ABSTRACT
Exercise in heat stress exacerbates performance decrements compared to normothermic environments. It has been documented that the performance decrements are associated with reduced efferent drive from the central nervous system (CNS), however, specific factors that contribute to the decrements are not completely understood. During exertional heat stress, blood flow is preferentially distributed away from the intestinal area to supply the muscles and brain with oxygen. Consequently, the gastrointestinal barrier becomes increasingly permeable, resulting in the release of lipopolysaccharides (LPS, endotoxin) into the circulation. LPS leakage stimulates an acute-phase inflammatory response, including the release of interleukin (IL)-6 in response to an increasingly endotoxic environment. If LPS translocation is too great, heat shock, neurological dysfunction, or death may ensue. IL-6 acts initially in a pro-inflammatory manner during endotoxemia, but can attenuate the response through signaling the hypothalamic pituitary adrenal (HPA)-axis. Likewise, IL-6 is believed to be a thermoregulatory sensor in the gut during the febrile response, hence highlighting its role in periphery – to – brain communication. Recently, IL-6 has been implicated in signaling the CNS and influencing perceptions of fatigue and performance during exercise. Therefore, due to the cascade of events that occur during exertional heat stress, it is possible that the release of LPS and exacerbated response of IL-6 contributes to CNS modulation during exertional heat stress. The purpose of this review is to evaluate previous literature and discuss the potential role for IL-6 during exertional heat stress to modulate performance in favor of whole body preservation.
KEYWORDS: CNS, cytokines, cycling, endurance, gut, LPS, running
Introduction
It is well accepted that exercise performance can be negatively affected in hot environments.1 Although this reduction in performance has been associated with perturbed cardiovascular,2 metabolic,3 perceived exertion4 and motivational5 responses, it still remains unclear as to the determining factor for the cessation of exercise. However, regardless of the individual or collective physiological responses during exercise heat stress, it is clear that central nervous system (CNS) down regulation plays a critical role as high core temperatures are associated with reduced central drive.6,7 This was also confirmed when core temperature was manipulated during passive heat stress and then systematically returned to resting values with the voluntary activation restored in an orderly fashion.8 These studies collectively show that exercise-induced hyperthermia evokes a central component, which reduces the capability of the brain to continue to drive motor output at a level that sustains exercise at a given intensity.
It is interesting to note that much of the research attempting to elucidate the mechanisms responsible for reduced exercise performance in the heat has largely dealt with understanding the role of the CNS, in particular the relationship between rising temperature and neuronal activity,9 cerebral blood flow10 and energy turnover.11,12 However, it has been known for some time that strenuous exercise also promotes alterations in gastrointestinal (GI) permeability,13,14 leading to increased leakage of lipopolysaccharides (LPS, endotoxin) from the intestinal lumen to the internal environment; although this leakage has been shown to be attenuated with L-Arginine supplementation.15 Nevertheless, this permeability was shown to be exacerbated in heat stress whereby athletes performing in warm conditions showed rises in plasma concentrations of LPS.16 In fact, it has been known for some time that reduced heat tolerance could be due to the differential endotoxin leakage from the gut, which distinguishes physically fit from unfit animals.17 Conversely, enhanced integrity of GI permeability which reduces endotoxin release from the gut is thought to minimize the likelihood of developing heat stroke.18 Under hyperthermic conditions, a number of factors could be responsible for the increased gut leakage including, but not limited to, reduced intestinal blood flow,19 tissue hypoxia,20 dehydration21 and nonsteroidal anti-inflammatory drugs.22 It is now well known that strenuous exercise leading to endotoxin release from the gut mucosa also triggers a cascade involving pro-inflammatory and anti-inflammatory cytokines including tumor necrosis factor α (TNF-a) and the interleukins (IL)-1 beta (β) and IL-6,23 with heat stress exacerbating the cytokine response.16
Given the possible avenues by which gut leakage could come about along with the cascade of associated inflammatory responses, Lambert24 has suggested that endotoxemia resulting from GI permeability could act as a warning for a more serious condition such as heatstroke. Heatstroke is a serious neurological condition which can occur in a range of settings but typically occurs in sporting competitions and in the field with military personnel.25 However, the down regulation of CNS drive during exercise occurs well before the development of hyperpyrexia or heat injury, which may suggest that GI permeability and the associated inflammatory cascade serve as a further mechanism for the development of premature fatigue, and to preserve homeostasis.
We have recently proposed a neuro-inflammatory model of fatigue during exercise26 based on the potential signaling to the brain by cytokines which could act as cellular messengers.27,28 In the present review, we consider the significance of the cytokine response, specifically the release of IL-6 associated with translocation of LPS during heat stress, as a possible candidate for the development of fatigue by acting on the CNS.
GI dysfunction in exercise heat stress
In resting, healthy individuals, the GI barrier is comprised of epithelial tissue and tight junctions that hold enterocytes together with mucous secretion and immune mediators, such as macrophages; maintaining the function of the epithelial wall.29 During either passive or exertional heat stress, GI permeability can be exacerbated due to rising core temperatures and preferential blood flow away from the splanchnic area, which can directly open the tight junctions and cause tissue hypoxia, oxidative stress, and damage the enterocyte membrane.29 Consequently, the damaged epithelial wall and tight junctions allow translocation of luminal LPS into the blood stream.30
To evaluate the consequence of heat stress on GI dysfunction, it is worth reviewing some of the known responses and the magnitudes of change in some key variables. First, splanchnic temperatures of subjects exercising in the heat have been shown to reach 41.7°C which was well above the rectal temperatures of 40.2°C.31 However, it seems that high core temperatures of this kind are not necessarily required to induce GI permeability. In fact, Moseley et al.32 have found that only small rises in temperature (in vitro) can induce permeability in a high-resistance clone of canine kidney epithelial cells. These high-resistance clones form a monolayer which eliminates the potential confounding effects of inflammation and bacteria, increasing the likelihood that the changes were in fact due to heat stress.32 These data suggest that commonly achieved core temperatures of 39–40°C during exercise heat stress are able to induce GI permeability both in vivo and in vitro, potentially leading to endotoxemia.15 Likewise, others have used surrogate measures to assess gut permeability under different exercise conditions as well.29
Figure 1a and b show data from two separate studies where GI permeability was assessed after running at 75%VO2max, post ingestion of two different non-steroidal anti-inflammatory drugs (NSAIDs), or a placebo (Fig. 1a), and in a further study following the ingestion of either a sweetened placebo, a 4% glucose solution or no fluid (Fig. 1b). The surrogate measure for GI permeability used in these studies was a ratio of lactulose to rhamnose, which was determined from urinary excretion of orally administered inert carbohydrate ‘probes’ (for review see Lambert et al.33). These figures indicate that ingestion of aspirin increases GI permeability more so than ibuprofen and significantly more than a placebo even when core temperature was relatively stable between conditions (Fig. 1a). NSAIDs are thought to induce several key changes including un-coupling of oxidative phosphorylation and reduced ATP production in the mitochondria of epithelial cells of the small intestine; increased membrane fluidity and permeability due to changes in the endoplasmic reticulum; and prostaglandin synthesis, hence exacerbating the permeability.34 Further to this, when subjects were restricted from fluid intake, compared to ingesting either a sweetened placebo or 4% glucose, GI permeability was further compromised, likely due to the combination of reduced blood flow to the GI and a decreased plasma volume from fluid restriction.21 These data show that GI permeability can be compromised during exercise when NSAIDs are ingested and when fluids are restricted, hence leading to translocation of LPS.
