Abstract
Background
Even with colonoscopy screening and preventive measures becoming more commonplace, colorectal cancer (CRC) remains the third leading cause of oncologic death in the United States as of 2014. Many chemotherapeutics exist for the treatment of colorectal cancer, though they often come with significant side effect profiles or narrow efficacy ranges in terms of patient profile. Dietary phytochemicals such as glucobrassicin and its metabolite Indole-3-carbinol (I3C) have been implicated in tumor prevention in many preclinical models across a variety of gastrointestinal tumors and represent an intriguing new class of natural chemotherapeutics for CRC. I3C has been identified as a ligand of the aryl hydrocarbon receptor (AHR), and we aimed to characterize this AHR activation in relation to its cytotoxic properties.
Methods
Human colorectal cancer cell lines DLD1, HCT116, HT-29, LS513, and RKO were treated with indole-3-carbinol or vehicle. Cell viability was assessed via a fluorescent product assay, and apoptotic activity was assessed via a luminescent signal tied to a ratio of caspase-3 and caspase-7 activity. Gene expression of AHR and CYP1A1 mRNA was measured using quantitative-real time-polymerase chain reaction. SiRNA stable expression lines were established on a HCT116 background using a lab-developed transfection protocol as published elsewhere.
Results
Multiple colorectal cancer cell types express increased CYP1A1 mRNA levels (a specific marker of AHR-driven activity) following treatment with I3C, characterizing I3C treatment as agonistic of this pathway. Also, I3C induced a dose-dependent decrease in cell viability as well as inducing apoptosis. Further, using siRNA interference to knock down AHR responsiveness generated a significant resistance to the chemotherapeutic actions of indole-3-carbinol regarding both cell viability and apoptotic activity.
Conclusion
Some degree of the cytotoxic and proapoptotic effects of indole-3-carbinol on colon cancer cells is dependent on activation of the aryl hydrocarbon receptor. This represents a novel mechanism for the molecular action of indole-3-carbinol and enhances our understanding of its effects in the context of colorectal cancer. Continued preclinical study of both indole-3-carbinol and the aryl hydrocarbon receptor pathway is warranted, which may one day lead to novel diet-derived colon cancer treatments that enlist the AHR.
Keywords: colorectal cancer, chemotherapy, aryl hydrocarbon receptor, indole-3-carbinol, phytochemical
1. Introduction
In 2014, colorectal cancer (CRC) was both the 3rd most common and 3rd most deadly cancer in the United States among both sexes (1). Due to the prevalence and deadly nature of the disease in our society, there is a demand for safer and more effective chemotherapeutics. The most common treatment modalities for colorectal cancer in addition to resection and radiation include FOLFOX (oxaliplatin, short- term infusional 5-flourouracil (5-FU), and leucovorin), CAPOX (A.K.A. XELOX: oxaliplatin, oral capecitabine), and FOLFIRI (leucovorin, 5-FU, irinotecan) (2). Newer monoclonal antibodies targeting the receptors of vascular endothelial growth factor (VEGFR) and of the epidermal growth factor (EGFR) have also come into clinical favor in the setting of metastatic colorectal cancer (2, 3). Unfortunately, most of these treatments have either significant side effect profiles, efficacies limited to certain types of patients, or both (4). This creates a niche for novel chemotherapeutics, which are targeted to the intrinsic disturbances of CRC. Various dietary, environmental, and mutagenic causal agents have been linked to the malignant process of CRC, yet a specific understanding of the complete molecular etiology of the disease has yet to be proven.
It has been reported that ingestion of diets rich in cruciferous vegetables serves to prevent many types of malignancies (5, 6). This effect seems to be most pronounced in the gastrointestinal tract, especially the large intestine (7, 8). Fortunately, colorectal cancer is almost always a luminal malignancy, which provides a unique circumstance for dietary chemotherapy to become efficacious (9). Research has indicated that indoles are the bioactive component of cruciferous vegetables (10, 11). Much of their cytotoxic effect in the context of colon and rectal cancer has been attributed to signaling events caused by the naturally occurring agent indole-3-carbinol (I3C) and its library of metabolic condensates (10, 12, 13). Dietary indoles are potent candidates for chemotherapeutic compounds, however the exact mechanism by which they exert these effects is not completely understood.
One potential mediator of the effects of I3C in colorectal cancer is the aryl hydrocarbon receptor (AHR) signaling pathway (14–16). The AHR is an orphan cytosolic protein that can only influence transcription after it dimerizes with a ligand and translocates to the nucleus. Once bound, the AHR can link to its binding partner ARNT (AHR-nuclear translocator) and induce transcription of a number of genes by binding to the xenobiotic response element (XRE) on the genome (17). Due to its ability to bind various toxins and xenobiotics, stimulation of AHR is thought to provoke malignant transformation in certain tissues, while imparting a protective role in others (18, 19). This bifunctional effect of AHR is partially linked to the upregulation of multiple gene targets involved in drug and toxin metabolism, including the P450 cytochrome (CYP) family of XMEs (xenobiotic metabolizing enzymes) (20–23). The universally accepted specific CYP surrogate for AHR activation is CYP1A1(24–26). The effects of I3C have not been fully characterized as a result of canonical AHR signaling via CYP1A1, and this remains an important avenue of investigation going forward. The mechanism(s) by which AHR activity contributes positively or negatively to colorectal carcinogenesis remain unknown, though it is known that the aryl hydrocarbon receptor activation plays multiple roles in the development of mucosal immunity, homeostasis, and lymphoid organogenesis in the colon (5, 27–29). Moreover, AHR signaling has documented downstream anti- inflammatory effects in the gut (30–32).
