Abstract
A relationship between thyroid hormones and the cardiovascular system has been well established in the literature. The present in vitro study aimed to investigate the mechanisms involved in the vasodilator effect produced by the acute application of 10-8–10-4 M triiodothyronine (T3) to isolated rat aortic rings. Thoracic aortic rings from 80 adult male Wistar rats were isolated and mounted in tissue chambers filled with Krebs-Henseleit bicarbonate buffer in order to analyze the influence of endothelial tissue, inhibitors and blockers on the vascular effect produced by T3. T3 induced a vasorelaxant response in phenylephrine-precontracted rat aortic rings at higher concentrations (10-4.5–10-4.0 M). This outcome was unaffected by 3.1×10-7 M glibenclamide, 10-3 M 4-aminopyridine (4-AP), 10-5 M indomethacin, or 10-5 M cycloheximide. Contrarily, vasorelaxant responses to T3 were significantly (P<0.05) attenuated by endothelium removal or the application of 10-6 M atropine, 10-5 M L-NG-nitroarginine methyl ester (L-NAME), 10-7 M 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 10-6 M (9S,10R,12R)-2,3,9,10,11,12-Hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i](1,6)benzodiazocine-10-carboxylic acid, methyl ester KT 5823, 10-2 M tetraethylammonium (TEA), or 10-7 M apamin plus 10-7 M charybdotoxin. The results suggest the involvement of endothelial mechanisms in the vasodilator effect produced by the acute in vitro application of T3 to rat aortic rings. Possible mechanisms include the stimulation of muscarinic receptors, activation of the NO-cGMP-PKG pathway, and opening of Ca2+-activated K+ channels.
Keywords: Triiodothyronine, Rat aorta, Vasorelaxation, NO-cGMP/PKG pathway, K+ channels
Introduction
Numerous experimental and clinical studies have demonstrated a relationship between thyroid hormones and the cardiovascular system, including reports of significant changes in cardiac function in patients with persistent subclinical thyroid dysfunction (1 –5). Triiodothyronine (T3) and thyroxine (T4) are thyroid hormones present in plasma and peripheral tissues (6). T3 is mostly generated by 5′-monodeiodination of T4 in peripheral tissues (6,7). The acute application of T3 has been linked to a vasorelaxant effect (3,8 –12), which has both an endothelium-independent and endothelium-dependent component. The endothelium-independent effect predominates in physiological concentrations and the endothelium-dependent effect in supraphysiological concentrations (13). The vasorelaxant endothelium-dependent effect produced by T3 has been linked to the activation of the endothelial nitric oxide synthase (eNOS), via thyroid hormone receptor/phosphatidylinositol 3-kinase/protein kinase-B pathway (TR/PI3-kinase/Akt pathway) (14). However, further research is needed about the possible involvement of muscarinic receptors, the nitric oxide-cyclic guanosine monophosphate-protein kinase G pathway (NO-cGMP-PKG pathway), and K+ channels in this vasorelaxant effect.
The present study aimed to analyze the effect of endothelium removal as well as the application of atropine, L-NG-nitroarginine methyl ester (L-NAME), 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), KT 5823, glibenclamide, 4-aminopyridine (4-AP), tetraethylammonium (TEA), apamin plus charybdotoxin, indomethacin and cycloheximide on the vasorelaxant response produced by supraphysiological concentrations (10-8–10-4 M) of T3 in phenylephrine-precontracted rat aortic rings.
Material and Methods
Animals
Experiments were performed on isolated thoracic aortic rings of adult male Wistar rats (body weight 250-300 g). Rats (n=80) were purchased from the bioterium of the Higher School of Medicine in the National Polytechnic Institute (Mexico City, Mexico). Animals were housed in plastic cages in a special temperature-controlled room (22±2°C, 50% humidity) on a 12:12 h light/dark cycle (lights on at 7:00 a.m.). The study was approved by the Animal Care Committee of the Higher School of Medicine and the protocol is in agreement with the 1986 Animals (Scientific Procedures) Act of the British Parliament (http://www.legislation.gov.uk/ukpga/1986/14/contents, accessed April 5, 2016).
Preparation of aortic rings
Animals were euthanized by decapitation and the aortas were immediately excised and placed in cold buffer, cleaned and freed from surrounding connective tissue. The isolated arteries were cut into rings (4–5 mm long) and placed in 10 mL tissue chambers filled with Krebs-Henseleit bicarbonate buffer (1.18×10-1 M NaCl; 4.7×10-3 M KCl; 1.2×10-3 M KH2 PO4; 1.2×10-3 M MgSO4.7H2O; 2.5 × 10-3 M CaCl2·2H2O; 2.5×10-2 M NaHCO3; 1.17×10-2 M dextrose, and 2.6×10-5 M calcium disodium EDTA). In some experiments, the KCl concentration was increased to 8×10-2 M and that of Na+ decreased to maintain osmotic equilibrium. Tissue baths, maintained at 37°C and pH 7.4, were bubbled with a mixture of 95% O2 and 5% CO2.