Figure 1.
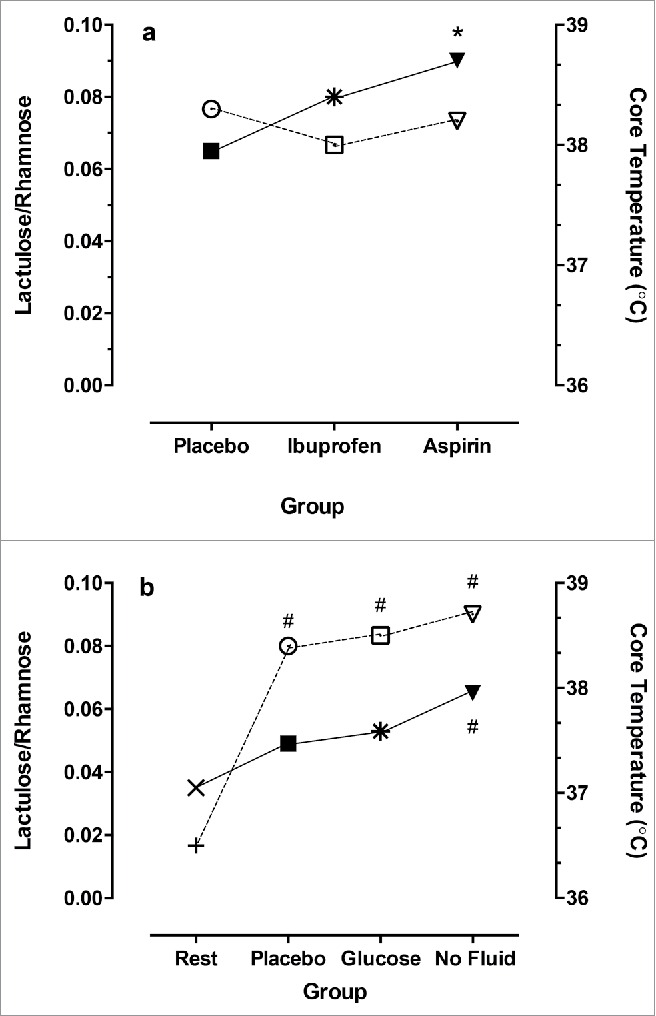
Shows the GI permeability based on ratio of lactulose to rhamnose (left ordinate, filled symbols) with ingestion of nonsteroidal anti-inflammatory drugs (a) and with ingestion of sweetened placebo, glucose or no fluid during running at 70% maximal oxygen consumption. Final core temperature (open symbols) for each condition (right ordinate). *Significantly different to placebo, #significantly different from rest. Data are medians and redrawn from21,22 for a and b, respectively.
We have already mentioned that GI permeability can lead to increased spillage of LPS into the internal environment. LPS are typically produced on the outer walls of the bacteria residing in the GI tract, and, while confined to the intestinal lumen, are harmless, though they can otherwise act as pyrogens.35 However, LPS leakage is able to elicit a strong immune (cytokine) response that can culminate in cardiovascular collapse, disseminated intravascular coagulation, and multiple-organ failure33 which, if left unchecked, can lead to life threatening sepsis.36 Although the leakage of LPS from the gut is known to be a serious pathological outcome during exercise heat stress, this view should be balanced by the very interesting findings presented by Brock-Utne et al.13 In this classic study, the authors collected random blood samples from 89 of 340 ultramarathon runners who had either collapsed or were taken to the medical tent after competing in wet bulb globe temperature (WBGT) of 20.3 – 22.3°C. The blood samples were analyzed for endotoxin (LPS) and endotoxin IgG (anti-LPS). The results of the blood analysis allowed the researchers to place runners into two groups; those with low and normal LPS and the other with high levels of LPS, including two runners with LPS in the lethal range. The interesting finding was that the runners with low or normal post-race LPS also had high levels of anti-LPS, had faster race times, reported less illness and recovered quicker. However, those with high LPS levels had low levels of anti-LPS, reported more illness and required longer recovery times. These findings raise at least two salient points to note. First, the presence of anti-LPS antibodies must have counteracted the LPS leakage in order that low levels of LPS resulted, and second, the low levels of LPS reduced the morbidity and attenuated the performance decrements, in contrast to those with low anti-LPS antibodies and high LPS levels.13 Taken collectively, these findings raise the important question as to whether training and exposure to heat stress can induce a protective mechanism against GI permeability whereby endotoxemia is attenuated to levels that do not compromise performance and whether high levels of LPS, or associated cytokine release, are indeed a signaling mechanism for the development of premature fatigue in order to preserve homeostasis. In fact, it has been hypothesized that endotoxemia can influence fatigue by increasing the perception of effort through the release of cytokines.37
It is now known that exposure to heat stress can indeed be protective to the GI barrier. Mosley et al.32 found that exposure of kidney epithelial cells to heat stress of 42°C in vitro for up to 90 min conditioned the cells to higher temperatures at which epithelial dysfunction occurred, and that cell survival to a subsequent dose of lethal heat exposure was improved. It has also been shown in a rat model that if heat stress preceded a lethal dose of LPS by 24 h, all rats survived the endotoxic insult, but only 29% survived if not exposed to previous heat stress.38 In addition to heat stress inducing a GI protective effect, exercise-induced muscle damage (EIMD) has also been shown to blunt heat strain whereby subjects exposed to both exercise heat stress and EIMD up to 14 days after the initial exposure, showed attenuated rises in core temperature and a reduced threshold for the initiation of sweating.39 These studies indicate that acute heat stress can provide some protection against GI permeability and counteract the leakage of LPS into the internal environment. This protection is likely related to the favorable alterations in heat shock proteins (HSP),40 which can thereby minimise the otherwise associated inflammatory response.