Indole-3-carbinol, as well as its diverse catalog of metabolic conjugates, are known ligands of the AHR (33–35) Additionally, many groups have identified the antiproliferative and proapoptotic actions of indole-3-carbinol in the context of colorectal cancer, however studies bridging these cell fate analyses to the ability of indole-3-carbinol to activate the AHR are lacking (36–38) In this way, the true extent to which the cytotoxic effects of I3C are mediated by AHR signaling remain to be elucidated. We hypothesized that some degree of the chemotherapeutic effects enacted by indole-3-carbinol in human colon cancer cells in vitro is dependent on AHR signaling.
2. Materials and Methods
2.1 Cell Culture and Reagents
DLD1 (colon adenocarcinoma), HCT116 (colon carcinoma), HT-29 (well-differentiated colorectal adenocarcinoma), LS513 (multidrug resistant cecal carcinoma), and RKO (poorly differentiated colon carcinoma) cell lines were obtained from ATCC (Manassas, VA). Cells were cultured in DMEM medium (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Life Technologies), 1% non-essential amino acids (Life Technologies), 1% penicillin-streptomycin (Life Technologies), and 1% glutamine (Life Technologies) at 37° C and 5% CO2. G418 (gentamicin) antibiotic supplemented media was employed to maintain stable siRNA colonies, as stable cells co-expressed a resistance vector for this agent. Both solid powder indole-3-carbinol (I3C) and its vehicle dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Cell images were taken with a Zeiss compound light microscope with digital camera adapter (Carl Zeiss, Oberkochen, Germany).
2.2 Cell Viability Assay
Cells were plated in monolayer in black clear top 96-well plates with approximately 1.0 × 104 cells/well in DMEM supplemented medium for 24 hours. Cells were then treated with indole-3-carbinol (Sigma-Aldrich, St. Louis, MO) (100 μM, 500 μM, 1 mM) or vehicle (DMSO) for 24 hours. The fluorescent cell viability assay CellTiter-FluorTM (Promega, Madison, WI) was used to assess cell fate following treatment. The fluorescence (excitation at 390 nm, emission at 460 nm) was measured using SpectraMax Plus 384 microplate reader (Molecular Devices).
2.3 Apoptosis Assay
Cells were plated in monolayer in black clear top 96-well plates with approximately 1.0 × 104 cells/well in DMEM supplemented medium for 24 hours. Cells were then treated with indole-3-carbinol (Sigma-Aldrich, St. Louis, MO) (1 mM) or vehicle (DMSO) for 24 hours. To assess the degree of apoptotic activity in indole-3-carbinol treated cells, we used the luminescent Caspase-Glo® 3/7 Reagent Assay (Promega). Luminescence was measured by MicroLumat 60 plus luminometer (Berthold Technologies, Switzerland).
2.4 Gene Expression Analysis
Cells were treated with indole-3-carbinol (500 μM) or vehicle (DMSO) for 24 hours as previously described. Total RNA was extracted from monolayer cultures and isolated using a combination of the Qiagen RNeasy Kit (Qiagen, Valencia CA) and the QiaCUBE automated spin column system (Qiagen) according to the manufacturer’s instructions. Reverse-transcription of the RNAs was conducted using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The mRNA expression levels were assessed with TaqMan Universal PCR Master Mix (Applied Biosystems), custom-designed RT-PCR probes (Assay ID: CYP1A1; Hs01054797_g1, Ahr: Hs00169233_m1, β-actin; Hs01060665_gl) and the automated gene expression protocol of the QuantStudio™ 7 Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The mRNA expression levels of β-actin were used as the internal housekeeping control.
2.5 Stable si-RNA Expression Cell Lines
Generation of small interfering RNA (siRNA) stable expression cell lines on a HCT116 colon carcinoma background was conducted according to an established lab protocol as published elsewhere (39). Si-AHR lines exhibit approximately 60% knockdown of AHR signaling (39). Si-Scramble lines stably express a scrambled siRNA transcript to act as a control to the Si-AHR lines.
2.6 Statistical Analysis
All statistical analyses were conducted within GraphPad Prism software (version 5, La Jolla CA) and MSTAT software (version 6.1.1, Madison, WI). Unless otherwise noted, all data are depicted as mean ± standard error. Two-tailed Wilcoxon rank sum test and the Jonckheere-Terpstra test were used to measure statistical significance. A p value <0.05 was considered significant.