Aortic rings were mounted on two stainless steel hooks, one fixed to the bottom of the chamber and the other to a BIOPAC TSD125C-50g force transducer connected to a BIOPAC MP100A-CE data acquisition system (Biopac Systems, Inc., USA) in order to record the isometric tension. Optimal tension, selected from preliminary experiments, was the one that gave the greatest response to 10-6 M phenylephrine. The rings were given 2 g (100%) of initial tension and allowed to equilibrate for 2 h. Thirty minutes after setting up the organ bath, tissues were contracted with 10-6 M phenylephrine to test their contractile responses.
Endothelium-denuded aortic strips were prepared by turning the rings gently several times on the distal portion of small forceps. Endothelial integrity was pharmacologically assessed with acetylcholine-induced vasodilatation (10-6 M). Segments showing no relaxation to acetylcholine were considered to be endothelium-denuded. After exposure to 10-6 M phenylephrine or 10-6 M acetylcholine, tissues were rinsed three times with Krebs solution to restore basal tension.
Drugs
All drugs except T3 were purchased from Sigma-Aldrich Co. (USA). T3 was a gift from Productos Medix, S.A. de C.V (Mexico). Atropine, L-NAME, 4-AP, TEA and cycloheximide were dissolved in distilled water. Solutions of 10-5 M ODQ, 10-4 M KT 5823, 10-5 M apamin plus 10-5 M charybdotoxin and 10-3 M indomethacin were prepared by using 1.39 M dimethyl sulfoxide, 1.01 M ethyl acetate, 1.73 M acetic acid and 9.4×10-3 M sodium bicarbonate, respectively. Solutions of T3 were prepared with serial dilutions as follows: a stock solution of 10-2 M T3 was made in an aqueous solution of 2.5 M NaOH, and subsequently dilutions of 10-2.5, 10-3, 10-3.5, 10-4, 10-4.5, 10-5, 10-5.5 and 10-6 M T3 were prepared in 1.38, 1.38×10-1, 1.38×10-2, 1.38×10-2, 1.38×10-3, 1.38×10-3, 1.38×10-4 and 1.38×10-4 M NaOH, respectively. Fresh solutions were made for each experiment.
Experimental protocol
To determine the mechanisms involved in the relaxant effect induced by T3 on phenylephrine-precontracted rat aortic rings, two main sets of experiments were performed.
First set of experiments. Thirty minutes after restoration of basal tension (see Preparation of aortic rings section), 10-6 M phenylephrine was added to rat aortic rings with or without endothelium. Twenty minutes later, the phenylephrine-induced contraction plateaued. Thirty minutes after adding phenylephrine, T3 began to be cumulatively added (10-8–10-4 M) in intervals of around 4 min. Tension is reported as a percentage of the phenylephrine-induced contraction (3.79±0.16 g = 100% for endothelium-intact rat aortic rings and 4.21±0.13 g = 100% for endothelium-denuded rings). With cumulative addition into the tissue chambers, dilutions of T3 (prepared in aqueous solutions of NaOH; see Drugs section) reached concentrations of 2.5×10-2, 1.38×10-2, 1.38×10-3, 1.38×10-3, 1.38×10-4, 1.38×10-4, 1.38×10-5, 1.38×10-5, 1.38×10-6 and 1.38×10-6 M NaOH.
Second set of experiments. Thirty min after adding 10-6 M phenylephrine (see first set of experiments), rat aortic rings with intact endothelium were preincubated for 30 min with one of the inhibitors, blockers or vehicles: i) 10-6 M atropine, ii) 10-5 M L-NAME, iii) 10-7 M ODQ, iv) 10-6 M KT 5823, v) 3.1×10-7 M glibenclamide, vi) 10-3 M 4-AP, vii) 10-2 M TEA, viii) 10-7 M apamin plus 10-7 M charybdotoxin, ix) 10-5 M indomethacin, x) 10-5 M cycloheximide, xi) distilled water (vehicle of atropine, L-NAME, 4-AP, TEA, and cycloheximide), xii) 1.39×10-2 M dimethyl sulfoxide (vehicle of ODQ), xiii) 1.01×10-2 M ethyl acetate (vehicle of KT 5823), xiv) 1.73×10-2 M acetic acid (vehicle of apamin plus charybdotoxin), or xv) 9.4×10-5 M sodium bicarbonate (vehicle of indomethacin). Subsequently, T3 was cumulatively added in approximately 4-min intervals to reach a concentration between 10-8 and 10-4 M. Once reaching the desired concentration, the vasorelaxant response of the rings was assessed.