The release of interleukin-6 during exercise in heat stress
IL-6 was first identified as an immune mediator which, along with other cytokines, activates the acute-phase inflammatory response.41 The pro-inflammatory actions of IL-6 involve activating T-cells and promoting B-cells and further inflammatory mediators to eliminate damaged tissue or foreign material from an immune challenge.41 However, IL-6 also mediates stress and inflammation in the periphery by directly signaling the HPA-axis and inducing adrenal corticosteroids which act to attenuate the inflammatory response, hence also rendering it a molecule with anti-inflammatory properties.42 IL-6 has since been identified as a regulator of blood glucose metabolism at rest43 and during exercise44,45 and in the febrile response as an endogenous pyrogen.46,47
In normothermic exercise conditions, IL-6 is released in an intensity and duration dependent fashion. Short bouts of high intensity exercise increase circulating IL-6 by ∼80% immediately post a 30 s Wingate test.48,49 Likewise, prolonged exercise results in similar responses with moderate cycling at ∼62% VO2peak for ∼1h resulting in a 44% increase in IL-6,40 while running a marathon (42 km) with a mean time of 206 min results in a 100% increase in IL-6.50 The total concentration of circulating IL-6 during exercise is primarily supplied by muscle glycogen break down,51-53 and neuroendocrine responses54 that may in turn help to regulate the neuroinflammatory response from within the HPA-axis.54,55
When exercise is performed with an additional heat stress or environmental load, the IL-6 response is exacerbated due to the GI permeability described above and the associated inflammatory assault. The insult of endotoxins initiates the release of IL-6 through an immune response for elimination,16,30 although hormonal, thermal and metabolic responses are likely to contribute as well.56 Starkie et al.16 report a significant increase in core temperature (∼36.5 – 39.1°C) and a 4 – fold increase in IL-6 after 90 min of cycling at 70% VO2max in 35°C compared to the control (15°C) condition (∼0.5 – 4 pg·mL−1 and ∼0.5 – 1 pg·mL−1, respectively). Similarly, Rhind et al.56 showed that 40 min of cycling at 65% VO2max in hot (39°C) water immersion increased core temperature to 39.1°C while IL-6 increased 150%, again with negligible changes in the cold water immersion (15°C) condition.56 However, environmental temperature alone is not necessarily required to increase IL-6 response. For instance, Fortes et al.57 showed that when subjects ran downhill on a treadmill at 10% gradient, EIMD was indeed enough to elevate circulating IL-6, which then exacerbated exercise-induced hyperthermia in a subsequent heat stress test 30 min, and to a lesser extent, 24 hours later. These authors suggested that EIMD could potentially be a risk factor for heat illness if exercise is undertaken in hot conditions within the time-frame they studied.
In the event that endotoxins are not removed from the circulation, exertional heat stroke, and potential death may ensue due to multi-organ failure from an increasingly complex pro-inflammatory environment.58 Accordingly, IL-6 has also been implicated as a thermal sensor within the muscle,59 and in the core, specifically believed to signal core temperature changes to the CNS during exercise, heat shock and illness56,58 and instigate behavioral modifications. It has been shown that centrally injecting IL-6 and IL-1 β separately into conscious rats both act to increase body temperature and decrease wheel running.60 When the two cytokines are injected together, they induce fever and anorexia.60 Further evidence suggests that injecting LPS intraperitoneally results in an increase in IL-6 and IL-1β, which can act on the CNS through afferent nerve signaling at the vagus nerve in the abdomen, or other humoral pathways.61 The signaling abilities of IL-6 make it an important molecule in bi-directional communication between the brain and the periphery, which then makes appropriate modifications for systemic or whole body preservation.
Interleukin-6 signaling and receptors
IL-6 signaling can occur at local or systemic levels.62 For instance, IL-6 has been shown to directly signal the HPA-axis in a febrile response using IL-6 knockout mice and cecal ligation.47 It is also a regulator of blood glucose homeostasis where circulating IL-6 signals the release of hepatic glucose in states of hypoglycaemia,63 and is implicated in arthritis and other chronic inflammatory disease, signaling tissues and exacerbating inflammatory activity,64 and pain.65 Importantly, circulating IL-6 can signal nociceptive muscle fibers,59,66 sensory nerves,67-69 and the circumventricular organs,70 all affecting the CNS.