3. Results
3.1 Indole-3-carbinol treatment stimulates the aryl hydrocarbon receptor in colorectal cancer cells in vitro
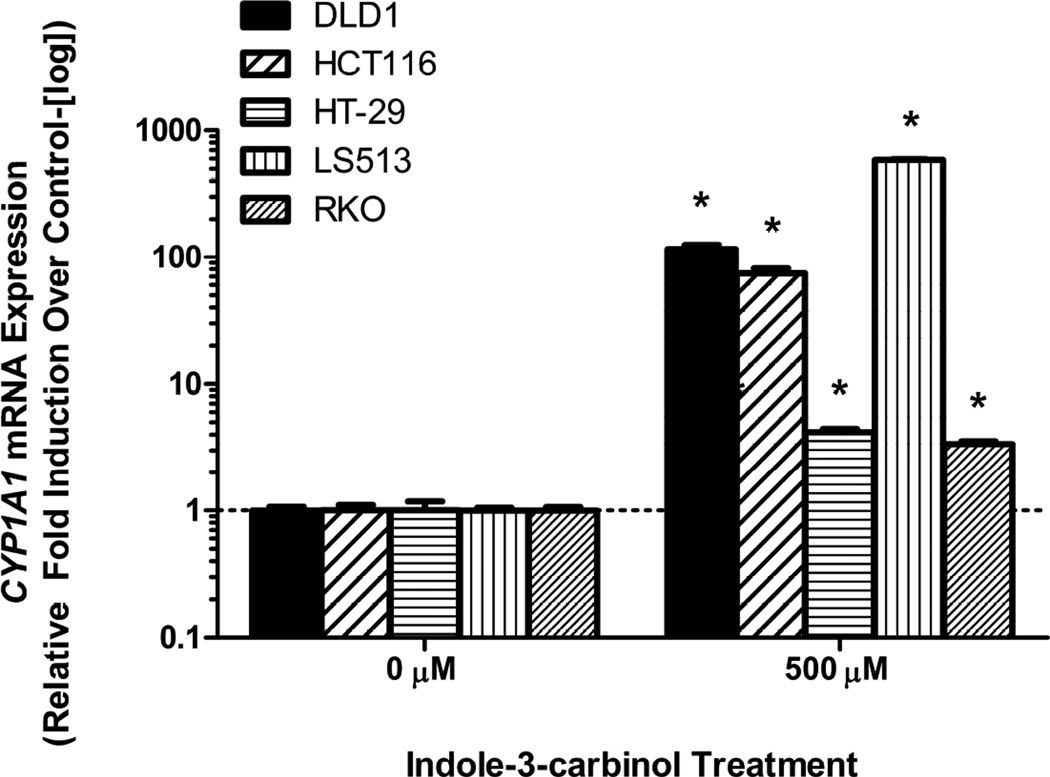
24 hour treatment with 500 μM I3C activated the AHR in all tested cell lines as illustrated by upregulation in CYP1A1 mRNA expression levels(p<0.05) (Figure 1). This effect was differential across our five selected cell lineages, suggesting an underlying AHR responsiveness unique to each cell type.
Figure 1. Indole-3-carbinol treatment activates the aryl hydrocarbon receptor in colorectal cancer cells.
A) DLD1, HCT116, HT-29, LS513, and RKO cells exhibited CYP1A1 mRNA upregulation following I3C treatment. With cell growth in logarithmic phase, cells were treated with either 500 μM I3C or 0.01% DMSO for 24 hours. Total RNA was harvested and reverse transcribed. Data are presented as relative fold induction of CYP1A1 mRNA of I3C treated cells over DMSO treated cells (controlled to internal levels of β-actin) ± standard error (representing at least three discrete experiments). “*” indicates statistical significance between I3C treated mRNA levels and vehicle (DMSO- μM) ) controls (p<0.05).
3.2 Indole-3-carbinol treatment decreases colorectal cancer cell viability and induces apoptosis
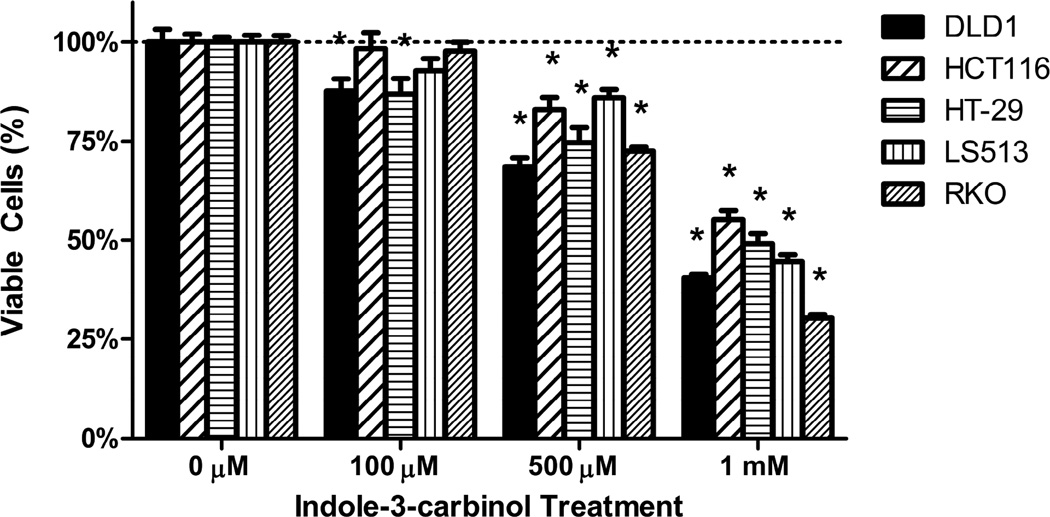
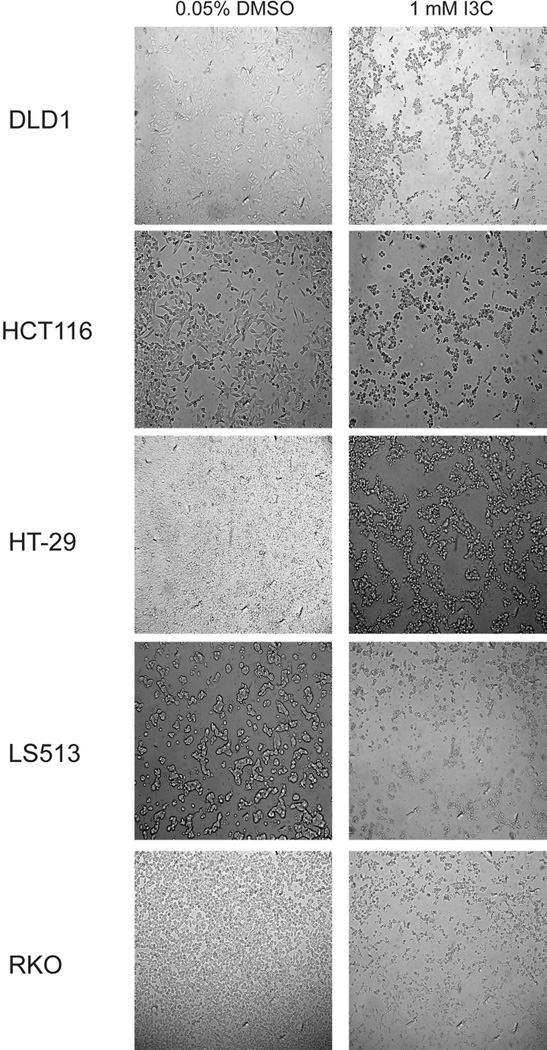
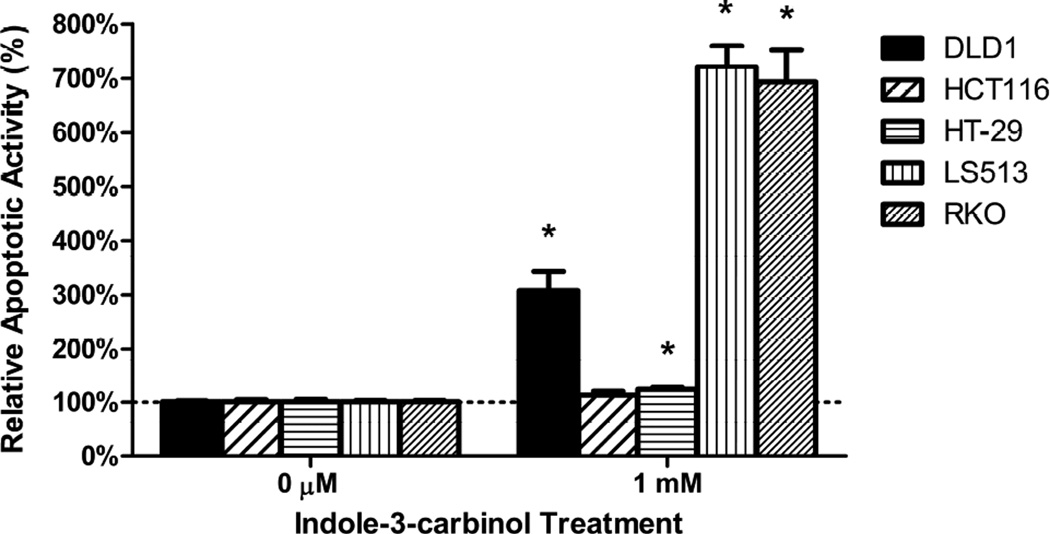
All colorectal cancer cells exhibited a dose-dependent decrease in cell viability following treatment with indole-3-carbinol over 24 hours (100 μM, 500 μM, 1 mM (p<0.05) (Figs 2A,2B). Maximally, at a dose of 1 mM indole-3-carbinol, the amount or remaining metabolically viable cells were 44.44%±0.82% of DLD1s, 55.21%±2.23 of HCT116s, 49.10%±2.52% of HT-29s, 44.55%±1.73% of