Abstract
BACKGROUND
We report on the status of imidacloprid and ethiprole resistance in Nilaparvata lugens Stål collected from across South and East Asia over the period 2005–2012.
RESULTS
A resistance survey found that field populations had developed up to 220‐fold resistance to imidacloprid and 223‐fold resistance to ethiprole, and that many of the strains collected showed high levels of resistance to both insecticides. We also found that the cytochrome P450 CYP6ER1 was significantly overexpressed in 12 imidacloprid‐resistant populations tested when compared with a laboratory susceptible strain, with fold changes ranging from ten‐ to 90‐fold. In contrast, another cytochrome P450 CYP6AY1, also implicated in imidacloprid resistance, was underexpressed in ten of the populations and only significantly overexpressed (3.5‐fold) in a single population from India compared with the same susceptible strain. Further selection of two of the imidacloprid‐resistant field strains correlated with an approximate threefold increase in expression of CYP6ER1.
CONCLUSIONS
We conclude that overexpression of CYP6ER1 is associated with field‐evolved resistance to imidacloprid in brown planthopper populations in five countries in South and East Asia. © 2015 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: brown planthopper, resistance, cytochrome P450, insecticides
1. INTRODUCTION
The brown planthopper (BPH), Nilaparvata lugens Stål, is an economically important pest of rice throughout both tropical and temperate zones of South and East Asia. It causes damage to the rice crop via direct phloem‐sap feeding, leading to nutrient depletion within the plant, which when infestation levels become high enough manifests as a characteristic stunting, wilting and browning of the affected crop, often referred to as ‘hopperburn’. BPH is also an effective vector of a number of rice pathogens, including ragged stunt virus and grassy stunt virus.1 The resulting cumulative damage to the rice crop can result in a significant (up to 60%) loss of yield in susceptible rice varieties.2 This is starkly illustrated by the observation that, between 2009 and 2011, rice production in Thailand suffered huge losses due to BPH, with more than 3 million ha infested and in excess of 1.1 million t of paddy, with an export value of an estimated $US 275 million, lost (data published by the International Rice Research Institute).
The control of BPH has for many years predominantly relied on the use of synthetic insecticides. This has resulted in the emergence of populations with high levels of resistance to many of the major classes of insecticides, including the organophosphates, carbamates, pyrethroids, neonicotinoids and phenylpyrazoles.3, 4, 5, 6 Since the early 1990s, the neonicotinoid insecticide imidacloprid has been widely applied throughout Asia for BPH control. Reduced efficacy/resistance to this insecticide emerged in populations across Asia over the period 2003–2006.7, 8 More recent monitoring across nine regions of China showed that imidacloprid resistance levels have again increased, with resistance ratios [LD50 field population/LD50 susceptible (1995 collected) strain] as high as 617‐fold being recorded in 2012.6 Similar levels of imidacloprid resistance in BPH immigrating into Japan have recently been reported, with resistance ratios of 616‐fold (comparing LD50 values of populations sampled in 1992 to 2012).9 Owing to the significant resistance to neonicotinoid insecticides, phenylpyrazole (fiprole) insecticides, such as ethiprole and fipronil, which target the gamma‐aminobutyric acid (GABA)‐gated chloride channel of the insect's central nervous system,10 have increasingly been used as a substitute for BPH control. However, emerging resistance to fipronil (23.8–43.3‐fold resistance) and cross‐resistance (47.1–100.9‐fold) to ethiprole in field populations of BPH have been reported in China,11, 12 and significant (308.5‐fold) levels of resistance to ethiprole in Thailand.5
Although the molecular mechanism(s) underlying resistance to fiproles have not been fully characterised,5 significant progress has been made in characterising the molecular basis of resistance to imidacloprid. Target‐site resistance to this compound was described in a laboratory‐selected strain of BPH before reports of control failure in the field; however, this mechanism has never been identified in any field‐collected population.13 In contrast, several studies have provided evidence that enhanced cytochrome P450 monooxygenase (P450) activity contributes to the neonicotinoid resistance of field‐collected populations of BPH.14, 15, 16 This detoxification mechanism was initially implicated by use of the metabolic enzyme inhibitor piperonyl butoxide (PBO) and the model substrate 7‐ethoxycoumarin.15, 17 More recently the overexpression of two candidate P450 enzymes, CYP6ER1 and CYP6AY1, has been linked with imidacloprid resistance.18, 19 In the first study, the expression levels of 32 tentative unique P450s, identified from two recent sequencing projects and by degenerate PCR, were examined in a susceptible N. lugens strain and moderately and highly resistant strains from China and Thailand, using quantitative real‐time PCR. A single P450 gene, CYP6ER1, was identified as highly overexpressed (up to 40‐fold) in all resistant strains compared with the susceptible strain, and the level of expression observed in the different strains was significantly correlated with the resistance phenotype.18 In the second study, the expression levels of 14 P450 genes were compared between a laboratory strain selected with imidacloprid for 40 generations and a susceptible strain, using quantitative RT‐PCR. Six genes were identified as significantly overexpressed in the resistant strain, with CYP6AY1 showing the highest level of overexpression (∼18‐fold) compared with the susceptible strain.19 Functional expression of CYP6AY1 and RNAi experiments provided evidence that CYP6AY1 has the capacity to metabolise imidacloprid and confer resistance.19
The aim of the present study was to analyse the changing levels of resistance to imidacloprid and ethiprole in N. lugens field strains collected from five countries in South and East Asia from 2005 through to 2012, and to investigate the relative roles of CYP6ER1 and CYP6AY1 in the resistance of these strains to imidacloprid.
2. EXPERIMENTAL METHODS
2.1. Insect strains
Baseline susceptibility data were generated using a laboratory‐maintained strain of N. lugens (Bayer‐S) provided by Bayer CropScience (Monheim, Germany). Bayer CropScience also organised the transfer to Rothamsted Research of field strains collected from across South and East Asia between 2005 and 2012. All strains were reared in the laboratory on whole rice plants (Oryza sativa L. ssp.) under controlled environmental conditions (26 °C/16 h photoperiod).
2.2. Laboratory selection
Two of the field strains, NL9 and NL39, demonstrating relatively high levels of resistance to imidacloprid, were placed under further selection with imidacloprid in the laboratory. NL9 was reared on rice plants treated with successively higher doses (concentrations ranging between 10 and 180 mg L−1) of imidacloprid over 13 generations, whereas NL39 was placed directly onto rice plants treated with 200 mg L−1 imidacloprid and selected over two generations.
