biosecurity reasons, treatment coyotes were given an acclimation period of 2 days before the start of the experiment. Dry diet was removed 12 hours prior to introduction of brain homogenate inoculum. A 50 ml aliquot of elk brain homogenate (normal or infected) was thawed for each coyote and placed in a clean bowl and mixed with approximately 1 g of red glitter to help visualize passage time through the alimentary canal. Relocation from outdoor to indoor kennels affected the coyotes' willingness to eat the brain homogenate. The addition of a small amount of diced raw chicken, or in one case, wet fish-flavored cat food was required to get them to eat. The afternoon after ingestion of the brain homogenate, they received dry dog food.
Feces Collection
Feces from all coyotes was collected the morning following elk brain homogenate consumption and at 6 time points: one day prior to the initiation of the study, and for 5 consecutive days following inoculum ingestion. After collection, fecal samples were frozen at −80°C.
Feces Protein Misfolding Cyclic Amplification (PMCA)
A 10% fecal homogenate was generated with 200 mg of feces placed into a 1.5 ml tube with 2.5 mm glass beads (BioSpec), and 1 ml of PMCA buffer (150 mM NaCl, 4 mM EDTA, in 1X PBS), then homogenized in a Blue Bullet homogenizer (Next Advance) for 2-4 min. Once homogenized, samples were centrifuged at 12,000 rpm for 20 sec, then 600 µl of supernatant was removed and mixed with 600 µl of the above PMCA buffer with 2% triton-X (Sigma Aldrich) added. Samples were mixed well and shaken on a heat block at 37°C for 20 min at 800 rpm, then centrifuged for 5 min at 2000 rpm. Supernatant was removed and stored at −80°C. To help develop an appropriate bioassay design, fecal samples were assessed for proteinase K resistance, a marker for infectivity, by PMCA as previously described by Pulford, et al.5 Amplified samples were visualized by western blot as previously described.25
Coyote Immunohistochemistry
At necropsy, retropharyngeal and mesenteric lymph nodes were preserved in 10% buffered formalin. One week after collection, tissues were placed in plastic cassettes and allowed to fix for an additional 2 days. Tissue slices 5 µm thick were mounted on positively charged glass slides (Fisher Scientific) for visualization and evaluation.26 Antigen retrieval with formic acid and hydrated autoclaving was performed prior to visualization of PrPCWD, a biomarker for CWD, by staining with F99/97.6.1 antibody. Following antibody, slides were by incubated with alkaline phosphatase-conjugated anti-mouse IgG secondary antibody and visualized using an automated immunostainer and an alkaline phosphatase red kit (Ventana). Slides were counterstained for 4 minutes with hematoxylin at 37°C. PrPCWD was visualized as brownish granular staining.
TG12 Transgenic Mice
To test the infectivity of the coyote feces, a transgenic mouse bioassay was conducted in which 77 transgenic cervidized TG12 mice of both sexes, between 2 and 5 months of age, were inoculated intracerebrally with coyote feces homogenate at 4 time points: 1- prior to ingestion of inoculum, 2- one day after ingestion, 3- two days after ingestion, 4- three days after ingestion (Table 2). TG12 transgenic mice were generated as previously described,27 and express the elk prion protein at twice the level of mouse prion protein in the FVB background strain.
TABLE 2.
Transgenic mouse bioassay results. The number of mice that died from CWD/total number of mice intracerebrally inoculated per group with coyote feces. Pre-ingestion indicates fecal samples collected prior to oral ingestion of CWD-positive elk brain homogenate, and days 1, 2, and 3 after ingestion. Day 1 post-ingestion resulted in 23% of the mice becoming terminally ill, day 2, 38% and day 3, 38%
| Coyote Number | CWD Status | Pre-Ingestion | Day 1 Post Ingestion | Day 2 Post Ingestion | Day 3 Post Ingestion |
|---|---|---|---|---|---|
| 132 | Control | 0/3 | 0/3 | 0/3 | 0/3 |
| 134 | Control | 0/3 | 0/3 | 0/3 | 0/3 |
| 133 | CWD | 0/3 | 1/3 | 0/4 | 0/4 |
| 135 | CWD | 0/3 | 0/4 | 0/4 | 0/4 |
| 136 | CWD | 0/3 | 0/3 | 3/4 | 3/3 |
| 137 | CWD | 0/3 | 2/2 | 3/4 | 2/2 |
Feces Preparation and Inoculation of Transgenic Mice
A 10% fecal homogenate (wt/vol) was generated in the same fashion as the brain homogenate with the exception of utilizing DI water instead of PMCA buffer, with 100 units/mL penicillin and 100 µg/mL streptomycin (Invitrogen) added. Inoculum was allowed to incubate at room temperature for 30 minutes, then inoculum was sonicated in a 3000 MP water bath sonicator (Misonix) for 30 seconds at power 70 prior to intracerebral inoculation. Mice were anesthetized with isoflurane gas until unresponsive to toe pinch. An insulin syringe was employed to intracerebrally inoculate 30 µl of the feces inoculum, 3 mm deep through the coronal suture, 3-5 mm lateral of the sagittal suture.
