Abstract
Primary biliary cholangitis (PBC) is an autoimmune hepatobiliary disease characterized by immune mediated destruction of the intrahepatic small bile ducts and the presence of antimitochondrial antibodies (AMAs). The mitochondrial autoantigens have been identified as the E2 subunits of the 2-oxo-acid dehydrogenase complex, including the E2 subunits of pyruvate dehydrogenase, branched-chain 2-oxo acid dehydrogenase complex, oxoglutarate dehydrogenase complex, E3 binding protein and PDC E1 alpha subunit. The AMA epitope is mapped within the E2 lipoic acid binding domain, which is particularly important for oxidative phosphorylation. In addition, lipoic acid, which serves as a swinging arm to capture electrons, is particularly susceptible to an electrophilic attack and may provide clues to the etiology of PBC. This review emphasizes the molecular characteristics of AMAs, including detection, immunochemistry and the putative role in disease. These data have significance not only specifically for PBC, but generically for autoimmunity.
Keywords: Antimitochondrial antibodies, E2 subunit of pyruvate dehydrogenase (PDC-E2), indirect immunofluorescence, immunoblotting, ELISA, Luminex bead assay, enzyme inhibition assay
Introduction
Antimitochondrial antibody (AMA) testing is a standard laboratory workup in patients suspected of hepatobiliary disease. In 1965, AMA was first detected in the sera of patients with primary biliary cholangitis (PBC) [1]. AMA is now a well-established serological marker of PBC. PBC is a female predominant chronic autoimmune cholestatic liver disease of unknown etiology [2]. Both genetics and environmental components are suspected to contribute to the pathogenesis of PBC [3-8]. Immunologically, PBC is characterized by high titer of AMA and immune mediated destruction of intrahepatic bile ducts. Clinically, patients with advanced stage PBC are presented with fatigue, generalized pruritus, obstructive jaundice, osteoporosis, fat-soluble vitamin deficiencies and portal hypertension. In recent years, the course of primary biliary cholangitis has improved substantially with ursodeoxycholic acid (UDCA) therapy [9,10]. For the diagnosis of PBC, the patient must have at least two of the following three parameters: clinical and/or biochemical characteristics of cholestasis, AMA reactivity, and histological changes associated with cholestasis, particularly florid biliary lesions and portal granulomas (Table 1) [11]. AMA can be detected in over 95% of patients with PBC and can be detected even before clinical symptoms or biochemical abnormalities [12-14]. The fact that AMA is present many years before disease manifestation and its high titer is persistent throughout the disease suggests that AMA has a contributory role in the immunopathology of PBC [15,16]. AMA can also be readily detected in animal models of PBC [17-30] even before the appearance of liver pathology.
Table 1. Criteria for Diagnosis of PBC*.
|
Positive diagnosis of PBC is required with at least two of the three above criteria
Molecular identification of PBC mitochondrial autoantigens
The cDNA sequence and molecular identities of the AMA autoantigens were first reported in the late 1980s [31-33]. AMA recognizes components of the 2-oxo-acid dehydrogenase complex within the mitochondria. Subsequent antigen specific isotype studies, epitope mapping, analysis of murine and PBC patient-derived monoclonal antibodies have revealed that AMAs are directed against a highly specific epitope within the lipoyl domain of the E2 subunits of the 2-oxo-acid dehydrogenase complex (2-OADC), with the E2 subunit of pyruvate dehydrogenase (PDC-E2) being the immunodominant mitochondrial antigen in PBC [33-44]. The other mitochondrial autoantigens in PBC include the E2 subunit of branched-chain 2-oxo acid dehydrogenase complex (BCOADC-E2), and E2 subunit of oxoglutarate dehydrogenase complex (OGDC-E2) (Table 2) [38,39]. Interestingly, AMA of IgA isotype can be found not only in the sera of patients with PBC, but also in their saliva, urine and bile [45-48], indicating the AMA of IgA isotype can be transported across the epithelial mucosa and hence has a role in mucosal immunity in PBC. The molecular identification of AMA autoantigens and their epitopes have generated specific reagents for the detection of AMA.
Table 2. Biochemical Characteristics of Mitochondrial Autoantigens in PBC.
| Autoantige n |
Molecular Weight |
Biochemical Function | Cofactors | Epitope | Predomina nt IgG isotype |
|---|---|---|---|---|---|
| PDC-E2 | 70 kDa | Transfers acetyl group from pyruvate to CoA to form acetyl-CoA |
Lipoic acid CoA |
Lipoyl domains | IgG3 |
| BCOADC- E2 |
52 kDa | Transfer acyl group from alpha keto acids to CoA to form acyl-CoA |
Lipoic acid CoA |
Lipoyl domain | IgG2 |
| OGDC-E2 | 48 kDa | Decarboxylation of alpha ketoglutarate and the addition of CoA to form succinyl-CoA |
Lipoic acid CoA |
Lipoyl domain | IgG2 |
| E3BP | 55KDa | Anchoring dihydrolipoamide dehydrogenase (E3) to PDC-E2 |
Lipoic Acid | Lipoyl domain | ND* |
| PDC-E1 alpha |
41KDa | Decarboxylation of pyruvate to form 2(-1-hydroxyethylidene)-TPP and reductive acetylation to form acetyl- lipoate |
Thiamine Pyrophosphate |
Phosphorylation site and the thiamine pyrophosphate binding site. |
ND |
ND: Non-determined
Biochemistry of AMA autoantigens and significance of lipoic acid
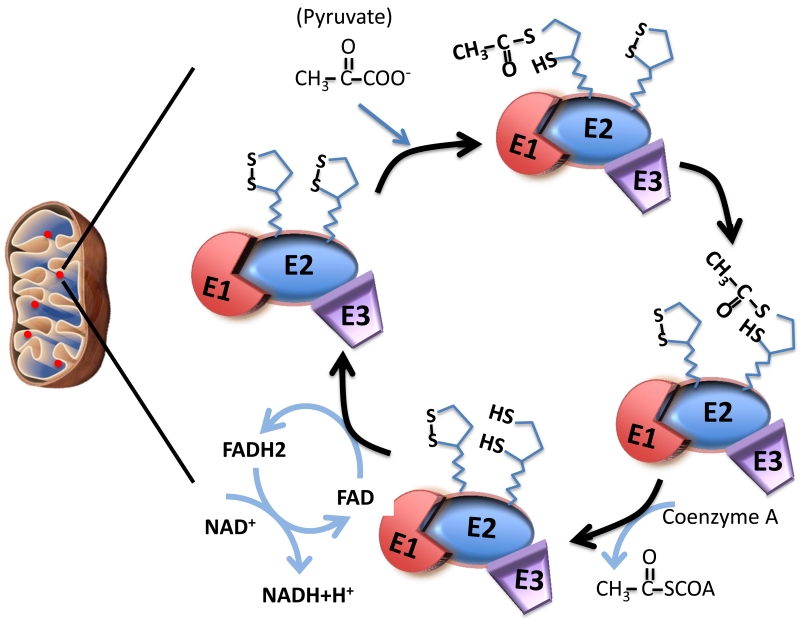
The 2-oxo-acid dehydrogenase complex (2-OADC) is among the best biochemically characterized enzyme. 2-OADC consists of three related complexes, the PDC, OGDC and BCOADC, which are located in the mitochondrial matrix. These enzyme complexes, although similar in structure, are distinct in their catalytic function for the oxidation of their specific 2-oxoacids to their corresponding acyl co-enzyme A, with reduction of NAD+ to NADH. The subunits E1, E2 and E3 are spatially arranged for a series of biochemical reactions in which E1 decarboxylates the substrate and transfers its product to E2; E2 produces acyl CoA through the reduction of the lipoic acid cofactor on E2 and E3 reoxidizes the lipoic acid, with the reduction of NAD+ to NADH (Figure 1). E3BP is responsible for binding of E3 to the core PDC.
