Abstract
Background
Amongst the challenges to improving care for depressive and anxiety disorders (the common mental disorders, CMD) in developing countries are the shortage of skilled human resources and the lack of availability of psychosocial interventions. The MANAS trial was implemented to test the effectiveness of a lay health worker led intervention in primary health care settings to improve outcomes of people with CMD in Goa, India.
Method
Cluster randomised trial with primary care facility as unit of randomisation. Twenty-four primary care facilities, with an equal proportion of public Primary Health Centres (PHC) and private General Practitioners (GP) practices, were randomly allocated within pre-defined strata, to intervention (collaborative stepped care, CSC) or control (enhanced usual care, EUC) groups. All adults presenting at a facility were screened for CMD with the General Health Questionnaire and those scoring above a cut-off point of five were regarded as eligible for participation in the trial. The CSC arm provided case management and psychosocial interventions, delivered by a trained Lay Health Counsellor, antidepressant medication by the primary care physician and supervision by a visiting mental health specialist. The primary outcome was recovery from ICD-10 defined CMD six months after recruitment. The secondary outcome was the severity of symptoms of depression and anxiety. Secondary analyses were stratified by facility type (public vs private).
Results
2796 (81% of the eligible population) were recruited (1360 in the CSC arm and 1436 in the EUC arm), of whom 1160 (85%) and 1269 (88%) respectively completed the outcome evaluation at the primary end-point of six months. Patients with ICD-10 definite CMD in the intervention arm were more likely to have recovered at 6 months (65% vs 53%; risk ratio=1.22, 95%CI 1.00–1.47; risk difference=12%, 95%CI 2%-23%). Secondary analyses showed that the intervention had a generally strong and consistent effect in PHC facility attenders but not GP facility attenders for all diagnostic groups apart from depression where no effect was found.
Implications
The MANAS trial is the largest effectiveness trial of a primary-care based intervention to integrate CMD treatments into routine primary care in a developing country. The trial demonstrates that an intervention led by a trained lay counsellor improves recovery from CMD, in particular among those attending primary health care facilities.
Study Registration
The MANAS project was registered with the National Institutes of Health sponsored clinical trials registry and has been assigned the identifier: NCT00446407
Background
Depressive and anxiety disorders are the leading neuropsychiatric cause of the global burden of disease[1], and are associated with an increased risk of suicide, increased health care costs and reduced economic productivity [2–5]. Although these disorders are classified as separate diagnostic categories in ICD-10[6], the broader category of common mental disorders (CMD)[7] is often used to describe them as a group because of the high level of co-morbidity and similarities in epidemiological characteristics and treatment responsiveness[7–10]. The majority of persons with CMD seek healthcare in primary care settings[1] where recognition is poor, with fewer than one third of clinically significant cases detected[11]. Primary care doctors tend to prescribe a range of medications for patients with CMD[12]; mainly tranquilizers (benzodiazepines) and vitamins[13].
A recent systematic review of the constituents of complex, collaborative care interventions which improve effectiveness for CMD management in primary care found that the use of routine screening, the professional skills of staff and specialist supervision predicted a favourable outcome[14]. Although evidence of the efficacy of antidepressants and brief psychological treatments has long been available, including trials from developing countries[15–18] [19], there are several obstacles to scaling up efficacious interventions to the ‘real-world’ primary care context in developing countries[20–22]. These comprise the low recognition rate of CMD by primary care doctors[23]; the inadequate use of evidence-based medications, including antidepressants and the frequent use of non evidence-based medications[24]; the inadequate use of psychosocial treatments; and low adherence with treatments (ref). Although training programmes for health workers often show an increase in knowledge, the improvement in recognition rates are transient[23], and translation to improved clinical outcomes has not been evaluated[21, 25].
The Manas project systematically developed an intervention for CMD which sought to address these barriers in routine primary health care in Goa, India [26]. Task shifting is an increasingly advocated method to address specialist health human resource shortages [27–28]. Community or lay health workers carry out functions related to health care delivery, trained in the context of the intervention and having no formal professional education.[29] The aim of the MANAS trial was to evaluate the effectiveness of a lay health counselor (LHC) led collaborative stepped care interventions for CMD in two types of primary health care settings. The intervention included psychoeducation and support by a lay health counselor supplemented by antidepressant Pharmacotherapy, structured psychotherapy, and/or psychiatric consultation. More than half of all primary care consultations in India take place in the private sector [30] and MANAS aimed to test the effectiveness of the intervention in both types of facilities.
Design And Methods
Setting
The trial was conducted in Goa, a state in West India with a population of 1.4 million. Goa has been the setting of studies on the epidemiology and treatment of CMD for eight years[15, 31–36]. Manas was implemented by Sangath, a Goan community mental health non-governmental organization, in collaboration with the London School of Hygiene & Tropical Medicine (LSHTM), the Government of Goa’s Directorate of Health Services, the Voluntary Health Association of Goa and private general practitioners.
Objective
To evaluate the effectiveness of a LHC led collaborative stepped care (CSC) intervention on recovery from CMD in public (government) Primary Health Centre (PHC) and private General Practitioner (GP) health care settings
Design
A randomised design with health facility as the unit of randomisation was chosen to prevent contamination between individuals. The trial was conducted in two consecutive phases from April 2007 to September 2009. Phase 1 involved 12 PHCs, while Phase 2 was conducted in 12 GP facilities.
Sample Size
Our sample size estimates have been described in detail in our protocol [37]. Briefly, we assumed: a coefficient of variation of 0.2, prevalence of ICD-10 of 66% among participants screened-positive; follow-up of 75% at 6 months. The resulting sample size of 100 screen-positive participants in 24 clusters provides over 90% power to detect a difference in recovery rates of 70% in the CSC arm versus 50% in the EUC control arm, with estimates based on earlier efficacy trials in Goa [15] and Chile [17]. There were no planned interim analyses or stopping rules. However, the higher rates of ICD10 cases and follow-up rates observed during Phase 1 led to a downward re-estimation of the sample sizes in Phase 2 to 80 screen-positive participants per cluster.
Selection of Facilities and Randomisation
The sampling frames included all PHC facilities with the space and privacy for LHCs and which were not involved in preliminary phases related to intervention development [26, 38]. For Phase 1 we collected the details of all the available PHC and larger Rural Medical Dispensaries (n=49) and assessed their suitability for inclusion in the trial based on the above criteria. 17 facilities met the inclusion criteria of which 12 were randomly selected for inclusion in the trial. For Phase 2, we sent out 400 letters to GPs from a list of all registered general medical practitioners in the state. However, the response rate was poor (only eight GPs responded of whom six were eligible). The research team then visited GPs who had not responded (n=60). Thus a total of 68 GPs were visited and assessed for eligibility of which 43 declined to participate and three did not meet the inclusion criteria. Twelve out of the 22eligible GP facilities were randomly selected for inclusion in the trialremaining ten. Within the PHC sector, facilities were first stratified by presence/absence of a visiting psychiatrist (part of the District Mental Health Program being implemented concurrently in one district) and then randomised within four strata; amongst those with a visiting psychiatrist, busy with approximately 150 patients per day (n=2), and less busy (n=2); and among the eight facilities without a visiting psychiatrist, urban (n=4) and rural (n=4). The 12 GP facilities were randomised within two strata; busy with more than 40 patients per day on average (n=7), and less busy (n=5). Facilities were randomly allocated within each stratum to either the intervention or control arm using a 1:1 allocation ratio by the trial statistician (HW) using the website www.randomization.com.