IL-6 signals tissues through both classical and trans-signaling pathways.71 Classical signaling occurs when IL-6 binds to a membrane bound IL-6 receptor (R), which then signals through an associated membrane bound glycoprotein (gp) 130 receptor.72 Membrane bound IL-6R, however, is only available on a small number of, primarily, immune tissues such as hepatocytes, monocytes and neutrophils, compared to the gp130 receptor which is located ubiquitously throughout the body.72 However, a soluble (s) receptor (R) (sIL-6R) is formed through alternative splicing73,74 or shedding from the membrane bound IL-6R,75 which enables further signaling. Trans-signaling occurs when sIL-6R binds to IL-6, and forms a sIL-6R/IL-6 complex. This complex then binds to a membrane bound gp130 receptor to initiate the designated response.76 Trans-signaling is known to occur in inflammatory arthritis64,77 and other autoimmune diseases78,79 and is implicated in both inflammation at the local area and in pain.80
Trans-signaling, however, can be inhibited by the antagonistic soluble receptor, sgp130,62 which does not act on IL-6 alone.81 Inhibition of trans-signaling using sgp130 to neutralise sIL-6R/IL-6 signaling in inflammatory conditions has been shown to reduce inflammation and pain in arthritis.65,77 Importantly, research suggests that the sIL-6R/IL-6 complex can bind to both the membrane bound gp130 receptor and sgp130, although sgp130 binds to the sIL-6R/IL-6 complex with a greater affinity.81 Therefore, it is posited that only in the event that there is a disproportionate increase in sIL-6R over that of sgp130, will an overall systemic response occur, otherwise, trans-signaling is likely limited to local areas.81 Systemic signaling is often a severe response such as that which occurs during sepsis and heat stroke, and so it is likely that local, trans-signaling through the gut, muscle, and other organs with sensory nerve fibers are responsible for signaling the CNS in order to prevent a septic-like response to extreme exertional heat stress.58
The exercise response of soluble interleukin-6 receptor and soluble glycoprotein 130
Most studies report increases in receptor concentrations alongside plasma IL-6 during prolonged exercise.82,83 For instance, 60 min of prolonged cycling at 90% lactate threshold resulted in a 5-fold increase in IL-6, a 1.2-fold increase in sIL-6R and a 2.1-fold increase in the sIL-6R/IL-6 complex immediately after exercise.82 Similarly, cycling at the same intensity to volitional exhaustion resulted in a 76% increase in IL-6 and a 10% increase in both sIL-6R and sgp130.83 Interestingly, despite high intensity interval training (HIIT) stimulating a significantly greater IL-6 response than moderate intensity cycling, the sIL-6R and sIL-6R/IL-6 complex response was not reported to differ between the two.84 Notably, both protocols were matched for workload, which suggests the type of exercise is not the determining factor in sIL-6R release, but the overall work completed is likely to be a key factor.84 Finally, in a study conducted during a mountain bike event covering 468 km in 6-days, IL-6 was elevated at baseline only after the first day, but increased significantly by the end of every day.28 On the other hand, sIL-6R was not elevated at baseline after the first day, but was in each consecutive day.28 Individuals reported greater levels of fatigue at baseline on days 4, 5 and 6, which was highly correlated to sIL-6R, therefore, the authors concluded that sIL-6R may be associated with perceptions of fatigue at rest.28
In contrast, some studies have revealed no changes in receptor concentrations despite significant increases in IL-6. For instance, cycling at 90% of lactate threshold to volitional exhaustion did not stimulate an increase in sIL-6R or sgp130 in individuals with chronic fatigue syndrome or healthy controls, despite an increase in IL-6.85 Similarly, 60 min cycling at 70% VO2max in normothermic (20°C) and cold (0°C) conditions, induced a significant increase in IL-6, while neither sIL-6R or sgp130 were altered by exercise or environment.86 The former study, specifically, suggests that sIL-6R may not be related to perceptions of fatigue, as it would be expected that individuals with chronic fatigue syndrome would have had greater levels compared with healthy controls. Still, the evaluation of the literature thus far, and the inherent assumptions in terms of signaling abilities highlights the need for further evidence to support each claim.
To the best of our knowledge, sIL-6R and sgp130 responses have not been studied during exertional heat stress. It has been reported, however, that individuals suffering from clinical heat stroke have resting sIL-6R concentrations that are lower compared to those suffering heat stress, potentially due to the binding of sIL-6R to IL-6,87 although these data were collected from migrants suffering from heat exposure, and thus there may have been variables that were not completely controlled for. Unfortunately, the discrepancies between results in the aforementioned studies, combined with the lack of sgp130 concentration data, limit the interpretation of whether IL-6 signaling can be implicated in the altered efferent drive of the CNS when fatigue ensues. Nevertheless, due to the known decrements in performance in exertional heat stress,16,88,90 and concomitant increase in intestinal permeability, it is plausible that the associated IL-6 response may be implicated in transient exercise induced fatigue and CNS modulation.
Effects of interleukin-6 signaling on the CNS
There is growing evidence that IL-6, along with other inflammatory cytokines, can stimulate neurons in the dorsal root ganglion,80,91,92 or through vagus afferent nerve endings in the abdomen,61,93 among other routes, and ultimately affect the CNS system as the signals travel to the brain. Peripheral sensory nerves possess the gp130 receptor which enables IL-6 signaling68 and indeed, research has highlighted a neural route of pyrogen signaling to the brain through the chemosensitive afferent fibers in the abdomen, specifically during a febrile response93 leading to sickness behaviors including fever, increased sleep and anorexia.61
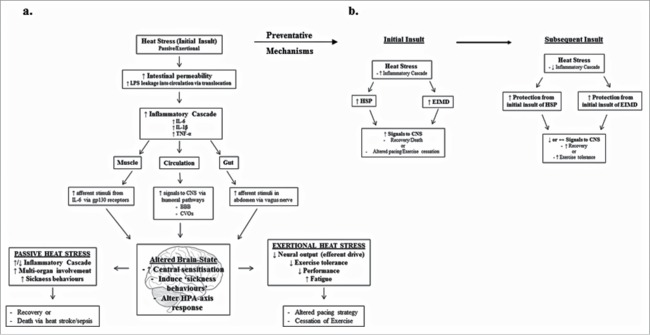
The signaling of nociceptive fibers can alter neuronal excitation and inhibition, which can further modify the threshold for pain tolerance80 and fatigue.94 This event is termed central sensitization and is well known to those researching in pain and fatigue paradigms,80,92,94 but less so in the exercise sciences, although inhibitory pathways have been implicated in down regulation of efferent drive through central fatigue.95 Central sensitization to afferent signaling is believed to have a cumulative effect on perceptions of pain and fatigue,80 which may, therefore, contribute to down regulation of the CNS during exercise and associated perceptions of fatigue. In contrast, it could be posited that increased exposure to heat stress, or acclimation, increases the tolerance to exercise and minimises performance decrements due to increased protection from HSP and an attenuated inflammatory cascade, as described above and depicted in the schematic Figure 2. Consequently, the aforementioned evidence in terms of the exacerbated response of GI permeability, the associated increase in circulating IL-6, and the potential for it to signal through nociceptive fibers in the periphery, suggests it may be an important regulator, or at least, contributor to transient feelings of fatigue during exertional heat stress (Fig. 2).
Figure 2.
Provides an overall representation of the model presented in the paper and how acute heat stress can provide preventative effects for subsequent heat stress. (a) shows how passive or exertional heat stress can effect resting or exercise behaviours through increased intestinal permeability and the associated inflammatory response signaling the CNS; (b) shows how acute heat stress can induce preventative mechanisms through HSP and EIMD in order to increase exercise tolerance and/or recovery from heat stress.