2.3. Topical application bioassay (imidacloprid)
Adult macropterous (long‐winged) females of N. lugens were taken from age‐structured populations and were less than 10 days old. Approximately 15 females were lightly anaesthetised and dosed with the required concentration of technical imidacloprid on the upper surface (pronotum) of the prothorax using 0.25 µL of acetone as the solvent carrier, delivered using a hand‐held Burkard microapplicator (Burkard Manufacturing Co. Ltd, Rickmansworth, UK) fitted with a 1 cm3 all‐glass syringe. Control insects were dosed with 0.25 µL of acetone only. Treated individuals were placed in 50 mL specimen tubes containing untreated five‐week‐old rice stems (cut into 10 cm lengths) and contained using a ventilated lid. A small hole (3 mm diameter) was drilled in the base of each of the tubes, which were then stored vertically in a water bath (submerging only the base of each rice stem) in a 16 h photoperiod at 26 °C for 48 h. Insect mortality at 48 h was assessed by eye; adults showing no sign of movement were scored as dead. Bioassays consisted of three replicates at each concentration. Diagnostic doses represented the LD95 (4 mg L−1) and 5 × LD95 (20 mg L−1) of the susceptible strain.
2.4. Leaf‐dip bioassay (ethiprole)
Adult females were taken from age‐structured populations and were less than 10 days old. Rice stems (10 cm cut lengths) were dipped into the required concentrations of formulated fiprol insecticide for 20 s, air dried and placed in a plastic specimen tube. Approximately 15 females were aspirated directly into each of the tubes, which were sealed with a ventilated lid. A small hole (3 mm diameter) was drilled in the base of each of the tubes, which were then stored vertically in a water bath (submerging only the base of each stem) in a 16 h photoperiod at 26 °C for 72 h. Mortality was assessed by eye; adults showing no sign of movement were scored as dead. Bioassays consisted of three replicates at each concentration.
2.5. Data analysis
Probit analysis with Genstat 16th Edition software (VSN International Ltd, Hemel Hempstead, UK) was conducted to generate estimated LC50 values. Resistance factors were calculated by dividing the LC50 of a resistant strain by that of the susceptible strain. Mortality rates at diagnostic concentrations were subjected to Abbott's correction for natural mortality.20 Standard errors for mortalities at diagnostic concentrations were calculated using a binomial model.
2.6. Real‐time quantitative RT PCR
In qRT‐PCR analysis of CYP6ER1 and CYP6AY1 expression, primers designed previously18 and the CYP6AY1 primers employed by Ding et al. 19 were used. PCR reactions (15 µL) contained 5 µL of cDNA (2.5 ng), 7.5 µL of SYBR Green JumpStart Taq Readymix (Sigma Aldrich) and 0.25 µM of each primer. Samples were run on a Rotor‐Gene 6000 (Corbett Research, Cambridge, UK) using the following temperature cycling conditions: 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s, 57 °C for 15 s and 72 °C for 20 s. A final melt‐curve step was included post‐PCR (ramping from 72 to 95 °C by 1 °C every 5 s) to check for non‐specific amplification. Each qRT‐PCR experiment consisted of three independent biological replicates, with two technical replicates for each. Technical replication was limited to two replicates, (1) as PCR reactions were set up using a liquid handling robot (CAS 1200; Corbett Research) which provided high levels of technical reproducibility, and (2) to allow us to employ a sample maximisation strategy (i.e. running as many samples as possible in the same run in order to minimise technical run‐to‐run variation). Data were analysed according to the ΔΔC t method.21 For normalisation, two reference genes were validated experimentally for each strain, actin and α2‐tubulin, with the geometric mean of the selected genes then used for normalisation according to the strategy described previously.22
3. RESULTS AND DISCUSSION
3.1. Development of imidacloprid resistance in N. lugens populations from 2005 to 2012
As previously reported,7 responses of 2005 field‐collected samples of N. lugens to imidacloprid showed variation, particularly at the lower (4 mg L−1) dose tested (Table 1), with some strains appearing susceptible but other strains showing the first indications of a resistance problem. Strains collected that exhibited a decreased susceptibility to imidacloprid at the higher (20 mg L−1) diagnostic concentration (IND‐6 and IND‐7) were analysed for the presence of the Y151S mutation, known to reduce the agonist potency of a range of neonicotinoid insecticides, including imidacloprid.13 Using PCR‐based techniques, it was shown that, at the Y151S mutation site, individuals of both strains expressed ‘wild‐type’ base pairings, i.e. there was no evidence for Y151S‐mediated target‐site resistance as recently described for a laboratory‐selected strain.
Table 1.
Mortalities (%) (± standard error) for all Nilaparvata lugens strains at two diagnostic doses (LD95 and 5 × LD95 of the susceptible strain) of imidacloprid topically applied to adult females. Highlighted data were previously reported in Gorman et al. 7
| Strain | Year | Country of origin | Region/area | Imidacloprida | |
|---|---|---|---|---|---|
| 4 mg L−1, 1 ng AI insect−1 | 20 mg L−1, 5 ng AI insect−1 | ||||
| Bayer‐S | — | — | 91.43(±4.48) | 100.00 ± nc | |
| CHN‐1 | 2005 | China | Nanjing | 53.45(±6.39) | 100.00 ± nc |
| IND‐1 | 2005 | India | East Godavari District, Andhra Pradesh | 85.21(±4.74) | 100.00 ± nc |
| IND‐2 | 2005 | India | Karnataka State | 91.23(±3.68) | 100.00 ± nc |
| IND‐3 | 2005 | India | Mumbai | 59.32(±6.09) | 100.00 ± nc |
| IND‐4 | 2005 | India | West Godavari District, Andhra Pradesh | 83.34(±5.02) | 100.00 ± nc |
| IND‐5 | 2005 | India | Bellary District, Karnataka State | 59.66(±7.57) | 100.00 ± nc |