Data analysis and statistics
Data are reported as means±SE. In all experiments, n equals the number of animals from which aortic segments were obtained (8 in each case). Values of maximal vasorelaxation (Emax) were analyzed by Student's t-test. Effects of inhibitors/blockers on the vasorelaxant responses produced by T3 on phenylephrine-precontracted aortic segments were analyzed by a two-way analysis of variance (ANOVA), which was followed by a Student-Newman-Keul's post hoc test. Statistical significance was considered at P<0.05 (15). Statistical analyses were performed with the SigmaPlot 12 program (Systat Software Inc., USA).
Results
Effect of T3 on endothelium-intact and -denuded phenylephrine-precontracted rat aortic rings
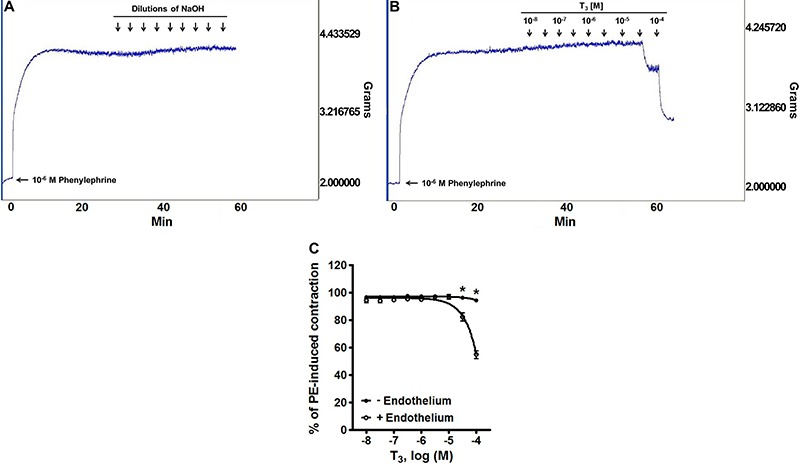
Figure 1A and B shows typical traces of the effect produced by the in vitro application of dilutions of NaOH (vehicle of T3) and 10-8–10-4 M T3 on phenylephrine-precontracted rat aortic rings with intact endothelium. The addition of 10-6 M phenylephrine to rat aortic rings produced a sustained contraction. The cumulative addition of T3 (10-8–10-4 M) produced a concentration-dependent vasorelaxant response, which was not observed with the vehicle (dilutions of NaOH). Figure 1C shows the effect of the cumulative addition of 10-8–10-4 M T3 to phenylephrine-precontracted rat aortic rings. When comparing endothelium-intact and -denuded rings, the Emax was 45.09±2.77 vs 5.44±0.97%, respectively, representing a significant difference (P<0.05).
Figure 1. Original experimental tracings illustrating the in vitro effect in phenylephrine (PE)-precontracted rat aortic rings produced by the application of: A, dilutions of NaOH (vehicle of T3), B, 10-8–10-4 M T3. A T3-induced vasorelaxant response can be seen at the highest concentrations of this hormone. Similar results were obtained in all assays (n=8). Endothelial denudation blocked the vasorelaxation produced by higher concentrations of T3 in PE-precontracted rat aortic rings (C). Data are reported as means±SE (n=8 for each group). *P<0.05 vs control (two-way ANOVA).
Effect of atropine on the vasorelaxation induced by T3 in phenylephrine-precontracted rat aortic rings
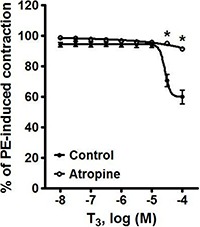
Figure 2 shows the effect of 10-6 M atropine on the vasorelaxation induced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings. When comparing the absence and presence of atropine, the values of Emax were 40.10±4.64 vs 8.51±1.07, respectively, representing a significant difference (P<0.05).
Figure 2. Pre-incubation with 10-6 M atropine blocked the vasorelaxation produced by higher concentrations of T3 in phenylephrine (PE)-precontracted rat aortic rings. Data are reported as means±SE (n=8). *P<0.05 vs control (two-way ANOVA).