Conclusions
While not a single physiological system can account for reductions in performance during exercise, it is likely that the afferent input from all internal and external stimuli culminate in a perceptual feeling and modification of behavior, or ceasing of exercise to avoid extreme danger to the system or organism as a whole. Due to the evidence that IL-6 is released during exercise and augmented in heat stress, the signaling pathways of IL-6 that lead to altered cortical inhibitory or excitatory mechanisms might highlight the contribution of circulating IL-6 to down regulated CNS function during exertional heat stress. Furthermore, understanding the implications of central sensitization from IL-6 in exertional heat stress may help to explain heat sensitivity and extreme fatigue that is known to occur in some autoimmune conditions such as multiple sclerosis.96,97 Hence, further research is warranted in terms of the endotoxemic response to exercise in heat stress and its potential to instigate transient and chronic perceptions of fatigue and behavior modifications in exercise and disease alike.
Abbreviations
- CNS
Central Nervous system
- EIMD
Exercise induced muscle damage
- GI
Gastrointestinal
- HPA
Hypothalamic pituitary adrenal
- HSP
Heat shock proteins
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- LPS
Endotoxin, Lipopolysaccharides
- NSAIDs
Non-steroidal anti-inflammatory drugs
- sIL-6R
Soluble Interleukin-6 receptor
- sgp130
Soluble glycoprotein 130
- TNF-α
Tumor necrosis factor - alpha
- VO2max
Maximal oxygen consumption
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Nicole Vargas was supported by a Charles Sturt University post-graduate scholarship. Frank Marino is supported by the Faculty of Science at Charles Sturt University.
References
- [1].Marino FE. Anticipatory regulation and avoidance of catastrophe during exercise-induced hyperthermia. Comp Biochem Physiol B Biochem Mol Biol 2004; 139:561-9; PMID:15581788; http://dx.doi.org/ 10.1016/j.cbpc.2004.09.010 [DOI] [PubMed] [Google Scholar]
- [2].González-Alonso J. Human thermoregulation and the cardiovascular system. Exp Physiol 2012; 97:340-6; http://dx.doi.org/ 10.1113/expphysiol.2011.058701 [DOI] [PubMed] [Google Scholar]
- [3].Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol 1999; 86:902-8; PMID:10066703; http://dx.doi.org/ 10.1063/1.370821 [DOI] [PubMed] [Google Scholar]
- [4].Nybo L, Nielsen B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 2001; 91:2017-23; PMID:11641339 [DOI] [PubMed] [Google Scholar]
- [5].Brück K, Olschewski H. Body temperature related factors diminishing the drive to exercise. Can J Physiol Pharmacol 1987; 65:1274-80; PMID:3621076; http://dx.doi.org/ 10.1139/y87-203 [DOI] [PubMed] [Google Scholar]
- [6].Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 2001; 91:1055-60; PMID:11509498 [DOI] [PubMed] [Google Scholar]
- [7].Sabiosky J, Marino F, Kay D, Cannon J. Exercise heat stress does not reduce central activation to non-exercised human skeletal muscle. Exp Physiol 2003; 88:783-90; PMID:14603378; http://dx.doi.org/ 10.1113/eph8802611 [DOI] [PubMed] [Google Scholar]
- [8].Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol 2004; 91:729-36; PMID:15015001; http://dx.doi.org/ 10.1007/s00421-004-1063-z [DOI] [PubMed] [Google Scholar]
- [9].Nielsen B, Hyldig T, Bidstrup F, González-Alonso J, Christoffersen GRJ. Brain activity and fatigue during prolonged exercise in the heat. Pflügers Arch 2001; 442:41-8; PMID:11374067; http://dx.doi.org/ 10.1007/s004240100515 [DOI] [PubMed] [Google Scholar]
- [10].Rasmussen P, Nybo L, Volianitis S, Møller K, Secher NH, Gjedde A. Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol (Oxf) 2010; 199:63-70; PMID:20102344; http://dx.doi.org/ 10.1111/j.1748-1716.2010.02084.x [DOI] [PubMed] [Google Scholar]
- [11].Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol 2002; 93:58-64; PMID:12070186; http://dx.doi.org/ 10.1152/japplphysiol.00049.2002 [DOI] [PubMed] [Google Scholar]
- [12].Trangmar SJ, Chiesa ST, Stock CG, Kalsi KK, Secher NH, González-Alonso J. Dehydration affects cerebral blood flow but not its metabolic rate for oxygen during maximal exercise in trained humans. J Physiol 2014; 592:3143-60; PMID:24835170; http://dx.doi.org/ 10.1113/jphysiol.2014.272104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brock-Utne J, Gaffin S, Wells M, Gathiram P, Sohar E, James M, Morrell D, Norman R. Endotoxaemia in exhausted runners after a long-distance race. South African Medical Journal/Suid-Afrikaanse Mediese Tydskrift 1988; 73:533-6 [PubMed] [Google Scholar]
- [14].Øktedalen O, Lunde OC, Opstad PK, Aabakken L, Kvernebo K. Changes in the Gastrointestinal Mucosa after Long-Distance Running. Scand J Gast 1992; 27:270-4; http://dx.doi.org/ 10.3109/00365529209000073 [DOI] [PubMed] [Google Scholar]
- [15].Costa KA, Soares ADN, Wanner SP, Santos RdGCd, Fernandes SOA, Martins FdS, Nicoli JR, Coimbra CC, Cardoso VN. l-Arginine Supplementation Prevents Increases in Intestinal Permeability and Bacterial Translocation in Male Swiss Mice Subjected to Physical Exercise under Environmental Heat Stress. J Nutr 2014; 144:218-23; PMID:24259555; http://dx.doi.org/ 10.3945/jn.113.183186 [DOI] [PubMed] [Google Scholar]
- [16].Starkie RL, Hargreaves M, Rolland J, Febbraio MA. Heat stress, cytokines, and the immune response to exercise. Brain Behav Immun 2005; 19:404-12; PMID:16061150; http://dx.doi.org/ 10.1016/j.bbi.2005.03.005 [DOI] [PubMed] [Google Scholar]
- [17].Sakurada S, Hales JRS. A role for gastrointestinal endotoxins in enhancement of heat tolerance by physical fitness. J Appl Physiol 1998; 84:207-14; PMID:9451637 [DOI] [PubMed] [Google Scholar]
