Abstract
This report highlights the discovery of a new polymorph of the anticonvulsant drug phenobarbital (PB) using polymer-induced heteronucleation (PIHn) and unravelling the crystal structure of the elusive form V. Both forms are characterized by structural, thermal and VT-Raman spectroscopy methods to elucidate phase transformation behavior and shed light on stability relationships.
Graphical Abstract
This report highlights the discovery of new polymorph ‘form XV’, and the crystal structure of the elusive form V of the anticonvulsant drug Phenobarbital.
Polymorphism, the ability of molecules to adopt more than one crystal structure, has attracted significant attention in the pharmaceutical industry due to its intellectual property and regulatory issues.1–3 Meticulous analysis of the polymorphic landscape provides valuable insights into structure-property relations which extend beyond pharmaceuticals into other areas such as explosives, pigments etc. On the other hand, polymorphism poses a major challenge to emerging crystal structure prediction methods and indeed represents a frontier for computational chemistry.4–6 The inability to predict the propensity of a molecule towards polymorphism, much less the details of structure and stability for these phases, is an “ongoing scandal” in solid state chemistry that is closely related to the much discussed problem of reliable crystal structure prediction from the knowledge of molecular structure.7 Although a recent review8 suggests that one in three molecules in CSD are polymorphic, the majority of them have one or two structurally characterized polymorphs. Molecules with three or more polymorphs are relatively rare. At the extreme end, our group was successful in tapping the polymorphic potential of flufenamic acid (FFA) through eight structurally characterized polymorphs.9 More recently, Zeidan and co-workers unraveled a case of dodecamorphism in aripiprazole (APZ) which now holds a new record with nine structurally characterized polymorphs.10 A CSD study and literature search revealed that other than FFA and APZ, a pharmaceutical precursor 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY)11 has seven forms elucidated structurally and to the best of our knowledge only eight other pharmaceuticals with five polymorphs (Fig. S1, ESI†). The polymorphic propensity of these pharmaceuticals may be attributed to various factors such as synthon diversity, flexible molecular conformation and packing differences in their crystal structures.12–25
In this study, we highlight conformational polymorphism3,26 of the anticonvulsant drug phenobarbital (PB, 5-ethyl-5-phenylpyrimidine-2,4,6(1H,3H,5H)-trione), Fig. 1. It is recorded on the list of essential medicines27 by the World Health Organization (WHO). PB is used widely for treating epilepsy and neonatal seizures. The polymorphic propensity of PB has intrigued researchers over the last six decades.28–37 In total, eleven polymorphs (I–XI), a hydrate (form XII), a hemihydrate (form XIII) and an amorphous phase (form XIV) are reported in the literature, making it one of the benchmark pharmaceuticals for studying polymorphism and crystal structure prediction.38,39 However, thus far only three polymorphs were amenable to structure elucidation, hinting at a molecule resistant to forming single crystals suitable for structure determination.35, 37
Figure 1.
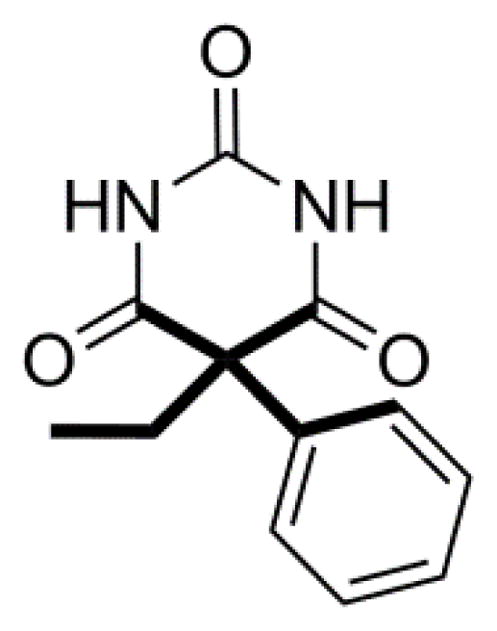
Chemical structure of Phenobarbital (PB).
