ABSTRACT
Exercise induces arterial flow patterns that promote functional and structural adaptations, improving functional capacity and reducing cardiovascular risk. While heat is produced by exercise, local and whole-body passive heating have recently been shown to generate favorable flow profiles and associated vascular adaptations in the upper limb. Flow responses to acute heating in the lower limbs have not yet been assessed, or directly compared to exercise, and other cardiovascular effects of lower-limb heating have not been fully characterized. Lower-limb heating by hot-water immersion (30 min at 42°C, to the waist) was compared to matched-duration treadmill running (65-75% age-predicted heart rate maximum) in 10 healthy, young adult volunteers. Superficial femoral artery shear rate assessed immediately upon completion was increased to a greater extent following immersion (mean ± SD: immersion +252 ± 137% vs. exercise +155 ± 69%, interaction: p = 0.032), while superficial femoral artery flow-mediated dilation was unchanged in either intervention. Immersion increased heart rate to a lower peak than during exercise (immersion +38 ± 3 beats·min-1 vs. exercise +87 ± 3 beats·min-1, interaction: p < 0.001), whereas only immersion reduced mean arterial pressure after exposure (−8 ± 3 mmHg, p = 0.012). Core temperature increased twice as much during immersion as exercise (+1.3 ± 0.4°C vs. +0.6 ± 0.4°C, p < 0.001). These data indicate that acute lower-limb hot-water immersion has potential to induce favorable shear stress patterns and cardiovascular responses within vessels prone to atherosclerosis. Whether repetition of lower-limb heating has long-term beneficial effects in such vasculature remains unexplored.
KEYWORDS: acute exercise, immersion, lower-limb heating, passive heat, shear stress
Introduction
Limb blood flow is affected by the temperature of local tissue, the limb and the body as a whole. Both the profile and the magnitude of arterial flow within limbs have important effects on that limb's arteries and potentially on downstream vessels, as well as on remote vasculature. Shear stress, the mechanical force of blood flow on the walls of arteries1 provides the stimulus for both acute2,3 and adaptive effects.4 Specifically, an increase in forward-directional (antegrade) shear acutely promotes vasodilation.5 Chronic exposure to increased antegrade shear (over several weeks), as in during repetitive bouts of exercise training, is known to improve endothelial function6 (responsiveness to shear stress) within the first 4 weeks, after which structural remodeling becomes evident (and thereby structurally normalizes shear stress within 6 to 8 weeks).7,8
Shear stress can be manipulated using various thermal and non-thermal interventions, including exercise, terrestrial heat exposure and hot-water immersion.5,9–12 Hot-water immersion can acutely increase antegrade shear in the arteries of immersed limbs9 as well as in remote limbs.12 Chronically, favorable adaptations in endothelial function10,12 and microvascular vasodilation13 have been demonstrated following repeated local heating in healthy individuals. Systemically-applied heat via sauna bathing has also been reported to improve endothelial function in patients with coronary risk factors14 and heart failure.15 Again, the suggested mechanism for these effects has been increased peripheral artery blood flow (+68% following 15 min of sauna14), although a causal relationship has not always been expounded in such studies. In support of the shear stress hypothesis, repeated sauna therapy (4-5 weeks) upregulates endothelial nitric oxide synthesis expression in animal models.16,17 Therefore, heat therapy, applied in various ways, appears to have potential as a stimulus for increasing blood flow and antegrade shear stress,12 in a manner similar to exercise.7,8
Heat is also implicated in other exercise-induced cardiovascular adaptations, which occur as a result of, or are amplified by, increased core and tissue temperature. Examples include blood volume expansion,18,19 muscle growth20,21 and glucose uptake,22 and a heightened cellular stress response conferring increased resilience to stress.23-25 Isolated heat stress also causes several other significant physiological responses similar to those induced by exercise (which is also heat stressful26). These include elevations in cutaneous and muscle blood flow, heart rate and sympathetic activity.27,28 Furthermore, repetitive heat exposure, independent of exercise, can improve cardiac function,15,29–31 reduce blood pressure32 and induce cardiac preconditioning,33 and is associated with reduced risk of cardiovascular and all-cause mortality.34
Despite this background, much remains unknown about the acute hemodynamic effects of heat and how these compare with traditional exercise, especially in the lower limbs. Therefore, the aim of this study was to directly compare the lower-limb artery shear rate pattern induced by an acute bout of lower-limb heating to that induced by running (i.e., the ubiquitous mode of exercise) – within the same young, healthy individuals. The duration of exposure was matched between interventions. Importantly, the novelty of this study was in the assessment of the lower-limb arterial response, as most investigations on arterial responses to exercise (acute and chronic) focus on the brachial artery and infer that this reflects global arterial responses. However, in cases where more than one anatomical location has been assessed, upper- and lower-limb arteries often show little resemblance in their respective flow profiles.35 It follows that upper-limb responses are unlikely to reflect local effects of lower-limb-specific interventions. Thus far, the superficial femoral artery (SFA) has not been studied in relation to acute hot-water immersion, and much also remains unknown about the acute vascular effects of exercise, especially in the lower limb.36 Assessing the hemodynamics in lower-limb arteries is important for 2 reasons: 1) to understand the response in the limbs involved in heat and exercise administration, and 2) atherosclerotic disease is far more prevalent in the lower than upper limbs,37 so understanding their shear profiles is important for considering future therapeutic prospects, particularly for people with a limited exercise capacity and concomitant high cardiovascular risk. Of secondary interest were systemic cardiovascular responses to the acute lower-limb heating protocol. There is a growing body of literature examining isolated stressors (e.g., heat) for their potential to induce beneficial cardiovascular and thermoregulatory strain. Understanding the complex acute effects of any such approach is therefore warranted to understand their potential as an alternative or complement to an exercise-training stimulus.
Participants and methods
Participant characteristics
Ten young, healthy individuals volunteered for this study (8 male and 2 female; age 27 ± 5 y, height 181 ± 8 cm, mass 81 ± 8 kg, BMI 24.4 ± 1.5 kg·m-2). Participants were not taking any medications or supplements, all were non-smokers, and all were recreationally active, typically engaging in moderate-intensity aerobic exercise (e.g. jogging) and resistance training (≥3 d per week). Written informed consent was obtained before participation. The study was approved by the University of Otago Human Ethics Committee, and conformed to the standards set by the Declaration of Helsinki.