When mice presented with severe ataxia or reached 405 DPI they were euthanized.
Results
Coyotes
Both control coyotes, and coyote #135 readily ate the brain homogenate. Two others consumed the homogenate after mixing it with a small amount of diced raw chicken and the fourth after mixing the homogenate with a tablespoon of fish-flavored wet cat food.
Treatment coyote #137 did not defecate on days post inoculation (DPI) 2, therefore the data reflects feces from DPI 3 and 4. Red glitter was utilized to give a general idea of the passage time from ingestion to defecation and was observable in feces on DPI 1 and 2.
Protein Misfolding Cyclic Amplification (PMCA)
To ascertain the appropriate number of days of feces collection to test in the transgenic mouse bioassay, PMCA was conducted to amplify minute levels of PK-resistant prions from the coyotes feces from each of the 6 collection time points. All fecal samples collected prior to ingestion were negative after 6 rounds of PMCA, as were all of the samples from coyotes in the control group (data not shown). The four coyotes fed CWD-positive brain homogenate had prion amplification on DPI 1, and only one coyote, #137, had amplification on DPI 2. No signal was detected in DPI3-5 (data not shown). Based on this information, the bioassay was designed to assess feces from DPI 1-3.
Coyote Immunohistochemistry
Immunohistochemistry for protease-resistant prions was conducted on head and mesenteric lymph nodes from the study coyotes to look for residual inoculum, and no evidence of CWD prions was detected in the tissues (Fig. 1).
Figure 1.
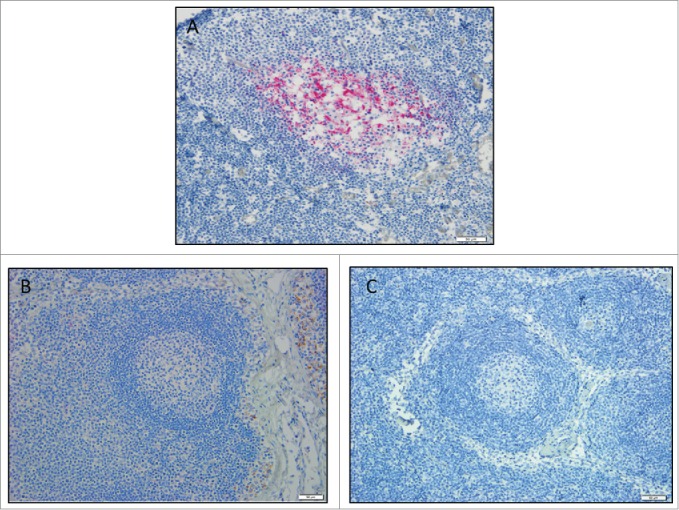
Coyote lymph node immunohistochemistry. Images are a representation of findings. (A) CWD-positive control elk retropharyngeal lymph node. Control coyote (B), and treatment coyote (C), retropharyngeal lymph node. 20X magnification.
Transgenic Mouse Bioassay
The transgenic mouse bioassay revealed that feces from coyotes fed infectious brain material could pass infectivity for at least 3 days after ingestion. Mice were euthanized at the presentation of severe ataxia, and disease was confirmed by western blot (data not shown). Several mice proved to be transgenic knockouts when their genetics were rechecked at the end of the study. As a results, these mice were excluded from results as they do not become sick in the absence of the prion protein. No mice became sick after being inoculated with pre-exposure feces. Half of the 4 coyotes fed infectious elk brain homogenate passed infectivity on DPI 1, 2 and 3. It is interesting to note that passage of infectivity varied greatly between animals, with one animals passing disease on only DPI 1, while one only began to pass infectivity on DPI 2 and 3, one coyote passed infectivity on all 3 days, however, this animals did not defecate on DPI 2 and feces collected represents 3 and 4 days after ingestion. And finally, one coyote, did not pass infectivity on any of the days (Table 2, Figure 2).