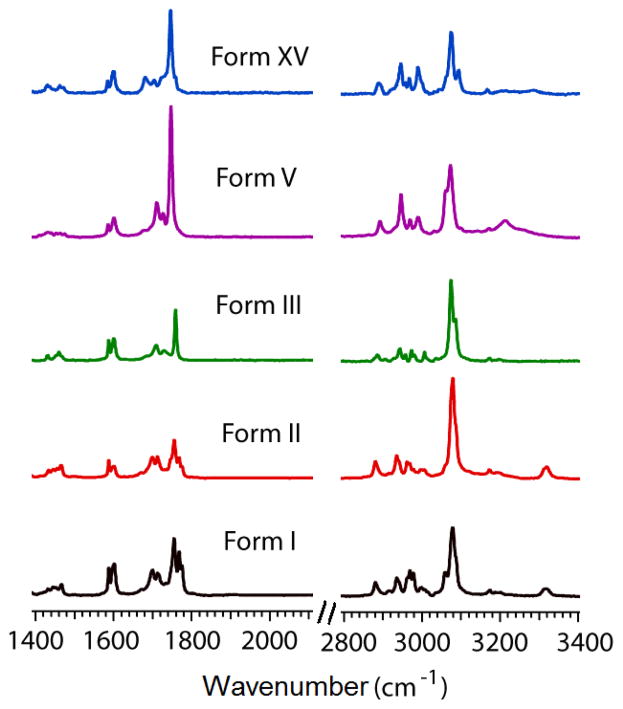
Discovery of polymorphs for such well-studied pharmaceuticals pose a major challenge to polymorph discovery techniques, such as polymer-induced heteronucleation (PIHn).40, 41 PIHn is a crystallization technique through which a single solvent/temperature condition leads to novel form discovery, enabled by a diverse array of insoluble polymers acting as heterogeneous growth sites.6, 13, 42–44 Initial crystallization utilizing PIHn resulted in forms I–III. Normal melt crystallization of PB yields an amorphous solid, which over the time converts to form I and II in the same experimental condition. However, melt crystallization of PB mixed with 4-(benzyloxy) benzonitrile modified polystyrene resulted in a novel phase, termed as form XV. Further, controlled sublimation of PB resulted in the crystallization of the elusive form V as very thin needles (SI. 2, experimental section, ESI†). Optical microscopy (Fig. S2, ESI†) and Raman spectroscopy (Fig. S3, ESI†) identified these five distinct polymorphs with characteristic Raman spectra in two main spectral regions at 3400–2900 and 1800–1400 cm−1 (Fig. 2). Powder X-ray diffraction (PXRD) patterns clearly reveal the difference between form V and XV and distinguish them from other forms (Fig. S4, ESI†). Form V exhibits unique 2θ peaks at 7.0, 8.0, 13.2, 14.1, 14.9 and 17.2, whereas novel form XV was identified from the peaks at 6.8, 7.3, 7.6, 11.9, 13.7, 14.7, 15.3, 19.6, 2θ (Table S1, ESI†).
Figure 2.
Raman spectra of PB polymorphs I, II, III, V and XV.
Single crystal X-ray diffraction was carried out on PB form V and XV. Form V is known since 1951 and unit cell dimensions were reported by Williams in 1973.36 Since then several researchers30,31 were able to produce only microcrystalline form V either by melt crystallization or sublimation. Through sublimation experiments using a hostage connected control processor, we were able to obtain diffraction quality form V crystals suitable for structure determination. Both form V and Form XV crystallized in monoclinic P21/n space group with two molecules in the asymmetric unit (Fig. S5, Table S2, ESI†). In the five crystal structures of PB, eleven symmetry independent molecules represent diverse conformational flexibility consistent with conformational polymorphism (Fig. 3). Such diversity is a major challenge for structure prediction by computational methods. In fact, a crystal structure prediction exercise was attempted by Day et al to predict form V.39 Though none of the predicted structures with Z′=2 matches with form V or form XV, structural analysis revealed that the predicted hydrogen bonding motifs of one of the structures matched with form V (Vide infra).
Figure 3.
