Abstract
Objectives
The Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT) is a Phase IIb randomized, placebo-controlled clinical trial, testing doxycycline (100 mg bid) for inhibition of growth in the greatest transverse, orthogonal diameter of small abdominal aortic aneurysms (AAA).
Methods
We will enroll 258 patients, ≥ 55 years of age who have AAA, men: 3.5–5.0 cm and women: 3.5–4.5 cm on CT scans confirmed centrally. The primary outcome is growth in maximal transverse, orthogonal diameter from baseline to 24-month follow-up. Secondary analyses address doxycycline effects on clinical events, aneurysm volume, and biomarkers. Primary analysis will be performed according to the principle of intention-to-treat accounting for death and ruptures by use of normal scores in analysis of covariance. At the time of the data file reported, 200 subjects have been randomized. We started enrollment in mid-2013 and will complete enrollment by mid-2016.
Results
Participant average age = 70.9 years, (SD = 7.6 years) and maximum transverse diameter = 4.3 cm for men (SD = 0.4) and 4.0 cm for women (SD = 0.3).
Conclusion
N-TA3CT is a critical experiment to determine whether doxycycline reduces growth of small AAA and systemic markers of inflammation previously seen in bench experiments and observational human studies to be associated with AAA growth. Our patient population baseline measurements agree with the design assumptions supporting our expectation of 90% power or greater to reject a null hypothesis in favor of an alternative hypothesis when growth is reduced by at least 40%. Registration: clinicaltrials.gov #NCT01756833.
INTRODUCTION
Prospective abdominal ultrasound screening by Alcorn et al for infrarenal aortic diameters greater than 3.0 cm, identified abdominal aortic aneurysms (AAA) in 9.5% of the population over the age of 65 years [1]. The natural history of AAA is one of asymptomatic, progressive expansion at 2.6 to 3.3 mm per year until rupture; the best predictor of rupture is aneurysm diameter [2–5]. Early detection and elective surgical repair [6–8], endovascular for most patients and open surgical repair for the remainder [9–11] prevents rupture. Endovascular repair risk is equivalent to or greater than the risk of rupture for AAAs less than 5.5 cm in men and 5.0 cm in women [12, 13].
Laboratory studies document that elastin and collagen degradation, mediated in large part by matrix metalloproteinases (MMPs) [14–19], are responsible for weakening and dilatation of the aortic wall and aneurysm rupture [20–22]. MMP-1, -2, -9 -12 and -13 are found in the extracellular matrix of aneurysm tissues, and MMP-9 is elevated in the plasma of patients with AAAs [16, 22]. Tetracyclines inhibit MMPs [23, 24]. Doxycycline suppresses inflammation and MMPs in degenerative human aortic aneurysms [22, 24, 25]. Doxycycline inhibits MMP-9 content through both translational (decreased mRNA) and post-translational mechanisms. The post-translational mechanisms are thought to be related to ribosome interactions with the message but this mechanism has not been fully elucidated. At dosages well above the therapeutic window, doxycycline can directly interfere with the activity of MMP-9.
In elastase-induced injury to the rat aorta, doxycycline suppresses aortic wall MMP activity, elastin degradation, and aneurysm development in a dose dependent fashion [26, 27]. In calcium chloride-induced injury to the mouse aorta, doxycycline demonstrates dose-dependent inhibition of aneurysm expansion [28]. In a 32-patient, placebo-controlled clinical trial of doxycycline (150 mg/day) for three months, ultrasound differences in expansion that could have occurred by chance were observed over 18 months follow-up [29]. Six-month interval analyses of growth found less expansion in the doxycycline patients at 12 months [29]. Consequently, we planned a placebo-controlled Phase IIb clinical trial to determine whether doxycycline (100 mg bid) will inhibit growth in the greatest transverse diameter of small AAAs (3.5–5.0 cm in men, 3.5–4.5 cm in women) as measured by CT scan over 24-months. Secondary analyses address doxycycline effects on interval aortic diameters and volume, on levels of MMP-9, interferon-gamma and high sensitivity C-Reactive Protein (hs-CRP). At the time we were about to initiate our clinical trial the results of a 286-patient, placebo-controlled trial of doxycycline (100 mg/day) for 18 months were published observing an accelerated growth rate on ultrasound associated with doxycycline and concluding that doxycycline therapy did not reduce aneurysm growth.[30]
METHODS
Administration and Organization
N-TA3CT has four principal entities: (1) a Clinical Coordinating Center (CCC) and Clinical Consortium (21 clinical sites, see appendix), (2) Imaging Core Laboratory (ICL), (3) Biomarkers Core Laboratory (BCL), and (4) Data Coordinating Center (DCC). Biomarker assays are performed by the BCL at Vanderbilt University, Nashville, TN, except for hs-CRP assays which are performed at Washington University in St. Louis. Circulating doxycycline levels are assayed in the University of Maryland School of Pharmacy. An independent Biostatistical Center, Axio Research LLC, Seattle, WA, and the DCC statistician at UMB perform interim analyses for data monitoring.
N-TA3CT is exempt from IND requirements of the US Food and Drug Administration (FDA) and is registered at ClinicalTrials.gov (NCT 01756833). The protocol has been approved by the Institutional Review Boards (IRBs) for each of the central units and each of the clinical and laboratory performance sites. The National Institute on Aging appointed an independent Data and Safety Monitoring Board (DSMB).
Patient Population
Eligible patients are 55 years of age or older and have AAAs that are not the result of known heritable diseases. Coordinators approach patients for consent to review CT scans centrally. Patients have small AAA on ICL reading of CT scans obtained within 30 days of randomization. Screened patients are excluded if they have health problems that compromise participation. Inclusion and exclusion criteria are below.