Effect of L-NAME, ODQ and KT 5823 on the vasorelaxation induced by T3 in phenylephrine-precontracted rat aortic rings
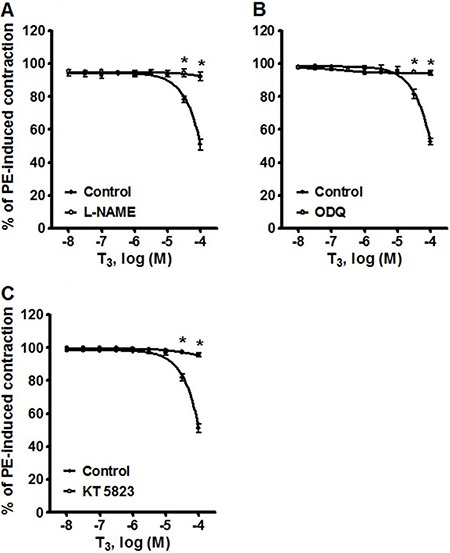
Figure 3 shows the effect of 10-5 M L-NAME (A), 10-7 M ODQ (B) and 10-6 M KT 5823 (C) on the vasorelaxation induced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings. The values of Emax from segments treated with T3 yielded a significant difference (P<0.05) when comparing the absence and presence, respectively, of each compound: 49.03±3.29 vs 7.74±2.62% for L-NAME, 47.42±1.74 vs 5.75±1.51% for ODQ, and 48.90±2.59 vs 4.43±1.33% for KT 5823.
Figure 3. Vasorelaxation produced by 10-8–10-4 M T3 in phenylephrine (PE)-precontracted rat aortic rings. Assays were carried out to test the effect of: A, 10-5 M L-NG-nitroarginine methyl ester (L-NAME), B, 10-7 M 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and C, 10-6 M (9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg: 3′,2′,1′-kl]pyrrolo[3,4-i](1,6) benzodiazocine-10-carboxylic acid, methyl ester (KT 5823). Data are reported as means±SE (n=8). *P<0.05 vs control (two-way ANOVA).
Effect of glibenclamide, 4-AP, TEA, and apamin plus charybdotoxin on the vasorelaxation induced by T3 in phenylephrine-precontracted rat aortic rings
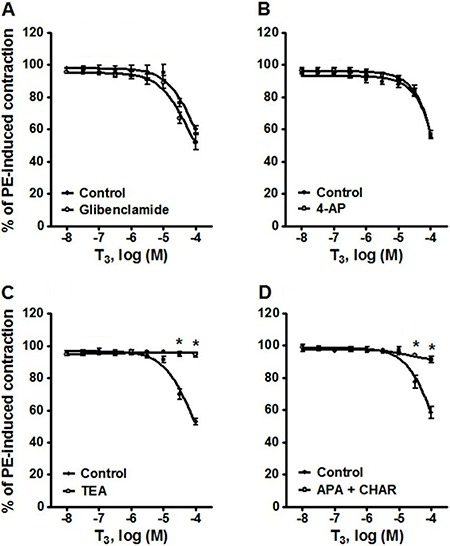
Figure 4 shows the effect of 3.1×10-7 M glibenclamide (A), 10-3 M 4-AP (B), 10-2 M TEA (C), and 10-7 M apamin plus 10-7 M charybdotoxin (D) on the vasorelaxation induced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings. The values of Emax represented a significant difference (P<0.05) only when comparing the absence and presence, respectively, of the latter two compounds: 39.87±2.29 vs 47.97±4.54% for glibenclamide, 42.97±2.44 vs 43.20±2.65% for 4-AP, 47.11±2.12 vs 5.34±1.50% for TEA, and 41.44±3.82 vs 8.69±1.97% for apamin plus charybdotoxin.
Figure 4. Vasorelaxation produced by 10-8–10-4 M T3 in phenylephrine (PE)-precontracted rat aortic rings. Assays were carried out to test the effect of: A, 3.1×10-7 M glibenclamide, B, 10-3 M 4-aminopyridine (4-AP), C, 10-2 M tetraethylammonium (TEA), and D, 10-7 M apamin plus 10-7 M charybdotoxin (APA+CHAR). Data are reported as means±SE (n=8). *P<0.05 vs control (two-way ANOVA).