LS513s, and 30.31%±0.92 of RKOs. In addition, at a dose of 1 mM indole-3-carbinol, nearly all tested cell types experienced a significantly increased amount of apoptotic activity compared to controls (p<0.05) (Figure 2C). Curiously, the HCT116 cell line experienced the smallest increase in apoptotic activity, and the difference was not significant.
Figure 2. Effects of indole-3-carbinol (I3C) on colorectal cancer cell viability.
A) I3C treatment of DLD1, HCT116, HT-29, LS513, and RKO cells lead to a dose-dependent decrease in cell viability. Cells were treated with I3C (100 μM, 500 μM, 1 mM) for 24 hours and cell viability was measured using a metabolically activated fluorogenic substrate. Data are depicted as mean viability percentage ± standard error, as normalized by DMSO treated control groups (representing at least three discrete experiments). “*” indicates statistical significance between I3C treated cell viability levels and vehicle (DMSO) controls in all cell types (p<0.05). B) Light microscope representation of 24 hour I3C treatment in all colorectal cancer cancer cell types. Images of monolayer cells are depicted with both vehicle (DMSO) and 1 mM I3C treatment at 24 hours. I3C treatment induced widespread pyknosis and fragmentation as compared to controls. C) I3C treatment stimulated apoptotic activity in all tested cell lines. Cells were treated with 1 mM I3C and luminescent caspase-3/7 activity was measured at 24 hours. Data are depicted as mean percentage of caspase-3/7 activity over DMSO treated controls ± standard error (representing at least three discrete experiments). “*” indicates statistical significance between I3C treated apoptotic activity levels and vehicle (DMSO) controls in all cell types (p<0.05).