- [18].Hales JRS. Hyperthermia and Heat Illness. Ann NY Acad Sci 1997; 813:534-44; PMID:9100931; http://dx.doi.org/ 10.1111/j.1749-6632.1997.tb51743.x [DOI] [PubMed] [Google Scholar]
- [19].Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 1974; 54:75-159; PMID:4587247 [DOI] [PubMed] [Google Scholar]
- [20].Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol Gastroint Liver Physiol 1999; 276:G1195-G203 [DOI] [PubMed] [Google Scholar]
- [21].Lambert G, Lang J, Bull A, Pfeifer P, Eckerson J, Moore G, Lanspa S, O'Brien J. Fluid restriction during running increases GI permeability. Int J Sports Med 2008; 29:194-8; PMID:17614027; http://dx.doi.org/ 10.1055/s-2007-965163 [DOI] [PubMed] [Google Scholar]
- [22].Lambert GP, Boylan M, Laventure JP, Bull A, Lanspa S. Effect of aspirin and ibuprofen on GI permeability during exercise. Int J Sports Med 2007; 28:722-6; PMID:17436199; http://dx.doi.org/ 10.1055/s-2007-964891 [DOI] [PubMed] [Google Scholar]
- [23].Pedersen BK. Exercise and cytokines. Immunol Cell Biol 2000; 78:532-5; PMID:11050536; http://dx.doi.org/ 10.1111/j.1440-1711.2000.t01-11-.x [DOI] [PubMed] [Google Scholar]
- [24].Lambert P. In: Marino F, ed. Thermoregulation and Human Performance. Basel (Switzerland: ): Karger, Medicine and Sport Exercise, 2008 [Google Scholar]
- [25].Casa DJ, Armstrong LE, Kenny GP, O'Connor FG, Huggins RA. Exertional Heat Stroke: New Concepts Regarding Cause and Care. Curr Sports Med Rep 2012; 11:115-23; PMID:22580488; http://dx.doi.org/ 10.1249/JSR.0b013e31825615cc [DOI] [PubMed] [Google Scholar]
- [26].Vargas NT, Marino F. A neuroinflammatory model for acute fatigue during exercise. Sports Med 2014; 44:1479-87; PMID:25164464; http://dx.doi.org/ 10.1007/s40279-014-0232-4 [DOI] [PubMed] [Google Scholar]
- [27].Robson-Ansley PJ, Milander Ld, Collins M, Noakes TD. Acute Interleukin-6 administration impairs athletic performance in healthy, trained male runners. Can J Appl Physiol 2004; 29:411-8; PMID:15317982; http://dx.doi.org/ 10.1139/h04-026 [DOI] [PubMed] [Google Scholar]
- [28].Robson-Ansley P, Barwood M, Canavan J, Hack S, Eglin C, Davey S, Hewitt J, Hull J, Ansley L. The effect of repeated endurance exercise on IL-6 and sIL-6R and their relationship with sensations of fatigue at rest. Cytokine 2009; 45:111-6; PMID:19097916; http://dx.doi.org/ 10.1016/j.cyto.2008.11.006 [DOI] [PubMed] [Google Scholar]
- [29].Lambert GP. Role of Gastrointestinal Permeability in Exertional Heatstroke. Exerc Sport Sci Rev 2004; 32:185-90; PMID:15604939; http://dx.doi.org/ 10.1097/00003677-200410000-00011 [DOI] [PubMed] [Google Scholar]
- [30].Zuhl M, Schneider S, Lanphere K, Conn C, Dokladny K, Moseley P. Exercise regulation of intestinal tight junction proteins. Br J Sports Med 2014; 48:980-6; PMID:23134759; http://dx.doi.org/ 10.1136/bjsports-2012-091585 [DOI] [PubMed] [Google Scholar]
- [31].Rowell LB, Brengelmann GL, Blackmon JR, Twiss RD, Kusumi F. Splanchnic blood flow and metabolism in heat-stressed man. J Appl Physiol 1968; 24:475-84; PMID:5643395 [DOI] [PubMed] [Google Scholar]
- [32].Moseley PL, Gapen C, Wallen ES, Walter ME, Peterson MW. Thermal stress induces epithelial permeability. Am J Physiol Cell Physiol 1994; 267:C425-C34 [DOI] [PubMed] [Google Scholar]
- [33].Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected Contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 2002; 92:1750-61; PMID:11896046; http://dx.doi.org/ 10.1152/japplphysiol.00787.2001 [DOI] [PubMed] [Google Scholar]
- [34].Somasundaram S, Hayllar H, Rafi S, Wrigglesworth JM, Macpherson AJS, Bjarnason I. Review: The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gast 1995; 30:289-99; http://dx.doi.org/ 10.3109/00365529509093280 [DOI] [PubMed] [Google Scholar]
- [35].Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 1994; 8:217-25; PMID:8119492 [DOI] [PubMed] [Google Scholar]
- [36].Krüttgen A, Rose-John S. Interleukin-6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res 2011; 32:60-5; http://dx.doi.org/ 10.1089/jir.2011.0062 [DOI] [PubMed] [Google Scholar]
- [37].Cheung SS, Sleivert GG. Multiple Triggers for hyperthermic fatigue and exhaustion. Exerc Sport Sci Rev 2004; 32:100-6; PMID:15243205; http://dx.doi.org/ 10.1097/00003677-200407000-00005 [DOI] [PubMed] [Google Scholar]
- [38].Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol 1992; 73:1517-22; PMID:1447099 [DOI] [PubMed] [Google Scholar]
- [39].Dolci A, Fortes MB, Walker FS, Haq A, Riddle T, Walsh NP. Repeated muscle damage blunts the increase in heat strain during subsequent exercise heat stress. Eur J Appl Physiol 2015; 115:1577-88; PMID:25736783; http://dx.doi.org/ 10.1007/s00421-015-3143-7 [DOI] [PubMed] [Google Scholar]
- [40].King YT, Lin CS, Lin JH, Lee WC. Whole-body hyperthermia-induced thermotolerance is associated with the induction of Heat Shock Protein 70 in mice. J Exp Biol 2002; 205:273-8; PMID:11821493 [DOI] [PubMed] [Google Scholar]
- [41].Shek PN, Shephard RJ. Physical exercise as a human model of limited inflammatory response. Can J Physiol Pharmacol 1998; 76:589-97; http://dx.doi.org/ 10.1139/y98-040 [DOI] [PubMed] [Google Scholar]
- [42].Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology 2002; 143:1571-4; PMID:11956136; http://dx.doi.org/ 10.1210/endo.143.5.8861 [DOI] [PubMed] [Google Scholar]
- [43].Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab 1997; 82:4167-70; PMID:9398733; http://dx.doi.org/ 10.1210/jcem.82.12.4422 [DOI] [PubMed] [Google Scholar]
- [44].Jeukendrup AE, Wagenmakers AJM, Stegen JHCH, Gijsen AP, Brouns F, Saris WHM. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Am J Physiol Endocrinol Metab 1999; 276:E672-E83 [DOI] [PubMed] [Google Scholar]