| IND‐6 | 2005 | India | West Godavari District, Andhra Pradesh | 17.98(±4.66) | 81.50(±4.74) |
| IND‐7 | 2005 | India | East Godavari District, Andhra Pradesh | 18.63(±4.79) | 71.40(±5.61) |
| ISA‐1 | 2005 | Indonesia | 96.36(±2.50) | 100.00 ± nc | |
| MAL‐1 | 2005 | Malaysia | 54.03(±6.91) | nt | |
| THAI‐1 | 2005 | Thailand | 87.01(±4.20) | 100.00 ± nc | |
| VTN‐1 | 2005 | Vietnam | 92.10(±3.67) | 100.00 ± nc | |
| CHN‐2 | October 2006 | China | Guandong Province | 41.41(±7.11) | 46.20(±10.40) |
| CHN‐3 | October 2006 | China | Guangxi Province | 23.34(±6.24) | 75.81(±6.69) |
| CHN‐4 | September 2006 | China | Jiangsu Province | 55.71(±6.64) | 75.11(±6.92) |
| CHN‐5 | October 2006 | China | Hunan Province | 35.00(±6.88) | 67.50(±6.76) |
| IND‐8 | April 2006 | India | Bellary District, Karnataka State | 57.53(±8.13) | 97.14(±2.36) |
| IND‐9 | April 2006 | India | East Kolkata, West Bengal | 50.00(±7.45) | 79.71(±5.15) |
| IND‐10 | October 2006 | India | West Godavari, Andhra Pradesh | 33.67(±6.68) | 48.04(±5.97) |
| IND‐11 | October 2006 | India | East Godavari, Andhra Pradesh | 0.00 ± nc | 5.75(±4.18) |
| MAL‐2 | December 2006 | Malaysia | Sabak Bernam District, Selangor | 13.87(±6.78) | 33.07(±5.23) |
| THAI‐2 | August 2006 | Thailand | Chainat Province, San Buri District | 22.41(±7.74) | 35.71(±8.10) |
| THAI‐3 | August 2006 | Thailand | Suphanburi Province | 35.00(±6.88) | 67.50(±6.76) |
| VTN‐2 | August 2006 | Vietnam | Đ ng Tháp Province, Tháp M ng Tháp Province, Tháp M
 i District i District |
2.27(±2.52) | 0.00 ± nc |
| VTN‐3 | August 2006 | Vietnam | Long An Province, B n L n L c District c District |
26.63(±7.70) | 42.11(±7.81) |
| NL2 | October 2008 | India | Bellary District, Karnataka State | 100.00 ± nc | 83.33(±6.80) |
| NL3 | October 2008 | India | Karnataka State | 66.67(±8.61) | 75.00(±7.91) |
| NL5 | October 2008 | Thailand | Samchuk District, Suphanburi Province | 86.67(±6.21) | 93.33(±4.55) |
| NL6 | October 2008 | India | West Medinapuri, West Bengal, East India | 51.11(±8.33) | 91.75(±4.35) |
| NL8 | December 2008 | Vietnam | Tantru District, Long An Province | 64.29(±8.75) | 82.14(±6.99) |
| NL9 | August 2009 | Thailand | 26.92(±8.10) | 53.85(±9.10) | |
| NL10 | September 2009 | Indonesia | Subang, West Java | 11.90(±5.40) | 73.57(±7.45) |
| NL11 | October 2009 | India | Sindhanoor, Southern India | 6.67(±4.55) | 23.33(±7.72) |
| NL12 | October 2009 | India | Karnataka State | 0.00 ± nc | 7.69(±4.87) |
| NL13 | October 2009 | India | Nadia District, West Bengal, East India | 0.00 ± nc | 3.70(±3.45) |
| NL14 | October 2009 | India | Hooghly District, West Bengal, East India | 0.00 ± nc | 68.00(±8.52) |
| NL15 | September 2009 | China | Nanning City, Guangxi Province | 34.38(±8.67) | 82.36(±6.85) |
| NL16 | September 2009 | China | Danyang City, Jiangsu Province | 2.32 ± nc | 3.43 ± nc |
| NL17 | November 2009 | China | Wuhan City, Hubei Province | 24.24(±7.14) | 91.98(±4.66) |
| NL18 | November 2009 | China | Fengxin County, Jiangxi Province | 39.50(±6.85) | 86.15(±6.01) |
| NL19 | December 2009 | Indonesia | East Java | 0.00 ± nc | 25.93(±8.00) |
| NL20 | December 2009 | Indonesia | Gabus Pati District, Central Java | 10.71(±5.65) | 60.71(±8.92) |
| NL21 | March 2010 | Thailand | Suphanburi Province, Sriprachan District | 16.78(±6.41) | 7.05(±3.69) |
| NL25 | October 2010 | India | Koppal District, Karnataka State | 16.27(±5.26) | 37.00(±5.04) |
| NL27 | September 2010 | China | Danyang City, Jiangsu Province | 66.52(±6.04) | 83.26(±4.78) |
| NL28 | September 2010 | China | Nanning City, Guanxi Province | 68.07(±6.95) | 76.48(±6.19) |
| NL29 | October 2010 | India | West Bengal | 85.51(±5.25) | 85.03(±5.14) |
| NL30 | September 2010 | China | Nanchang City, Jianxi Province | 62.40(±7.96) | 79.35(±5.78) |
| NL31 | October 2010 | Taiwan | Yulin County | 9.94(±4.85) | 37.32(±7.75) |
| NL32 | October 2010 | China | Foshan City, Guandong Province | 75.18(±6.30) | 74.64(±6.42) |
| NL33 | November 2010 | Vietnam | Trà Vinh Province, Southern Vietnam | 2.86(±2.64) | 21.80(±6.70) |
| NL34 | April 2011 | India | Koppal District, Karnataka State | 61.38(±6.34) | 85.96(±4.52) |
| NL35 | April 2011 | India | Koppal District, Karnataka State | 100.00 ± nc | 96.50(±2.56) |
| NL39 | August 2011 | Vietnam | Hau Giang | 2.00 ± nc | 1.00 ± nc |
| NL40 | August 2011 | Indonesia | Anjatan District, Indramayu | 8.90(±4.96) | 27.32(±7.53) |
| NL41 | August 2011 | Indonesia | Binong District, Subang | 23.75(±6.42) | 45.67(±8.08) |
| NL42 | August 2011 | Indonesia | Gegesik District, Cirebon | 26.67(±6.99) | 41.09(±7.50) |
| NL43 | August 2011 | Indonesia | Binong District, Subang | 19.44(±5.90) | 46.02(±7.51) |
| NL44 | August 2011 | Indonesia | Parnanukan District, Subang | 2.48(±2.52) | 4.45(±3.22) |
| NL45 | September 2011 | India | Raipur, Chhattisgarth | 30.14(±8.11) | 34.25(±8.14) |
| NL46 | October 2011 | India | Mohanpur, West Bengal | 10.00(±5.30) | 28.00(±8.20) |
| NL47 | September 2011 | China | Xi Jiao District, Danyang City, Jiangsu Province | 18.92(±6.44) | 50.00(±8.45) |
| NL52 | March 2012 | India | Koppal District, Karnataka State | 15.13(±5.67) | 58.31(±8.00) |
| NL53 | March 2012 | India | West Godavari District, Andhra Pradesh | 45.88(±7.88) | 67.00(±7.34) |
| NL54 | March 2012 | India | Karimnagar, Warangar District | 36.83(±7.58) | 78.06(±6.63) |
| NL55 | April 2012 | India | East Godavari District, Andhra Pradesh | 40.00(±7.95) | 60.00(±7.95) |
| NL56 | April 2012 | India | East Godavari District, Andhra pradesh | 62.11(±7.77) | 67.33(±7.24) |
| NL57 | October 2012 | India | Kanagala District, Karnataka State | 24.51(±7.38) | 31.06(±6.98) |
| NL58 | October 2012 | India | Mudhapur, Karnataka State | 29.41(±7.03) | 32.86(±6.93) |
| NL59 | October 2012 | India | Sidhikerra, Karnataka State | 15.74(±5.69) | 42.42(±8.02) |
nt = not tested; nc = not calculable.