Inclusion Criteria
Patients 55 years of age or older, women post-surgical menopause or at least two years since last menses if natural menopause.
CT scan documented infrarenal abdominal aortic aneurysm with maximum transverse diameter larger than 35 mm and no greater than 50 mm, in men, and larger than 35 mm and no greater than 45 mm in women.
Exclusion Criteria
Prior repair of the abdominal aortic aneurysm.
Renal artery involvement or suprarenal extension of the aneurysm.
Documented failure of the aneurysm to increase in size over the two years prior to enrollment if the aneurysm is < 4.0 cm in maximal transverse diameter.
Iliac artery aneurysm > 2.9 cm in diameter.
Iliac artery occlusive disease planned for repair.
Renal artery stenosis with planned open repair.
Known thoracic aortic aneurysm > 3.5 cm, for aneurysms that are saccular or observed to be expanding at a rate greater than ordinary and > 4.0 cm, for aneurysms that are not saccular and are expanding at accustomed rates.
Known connective tissue disease (e.g., collagen vascular disorder), heritable or genetic syndrome (e.g., Marfan Syndrome, Ehlers-Danlos Syndrome) underlying the abdominal aortic aneurysm.
Stage II hypertension (patients whose blood pressure is persistently in the range of systolic > 160 mm Hg or diastolic > 100 mm Hg despite personal physician’s best effort to achieve adequate therapy).
Creatinine > 2.0 g/dL or creatinine clearance < 30 ml/min.
Allergy or intolerance of tetracyclines.
Use of tetracyclines within past six months.
Taking anti-epileptic pharmaceutical agents (e.g., carbamazepine, diphenylhydantoin).
Current or planned treatment with chemotherapy or radiation therapy for cancer other than squamous cell cancer of the skin.
Current or planned treatment with systemic immunosuppressive agents (e.g., prednisone, azathioprine, methotrexate, cyclosporine for autoimmune disease or following transplantation of bone marrow, heart, liver, lung or other solid organ).
Chronic infection managed with long-term antibiotics, frequent courses of antibiotic therapy or self-administration of antibiotic therapy.
Personal physician/surgeon is not willing to follow the protocol.
Prognosis less than two-year survival or other reason the Clinical Site director believes the patient is not a suitable candidate for N-TA3CT (e.g., history of repeatedly missing follow-up appointments or regular residence outside of the U.S.)
Enrollment in another, concurrent clinical trial study.
Refusal or inability of patient to provide written informed consent.
Most patients are identified through vascular surgery clinics, referral from vascular surgeons/interventional radiologists, or direct patient contact after search of medical center data bases of radiology reports. Some patients have been recruited through community publicity (e.g., newspaper articles, newspaper advertisements, radio interviews, radio advertisements), and direct mail to patients agreeing to research in a commercial screening program (Life Line Screening, Austin, TX).
Imaging Studies
Our acquisition protocol for CT scans for multi-detector, helical CT is: 1) narrow field-of-view centered on aorta, <36 cm encouraged; 2) tube current and energy to be set to best clinical standards (least radiation exposure) for image quality; 3) CT scout without contrast, study without contrast unless contrast is judged to be clinically necessary by the treating physician; 4) scan from top of diaphragm to symphysis pubis; 5) section thickness less than or equal to 2.5 mm; 6) reconstruction overlap minimum 50%, i.e., 1.25 mm intervals, 2.5 mm thickness.
CT scans are transferred from clinical sites to the ICL on DICOM formatted CDs. The study assigned patient ID number (PID) and alphabetic code (Letcode) are the only identifiers that appear on the CD. Visit (Baseline, six-month, 12-month, 18-month, 24-month) appears in the electronic header.
CT scans are more expensive than ultrasound studies and require ionizing radiation. Some otherwise eligible patients have refused enrollment because of the cost of clinically indicated CT scans and others because of recent publicity concerning the exposure to ionizing radiation involved in CT scans. CT scans do provide a much more complete picture of the aneurysm because of their three dimensional nature. Abdominal aortic aneurysm (AAA) diameter can be measured relative to the longitudinal axis which is critical because many AAA are tortuous. The largest transverse diameter can be measured in the 360 degree radius relative to this axis. With ultrasound studies, the reader is dependent on the images captured and recorded by the technologist. Obesity and bowel gas may obscure the best images. Because of the inability to account for tortuosity, most standardized ultrasound investigations used the AP diameter. This single plane image compared to the 360 degree view measurable by CT scan is less likely to capture small changes in diameter.
Study Treatments
Study treatments consist of 100 mg capsules of doxycycline hyclate purchased from West Ward Pharma (NDC 00143-3142-05) and placebo (over)encapsulated at Catalent Pharma Solutions, Philadelphia, PA. Numbered treatment kits are shipped by Catalent to investigational pharmacies or dispensing physicians at clinical sites, under the direction of Axio Research, LLC, which is responsible for random assignment, distribution and tracking of treatment kits as part of the DCC.
Returned capsules are counted at clinic visits as part of adherence documentation. At the time of monitoring visits by CCC staff, capsule counts are verified and returned kits shipped to the University of Nebraska Medical Center Pharmacy for accountability, disposition and quality control.
Among tetracyclines, doxycycline was selected because: 1) animal and human preliminary data suggest that it is safe and possibly efficacious for reducing the rate of growth of small AAA; 2) it has a high level of absorption from the upper gastrointestinal tract; 3) its half-life allows for sustained blood levels with daily or bid dosing; 4) its clearance by both renal and hepatic/intestinal mechanisms reduces the possibility of overdose toxicity; 5) irreversible, severe or serious adverse effects were found to be rare after decades of use.