Figure 1.
Biochemistry of pyruvate dehydrogenase in decarboxylation and acetyl transfer. PDC is located near the mitochondrial inner membrane within the mitochondrial matrix. The subunits E1, E2 and E3 are spatially arranged for a series of biochemical reactions. Inside the mitochondria, E1 decarboxylates pyruvate, the acetyl group is transferred to one of the lipoic acids on PDC-E2. The opening and closing of the S-S bond together with the swinging arm of the lipoic acid moiety allow for transfer the acetyl group to coenzyme A to form acetyl-coA through the reduction of the lipoic acid cofactor on E2. The reduced form of lipoic acid (lipoamide) is re-oxidized to the disulfide form, as 2 e− + 2 H+ are transferred to a disulfide on E3 (disulfide interchange), which subsequently transfer 2 e− + 2 H+ to reduce FAD to FADH2. FADH2 is then re-oxidized by transferring its electron to NAD+, to yield NADH + H+. Through this process, lipoic acid is also re-oxidized.
In PDC, the E2 subunits and E3BP are both autoantigens in PBC. Structurally, both PDC-E2 and E3BP are composed of a central core region, a binding domain and lipoyl domain. There are two lipoyl domains in PDC-E2 and only one lipoyl domain in E3BP. High resolution structural analysis and modeling studies indicated that the lipoyl domains of PDC-E2 are exposed on the surface of the molecule with lipoic acid attached to a lysine residue, rendering lipoic acid exposed at the tip of the β-turn structure [49-51]. The antigenicity of the lipoyl domain may be partly explained by the distinct structure of the lipoyl domain together with the ability of lipoic acid to rotate by its “swinging arms” with respect to the bulk of the PDC-E2 molecule and the opening and closing of the lipoic acid S-S bond for electron transfer reduction acylation (Figure 1). Importantly, quantitative structure-activity relationship (QSAR) analysis demonstrated that when the lipoyl domain of PDC-E2 is modified with specific synthetic small molecule lipoyl mimics, some of the modified PDC-E2 are more reactive to AMA than lipoyl PDC-E2 [52,53]. Further, QSAR analysis on a focused panel of lipoic acid mimics in which the lipoyl S-S bond are modified provided evidence suggesting direct alteration of the lipoyl ring by xenobiotics – that is, disruption of the S-S linkage activates the lipoic acid and makes it receptive for xenobiotic modification and subsequent AMA recognition [52-57]. Furthermore, mutagenesis analysis of the PDC-E2 lipoyl domain revealed that specific amino acid residues are critical in maintaining the lipoyl loop conformation necessary for AMA recognition [58].
Biochemically, lipoic acid is a cofactor essential for the electron transfer function of the E2 subunits of PDC-E2, BCOADC-E2, OGDC-E2 and E3BP. Fregeau, et al. studied the AMA reactivity to BCOADC-E2 and OGDC-E2 and showed that affinity-purified PBC antibodies to BCOADC-E2 and OGDC-E2 self-recognize and do not cross-react to either PDC-E2, BCOADC-E2 or OGDC-E2. Thus, the absence of cross-reactivity between the three antigens suggests that, despite their structural and functional similarity, they each have distinct epitopes and are recognized by different subpopulations of PBC-specific AMA. Fregeau, et al. also showed that affinity-purified PBC sera specifically inhibited the enzyme function of each of these antigens specifically. Epitope mapping demonstrated that epitopes in each antigen are within the lipoic acid binding domain suggesting the binding of AMA disrupts the lipoic acid domain and inhibits enzymatic function [37,38].
Additional antigens have been identified in the PDC including the E3 binding protein (previously called Protein X) and the E1α subunit [43,59]. Almost all anti-PDC-E2 sera react with E3BP, suggesting the presence of cross-reactive epitopes [43]. Fregeau, et al. have found that 66% of PBC sera reacted with purified PDC-E1α and Kuroda, et al. found that levels of anti-PDC-E2 sera strongly correlated with levels of anti-E1 sera [59,60]. Like the other autoantigens of PBC, AMA is directed against the functional domain of PDC-E1α but this enzyme does not contain lipoic acid [61].
Other autoantigens in PBC
In addition to mitochondrial autoantigens, nuclear antigens are also present in PBC patients. 53% of PBC patients carried antinuclear antibodies (ANA), mainly against gp210 and sp100 [62]. Patients with persistent high titer anti-gp210 antibodies are associated with more severe interface hepatitis lobular inflammation, and progression to end stage liver failure [63-65]. Screening of protein microarrays with serum samples from patients with PBC have identified six novel PBC autoantigens, including hexokinase-1 (HK1), Kelch-like protein 7, Kelch-like protein 12 (KLHL12) zinc finger and BTB domain-containing protein 2, and eukaryotic translation initiation factor 2C, subunit 1 [66]. These new autoantigens may serve as useful serological biomarkers for the diagnosis of PBC. Interestingly, another study using an independent cohort of serum samples from patients with PBC and controls validated the specificity of anti-KLHL12 antibodies and anti-HK1 antibodies in both AMA-positive and AMA-negative PBC patients. In addition, antibodies to KLHL12 and anti-HK1 have higher sensitivity than anti-gp210 and anti-sp100 [67].
Detection of AMA
There are five common strategies for detecting AMA: indirect immunofluorescence (IIF), immunoblotting, enzyme linked immunosorbent assay, (ELISA), luminex beads assay and enzyme inhibition assay (EI). These strategies are based on the specific recognition of AMA to mitochondrial proteins. The readouts of these methods range from identifying AMA specificities from cellular level to the molecular level and can be applied accordingly depending on the goal of the study and facility available.