- [45].McConell G, Fabris S, Proietto J, Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise. J Appl Physiol 1994; 77:1537-41; PMID:7836162 [DOI] [PubMed] [Google Scholar]
- [46].Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol Reg Integr Comp Physiol 1998; 275:R269-R77 [DOI] [PubMed] [Google Scholar]
- [47].Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol 2002; 92:2648-55; PMID:12015385; http://dx.doi.org/ 10.1152/japplphysiol.01005.2001 [DOI] [PubMed] [Google Scholar]
- [48].Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol 2013; 113:241-8; PMID:22677919; http://dx.doi.org/ 10.1007/s00421-012-2432-7 [DOI] [PubMed] [Google Scholar]
- [49].Leggate M, Nowell MA, Jones SA, Nimmo MA. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones 2010; 15:827-33; PMID:20396982; http://dx.doi.org/ 10.1007/s12192-010-0192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ostrowski K, Schjerling P, Pedersen KB. Physical activity and plasma interleukin-6 in humans – effect of intensity of exercise. Eur J Appl Physiol 2000; 83:512-5; PMID:11192058; http://dx.doi.org/ 10.1007/s004210000312 [DOI] [PubMed] [Google Scholar]
- [51].Febbraio M, Steensberg A, Keller C, Starkie R, Nielsen H, Krustrup P, Ott P, Secher N, Pedersen B. Glucose Ingestion Attenuates Interleukin-6 Release from Contracting Skeletal Muscle in Humans. J Physiol 2003; 549:607-12; PMID:12702735; http://dx.doi.org/ 10.1113/jphysiol.2003.042374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Starkie R, Arkinstall M, Koukoulas I, Hawley J, Febbraio M. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol 2001; 533:585-91; PMID:11389214; http://dx.doi.org/ 10.1111/j.1469-7793.2001.0585a.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 2001; 529:237-42; http://dx.doi.org/ 10.1111/j.1469-7793.2000.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Steensberg A, Toft AD, Schjerling P, Halkjær-Kristensen J, Pedersen BK. Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am J Physiol Cell Physiol 2001; 281:C1001-C4; PMID:11502577 [DOI] [PubMed] [Google Scholar]
- [55].Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003; 285:E433-7; PMID:12857678; http://dx.doi.org/ 10.1152/ajpendo.00074.2003 [DOI] [PubMed] [Google Scholar]
- [56].Rhind SG, Gannon GA, Shephard RJ, Buguet A, Shek PN, Radomski MW. Cytokine induction qduring exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int J Hyperthermia 2004; 20:503-16; PMID:15277023; http://dx.doi.org/ 10.1080/02656730410001670651 [DOI] [PubMed] [Google Scholar]
- [57].Fortes MB, Di Felice U, Dolci A, Junglee NA, Crockford MJ, West L, Hillier-Smith R, Macdonald JH, Walsh NP. Muscle-damaging exercise increases heat strain during subsequent exercise heat stress. Med Sci Sports Exerc 2013; 45:1915-24; PMID:23559121; http://dx.doi.org/ 10.1249/MSS.0b013e318294b0f8 [DOI] [PubMed] [Google Scholar]
- [58].Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke. Sports Med 2006; 36:39-64; PMID:16445310; http://dx.doi.org/ 10.2165/00007256-200636010-00004 [DOI] [PubMed] [Google Scholar]
- [59].Welc SS, Phillips NA, Oca-Cossio J, Wallet SM, Chen DL, Clanton TL. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol 2012; 303:C455-C66; PMID:22673618; http://dx.doi.org/ 10.1152/ajpcell.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Harden LM, Plessis Id, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1β act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun 2008; 22:838-49; PMID:18255258; http://dx.doi.org/ 10.1016/j.bbi.2007.12.006 [DOI] [PubMed] [Google Scholar]
- [61].Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RPA, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Reg Integr and Comp Physiol 2000; 278:R331-R6 [DOI] [PubMed] [Google Scholar]
- [62].Rose-John S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int J Biol Sci 2012; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004; 53:1643-8; PMID:15220185; http://dx.doi.org/ 10.2337/diabetes.53.7.1643 [DOI] [PubMed] [Google Scholar]
- [64].Nowell MA, Richards PJ, Horiuchi S, Yamamoto N, Rose-John S, Topley N, Williams AS, Jones SA. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J Immunol 2003; 171:3202-9; PMID:12960349; http://dx.doi.org/ 10.4049/jimmunol.171.6.3202 [DOI] [PubMed] [Google Scholar]
- [65].Boettger MK, Leuchtweis J, Kummel D, Gajda M, Brauer R, Schaible HG. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res Ther 2010; 12:R140; PMID:20626857; http://dx.doi.org/ 10.1186/ar3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang Y, Pilon G, Marette A, Baracos VE. Cytokines and endotoxin induce cytokine receptors in skeletal muscle. Am J Physiol Endocrinol Metab 2000; 279:E196-E205; PMID:10893340 [DOI] [PubMed] [Google Scholar]
- [67].Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain 2005; 114:168-76; PMID:15733642; http://dx.doi.org/ 10.1016/j.pain.2004.12.020 [DOI] [PubMed] [Google Scholar]
- [68].Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Üceyler N, Brockhaus J, et al.. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009; 29:13473-83; PMID:19864560; http://dx.doi.org/ 10.1523/JNEUROSCI.1822-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain 2012; 13:10-20; PMID:22172450; http://dx.doi.org/ 10.1016/j.jpain.2011.10.003 [DOI] [PubMed] [Google Scholar]
- [70].Roth J, Harre EM, Rummel C, Gerstberger R, Hubschle T. Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci 2004; 9:290-300; PMID:14766367; http://dx.doi.org/ 10.2741/1241 [DOI] [PubMed] [Google Scholar]
- [71].Kovacs E. Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed Pharmacother 2001; 55:391-6; PMID:11669502; http://dx.doi.org/ 10.1016/S0753-3322(01)00079-8 [DOI] [PubMed] [Google Scholar]