Randomization and Treatment Allocation
Treatment assignments are based on randomization schedules prepared separately for each clinical site, stratified for sex (male/female), and in randomly varying block sizes of 2, 4, and 6 with 1:1 ratio of doxycycline: placebo. After complete baseline information is entered via electronic data capture (eDC), clinical site personnel log onto the randomization web site to obtain the number of a blinded treatment kit in the clinical site inventory to be dispensed to the subject.
Follow-Up
After randomization, subjects return for clinic visits every three months, to: 1) review health status with special reference to expected adverse effects and to serious or unexpected adverse effects; 2) return used treatment kits for capsule counts; 3) discuss adherence and, 4) obtain new treatment kits. At the 6-, 12-, 18- and 24-month visits, CT scans are obtained for reading in the ICL. Serum and plasma samples are forwarded to the BCL for later analysis; participants complete the SF-36 quality of life assessment. CT scans obtained in the intervals between visits for clinical indication may replace scheduled CT scans to avoid unnecessary radiation exposure. Clinical site staff may conduct visits by telephone in preference to missing visits altogether. When a patient cannot return for a visit involving a CT scan, efforts are made to schedule CT scans off-site. Study treatments are discontinued for any patient whose aneurysm grows to a size or manifests symptoms that lead to repair, but follow-up continues at six-month intervals.
Primary Outcome
We used growth in AAA maximum transverse orthogonal diameter on CT scan from baseline to follow-up assessment two years after randomization as measured in the ICL for power calculations as the metric of treatment effect. In a pilot investigation of ICL measurement of maximum transverse diameters, intra- and inter-observer intraclass correlation coefficients (ICC) exceeded 0.99.
Data Analysis of the Primary Outcome
We will compare the changes in AAA maximum transverse diameter from baseline to two years after randomization according to treatment assignment consistent with the principle of intention-to-treat, using linear regression (ANCOVA) with baseline AAA maximum transverse diameter, sex, and an indicator of treatment assignment in the model. The test for differences between treatments will be conducted at an overall one-sided alpha-level of 0.025. Prior to performing the primary outcome analysis, an interaction term for the randomization stratum (sex) with treatment assignment will be assessed in the ANCOVA model. If there is significant (at two-sided alpha = 0.05) evidence of difference in treatment effect according to sex, other than a small difference in the same direction, the analysis will be performed separately for men and women.
The primary analysis will be performed on normal scores corresponding to percentile rank of each patient at baseline and follow-up [31, 32]. At baseline all scores will be based on rank of AAA maximum transverse diameter. At two-year follow-up, ranks worse than any CT scan based rank will be assigned according to survival and repair status for patients who did not undergo a two-year CT scan, with deaths being ranked worst. After deaths, next worst ranks will be assigned according to patient condition at the time of surgical intervention, from rupture to symptoms or signs of imminent rupture to undergoing repair for reason of maximum transverse diameter exceeding 5.5 cm. for men or 5.0 cm. for women to other less urgent indications. Within each category, ranks will be assigned in order of time from randomization to occurrence. Multiple imputation methods will be used for those few patients who do not, for unanticipated reasons, complete a CT scan at two years.
Interim Analysis Plan and Critical Values
The DSMB will convene to review N-TA3CT conduct and safety at six-month intervals, performing interim analyses with a possibility of early termination for efficacy when one third (approximately) of subjects have completed two-year follow-up, at a critical z-boundary of 3.291 (p = 0.0005, one-sided) and when two thirds (approximately) of subjects have completed follow-up at the same critical z-boundary, leading us to adjust the final analysis critical value to z = 1.965 (p = 0.0247). These interim analysis boundaries are of the Haybittle-Peto type [33,34] but with an adjustment at the final analysis. An adjustment in the final critical values will be made as necessary if the times of the interim analyses change. At the time of the second interim analysis for efficacy, we propose a futility analysis to terminate the clinical trial if there is less than a 20% chance (conditional power) to reject the null hypothesis of no difference favorable to doxycycline in comparison to placebo at a one-tailed alpha level of 0.025, under the assumption of a 40% reduction in growth (the alternative hypothesis) for the remaining one third of patients [35].
The distinction between the correlation of measurements of abdominal aorta diameter from the correlation of growth in abdominal aorta diameter is important to N-TA3CT design and analysis. The correlation of abdominal aorta diameter measurements over time (r) is expected to be large, 0.80–0.94, as observed in longitudinal studies. The correlation of measurements of growth in abdominal aorta diameter over six-month intervals is not well known, and is expected to be small, especially between early and later intervals. For example, we anticipate, as Mosorin et al. [29] observed, that six-month CT scan measurements will reflect random variation in growth from baseline and be little correlated with growth over the course of two years.
After the start of N-TA3CT, the results of the Pharmaceutical Aneurysm Stabilisation Trial (PHAST) were published, with the finding that, “Doxycycline treatment was associated with increased aneurysm growth (4.1 mm in the doxycycline group vs. 3.3 mm in the placebo group at 18 months...P = 0.016...) [30].” Although the results were difficult to interpret because patients who stopped taking study medication withdrew from the study, which affected analyses based on intention-to-treat, we took seriously the possibility that doxycycline might be harmful instead of beneficial to individuals who have small AAAs. Most (75%) of the difference in growth unfavorable to doxycycline in PHAST was observed in the first six months of treatment. We negotiated with our Data and Safety Monitoring Board a one-sided monitoring boundary at z =1.645 (p = 0.05), an approach similar to that of Pocock [36], to be assessed at every interim analysis in which new six-month CT scan data are available, using the same analysis methods as for the primary outcome analysis. If six-month CT scans document growth unfavorable to doxycycline treatment at that critical boundary, termination of the clinical trial will be considered.