Experimental procedures
This cross-over study involved 2 experimental interventions, namely exercise and water immersion, which were performed in a randomized and balanced order. Upon arrival to the laboratory, participants rested supine for 15 min before initial baseline data collection. They then completed the assigned intervention (exercise or immersion), with measures recorded during and immediately afterward as outlined below. The interventions were matched for parameters of stress rather than strain; i.e., 30-min duration of exposure; being realistic/tolerable for both interventions as well as meeting the current health-related exercise guidelines.38 Similarly, intensities were chosen to be strenuous but tolerable. Neither intervention provides an exclusively lower-limb or whole-body stimulus per se, so the intent was for interventions to be somewhat matched for the extent of tissue exposed to the stimulus provided. Exercise consisted of 30-min treadmill running at 65-75% age-predicted heart rate maximum. Running speeds ranged from 9.5 to 12 km·h-1 with an average of 10 km·h-1. Exercise was performed in a temperature-controlled environment at 22-23°C. Water Immersion consisted of 30-min seated, immersed to the waist in hot water (42.0 ± 0.4°C). Water temperature was checked continually and adjusted throughout the 30-min immersion. This temperature was chosen as ‘a tolerable maximum’ based on pilot experiments.
Each session was performed at the same time of day (>10 :00 h because of the known early-morning attenuation of endothelial function),39 and 3-7 d apart, sufficient to ensure wash-out. All participants were instructed to abstain from exercise36 and alcohol40 for 24 h prior to the test, and to avoid caffeine on the morning of testing.41 Participants were also instructed to maintain their normal diet during the study period. Female participants were tested in days 1 – 7 of the menstrual cycle.42
Experimental measures
Temperature measurements
Core body temperature (Tc) was measured using a thermistor in the esophagus at a depth 48% of sitting height, minus 4.44 cm.43 Muscle temperature (Tm) of the medial gastrocnemius was measured using a needle thermocouple (YSI 525, Yellow Springs Instruments, Yellow Springs, OH, USA) at a depth of 2 cm below the skin surface. Muscle temperature was measured throughout the water immersion protocol, and after exercise (<5 min post-exercise). All temperatures were recorded at 30-s intervals using portable, battery-operated loggers (Squirrel SQ2010, Grant Instruments, Cambridge, UK). Perceived ratings of body temperature and thermal discomfort were ascertained from a 13- and 5-point scale respectively (extended from Gagge44), at baseline and at 10, 20 and 30 min through the intervention.
Superficial femoral artery hemodynamics
Superficial femoral artery (SFA) diameter and blood velocity were measured using ultrasound (Aplio XG, Toshiba, Nasu, Japan) with a 7 MHz linear array transducer (bandwidth 4-11 MHz) by simultaneously recording a longitudinal section B-mode image and a spectral Doppler trace of blood velocity. The Doppler angle of insonation was maintained at 60°. Participants were supine during this procedure. Measurements were made 2-3 cm distal to the bifurcation of the common femoral artery. The location of the transducer was recorded and marked on the skin using indelible ink and reused for the repeat test. Ultrasound settings (depth, focus position and gain) were optimized for each participant, and reused for the repeat test. All ultrasound scans were performed by the same vascular sonographer (K.T.). Video clips were recorded using a VGA to USB screen capture device at 21 Hz (VGA2USB LR, Epiphan Systems Inc., Palo Alto, California, USA). Analysis of diameter and velocity, and the calculation of shear rate (SR = 4* velocity / diameter)45 were performed using wall-tracking software (Cardiovascular Suite UE v 2.5, Quipu, Pisa, Italy),46,47 which reduces investigator bias. Our test-retest reliability using this software for measures of diameter and velocity were 0.4% and 2.1% respectively (n = 10).
Flow-mediated dilation (FMD)
FMD is predominantly an endothelium-dependent measure of vascular function based on the ability of the vessel to respond to transient ischemia with reactive hyperemia. The resting hemodynamics (diameter, velocity, shear rate) and FMD of the SFA were assessed before and 5-10 min after the intervention, following 2-min baseline recording, according to international guidelines.48 To perform the FMD, arterial flow was blocked at the distal thigh using a 17-cm contoured cuff inflated to 200 mmHg within 2 seconds (CC17 contoured leg cuff, E20 Rapid Cuff Inflator and AG101 Cuff Inflator Air Source, Hokanson, Bellevue WA, USA). Occlusion was maintained for 5 min. Recording resumed for the final 30 s of occlusion and continued for 3 min following rapid release of the cuff (<2 s).
Systemic hemodynamics
Heart rate (HR) was obtained continuously using detection of the R-R wave of ventricular depolarization frequency (Polar S810i, Polar, Finland). Blood pressure (BP) was measured using finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Stroke volume (SV) and cardiac output () were calculated using the Modelflow method, which incorporates sex, age, height and mass (BeatScope 1.0 software, Finapres Medical Systems, Amsterdam, The Netherlands). Continuous recording of the above variables was obtained at 200 Hz using an analog-to-digital converter (Powerlab/16SP, ADInstruments, Dunedin, New Zealand) and later analyzed using Chart software (LabChart Pro v 7.2.5, ADInstruments). Baseline data were collected over 5 min before each intervention, and post-intervention recording began within 5 min of finishing exercise or exiting the water.
Data analysis
Calculations: Core temperature (Tc) was calculated as the maximum change from baseline during the intervention (ΔT), while muscle temperature (Tm) was calculated as either the maximum change from baseline during immersion, or immediately post-exercise (ΔT). Heat impulse was calculated using ΔT * time at that temperature for both Tc and Tm49, to approximate the volume of heat strain and thus a unitary but admittedly simplistic index of the thermal stimulus for adaptation.
Mean arterial blood pressure (MAP) was calculated as one-third systolic (SBP) plus two-thirds diastolic blood pressure (DBP). Baseline blood pressure, HR, SV and data were obtained as an average of a 5-min period. MAP, SBP, DBP, SV and are presented as pre vs. post-intervention (within 5 min of completion); HR is presented as pre vs. peak HR attained during intervention.
Baseline diameter (Dbase), blood flow velocity (v) and shear rate (SR) were calculated as the mean of the last minute of the baseline period, pre-cuff inflation. Peak diameter post-deflation was determined automatically using the edge-detection software. FMD was calculated as the percentage increase (FMD%) in diameter from the baseline (FMD = (Dpeak – Dbase) / Dbase * 100). Recent publications50,51 have highlighted the biased nature of using the FMD% due to its reliance on Dbase and the known negative correlation between FMD% and Dbase.51 We therefore followed guidelines51,52 utilizing allometric scaling to adjust for Dbase with a covariate-controlled approach. These results are presented as “Dbase-adjusted FMD%.”