Trial Participants
A trained community worker used the 12 item General Health Questionnaire (GHQ-12) with a cut-off score of 5/6 to screen for CMD. This threshold was determined on the basis of the psychometric properties of this tool in similar settings [38]. Eligibility criteria for screening were: age >17 years, speaking Konkani, Marathi, Hindi, or English, not requiring urgent medical attention, not having difficulty with hearing, speaking or cognition which makes interviewing difficult, , not already screened in the previous two weeks, and not already receiving the intervention. Those who screened positive for CMD and who expected to be resident in Goa for the subsequent 12 months were invited to participate in the trial. If the patient gave written or verbal consent, a structured clinical diagnostic interview (the Revised Clinical Interview Schedule or CIS-R) [39] (see below) was administered to provide a baseline assessment of severity and diagnostic categorization. Patients who screened positive but did not meet the inclusion criteria were offered the intervention but were not followed-up in the trial.
The Interventions
All interventions were implemented at the individual level within clusters.
The Collaborative Stepped Care (CSC) Intervention
The formative and piloting work leading to the design of the CSC intervention has been described previously [26]. In brief, the intervention is based on the stepped-care approach used in a Chilean trial [17] which emphasizes that while simple interventions such as psycho-education may be provided to all patients, more resource-intensive interventions may be reserved for participants who are severely ill or not responding to the simpler interventions. Thus, the approach focuses o efficient use of limited resources. The collaborative approach involves three key team members: the LHC, the primary care physician and a visiting psychiatrist (“clinical specialist”). The locally-recruited LHC had non-health backgrounds and underwent a structured two month training course (http://www.sangath.com/sangath/node/88). The LHC acted as a case-manager for all who screened positive for CMD and took overall responsibility for delivering the intervention for all non-drug treatments, in close collaboration with the primary care physician and the clinical specialist. The steps of the intervention are presented in Table 1 and individual components are briefly described below.
Table 1. Steps of the Collaborative Stepped Care Intervention.
| Step | For whom | Timing | Treatment | Human Resource |
|---|---|---|---|---|
| Treatment Initiation | Patients screened with CMD | At first consultation | Advice regarding screening questionnaire results; advice regarding seeing LHC | Primary care physician |
| Psycho-education | LHC | |||
| Managing moderate or severe cases | Patients who are severely ill at first consultation, or whose symptoms persist at follow-up | At first consultation or at follow up if not responding to Step 1 | Antidepressants | Primary care physician |
| Adherence Management | LHC | |||
| Monitoring outcomes | Patients who remain unwell, or are not adherent | Patients who do not respond to Step 2 despite taking the treatment | Antidepressants & | Primary care physician |
| IPT & | LHC | |||
| Adherence Management | LHC | |||
| Referral to specialists | Patients who do not respond despite good adherence or are judged to be at high suicide risk | Patients who do not respond to Step 2 or 3
despite taking the treatment & Patients who are at high suicidal risk at any time |
Continue all existing treatments
Referral to clinical specialist |
LHC & Primary care
physician Clinical specialist |
Psycho-education provided by the LHC to all patients screened positive for CMD focused on educating the person about their symptoms, the association of CMD with inter-personal difficulties (derived from the initial phase of Inter-personal Psychotherapy (IPT), see below) and the need to share emotional symptoms with the doctor and to share personal difficulties with caring family members or other key persons in their social network. Psycho-education taught patients simple strategies for symptom alleviation, for example breathing exercises for anxiety symptoms. Encouraging adherence to CMD treatments and providing information about social/welfare agencies when required were other key components of psycho-education. one
Antidepressants were recommended only for moderate or severe CMD (i.e. GHQ score>7) and for those who did not respond to psycho-education alone on the basis of routine clinical assessments by the LHC. The antidepressant of choice, fluoxetine,?? is not available in state PHCs and was provided by the project to integrate with the existing model of free medicines prescribed by the PHC doctor. In the GP clinics doctors could prescribe antidepressants of their choice which were purchased by patients as normal. Once initiated, antidepressants were recommended for a minimum of 90 days at an adequate dose (at least 20mg per day of fluoxetine or the equivalent). Physicians were given training over half a day and a manual. The other key roles of the physicians were to encourage patients to meet the LHC, to avoid the use of unnecessary medications (such as vitamins) and to provide usual care for any co-existing physical health problems.
IPT, delivered by the LHC, was the structured psychological intervention chosen due to its demonstrated feasibility and effectiveness in another low income country [41], and on its focus on interpersonal problems such as grief, disputes and role transitions which were common themes in the adverse life experiences of participants in earlier research in Goa [42]. A minimum of six sessions, with an optimum of eight and a maximum of 12 sessions, were offered. IPT was reserved only for patients who had moderate or severe CMD, and was offered as an alternative to antidepressants or in addition to antidepressants for those who did not respond to antidepressants.
Referral to the clinical specialist was reserved for patients who were assessed as high suicide risk at any stage; were unresponsive to the earlier treatments; posed diagnostic dilemmas; had significant co-morbidity with alcohol dependence; had associated significant other medical problems; or for whom the primary care physician requested a consultation. Each facility team was supported by a clinical specialist who visited at least once a month and was also available for consultation on the phone to discuss cases and to assure supervision and quality assurance of the program.
Patients could be discharged either in a planned manner (for example, recovered) or unplanned (for example, did not return for reviews despite adherence management procedures).
Control intervention - Enhanced usual care control (EUC)
Physicians and patients in usual care practices received screening results and were given the treatment manual prepared for primary care physicians. They were allowed to initiate treatments of their choice.
Outcomes
As per the trial protocol[37] (http://clinicaltrials.gov/ct2/show/NCT00446407?term=MANAS&rank=1), the primary outcome was the proportion recovered from ICD-10 CMD, and the secondary outcome the severity of symptoms (see following paragraph), assessed at six months.
Measurement
The primary and secondary outcomes were assessed using the CIS-R, a structured interview for use by trained lay researchers, which generates two outputs: a ICD-10 diagnosis derived from a computer algorithm and a total score reflecting the overall severity of symptoms [39]. The CIS-R is one of the most widely used measures of CMD globally with extensive prior use in the study setting [15, 34, 43]. Research assessors underwent two weeks training in the use of the interview and quality assurance including using hand-held PDAs to collect data.