Figure 2.
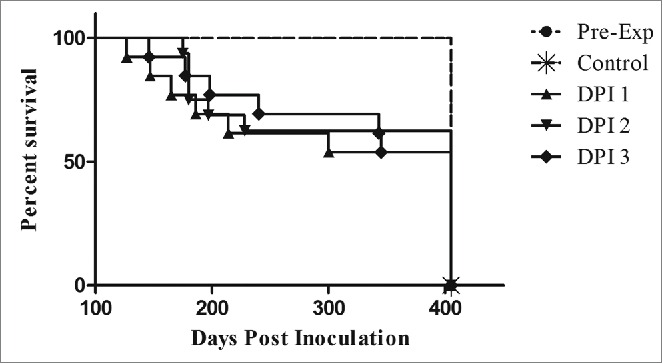
Transgenic mouse bioassay of coyote feces. All control mice and mice inoculated with feces collected prior to ingestion of CWD-positive elk brain remained disease-free for the duration of the study. Deaths occurred in all of the DPI tested, however, disease penetrance was incomplete. Mice inoculated with DPI 3 feces lived slightly longer than DPI 1 and 2. Each DPI group represented above combines survival times of mice from each of the study coyotes.
No significant difference was seen in mouse survival times between DPI 1, 2, and 3 (one way ANOVA, p = 0.1212), however, the study mice lived significantly longer (Student's T-test, p= <0.0001) than the documented time after IC inoculation with a 1:100 dilution of infected elk brain (118 ± 6 DPI), suggesting a lower infectious dose present in the fecal samples.27 The study mice lived an average of 214 days, with a large variability in survival days (± 87 days).
Discussion
The continued spread of CWD is of concern to the health of both wild and captive cervid populations. Indirect transmission through the environment has been demonstrated in captive animals living in paddocks where CWD-positive animals had lived,3 and is a particular challenge due to the long persistence of CWD within the environment.7,28 Infectious material can be deposited in the environment by the decay of infected carcasses, from urine, feces, and saliva,5,6,29 and the spread of infected material may be aided by scavengers and predators. In this study we illustrated the ability of coyotes to pass infectivity in their feces after the ingestion of CWD-infected brain homogenate.
Coyotes have the ability to travel significant distances. This distance, however, is based upon social structure, which is generally placed in 2 categories; resident or transient.30 Resident animals are those that utilize a specific territory and are comprised of a mated pair and sometimes pups from a previous year, while transient animals are individuals that are nomadic, more commonly male, and have no affinity for a specific territory.30 In a study evaluating the range of coyotes in southern Colorado, transient animals, which represented 22% of the population, ranged over 106.5 ± 27 km2, versus resident groups which ranged over 11.3 ± 5.8 km.2,30 Transient coyotes are therefore provided an opportunity to translocate disease to previously CWD-negative localities.
Control coyotes readily consumed the homogenized elk brain. Of the treatment coyotes, which were moved indoors 2 days prior to the initiation of the study, only one (#135) immediately ate the brain homogenate. The other coyotes required supplementation with diced, raw chicken, or fish-flavored soft cat food. Although the numbers are too small to come to any definitive conclusions, it is interesting to note that the coyote that ingested the brain homogenate without chicken or cat food supplementation did not appear to transfer infectivity to any of the mice in the bioassay. Neither age nor sex appeared to have any effect on fecal shedding. However, it is possible that individual variation within the stomach environment, such as pH and flora could have influenced the passage of the infectious prions through the gastrointestinal tract.
Our experimental design was based on detection of CWD in coyote feces by PMCA prior to initiation of the bioassay. PMCA was able to repeatedly detect the presence of proteinase K-resistant prions signal in feces from DPI 1, so the bioassay was designed to evaluate feces for 2 days following, to account for any uncertainty in prion detection in feces. Results from the bioassay showed transmission of disease to 2/4 mouse groups in DPI 3, suggesting that infectivity may continue to be present in the feces more than 3 days after ingestion. We were unable to go back and increase the bioassay to include DPI 4 and 5, due to logistical reasons.