Statistics: All descriptives are reported as mean ± SD and all estimates are presented as mean ± SE unless stated otherwise. The cardiovascular and shear stress responses to the 2 interventions were analyzed using mixed models with random effects at the participant and participant-intervention levels, with an intervention-time interaction used to identify differences between intervention effects. Within-intervention changes are presented to assist interpretation of between intervention tests. Mixed models were used to compare between-intervention changes in FMD in 3 ways: using the raw data, FMD adjusted for baseline FMD, and FMD adjusted for Dbase. Period effects (first or second) were included in all mixed models and a lack of carryover was assumed based on the study design rather than being formally tested for. Analyses were performed using Graphpad Prism 6 (Graphpad Software, Inc., La Jolla, California, USA), SPSS (v 19.0, SPSS Inc., Chicago, Illinois, USA), and Stata (v 13.1, StataCorp, College Station, Texas, USA) statistical software. All statistical tests were performed at the 2-sided 0.05 level with no adjustment for multiple comparisons.
Results
All participants completed both conditions. All data are of n = 10 for all variables except post-exercise Tm, for which n = 8.
Temperature
The increase in Tc during 30 min of water immersion was approximately twice as much as during 30 min of exercise (p < 0.001; Table 1 and Fig. 1). Peak Tm, measured as soon as possible (<5 min) following exercise and at the equivalent time following immersion, was higher following immersion (mean ± SD: 38.5 ± 0.4 vs. 38.1 ± 0.4°C, p = 0.007). The Tm at this time post-immersion was within 0.2 ± 0.3°C of the peak recorded for the entire immersion trial. Consequently, the heat impulse generated for both Tc and Tm was larger for water immersion than exercise (Tc, as calculated from the entire trial: 19.0 ± 2.2 vs. 6.0 ± 2.2°C·min, p < 0.001; Tm, as calculated from 10 min of recovery: 44.1 ± 1.8 vs. 30.4 ± 2.0°C·min, p < 0.001). Perceived body temperature was “hot” (i.e., 10 on the 13-point sensation scale) at completion of both interventions, which was rated as ‘slightly uncomfortable’ (2/5) for exercise and ‘slightly uncomfortable’-to-‘uncomfortable’ (2.5) for water immersion on the discomfort scale.
Table 1.
Thermoregulatory and systemic cardiovascular variables at baseline and immediately post-intervention (<5 min). Data are mean ± SD for baseline and post values, and mean ± SE for change scores. Baseline and post-intervention data were averaged over 5 min. Tc, core temperature; Tm, muscle temperature; SV, stroke volume; , cardiac output; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.a HR post is peak HR reached during intervention.
| Baseline |
Post |
Change |
||||
|---|---|---|---|---|---|---|
| Variable | Exercise | Immersion | Exercise | Immersion | Exercise | Immersion |
| Tc (°C) | 36.3 ± 0.5 | 36.5 ± 0.3 | 37.0 ± 0.7 | 37.7 ± 0.6 *† | +0.6 ± 0.4 | +1.3 ± 0.4 |
| Tm (°C) | – | 33.7 ± 0.7 | 38.1 ± 0.4 | 38.5 ± 0.4 ‡ | – | +4.7 ± 0.9 |
| SV (mL·min-1) | 89 ± 22 | 109 ± 24 | 89 ± 16 | 98 ± 38 | +1 ± 9 | −10 ± 9 |
| (L·min-1) | 4.8 ± 1.6 | 5.6 ± 1.5 | 6.9 ± 2.0 † | 6.6 ± 1.1 | +2.1 ± 0.5 | +1.0 ± 0.5 |
| MAP (mm Hg) | 88 ± 7 | 89 ± 9 | 88 ± 11 | 82 ± 12 † | 0 ± 3 | −8 ± 3 |
| SBP (mm Hg) | 119 ± 11 | 125 ± 11 | 120 ± 13 | 116 ± 16 † | +1 ± 4 | −10 ± 4 |
| DBP (mm Hg) | 72 ± 6 | 71 ± 9 | 72 ± 10 | 65 ± 11 † | 0 ± 3 | −6 ± 3 |
| HR (beats·min-1)a | 54 ± 7 | 54 ± 7 | 141 ± 12 †* | 93 ± 8 † | +87 ± 3 | +38 ± 3 |
interaction: intervention x time (p < 0.05)
different from baseline (p < 0.05)
different from post-exercise (p < 0.05).
Figure 1.
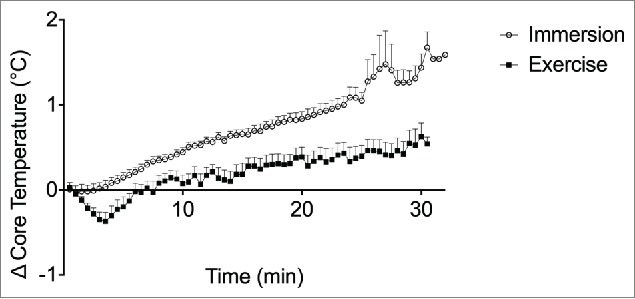
Change in core temperature from baseline throughout exercise and water immersion measured at 30-s intervals. Data points represent the group mean and error bars are SD.
Shear rate patterns
Total shear rate was increased to a greater extent after immersion than exercise (measured 5-10 min following cessation of intervention; immersion: +181 s-1 ± 23 s-1; exercise: +104 s-1 ± 23 s-1, both p<0.001, interaction p = 0.032, see Table 2 and Fig. 2). This represents an increase of ∼250% following immersion and ∼150% following exercise. Similarly, antegrade shear rate was increased differentially between interventions (immersion: +157 s-1 ± 22 s-1; exercise: +85 s-1 ± 22 s-1, both p < 0.001, interaction p=0.004). Retrograde shear rate was attenuated by 24 s-1 ± 4 s-1 following both interventions (both p < 0.001) but not differentially so (p = 0.862).
Table 2.