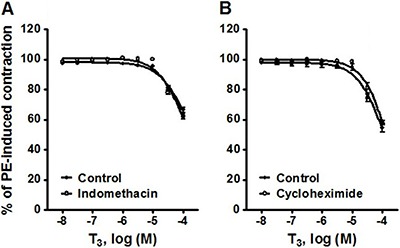
Effect of indomethacin and cycloheximide on the vasorelaxation induced by T3 in phenylephrine-precontracted rat aortic rings
Figure 5 shows the effect of 10-5 M indomethacin (A) and 10-5 M cycloheximide (B) on the vasorelaxation induced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings. The difference in the values of Emax when comparing the absence and presence, respectively, of each compound were not significant: 33.54±1.80 vs 37.77±1.85% for indomethacin and 45.44±2.88 vs 42.08±1.50% for cycloheximide.
Figure 5. Vasorelaxation produced by 10-8–10-4 M T3 in phenylephrine (PE)-precontracted rat aortic rings. Assays were carried out to test the effect of: A, 10-5 M indomethacin, and B, 10-5 M cycloheximide. Data are reported as means±SE (n=8).
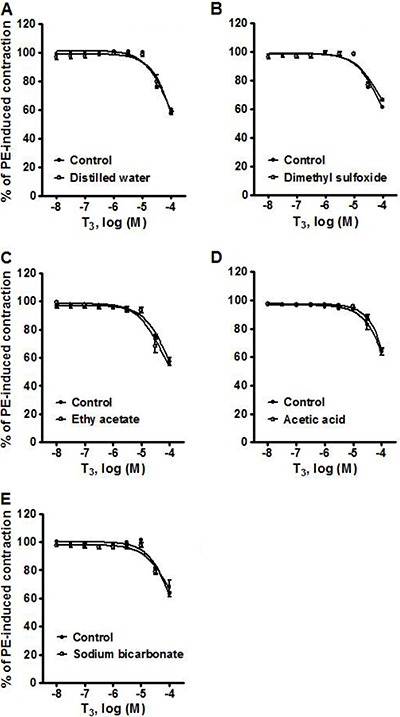
Effect of distilled water, dimethyl sulfoxide, ethyl acetate, acetic acid and sodium bicarbonate on the vasorelaxation induced by T3 in phenylephrine-precontracted rat aortic rings
Figure 6 shows the effect on the vasorelaxation induced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings produced by distilled water (vehicle of atropine, L-NAME, 4-AP, TEA and cycloheximide (A), 1.39×10-2 M dimethyl sulfoxide (vehicle of ODQ; B), 1.01×10-2 M ethyl acetate (vehicle of KT 5823; C), 1.73×10-2 M acetic acid (vehicle of apamin plus charybdotoxin; D), and 9.4×10-5 M sodium bicarbonate (vehicle of indomethacin; E). The difference in the values of Emax in the absence and presence, respectively, of each compound was not significant in any case: 39.56±1.27 vs 42.36±1.31% for distilled water, 38.73±0.48 vs 33.47±1.10% for dimethyl sulfoxide, 41.45±1.51 vs 44.12±1.60% for ethyl acetate, 36.31±2.58 vs 34.99±1.01% for acetic acid, and 37.20±1.89 vs 31.93±4.67% for sodium bicarbonate.
Figure 6. Vasorelaxation produced by 10-8–10-4 M T3 in phenylephrine (PE)-precontracted rat aortic rings. Assays were carried out to test the effect of: A: distilled water, B, 1.39×10-2 M dimethyl sulfoxide, C, 1.01×10-2 M ethyl acetate, D, 1.73×10-2 M acetic acid, and E, 9.4×10-5 M sodium bicarbonate. Data are reported as means±SE (n=8).
Discussion
The acute (immediate) application of T3 produced an immediate vasorelaxant effect in endothelium-intact but not in endothelium-denuded phenylephrine-precontracted rat aortic rings, suggesting that this thyroid hormone produces an endothelium-dependent vasorelaxation. This vasorelaxant effect was statistically significant at higher concentrations of T3 (10-4.5–10-4.0 M), in line with previous findings in which the endothelium-dependent effect produced by T3 was most obvious in supraphysiological concentrations (13). However, our findings are in contrast with a previous report in segments of endothelium-denuded rat thoracic aorta, in which incubation for 30 min with 10-7 M T3 decreased the phenylephrine-induced contractile response (16). A possible explanation for this discrepancy could be that 10-7 M T3 cannot produce an immediate endothelium-dependent vasorelaxation and it is necessary to incubate for 30 min to see endothelium-independent vasorelaxant effects on the aortic tissues. Since the vehicle did not produce a concentration-dependent vasorelaxant effect in phenylephrine-precontracted rat aortic rings, it can be ruled out that the T3-induced vasorelaxation was due to tachyphylactic effects caused by the repeated application of dilutions of NaOH to aortic segments.