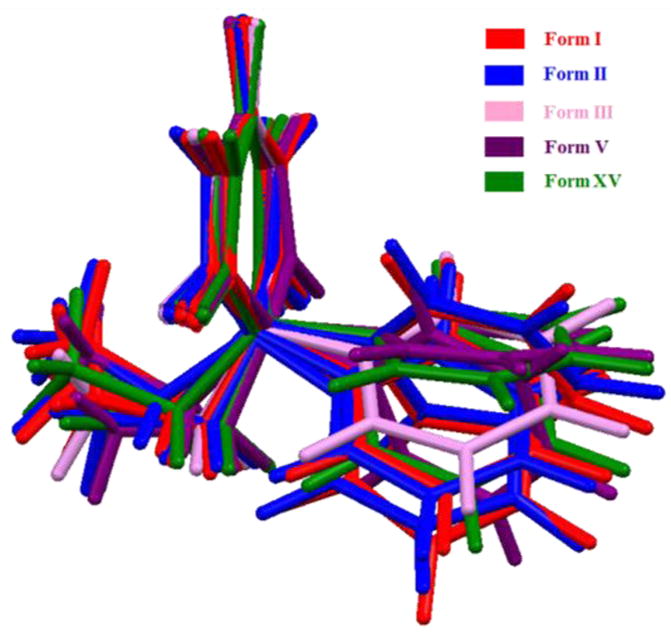
Overlay of 11 symmetry-independent molecules present in form I, II, III, V and XV of PB.
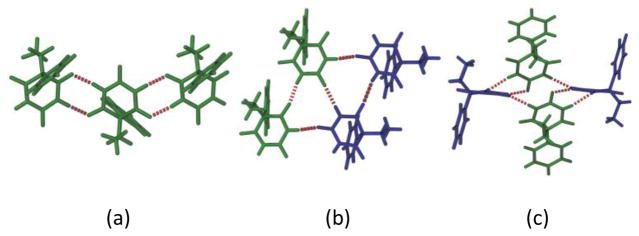
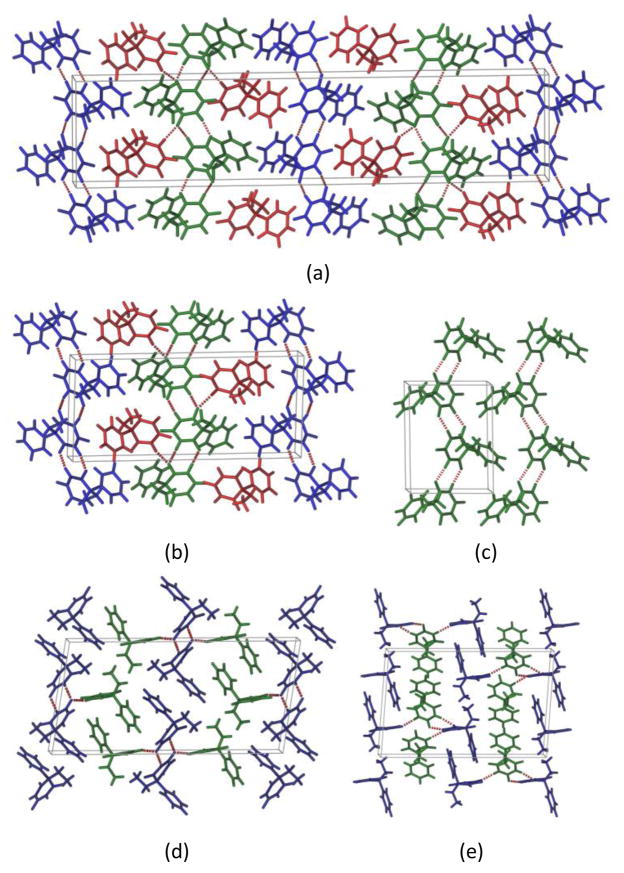
Structural analysis of the five PB forms display the presence of N–H···O hydrogen bonded R22(8) dimers (Fig. 4a). These dimers propagate through various symmetry elements (inversion, glide or screw axes) to form 1D ribbon chains in different forms. In form I and form II (both with Z′=3) two independent chains propagate through two symmetry independent molecules (blue and green molecules in Fig. 5a and 5b) connected by a third molecule (red) acting as bridge between the two chains. Overall both form I and form II exhibit very similar crystal packing (Fig. 5a and 5b). In form III (Z′=1), 21 screw axis extend these ribbon-like chains which are further supported by C–H···O hydrogen bonds (Fig. 5c). In Form V (Z′=2), one type of symmetry independent molecule (blue) extend as a molecular chain, while the other molecule (green) connects this chain through N–H···O hydrogen bonds. The N–H···O catemer chains of crystallographically inequivalent molecules interact with each other forming an R33(12) tape motif (Fig. 4b), completing the 3D packing (Fig. 5d). Presence of this hydrogen bonded tape motif was predicted in the PB crystal structure prediction exercise by Day et al.39 The hydrogen bond dimer chains are notably different in form XV, where N–H···O dimers involve two symmetry independent molecules (Fig. 4c). These dimers further extend through N–H···O hydrogen bonds enabling a significantly different packing for form XV in comparison to other polymorphs (Fig. 5e).