Masking
Masking of the research assessor was maximized by: carrying out evaluations at home; randomly allocating unique patient IDs so that there was no association between the ID number and the facility identity; outcome evaluation being carried out by an independent institution whose team was not privy to the randomization allocation; and carrying out the primary outcome assessment prior to all other assessments.
Process evaluation
Process indicators assessing the fidelity and quality of the CSC intervention were obtained from four sources: the separate clinical records maintained by the LHC and the clinical specialist; antidepressant use from the clinic records; and quality assessments carried out for each component of the intervention. Quality assessments for intervention components were made by direct observation or through transcripts of sessions and were rated by senior clinicians. The only possible process indicator in the EUC arm was antidepressant use.
Statistical methods
All analyses were conducted in Stata 11.0. Baseline comparability was assessed for individuals who did not consent to be part of the trial, and of participants who did not complete review assessments. Comparability of participants in the two arms was assessed for potential confounding factors, notably: age, sex, education, severity of CIS-R scores and ICD-10 diagnostic distribution. The primary analyses compared participants in their original assigned groups, regardless of adherence to the intervention. Patients were divided into four diagnostic groups based on their clinical diagnosis at baseline:
ICD-10 CMD cases assessed using the CIS-R - the primary analysis group.
Screen positive cases assessed using the GHQ-12.
Sub-threshold cases – patients who screened positive for CMD on the GHQ but who did not meet ICD-10 diagnostic criteria for CMD on the CIS-R.
ICD-10 depression cases assessed using the CIS-R.
As per the trial protocol [37], the primary analysis was the difference across arms in the proportion of ICD-10 cases at baseline who recover at six months. Secondary analyses were also evaluated at six months and comprised the differences across arms in: the proportion of depression cases who recover; the prevalence of ICD-10 CMD among screen positive cases and sub-threshold cases; and the mean total CIS-R score in each diagnostic groups.
Analyses were based on cluster-level summary measures, as individual-level regression methods do not perform robustly when there are relatively few clusters per arm, especially for stratified cluster randomized trials [44]. For binary outcomes, the impact was measured by the risk ratio (RR) and risk difference (RD). The stratum-specific risk ratios were calculated as the ratio of the geometric mean risks between arms for each of the six strata, and the overall RR was estimated as the weighted-average of these stratum-specific risk ratios. An approximate variance for the log(mean risk) in each arm was obtained from the residual mean square from a two-way analysis of variance of community log-risk on strata and study arm. A 95% confidence interval (CI) for the RR was calculated from this variance using a stratified t-test with 12 degrees of freedom [44]. Similarly, a 95%CI for the RD was obtained from an analysis of variance of the mean risk on strata and study arm. For continuous outcomes (CIS-R score), the measure of effect was the mean difference between arms, and these were analysed in an analogous method based on mean scores in each facility. As there were no substantial baseline imbalances of key covariates, the primary analyses did not adjust for potential confounders. Secondary planned analyses examined the impact of the intervention separately in the two types of facilities, with assessment of effect-modification of the intervention effect by facility type [45]. Sensitivity analyses included adjustment for factors imbalanced at baseline.
Ethics
Details of trial protocol approval and consent have been published previously [Trials paper].
Results
Participant flow
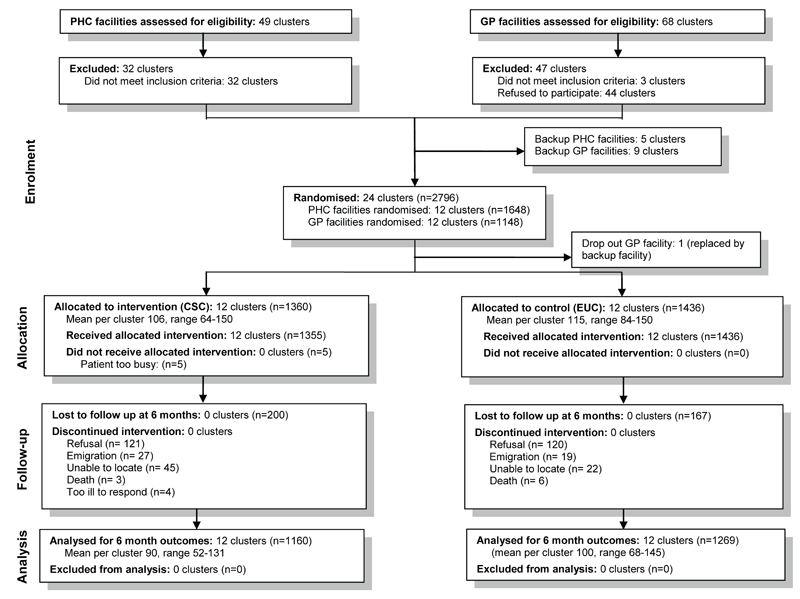
(Figure 1): One GP cluster was replaced by a back-up within a month because of small patient numbers. All 24 clusters were followed to the end of the trial. Altogether 20,352 patients were screened, of whom 3,816 (18.8%) screened positive for CMD and 3,434 were eligible to participate. Of these, 2,796 (81%) consented to participate and were enrolled (1,360 in CSC arm and 1,436 in the EUC arm). Participants who did not consent tended to be slightly younger and have more severe CIS-R scores than those who consented. A total of 1,160 participants in the CSC arm (85%) and 1,269 (88%) in the EUC arm completed the six month outcome evaluation. Reasons for loss to follow up were refusal (n=241; 121 in the CSC arm and 120 in the EC arm), emigration (n=46; 27, 19), unable to locate (n=67; 45, 22), death (n=9; 3, 6) and other (n=4; 4, 0). Participants who were not followed up at six months were more likely to be younger and male. However there were no differences in terms of intervention arm, facility type or baseline diagnostic group (Web Table 1).
Figure 1. Participant flow chart.
Web Table 1.
Characteristics of participants reviewed and not reviewed at 6 months
| Attended 6 month
review |
|||||
|---|---|---|---|---|---|
| No | Yes | ||||
| N=367 | N=2429 | ||||
|
| |||||
| n | % | n | % | p-value1 | |
| Intervention arm | |||||
| CSC | 200 | (15) | 1160 | (85) | |
| EUC | 161 | (12) | 1269 | (88) | 0.101 |
| Age (years) | |||||
| 18-29 years | 66 | (18.0) | 228 | (9.4) | |
| 30-39 years | 77 | (21.0) | 494 | (20.3) | |
| 40-49 years | 91 | (24.8) | 642 | (26.4) | |
| 50-59 years | 63 | (17.2) | 471 | (19.4) | |
| Over 60 years | 70 | (19.1) | 594 | (24.5) | 0.000 |
| Sex | |||||
| Male | 97 | (26.4) | 394 | (16.2) | |
| Female | 270 | (73.6) | 2035 | (83.8) | 0.000 |
| Facility type | |||||
| PHC | 232 | (63.2) | 1416 | (58.3) | |
| GP | 135 | (36.8) | 1013 | (41.7) | 0.129 |
| Baseline diagnostic category | |||||
| Sub-threshold case | 86 | (23) | 468 | (19) | 0.173 |
| ICD-10 case | 281 | (77) | 1961 | (81) | 0.173 |
| Depression case | 101 | (28) | 673 | (28) | 0.834 |
P-value is likelihood ratio test of contribution of the variable to the model from a logistic regression adjusted for clinic to account for clustering.