- [72].Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta Mol Cell Res 2002; 1592:323-43; http://dx.doi.org/ 10.1016/S0167-4889(02)00325-7 [DOI] [PubMed] [Google Scholar]
- [73].Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine 1992; 4:96-100; PMID:1633265; http://dx.doi.org/ 10.1016/1043-4666(92)90043-Q [DOI] [PubMed] [Google Scholar]
- [74].Horiuchi S, Koyanagiu Y, Zhouu Y, Miyamotou H, Tanakau Y, Waki M, Matsumoto A, Yamamotou M, Yamamotof N. Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur J Immun 1994; 24:1945-8; http://dx.doi.org/ 10.1002/eji.1830240837 [DOI] [PubMed] [Google Scholar]
- [75].Mülberg J, Schooltink H, Stoyan T, Günther M, Graeve L, Buse G, Mackiewicz A, Heinrich PC, Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol 1993; 23:473-80; PMID:8436181; http://dx.doi.org/ 10.1002/eji.1830230226 [DOI] [PubMed] [Google Scholar]
- [76].Rose-John S, Neurath MF. IL-6 trans-Signaling: The Heat Is On. Immunity 2004; 20:2-4; PMID:14738759; http://dx.doi.org/ 10.1016/S1074-7613(04)00003-2 [DOI] [PubMed] [Google Scholar]
- [77].Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67:1516-23; PMID:18625622; http://dx.doi.org/ 10.1136/ard.2008.092932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cyt Growth Factor Rev 2002; 13:357-68; http://dx.doi.org/ 10.1016/S1359-6101(02)00027-8 [DOI] [PubMed] [Google Scholar]
- [79].Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012; 122:143-59; PMID:22029668; http://dx.doi.org/ 10.1042/CS20110340 [DOI] [PubMed] [Google Scholar]
- [80].Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28:5189-94; PMID:18480275; http://dx.doi.org/ 10.1523/JNEUROSCI.3338-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath M, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor trans signalling responses. Eur J Biochem 2001; 268:160-7; PMID:11168360 [DOI] [PubMed] [Google Scholar]
- [82].Gray SR, Clifford M, Lancaster R, Leggate M, Davies M, Nimmo MA. The response of circulating levels of the interleukin-6/interleukin-6 receptor complex to exercise in young men. Cytokine 2009; 47:98-102; PMID:19527938; http://dx.doi.org/ 10.1016/j.cyto.2009.05.011 [DOI] [PubMed] [Google Scholar]
- [83].Gray SR, Robinson M, Nimmo MA. Response of plasma IL-6 and its soluble receptors during submaximal exercise to fatigue in sedentary middle-aged men. Cell Stress Chaperones 2008; 13:247-51; PMID:18320358; http://dx.doi.org/ 10.1007/s12192-008-0019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Robson-Ansley P, Blannin A, Gleeson M. Elevated plasma interleukin-6 levels in trained male triathletes following an acute period of intense interval training. Eur J Appl Physiol 2007; 99:353-60; PMID:17165057; http://dx.doi.org/ 10.1007/s00421-006-0354-y [DOI] [PubMed] [Google Scholar]
- [85].Robinson M, Gray SR, Watson MS, Kennedy G, Hill A, Belch JJ, Nimmo MA. Plasma IL-6, its soluble receptors and F2-isoprostanes at rest and during exercise in chronic fatigue syndrome. Scand J Med Sci Sports 2010; 20:282-90; PMID:19422646; http://dx.doi.org/ 10.1111/j.1600-0838.2009.00895.x [DOI] [PubMed] [Google Scholar]
- [86].Patterson S, Reid S, Gray S, Nimmo M. The response of plasma interleukin-6 and its soluble receptors to exercise in the cold in humans. J Sports Sci 2008; 26:927-33; PMID:18569558; http://dx.doi.org/ 10.1080/02640410801885941 [DOI] [PubMed] [Google Scholar]
- [87].Hammami MM, Bouchama A, Al-Sedairy S, Shail E, AlOhaly Y, Mohamed GE. Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heatstroke and heatstress. Crit Care Med 1997; 25:1314-9; PMID:9267943; http://dx.doi.org/ 10.1097/00003246-199708000-00017 [DOI] [PubMed] [Google Scholar]
- [88].Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Pysiol 2006; 574:905-15; http://dx.doi.org/ 10.1113/jphysiol.2005.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].González-Alonso J, Teller C, Andersen S, Jensen F, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercsie in the heat. J Appl Physiol 1999; 83:1032-9 [DOI] [PubMed] [Google Scholar]
- [90].Kay D, Marino FE, Cannon J, St Clair Gibson A, Lambert MI, Noakes TD. Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur J Appl Physiol 2001; 84:115-21; PMID:11394239; http://dx.doi.org/ 10.1007/s004210000340 [DOI] [PubMed] [Google Scholar]
- [91].Coderre TJ, Melzack R. Central neural mediators of secondary hyperalgesia following heat injury in rats: Neuropeptides and excitatory amino acids. Neurosci Lett 1991; 131:71-4; PMID:1686478; http://dx.doi.org/ 10.1016/0304-3940(91)90339-U [DOI] [PubMed] [Google Scholar]
- [92].Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Prac Res Clin Rheumatol 2007; 21:481-97; http://dx.doi.org/ 10.1016/j.berh.2007.03.006 [DOI] [PubMed] [Google Scholar]
- [93].Romanovsky AA, Ivanov AI, Székely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis 2000; 31:S162-S7; PMID:11113019; http://dx.doi.org/ 10.1086/317515 [DOI] [PubMed] [Google Scholar]
- [94].Nijs J, Meeus M, Van Oosterwijck J, Ickmans K, Moorkens G, Hans G, De Clerck LS. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur J Clin Invest 2012; 42:203-12; PMID:21793823; http://dx.doi.org/ 10.1111/j.1365-2362.2011.02575.x [DOI] [PubMed] [Google Scholar]
- [95].Taylor JL, Gandevia S In: Marino F, ed. Regulation of Fatigue in Exercise. New York: Nova Science Publishers, Inc, 2011:63-77 [Google Scholar]
- [96].Nelson DA, McDowell F. The effects of induced hyperthermia on patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1959; 22:113-6; PMID:13655099; http://dx.doi.org/ 10.1136/jnnp.22.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Marino FE. Heat reactions in multiple sclerosis: An overlooked paradigm in the study of comparative fatigue. Int J Hyperth 2009; 25:34-40; http://dx.doi.org/ 10.1080/02656730802294020 [DOI] [PubMed] [Google Scholar]