The vasorelaxant effect produced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings is in agreement with numerous studies. It has been reported that: i) the bolus injection of T3 elicited an immediate and transient dose-dependent vasodilator effect in rat coronary arteries (11), ii) the acute application of 10-8–10-4 M L-T3 or 10-8–10-4 M D-T3 on rat mesenteric arteries produced a concentration-dependent vasorelaxant effect (10), iii) the acute application of 10-10–10-7 M T3 on rat skeletal muscle resistance arteries produced a concentration-dependent vasorelaxant effect (13), and iv) the acute application of 10-6–10-4 M L-T3 or 10-6–10-4 M D-T3 on rabbit mesenteric arteries produced a concentration-dependent vasorelaxant effect (8). However, the current results are in contrast with a previous study in which the cumulative application of 10-7–10-4.5 M T3 did not produce significant changes in rat aortic segments (3). Discrepancies in the reported vascular effect of T3 may be related to differences in experimental conditions, such as the concentrations of T3 applied and the type of vascular tissue studied (i.e., conductance or resistance vessels).
The current findings suggest that endothelium-independent mechanisms were not involved in this vasorelaxant effect. These findings contrast with two previous studies in which: after endothelial denudation, 10-10–10-7 M T3 produced a moderate vasorelaxant effect in rat skeletal muscle arteries (13), and the endothelial denudation of rat mesenteric and femoral arteries did not modify the vasorelaxant effect produced by 3×10-7-3×10-5 M T3 (3). These discrepancies could be due to: i) the time frame for the T3 stimulation of the endothelium-independent mechanisms (the application of all concentrations of T3 was herein performed in about 40 min, while previous studies took over 60 min for T3 application), and ii) the endothelium-independent mechanisms are more sensitive to T3 in resistance than in capacitance vessels.
An attempt was made to determine the endothelial mechanisms involved in the vasorelaxant effect found in endothelium-intact but not -denuded aortic rings. It is known that in the vasculature, the endothelial stimulation of muscarinic M3 and M5 receptors produces a vasorelaxant effect (17). The concentration of atropine employed herein (10-6 M), known to completely block muscarinic receptors (18), impeded the vasorelaxation produced by the acute application of supraphysiological concentrations of T3 in endothelium-intact aortic rings. However, distilled water (vehicle of atropine) did not impede such vasorelaxation (Figure 2), suggesting the possible involvement of muscarinic receptors. Further experiments, which fall beyond the scope of this investigation, are needed to identify the specific muscarinic receptor subtype(s) involved in the vasorelaxant effect produced by T3.
The current results also suggest the involvement of the NO-cGMP-PKG pathway in the vasorelaxant effect produced by 10-8–10-4 M T3 in rat aortic rings, since this effect was significantly attenuated by 10-5 M L-NAME (a direct inhibitor of NOS) (19), 10-7 M ODQ (an inhibitor of nitric oxide-sensitive guanylyl cyclase) (20), and 10-6 M KT 5823 (an inhibitor of protein kinase G) (21), but unaffected by the respective vehicles (distilled water), 1.39×10-2 M dimethyl sulfoxide) and 1.01×10-2 M ethyl acetate. These findings exclude the possibility that the attenuating effect produced by L-NAME, ODQ and KT 5823 were due to tachyphylactic effects induced by their respective vehicles.
On the other hand, 10-7 M ODQ and 10-6 M KT 5823, but not 10-5 M L-NAME inhibited the vasorelaxant responses to 10-11–10-5 M sodium nitroprusside (data not shown). These results suggest that the concentrations of ODQ and KT 5823 were high enough to inhibit the nitric oxide-sensitive guanylyl cyclase and the protein kinase G, respectively. Moreover, the fact that L-NAME did not modify the vasorelaxant effect to sodium nitroprusside suggests that this inhibitor acts specifically on the vasorelaxation dependent of the synthesis of nitric oxide.
The probable involvement of NO in the vasorelaxation produced by 10-8–10-4 M T3 in rat aortic rings is in line with previous studies suggesting that T3 exerts a direct effect on the regulation of vascular tone through non-genomic activation of eNOS, via the TR/PI3-kinase/Akt pathway (14). NO produced in endothelial cells by eNOS diffuses into vascular smooth muscle and directly activates soluble guanylate cyclase (22,23), leading to increased formation of cGMP. The resulting synthesis of cGMP is critical in mediating vasodilation through activation of PKG (24,25).