Indirect immunofluorescence (IIF)
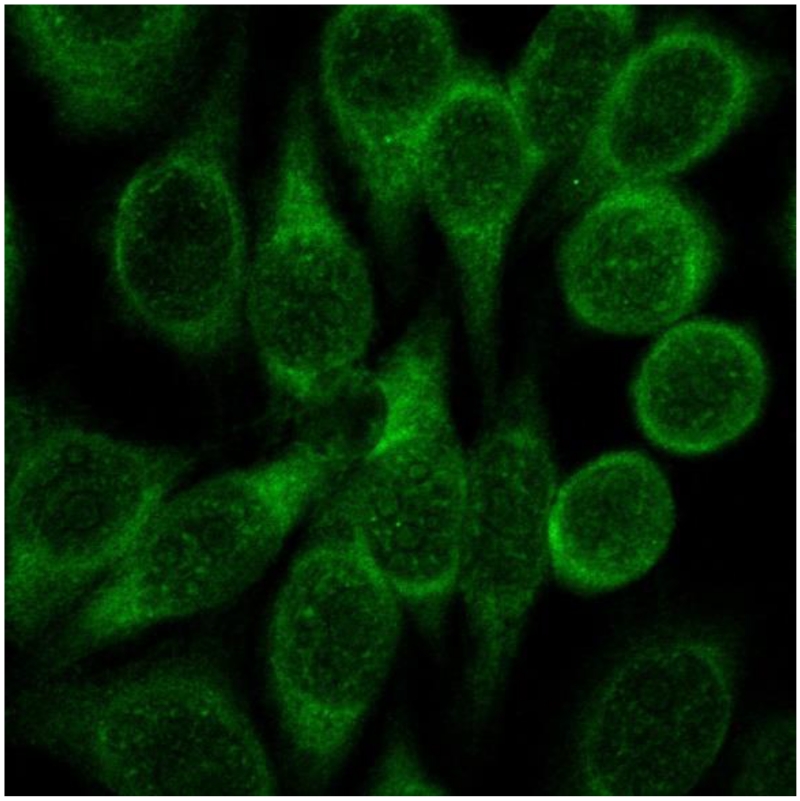
IIF is a routine method for AMA detection. Indirect immunofluorescence is a two-step technique in which unlabeled primary antibodies first bind to the target antigen in the substrate followed by binding of fluorescent-labeled secondary antibodies to the primary antibodies. Detection of AMA by immunofluorescence can be performed using rat multiorgan (kidney, liver, stomach) substrates or HEp-2 cells and examined by fluorescent microscopy [68]. Positivity in HEp-2 cells show granular cytoplasmic staining (Figure 2). However, it may be difficult to discern AMA from concurrent antibodies e.g. liver/kidney microsomal antibodies when using HEp-2 cells [69]. With the multiorgan substrate, AMA will preferentially stain distal over proximal renal tubules as well as the gastric cells. The screening serum dilution for PBC-specific AMA begins with sera dilution of 1/40 and subsequent higher dilutions to determine the titer [68].
Figure 2.
Immunofluorescence of AMA positive PBC sera on HEp-2 cells. Note the presence of strong cytoplasmic staining in the cells.
Accuracy of results from IIF largely depends on the observer’s skill in recognizing the characteristic patterns for AMA. The method is time-consuming and requires extra attention in the preparation of sera, tissue substrate, and the fluorescent antiserum samples. Undiluted sera, high fluorescein-protein ratio, and fixed tissues can all cause non-specific binding [68,70]. To circumvent these constraints, commercial AMA detection kits included with HEp-2 cells fixed on a microscope slide, pretested reagents and positive control sera samples are available (e. g. INOVA Diagnostics). It should be noted that low specificity of IIF is one of its disadvantages in screening for AMA. For example, in a study performed by Provenzano, et al., 16 non-PBC patients had positive AMA by IIF but only 3 were confirmed to be AMA positive by immunoblotting. In addition, over the course of two years, 18 out of 34 patients with positive AMA by IIF were subsequently diagnosed as PBC [69]. In spite of such limitations, IIF is still a sufficiently sensitive method that will be useful in diagnosis when utilized with other assays [71].
Immunoblotting
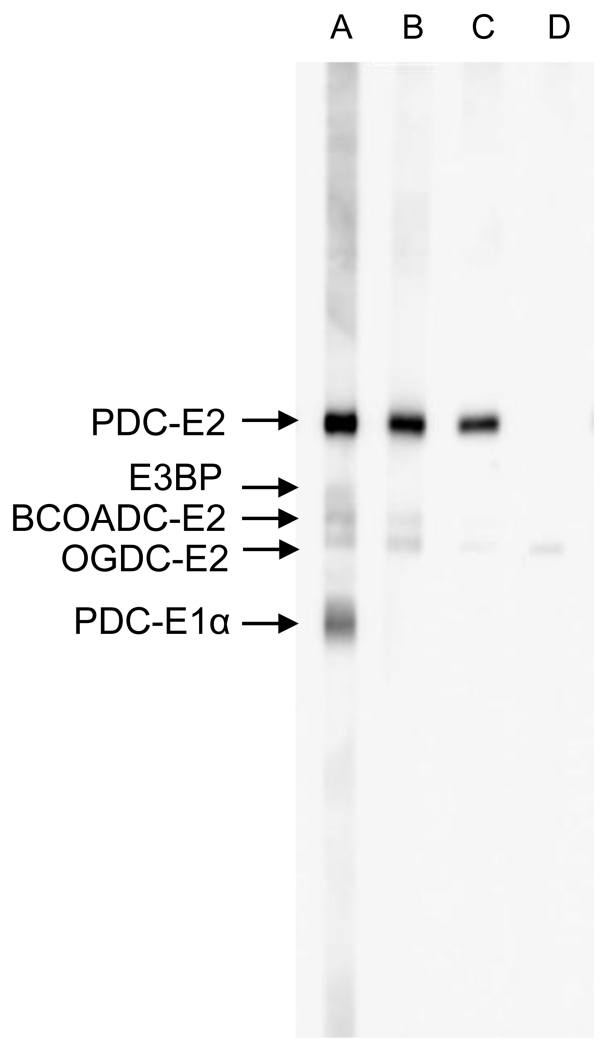
Immunoblotting, also known as Western blotting, tests the reactivity of antibodies in sera with different antigens separated out by their molecular weight via gel electrophoresis. Briefly, proteins are resolved by SDS-PAGE, transferred onto membranes and incubated with primary antibodies. Secondary antibodies conjugated with enzymes are added to bind to the primary antibodies. After washing off non-specific bindings, a chromogenic substrate is added to visualize enzyme labeled secondary antibody bound protein complexes. The degree of positivity can be measured by the band intensity of the target protein. Sources of antigen include mitochondrial preparations isolated from bovine heart and more recently recombinant autoantigens. Immunoblotting is a highly sensitive and specific method, depending on the source of antigen used. Recombinant proteins are preferred over crude mitochondria from bovine heart, which can result in greater background (Figure 3). AMA ELISA against PDC-E2, BCOADC-E2, OGDC-E2, E3BP and PDC E1α have been demonstrated to be highly selective in the detection of AMA reactivity to each of these mitochondrial autoantigens [40,42,58,61,72,73]. Higher sensitivity can be achieved with the use of recombinant hybrid molecules, such as MIT3, which co-expresses the three immunodominant epitopes of PDC-E2, BCOADC-E2, and OGDC-E2 [73]. By immunoblotting, 91% of PBC sera reacted against the recombinant protein, compared to 81% against the recombinant fusion protein of PDC-E2 [73]. The difference in sensitivity can be attributed to the fact that there is a small percentage of PBC patients with AMA reacting only to BCOADC-E2 and/or OGDC-E2 [40,74,75]. This is also true for ELISA showing lower sensitivity when recombinant PDC-E2 is used alone and higher sensitivity with the recombinant molecule containing all three antigens [74]. Overall, immunoblotting is a highly sensitive and specific method. However, it is not the most practical assay for clinical laboratories since it is time-consuming and involves a laborious and complicated technique.
Figure 3.
Immunoblot analysis of PBC sera. Mitochondrial extract was resolved on SDS-PAGE, transferred to nitrocellulose membrane and probed with AMA positive sera. Note AMA reactivity to PDC-E2, E3BP, BCOADC-E2 and OGDC-E2 (lane A), AMA reactivity to PDC-E2, BCOADC-E2 and OGDC-E2 (lane B), AMA reactivity to PDC-E2 and OGDC-E2 (lane C) and AMA reactivity to OGDC-E2 only (lane D). Note: To minimize background, recombinant proteins are preferred over mitochondrial extracts for AMA detection.