In contrast to 2005, all 13 field samples collected in 2006 showed reduced susceptibility to imidacloprid at both diagnostic doses. Responses at 4 mg L−1 ranged from 0 to 60% mortality, and those at 20 mg L−1 from 0 to 97% mortality. The most resistant samples, IND‐11, VTN‐2, MAL‐2, THAI‐2 and CHN‐2, originated from different countries (India, Vietnam, Malaysia, Thailand and China), leading to the conclusion that resistance was neither confined to nor focused within a specific geographical region. This widespread distribution is, however, consistent with the migratory behaviour of N. lugens. To assess the potency of the mechanism(s) responsible, dose–response data for one of the most resistant samples (IND‐11) were generated. A comparison between the laboratory susceptible strain (S) and strain IND‐11 showed near‐parallel response lines,7 with a resistance ratio of 96.7 at LD50. Approximately 30% of the IND‐11 individuals were capable of surviving 100 mg L−1 (25 ng AI insect−1), which relates to 25 × LD95 of the susceptible strain. As in 2005, results for two of the most imidacloprid‐resistant strains collected in 2006 (CHN‐2 and THAI‐2) also disclosed ‘wild‐type’ sequences at the Y151S mutation site.
A limited number of field samples collected in 2008 from India, Thailand and Vietnam suggested that resistance was not as high in individual strains as in 2006. However, for field samples collected in 2009, responses at 4 mg L−1 ranged from 0 to 40% mortality, and those at 20 mg L−1 from 4 to 92% mortality. Again, highly resistant samples were identified as originating from different countries (India, China, Indonesia and Thailand), suggesting that the resistance problem across South and East Asia had not really abated. LD50 analysis of strain NL9 from Thailand indicated a resistance ratio of 139 (Table 2), roughly comparable with that reported for IND‐11 in 2006. Resistance to imidacloprid appeared to stabilise in 2010, but a highly resistant sample NL30, with an LD50 resistance ratio of 220, was collected from China, indicating that in some strains the potency of resistance to imidacloprid was continuing to increase. Since 2010, resistance to imidacloprid has continued to persist in field‐collected strains (Table 1), and is clearly entrenched in BPH populations.
Table 2.
Dose–response data for Nilaparvata lugens laboratory susceptible (Bayer‐S) and imidacloprid‐resistant strains against imidacloprid topically applied to adult females
| Strain | Year | Country | Imidacloprid | |
|---|---|---|---|---|
| LD50 (95% limits) (ng AI insect−1) | RRa | |||
| Bayer‐S | 0.61 (0.46–0.79) | 1.0 | ||
| CHIN‐1 | 2005 | China | 6.06 (4.82–7.55) | 10.0 |
| IND‐3 | 2005 | India | 4.47 (3.41–5.71) | 7.4 |
| IND‐5 | 2005 | India | 7.20 (6.60–7.83) | 11.9 |
| IND‐6 | 2005 | India | 11.09 (9.62–12.78) | 18.3 |
| IND‐7 | 2005 | India | 13.65 (11.42–16.07) | 22.5 |
| MAL‐1 | 2005 | Malaysia | 3.46 (3.04–3.92) | 5.7 |
| IND‐11 | 2006 | India | 58.68 (31.83–97.77) | 96.7 |
| NL2 | 2008 | India | 0.80 (0.15–2.35) | 1.3 |
| NL3 | 2008 | India | 0.86 (0.06–3.53) | 1.4 |
| NL6 | 2008 | India | 24.10 (1.15–259.09) | 39.7 |
| NL8 | 2008 | Vietnam | 2.52 (0.17–9.77) | 4.2 |
| NL9 | 2009 | Thailand | 97.00 (3.40–434.00) | 139.0 |
| NL11 | 2009 | India | 10.98 (2.18–31.00) | 18.0 |
| NL15 | 2009 | China | 20.12 (1.14–243.60) | 33.1 |
| NL16 | 2009 | China | 29.80 (5.98–64.50) | 49.1 |
| NL25 | 2010 | India | 38.88 (1.06–323.60) | 64.1 |
| NL27 | 2010 | China | 42.41 (15.61–87.62) | 69.1 |
| NL30 | 2010 | China | 133.80 (59.9–277.00) | 220.4 |
| NL32 | 2010 | China | 60.59 (31.25–85.59) | 99.8 |
RR = resistance ratio (R/S).
Analysis of imidacloprid resistance development in the individual countries of India, Thailand, Indonesia and Vietnam, based on the responses of collected field strains to discriminating doses of imidacloprid, indicates a clear trend towards high resistance (Fig. 1). For China, however, the trend is less clear. This may be because BPH cannot overwinter in subtropical and temperate regions north of 22° N, and immigrate into China from other regions during the autumn months.
Figure 1.
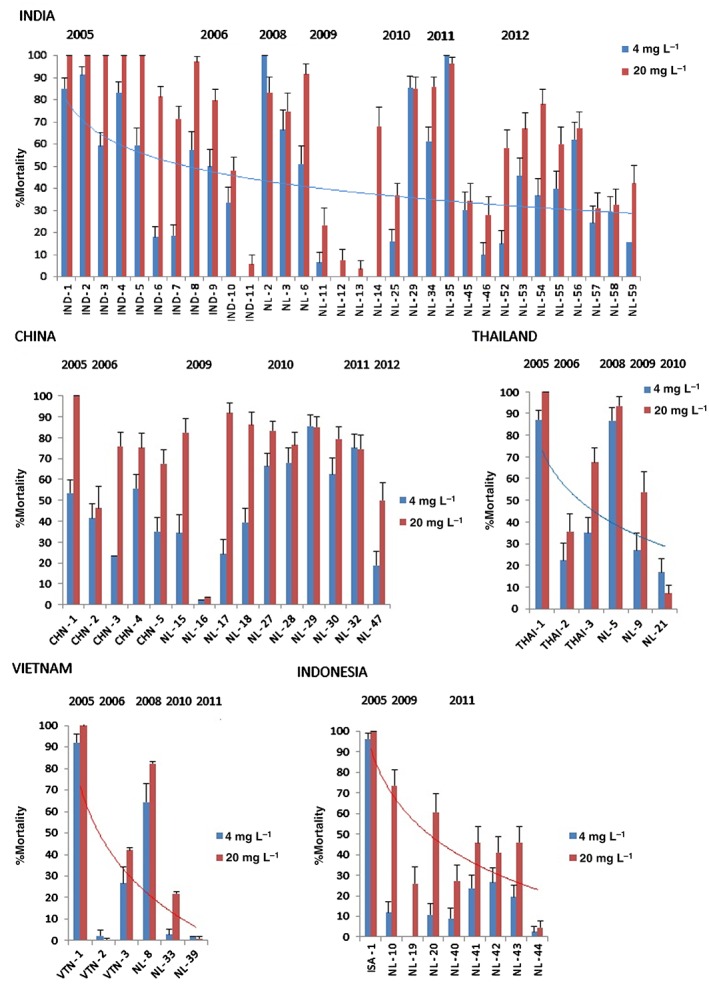
Mortalities (%) (± standard error) at two discriminating doses of imidacloprid for field‐collected strains of N. lugens.