3.3 Inhibition of AHR expression abrogates AHR activity induced by indole-3-carbinol
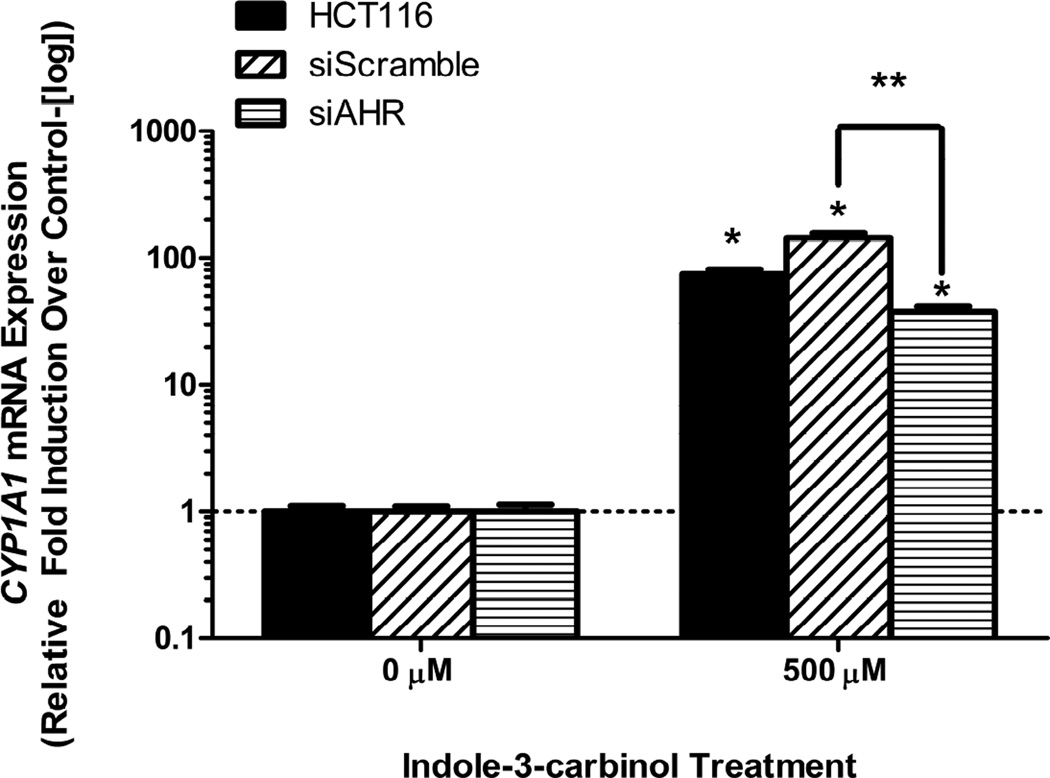
As previously shown, indole-3-carbinol is an agonist/ligand of the aryl hydrocarbon receptor in colorectal cancer cell lines in vitro. Although indole-3-carbinol activates the AHR, the relationship between this activation and cell death remains unclear. The dependence of both indole-3-carbinol-induced CYP1A1 mRNA expression and induction of apoptosis on AHR activation was assessed via the use of stable siRNA transfection of HCT116 cell lines. Thus, si-AHR expression vector lines were created as well as si-Scramble vector lines to act as a control. Following treatment with 500 μM I3C for 24 hours, si-AHR cells exhibited a 3.81-fold lower CYP1A1 mRNA expression than si-Scramble cells, illustrating successful knock-down of AHR signaling (p<0.05)(Figure 3).
Figure 3. Inhibition of AHR mRNA expression abrogates AHR activity induced by indole-3-carbinol (I3C).
Si-scramble cells display a more robust activation AHR signaling (in the form of CYP1A1 mRNA expression) than si-AHR cells following I3C treatment. Cells were treated with either 500 μM I3C or 0.01% DMSO for 24 hours. Wild type HCT116 mRNA response is depicted as an additional model. Data are presented as relative fold induction of CYP1A1 mRNA of I3C treated cells over DMSO treated cells (controlled to internal levels of β-actin) ± standard error (representing at least three discrete experiments). “*” indicates statistical significance between I3C treated mRNA levels and vehicle (DMSO) controls in all cell types (p<0.05). “**” with brackets indicate statistical significance of I3C treated mRNA level differences between si-scramble and si-AHR cells (p<0.05).