Enzyme-linked immunosorbent assay (ELISA)
Enzyme-linked immunosorbent assay (ELISA) is a quantitative method that can measure the titer of antibodies and reactivity of sera to the target antigen. The technique is similar to immunoblotting, in which the target antigen is first coated onto well plates and incubated with primary antibodies, which are then bound by enzyme-conjugated secondary antibodies. In the detection step, positivity is determined by adding a chromogenic substrate in each well and the color intensity measured as optical density (OD). Positive AMA in ELISA is defined by OD value of 2-10 SD above the mean control depending on the laboratory [75]. ELISA is one of the most practical assays for AMA screening in clinical laboratories because it is rapid, objective, automated, and can test a large number of samples at once. Recently, sensitive ELISAs using MIT3 or a mixture of purified PDC and MIT3 as antigenic targets have been developed [76]. In the IgG and IgA MIT3-based ELISAs, the sensitivity and specificity for AMA detection in PBC patients was higher than IIF and the conventional anti-M2. This MIT3 ELISA could detect the presence of AMA in nearly half of the AMA-negative sera by IIF [74]. Dähnrich et. al. developed an ELISA for AMA detection (anti-M2-3E), using a mixture of purified PDC and the recombinant hybrid MIT3. Their data demonstrated a 93.6% of sensitivity for AMA detection compared with 91.3%, 83.8%, and 87.3% for MIT3, purified PDC, or IIF, when all specificities are set to 98.8% [76]. Gabeta, et al. found positive AMA in 12/27 patients with negative AMA by IIF through IgG MIT3 ELISA, and 13/27 through IgG/IgA MIT3 ELISA [74]. The availability of recombinant proteins using cloned antigens from human sources greatly increased the sensitivity of ELISA (94%, compared with 84% by IIF) in the detection of AMA, with 73% of PBC patients with negative AMA by IIF showing positive reactivity [75].
Luminex bead assay
The bead assay using the Luminex principle is a highly sensitive and specific assay comparable to ELISA. The assay uses beads pre-coated with target antigens, to which primary antibodies from a serum sample are added and attached. Detection antibodies are used to capture the primary antibodies and visualize them under a dual-laser flow-based detection instrument. The method is quantitative as the readout is in pixel intensity and can be titered out by sera dilution. The bead assay has its advantages over other assays in that it allows for higher throughput screening of multiple serum samples, increased antigen specificity and sensitivity and the spatial presentation of conformational epitopes. Oertelt, et al. developed a bead assay with PDC-E2, BCOADC-E2 and OGDC-E2. Using this method, 6 out of 30 AMA-negative patients were found to react with at least one of the three mitochondrial antigens in PBC [77].
Enzyme inhibition assay (EI)
One of serological assays to detect AMA is PDC enzyme inhibition. Mackay et. al. demonstrated that the binding of serum autoantibodies to mitochondrial autoantigens in vitro inhibited the enzymatic function of PDC [78]. Such inhibitory function was also found to affect the function of other 2-OADC enzymes [79,80]. The standard assay of the enzyme inhibition was performed in cuvettes and PDC activity was determined by the spectrophotometric measurement of the formation of NADH from NAD+ in the presence of the substrate pyruvate and the cofactors cocarboxylase, NAD+, and coenzyme A. (Pyruvate + NAD+ + CoA → Acetyl-CoA + NADH + H+ + CO2). The inhibitory effect of the PBC serum on the enzyme activity was measured by adding test serum pre-incubated with PDC [44,81]. However, this assay is time-consuming and labor-intensive. An automated enzyme inhibition (EI) microtiter plate assay kit manufactured by TRACE Scientific (Victoria, Australia), which provides quick and reliable titration of multiple samples without loss of specificity [82] is commercially available. This assay is based on the extent to which anti-PDC antibody can inhibit the catalytic activity of PDC complex in vitro. The substrate reagent (250μl), consisting of sodium pyruvate, magnesium acetate, cocarboxylase, CoA, and NAD, is added to flat-bottomed microtiter wells. Subsequently, undiluted serum sample (4μl) is added to each well and incubated for 1 min at 37°C before adding the “enzyme reagent” consisting of pyruvate dehydrogenase and dithiothreitol. The rate of reaction can be monitored by measuring the rate of increase in absorbance at 340 nm as NADH is formed as an end product of the catalytic degradation of pyruvate. The rate of reaction for both macro- and microassays can be calculated as: (final absorbance - initial absorbance)/time. The percentage of inhibition is calculated as: {(control rate - final rate)/control rate} × 100. The control rate is derived from wells that do not contain anti-PDC antibody (100% activity, equivalent to 0% inhibition) [80]. The level of inhibition of catalytic activity of PDC for discriminating between PBC sera and non-PBC sera is considered 30% as a positive cutoff (PDC activity < 70%). Jensen et al. reported that the automated enzymatic mitochondrial antibody assay differentiated PBC patients from healthy controls with a sensitivity of 83% and a specificity of 100%. This kit method was also compared to a commercial ELISA, which had a sensitivity of 73% and specificity of 100% [83]. Comparative studies of three AMA detection methods (IIF, ELISA, and EI) reported that the sensitivity and specificity of the EI kit assay are relatively close to one another, especially in specificity (100%) [84,85]. A positive predictive value of EI assay is 100%, which means that subjects with a positive EI result truly have PBC [84]. To conclude, the EI assay is a useful test for the detection of AMA and the diagnosis of AMA-positive PBC. Furthermore, the EI assay kit method is an economical method that offers simplicity, rapidity and objectivity .
Diagnosis of PBC is based largely on clinical data and laboratory work up. Earlier studies have shown that there are about 5%-10% of patients who show the clinical and pathological features of PBC but are negative for AMA by IIF. However, Nakanuma et al. reported that majority of IIF AMA negative positive sera reacted positively to recombinant 2-OADCE2 polypeptides [86]. Similar observations were reported in a Greek study using ELISA against MIT3 [74] and a study by Dahnrich et. al. using a mixture of MIT3 and purified PDC [76]. More recently, Bizzaro et. al. studied 100 IIF AMA negative PBC sera and 104 sera samples from patients with other chronic liver diseases by an ELISA (PBC screen INOVA) and purified coating antigens of gp210 and sp100. Among the 100 IIF AMA negative PBC sera, 43 were positive by PBC screen assay and 11/100 recognized MIT3 [87]. This study demonstrated that a combination of recombinant antigens including MIT3 and new laboratory methods could markedly improve the sensitivity of AMA detection over IIF. With improved sensitivity and specificity using recombinant proteins, the diagnosis of PBC can be made before the patient becomes symptomatic with liver biochemistry and/or AMA.
AMA and Pathogenesis of PBC
While the specificity of AMA in PBC is well established, is there any association of AMA with severity of disease in PBC? Using IIF, 95 AMA positive PBC patients from Greece were assayed for IgG subclass. IgG1, IgG2, IgG3 AMA were detected but not IgG4. In addition, there was a positive correlation between IgG3 and the Mayo risk score (r=0.55, p=0.009). Spearman’s correlation suggests that IgG3 is associated with a more severe disease course [88]. Using recombinant MIT3 ELISA, Gabeta et. al. reported that IgG and IgA AMA titers are positively associated with Mayo risk score in a cohort of 103 patients with PBC but none of the isotypes could be used to predict disease outcome [74]. Analysis of AMA reactivity by IIF and PDC-E2 ELISA between serum samples from AMA positive PBC patients and individuals with AMA without clinical or biochemical evidence of disease, showed that high-titer IIF-AMA and high-avidity anti-PDC-E2 were associated with established PBC [89]. In another study, the presence of intense granular staining of PDC-E2 and an autophagy marker, microtubule-associated protein-light chain 3β in damaged small bile ducts of PBC patients suggests that dysregulated autophagy together with abnormal expression of mitochondrial antigens may be involved in the bile duct pathology of PBC [90]. However, other studies suggest that the levels of AMA do not correlate with disease stage and prognosis of PBC [91,92]. Recently, Tana et. al. studied whether autoantibody levels changed over time and correlated with clinical outcomes in a cohort of patients with PBC [93]. They observed that over time changes in AMA do not correlate with clinical outcomes in PBC.