There were 1,098 ICD-10 cases at baseline in the CSC arm (81% of screened positives), and 1,144 (80%) in the EUC arm. Of these, 944 (86%) of CSC arm participants and 1,017 (89%) of EUC arm participants were seen at the six month outcome evaluation. The coefficient of variation (k) for CMD prevalence at baseline among all screen positive cases was 0.08, indicating relatively little intra-cluster correlation (ICC=0.03).
Study population
Table 2 shows the socio-demographic and clinical characteristics of the two arms. The trial population was predominantly female (82%), with mean age 46.3 years (SD 13.3 years). Of the 2,242 ICD-10 cases at baseline (81%), 774 (35%) were depression cases. In general, there was good balance between arms; although participants in the EUC arm were more likely to have depression, the proportion of ICD-10 CMD cases and mean CIS-R scores were similar.
Table 2. Baseline characteristics of trial participants, by arm.
| Intervention arm |
||||
|---|---|---|---|---|
| CSC | EUC | |||
|
| ||||
| Clusters=12 | Clusters=12 | |||
| N=1360 | N=1436 | |||
|
| ||||
| n | % | n | % | |
| Facility type | ||||
| PHC | 823 | (61) | 825 | (57) |
| GP | 537 | (39) | 611 | (43) |
| Baseline diagnostic category | ||||
| Sub-threshold case | 262 | (19) | 292 | (20) |
| ICD-10 case | 1098 | (81) | 1144 | (80) |
| Depression case (including co-morbid with anxiety) | 304 | (22) | 470 | (33) |
| CIS-R score (mean, SD) | 19.9 | (9.1) | 19.4 | (9.1) |
| Age | ||||
| 18-29 years | 147 | (11) | 147 | (10) |
| 30–39 years | 296 | (22) | 275 | (19) |
| 40-49 years | 365 | (27) | 368 | (26) |
| 50-59 years | 256 | (19) | 278 | (19) |
| over 60 years | 296 | (22) | 368 | (26) |
| Sex | ||||
| Male | 246 | (18) | 245 | (17) |
| Female | 1114 | (82) | 1191 | (83) |
| Marital status 1 | ||||
| Never married | 96 | (8) | 63 | (5) |
| Married | 761 | (64) | 857 | (65) |
| Widowed | 330 | (28) | 378 | (29) |
| Separated/divorced | 8 | (1) | 18 | (1) |
| Ethnicity 1 | ||||
| Goan | 1139 | (95) | 1262 | (96) |
| Non-Goan | 55 | (5) | 54 | (4) |
| Language 1 | ||||
| Konkani | 1173 | (98) | 1303 | (99) |
| Other | 23 | (2) | 13 | (1) |
| Education 1 | ||||
| <1 year | 493 | (41) | 664 | (50) |
| 1-4 years | 222 | (19) | 200 | (15) |
| >=5 years | 478 | (40) | 451 | (34) |
These variables were asked at the 2 month follow-up and are based on 2491 (89%) of participants: 1186 (87%) of those in the CSC arm and 1305 (91%) in the EUC arm
Impact of the intervention
Recovery in ICD-10 cases: (Table 3; Figure 2)
Table 3. Impact of intervention on recovery from ICD-10 CMD at 6 months among ICD-10 definite CMD cases at baseline (primary analysis), and ICD-10 depression and anxiety cases at baseline (secondary analyses), by facility type.
| Diagnostic group at baseline | Type of facility |
p-value for effect modification | ||
|---|---|---|---|---|
| All facilities | PHC | GPs | ||
| Primary: ICD-10 cases (all) (n=1961) | ||||
| Collaborative Stepped Care | 620 (65.0%) | 369 (65.9%) | 251 (64.1%) | |
| Enhanced Usual Care | 553 (52.9%) | 267 (42.5%) | 286 (65.9%) | |
| Risk difference (95% CI) | 12.1% (1.6%, 22.5%) | 23.4% (5.0%, 41.7%) | -1.8% (-17.7%, 14.1%) | 0.001 |
| Risk ratio (95% CI) | 1.22 (1.00, 1.47) | 1.55 (1.02, 2.35) | 0.95 (0.74, 1.22) | 0.001 |
| P-value | 0.05 | 0.04 | 0.63 | |
|
| ||||
| Secondary: Depression cases (n=673) | ||||
| Collaborative Stepped Care | 142 (53.6%) | 80 (52.1%) | 62 (55.1%) | |
| Enhanced Usual Care | 216 (50.4%) | 114 (38.8%) | 102 (65.5%) | |
| Risk difference (95% CI) | 3.2% (-9.4%, 15.7%) | 13.3% (-10.1%, 36.6%) | -10.4% (-29.0%, 8.1%) | 0.008 |
| Risk ratio (95% CI) | 1.05 (0.81, 1.36) | 1.34 (0.73, 2.45) | 0.82 (0.60, 1.12) | 0.007 |
| P-value | 0.69 | 0.25 | 0.18 | |
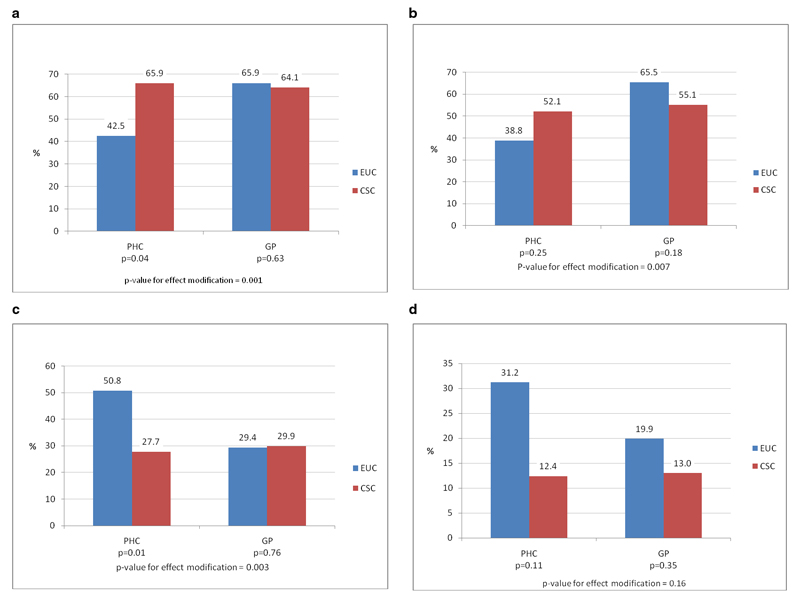
Figure 2. Effect of intervention on baseline diagnostic groups.
a) Proportion of ICD10 cases who recovered at 6 months (n=1961) b) Proportion of depression cases who recovered at 6 months (n=673) c) Prevalence of CMD at 6 months among screen positive cases (n=2429) d) Prevalence of CMD at 6 months among sub-threshold cases (n=468)
There was modest evidence of an effect of the intervention on recovery from ICD-10 cases at six months (proportion recovered: 65.0% vs. 52.9% in CSC and EUC arms respectively; RR=1.22, 95%CI 1.00,1.47). The effect was larger among PHC participants (65.9% vs. 42.5%, RR=1.55, 95%CI 1.02,2.35) but not GP participants (64.1% vs. 65.9%, RR=0.95, 95%CI 0.74, 1.22, p-value for effect-modification=0.001). However, there was little evidence of an effect of the intervention on recovering from CMD among depression cases (RR=1.05, 95%CI 0.81,1.36).