3.4 Inhibition of AHR expression blunts the cytotoxic action of indole-3-carbinol
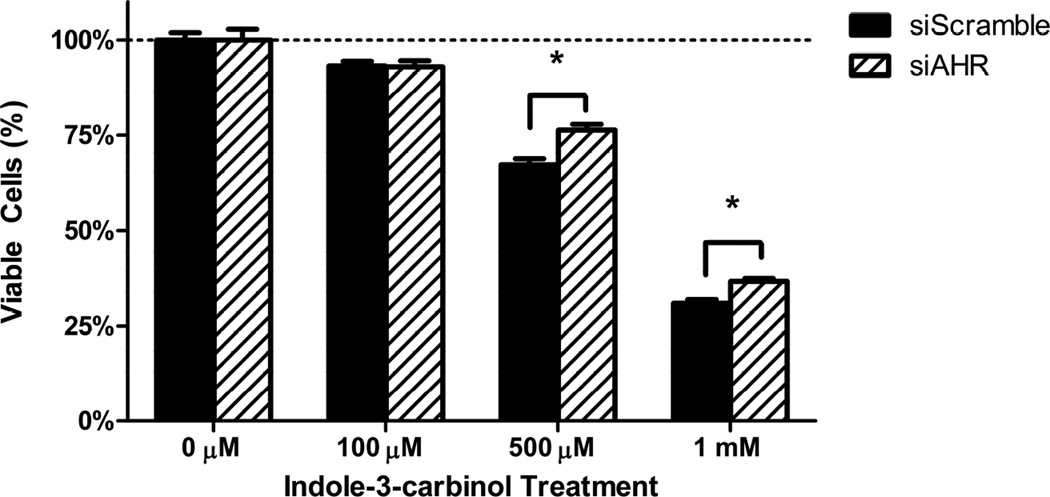
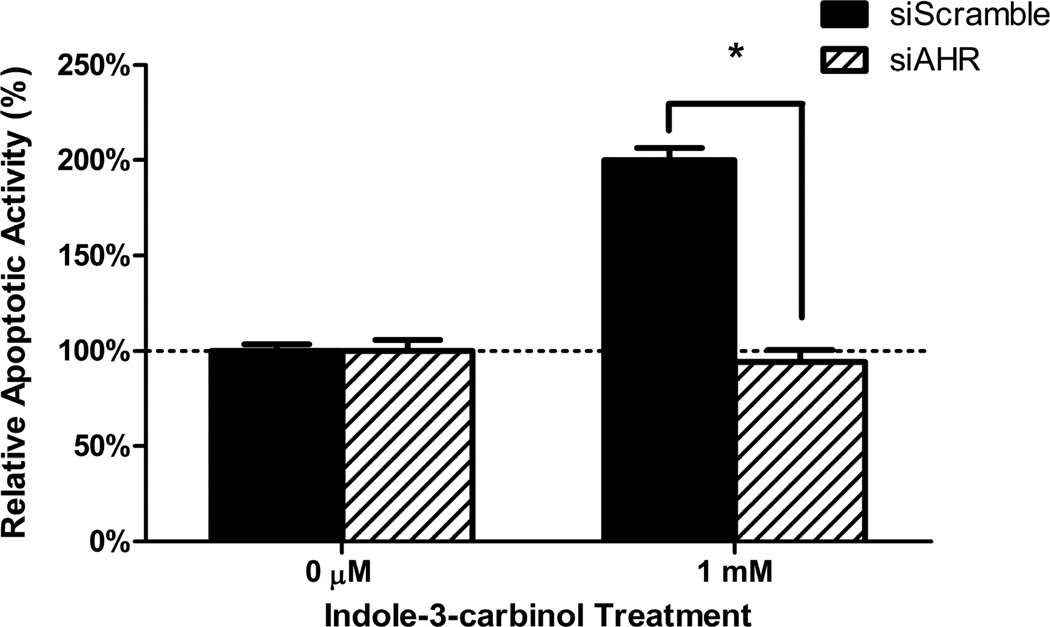
To examine the effects of AHR expression levels have on the cytotoxic action of indole-3-carbinol, we subjected the siRNA lines si-AHR and si-Scramble lines to the identical cell fate assays as found above in our wild type colorectal cancer cell lines. At both the 500 μM and 1 mM dosage intervals, the si-AHR lines demonstrated a relative resistance to I3C as compared to the si-Scramble controls. The differences in cell viability between the cell lines were as follows: 500 μM: 9.12% 1 mM: 5.73% (p<0.05)(Figure 4A). Additionally, at a dose of 1mM I3C, the si-Scramble cells exhibited a greater than 2-fold increase in caspase-3/7 apoptotic activity as compared to the si-AHR lines. (p<0.001)(Figure 4B).
Figure 4. Inhibition of AHR expression blunts the cytotoxic action of indole-3-carbinol.
A) I3C treatment of si-Scramble and si-AHR cells lead to a dose-dependent decrease in cell viability. This effect was more robust in si-Scramble cells. Cells were treated with I3C (100 μM, 500 μM, 1 mM) for 24 hours and cell viability was measured using a metabolically activated fluorogenic substrate. Data are depicted as mean viability percentage ± standard error, as normalized by DMSO treated control groups (representing at least three discrete experiments). “*” with brackets indicate statistical significance between the cell viability of si-scramble and si-AHR cells (p<0.05). B) I3C treatment stimulated apoptotic activity more so in si-Scramble cells than si-AHR cells. Cells were treated with 1 mM I3C and luminescent caspase-3/7 activity was measured at 24 hours. Data are depicted as mean percentage of caspase-3/7 activity over DMSO treated controls ± standard error (representing at least two discrete experiments). “*” with brackets indicates statistical significance between I3C treated levels of apoptotic activity between si-scramble and si-AHR cells (p<0.05).