3.2. Association of overexpression of CYP6ER1 and CYP6AY1 with resistance to imidacloprid
As detailed in the introduction, two cytochrome P450s have previously been linked with imidacloprid resistance in a small number of BPH laboratory and field populations. In the present study the expression levels of these two P450s were explored in 12 field populations collected from a range of countries in Asia (from 2009 to 2012) that exhibited clear resistance to imidacloprid in discriminating dose bioassays (Fig. 1, Table 1). As shown in Fig. 2, CYP6ER1 was significantly overexpressed in all 12 resistant populations when compared with a lab susceptible strain, with fold changes ranging from ten‐ to 90‐fold. In contrast, CYP6AY1 was underexpressed in ten of the populations compared with the same susceptible strain, and was only significantly overexpressed (3.5‐fold) in a single population from India (NL59). To see whether selection of the field strains with imidacloprid caused any increase in the expression levels of CYP6ER1 or CYP6AY1, two field strains (NL9 and NL39) were selected with imidacloprid up to final concentrations of 180 and 200 mg L−1 imidacloprid respectively. When the expression levels of CYP6ER1 were compared between NL9 (unselected) and NL9‐180 (selected), the expression level was found to have significantly increased threefold after selection, rising from ∼11‐ to 33‐fold. A similar effect was seen for NL39 (unselected) versus NL39‐200 (selected), with an increase from 43‐ to 103‐fold overexpression. Also noteworthy is that variation in the level of expression of CYP6ER1 among individual biological replicates decreased considerably after selection (as indicated by significantly reduced 95% confidence limits – see Fig. 2), suggesting that selection has reduced genetic heterogeneity in this strain and that all replicates overexpress this CYP at a universally high level. After selection, CYP6AY1 expression increased (see Fig. 2) from 0.24 in NL9 to 0.29 in NL9‐180 and from 0.28 in NL39 to 0.91 in NL39‐200; however, the difference in expression between both unselected/selected strains was not statistically significant as a result of significant variation in the expression levels of this CYP observed between biological replicates, particularly in the case of NL39‐200, and expression levels remained below that of the susceptible strain.
Figure 2.
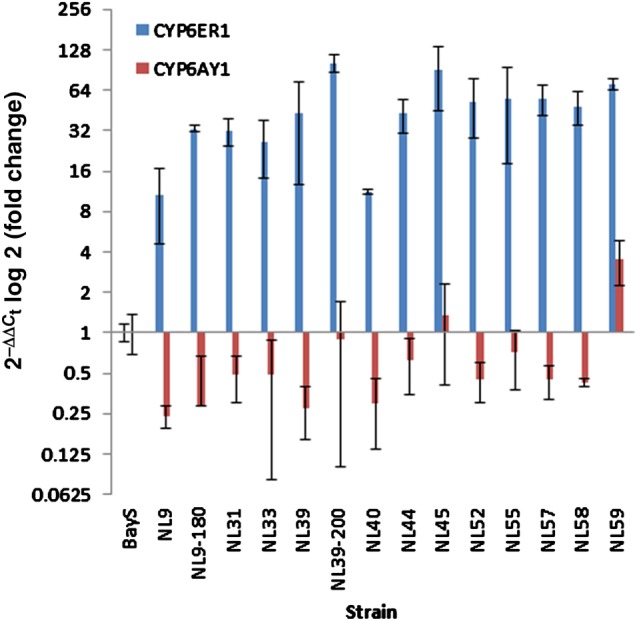
Fold change in expression of CYP6ER1 and CYP6AY1 in 14 resistant N. lugens strains compared with the susceptible reference Bayer‐S as determined by quantitative real‐time PCR. Error bars display 95% confidence intervals.
These results provide further evidence that overexpression of CYP6ER1 contributes to imidacloprid resistance in BPH throughout South and East Asia. The results for CYP6AY1 were surprising, and so to confirm this finding we ordered the primer pair used previously to measure CYP6AY1 expression in the study by Ding et al. 19 and repeated the qPCR experiments on the NL9, NL9‐180, NL39, NL39‐200 and NL59 strains. The results of this experiment confirmed our initial findings, with CYP6AY1 downregulated in all strains compared with the susceptible strain, including NL9‐180 and NL39‐200, the two selected strains (see Table 3). The previous study reporting this P450 as overexpressed used a resistant strain, originally collected from a field population in China that had been continuously selected in the laboratory with imidacloprid over 40 generations. Expression of CYP6AY1 in this strain was compared with a lab susceptible strain, and no comparison was made with the ‘unselected’ parental line of the resistant strain. However, screening of four field populations from China also showed that CYP6AY1 was significantly overexpressed (4–9‐fold). It is possible that CYP6AY1 is overexpressed in N. lugens populations in China and not the rest of Asia. In our study, all resistant field strains were compared with a single reference lab susceptible strain, as it is now very difficult to obtain BPH field strains that are susceptible to imidacloprid. Further investigation of the relative roles of CYP6ER1 and CYP6AY1 in imidacloprid resistance by comparing resistant strains with additional susceptible laboratory strains, or field strains if they can be sourced, is required to confirm our findings. Finally, although the results of the present study provide further evidence of a role for CYP6ER1 in imidacloprid resistance, functional characterisation of this P450 to confirm its ability to detoxify imidacloprid is now required.
Table 3.
Fold change in expression of CYP6AY1 in five imidacloprid‐resistant N. lugens strains compared with the susceptible reference Bayer‐S as determined by quantitative real‐time PCR
| Strain | Fold change () | 95% confidence level |
|---|---|---|
| Bayer‐S | 1.06 | 0.45 |
| NL9 | 0.30 | 0.10 |
| NL9‐180 | 0.20 | 0.28 |
| NL39 | 0.25 | 0.06 |
| NL39‐200 | 0.50 | 0.34 |
| NL59 | 0.78 | 0.35 |
3.3. Development of ethiprole resistance in N. lugens populations from 2005 to 2012
There was no significant variation in the responses of field samples collected in 2005 to the diagnostic concentrations of ethiprole (Table 4). Mortality of all strains was over 85% at 3 mg L−1 (LC95 of the susceptible strain) and 100% at 15 mg L−1 (5 × LC95 of the susceptible strain). In 2006, a field sample from India (IND‐11) displaying high levels of resistance to imidacloprid also survived a 3 mg L−1 discriminating dose bioassay with ethiprole (34% mortality), indicating an emerging resistance problem. This was confirmed in 2008, when field samples NL5 and NL8 from Thailand and Vietnam had a significant number of survivors (0 and 27% mortality respectively) when bioassayed with 3 mg L−1 of ethiprole. A full dose–response analysis of these two strains indicated LC50‐based resistance ratios for ethiprole of 67‐ and 100‐fold respectively, and 28.5‐ and 21‐fold respectively for fipronil (Table 5). In 2009, all four field samples collected from China had significant ethiprole resistance (0–14% mortality at a discriminating dose of 3 mg L−1), with LC50 resistance ratios for ethiprole ranging from 81‐ to 223‐fold and the corresponding resistance ratios for fipronil ranging from 14‐ to 68‐fold (Table 4).