The present findings suggest the involvement of yet another mechanism – that of Ca2+-activated K+ channels – in the vasorelaxant response produced by 10-8–10-4 M T3 in phenylephrine-precontracted rat aortic rings. Vasorelaxation was unaffected by 3.1×10-7 M glibenclamide (an ATP-sensitive K+ channel blocker) (26) and 10-3 M 4-AP (a voltage-activated K+ channel blocker) (27,28), but was significantly (P<0.05) attenuated by 10-2 M TEA (a Ca2+-activated K+ channel blocker and non-specific voltage-activated K+ channel blocker) (27,29) and 10-7 M apamin plus 10-7 M charybdotoxin (blockers of small- and large-conductance Ca2+-activated K+ channels, respectively) (30 –32). Moreover, the vasorelaxant response was unaffected by distilled water (vehicle of L-NAME, 4-AP and TEA) and acetic acid (vehicle of apamin plus charybdotoxin). This emphasizes the reproducibility of these results and rules out the possibility that attenuations produced by the K+ channel blockers are due to tachyphylactic effects.
The combination of apamin plus charybdotoxin was used because it was previously reported that a complete blockage of Ca2+-activated K+ channels is necessary to produce a pharmacological response (31 –33). In this sense, a pilot experiment conducted in our laboratory showed that apamin alone did not modify the vasorelaxant response to 10-8–10-4 M T3 (data not shown). These observations suggest, but do not prove, that T3 produces vascular hyperpolarization attributable to the release of an endothelium-dependent hyperpolarizing factor. The above effect and mechanism was previously reported for acetylcholine (34,35). Certainly, this idea is still speculative and requires additional experiments that are beyond the scope of the present study.
There is a large body of evidence suggesting that prostacyclins (36) and protein synthesis (37) are involved in the endothelial control of vascular tone. However, the possible involvement of prostaglandin/protein synthesis in the vasorelaxation produced by T3 (38) is excluded by the current results in regard to indomethacin (a prostaglandin synthesis inhibitor) (39) and cycloheximide (a general protein synthesis inhibitor). Moreover, the lack of effect of cycloheximide on T3-induced vasorelaxation suggests that genomic mechanisms are not involved.
The present study showed that an acute in vitro application of supraphysiological concentrations of T3 in endothelium-intact rat aortic rings produced an immediate vasorelaxant effect. The in vitro character of this study represents a limitation. Although the current findings suggest an immediate vasorelaxant effect of T3, in vivo studies are needed to establish whether the administration of higher doses of T3 produces vasodepressor effects. Overall, the present results suggest some possible non-genomic mechanisms for the vasorelaxant effect observed – the NO-cGMP-PKG pathway and Ca2+-activated K+ channels via activation of muscarinic receptors.
Acknowledgments
The authors greatly appreciate the technical assistance of Lizeth Ledesma Rodea and Oscar Martín Boche Olivan.
References
- 1.Biondi B, Palmieri EA, Lombardi G, Fazio S. Subclinical hypothyroidism and cardiac function. Thyroid. 2002;12:505–510. doi: 10.1089/105072502760143890. [DOI] [PubMed] [Google Scholar]
- 2.Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol. 2008;52:1152–1159. doi: 10.1016/j.jacc.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Y, Manio MM, Leung GP, Xu A, Tang EH, Vanhoutte PM. Thyroid hormone affects both endothelial and vascular smooth muscle cells in rat arteries. Eur J Pharmacol. 2015;747:18–28. doi: 10.1016/j.ejphar.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson JH. Recent work on thyroid hormones. Postgrad Med J. 1957;33:333–337. doi: 10.1136/pgmj.33.381.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 2005;115:2524–2533. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa T, Chijiwa T, Hagiwara M, Mamiya S, Hidaka H. Thyroid hormones directly interact with vascular smooth muscle strips. Mol Pharmacol. 1989;35:760–765. [PubMed] [Google Scholar]
- 9.Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid. 1996;6:505–512. doi: 10.1089/thy.1996.6.505. [DOI] [PubMed] [Google Scholar]
- 10.Zwaveling J, Pfaffendorf M, van Zwieten PA. The direct effects of thyroid hormones on rat mesenteric resistance arteries. Fundam Clin Pharmacol. 1997;11:41–46. doi: 10.1111/j.1472-8206.1997.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda K, Takasu N, Higa S, Oshiro C, Oshiro Y, Shimabukuro M, et al. Direct effects of thyroid hormones on rat coronary artery: nongenomic effects of triiodothyronine and thyroxine. Thyroid. 1998;8:609–613. doi: 10.1089/thy.1998.8.609. [DOI] [PubMed] [Google Scholar]