Secondary Analyses
The interaction of baseline aneurysm size with treatment group in effect on growth is of interest. Linear regression models will be used to explore age, statin use and other factors for effects on aneurysm growth. Secondary outcomes of interest include changes in aneurysm volume, the occurrence of clinical events such as death, the occurrence of myocardial infarction or death, measures of health related quality of life, high sensitivity C-reactive protein, MMP-9 levels, interferon gamma levels, other measurements made on CT scans and outcomes in clinically defined subgroups. Analyses of adherence and per protocol analyses will be used for Phase III clinical trial planning. To take account of multiplicity in secondary analyses, a p-value <0.01 will be required for some evidence of difference, and P<0.001 for strong evidence.
Power Calculations and Study Size
We have specified the alternative hypothesis as a 40% reduction in maximal transverse diameter growth, from 2.5 mm/year to 1.5 mm/year over two years, a minimal clinically important difference, based on a Monte Carlo simulation. Using age-stratified mortality in the US male population in 2006 and an age and aneurysm size distribution from our clinical sites, the proportion of patients whose aneurysms would indicate surgical repair over the course of a lifetime changes from just over 3/4 to just under 2/3.
Our Monte Carlo simulation experiment [37, 38] (SAS 9.1.3, SAS Institute, Inc) was designed to project the number of abdominal aortic aneurysm invasive repairs that could be prevented by doxycycline treatment in an older U.S. AAA prevalence sample, within a 20-year time frame.
Simulation settings
A prevalence sample of 60,000 older AAA patients (>=55 years old) in the U.S. was simulated using data from the preliminary survey described above. Age and AAA diameter distributions are displayed in Table 1.
-
Follow-up was assessed in 6 month intervals:
The impact of treatment was assessed by simulating different effect sizes and other parameters (Table 3, Table 4, Figure 1).
Table 1.
Age and AAA Diameter Distribution of Simulated Sample
| Age Groups | Mean AAA Diameter | Standard Deviation | Percentage of the Whole Sample % |
|---|---|---|---|
| 56–60 | 4.5 | 0.7 | 8 |
| 61–65 | 4.5 | 0.6 | 12 |
| 66–70 | 4.9 | 1.2 | 16 |
| 71–75 | 4.9 | 1.2 | 18 |
| 76–80 | 4.8 | 0.8 | 20 |
| 81 or older | 5.0 | 0.9 | 26 |
Table 2.
U.S. Age-Specific Mortality
| Age Groups | Estimated Mortality per 6 Month |
|---|---|
| 55–64 | 0.006 |
| 65–74 | 0.013 |
| 75–84 | 0.032 |
| 85 or older | 0.075 |
Table 3.
Assuming 0.15 cm/year Growth Rate in Treatment Group
| Follow-up period=6 months | ||||
|---|---|---|---|---|
| Stratum | Total No. of simulated patients | No. of patients whose diameter were larger than 5.5cm at baseline | No. of patients whose diameter grows >= 5.5 cm during follow-ups | No. of patients censored by mortality |
| Control group: Growth rate=0.25 cm per year | 60,000 | 13,760 | 35,929 | 10,311 |
| Doxycycline group: Growth rate=0.15 cm per year | 60,000 | 13,760 | 30,064 | 16,176 |
| --------- | --------- | −16.3% | +56.9% | |
Table 4.
Assuming Different Growth Rates in Treatment Group
| % Reduction in 0.250 cm/year growth rate | % Operated | |||
|---|---|---|---|---|
| Placebo | Doxycycline | Absolute Reduction | Relative Reduction | |
| 40% (to 0.150 cm/year) | 78% | 65% | 13% | 16% |
| 30% (to 0.175 cm/year) | 78% | 70% | 8% | 11% |
| 20% (to 0.200 cm/year) | 78% | 73% | 5% | 7% |
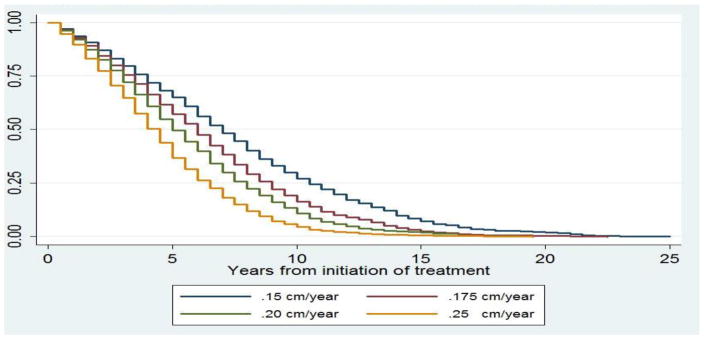
Figure 1.
Surgery Free Probability Assuming Different Aneurysm Growth Rates
At the end of 20-year follow-up, almost all patients will have surgical repair or be censored by mortality, Figure 1. Assuming doxycycline treatment reduces the average aneurysm expansion rate from 2.5 mm (0.25 cm) per year to 1.5 mm (0.15 cm) per year, doxycycline could prevent 5865 (16.3%) of 35,929 elective invasive repair procedures, Table 3. Fixing the growth rate of AAA in the placebo group at 2.5 mm (0.25 cm) per year, 40%, 30%, and 20% reductions in growth rate in the doxycycline group result in 13%, 8%, and 5% absolute decreases in the number of invasive repairs made during a 20-year follow-up, Table 4.
A 5% difference between 78% and 73% undergoing surgery as we project for a 20% reduction in growth rate is unimpressive. The difference between a probability of about 3/4 of surgical repair and a probability of 2/3 appeared to our vascular surgeons to be the smallest that would convince patients to take daily medication for the rest of their lives. Therefore, we predicated N-TA3CT power calculations on a 40% reduction in growth rate as the alternative hypothesis.