Results for recovery from CMD were similar when adjusted for available baseline data (age, sex, diagnostic category and education; adjusted RR =1.16, 95%CI 0.98-1.38).
Prevalence of ICD-10 CMD among screen positive cases and sub-threshold cases (Table 4; Figure 2)
Table 4. Impact of intervention on prevalence of ICD-10 CMD at 6 months, by baseline diagnostic group and facility type (secondary analyses).
| Diagnostic group at baseline | Type of facility |
p-value for effect modification | ||
|---|---|---|---|---|
| All facilities | PHC | GPs | ||
| Screen positive cases (n=2429) | ||||
| Collaborative Stepped Care | 354 (28.8%) | 196 (27.7%) | 158 (29.9%) | |
| Enhanced Usual Care | 541 (38.6%) | 375 (50.8%) | 166 (29.4%) | |
| Risk difference (95% CI) | -9.9% (-19.4%, -0.03%) | -23.1% (-42.1%, -4.2%) | -0.05% (-12.9%, 14.0%) | <0.001 |
| Risk ratio (95% CI) | 0.76 (0.57–1.02) | 0.54 (0.37–0.81) | 1.07 (0.66–1.74) | 0.003 |
| P-value | 0.06 | 0.01 | 0.76 | |
|
| ||||
| Sub-threshold cases (n=468) | ||||
| Collaborative Stepped Care | 30 (12.7%) | 20 (12.4%) | 10 (13.0%) | |
| Enhanced Usual Care | 77 (25.0%) | 49 (31.2%) | 28 (19.9%) | |
| Risk difference (95% CI) | -12.3% (-23.5%, -1.1%) | -18.8% (-46.6%, 9.0%) | -6.9% (-19.3%, 5.5%) | 0.18 |
| Risk ratio (95% CI) | 0.52 (0.29–0.96) | 0.40 (0.11–1.38) | 0.70 (0.31–1.59) | 0.16 |
| P-value | 0.04 | 0.11 | 0.35 | |
There was modest evidence of an effect on prevalence of ICD-10 at six months among screen positive cases, with a halving of prevalence in PHCs (RR=0.54, 95%CI 0.34, 0.81) but no effect in GP facilities (p-value for effect-modification=0.003). Among sub-threshold cases, there was evidence of a protective effect of the intervention overall, and this did not differ by facility type.
Severity of psychiatric symptoms at six months (Table 5)
Table 5. Impact of intervention on severity of symptoms (mean CIS-R score) at 6 months, by facility type and diagnostic group at baseline (secondary analyses).
| Diagnostic group at baseline | Type of facility |
p-value for effect modification | ||
|---|---|---|---|---|
| All facilities | PHC | GPs | ||
| ICD-10 cases (n=1961) | ||||
| Collaborative Stepped Care | 9.30 | 9.28 | 9.32 | |
| Enhanced Usual Care | 11.61 | 14.12 | 9.10 | |
| Mean difference (95% CI) | -2.14 (-4.32, 0.04) | -4.84 (-8.48, -1.20) | 0.63 (-2.76, 4.02) | .001 |
| P-value | 0.05 | 0.02 | 0.68 | |
|
| ||||
| Depression cases (n=673) | ||||
| Collaborative Stepped Care | 11.38 | 11.30 | 11.47 | |
| Enhanced Usual Care | 12.54 | 15.60 | 9.48 | |
| Mean difference (95% CI) | -1.01 (-3.63, 1.61) | -4.30 (-9.58, 0.98) | 2.38 (-1.29, 6.05) | <0.001 |
| P-value | 0.42 | 0.09 | 0.17 | |
|
| ||||
| Screen positive cases (n=2429) | ||||
| Collaborative Stepped Care | 8.56 | 8.39 | 8.72 | |
| Enhanced Usual Care | 10.87 | 13.13 | 8.60 | |
| Mean difference (95% CI) | -2.15 (-4.20, -0.10) | -4.75 (-8.86, -0.64) | 0.52 (-2.36, 3.40) | 0.001 |
| P-value | 0.04 | 0.03 | 0.69 | |
|
| ||||
| Sub-threshold cases (n=468) | ||||
| Collaborative Stepped Care | 5.62 | 5.69 | 5.56 | |
| Enhanced Usual Care | 7.62 | 8.56 | 6.68 | |
| Mean difference (95% CI) | -1.90 (-4.01, 0.20) | -2.87 (-8.09, 2.34) | -0.91 (-3.25, 1.44) | 0.20 |
| P-value | 0.07 | 0.20 | 0.40 | |
For each diagnostic group, the mean CIS-R score was lower in the CSC than the EUC arm. Further, in the screen-positive cases, ICD-10 cases and depression cases, the intervention had a strong effect in PHCs and no effect in GP facilities (p-value for effect modification<0.001).
including imputation using …..
Process indicators (Table 6)
Table 6. Process indicators for the intervention facilities.
| Process indicator | Original benchmark | Trial performance | ||
|---|---|---|---|---|
| PHC phase | GP phase | Weighted average (95% confidence interval) | ||
| Proportion of screen positive patients who meet ICD-10 criteria for CMD | Minimum 66% | 78% | 84% | 81 (79-83) |
| Proportion of patients who receive at least first psychoeducation session | Minimum 90% | 95% | 98% | 96 (95-97) |
| Proportion of moderate-severe cases who receive antidepressants | Minimum 80% | 83% | 88% | 85 (82-88) |
| Proportion of all patients who receive ADT | NA | 48% | 64% | 54 (51-57) |
| Proportion of patients receiving antidepressants who complete at least 3 months treatment | Minimum 50% | 53% | 52% | 53 (49-56) |
| Proportion of moderate-severe cases who receive IPT | NA | 5% | <1% | - |
| Proportion of patients receiving IPT who complete at least 6 sessions | Minimum 50% | 33% | nil | - |
| Proportion of patients who had a planned discharge | Minimum 60% | 51% | 67% | 57 (54-60) |
| Proportion of patients referred to psychiatrist | Maximum 5% | <1% | <1% | 0.5 (0.3-1.1) |
Table 6 shows process indicators overall and by type of facility. The original target for coverage was reached for most indicators, although among patients receiving IPT the proportion who completed at least 6 session was lower than expected. This led to a modification of how IPT was used in Phase 2 in the GP facilities, in which IPT was reserved as step three treatment for patients not improving with ADT, and components of IPT were integrated with the psycho-education. Over half of all patients had a planned discharge from the program. These process indicators show that, apart from the IPT, the intervention was delivered with high fidelity to the original protocol. The number of quality assessments also exceeded the targets set for the trial (detailed results available from corresponding author).