Table 4.
Mortalities (%) (± standard error) for all Nilaparvata lugens strains at two diagnostic doses (LC95 and 5 × LC95 of the susceptible strain) of ethiprole by leaf‐dip bioassay
| Strain | Year | Country of origin | Region/area | Ethiprolea | |
|---|---|---|---|---|---|
| 3 mg L−1 | 15 mg L−1 | ||||
| Bayer‐S | — | — | 100.00 ± nc | 100.00 ± nc | |
| CHN‐1 | 2005 | China | Nanjing | 100.00 ± nc | nt |
| IND‐1 | 2005 | India | East Godavari District, Andhra Pradesh | 98.04(±1.90) | 100.00 ± nc |
| IND‐2 | 2005 | India | Karnataka State | 100.00 ± nc | 100.00 ± nc |
| IND‐3 | 2005 | India | Mumbai | 96.55(±2.37) | 100.00 ± nc |
| IND‐4 | 2005 | India | West Godavari District, Andhra Pradesh | 96.23(±2.59) | 100.00 ± nc |
| IND‐5 | 2005 | India | Bellary District, Karnataka State | 100.00 ± nc | nt |
| IND‐6 | 2005 | India | West Godavari District, Andhra Pradesh | 94.90(±4.58) | 100.00 ± nc |
| IND‐7 | 2005 | India | East Godavari District, Andhra Pradesh | 85.08(±6.23) | 100.00 ± nc |
| ISA‐1 | 2005 | Indonesia | 98.14(±1.82) | 100.00 ± nc | |
| MAL‐1 | 2005 | Malaysia | 95.00(±4.95) | nt | |
| THAI‐1 | 2005 | Thailand | 89.98(±4.13) | 100.00 ± nc | |
| VTN‐1 | 2005 | Vietnam | 100.00 ± nc | 100.00 ± nc | |
| IND‐11 | October 2006 | India | East Godavari, Andhra Pradesh | 33.59(±8.35) | nt |
| NL2 | October 2008 | India | Bellary District, Karnataka State | 92.00(±4.95) | nt |
| NL3 | October 2008 | India | Karnataka State | 80.00(±7.30) | nt |
| NL5 | October 2008 | Thailand | Samchuk District, Suphanburi Province | 0.00 ± nc | nt |
| NL6 | October 2008 | India | West Medinapuri, West Bengal, East India | 83.33(±6.80) | nt |
| NL8 | December 2006 | Vietnam | Tantru District, Long An Province | 26.67(±8.07) | nt |
| NL9 | August 2009 | Thailand | 41.67(±9.00) | nt | |
| NL10 | September 2009 | Indonesia | Subang, West Java | 24.35(±7.84) | nt |
| NL11 | October 2009 | India | Sindhanoor, Southern India | 77.26(±6.39) | nt |
| NL12 | October 2009 | India | Karnataka State | 42.86(±8.89) | nt |
| NL13 | October 2009 | India | Nadia District, West Bengal, East India | 96.72(±2.97) | nt |
| NL14 | October 2009 | India | Hooghly District, West Bengal, East India | 76.13(±8.20) | nt |
| NL15 | September 2009 | China | Nanning City, Guangxi Province | 0.00 ± nc | nt |
| NL16 | September 2009 | China | Danyang City, Jiangsu Province | 7.41(±4.78) | nt |
| NL17 | November 2009 | China | Wuhan City, Hubei Province | 14.81(±6.49) | nt |
| NL18 | November 2009 | China | Fengxin County, Jiangxi Province | 7.41(±4.78) | nt |
| NL19 | December 2009 | Indonesia | East Java | 23.08(±7.69) | nt |
| NL20 | December 2009 | Indonesia | Gabus Pati District, Central Java | 48.53(±9.28) | nt |
| NL21 | March 2010 | Thailand | Suphanburi Province, Sriprachan District | 24.29(±7.07) | nt |
| NL25 | October 2010 | India | Koppal District, Karnataka State | 49.87(±7.07) | nt |
| NL27 | September 2010 | China | Danyang City, Jiangsu Province | 39.20(±7.28) | nt |
| NL28 | September 2010 | China | Nanning City, Guangxi Province | 42.95(±7.38) | nt |
| NL29 | October 2010 | India | West Bengal | 100.00 ± nc | nt |
| NL30 | September 2010 | China | Nanchang City, Jiangxi Province | 36.30(±7.17) | nt |
| NL32 | October 2010 | China | Foshan City, Guandong Province | 33.58(±6.96) | nt |
| NL33 | November 2010 | Vietnam | Trà Vinh Province, Southern Vietnam | 6.72(±3.82) | nt |
| NL34 | April 2011 | India | Koppal District, Karnataka State | 58.35(±6.65) | nt |
| NL35 | April 2011 | India | Koppal District, Karnataka State | 90.71(±4.38) | nt |
| NL39 | August 2011 | Vietnam | Hau Giang | 3.64(±3.12) | 0.00 ± nc |
| NL40 | August 2011 | Indonesia | Anjatan District, Indramayu | 8.59(±4.55) | 5.82(±3.80) |
| NL41 | August 2011 | Indonesia | Binong District, Subang | 34.80(±7.94) | 39.82(±7.84) |
| NL42 | August 2011 | Indonesia | Gegesik District, Cirebon | 25.93(±7.30) | 24.32(±7.05) |
| NL43 | August 2011 | Indonesia | Binong District, Subang | 11.42(±5.30) | 38.44(±8.00) |
| NL44 | August 2011 | Indonesia | Parnanukan District, Subang | 34.15(±7.32) | 46.12(±7.69) |
| NL45 | September 2011 | India | Raipur, Chhattisgarth | 68.66(±7.43) | 89.14(±4.64) |
| NL46 | October 2011 | India | Mohanpur, West Bengal | 56.87(±7.47) | 82.64(±5.65) |
| NL47 | September 2011 | China | Xi Jiao District, Danyang City, Jiangsu City | 15.15(±5.41) | 21.92(±6.17) |
| NL52 | March 2012 | India | Koppal District, Karnataka State | 12.50(±5.51) | 50.00(±8.33) |
| NL53 | March 2012 | India | West Godavari District, Andhra Pradesh | 74.13(±7.01) | 89.06(±4.88) |
| NL54 | March 2012 | India | Karimnagar, Warangal District | 75.85(±6.53) | 81.22(±5.96) |
| NL55 | April 2012 | India | East Godavari District, Andhra Pradesh | 13.89(±5.69) | 8.64(±4.68) |
| NL56 | April 2012 | India | East Godavari District, Andhra Pradesh | 36.11(±7.69) | 30.79(±7.69) |
| NL57 | October 2012 | India | Kanagala Camp, Karnataka State | 30.00(±7.07) | 65.00(±7.36) |
| NL58 | October 2012 | India | Mudhapur, Karnataka State | 45.95(±8.19) | 55.26(±8.07) |
| NL59 | October 2012 | India | Sidhikerra, Karnataka State | 35.82(±7.78) | 63.33(±7.82) |
RR = resistance ratio (R/S).