Superficial femoral artery (SFA) hemodynamic responses at baseline and post-intervention (< 10 min). Dbase, baseline diameter; SR, shear rate; Dpeak, peak diameter; Ddiff, change in diameter; FMD, flow-mediated dilation. Data are mean ± SD for baseline and post values, except for adjusted FMD, which are mean ± SE. Change scores are mean ± SE.
| Baseline |
Post |
Change |
||||
|---|---|---|---|---|---|---|
| Variable | Exercise | Immersion | Exercise | Immersion | Exercise | Immersion |
| Dbase (mm) | 6.6 ± 0.8 | 6.8 ± 0.9 | 7.0 ± 0.9 † | 6.7 ± 1.0 | +0.4 ± 0.1 | −0.0 ± 0.1 |
| Total SR (s−1) | 71 ± 28 | 78 ± 34 | 175 ± 74 † | 259 ± 118 *† | +104 ± 23 | +181 ± 23 |
| Antegrade SR (s−1) | 101 ± 32 | 108 ± 38 | 185 ± 70 † | 265 ± 112 *† | +85 ± 22 | +157 ± 22 |
| Retrograde SR (s−1) | −30 ± 9 | −30 ± 10 | −6 ± 12 † | −6 ± 7 † | +24 ± 4 | +24 ± 4 |
| Dpeak (mm) | 6.9 ± 0.9 | 7.1 ± 0.9 | 7.3 ± 1.0 † | 7.0 ± 1.0 | +0.4 ± 0.1 | −0.1 ± 0.1 |
| Ddiff (mm) | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 | −0.0 ± 0.1 | −0.0 ± 0.1 |
| Dbase-adjusted FMD (%) | 4.75 ± 0.83 | 4.69 ± 0.83 | 4.31 ± 0.83 | 4.71 ± 0.83 | −0.41 ± 1.11 | +0.01 ± 1.10 |
interaction: intervention x time (p<0.05)
different from baseline (p<0.05).
Figure 2.
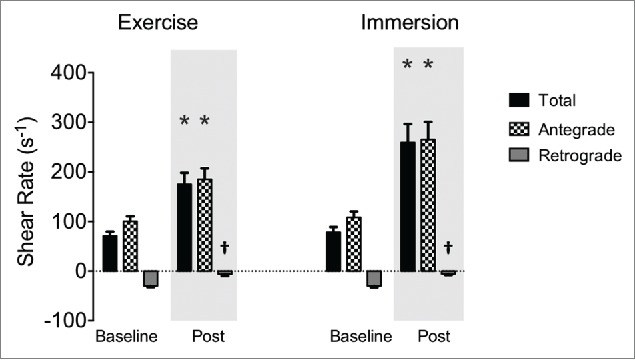
Superficial femoral artery total (black bars), antegrade (checked bars) and retrograde (gray bars) shear rate at baseline and post-intervention. Bars represent group mean, error bars are SE. * interaction: intervention x time (p<0.05); † different from baseline (p<0.05).
Flow-mediated dilation
Baseline diameter (Dbase, i.e., before FMD) was increased following exercise (+0.40 ± 0.11 mm, p < 0.001, see Table 2 and Fig. 3), but did not change following immersion (−0.04 ± 0.11 mm, p = 0.713, interaction: p = 0.005, Fig. 3). The FMD was unrelated to Dbase before or after either intervention (all p ≥ 0.256). Irrespective of analyses used, FMD was not reliably affected between interventions (all p ≥ 0.640) or across time (all p ≥ 0.584), and showed large individual variability (Fig. 4). FMD results are presented as Dbase-adjusted FMD% based on the methods suggested by Atkinson and Batterham.51,52
Figure 3.
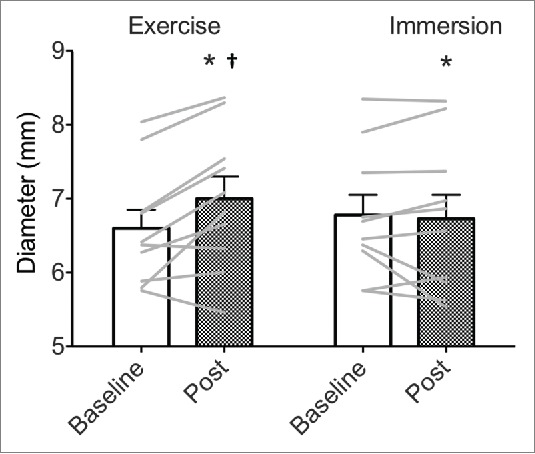
Absolute superficial femoral artery diameter (mm) at baseline and in response to exercise and water immersion. Bars represent group mean, error bars are SE, gray lines are individual data. * interaction: intervention x time (p < 0.05); † different from baseline (p < 0.05).
Figure 4.
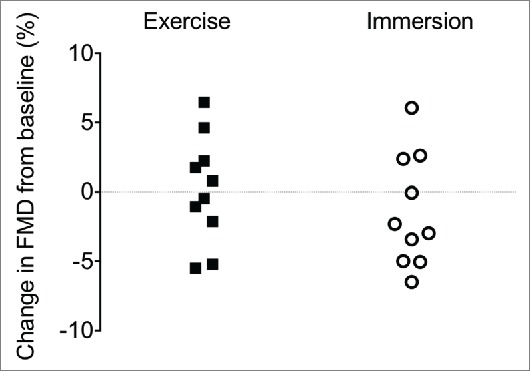
Individual absolute change in superficial femoral artery flow-mediated dilation (FMD, %) in response to exercise and water immersion.
Systemic cardiovascular responses
The stress-induced rise in HR was approximately twice as large during exercise than immersion (interaction p < 0.001; Table 1). was elevated following exercise (+2.1 ± 0.5 L·min-1, p < 0.001) but the changes post-immersion did not reach statistical significance (+1.0 ± 0.5 L·min-1, p = 0.056). For SV, there was no differential response following each intervention (interaction: p = 0.400), nor an effect of intervention per se (p ≥ 0.275). The MAP tended to show a hypotensive response following immersion only (interaction: p = 0.068, immersion MAP: −8 ± 3 mmHg, p = 0.012; exercise: 0 ± 3 mmHg, p = 0.944), with 9 out of 10 participants having a reduction in MAP following immersion. The SBP was reduced by ∼10 ± 4 mmHg following immersion (p = 0.008) but was unchanged following exercise (1 ± 4 mmHg; interaction: p = 0.041). The DBP responses to interventions were unclear (interaction: p = 0.134).