Adverse events
There were a total of seven serious adverse events (three deaths and four suicide attempts) in the CSC arm compared with 12 in the EUC arm (six deaths and six suicide attempts).
Discussion
The Manas trial is the largest evaluation of the effectiveness of a lay health worker led intervention for any mental disorder. The primary analysis showed that overall there was some evidence of an effect of the intervention on recovery from ICD-10 cases of CMD at six months, with and a clear -type effect in PHCs but no effect in GP private facilities. In keeping with this finding secondary analyses showed that the intervention had a consistent effect in PHC but not GP for all baseline diagnostic groups included apart from depression.
These findings support the primary hypothesis that a LHC led intervention improves recovery rates among CMD patients, although only among those attending PHCs. The observed effects may under-estimate the true effect as two key components of the intervention (screening of all patients and provision of screening results and evidence-based guidelines to the patient and physician) were offered in both arms. Neither would be available in routine care. The prevalence of outcomes was generally similar in both arms of GP facilities and the CSC arm of the PHC facilities. Indeed, the recovery rates in these three arms are similar to those reported by other trials (ref) and to our originally hypothesis [37]. Thus, it appears that GPs perform well irrespective of presence of a LHC, and as well as PHCs with a LHC was available. In contrast, PHCs benefit from the addition of a LHC, and the intervention was effective at preventing CMD as well as treating it, as seen by the benefit found among sub-threshold cases.
There are several possible explanations for the good performance of the EUC GP facilities in the trial. First, the style of GP interactions with patients may be modified once they obtained the diagnosis of CMD through screening,. After this they may have offering care similar to the LHCs (e.g. better continuity of care same treating physician, more privacy in the office, or longer consultations?). In contrast, in the PHCs, large numbers of patients tend to be seen for short periods by a given doctor and the privacy required to discuss interpersonal difficulties, is not assured. Second, there may be specific therapeutic ingredients in GP consultations which deserve inclusion in the intervention to enhance its effectiveness; we will be exploring these hypotheses through our qualitative interviews with GPs and participants. Third, there were differences in patient characteristics by clinic type (see Web Table 2). Patients attending PHCs had poorer socio-economic indicators than those attending GP facilities. Finally, whereas the PHC facilities were representative of the typical PHC having been selected randomly from the eligible sampling frame, the GP facilities were a highly selected group and likely to represent a sub-group of highly motivated physicians who were eager to improve the quality of care for CMD in their clinics. The lack of an overall effect among the subgroup of depression patients may be partly due to lower power to detect a clinically significant effect in this sub-group, and inadequate intensity of the treatments, in particular the failure to deliver IPT as planned.
Web Table 2. Characteristics of respondents by type of facility.
| Facility type |
|||||
|---|---|---|---|---|---|
| PHC | GP | ||||
|
| |||||
| Clusters=12 | Clusters=12 | ||||
| N=1648 | N=1148 | ||||
|
| |||||
| n | % | n | % | p-value2 | |
| Age | |||||
| 18-29 years | 202 | (12.3) | 92 | (8.0) | |
| 30-39 years | 383 | (23.2) | 188 | (16.4) | |
| 40-49 years | 439 | (26.6) | 294 | (25.6) | |
| 50-59 years | 292 | (17.7) | 242 | (21.1) | |
| over 60 years | 332 | (20.2) | 332 | (28.9) | 0.000 |
| Sex | |||||
| Male | 319 | (19.4) | 172 | (15.0) | |
| Female | 1329 | (80.6) | 976 | (85.0) | 0.002 |
| Marital status 1 | |||||
| Never married | 102 | (7.0) | 57 | (5.4) | |
| Married | 965 | (66.6) | 653 | (61.6) | |
| Widowed | 367 | (25.3) | 341 | (32.1) | |
| Separated/divorced | 16 | (1.1) | 10 | (0.9) | 0.002 |
| Ethnicity 1 | |||||
| Goan | 1374 | (94.8) | 1027 | (96.9) | |
| Non-Goan | 76 | (5.2) | 33 | (3.1) | 0.007 |
| Language 1 | |||||
| Konkani | 1431 | (98.7) | 1045 | (98.4) | |
| Other | 19 | (1.3) | 17 | (1.6) | 0.336 |
| Education 1 | |||||
| <1 year | 653 | (45.1) | 504 | (47.6) | |
| 1-4 years | 271 | (18.7) | 151 | (14.3) | |
| >=5 years | 525 | (36.2) | 404 | (38.2) | 0.042 |
| Baseline diagnostic category | |||||
| Sub-threshold case | 341 | (21) | 213 | (19) | 0.094 |
| ICD-10 case | 1307 | (79) | 935 | (82) | 0.094 |
| Depression case | 470 | (29) | 304 | (27) | 0.1954 |
These variables were asked at the 2 month follow-up and are based on 2491 (89%) of participants: 1186 (87%) of those in the CSC arm and 1305 (91%) in the EUC arm
P-value is likelihood ratio test of contribution of the variable to the model from a logistic regression adjusted for clinic to account for clustering.
Trial strengths include: large samples drawn from rural and urban populations and including both government and private facilities; high follow-up rates; high levels of fidelity and quality of the intervention (with the exception of IPT this reads like the quality of IPT delivery was low, which was not the case ); and consistent demonstration of impact in PHCs for each diagnostic group apart from depression. In addition, we were able to confirm the high specificity of our screening procedure in a ‘real-world’ context. Around 13% of patients were not seen at the 6 month outcome, and these were more likely to be younger and male. However, separate models by age group and sex were fitted, and showed no evidence of differential recovery rates by gender or age. It is therefore unlikely that this missing data affects the results.
In light of our findings, we recommend that the intervention be extended to clinics run by government facilities. which constitute the majority of clinics? and are likely to be influenced by state policies. Screening is feasible because of the high prevalence of CMDs in primary care attenders, the brevity of screening instruments[47] and increasing literacy rates in many countries which makes self-completion feasible. Screening may have been a critically important component accounting for the impressive outcomes in the control GP facilities. Those acting as recruited as LHCs could perform several roles, are were relatively low-cost and readily available in most developing countries. However, the fact that the intervention had no effect at all on the sub-group of patients with depression also indicates the need for more intensive treatments for these patients, for example greater emphasis on the delivery of the structured psychological treatments or more aggressive pharmacotherapy[48].
In conclusion, the Manas trial has demonstrated the effectiveness of a lay health counsellor led collaborative stepped care intervention for CMD in primary health care facility attenders in India. This evidence should be used to scale-up services for common mental disorders in settings where mental health professionals are scarce.