Table 5.
Dose–response data for Nilaparvata lugens laboratory susceptible (S) and fiprol‐resistant strains against ethiprole applied as a leaf dip to adult females
| Strain | Year | Country | Ethiprole | Fipronil | ||
|---|---|---|---|---|---|---|
| LC50 (95% limits) | RRa | LC50 (95% limits) | RR | |||
| Bayer‐S | 0.41 (0.29–0.54) | 1 | 1.16 (0.70–1.66) | 1 | ||
| NL3 | 2008 | India | 0.74 (0.52–1.06) | 1.8 | 1.61 (1.27–2.03) | 1.4 |
| NL5 | 2008 | Thailand | 27.35 (10.56–55.50) | 66.8 | 33.12 (9.70–76.46) | 28.5 |
| NL6 | 2008 | India | 0.21 (0.12–0.35) | 0.51 | 1.48 (0.15–6.30) | 1.3 |
| NL8 | 2008 | Vietnam | 41.01 (13.05–116.75) | 100 | 24.33 (6.28–79.06) | 20.9 |
| NL9 | 2009 | Thailand | 25.56 (5.23–62.57) | 62.8 | 14.49 (7.34–27.56) | 12.5 |
| NL10 | 2009 | Indonesia | 8.06 (3.38–17.73) | 19.8 | 50.17 (16.52–125.30) | 43.3 |
| NL11 | 2009 | India | 0.30 (0.002–1.85) | 0.72 | 4.28 (1.79–7.79) | 3.7 |
| NL12 | 2009 | India | 21.01 (7.67–49.19) | 51.6 | 1.45 (0.87–2.18) | 1.3 |
| NL13 | 2009 | India | 0.06 (0.00–0.26) | 0.15 | 0.25 (0.16–0.35) | 0.2 |
| NL14 | 2009 | India | 1.06 (0.30–3.09) | 2.6 | 2.61 (0.73–4.77) | 2.3 |
| NL15 | 2009 | China | 56.30 (29.10–108.20) | 138.3 | 70.07 (2.35–356.30) | 60.5 |
| NL16 | 2009 | China | 90.73 (20.55–205.50) | 222.9 | 78.41 (18.71–203.60) | 67.7 |
| NL17 | 2009 | China | 74.23 (33.43–132.80) | 182.4 | 16.37 (14.20–18.34) | 14.1 |
| NL18 | 2009 | China | 33.06 (5.974–222.77) | 81.2 | 16.61 (12.94–19.43) | 14.3 |
| NL19 | 2009 | Indonesia | 33.66 (3.62–105.50) | 82.70 | 6.92 (1.27–21.85) | 6.0 |
| NL20 | 2009 | Indonesia | 42.10 (2.59–142.10) | 103.4 | 47.71 (11.93–122.40) | 41.2 |
| NL21 | 2009 | Thailand | 13.02 (5.76–21.95) | 32.0 | 8.21 (1.61–22.94) | 7.1 |
RR = resistance ratio (R/S).
For the 2010 and 2011 seasons, some apparently susceptible populations (NL29, NL35) were collected from India, but the general trend across South and East Asia indicated a developing resistance problem. Sample NL39, collected in 2011 from Vietnam (and having high levels of imidacloprid resistance), had 0% mortality at a higher (15 mg L−1) discriminating dose of ethiprole. Similarly, sample NL40, collected from Indonesia, also displayed good survivability (6% mortality) at the higher discriminating dose.
As for imidacloprid resistance, analysis of ethiprole resistance development in the individual countries of India, Thailand, Indonesia and Vietnam, based on the responses of collected field strains to discriminating doses of ethiprole (Fig. 3), indicates a clear trend towards high resistance. For China, however, the trend is again less clear, but ethiprole resistance is undoubtedly a major problem in this country.
Figure 3.
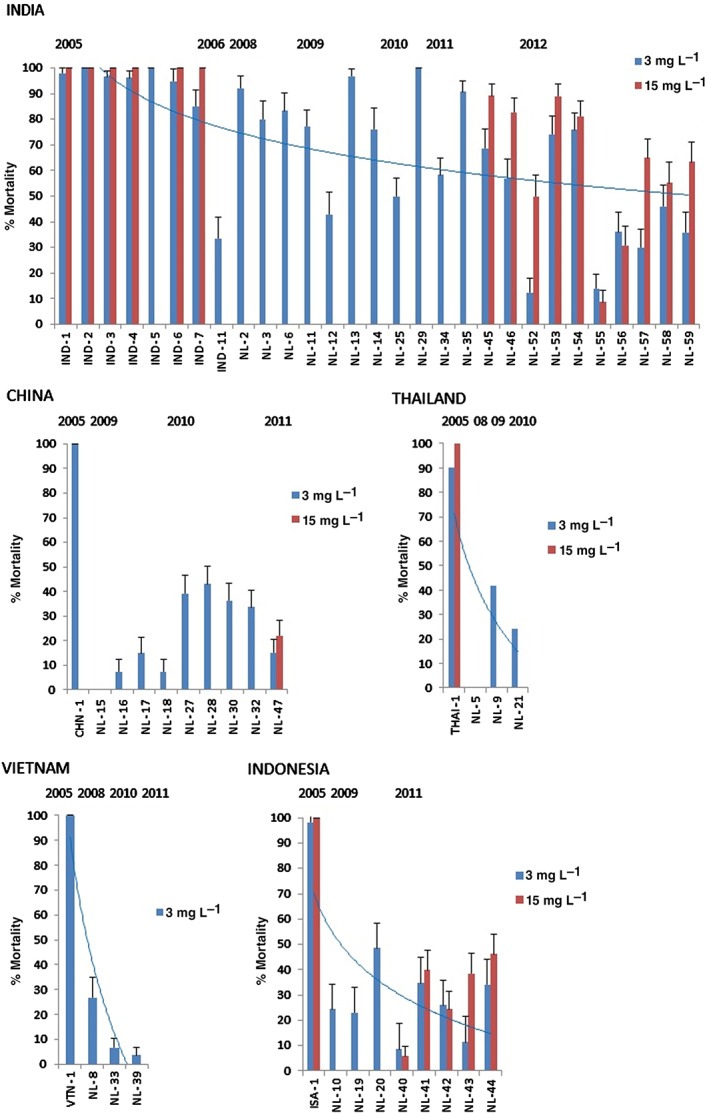
Mortalities (%) (± standard error) at two discriminating doses of ethiprole for field‐collected strains of N. lugens.