Discussion
Shear stress is a principal determinant of arteries' acute responses and adaptive remodeling (reviewed in Laughlin4 and Newcomer53). The shear stress effects of heating and exercise have been studied mostly in the upper limb yet the lower limb is susceptible to arterial disease,37 so we examined SFA shear rate responses to acute lower-limb heating via hot-water immersion, and also relative to a typical bout of predominantly lower-limb exercise. Understanding the acute responses during and/or following transient stress such as heat or exercise is important for at least 3 reasons. First, heat is part of exercise, so delineating effects of heat within or apart from exercise has mechanistic value. Second, acute responses mediate long-term adaptation, so understanding these responses improves knowledge of adaptation. Third, a major portion of the health-related benefits of regular bouts of stress is attributable to the recovery period itself, (e.g., prolonged post-exercise hypotension is likely more important in cardiovascular risk reduction than is the small adaptive reduction in resting blood pressure induced by exercise training). We were interested also in systemic cardiovascular responses because the potential for lower-limb heating to provide therapeutic benefit is largely unknown as this intervention has not been comprehensively described or directly compared to exercise.
Our main findings were that: 1) antegrade shear rate in the SFA increased at least 2-fold following each of the stressors, and to a greater extent following hot-water immersion than exercise; 2) core and muscle temperatures were also higher after immersion; and 3) acute endothelium-dependent vasodilation in the SFA was not consistently altered by either intervention.
Shear rate
Increased antegrade shear rate is an important driver in vascular adaptation. To our knowledge, this is the first time that SFA shear rate has been assessed and demonstrated to be increased substantially in response to lower-limb heating. The peak SFA shear rate induced by lower-limb heating in this study (265 s−1, 95%CI: 196 to 335 s−1) was within the range of brachial shear rates that have been associated with acute and chronic improvements in FMD (∼200 s−1 during handgrip exercise7 and forearm heating,5 ∼230 s−1 during cycling5), but lower than others (∼350 s−1 during forearm heating,10 ∼450 s−1 during cycling8). However, in the literature there is considerable variation in brachial shear rate between very similar forearm heating protocols (∼200 s−15 vs. ∼350 s−110). Contextualising the shear changes demonstrated here is therefore challenging, as comparative data in the lower-limb vessels are scarce – the lower-limb arteries appear to be persistently overlooked in studies of shear rate manipulation. However, a human MRI study found smaller mean and peak shear rates for the SFA than brachial artery, by ∼33% and ∼20%, respectively,54 so the lower SFA shear rates found in our study than in some studies on the brachial artery may be anticipated.
It remains unknown whether a threshold exists above which shear rate must be increased to induce adaptation, and if so, whether it differs between limbs or in healthy versus diseased vessels. Until such evidence is available in humans, the implications of our shear stress findings (Table 2) are difficult to quantify or comment on. Importantly though, in vitro studies on animal models indicate that endothelial nitric oxide synthase upregulation in endothelial cells responds to shear stress in a dose-dependent manner.55 Therefore, any increase in antegrade shear rate may induce some adaptation, if repeated appropriately.
The lesser increase in shear rate when measured after exercise than after immersion possibly reflects a methodological bias. The peak of the exercise induced-shear response was likely to have been missed by our protocol because the largely-metabolic stimulus was already decaying following exercise, whereas the heat stimulus following lower-limb heating was still mostly present. However, the range of shear rates reported in this study for both immersion and exercise were not dissimilar to those exhibited in other studies manipulating shear in conduit vessels,5,7 as mentioned above. The lower shear rate after exercise could also be partly due to the increased Dbase induced by the exercise. Furthermore, the difference in Tc and/or Tm could contribute to the shear rates generated, as the immersion protocol resulted in higher temperatures in both the core and muscle; however a matched-temperature study design would be required to resolve this, and is a possible future research direction. Regardless of the comparison between interventions however, the finding of an elevated shear rate, in particular antegrade, following lower-limb heating is significant due to the important role of repetitive antegrade shear stress in promoting vascular adaptation.
Systemic cardiovascular strain
Knowledge of the MAP-lowering effects of hot-water immersion alone, regardless of the comparison with exercise, is valuable because post-stress hypotension is important in its own right and for mediating cardiovascular adaptations, as mentioned above and described below. The heating protocol was effective in inducing a hypotensive effect in recovery whereas exercise was not in this study. The average MAP reduction post-immersion of 8 mmHg (95%CI: −3 to −12 mmHg) was similar to that found previously following passive heating.24 Whether repetitive heating (e.g., a 6-week conditioning intervention) would lower baseline blood pressure is unknown.
As hypotension appears necessary for expanding plasma volume,56 heat stress may be a useful such stimulus. Convertino et al.19 demonstrated that thermal effects alone accounted for 40% of the exercise-induced plasma volume expansion. Larger blood volume, even via the plasma alone, improves cardiovascular function at rest and across the range of cardiovascular capacity, by increasing stroke volume,57 lowering heart rate and increasing aerobic capacity.58,59 The significant reductions in MAP demonstrated here may be important in this role, although the dose-response relations of acute hypotensive duration and magnitude required to provoke this hypervolemia effect are not yet fully known.
Hot-water immersion additionally increased heart rate and tended to increase cardiac output, as occurs with various forms of passive heat stress.24,28 Increased heart rate and cardiac output with a concomitant, albeit non-significant, reduction in blood pressure would indicate the heat stimulus provides an attenuated increase in cardiac workload compared to that for an equivalent increase in heart rate during exercise.27 This increased cardiac work plus the increased heart temperature as discussed below, could be interpreted as a gentler but perhaps appropriate stressor for those with contraindications to exercise, i.e., increased cardiac work pertaining to volume and temperature changes rather than pressure changes. Overall, these results indicate that hot-water immersion may have potential to induce at least some of the acute hemodynamic and cardiovascular effects associated with exercise.
Temperature
Core and muscle temperature increased significantly, and to a greater extent upon completing immersion than exercise. As mentioned above, this difference may have impacted on the shear rate generated following interventions, but regardless of the temperature-dependence of the shear rate response, heating of the heart and skeletal muscle can mediate other beneficial adaptations including muscle growth and stress response proficiency.20,24,25 The thermal impulse for the ‘core’ and gastrocnemius during early recovery was ∼3 times larger following the passive warming, but as for the shear stress response, it is unresolved as to whether this would confer a similarly-larger stimulus for adaptation; e.g., what is the threshold perturbation, and for which adaptation in which tissues? Nevertheless, it seems reasonable to conclude that lower-limb heating may be a suitable stimulus to induce such temperature elevations independent of exercise.