Acknowledgements
The MANAS Project is entirely funded by a Wellcome Trust Senior Clinical Research Fellowship to VP. We are grateful to the members of the Trial Steering and Data Monitoring & Ethics Committees for their inputs which led to the final protocol described in this paper: KS Jacob, Prathap Tharyan, Nilesh Shah, Amar Jesani, Amit Dias, Matthew Varghese and G Gururaj. We are grateful to the managements of the Directorate of Health Services, Government of Goa and of Sangath and VHAG. We are grateful to the staff of the 12 PHCs and 12 GP practices (Aman B. Prabhu Gaonkar; Ashok Amshekar; Deepak D.Lotliker; Dennis L. S. Vas; Gajanan S.Prabhudesai; Irwin S. De Barros; K. Ramananda Kamath; Naraina S.Edo; Sandesh N. Dharwadker; V. S. Mardolkar; Vasudev V. Dukle; & Vishnu R. P.Vaidya). The funding body has played no role in the study design.
Footnotes
Competing Interests
None of the authors have declared any competing interests.
Authors Contributions
All authors have made substantive contributions to the study design or analysis of the trial data, have been involved in drafting the manuscript and have approved the final submitted version.
Contributor Information
Vikram Patel, London School of Hygiene & Tropical Medicine, UK & Sangath, India (vikram.patel@lshtm.ac.uk).
Helen A Weiss, London School of Hygiene and Tropical Medicine, UK.
Neerja Chowdhary, Sangath, India.
Smita Naik, Sangath, India.
Sulochana Pednekar, Sangath, India.
Sudipto Chatterjee, Sangath, India.
Mary De Silva, London School of Hygiene and Tropical Medicine, UK.
Bhargav Bhat, Voluntary Health Association of Goa, India.
Ricardo Araya, University of Bristol, UK.
Michael King, Royal Free and University College Medical School, UK.
Gregory Simon, Centre for Health Studies, Group Health Cooperative, Seattle, USA.
Helena Verdeli, Teachers College,, Columbia University; Columbia College of Physicians & Surgeons, New York, USA.
Betty Kirkwood, London School of Hygiene & Tropical Medicine, UK.
References
- 1.World Health Organization. Geneva: WHO; 2001. The World Health Report 2001: Mental health: New Understanding, New Hope. [Google Scholar]
- 2.Patel V, Kleinman A. Poverty and Common Mental Disorders in Developing Countries. Bulletin of the World Health Organization. 2003;81:609–615. [PMC free article] [PubMed] [Google Scholar]
- 3.Chisholm D, Sekar K, Kumar K, Saeed S, James S, Mubbashar M, et al. Integration of mental health care into primary care. Demonstration cost-outcome study in India and Pakistan. British Journal of Psychiatry. 2000;176:581–588. doi: 10.1192/bjp.176.6.581. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Mental Illness in General Health Care: an international study. Chichester: John Wiley & Sons; 1995. [Google Scholar]
- 5.Patel V, Chisholm D, Kirkwood B, Mabey D. Prioritising Health Problems In Women In Developing Countries: Comparing The Financial Burden Of Reproductive Tract Infections, Anaemia And Depressive Disorders In A Community Survey In India. Trop Med Int Health. doi: 10.1111/j.1365-3156.2006.01756.x. In Press. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Geneva: World Health Organization; 1992. [Google Scholar]
- 7.Goldberg D, Huxley P. Common Mental Disorders: A Biosocial Model. London: Tavistock/Routledge; 1992. [Google Scholar]
- 8.Jacob K, Everitt BS, Patel V, Weich S, Araya R, Lewis G. A comparison of latent variable models of nonpsychotic psychiatric morbidity in four culturally different populations. Psychological Medicine. 1998;28:145–152. doi: 10.1017/s0033291797005710. [DOI] [PubMed] [Google Scholar]
- 9.Lewis G. Dimensions of Neurosis. Psychological Medicine. 1992;22:1011–1018. doi: 10.1017/s0033291700038575. [DOI] [PubMed] [Google Scholar]
- 10.Tyrer P. The case for cothymia:mixed anxiety and depression as a single diagnosis. British Journal of Psychiatry. 2001;179:191–193. doi: 10.1192/bjp.179.3.191. [DOI] [PubMed] [Google Scholar]
- 11.Ustun TB, Von Korff M. Primary Mental Health Services: access and provision of care. In: Ustun TB, Sartorius N, editors. Mental Illness in General Health Care: an international study. Chichester: John Wiley & Sons; 1995. pp. 347–360. [Google Scholar]
- 12.Patel V, Pereira J, Coutinho L, Fernandes R, Fernandes J, Mann A. Poverty, Psychological Disorder & Disability in Primary Care Attenders in Goa, India. British Journal of Psychiatry. 1998;171:533–536. doi: 10.1192/bjp.172.6.533. [DOI] [PubMed] [Google Scholar]
- 13.Linden M, Lecrubier Y, Bellantuono C, Benkert O, Kisely S, Simon G. The prescribing of psychotropic drugs by primary care physicians: an international collaborative study. Journal of Clinical Psychopharmacology. 1999;19:132–140. doi: 10.1097/00004714-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care: Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry. 2006;189:484–493. doi: 10.1192/bjp.bp.106.023655. [DOI] [PubMed] [Google Scholar]
- 15.Patel V, Chisholm D, Rabe-Hesketh S, Dias-Saxena F, Andrew G, Mann A. The efficacy and cost-effectiveness of a drug and psychological treatment for common mental disorders in general health care in Goa, India: a randomised controlled trial. Lancet. 2003;361:33–39. doi: 10.1016/S0140-6736(03)12119-8. [DOI] [PubMed] [Google Scholar]
- 16.Bolton P, Bass J, Neugebauer R, Verdeli H, Clougherty K, Wickramaratne P, et al. Group Interpersonal Psychotherapy for Depression in Rural Uganda. Journal of the American Medical Association. 2003;289:3117–3124. doi: 10.1001/jama.289.23.3117. [DOI] [PubMed] [Google Scholar]
- 17.Araya R, Rojas G, Fritsch R, Gaete J, Simon G, Peters TJ. Treating Depression In Primary Care Among Low-Income Women In Santiago, Chile: A Randomised Controlled Trial. Lancet. 2003;361:995–1000. doi: 10.1016/S0140-6736(03)12825-5. [DOI] [PubMed] [Google Scholar]
- 18.Bass J, Neugebauer R, Clougherty KF, Verdeli H, Wickramaratne P, Ndogoni L, et al. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes: randomised controlled trial. Br J Psychiatry. 2006;188:567–573. doi: 10.1192/bjp.188.6.567. [DOI] [PubMed] [Google Scholar]
- 19.Paykel ES, Priest R. Recognition and management of depression in general practice: consensus statement. British Medical Journal. 1992;305:1198–1202. doi: 10.1136/bmj.305.6863.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abas M, Baingana F, Broadhead J, Iacoponi E, Vanderpyl J. Common Mental Disorders and Primary Health Care: Current Practice in Low-income Countries. Harvard Review of Psychiatry. 2003;11:166–173. doi: 10.1080/10673220303954. [DOI] [PubMed] [Google Scholar]