The 50 mL oral dose ingested by coyotes in this study was comprised solely of infected brain tissue and represented a high dose. In the wild, coyotes would opportunistically consume a wide variety of tissues from a kill or scavenged deer or elk carcass, likely making their actual ingested infective dose much smaller. This study was not designed to mimic a naturally consumed dose of CWD, but rather as a proof of concept to determine if infectivity could pass into coyote feces. The passage of disease in feces is a common route of translocation for many viral, bacterial and parasitic diseases.
The results of this bioassay indicate that infectious CWD prions are able to be passed in the feces of coyotes fed infected elk brain homogenate for at least 3 DPI, making them a potential vector for CWD prion transport and contamination within the environment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to extend their deepest gratitude to the USDA/APHIS National Wildlife Research Center, Logan Utah field station for providing the coyotes for this study, as well as Dr. Thomas Gidlewski for assistance with necropsy and tissue collection.
REFERENCES
- 1.Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 1980; 16:89-98; PMID:7373730; http://dx.doi.org/ 10.7589/0090-3558-16.1.89 [DOI] [PubMed] [Google Scholar]
- 2.Miller MW, Williams ES. Prion disease: horizontal prion transmission in mule deer. Nature 2003; 425:35-6; PMID:12955129; http://dx.doi.org/ 10.1038/425035a [DOI] [PubMed] [Google Scholar]
- 3.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 2004; 10:1003-6; PMID:15207049; http://dx.doi.org/ 10.3201/eid1006.040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 2009; 4:e4848; PMID:19293928; http://dx.doi.org/ 10.1371/journal.pone.0004848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulford B, Spraker TR, Wyckoff AC, Meyerett C, Bender H, Ferguson A, Wyatt B, Lockwood K, Powers J, Telling GC, et al.. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis 2012; 48:425-34; PMID:22493117; http://dx.doi.org/ 10.7589/0090-3558-48.2.425 [DOI] [PubMed] [Google Scholar]
- 6.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, et al.. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 2006; 314:133-6; PMID:17023660; http://dx.doi.org/ 10.1126/science.1132661 [DOI] [PubMed] [Google Scholar]
- 7.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2006; 2:e32; PMID:16617377; http://dx.doi.org/ 10.1371/journal.ppat.0020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols TA, Spraker TR, Rigg TD, Meyerett-Reid C, Hoover C, Michel B, Bian J, Hoover E, Gidlewski T, Balachandran A, et al.. Intranasal Inoculation of White-Tailed Deer (Odocoileus virginianus) with Lyophilized Chronic Wasting Disease Prion Particulate Complexed to Montmorillonite Clay. PLoS One 2013; 8:e62455; PMID:23671598; http://dx.doi.org/ 10.1371/journal.pone.0062455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts JC, Balachandran A, Westaway D. The expanding universe of prion diseases. PLoS Pathog 2006; 2:e26; PMID:16609731; http://dx.doi.org/ 10.1371/journal.ppat.0020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VerCauteren KC, Pilon JL, Nash PB, Phillips GE, Fischer JW. Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos). PLoS One 2012; 7:e45774; PMID:23082115; http://dx.doi.org/ 10.1371/journal.pone.0045774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennelle CS, Samuel MD, Nolden CA, Keane DP, Barr DJ, Johnson C, Vanderloo JP, Aiken JM, Hamir AN, Hoover EA. Surveillance for transmissible spongiform encephalopathy in scavengers of white-tailed deer carcasses in the chronic wasting disease area of Wisconsin. J Toxicol Environ Health A 2009; 72:1018-24; PMID:19697235; http://dx.doi.org/ 10.1080/15287390903084249 [DOI] [PubMed] [Google Scholar]
- 12.Jennelle CS, Samuel MD, Nolden CA, Berkley EA. Deer carcass decomposition and potential scavenger exposure to chronic wasting disease. The Journal of Wildlife Management 2009; 73:655-62; http://dx.doi.org/ 10.2193/2008-282 [DOI] [Google Scholar]