On the other hand, other observations suggest that AMA plays a significant role in the development and progression of PBC and is not an epiphenomenon. First, AMAs can be detected before the clinical symptoms or biochemical abnormalities that are indicative of PBC. Second, AMA-autoantigen complexes can be detected in biliary cells in PBC. The deposition of immune complexes is related to severe organ damage in many antibody-mediated chronic autoimmune diseases. In PBC patients, but not in disease controls, AMA-IgA has been reported to co-localize with PDC-E2 both inside the cell cytoplasm and in the apical membrane of cholangiocytes, suggesting that IgA AMA/PDC-immune complexes are deposited in the epithelium [94,95]. These immune complexes lead to cellular dysfunction and subsequent biliary injury [96]. Third, PDC-E2 are present in the apoptotic blebs of human intrahepatic bile duct cells but not in the blebs of other epithelial cell lines [97,98]. Furthermore, a triad containing AMA positive sera, monocyte-derived macrophages and apoptotic biliary cell blebs could mount a burst of inflammatory cytokines and chemokines [99]. This further highlights the contribution of AMA in the perpetuation of autoimmune injury in PBC.
Expert Commentary
Primary biliary cirrhosis has recently been renamed as primary biliary cholangitis (PBC) by the European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Disease (AASLD), the Asian Pacific Association for the Study of the Liver (APASL) and the American Gastroenterological Association (AGA) [100]. The diagnosis of PBC is based largely on clinical data and laboratory work up. With the advent in molecular biology and identification of autoantigens in PBC, the diagnosis of PBC is greatly improved using recombinant proteins and can be made before the patient becomes symptomatic with liver biochemistry and/or AMA. Over the years, research laboratories have been using immunoblotting and ELISA based on recombinant proteins as the preferred methods for AMA determination over IIF due to their increased sensitivity and specificity. Assays, which are automated, standardized, rapid, and simple are preferred for more reliable detection of PBC-specific AMA. New types of ELISA and bead assays are simple, efficient, and have increased sensitivity to detect AMA in previously AMA-negative PBC patients.
Although AMA are highly reliable for the diagnosis of PBC, some limitations and precautions are noted below. Firstly, the high titer of AMA and the sensitivity of the test demand extreme caution to avoid cross-contamination of sera and false positives. Secondly, about 5-10% of PBC patients are AMA negative. Other clinical diagnostic features are also critical for differential diagnosis. Thirdly, neither the titer nor the specificity of autoantigens are related to the severity of the disease and therefore cannot be used to monitor the clinical course or give any indication on prognosis. Most importantly, positive and negative antibody controls should be included in the assays. All factors taken together, the availability of cloned autoantigens and their recombinant proteins are nevertheless extremely valuable diagnostic tools for PBC.
Five Year View
An accurate and early diagnosis is critical in the clinical management of PBC. Early detection and subsequent treatment can slow progression, delay liver failure and improve the survival rate of PBC. AMA is a serological marker in PBC that can be detected years before any clinical symptoms of PBC. Although AMA is not associated with the severity of PBC, combination of AMA and discovery of other biomarkers in PBC [67,101-104] will increase the sensitivity and specificity in the diagnosis, disease prediction and prognosis of PBC. Finally, increasing knowledge on antigen specificity of AMA to lipoylated and non-lipoylated forms of the mitochondrial autoantigens, lipoic acid, xenobiotics and isotypes of the Ig molecule [52,58,101,105], may lead to discovery of AMA subpopulations which can be of indicative value for disease progression and prognosis.
Table 3. Comparison of AMA Detection Methods.
| Methods | Sensitivity | Specificity | Remarks |
|---|---|---|---|
|
| |||
| Immunofluorescence | 43.8-100% | 83.3-100% | [75,76,106] |
|
| |||
| ELISA: | 72.9-100% | 76.5-100% | [106] |
| Purified PDC | 83.8% | 98.8% | [76] |
| MIT3 | 91.3% | 98.8% | [76] |
| M2-3E* | 93.6% | 98.8% | [76] |
| Cloned proteins from humans | 94.0% | 100% | [78] |
|
| |||
| Immunoblotting | 85.0-94.9% | 84.8-100% | [106] |
|
| |||
| Enzyme inhibition | 72-100% | 100% | [83-85] |
M2-3E is a mixture of purified PDC and MIT3 containing a combination of three E2-subunits.
Key Issues.
AMA is a serological hallmark of PBC and was first detected in the sera of patients with primary biliary cirrhosis by indirect immunofluorescence.
AMA can be detected in over 95% of patients with PBC and can be detected even before clinical symptoms or biochemical abnormalities. However, the titer of AMA is not associated with the severity of PBC or the prognosis of PBC.
The major PBC mitochondrial autoantigens have been cloned, expressed and identified as the E2 subunits of the 2-oxo-acid dehydrogenase complex (2-OADC), which includes the E2 subunits of pyruvate dehydrogenase (PDC-E2), branched-chain 2-oxo acid dehydrogenase complex (BCOADC-E2), oxoglutarate dehydrogenase complex (OGDC-E2), E3 binding protein and PDC E1 alpha subunit.
The AMA epitopes are mapped within the E2 lipoic acid binding domains, with PDC-E2 as the major mitochondrial autoantigen.
There are five common strategies for detecting AMA: indirect immunofluorescence, immunoblotting, enzyme linked immunosorbent assay, luminex beads assay and enzyme inhibition assay. Among these methods, ELISA and bead assays with recombinant mitochondrial autoantigens are simple, efficient, and have increased sensitivity that can even detect AMA in previously AMA-negative PBC patients.
Acknowledgement
This work is supported in part by a National Institutes of Health grant DK39588 to ME Gershwin.