4. Discussion
Colorectal cancer is a common and deadly cancer in the United States and the aryl hydrocarbon receptor may represent a novel anticancer pathway to combat this malignancy. In this investigation we examined the role that AHR signaling plays in the cytotoxic effects wrought by the natural phytochemical indole-3-carbinol. We found that I3C is an agonist of the AHR in multiple lineages of colorectal cancer as illustrated by its ability to upregulate CYP1A1 mRNA levels. Additionally, we found that I3C treated led to a dose-dependent decrease in cell viability as well as the induction of apoptotic activity in these cell lines. Interestingly, the HCT116 cell line did not experience a significant increase in apoptotic activity at 24 hours, and we suspect that this indicates that the caspase-3/7 activity at peaked out earlier. Upon investigation at 12 and 18 hour time points, the result remained the same. A metabolic abnormality or increased I3C efflux is suspected. To further examine the role that AHR activation plays in the cytotoxic effects of I3C, we established a model of AHR knock down with stable expression of siRNA interference. This model proved to successfully inhibit AHR signaling driven by I3C exposure. Most importantly, interfering with AHR signaling led to a relative resistance to the effects of I3C on cell viability and induction of apoptosis in colon cancer cells.
I3C has long been recognized as an anticancer agent across various colorectal cancer cell lines (10, 13, 37, 40, 41). These negative effects on cell fate have been attributed to cell-cycle arrest, cytotoxic necrosis, and apoptosis (10, 13, 41). In this way, the concept of indole-3-carbinol chemotherapy for malignancies of the large bowel is not novel. Additionally, the fact that the AHR can be stimulated by dietary indoles such as I3C is also well established in many cell and tissue types (33, 35, 42) To date, only one group has concomitantly investigated the role of I3C as a ligand of the AHR in this context. However in this study, Bonnesen et al. examined CYP1A1 induction following exposure to I3C when considering the ability of CYP enzymes to augment carcinogen metabolism, not to drive cell death (10). One future direction of study emphasized in that investigation was possible AHR-driven signaling that is somehow linked to the ability of dietary indoles such as I3C to induce colon cancer cell death. To our knowledge, our study represents the first time that colorectal cancer cell death by indole-3-carbinol has been even partially linked to AHR activation. Additionally, our group recently established that chrysin, another natural ligand of the AHR, induces apoptosis in human colon cancer cell cells in an AHR-dependent fashion (39). Together, these works represent an initial characterization of the AHR as a novel putative target in both chemoprevention as well as chemotherapy for colorectal cancer. Further study should identify other dietary indoles, specifically the metabolic condensates of I3C, as targeted cytotoxic agents which activate the AHR.
An important gap left in our understanding at the conclusion of this study is identification of the downstream signaling events driven by the AHR that eventually lead to apoptotic cell death. We have previously identified Tnf and c-fos activity as the means by which aryl hydrocarbon receptor activation by the flavonoid chrysin leads to apoptosis (39). Also, the aryl hydrocarbon receptor has been found to mediate raloxifene-induced apoptosis in triple-negative breast cancer cells and estrogen receptor-deficient hepatoma cells (43). These studies represent instances in which the apoptogenic cascades activated by the AHR have been further characterized. Further work regarding indole-3-carbinol should focus on elucidating similar mechanisms in colorectal cancer cells, especially the activity of Tnf and c-fos.
Another avenue to pursue would be to examine if any of the currently accepted mechanisms by which I3C affects colon cancer cell fate are modulated in any fashion by the AHR. The chemopreventive NSAID-activated gene 1 (NAG-1) has been found to be activated during indole-3-carbinol associated colon cancer cell death. (40) This pathway is firmly established as a driver of apoptosis in the gut (44). Even more, NAG-1 has been found to be involved in colon cancer cell apoptosis induced by exposure to highly bioactive natural chemicals such as various flavonones and the major bioactive constituent of green tea extract, epicatechin gallate (45, 46). The possibility of crosstalk between AHR activation and NAG-1 expression contributing to I3C-induced cell death expression warrants further investigation.