We found a Pearson correlation, r, of 0.88 between baseline and 18-month AAA measurements in 12 placebo recipients and r = 0.94 in doxycycline recipients in a small randomized clinical trial [29]. In our pilot study r was 0.88 [40], and in an unpublished data set provided by Dr. Frank Lederle, r = 0.94 for measurements one year apart. In these same data sets we found standard deviations (SD) ranging from 4.6 to 9.6 mm. Predicating effective sample size on r = 0.82 and SD = 7 mm, we arrived at treatment group sizes of 85 each, for a one-sided alpha level = 0.025 and power 0.90, based on a t-test and equal-sized groups, using a standard formula for the efficiency of ANCOVA [41]. We calculated the power of a study with 85 subjects per treatment group for several values of r (0.80, 0.88, 0.96) and SD (5 mm, 7 mm, 9 mm).
Power exceeds 0.99 when r ≥ 0.88 and SD = 5 mm or when r ≥ 0.96 and SD is between 5 mm and 9 mm. If r = 0.80, power > 0.80 for SD ≤ 7 mm; for r = 0.80 and SD = 9 mm, power is approximately = 0.67. The normal scores analysis we plan is asymptotically equivalent in power to a t-test for normal distributions [31, 32]. Allowing for noise introduced into the analysis by non-adherence of 10% in each treatment group and 15% unavailable for CT scan at two-year follow-up, we increased the group size from 85 to 85/(1−0.1)2/(1−0.15) = 124. To allow for recruitment of 10 extra women, we increased the group size again to 129. Representation of women in N-TA3CT is important even though there is not enough power to analyze data separately for women. Abdominal aortic aneurysm is not as well studied in women as in men, and we believe that information will be valuable.
Laboratory Methods
Doxycycline and biomarker assays are performed on specimens frozen in cryovials labeled with numbers that cannot be linked to PID/Letcode except through the DCC and to individuals in the clinical sites only. We use an ultra high performance liquid chromatography (UPLC) tandem mass spectrometry (MS/MS) method at the University of Maryland School of Pharmacy to measure serum doxycycline levels.
Analysis of hs-CRP will be performed using an immunoturbidimetric latex agglutination method (K-Assay [KAI-060], Kamiya Biomedical Co., Seattle, WA). Serum cotinine will be measured with an ELISA (Calbiotech, Spring Valley, CA). Plasma MMP-9 concentrations will be measured by an ELISA, two-site sandwich method that is commercially available (R & D Systems, Quantikine, DMP900). Interferon-gamma will be measured by a sandwich-type ELISA also (R&D Systems, Quantikine, DMP900).
Current Status of the Clinical Trial
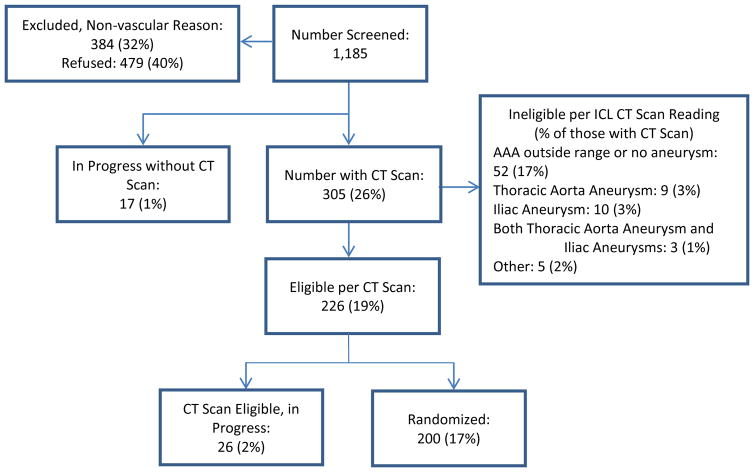
As of December 14, 2015, 1,152 patients -- 929 (82%) male, 210 (18%) female, 6 (0.5%) missing data -- had been screened, resulting in 200 (17%) -- 179 (90%) male and 21 (10%) female -- randomized in the clinical trial, Figure 2.
Figure 2.
Interim Consort Diagram
The average age was 72.8 years (SD = 8.3) for those screened and 70.9 (SD = 7.6) for those randomized. Among randomized patients, the average maximum transverse diameter for men was 4.3 cm (SD = 0.4) and for women 4.0 cm (SD = 0.3). Anti-hypertensive medications were being taken by 157 (79%), and aspirin or other anti-platelet agents by 150 (75%). HMG-CoA reductase inhibitors (statins) were being taken by 163 (82%). We started enrollment in mid-2013 and will complete enrollment by mid-2016.
Discussion
N-TA3CT is a Phase IIb clinical trial because the primary outcome is a continuous, anatomical measurement that patients do not perceive, instead of a clinical event important to patients (i.e., repair, rupture, death) that would be required for a Phase III clinical trial. We are gaining information about the distribution, growth and correlations of CT scan measurements of AAA maximum transverse diameter, adherence to study treatment, adverse effects of study treatment, retention and completeness of follow-up that will be necessary for a Phase III clinical trial. Our analysis plan addresses concerns with the vagaries of randomization in Mosorin’s work [29, 42] and criticism of PHAST for lack of intention-to-treat analysis [30].
N-TA3CT is designed as a critical experiment that will determine whether doxycycline can reduce MMP-9 levels and reduce systemic markers of inflammation (interferon- gamma and hs-CRP) in ways that we associate in bench experiments and observational human studies with AAA growth. We will learn whether or not we are pursuing a pathogenesis that applies in humans and whether we are pursuing an efficacious or an inefficacious treatment. If doxycycline has an effect of marginal clinical relevance, the search for more potent or specific MMP inhibitors could be the next step. Even if doxycycline has no effect on aneurysm growth, we will be assaying specimens for candidate biomarkers and banking specimens for possible assay of new biomarker candidates that could be surrogates for expansion.