Flow-mediated dilation
FMD has been used as a “vascular health” outcome in thousands of studies. Despite its widespread usage, a systemic understanding of FMD (and its components) to different stimuli (exercise36 and discrete stressors) is still lacking. The lower-limb arteries are seldom studied; FMD is assessed predominantly in the brachial artery, yet we are often most interested in the lower limbs for reasons described in the Introduction. Where it has been looked at, the arterial function in the upper limb appears to be a poor predictor of that in the lower limb.64 Furthermore, much remains unknown about the acute vascular effects of exercise (or other stressors); in particular the relationship of the shear stimulus, the resultant acute FMD response, and the associated long-term adaptation. This study provided a unique opportunity to assess the acute responses of 2 different interventions targeting the same outcome response (i.e., increased antegrade shear in the SFA). Moreover, no comparative data exist for the effect of heating on lower-limb FMD. Only one study to our knowledge has looked at the effect of acute exercise on lower-limb FMD; this was within one hour of finishing a marathon, and femoral FMD was reduced.60
With this background in mind, a hypothesis regarding the likely SFA FMD change was difficult, as the acute effects of exercise (and even more so, other interventions) on FMD (in the SFA as well as other arteries) are both equivocal and insufficiently characterized at present. These effects depend on timing of measurement, intensity, duration and mode of exercise, the cohort studied and factors such as diameter changes intrinsic to the study.36 Indeed, conflicting data exist, with several acute exercise studies reporting no change in FMD with an increase in Dbase,60,61 as in this study, although others have described a reduction in FMD with increased Dbase following exercise.62,63 Due to the paucity of comparative data for our study, we did not expect to be able to formulate a hypothesis on the lower-limb responses based on previous findings of upper-limb FMD responses during similar stress.
Several factors may explain the lack of change in FMD. Oxidative stress generated during exercise, particularly higher-intensity exercise, has been suggested to explain the reported reduction in FMD following exercise. However, exercise training improves antioxidant status;64 therefore, given that our participants typically exercised at least 3 d per week, they may have been better able to tolerate the acute oxidative stimulus generated by the intervention, resulting in little or no perturbation of FMD. Similarly, the exercise was of moderate intensity (65-75% age-predicted HR maximum), which may not have induced much oxidative response in this cohort. Substantial variability in both Dbase and FMD responses would also conceal any small effect of either intervention. The variation shown here seemed large (Table 2 and Fig. 4); however most studies of this nature do not adequately present individual variation, making comparison difficult. There were no significant correlations evident between FMD and shear rate or diameter to help explain this variability (data not presented). Acute changes in diameter, although controlled for here with the allometric scaling, may present a limitation for the use and interpretation of FMD in an experimental crossover setting.
Application
Some individuals may stand to benefit from such a stressor more than others; for example, patients with peripheral arterial disease, who have a severely limited exercise capacity as a consequence of their condition. Repeated dry sauna therapy in a peripheral arterial disease cohort demonstrated a significant reduction in symptoms and an improvement in several measures of leg perfusion;65,66 however, mechanisms for this and measures of cardiovascular response were not included. Patients with heart failure have also been treated with sauna resulting in improved cardiac function and reduction of arrhythmias.15,30,31 The extent to which heat can provide health benefits in clinical groups appears promising but has not yet been fully explored.
Conclusion
Heat, administered by sitting with the lower limbs immersed in hot water may have potential to be used as a stand-alone stressor, at least as a way to induce transient increased peripheral artery shear rate, increased core and muscle temperature, and transitory hypotension. The lower-limb heating protocol was well-tolerated in this young, healthy group. Future studies should focus on the tolerance and physiological responses to a passive heat stimulus such as this in other population groups, and importantly, the adaptations to repetitive exposure. Patient populations, who have much to gain from exercise but often have a compromised ability to perform exercise, would benefit from a potent and time-efficient means of inducing the health-related adaptations of exercise by such alternative methods.
Abbreviations
- BP
blood pressure
- Dbase
baseline diameter
- DBP
diastolic blood pressure
- Dpeak
peak diameter
- FMD
flow-mediated dilation
- HR
heart rate
- MAP
mean arterial pressure
- Q˙
cardiac output
- SBP
systolic blood pressure
- SFA
superficial femoral artery
- SR
shear rate
- SV
stroke volume
- Tc
core temperature
- Tm
muscle temperature
- ΔT
change in temperature
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol 2005; 46:9-15; PMID:15807389 [PubMed] [Google Scholar]
- [2].Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 1986; 250:H1145-9; PMID:3487253 [DOI] [PubMed] [Google Scholar]
- [3].Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodi7lator response to increased flow in vivo. Hypertension 1986; 8:37-44; PMID:3080370; http://dx.doi.org/ 10.1161/01.HYP.8.1.37 [DOI] [PubMed] [Google Scholar]
- [4].Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 2008; 104:588-600; PMID:18063803; http://dx.doi.org/ 10.1152/japplphysiol.01096.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 2009; 54:278-85; PMID:19546374; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.109.134361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 2008; 586:5003-12; PMID:18755749; http://dx.doi.org/ 10.1113/jphysiol.2008.158014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010; 55:312-8; PMID:20048193; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.109.146282 [DOI] [PubMed] [Google Scholar]