The molecular mechanisms underlying resistance to ethiprole have not been characterised; however, work on a resistant strain from Thailand suggested that enhanced expression of P450s and esterases may contribute to resistance.5 Although many of the samples analysed in the present study were highly resistant to imidacloprid, there is no evidence to date for a cross‐resistance problem involving CYP6ER1.
4. CONCLUSIONS
At present there is no evidence of a common cross‐resistance resistance between these two chemical classes of insecticide; however, there is evidence that individual planthoppers may exhibit multiple mechanisms of resistance to the different insecticide modes of action. Our results reveal that overexpression of the cytochrome P450 CYP6ER1 is associated with imidacloprid resistance in BPH populations in five countries in South and East Asia.
ACKNOWLEDGEMENTS
This work was funded by Bayer CropScience. Rothamsted Research receives grant‐aided support from the Biotechnology and Biosciences Research Council of the United Kingdom. The authors would like to thank Linda Oliphant, Amalia Kati and Neil Mason, the technicians who worked on this project.
REFERENCES
- 1. Cabauatan PQ, Cabunagan RC and Choi IL‐R, Rice viruses transmitted by the brown planthopper Nilaparvata lugens Stål, in Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia, ed. by Heong KL. and Hardy B. International Rice Research Institute, Los Baños, Philippines, pp. 357–368 (2009). [Google Scholar]
- 2. Cheng JA, Rice planthopper problems and relevant causes in China, in Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia, ed. by Heong KL. and Hardy B. International Rice Research Institute, Los Baños, Philippines, pp. 157–178 (2009). [Google Scholar]
- 3. Hemingway J, Karunaratne SHPP and Claridge MF, Insecticide resistance spectrum and underlying resistance mechanisms in tropical populations of the brown planthopper (Nilaparvata lugens) collected from rice and the wild grass Leersia hexandra . Int J Pest Manag 45:215–223 (1999). [Google Scholar]
- 4. Nagata T, Kamimuro T, Wang YC, Han SG and Noor NM, Recent status of insecticide resistance of long‐distance migrating rice planthoppers monitored in Japan, China and Malaysia. J Asia‐Pacif Entomol 5:113–116 (2002). [Google Scholar]
- 5. Punyawattoe P, Han ZJ, Sriratanasa W, Arunmit S, Chaiwong J and Bullangpoti V, Ethiprole resistance in Nilaparvata lugens (Hemiptera: Delphacidae): possible mechanisms and cross‐resistance. Appl Entomol Zool 48:205–211 (2013). [Google Scholar]
- 6. Zhang XL, Liu XY, Zhu FX, Li JH, You H and Lu P, Field evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) in China. Crop Prot 58:61–66 (2014). [Google Scholar]
- 7. Gorman KJ, Liu Z, Denholm I, Bruggen K‐U and Nauen R, Neonicotinoid resistance in rice brown planthopper, Nilaparvata lugens . Pest Manag Sci 64:1122–1125 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Matsumura M, Takeuchi M, Satoh M, Sanada‐Morimura S, Otuka A, Watanabe T et al, Species‐specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South‐east Asia. Pest Manag Sci 64:1115–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 9. Matsumura M, Sanada‐Morimura S, Otuka A, Ohtsu R, Sakumoto S, Takeuchi H et al, Insecticide susceptibilities in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera, immigrating into Japan in the period 2005–2012. Pest Manag Sci 70:615–622 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Cole LM, Nicholson R and Casida JE, Action of phenylpyrazole insecticides at the GABA‐gated chloride channel. Pestic Biochem Physiol 46:47–54 (1993). [Google Scholar]
- 11. Wang Y‐H, Wu C‐X, Zhao X‐P, Chen L‐P, Yu R‐X, Cang T et al, Current status of the pest resistance to fipronil. Chin Bull Entomol 46:846–854 (2009). [Google Scholar]
- 12. Zhao X, Ning Z, He Y, Shen J, Su J, Gao C et al, Differential resistance and cross‐resistance to three phenylpyrazole insecticides in the planthopper Nilaparvata lugens (Hemiptera: Delphacidae). J Econ Entomol 104:1364–1368 (2011). [DOI] [PubMed] [Google Scholar]
- 13. Liu ZW, Williamson MS, Lansdell SJ, Denholm I, Han ZJ and Millar NS, A nicotinic acetylcholine receptor mutation conferring target‐site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc Natl Acad Sci USA 102:8420–8425 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nauen R and Denholm I, Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch Insect Biochem Physiol 58:200–215 (2005). [DOI] [PubMed] [Google Scholar]
- 15. Wen Y, Liu Z, Bao H and Han Z, Imidacloprid resistance and its mechanisms in field populations of brown planthopper, Nilaparvata lugens Stål, in China. Pestic Biochem Physiol 94:36–42 (2009). [Google Scholar]
- 16. Zewen L, Zhaojun H, Yinchang W, Lingchun Z, hongwei Z and Chengjun L, Selection for imidacloprid resistance in Nilaparvata lugens: cross‐resistance patterns and possible mechanisms. Pest Manag Sci 59:1355–1359 (2003). [DOI] [PubMed] [Google Scholar]
- 17. Puinean AM, Denholm I, Millar NS, Nauen R and Williamson MS, Characterisation of imidacloprid resistance mechanisms in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pestic Biochem Physiol 97:129–132 (2010). [Google Scholar]
- 18. Bass C, Carvalho RA, Oliphant L, Puinean AM, Field LM, Nauen R et al, Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol Biol 20:763–773 (2011). [DOI] [PubMed] [Google Scholar]
- 19. Ding Z, Wen Y, Yang B, Zhang Y, Liu S, Liu Z et al, Biochemical mechanisms of imidacloprid resistance in Nilaparvata lugens: over‐expression of cytochrome P450 CYP6AY1. Insect Biochem Mol Biol 43:1021–1027 (2013). [DOI] [PubMed] [Google Scholar]
- 20. Abbott WS, A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267 (1925). [Google Scholar]
- 21. Pfaffl MW, A new mathematical model for relative quantification in real‐time RT‐PCR. Nucl Acids Res 29(9):e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD et al, Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):0034.1–0034.11 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]