Based on the present state of knowledge, a hypothesis that inflammation results in AAA growth and elevates MMP-9 and hs-CRP levels as systemic markers of this inflammation is plausible. Under this scenario, we hypothesize doxycycline will produce a series of events: 1) therapeutic levels of doxycycline are achieved in the aneurysm; 2) a reduction of MMP-9 levels is observed; 3) a reduction in hs-CRP is observed; 4) a reduction in aneurysm expansion is observed. This sequence of events takes time to change the course of aneurysm growth. No effect may be seen at early time points while doxycycline may prove effective in the longer term. Consequently, we designed the primary end point of this trial to be measured after 24 months.
The publication of PHAST results caused us to re-examine the assumptions in N-TA3CT design. The subjects in PHAST received only 100 mg daily of doxycycline once per day. Our dose (100 mg BID; 200 mg daily) of doxycycline has been shown 1) to reduce AAA tissue MMP levels and 2) produce circulating levels of doxycycline in humans similar to the circulating levels required in mice to inhibit aneurysm expansion. We would not have expected PHAST to demonstrate a reduction in growth of AAAs. Abdul-Hussien et al found 100 mg daily reduced AAA inflammation [25]. Recently, Kroom and Taanman [43] suggested doxycycline may inhibit clonal expansion of T-cells, and once a day dosing leads to troughs in doxycycline levels that reduce this effect. Unlike previous human AAA studies, we are measuring plasma doxycycline levels. These measurements will allow us to compare levels to those in our animal studies and to effects on aneurysm expansion and circulating biomarkers.
Previous studies of doxycycline have measured AAA with ultrasound instead of CT scan. In PHAST “the largest ultrasonographic measurement was taken of the antero-posterior diameter perpendicular to the blood flow and the diameter measured from inner-to-inner aortic wall [30].” The effects of doxycycline could differentially affect the major and minor diameters [44]. Also, ultrasound measurements do not assure ascertainment of the maximal aortic diameter and make taking aortic tortuosity into account difficult.
We agree with the interpretation of PHAST that 100 mg of doxycycline per day does not lead to favorable ultrasound-measured AP diameter effects in doxycycline-treated patients. Still, the lower dose of doxycycline used in PHAST could be appropriate for prevention of aneurysm rupture. In PHAST, the count of deaths and ruptures favored doxycycline over placebo, although not more than one could expect by chance. A randomized trial with the power to show a difference in clinical event endpoints would be necessary to know whether or not there is a clinical event benefit to be obtained from doxycycline at either the PHAST dose (100 mg daily) or our dose (200 mg daily).
We look forward to concluding N-TA3CT recruitment and follow-up, obtaining new information important for patient care and future research. If doxycycline reduces aneurysm growth by 40% or more, changes in medical practice might save a portion of AAA patients from invasive treatments that carry inescapable if infrequent adverse outcomes. Biomarker data will be helpful in future trials. Finally, we believe that the information obtained from this trial will result in new understanding of the biology of human AAA.
Acknowledgments
GRANT SUPPORT
N-TA3CT was supported by awards 1R34AG028684, 1R01AG037120, T32AG000262 and P30AG028747 from the National Institute on Aging (NIA), National Institutes of Health (NIH).
APPENDIX. Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT): Institutions and Investigators
-
Clinical Coordinating Center, University of Nebraska School of Medicine, Omaha, NE
Tim Baxter (PI), John Beck, LuAnn Larson, Faye Park, Mary Phillips, Greg Prorok, Brigette Vaughan
-
Imaging Core Laboratory, University of Wisconsin School of Medicine, Madison, WI
Jon Matsumura (PI), Jennifer Grudzinski, Wendy Meadows
-
Biomarkers Core Laboratory, Vanderbilt University/Washington University School of Medicine
John Curci (PI), Licia Rowe
-
Data Coordinating Center, University of Maryland School of Medicine, Baltimore, MD
Michael Terrin (PI), Liz Casher, Andrea Lefever, Ling Tang, William Blackwelder
-
Doxycycline Assay Laboratory, University of Maryland School of Pharmacy, Baltimore, MD
Hazem Hassan, Raghunadha-Reddy Seelam
-
Biostatistical Center, Axio Research LLC, Seattle, WA
Ruth McBride, Anna Leonen, Navneet Hakhu, Ann Ngo
-
SAE Classification Committee
Michael Dalsing, Matthew Eagleton, Mark Morasch, Gilbert Upchurch
Clinical Sites
-
Beth Israel Deaconess Medical Center
Mark Wyers, Theresa Bishop, Eliot Chaikof, Allen Hamdan, Lauren Mills, Audrey Nathanson, Vassilios Raptopoulos, Marc Schermerhorn, Mary Trovato
-
Carondelet Heart and Vascular Institute
Scott Berman, Emily Taylor, Shonda Banegas, Marcie Ford-Tarleton, Rhonda Quick
-
Columbia University Medical Center
Richard Green, Diana Catz, Timothy Crimmins, Enkela Gragjevi, James McKinsey
-
Geisinger Medical Center
James Elmore, Elisabeth Deetz, Kay Blyler, Cathy Miller, Samantha Moyer, Trudy Snyder
-
McLaren Northern Michigan
Andris Kazmers, Denise Antonishen, William Riordan, Jason Ricci, Colleen Shaw
-
Miami Cardiac and Vascular Institute
Barry Katzen, Susan Arp, Ivette Cruz, Ripal Gandhi, Constantino Pena, Vicki Garabedian
-
Northwestern University
Mark Eskandari, Julie Blaisdell, Michelle Endo, Andrew Hoel, Anna Karas, William Pearce, Heron Rodriguez, Natali Rutiaga, Kaitlynn Williams
-
Omaha Veterans Administration Hospital
Iraklis Pipinos, Holly DeSpiegelaere
-
Oregon Health and Science University
Amir Azarbal, Sharon Kryger, Ashley Price, Alyssa Ward
-
Portland Veterans Administration Hospital