- [8].Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 2012; 112:1653-8; PMID:22403347; http://dx.doi.org/ 10.1152/japplphysiol.01489.2011 [DOI] [PubMed] [Google Scholar]
- [9].Carter HH, Dawson EA, Birk GK, Spence AL, Naylor LH, Cable NT, Thijssen DH, Green DJ. Effect of SR manipulation on conduit artery dilation in humans. Hypertension 2013; 61:143-50; PMID:23150517; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.112.197277 [DOI] [PubMed] [Google Scholar]
- [10].Naylor LH, Carter H, Fitzsimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 2011; 300: H664–9; PMID:21131471. [DOI] [PubMed] [Google Scholar]
- [11].Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc 2009; 41:1072-9; PMID:19346980; http://dx.doi.org/ 10.1249/MSS.0b013e3181923957 [DOI] [PubMed] [Google Scholar]
- [12].Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 2014; 114:859-65; PMID:24399113 [DOI] [PubMed] [Google Scholar]
- [13].Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 2010; 588:1571-7; PMID:20211982; http://dx.doi.org/ 10.1113/jphysiol.2010.186965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 2001; 38:1083-8; PMID:11583886; http://dx.doi.org/ 10.1016/S0735-1097(01)01467-X [DOI] [PubMed] [Google Scholar]
- [15].Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 2002; 39:754-9; PMID:11869837; http://dx.doi.org/ 10.1016/S0735-1097(01)01824-1 [DOI] [PubMed] [Google Scholar]
- [16].Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, Orihara K, Kihara T, Tei C. Repeated thermal therapy upregulates arterial endothelial nitric oxide synthase expression in Syrian golden hamsters. Jpn Circ J 2001; 65:434-8; PMID:11348049; http://dx.doi.org/ 10.1253/jcj.65.434 [DOI] [PubMed] [Google Scholar]
- [17].Akasaki Y, Miyata M, Eto H, Shirasawa T, Hamada N, Ikeda Y, et al.. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J 2006; 70:463-70; PMID:16565566 [DOI] [PubMed] [Google Scholar]
- [18].Scoon GS, Hopkins WG, Mayhew S, Cotter JD. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J Sci Med Sport 2007; 10:259-62; PMID:16877041; http://dx.doi.org/ 10.1016/j.jsams.2006.06.009 [DOI] [PubMed] [Google Scholar]
- [19].Convertino VA, Greenleaf JE, Bernauer EM. Role of thermal and exercise factors in the mechanism of hypervolemia. J Appl Physiol 1980; 48:657-64. [DOI] [PubMed] [Google Scholar]
- [20].Oishi Y, Hayashida M, Tsukiashi S, Taniguchi K, Kami K, Roy RR, Ohira Y. Heat stress increases myonuclear number and fiber size via satellite cell activation in rat regenerating soleus fibers. J Appl Physiol 2009; 107:1612-21; PMID:19556452; http://dx.doi.org/ 10.1152/japplphysiol.91651.2008 [DOI] [PubMed] [Google Scholar]
- [21].Yamane M, Teruya H, Nakano M, Ogai R, Ohnishi N, Kosaka M. Post-exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. Eur J Appl Physiol 2006; 96:572-80; PMID:16372177; http://dx.doi.org/ 10.1007/s00421-005-0095-3 [DOI] [PubMed] [Google Scholar]
- [22].Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol 2011; 110:451-7; PMID:21148343; http://dx.doi.org/ 10.1152/japplphysiol.00849.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 2009; 39:643-62; PMID:19769414; http://dx.doi.org/ 10.2165/00007256-200939080-00003 [DOI] [PubMed] [Google Scholar]
- [24].Iguchi M, Littmann AE, Chang SH, Wester LA, Knipper JS, Shields RK. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train 2012; 47:184-90; PMID:22488284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Staib JL, Quindry JC, French JP, Criswell DS, Powers SK. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am J Physiol Regul Integr Comp Physiol 2007; 292:R432-9; PMID:16990482; http://dx.doi.org/ 10.1152/ajpregu.00895.2005 [DOI] [PubMed] [Google Scholar]
- [26].Laughlin MH. Cardiovascular response to exercise. Am J Physiol 1999; 277:S244-59; PMID:10644251 [DOI] [PubMed] [Google Scholar]
- [27].Vuori I. Sauna bather's circulation. Ann Clin Res 1988; 20:249-56; PMID:3218896 [PubMed] [Google Scholar]
- [28].Crandall CG, Gonzalez-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf) 2010; 199:407-23; PMID:20345414; http://dx.doi.org/ 10.1111/j.1748-1716.2010.02119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 2008; 586:293-301; PMID:17962331; http://dx.doi.org/ 10.1113/jphysiol.2007.143057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kihara T, Biro S, Ikeda Y, Fukudome T, Shinsato T, Masuda A, Miyata M, Hamasaki S, Otsuji Y, Minagoe S, et al.. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J 2004; 68:1146-51; PMID:15564698; http://dx.doi.org/ 10.1253/circj.68.1146 [DOI] [PubMed] [Google Scholar]
- [31].Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 1995; 91:2582-90; PMID:7743620; http://dx.doi.org/ 10.1161/01.CIR.91.10.2582 [DOI] [PubMed] [Google Scholar]
- [32].Miyamoto H, Kai H, Nakaura H, Osada K, Mizuta Y, Matsumoto A, Imaizumi T. Safety and efficacy of repeated sauna bathing in patients with chronic systolic heart failure: a preliminary report. J Card Fail 2005; 11:432-6; PMID:16105634; http://dx.doi.org/ 10.1016/j.cardfail.2005.03.004 [DOI] [PubMed] [Google Scholar]
- [33].Joyeux-Faure M, Arnaud C, Godin-Ribuot D, Ribuot C. Heat stress preconditioning and delayed myocardial protection: what is new? Cardiovasc Res 2003; 60:469-77; PMID:14659792; http://dx.doi.org/ 10.1016/j.cardiores.2003.08.012 [DOI] [PubMed] [Google Scholar]
- [34].Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 2015; 175:542-8; PMID:25705824; http://dx.doi.org/ 10.1001/jamainternmed.2014.8187 [DOI] [PubMed] [Google Scholar]
- [35].Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ . Relationship between Upper and Lower Limb Conduit Artery Vasodilator Function in Humans. J Appl Physiol 2011; 111:244-50; PMID:21512151; http://dx.doi.org/ 10.1152/japplphysiol.00290.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 2013; 115:1589-98; PMID:24030665; http://dx.doi.org/ 10.1152/japplphysiol.00450.2013 [DOI] [PubMed] [Google Scholar]
- [37].McMillan DE. Blood flow and the localization of atherosclerotic plaques. Stroke 1985; 16:582-7; PMID:2411027; http://dx.doi.org/ 10.1161/01.STR.16.4.582 [DOI] [PubMed] [Google Scholar]