PSC Leung and ME Gershwin have a United States patent (US6111071), ‘Recombinant Fusion Protein Comprising PDC-E2, BCOADC-E2 and OGDC-E2 and uses thereof’.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Walker JG, Doniach D, Roitt IM, Sherlock S. Serological Tests in Diagnosis of Primary Biliary Cirrhosis. Lancet. 1965;1(7390):827–831. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Haapanen K, Li B, Zhang W, Van de Water J, Gershwin ME. Women and primary biliary cirrhosis. Clinical reviews in allergy & immunology. 2015;48(2-3):285–300. doi: 10.1007/s12016-014-8449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong M, Li J, Tang R, et al. Multiple genetic variants associated with primary biliary cirrhosis in a Han Chinese population. Clinical reviews in allergy & immunology. 2015;48(2-3):316–321. doi: 10.1007/s12016-015-8472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths L, Dyson JK, Jones DE. The new epidemiology of primary biliary cirrhosis. Seminars in liver disease. 2014;34(3):318–328. doi: 10.1055/s-0034-1383730. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfield GM, Siminovitch KA. Genetics in PBC: what do the “risk genes” teach us? Clinical reviews in allergy & immunology. 2015;48(2-3):176–181. doi: 10.1007/s12016-014-8419-x. [DOI] [PubMed] [Google Scholar]
- 6.Selmi C, Cavaciocchi F, Lleo A, et al. Genome-wide analysis of DNA methylation, copy number variation, and gene expression in monozygotic twins discordant for primary biliary cirrhosis. Frontiers in immunology. 2014;5:128. doi: 10.3389/fimmu.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb GJ, Siminovitch KA, Hirschfield GM. The immunogenetics of primary biliary cirrhosis: A comprehensive review. Journal of autoimmunity. 2015;64:42–52. doi: 10.1016/j.jaut.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie YQ, Ma HD, Lian ZX. Epigenetics and Primary Biliary Cirrhosis: a Comprehensive Review and Implications for Autoimmunity. Clinical reviews in allergy & immunology. 2015 doi: 10.1007/s12016-015-8502-y. [DOI] [PubMed] [Google Scholar]
- 9.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 10.Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 11.Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmunity reviews. 2014;13(4-5):441–444. doi: 10.1016/j.autrev.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial analysis of antimitochondrial antibody in patients with primary biliary cirrhosis. Clin Dev Immunol. 2004;11(2):129–133. doi: 10.1080/10446670410001722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayo MJ. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2008;12(2):277–288. viii. doi: 10.1016/j.cld.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Yang G, Dubrovsky AM, Choi J, Leung PS. Xenobiotics and loss of tolerance in primary biliary cholangitis. World J Gastroenterol. 2016;22(1):338–348. doi: 10.3748/wjg.v22.i1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisand KE, Metskula K, Kisand KV, Kivik T, Gershwin ME, Uibo R. The follow-up of asymptomatic persons with antibodies to pyruvate dehydrogenase in adult population samples. Journal of gastroenterology. 2001;36(4):248–254. doi: 10.1007/s005350170111. [DOI] [PubMed] [Google Scholar]
- 16.Uibo R, Kisand K, Yang CY, Gershwin ME. Primary biliary cirrhosis: a multi-faced interactive disease involving genetics, environment and the immune response. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2012;120(11):857–871. doi: 10.1111/j.1600-0463.2012.02914.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang CH, Chen YC, Yu YH, et al. Innate immunity drives xenobiotic-induced murine autoimmune cholangitis. Clinical and experimental immunology. 2014;177(2):373–380. doi: 10.1111/cei.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Kachapati K, Adams D, et al. Murine autoimmune cholangitis requires two hits: cytotoxic KLRG1(+) CD8 effector cells and defective T regulatory cells. Journal of autoimmunity. 2014;50:123–134. doi: 10.1016/j.jaut.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irie J, Wu Y, Wicker LS, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. The Journal of experimental medicine. 2006;203(5):1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsumi T, Tomita K, Leung PS, Yang GX, Gershwin ME, Ueno Y. Animal models of primary biliary cirrhosis. Clinical reviews in allergy & immunology. 2015;48(2-3):142–153. doi: 10.1007/s12016-015-8482-y. [DOI] [PubMed] [Google Scholar]
- 21.Kawata K, Yang GX, Ando Y, et al. Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGFbetaRII mice. Hepatology. 2013;58(3):1094–1104. doi: 10.1002/hep.26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oertelt S, Lian ZX, Cheng CM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. Journal of immunology. 2006;177(3):1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 23.Salas JT, Banales JM, Sarvide S, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134(5):1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Zhang W, Yang GX, et al. Deletion of interleukin (IL)-12p35 induces liver fibrosis in dominant-negative TGFbeta receptor type II mice. Hepatology. 2013;57(2):806–816. doi: 10.1002/hep.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakabayashi K, Lian ZX, Leung PS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48(2):531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakabayashi K, Lian ZX, Moritoki Y, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44(5):1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi K, Yoshida K, Leung PS, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clinical and experimental immunology. 2009;155(3):577–586. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Yang GX, Tsuneyama K, Gershwin ME, Ridgway WM. Leung PS. Animal models of primary biliary cirrhosis. Seminars in liver disease. 2014;34(3):285–296. doi: 10.1055/s-0034-1383728. [DOI] [PubMed] [Google Scholar]
- 29.Wu SJ, Yang YH, Tsuneyama K, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53(3):915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y, Yang W, Yang YQ, et al. Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Ralpha(−/−) mice. Journal of autoimmunity. 2014;51:99–108. doi: 10.1016/j.jaut.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. Journal of immunology. 1987;138(10):3525–3531. [PubMed] [Google Scholar]
- 32.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. The Journal of experimental medicine. 1988;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppel RL, McNeilage LJ, Surh CD, et al. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(19):7317–7321. doi: 10.1073/pnas.85.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha S, Leung PS, Coppel RL, Van de Water J, Ansari AA, Gershwin ME. Heterogeneity of combinatorial human autoantibodies against PDC-E2 and biliary epithelial cells in patients with primary biliary cirrhosis. Hepatology. 1994;20(3):574–583. [PubMed] [Google Scholar]
- 35.Cha S, Leung PS, Gershwin ME, Fletcher MP, Ansari AA, Coppel RL. Combinatorial autoantibodies to dihydrolipoamide acetyltransferase, the major autoantigen of primary biliary cirrhosis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2527–2531. doi: 10.1073/pnas.90.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha S, Leung PS, Van de Water J, et al. Random phage mimotopes recognized by monoclonal antibodies against the pyruvate dehydrogenase complex-E2 (PDC-E2) Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):10949–10954. doi: 10.1073/pnas.93.20.10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fregeau DR, Davis PA, Danner DJ, et al. Antimitochondrial antibodies of primary biliary cirrhosis recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha-ketoacid dehydrogenase complex. Journal of immunology. 1989;142(11):3815–3820. [PubMed] [Google Scholar]
- 38.Fregeau DR, Prindiville T, Coppel RL, Kaplan M, Dickson ER, Gershwin ME. Inhibition of alpha-ketoglutarate dehydrogenase activity by a distinct population of autoantibodies recognizing dihydrolipoamide succinyltransferase in primary biliary cirrhosis. Hepatology. 1990;11(6):975–981. doi: 10.1002/hep.1840110611. [DOI] [PubMed] [Google Scholar]