Figure 4.
Hydrogen bonding motifs observed in PB polymorphs (a) R22(8) chain motif (b) R33(12) tape motif and (c) ladder.
Figure 5.
Packing of (a) form I (b) form II (c) form III view along ab plane (d) form V and (e) form XV view along ac plane.
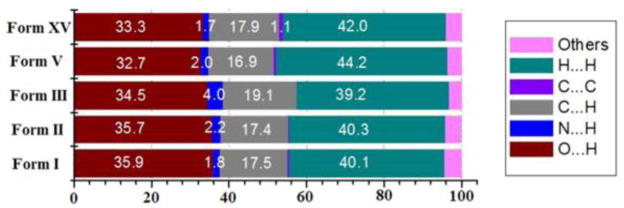
Hirshfeld surface45 analysis of the polymorphs of PB (Fig. 6) revealed that form I, II and III exhibit greater contribution from O···H contacts (35.9%, 35.7%and 34.7%) than form V (32.7%) and form XV (33.3%). However, form III has more N···H (4%) and C···H (19%) contacts than other polymorphs. Overall, the analysis suggests that form V and XV have comparatively lower contribution from strong hydrogen bonding contacts than the other three polymorphs (Fig. S6, ESI†).
Figure 6.
Average relative contribution to the Hirshfeld surface for the various intermolecular contacts for the polymorphs of PB.
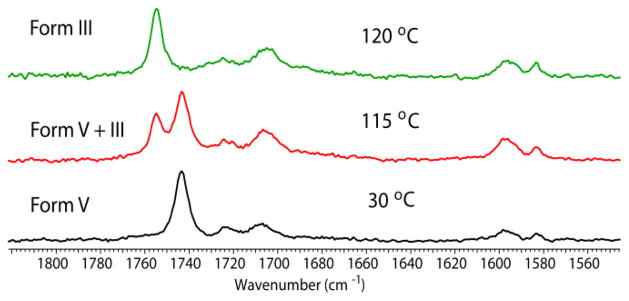
Differential scanning calorimetry (DSC) confirmed that form I exhibits the highest melting point (176 °C), while all the other polymorphs convert to form I during heating (Fig. S7, ESI†). This thermal conversion was monitored through variable temperature Raman spectroscopy. Form I does not show any phase transformation on heating whereas form II converts to form I before melting at 170 °C (Fig. S8a, ESI†). Form V converts completely to form III at 120 °C (Fig. 7), which in turn transforms to form II and I above 165 °C (Fig. S8b, ESI†). Form XV converts to form II and I above 80 °C (Fig. S8c, ESI†). In order to determine the relative free energy relationship, slurry conversion experiments were carried out with PB polymorphs in ethanol. This experiment resulted in the complete conversion of all polymorphs to form I. Thus at room temperature form I is the most stable modification of PB. Equilibrium solubility experiments in heptane suggested that form I and form II are similar in energy, whereas form XV is the least stable modification among forms I, II, III and XV (Fig. S9, ESI†).
Figure 7.
Variable temperature Raman spectroscopy shows partial conversion of form V to form III at 115 °C, with complete conversion occurring at 120 °C.