- [38].2008 Physical Activity Guidelines for Americans US. In: Services. DoHaH, editor 2008. [Google Scholar]
- [39].Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 2004; 109:2507-10; PMID:15136499; http://dx.doi.org/ 10.1161/01.CIR.0000128207.26863.C4 [DOI] [PubMed] [Google Scholar]
- [40].Hijmering ML, de Lange DW, Lorsheyd A, Kraaijenhagen RJ, van de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med 2007; 65:29-35; PMID:17293637 [PubMed] [Google Scholar]
- [41].Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 2005; 109:55-60; PMID:15799717; http://dx.doi.org/ 10.1042/CS20040358 [DOI] [PubMed] [Google Scholar]
- [42].Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010; 235:111-8; PMID:20404025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mekjavic IB, Rempel ME. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol 1990; 69:376-9; PMID:2394660 [DOI] [PubMed] [Google Scholar]
- [44].Gagge AP, Stolwijk JA, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 1969; 2:209-29; PMID:5788908; http://dx.doi.org/ 10.1016/0013-9351(69)90037-1 [DOI] [PubMed] [Google Scholar]
- [45].Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 2004; 97:499-508; PMID:15064302; http://dx.doi.org/ 10.1152/japplphysiol.01245.2003 [DOI] [PubMed] [Google Scholar]
- [46].Gemignani V, Bianchini E, Faita F, Giannarelli C, Plantinga Y, Ghiadoni L, Demi M. Ultrasound measurement of the brachial artery flow-mediated dilation without ECG gating. Ultrasound Med Biol 2008; 34:385-91; PMID:17964069; http://dx.doi.org/ 10.1016/j.ultrasmedbio.2007.08.006 [DOI] [PubMed] [Google Scholar]
- [47].Gemignani V, Faita F, Ghiadoni L, Poggianti E, Demi M. A system for real-time measurement of the brachial artery diameter in B-mode ultrasound images. IEEE Trans Med Imaging 2007; 26:393-404; PMID:17354644; http://dx.doi.org/ 10.1109/TMI.2006.891477 [DOI] [PubMed] [Google Scholar]
- [48].Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300:H2-12; PMID:20952670; http://dx.doi.org/ 10.1152/ajpheart.00471.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Taylor NAS, Cotter JD. Heat adaptation: Guidelines for the optimisation of human performance. International SportMed J 2006; 7:33-57. [Google Scholar]
- [50].Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 2013; 226:425-7; PMID:23261170; http://dx.doi.org/ 10.1016/j.atherosclerosis.2012.11.027 [DOI] [PubMed] [Google Scholar]
- [51].Atkinson G, Batterham AM. The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med 2013; 18:354-65; PMID:24172228; http://dx.doi.org/ 10.1177/1358863X13508446 [DOI] [PubMed] [Google Scholar]
- [52].Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 2013; 31:287-91; PMID:23169234; http://dx.doi.org/ 10.1097/HJH.0b013e32835b8164 [DOI] [PubMed] [Google Scholar]
- [53].Newcomer SC, Thijssen DH, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle crosstalk: Role of exercise-induced hemodynamics. J Appl Physiol (1985) 2011; 111:311-20. [DOI] [PubMed] [Google Scholar]
- [54].Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging 2004; 19:188-93; PMID:14745752; http://dx.doi.org/ 10.1002/jmri.10441 [DOI] [PubMed] [Google Scholar]
- [55].Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG . Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 1995; 269:C1371-8; PMID:8572165 [DOI] [PubMed] [Google Scholar]
- [56].Hayes PM, Lucas JC, Shi X. Importance of post-exercise hypotension in plasma volume restoration. Acta Physiol Scand 2000; 169:115-24; PMID:10848641; http://dx.doi.org/ 10.1046/j.1365-201x.2000.00728.x [DOI] [PubMed] [Google Scholar]
- [57].Hopper MK, Coggan AR, Coyle EF. Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol (1985) 1988; 64:404-8; PMID:2451658 [DOI] [PubMed] [Google Scholar]
- [58].Luetkemeier MJ, Thomas EL. Hypervolemia and cycling time trial performance. Med Sci Sports Exerc 1994; 26:503-9; PMID:7515456; http://dx.doi.org/ 10.1249/00005768-199404000-00016 [DOI] [PubMed] [Google Scholar]
- [59].Berger NJ, Campbell IT, Wilkerson DP, Jones AM. Influence of acute plasma volume expansion on VO2 kinetics, VO2 peak, and performance during high-intensity cycle exercise. J Appl Physiol (1985) 2006; 101:707-14; PMID:16690793; http://dx.doi.org/ 10.1152/japplphysiol.00154.2006 [DOI] [PubMed] [Google Scholar]
- [60].Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, Gaze D, Shave RE, Wilson M, George KP, et al.. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 2008; 105:1562-8; PMID:18719231; http://dx.doi.org/ 10.1152/japplphysiol.90837.2008 [DOI] [PubMed] [Google Scholar]
- [61].Rognmo O, Bjornstad TH, Kahrs C, Tjonna AE, Bye A, Haram PM, Stølen T, Slørdahl SA, Wisløff U. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res 2008; 22:535-42; PMID:18550971; http://dx.doi.org/ 10.1519/JSC.0b013e31816354b1 [DOI] [PubMed] [Google Scholar]
- [62].Bailey TG, Birk GK, Cable NT, Atkinson G, Green DJ, Jones H, Thijssen DH. Remote Ischemic Preconditoning Prevents Reduction in Brachial Artery Flow Mediated Dilation after Strenuous Exercise. Am J Physiol Heart Circ Physiol 2012; 303:H533-8; PMID:22730390; http://dx.doi.org/ 10.1152/ajpheart.00272.2012 [DOI] [PubMed] [Google Scholar]
- [63].Hwang IC, Kim KH, Choi WS, Kim HJ, Im MS, Kim YJ, Kim SH, Kim MA, Sohn DW, Zo JH. Impact of acute exercise on brachial artery flow-mediated dilatation in young healthy people. Cardiovasc Ultrasound 2012; 10:39; PMID:23031621; http://dx.doi.org/ 10.1186/1476-7120-10-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Finaud J, Lac G, Filaire E. Oxidative stress : relationship with exercise and training. Sports Med 2006; 36:327-58; PMID:16573358; http://dx.doi.org/ 10.2165/00007256-200636040-00004 [DOI] [PubMed] [Google Scholar]
- [65].Tei C, Shinsato T, Miyata M, Kihara T, Hamasaki S. Waon therapy improves peripheral arterial disease. J Am Coll Cardiol 2007; 50:2169-71; PMID:18036456; http://dx.doi.org/ 10.1016/j.jacc.2007.08.025 [DOI] [PubMed] [Google Scholar]
- [66].Shinsato T, Miyata M, Kubozono T, Ikeda Y, Fujita S, Kuwahata S, Akasaki Y, Hamasaki S, Fujiwara H, Tei C. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol 2010; 56:361-6; PMID:20843662; http://dx.doi.org/ 10.1016/j.jjcc.2010.08.004 [DOI] [PubMed] [Google Scholar]