- 21.Cohen A. Geneva: World Health Organization; 2001. The effectiveness of mental health services in primary care: the view from the developing world. [Google Scholar]
- 22.Petersen I. From policy to praxis: Rethinking comprehensive integrated primary mental health care. University of Cape Town: 2000. [Google Scholar]
- 23.Patel V. Recognizing common mental disorders in primary care in African countries: should “mental” be dropped? Lancet. 1996;347:742–744. doi: 10.1016/s0140-6736(96)90083-5. [DOI] [PubMed] [Google Scholar]
- 24.Patel V, Andrade C. Pharmacological treatment of severe psychiatric disorders in the developing world : lessons from India. CNS Drugs. 2003;17:1071–1080. doi: 10.2165/00023210-200317150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Abas M, Mbengeranwa OL, Chagwedera IVS, Maramba P, Broadhead J. Primary Care Services for Depression in Harare, Zimbabwe. Harvard Review of Psychiatry. 2003;11:157–165. doi: 10.1080/10673220303952. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S, Chowdhary N, Pednekar S, Cohen A, Andrew G, Araya R, et al. Integrating evidence-based treatments for common mental disorders in routine primary care: feasibility and acceptability of the MANAS intervention in Goa, India. World Psychiatry. 2008;7:45–53. doi: 10.1002/j.2051-5545.2008.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn JE, Rohde J, Rifkin S, Were M, Paul VK, Chopra M. Alma-Ata 30 years on: revolutionary, relevant, and time to revitalise. Lancet. 2008;372:917–927. doi: 10.1016/S0140-6736(08)61402-6. [DOI] [PubMed] [Google Scholar]
- 28.Lewin SA, Dick J, Pond P, Zwarenstein M, Aja G, van Wyk B, et al. Lay health workers in primary and community health care. Cochrane Database Syst Rev. 2005:CD004015. doi: 10.1002/14651858.CD004015.pub2. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Geneva: WHO; 2007. Community health workers: What do we know about them? [Google Scholar]
- 30.Brugha R, Zwi A. Improving the Quality of Private Sector Delivery of Public health Services: Challenges and Strategies. Health Policy & Planning. 1998;13:107–120. doi: 10.1093/heapol/13.2.107. [DOI] [PubMed] [Google Scholar]
- 31.Gaunekar G, Patel V, Rane A. The impact and patterns of hazardous drinking amongst male industrial workers in Goa, India. Soc Psychiatry Psychiatr Epidemiol. 2005;40:267–275. doi: 10.1007/s00127-005-0886-1. [DOI] [PubMed] [Google Scholar]
- 32.Patel V, Andrew G. Gender, Sexual Abuse & Risk Behaviours: a cross-sectional survey in schools in Goa. National Medical Journal of India. 2001;14:263–267. [PubMed] [Google Scholar]
- 33.Patel V, De Souza N, Rodrigues M. Postnatal Depression and Infant Growth & Development in low-income countries: a cohort study from Goa, India. Archives of Disease in Childhood. 2003;88:34–37. doi: 10.1136/adc.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel V, Kirkwood BR, Pednekar S, Pereira B, Barros P, Fernandes J, et al. Gender disadvantage and reproductive health risk factors for common mental disorders in women: a community survey in India. Arch Gen Psychiatry. 2006;63:404–413. doi: 10.1001/archpsyc.63.4.404. [DOI] [PubMed] [Google Scholar]
- 35.Patel V, Kirkwood BR, Pednekar S, Weiss H, Mabey D. Risk factors for common mental disorders in women: Population-based longitudinal study. Br J Psychiatry. 2006;189:547–555. doi: 10.1192/bjp.bp.106.022558. [DOI] [PubMed] [Google Scholar]
- 36.Patel V, Kirkwood BR, Weiss H, Pednekar S, Fernandes J, Pereira B, et al. Chronic fatigue in developing countries: population based survey of women in India. BMJ. 2005;330:1190–1193. doi: 10.1136/bmj.38442.636181.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel VH, Kirkwood BR, Pednekar S, Araya R, King M, Chisholm D, et al. Improving the outcomes of primary care attenders with common mental disorders in developing countries: a cluster randomized controlled trial of a collaborative stepped care intervention in Goa, India. Trials. 2008;9:4. doi: 10.1186/1745-6215-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel V, Araya R, Chowdhary N, King M, Kirkwood B, Nayak S, et al. Detecting common mental disorders in primary care in India: a comparison of five screening questionnaires. Psychol Med. 2008;38:221–228. doi: 10.1017/S0033291707002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis G, Pelosi A, Araya R, Dunn G. Measuring psychiatric disorder in the community : a standardized assessment for use by lay interviewers. Psychological Medicine. 1992;22:465–486. doi: 10.1017/s0033291700030415. [DOI] [PubMed] [Google Scholar]
- 40.Pereira J, Patel V. Which antidepressants are best tolerated in primary care? A pilot randomized trial in Goa. Indian Journal of Psychiatry. 1999;41:358–363. [PMC free article] [PubMed] [Google Scholar]
- 41.Verdeli H, Clougherty K, Bolton P, Speelman L, Ndogoni L, Bass J, et al. Adapting group Interpersonal Psychotherapy for a developing country: experience in rural Uganda. World Psychiatry. 2004;2:113–120. [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira B, Andrew G, Pednekar S, Pai R, Pelto P, Patel V. The Explanatory Models Of Depression In Low Income Countries: Listening To Women In India. J Affect Disord. doi: 10.1016/j.jad.2006.09.025. In Press. [DOI] [PubMed] [Google Scholar]
- 43.Patel V, Pereira J, Mann A. Somatic and Psychological Models of Common Mental Disorders in India. Psychological Medicine. 1998;28:135–143. doi: 10.1017/s0033291797005941. [DOI] [PubMed] [Google Scholar]
- 44.Hayes R, Moulton LH. Cluster Randomised Trials: A Practical Approach. USA: CRC Press LLC; 2009. [Google Scholar]
- 45.Cheung YB, Jeffries D, Thomson A, Milligan P. A simple approach to test for interaction between intervention and an individual-level variable in community randomized trials. Trop Med Int Health. 2008;13:247–255. doi: 10.1111/j.1365-3156.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 46.Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, et al. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. 2007;370:991–1005. doi: 10.1016/S0140-6736(07)61240-9. [DOI] [PubMed] [Google Scholar]
- 47.Whooley MA, Stone B, Soghikian K. Randomized trial of case-finding for depression in elderly primary care patients. J Gen Intern Med. 2000;15:293–300. doi: 10.1046/j.1525-1497.2000.04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institute for Clinical E. London: DoH; 2004. Depression: the management of depression in primary and secondary care. [Google Scholar]