4.1 Conclusions
In summation, we have confirmed numerous prior reports underscoring the chemotherapeutic and proapoptotic abilities of indole-3-carbinol against colorectal cancer cells. Additionally, we have proven that I3C treatment in vitro upregulates AHR activity in five separate colorectal cancer cell lines, solidifying its agonist nature. Further, we have demonstrated that siRNA knockdown of AHR levels corresponds to a relative resistance to I3C-induced decreases in cell viability and induction of apoptosis. These results indicate that aryl hydrocarbon receptor activation deserves continued preclinical investigation as a novel chemotherapeutic mechanism in colorectal cancer. This line of inquiry could potentially lead to the development of novel chemotherapeutics and current treatment adjuncts that harness the AHR pathway to combat colorectal cancer.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health Grant R01ES020900 (GDK) and the University of Wisconsin School of Medicine and Public Health Shapiro Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: No competing interests, financial or otherwise, exist for any author
- Conception and design: Megna BW, Nukaya M, Kennedy GD
- Development of methodology: Megna BW, Carney PR
- Data acquisition: Megna BW, Carney PR
- Data analysis and interpretation: Megna BW, Carney PR
- Writing, review, revision of manuscript: Megna BW, Carney PR, Kennedy GD
- Administrative, technical, or material support: Geiger P, Kennedy GD
- Study Supervision: Nukaya M, Kennedy GD
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Stintzing S. Management of colorectal cancer. F1000prime reports. 2014;6:108. doi: 10.12703/P6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun MS, Seymour MT. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Therapeutic advances in medical oncology. 2011;3:43–52. doi: 10.1177/1758834010388342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012;14:e31–e47. doi: 10.1111/j.1463-1318.2011.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leavy O. Mucosal immunology: the 'AHR diet' for mucosal homeostasis. Nat Rev Immunol. 2011;11:806. doi: 10.1038/nri3115. [DOI] [PubMed] [Google Scholar]
- 6.Kapusta-Duch J, Kopec A, Piatkowska E, Borczak B, Leszczynska T. The beneficial effects of Brassica vegetables on human health. Roczniki Panstwowego Zakladu Higieny. 2012;63:389–395. [PubMed] [Google Scholar]
- 7.Arikawa AY, Gallaher DD. Cruciferous vegetables reduce morphological markers of colon cancer risk in dimethylhydrazine-treated rats. J Nutr. 2008;138:526–532. doi: 10.1093/jn/138.3.526. [DOI] [PubMed] [Google Scholar]
- 8.Hara M, Hanaoka T, Kobayashi M, Otani T, Adachi HY, et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46:138–147. doi: 10.1207/S15327914NC4602_06. [DOI] [PubMed] [Google Scholar]
- 9.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 11.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr. 2008;47(Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 13.Neave AS, Sarup SM, Seidelin M, Duus F, Vang O. Characterization of the N-methoxyindole-3-carbinol (NI3C)--induced cell cycle arrest in human colon cancer cell lines. Toxicol Sci. 2005;83:126–135. doi: 10.1093/toxsci/kfi008. [DOI] [PubMed] [Google Scholar]
- 14.Safe S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol Lett. 2001;120:1–7. doi: 10.1016/s0378-4274(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 15.Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135:1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicol Sci. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, et al. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70:212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–4710. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly AK. Polymorphic Variants of Cytochrome P450: Relevance to Cancer and Other Diseases. Advances in pharmacology. 2015;74:85–111. doi: 10.1016/bs.apha.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 24.Israel DI, Whitlock JP., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984;259:5400–5402. [PubMed] [Google Scholar]
- 25.Gonzalez FJ, Tukey RH, Nebert DW. Structural gene products of the Ah locus. Transcriptional regulation of cytochrome P1-450 and P3-450 mRNA levels by 3-methylcholanthrene. Mol Pharmacol. 1984;26:117–121. [PubMed] [Google Scholar]
- 26.Fisher JM, Jones KW, Whitlock JP., Jr Activation of transcription as a general mechanism of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Mol Carcinog. 1989;1:216–221. doi: 10.1002/mc.2940010403. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248. 248, e231. doi: 10.1053/j.gastro.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310–313. doi: 10.1097/MOG.0b013e328352ad69. [DOI] [PubMed] [Google Scholar]
- 32.Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunology and cell biology. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 33.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ociepa-Zawal M, Rubis B, Lacinski M, Trzeciak WH. The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim Pol. 2007;54:113–117. [PubMed] [Google Scholar]
- 35.Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci. 2011;124:327–338. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronbak R, Duus F, Vang O. Effect of 4-methoxyindole-3-carbinol on the proliferation of colon cancer cells in vitro, when treated alone or in combination with indole-3-carbinol. J Agric Food Chem. 2010;58:8453–8459. doi: 10.1021/jf101806t. [DOI] [PubMed] [Google Scholar]
- 37.Frydoonfar HR, McGrath DR, Spigelman AD. Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Colorectal Dis. 2002;4:205–207. doi: 10.1046/j.1463-1318.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 38.Safe S, Papineni S, Chintharlapalli S. Cancer chemotherapy with indole-3-carbinol, bis(3'-indolyl)methane and synthetic analogs. Cancer Lett. 2008;269:326–338. doi: 10.1016/j.canlet.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronnekleiv-Kelly SM, Nukaya M, Diaz-Diaz CJ, Megna BW, Carney PR, et al. Aryl hydrocarbon receptor-dependent apoptotic cell death induced by the flavonoid chrysin in human colorectal cancer cells. Cancer Lett. 2016 doi: 10.1016/j.canlet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3'-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328:63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 41.Hudson EA, Howells LM, Gallacher-Horley B, Fox LH, Gescher A, et al. Growth-inhibitory effects of the chemopreventive agent indole-3-carbinol are increased in combination with the polyamine putrescine in the SW480 colon tumour cell line. BMC Cancer. 2003;3:2. doi: 10.1186/1471-2407-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell EF, Koch DC, Bisson WH, Jang HS, Kolluri SK. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell death & disease. 2014;5:e1038. doi: 10.1038/cddis.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, et al. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology. 2002;122:1388–1398. doi: 10.1053/gast.2002.32972. [DOI] [PubMed] [Google Scholar]
- 45.Shin SY, Kim JH, Lee JH, Lim Y, Lee YH. 2'-Hydroxyflavanone induces apoptosis through Egr-1 involving expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr Food Res. 2012;56:761–774. doi: 10.1002/mnfr.201100651. [DOI] [PubMed] [Google Scholar]
- 46.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, et al. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]