Amir Azarbal, Kathy Avalos
-
Stanford University
Ronald Dalman, Oliver Aalami, George Lee, Lori McDonnell
-
University of Arizona Health Sciences Center
Joseph Mills, David Armstrong, Marivec Hansen, Kay Goshima, Julie Kransler, Marcy Watchman
-
University of Maryland Medical Center
Robert Crawford, Carly Goldstein, Gregory Kowalewski, Rishi Kundi, Brajesh Lal, Debbie Nesbitt, Shahab Toursavadkohi
-
University of Michigan Medical Center
Jonathan Eliason, Susan Blackburn, Nickole Garvey, Jacquelyn Madden, Molly Oberdoerster
-
University of Nebraska Medical Center
Jason MacTaggart, Andrea Anderson, Marcus Balters, Karen Taylor
-
University of Southern California
Fred Weaver, Pui Yan, Sukgu Han, Leonardo Clavijo, William Lee, Valentina Rodina, Edward Lozano
-
University of South Florida
Murray Shames, Michelle Jung, Rachel Karlnoski, Stephenie Yapchanyk
-
University of Utah Health Sciences Center
Larry Kraiss, Benjamin Brooke, Monica Hatch, Joanna Lynch, Maria Maloney, Julie Beckstrom, Claire Griffin, Dee Jost, Dan Kinikini, Michelle Mueller, Katherine Reigstad, Mark Sarfati, Brigette Smith, Sarah Rogers, Lindsay Schlotfeldt
-
Utah VA Medical Center
Larry Kraiss, Benjamin Brooke, Maria Maloney, Julie Beckstrom, Dan Kinikini, Michelle Mueller, Katherine Reigstad, Denny Schumacher, Gerald Treiman, Robin Vezeau
-
Vanderbilt University
Thomas Naslund, Nikki Bratcher, Carol Madison, Lori Michalowski, Susan Sommers, Cheri Stewart, James Valentine, Louis Garrard
-
Washington University School of Medicine
Robert Thompson, Kathy Dodds, Christine Keller, Theresa Larose, Courtney Porter, Jennifer Wille
-
National Institute on Aging, National Institutes of Health, Bethesda, MD
Barbara Radziszewska, Susan Zieman
-
Data and Safety Monitoring Board
Dalane Kitzman (Chair), Christopher S. Coffey, Vikram Kashyap, Joao Lima, Jeremy Walston
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk Factors for Abdominal Aortic Aneurysms in Older Adults Enrolled in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 2.The UK Small Aneurysm Trial Participants. Mortality Results for Randomised Controlled Trial of Early Elective Surgery or Ultrasonographic Surveillance for Small Abdominal Aortic Aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 3.Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Jr, Blebea J, et al. Rupture Rate of Large Abdominal Aortic Aneurysms in Patients Refusing or Unfit for Elective Repair. JAMA. 2002;287:2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 4.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate Repair Compared with Surveillance of Small Abdominal Aortic Aneurysms. N Engl J Med. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 5.Schlosser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, et al. Growth Predictors and Prognosis of Small Abdominal Aortic Aneurysms. J Vasc Surg. 2008;47:1127–1133. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of Screening on the Incidence of Ruptured Abdominal Aortic Aneurysm: 5-Year Results of a Randomized Controlled Study. Br J Surg. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 7.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 8.Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital Costs and Benefits of Screening for Abdominal Aortic Aneurysms. Results from a Randomised Population Screening Trial. Eur J Vasc Endovasc Surg. 2002;23:55–60. doi: 10.1053/ejvs.2001.1534. [DOI] [PubMed] [Google Scholar]
- 9.United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus Open Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 10.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. DREAM Study Group. Long-term Outcome of Open or Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2010;362:1881–1889. doi: 10.1056/NEJMoa0909499. [DOI] [PubMed] [Google Scholar]
- 11.Lederle F, Freischlag J, Kyriakides TC, Matsumura JS, Padberg F, Kohler TR, et al. OVER Veterans Affairs Cooperative Study Group. Long-Term Comparison of Endovascular and Open Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2012;367(21):1988–1997. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 12.Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E CAESAR Trial Group. Comparison of Surveillance versus Aortic Endografting for Small Aneurysm Repair (CAESAR): Results from a Randomised Trial. Eur J Vasc Endovasc Surg. 2011;41:13–25. doi: 10.1016/j.ejvs.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Ouriel K, Clair DG, Kent KC, Zarins CK Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular Repair Compared with Surveillance for Patients with Small Abdominal Aortic Aneurysms. J Vasc Surg. 2010;51:1081–1087. doi: 10.1016/j.jvs.2009.10.113. [DOI] [PubMed] [Google Scholar]
- 14.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix Metalloproteinases 2 and 9 Work in Concert to Produce Aortic Aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrin PB, Mrkvicka R. Failure of Elastin or Collagen as Possible Critical Connective Tissue Alterations Underlying Aneurysmal Dilatation. Cardiovasc Surg. 1994;2:484–488. [PubMed] [Google Scholar]
- 16.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, et al. Matrix Metalloproteinase-2 Production and its Binding to the Matrix are Increased in Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 17.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and Localization of Macrophage Elastase (Matrix Metalloproteinase-12) in Abdominal Aortic Aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster MW, McAuley CE, Steed DL, Miller DD, Evans CH. Collagen Stability and Collagenolytic Activity in the Normal and Aneurysmal Human Abdominal Aorta. Am J Surg. 1991;161:635–638. doi: 10.1016/0002-9610(91)91246-f. [DOI] [PubMed] [Google Scholar]