In conclusion, new polymorphic landscape has been accessed for a compound that is well studied over the last six decades. This study highlights the importance of crystal growth techniques like PIHn in expanding the polymorphic propensity of a molecule that is previously known to exhibit several polymorphs. Moreover we were able to solve the half a century old puzzle of elucidating form V crystal structure. The presence of diverse hydrogen bonding motifs, multiple molecules in the asymmetric unit, and a high degree of conformational flexibility, project PB as a challenging benchmark molecule for crystal structure prediction. Unraveling the crystal structures of other polymorphs of PB, augmented by better crystal growth methods and more powerful diffraction techniques may further clarify the intricate relationship between molecular conformation and polymorphism.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grant Number GM106180. We thank Jeff W. Kampf for his help with structure determination of form V.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental and characterization. CCDC numbers: 1450610, 1450611 See DOI: 10.1039/x0xx00000x
References
- 1.Byrn SR, Pfeiffer RR, Stowell JG. Solid-State Chemistry of Drugs. SSCI; West Lafayette, IN: 1999. [Google Scholar]
- 2.Hilfiker R. Polymorphism in the Pharmaceutical Industry. Wiley-VCH, Weinheim; Weinheim: 2006. [Google Scholar]
- 3.Bernstein J. Polymorphism in Molecular Crystals. Clarendon: Oxford; 2002. [Google Scholar]
- 4.Day GM, Cooper TG, Cruz-Cabeza AJ, Hejczyk KE, Ammon HL, Boerrigter SXM, Tan JS, Della Valle RG, Venuti E, Jose J, Gadre SR, Desiraju GR, Thakur TS, van Eijck BP, Facelli JC, Bazterra VE, Ferraro MB, Hofmann DWM, Neumann MA, Leusen FJJ, Kendrick J, Price SL, Misquitta AJ, Karamertzanis PG, Welch GWA, Scheraga HA, Arnautova YA, Schmidt MU, van de Streek J, Wolf AK, Schweizer B. Acta Crystallogr Sect B: Struct Sci. 2009;65:107. doi: 10.1107/S0108768109004066. [DOI] [PubMed] [Google Scholar]
- 5.Neumann MA, Leusen FJJ, Kendrick J. Angew Chem Int Ed. 2008;47:2427. doi: 10.1002/anie.200704247. [DOI] [PubMed] [Google Scholar]
- 6.Roy S, Matzger AJ. Angew Chem Int Ed. 2009;48:8805. doi: 10.1002/anie.200903285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddox J. Nature. 1988;335:201. doi: 10.1038/335760a0. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Cabeza AJ, Reutzel-Edens SM, Bernstein J. Chem Soc Rev. 2015;44:8619. doi: 10.1039/c5cs00227c. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Mejias V, Kampf J, Matzger AJ. J Am Chem Soc. 2012;134:9872. doi: 10.1021/ja302601f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidan TA, Trotta JT, Tilak PA, Oliveira MA, Chiarella RA, Foxman BM, Almarsson Ö, Hickey MB. CrystEngComm. doi: 10.1039/c5ce02467f. [DOI] [Google Scholar]
- 11.Chen S, Guzei IA, Yu L. J Am Chem Soc. 2005;127:9881. doi: 10.1021/ja052098t. [DOI] [PubMed] [Google Scholar]
- 12.Blagden N, Davey RJ, Lieberman HF, Williams L, Payne R, Roberts R, Rowe R, Docherty R. J Chem Soc Faraday Trans. 1998;94:1035. [Google Scholar]
- 13.Lopez-Mejas V, Kampf J, Matzger AJ. J Am Chem Soc. 2009;131:4554. doi: 10.1021/ja806289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystallogr Sect E: Struct Rep Online. 2006;62:o4393. [Google Scholar]
- 15.Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystallogr Sect C: Struct Chem. 2007;63:o355. doi: 10.1107/S010827010701952X. [DOI] [PubMed] [Google Scholar]