- 39.Leung PS, Chuang DT, Wynn RM, et al. Autoantibodies to BCOADC-E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatology. 1995;22(2):505–513. [PubMed] [Google Scholar]
- 40.Moteki S, Leung PS, Dickson ER, et al. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2-oxoglutarate dehydrogenase complex. Hepatology. 1996;23(3):436–444. doi: 10.1002/hep.510230307. [DOI] [PubMed] [Google Scholar]
- 41.Surh CD, Ahmed-Ansari A, Gershwin ME. Comparative epitope mapping of murine monoclonal and human autoantibodies to human PDH-E2, the major mitochondrial autoantigen of primary biliary cirrhosis. Journal of immunology. 1990;144(7):2647–2652. [PubMed] [Google Scholar]
- 42.Surh CD, Cooper AE, Coppel RL, et al. The predominance of IgG3 and IgM isotype antimitochondrial autoantibodies against recombinant fused mitochondrial polypeptide in patients with primary biliary cirrhosis. Hepatology. 1988;8(2):290–295. doi: 10.1002/hep.1840080217. [DOI] [PubMed] [Google Scholar]
- 43.Surh CD, Roche TE, Danner DJ, et al. Antimitochondrial autoantibodies in primary biliary cirrhosis recognize cross-reactive epitope(s) on protein X and dihydrolipoamide acetyltransferase of pyruvate dehydrogenase complex. Hepatology. 1989;10(2):127–133. doi: 10.1002/hep.1840100202. [DOI] [PubMed] [Google Scholar]
- 44.Van de Water J, Fregeau D, Davis P, et al. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. Journal of immunology. 1988;141(7):2321–2324. [PubMed] [Google Scholar]
- 45.Nishio A, Van de Water J, Leung PS, et al. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 1997;25(5):1085–1089. doi: 10.1002/hep.510250506. [DOI] [PubMed] [Google Scholar]
- 46.Reynoso-Paz S, Leung PS, Van De Water J, et al. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31(1):24–29. doi: 10.1002/hep.510310106. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka A, Nalbandian G, Leung PS, et al. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32(5):910–915. doi: 10.1053/jhep.2000.19254. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka A, Nezu S, Uegaki S, et al. The clinical significance of IgA antimitochondrial antibodies in sera and saliva in primary biliary cirrhosis. Annals of the New York Academy of Sciences. 2007;1107:259–270. doi: 10.1196/annals.1381.028. [DOI] [PubMed] [Google Scholar]
- 49.Howard MJ, Fuller C, Broadhurst RW, et al. Three-dimensional structure of the major autoantigen in primary biliary cirrhosis. Gastroenterology. 1998;115(1):139–146. doi: 10.1016/s0016-5085(98)70375-0. [DOI] [PubMed] [Google Scholar]
- 50.Jones DD, Stott KM, Howard MJ, Perham RN. Restricted motion of the lipoyl-lysine swinging arm in the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 2000;39(29):8448–8459. doi: 10.1021/bi992978i. [DOI] [PubMed] [Google Scholar]
- 51.Stott KM, Yusof AM, Perham RN, Jones DD. A surface loop directs conformational switching of a lipoyl domain between a folded and a novel misfolded structure. Structure. 2009;17(8):1117–1127. doi: 10.1016/j.str.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Amano K, Leung PS, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. Journal of immunology. 2005;174(9):5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 53.Rieger R, Leung PS, Jeddeloh MR, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. Journal of autoimmunity. 2006;27(1):7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Leung PS, Wang J, Naiyanetr P, et al. Environment and primary biliary cirrhosis: electrophilic drugs and the induction of AMA. Journal of autoimmunity. 2013;41:79–86. doi: 10.1016/j.jaut.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long SA, Quan C, Van de Water J, et al. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. Journal of immunology. 2001;167(5):2956–2963. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 56.Naiyanetr P, Butler JD, Meng L, et al. Electrophile-modified lipoic derivatives of PDC-E2 elicits anti-mitochondrial antibody reactivity. Journal of autoimmunity. 2011;37(3):209–216. doi: 10.1016/j.jaut.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen RC, Naiyanetr P, Shu SA, et al. Antimitochondrial antibody heterogeneity and the xenobiotic etiology of primary biliary cirrhosis. Hepatology. 2013;57(4):1498–1508. doi: 10.1002/hep.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Budamagunta MS, Voss JC, et al. Antimitochondrial antibody recognition and structural integrity of the inner lipoyl domain of the E2 subunit of pyruvate dehydrogenase complex. Journal of immunology. 2013;191(5):2126–2133. doi: 10.4049/jimmunol.1301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fregeau DR, Roche TE, Davis PA, Coppel R, Gershwin ME. Primary biliary cirrhosis. Inhibition of pyruvate dehydrogenase complex activity by autoantibodies specific for E1 alpha, a non-lipoic acid containing mitochondrial enzyme. The Journal of Immunology. 1990;144(5):1671–1676. [PubMed] [Google Scholar]
- 60.Kuroda M, Morito T, Takagi T, et al. Antibodies to E1 and E2/Protein X components of pyruvate dehydrogenase complex in sera of patients with primary biliary cirrhosis. J Hepatol. 1996;25(6):867–876. doi: 10.1016/s0168-8278(96)80291-1. [DOI] [PubMed] [Google Scholar]
- 61.Iwayama T, Leung PS, Coppel RL, et al. Specific reactivity of recombinant human PDC-E1 alpha in primary biliary cirrhosis. Journal of autoimmunity. 1991;4(5):769–778. doi: 10.1016/0896-8411(91)90172-9. [DOI] [PubMed] [Google Scholar]
- 62.Muratori P, Muratori L, Ferrari R, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98(2):431–437. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura M, Komori A, Ito M, et al. Predictive role of anti-gp210 and anticentromere antibodies in long-term outcome of primary biliary cirrhosis. Hepatol Res. 2007;37(Suppl 3):S412–419. doi: 10.1111/j.1872-034X.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura M, Kondo H, Mori T, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45(1):118–127. doi: 10.1002/hep.21472. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura M, Shimizu-Yoshida Y, Takii Y, et al. Antibody titer to gp210-C terminal peptide as a clinical parameter for monitoring primary biliary cirrhosis. J Hepatol. 2005;42(3):386–392. doi: 10.1016/j.jhep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Hu CJ, Song G, Huang W, et al. Identification of new autoantigens for primary biliary cirrhosis using human proteome microarrays. Mol Cell Proteomics. 2012;11(9):669–680. doi: 10.1074/mcp.M111.015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norman GL, Yang CY, Ostendorff HP, et al. Anti-kelch-like 12 and anti-hexokinase 1: novel autoantibodies in primary biliary cirrhosis. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(2):642–651. doi: 10.1111/liv.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vergani D, Alvarez F, Bianchi FB, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. Journal of Hepatology. 2004;41(4):677–683. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Provenzano G, Diquattro O, Craxi A, et al. Immunoblotting as a confirmatory test for antimitochondrial antibodies in primary biliary cirrhosis. Gut. 1993;34(4):544–548. doi: 10.1136/gut.34.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter SL, Kraft SC, Boyer JL. Antimitochondrial antibodies: reagent variables may lead to diagnostic error. Digestive diseases and sciences. 1979;24(1):15–20. doi: 10.1007/BF01297232. [DOI] [PubMed] [Google Scholar]
- 71.Miura R, Tanaka A, Fukami M, et al. Indirect immunofluorescence and ELISA for testing of antimitochondrial antibodies -Which is better? Kanzo. 2010;51(9):531–533. [Google Scholar]
- 72.Dubel L, Tanaka A, Leung PS, et al. Autoepitope mapping and reactivity of autoantibodies to the dihydrolipoamide dehydrogenase-binding protein (E3BP) and the glycine cleavage proteins in primary biliary cirrhosis. Hepatology. 1999;29(4):1013–1018. doi: 10.1002/hep.510290403. [DOI] [PubMed] [Google Scholar]