- 19.Carrell TW, Burnand KG, Wells GM, Clements JM, Smith A. Stromelysin-1 (Matrix Metalloproteinase-3) and Tissue Inhibitor of Metalloproteinase-3 are Overexpressed in the Wall of Abdominal Aortic Aneurysms. Circulation. 2002;105:477–482. doi: 10.1161/hc0402.102621. [DOI] [PubMed] [Google Scholar]
- 20.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, et al. MMP-12 has a Role in Abdominal Aortic Aneurysms in Mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Cho BS, Roelofs KJ, Ford JW, Henke PK, Upchurch GR., Jr Decreased Collagen and Increased Matrix Metalloproteinase-13 in Experimental Abdominal Aortic Aneurysms in Males Compared with Females. Surgery. 2010;147:258–267. doi: 10.1016/j.surg.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size Matters: The Relationship between MMP-9 Expression and Aortic Diameter. Circulation. 1997;96:2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- 23.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines Inhibit Connective Tissue Breakdown: New Therapeutic Implications for an Old Family of Drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 24.Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, et al. Preoperative Treatment with Doxycycline Reduces Aortic Wall Expression and Activation of Matrix Metalloproteinases in Patients with Abdominal Aortic Aneurysms. J Vasc Surg. 2000;31:325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The Pathophysiology of Abdominal Aortic Aneurysm Growth: Corresponding and Discordant Inflammatory and Proteolytic Processes in Abdominal Aortic and Popliteal Artery Aneurysms. J Vasc Surg. 2010;51:1479–1487. doi: 10.1016/j.jvs.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 26.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline Inhibition of Aneurysmal Degeneration in an Elastase-Induced Rat Model of Abdominal Aortic Aneurysm: Preservation of Aortic Elastin Associated with Suppressed Production of 92 kD Gelatinase. J Vasc Surg. 1996;23:336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 27.Curci JA, Petrinec D, Liao S, Golub LM, Thompson RW. Pharmacologic Suppression of Experimental Abdominal Aortic Aneurysms: Acomparison of Doxycycline and Four Chemically Modified Tetracyclines. J Vasc Surg. 1998;28:1082–1093. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- 28.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, et al. Doxycycline in Patients with Abdominal Aortic Aneurysms and in Mice: Comparison of Serum Levels and Effect on Aneurysm Growth in Mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 29.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, et al. Use of Doxycycline to Decrease the Growth Rate of Abdominal Aortic Aneurysms: a Randomized, Double-blind, Placebo-controlled Pilot Study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 30.Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH. Doxycycline for Stabilization of Abdominal Aortic Aneurysms: a Randomized Trial. Pharmaceutical Aneurysm Stabilisation Trial Study Group. Annals of Internal Medicine. 2013;159:815–823. doi: 10.7326/0003-4819-159-12-201312170-00007. [DOI] [PubMed] [Google Scholar]
- 31.Conover WJ. Practical Nonparametric Statistics. 3. New York: Wiley; 1999. p. 396. [Google Scholar]
- 32.McMahon RP, Harrell FE., Jr Power Calculation for Clinical Trials when the Outcome is a Composite Ranking of Survival and a Nonfatal Outcome. Controlled Clinical Trials. 2000;21:305–312. doi: 10.1016/s0197-2456(00)00052-0. [DOI] [PubMed] [Google Scholar]
- 33.Haybittle JL. Repeated Assessments of Results in Clinical Trials of Cancer Treatment. Brit J Radiol. 1971;44(526):793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 34.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, I. Introduction and design. Brit J Cancer. 1976;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachin JM. A Review of Methods for Futility Stopping Based on Conditional Power. Stat Med. 2005;24(18):2747–64. doi: 10.1002/sim.2151. [DOI] [PubMed] [Google Scholar]
- 36.Pocock SJ. Group Sequential Methods in the Design and Analysis of Clinical Trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 37.Fan Xitao, Felsovalyi Akos, Sivo Stephen A, Keenan Sean C. SAS for Monte Carlo Studies: A Guide for Quantitative Researchers. SAS Institute Inc; 2002. [Google Scholar]
- 38.Metropolis N, Ulam S. The Monte Carlo Method. Journal of the American Statistical Association. 1949;44(247):335–341. doi: 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- 39.Center for Disease Control and Prevention, National Vital Statistics System. Death Rates by 10 year Age Groups: United States and Each State, 1999–2006. http://www.cdc.gov/nchs/nvss/mortality/gmwk23r.htm.
- 40.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, et al. Prolonged Administration of Doxycycline in Patients with Small Asymptomatic Abdominal Aortic Aneurysms: Report of a Prospective (Phase II) Multicenter Study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 41.Frison L, Pocock SJ. Repeating Measures in Clinical Trials: Analysis using Mean Summary Statistics and its Implications for Design. Statistics in Medicine. 1992:1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 42.Lederle FA. Abdominal Aortic Aneurysm: Still No Pill. Annals of Internal Medicine. 2013;159:852–853. doi: 10.7326/0003-4819-159-12-201312170-00012. [DOI] [PubMed] [Google Scholar]
- 43.Kroon AM, Taanman JW. Clonal expansion of T cells in abdominal aortic aneurysm: a role for doxycycline as drug of choice? Int J Mol Sci. 2015;16(5):11178–95. doi: 10.3390/ijms160511178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F- Fluorodeoxyglucose Uptake in Abdominal Aortic Aneurysms in Positron Emission/Computed Tomography is Associated with Inflammation, Aortic Wall Instability, and Acute Symptoms. J Vasc Surg. 2008;48:417–423. doi: 10.1016/j.jvs.2008.03.059. [DOI] [PubMed] [Google Scholar]