- 12.Ojamaa K, Balkman C, Klein IL. Acute effects of triiodothyronine on arterial smooth muscle cells. Ann Thorac Surg. 1993;56:S61–S66. doi: 10.1016/0003-4975(93)90556-W. [DOI] [PubMed] [Google Scholar]
- 13.Park KW, Dai HB, Ojamaa K, Lowenstein E, Klein I, Sellke FW. The direct vasomotor effect of thyroid hormones on rat skeletal muscle resistance arteries. Anesth Analg. 1997;85:734–738. doi: 10.1097/00000539-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steel RGD, Torrie JH. Principles and procedures of statistic: a biomedical approach. New York: McGraw-Hill; 1997. [Google Scholar]
- 16.Carrillo-Sepulveda MA, Ceravolo GS, Fortes ZB, Carvalho MH, Tostes RC, Laurindo FR, et al. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res. 2010;85:560–570. doi: 10.1093/cvr/cvp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey RD. Muscarinic receptor agonists and antagonists: effects on cardiovascular function. Handb Exp Pharmacol. 2012:299–316. doi: 10.1007/978-3-642-23274-9_13. [DOI] [PubMed] [Google Scholar]
- 18.Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palacios M, Knowles RG, Palmer RM, Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989;165:802–809. doi: 10.1016/S0006-291X(89)80037-3. [DOI] [PubMed] [Google Scholar]
- 20.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 21.Gadbois DM, Crissman HA, Tobey RA, Bradbury EM. Multiple kinase arrest points in the G1 phase of nontransformed mammalian cells are absent in transformed cells. Proc Natl Acad Sci U S A. 1992;89:8626–8630. doi: 10.1073/pnas.89.18.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 24.Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- 25.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–830. doi: 10.1161/01.RES.87.9.825. [DOI] [PubMed] [Google Scholar]
- 26.Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The antidiabetic sulfonylurea glibenclamide is a potent blocker of the ATP-modulated K+ channel in insulin secreting cells. Biochem Biophys Res Commun. 1987;146:21–25. doi: 10.1016/0006-291X(87)90684-X. [DOI] [PubMed] [Google Scholar]
- 27.Ok SH, Han JY, Sung HJ, Yang SM, Park J, Kwon SC, et al. Ropivacaine-induced contraction is attenuated by both endothelial nitric oxide and voltage-dependent potassium channels in isolated rat aortae. Biomed Res Int. 2013;2013:565271. doi: 10.1155/2013/565271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shieh CC, Kirsch GE. Mutational analysis of ion conduction and drug binding sites in the inner mouth of voltage-gated K+ channels. Biophys J. 1994;67:2316–2325. doi: 10.1016/S0006-3495(94)80718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/S0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 30.Zografos P, Li JH, Kau ST. Comparison of the in vitro effects of K+ channel modulators on detrusor and portal vein strips from guinea pigs. Pharmacology. 1992;45:216–230. doi: 10.1159/000139000. [DOI] [PubMed] [Google Scholar]
- 31.Pataricza J, Marton Z, Lengyel C, Toth M, Papp JG, Varro A, et al. Potassium channels sensitive to combination of charybdotoxin and apamin regulate the tone of diabetic isolated canine coronary arteries. Acta Physiol. 2008;194:35–43. doi: 10.1111/j.1748-1716.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 32.Qiu Y, Quilley J. Apamin/charybdotoxin-sensitive endothelial K+ channels contribute to acetylcholine-induced, NO-dependent vasorelaxation of rat aorta. Med Sci Monit. 2001;7:1129–1136. [PubMed] [Google Scholar]
- 33.Lopez-Canales JS, Lozano-Cuenca J, Lopez-Canales OA, Aguilar-Carrasco JC, Aranda-Zepeda L, Lopez-Sanchez P, et al. Pharmacological characterization of mechanisms involved in the vasorelaxation produced by rosuvastatin in aortic rings from rats with a cafeteria-style diet. Clin Exp Pharmacol Physiol. 2015;42:653–661. doi: 10.1111/1440-1681.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tottrup A, Kraglund K. Endothelium-dependent responses in small human mesenteric arteries. Physiol Res. 2004;53:255–263. [PubMed] [Google Scholar]
- 35.Corriu C, Feletou M, Canet E, Vanhoutte PM. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br J Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977;11:323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- 37.Blose SH, Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J Cell Biol. 1976;70:459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satav JG, Katyare SS, Fatterparker P, Sreenivasan A. Study of protein synthesis in rat liver mitochondria use of cycloheximide. Eur J Biochem. 1977;73:287–296. doi: 10.1111/j.1432-1033.1977.tb11318.x. [DOI] [PubMed] [Google Scholar]
- 39.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]