- 13.Spraker TR, O'Rourke KI, Gidlewski T, Powers JG, Greenlee JJ, Wild MA. Detection of the abnormal isoform of the prion protein associated with chronic wasting disease in the optic pathways of the brain and retina of Rocky Mountain elk (Cervus elaphus nelsoni). Vet Pathol 2010; 47:536-46; PMID:20382822; http://dx.doi.org/ 10.1177/0300985810363702 [DOI] [PubMed] [Google Scholar]
- 14.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 1999; 80 (Pt 10):2757-64; PMID:10573172; http://dx.doi.org/ 10.1099/0022-1317-80-10-2757 [DOI] [PubMed] [Google Scholar]
- 15.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 2001; 82:2327-34; PMID:11562526; http://dx.doi.org/ 10.1099/0022-1317-82-10-2327 [DOI] [PubMed] [Google Scholar]
- 16.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. Prions in skeletal muscles of deer with chronic wasting disease. Science 2006; 311:1117; PMID:16439622; http://dx.doi.org/ 10.1126/science.1122864 [DOI] [PubMed] [Google Scholar]
- 17.Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol 2006; 87:3443-50; PMID:17030881; http://dx.doi.org/ 10.1099/vir.0.81777-0 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald JP, Meaney CA, Armstrong DM. Mammals of Colorado. Niwot, Colorado: University Press of Colorado; 1994. [Google Scholar]
- 19.MacCracken JG, Hansen RM. Coyote feeding strategies in Southeastern Idaho: Optimal foraging by an opportunistic predator? Journal of Wildlife Management 1987; 51:278-85; http://dx.doi.org/ 10.2307/3801003 [DOI] [Google Scholar]
- 20.O'Donoghue M, Boutin S, Krebs CJ, Zuleta G, Murray DL, Hofer EJ. Functional responses of coyotes ad lynx to the snowshoe hare cycle. Ecology 1998; 79:1193-208; http://dx.doi.org/ 10.2307/176736 [DOI] [Google Scholar]
- 21.MacCracken JG. Coyote foods in the Black Hills, South Dakota. Journal of Wildlife Management 1984; 48:1420-3; http://dx.doi.org/ 10.2307/3801809 [DOI] [Google Scholar]
- 22.Wilmers CC, Stahler DR, Crabtree RL, Smith DW, Getz WM. Resource dispersion and consumer dominance: scavenging at wolf- and hunter-killed carcasses in Greater Yellowstone, USA. Ecol Lett 2003; 6:996-1003; http://dx.doi.org/ 10.1046/j.1461-0248.2003.00522.x [DOI] [Google Scholar]
- 23.Korschgen LJ. Food habits of the coyote in Missouri. Journal of Wildlife Management 1957; 21:424-35; http://dx.doi.org/ 10.2307/3796675 [DOI] [Google Scholar]
- 24.Paquet PC. prey use strategies of sympatric wolves and coyotes in Riding Mountain National Park, Manitoba. J Mammol 1992; 73:337-43; http://dx.doi.org/ 10.2307/1382067 [DOI] [Google Scholar]
- 25.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, Hoover EA, Jewell JE, Telling GC, Zabel MD. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 2009; 3:171-83; PMID:19823039; http://dx.doi.org/ 10.4161/pri.3.3.9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spraker TR, O'Rourke KI, Balachandran A, Zink RR, Cummings BA, Miller MW, Powers BE. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest 2002; 14:3-7; PMID:12680636; http://dx.doi.org/ 10.1177/104063870201400102 [DOI] [PubMed] [Google Scholar]
- 27.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, et al.. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 2005; 25:7944-9; PMID:16135751; http://dx.doi.org/ 10.1523/JNEUROSCI.2467-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiggins RC. Prion stability and infectivity in the environment. Neurochem Res 2009; 34:158-68; PMID:18483857; http://dx.doi.org/ 10.1007/s11064-008-9741-6 [DOI] [PubMed] [Google Scholar]
- 29.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 2009; 4:e7990; PMID:19956732; http://dx.doi.org/ 10.1371/journal.pone.0007990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gese EM, Rongstad OJ, Mytton WR. Home range and habitat use of coyotes in southeastern Colorado. Journal of Wildlife Management 1988; 52:640-6; http://dx.doi.org/ 10.2307/3800923 [DOI] [Google Scholar]