- 16.Drebushchak TN, Chukanov NV, Boldyreva EV. Acta Crystallogr Sect C: Struct Chem. 2008;64:o623. doi: 10.1107/S0108270108034550. [DOI] [PubMed] [Google Scholar]
- 17.Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. J Pharm Sci. 2009;98:2010. doi: 10.1002/jps.21574. [DOI] [PubMed] [Google Scholar]
- 18.Arlin JB, Price LS, Price SL, Florence AJ. Chem Commun. 2011;47:7074. doi: 10.1039/c1cc11634g. [DOI] [PubMed] [Google Scholar]
- 19.Grzesiak AL, Lang MD, Kim K, Matzger AJ. J Pharm Sci. 2003;92:2260. doi: 10.1002/jps.10455. [DOI] [PubMed] [Google Scholar]
- 20.Chekal BP, Campeta AM, Abramov YA, Feeder N, Glynn PP, McLaughlin RW, Meenan PA, Singer RA. Org Process Res Dev. 2009;13:1327. [Google Scholar]
- 21.Surov AO, Solanko KA, Bond AD, Perlovich GL, Bauer-Brandl A. Cryst Growth Des. 2012;12:4022. [Google Scholar]
- 22.Mishra MK, Desiraju GR, Ramamurty U, Bond AD. Angew Chem Int Ed. 2014;53:13102. doi: 10.1002/anie.201406898. [DOI] [PubMed] [Google Scholar]
- 23.Thirunahari S, Aitipamula S, Chow Pui Shan, Tan RBH. J Pharm Sci. 2010;99:2975. doi: 10.1002/jps.22061. [DOI] [PubMed] [Google Scholar]
- 24.Nath NK, Nangia A. CrystEngComm. 2011;13:47. [Google Scholar]
- 25.Karanam M, Dev S, Choudhury AR. Cryst Growth Des. 2012;12:240. [Google Scholar]
- 26.Nangia A. Acc Chem Res. 2008;41:595. doi: 10.1021/ar700203k. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Model List of Essential Medicines. 19. World Health Organization (WHO); May, 2015. [Google Scholar]
- 28.Huang TY. Acta Pharm Intern. 1951;2:95. [PubMed] [Google Scholar]
- 29.Huang TY. Acta Pharm Intern. 1951;2:43. [PubMed] [Google Scholar]
- 30.Zencirci N, Gelbrich T, Apperley DC, Harris RK, Kahlenberg V, Griesser UJ. Cryst Growth Des. 2010;10:302. [Google Scholar]
- 31.Zencirci N, Gelbrich T, Kahlenberg V, Griesser UJ. Cryst Growth Des. 2009;9:3444. doi: 10.1021/acs.cgd.0c00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesley RJ, Clements RL. J Pharm Pharmacol. 1968;20:341. doi: 10.1111/j.2042-7158.1968.tb09757.x. [DOI] [PubMed] [Google Scholar]
- 33.Mesley RJ, Clements RL, Flaherty B, Goodhead KJ. J Pharm Pharmacol. 1968;20:329. doi: 10.1111/j.2042-7158.1968.tb09756.x. [DOI] [PubMed] [Google Scholar]
- 34.Mesley RJ. Spectrochim Acta. 1970;26A:1427. [Google Scholar]
- 35.Williams PP. Acta Crystallogr Sect B: Struct Sci. 1974;30:12. [Google Scholar]
- 36.Williams PP. Acta Crystallogr Sect B: Struct Sci. 1973;29:1572. [Google Scholar]
- 37.Platteau C, Lefebvre J, Hemon S, Baehtz C, Danede F, Prevost D. Acta Crystallogr Sect B: Struct Sci. 2005;61:80. doi: 10.1107/S0108768104031143. [DOI] [PubMed] [Google Scholar]
- 38.Otsuka M, Nakanishi M, Matsuda Y. Drug Dev Ind Pharm. 1999;25:205. doi: 10.1081/ddc-100102161. [DOI] [PubMed] [Google Scholar]
- 39.Day GM, Motherwell WDS, Jones W. Phys Chem Chem Phys. 2007;9:1693. doi: 10.1039/b612190j. [DOI] [PubMed] [Google Scholar]
- 40.Lang MD, Grzesiak AL, Matzger AJ. J Am Chem Soc. 2002;124:1484. doi: 10.1021/ja0286526. [DOI] [PubMed] [Google Scholar]
- 41.Price CP, Grzesiak AL, Matzger AJ. J Am Chem Soc. 2005;127:5512. doi: 10.1021/ja042561m. [DOI] [PubMed] [Google Scholar]
- 42.Grzesiak AL, Uribe FJ, Ockwig NW, Yaghi OM, Matzger AJ. Angew Chem, Int Ed Engl. 2006;45:2553. doi: 10.1002/anie.200504312. [DOI] [PubMed] [Google Scholar]
- 43.Grzesiak AL, Matzger AJ. J Pharm Sci. 2007;96:2978. doi: 10.1002/jps.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foroughi LM, Kang YN, Matzger AJ. Cryst Growth Des. 2011;11:1294. [Google Scholar]
- 45.Speckman MA, Jayatilaka D. CrystEngComm. 2009;11:19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.