- 73.Moteki S, Leung PS, Coppel RL, et al. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24(1):97–103. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 74.Gabeta S, Norman GL, Liaskos C, et al. Diagnostic relevance and clinical significance of the new enhanced performance M2 (MIT3) ELISA for the detection of IgA and IgG antimitochondrial antibodies in primary biliary cirrhosis. Journal of clinical immunology. 2007;27(4):378–387. doi: 10.1007/s10875-007-9092-0. [DOI] [PubMed] [Google Scholar]
- 75.Miyakawa H, Tanaka A, Kikuchi K, et al. Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology. 2001;34(2):243–248. doi: 10.1053/jhep.2001.26514. [DOI] [PubMed] [Google Scholar]
- 76.Dahnrich C, Pares A, Caballeria L, et al. New ELISA for detecting primary biliary cirrhosis-specific antimitochondrial antibodies. Clinical chemistry. 2009;55(5):978–985. doi: 10.1373/clinchem.2008.118299. [DOI] [PubMed] [Google Scholar]
- 77.Oertelt S, Rieger R, Selmi C, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45(3):659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 78.Mackay IR, Rowley MJ, Armstrong JM. Identity of the M2 autoantigens in primary biliary cirrhosis. Hepatology. 1989;10(3):389–390. doi: 10.1002/hep.1840100325. [DOI] [PubMed] [Google Scholar]
- 79.Rowley MJ, McNeilage LJ, Armstrong JM, Mackay IR. Inhibitory autoantibody to a conformational epitope of the pyruvate dehydrogenase complex, the major autoantigen in primary biliary cirrhosis. Clinical immunology and immunopathology. 1991;60(3):356–370. doi: 10.1016/0090-1229(91)90093-p. [DOI] [PubMed] [Google Scholar]
- 80.Uibo R, Mackay IR, Rowley M, Humphries P, Armstrong JM, McNeilage J. Inhibition of enzyme function by human autoantibodies to an autoantigen pyruvate dehydrogenase E2: different epitope for spontaneous human and induced rabbit autoantibodies. Clinical and experimental immunology. 1990;80(1):19–24. doi: 10.1111/j.1365-2249.1990.tb06435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pettit FH, Reed LJ. Pyruvate dehydrogenase complex from bovine kidney and heart. Methods in enzymology. 1982;89(Pt D):376–386. doi: 10.1016/s0076-6879(82)89067-8. [DOI] [PubMed] [Google Scholar]
- 82.Teoh KL, Rowley MJ, Zafirakis H, et al. Enzyme inhibitory autoantibodies to pyruvate dehydrogenase complex in primary biliary cirrhosis: applications of a semiautomated assay. Hepatology. 1994;20(5):1220–1224. [PubMed] [Google Scholar]
- 83.Jensen WA, Jois JA, Murphy P, et al. Automated enzymatic mitochondrial antibody assay for the diagnosis of primary biliary cirrhosis. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2000;38(8):753–758. doi: 10.1515/CCLM.2000.107. [DOI] [PubMed] [Google Scholar]
- 84.Omagari K, Hazama H, Kohno S. Enzyme inhibition assay for pyruvate dehydrogenase complex: clinical utility for the diagnosis of primary biliary cirrhosis. World journal of gastroenterology. 2005;11(43):6735–6739. doi: 10.3748/wjg.v11.i43.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmit P, Gilson G, Humbel RL. Evaluation of an automated enzyme inhibition assay for the detection of anti-mitochondrial M2 autoantibodies. Clinical chemistry. 1999;45(12):2287–2289. [PubMed] [Google Scholar]
- 86.Nakanuma Y, Harada K, Kaji K, et al. Clinicopathological study of primary biliary cirrhosis negative for antimitochondrial antibodies. Liver. 1997;17(6):281–287. doi: 10.1111/j.1600-0676.1997.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 87.Bizzaro N, Covini G, Rosina F, et al. Overcoming a "probable" diagnosis in antimitochondrial antibody negative primary biliary cirrhosis: study of 100 sera and review of the literature. Clinical reviews in allergy & immunology. 2012;42(3):288–297. doi: 10.1007/s12016-010-8234-y. [DOI] [PubMed] [Google Scholar]
- 88.Rigopoulou EI, Davies ET, Bogdanos DP, et al. Antimitochondrial antibodies of immunoglobulin G3 subclass are associated with a more severe disease course in primary biliary cirrhosis. Liver international : official journal of the International Association for the Study of the Liver. 2007;27(9):1226–1231. doi: 10.1111/j.1478-3231.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 89.Dellavance A, Cancado EL, Abrantes-Lemos CP, Harriz M, Marvulle V, Andrade LE. Humoral autoimmune response heterogeneity in the spectrum of primary biliary cirrhosis. Hepatol Int. 2013;7(2):775–784. doi: 10.1007/s12072-012-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Increased expression of mitochondrial proteins associated with autophagy in biliary epithelial lesions in primary biliary cirrhosis. Liver Int. 2013;33(2):312–320. doi: 10.1111/liv.12049. [DOI] [PubMed] [Google Scholar]
- 91.Cancado EL, Harriz M. The Importance of Autoantibody Detection in Primary Biliary Cirrhosis. Frontiers in immunology. 2015;6:309. doi: 10.3389/fimmu.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clinics in liver disease. 2003;7(4):741–758. doi: 10.1016/s1089-3261(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 93.Tana MM, Shums Z, Milo J, et al. The Significance of Autoantibody Changes Over Time in Primary Biliary Cirrhosis. American journal of clinical pathology. 2015;144(4):601–606. doi: 10.1309/AJCPQV4A7QAEEFEV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukushima N, Nalbandian G, Van De Water J, et al. Characterization of recombinant monoclonal IgA anti-PDC-E2 autoantibodies derived from patients with PBC. Hepatology. 2002;36(6):1383–1392. doi: 10.1053/jhep.2002.37140. [DOI] [PubMed] [Google Scholar]
- 95.Malmborg AC, Shultz DB, Luton F, et al. Penetration and co-localization in MDCK cell mitochondria of IgA derived from patients with primary biliary cirrhosis. Journal of autoimmunity. 1998;11(5):573–580. doi: 10.1006/jaut.1998.0220. [DOI] [PubMed] [Google Scholar]
- 96.Matsumura S, Van De Water J, Leung P, et al. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39(5):1415–1422. doi: 10.1002/hep.20175. [DOI] [PubMed] [Google Scholar]
- 97.Lleo A, Selmi C, Invernizzi P, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49(3):871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rong G, Zhong R, Lleo A, et al. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54(1):196–203. doi: 10.1002/hep.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lleo A, Bowlus CL, Yang GX, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52(3):987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beuers U, Gershwin ME, Gish RG, et al. Changing Nomenclature for PBC: From ‘Cirrhosis’ to ‘Cholangitis’. Gastroenterology. 2015;149(6):1627–1629. doi: 10.1053/j.gastro.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 101.Maverakis E, Kim K, Shimoda M, et al. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. Journal of autoimmunity. 2015;57:1–13. doi: 10.1016/j.jaut.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ninomiya M, Kondo Y, Funayama R, et al. Distinct microRNAs expression profile in primary biliary cirrhosis and evaluation of miR 505-3p and miR197-3p as novel biomarkers. PloS one. 2013;8(6):e66086. doi: 10.1371/journal.pone.0066086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padgett KA, Lan RY, Leung PC, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. Journal of autoimmunity. 2009;32(3-4):246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomiyama T, Yang GX, Zhao M, et al. The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cellular & molecular immunology. 2015 doi: 10.1038/cmi.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bruggraber SF, Leung PS, Amano K, et al. Autoreactivity to lipoate and a conjugated form of lipoate in primary biliary cirrhosis. Gastroenterology. 2003;125(6):1705–1713. doi: 10.1053/j.gastro.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 106.Hu S, Zhao F, Wang Q, Chen WX. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: a meta-analysis. Clinical chemistry and laboratory medicine. 2014;52(11):1533–1542. doi: 10.1515/cclm-2013-0926. [DOI] [PubMed] [Google Scholar]