ABSTRACT
The present study determined whether 0.8g/kg bodyweight sago ingested before (Pre-Sago) or during (Dur-Sago) exercise under warm-humid conditions (30 ± 2°C, 78 ± 3 % RH; 20 km·h−1 frontal airflow) conferred a performance and/or physiological benefit compared to a control (Control) condition. Eight trained, male cyclists/triathletes (45 ± 4 y, VO2peak: 65 ± 10 ml·kg−1·min−1, peak aerobic power: 397 ± 71 W) completed 3 15-min time-trials (∼75% VO2peak) pre-loaded with 45 min of steady-state (∼55% VO2peak) cycling following > 24 h standardization of training and diet. Measures of work completed, rectal and mean skin temperatures, heart rate, expiratory gases and venous blood samples were taken. Compared to Control, Pre-Sago resulted in a smaller rise in rectal temperature (0.3 ± 0.5°C) while heart rate increased to a greater extent (6 ± 13 beats·min−1) during exercise (both P < 0.05), however, compared to Control time-trial performance remained unaffected (Pre-Sago: −0.5 ± 4.0%, P > 0.05). During exercise, plasma glucose concentrations were maintained higher for Dur-Sago than Control (P < 0.05), however substrate oxidation rates remained similar (P > 0.05). Dur-Sago also resulted in a higher plasma sodium concentration (2 ± 2 mmol·l1) and lower whole-body sweat loss (544 ± 636 g) and, therefore, reduced plasma volume contraction (all P < 0.05). Heart rate increased to a greater extent (5 ± 13 beats·min−1) during Dur-Sago, yet compared to Control time-trial performance remained unaffected (+0.9 ± 2.3%, P > 0.05). Uniquely, these results indicate that during exercise heat stress feeding sago can result in some ‘beneficial’ physiological responses, however these do not translate to changes in exercise performance when performed in a post-prandial state.
KEYWORDS: exercise, malaysia, starch, tropical heat
Introduction
Consuming carbohydrate (CHO) before and/or during prolonged exercise can delay the onset of fatigue and improve work output and capacity.1 The main goals for CHO supplementation are to fill skeletal muscle and liver glycogen stores prior to exercise and to provide exogenous glucose during prolonged exercise; the latter to partly offset skeletal muscle and central nervous system CHO requirements when glycogen stores run low. The combination of an appropriate CHO composition and administration regimen can subsequently deliver major benefits to endurance sport performance; 2 hence the prescriptive use of CHO-containing sports drinks.3
Glycogen takes some hours to form following CHO ingestion, and cannot be manufactured and stored while a muscle fiber is contracting. Thus, CHO ingestion in the hour before exercise practically provides the only opportunity to “top up” hepatic glycogen stores and maximize exogenous carbohydrate availability as exercise begins. The performance effects of CHO ingestion in the hour prior to exercise are varied but are generally positive despite an oft-seen transient hypoglycaemia as exercise begins.4
Many major sporting events take place during the summer, in warm environments or at the hottest part of a day.5 During exercise with heat stress, there is consensus that performance is decreased and there is an increased risk of heat illness, especially with high humidity.6 Heat stress during exercise also results in alterations in CHO metabolism. Febbraio7 concluded that heat stress increases CHO and decreases fat utilization, while Jeukendrup8 concurs that the ambient environmental conditions can affect substrate utilization at rest or during exercise. For example, Yaspelkis and Ivy9 demonstrated that exercise in the heat accelerated fatigue because of an increase in reliance upon CHO as a substrate, while Jentjens et al.10 demonstrated that when ambient temperatures increase so does CHO oxidation during exercise largely due to an increased muscle glycogen use. There have been consistent reports of CHO supplementation proving ergogenic during exercise heat stress, even for shorter (< 1h), more intense (∼80% VO2max) bouts (see refs. 11-12), however the mechanism(s) responsible remain poorly understood and others have found no such effect (see ref. 13).
Where commercially available CHO products are not necessarily affordable or accessible to those competing in sport or exercise, there is a need to investigate local food sources as suitable alternatives. Sago (Metroxylin sagu) palms grow all over Southeast Asia, a region with over 600,000,000 inhabitants and a year-round tropical climate. Where there is insufficient rain to grow wet rice, sago palms are used as staple foods. For example, in Malaysia sago starch is an important dietary CHO source14 with Malaysia, Indonesia and Papua New Guinea being the world's leading countries in the production of sago.15 In Sarawak, Malaysia, sago is widely used to produce sago pearls that can be boiled and consumed directly as a CHO source.
To date, there has been no investigation of sago meals ingested before or during exercise although some studies have tested starch meals such as waxy and corn starch (see refs. 16-17). Sago starch contains 27% amylose and 73% amylopectin18 and CHO supplementation consisting of a high amylopectin proportion before exercise has been shown to be equally beneficial as glucose, with less carbohydrate available to the active musculature when the starch is higher in amylose content due to a lower exogenous carbohydrate oxidation.16,17 Starch can be separated into 3 categories based on its digestibility19 and in this context sago is known as being rapidly digestible and quickly absorbed,20 again suggesting it should be suitable for consumption before/during exercise.
Therefore, the purpose of the present study was to determine whether sago ingestion before or during exercise under conditions of heat stress conferred a performance and/or physiological benefit(s) compared to a control condition. In order to collect both meaningful physiological data during a controlled steady-state period and include a measure of performance, in combination lasting greater than 45 minutes, the protocol developed and tested previously21 was used knowing that it is highly reliable (CV = 3.6%, ICC = 0.96) when using the same cohort and experimental control.
Materials and methods
Participants
Eight healthy, male cyclists and/or triathletes provided their informed, written consent to participate in the study. Their mean (SD) physical characteristics were, age: 45 (4) y, height: 1.77 (0.07) m, weight: 77 (10) kg, VO2peak: 65 (10) ml·kg−1·min−1, maximal heart rate: 176 (7) beats·min−1 and peak aerobic power: 397 (71) W. All participants were regularly cycling > 200 km·week−1 and participated in club-level cycling races. The study was approved by the Massey University Human Ethics Committee and performed in accordance with the 1975 Helsinki Declaration.
Experimental overview
All the participants visited the laboratory on 5 separate occasions: 1) preliminary submaximal and maximal tests, 2) experimental familiarization, 3-5) experimental trials. The experimental trials were completed using a randomized crossover design, with these trials separated by 7 days, conducted at the same time of day (± 1 h), and following 24 h of dietary and exercise control (see below for details). Trials consisted of a Control (nothing consumed), Pre-Sago (Sago consumed before exercise) and Dur-Sago (Sago consumed during exercise); a schematic diagram accompanying the following sections can be seen in Figure 1. All trials were completed on an electronically-braked cycle ergometer (Lode Excalibur, The Netherlands), where participants' set-up (e.g., seat/handle bar height and horizontal position, etc.) was customised and replicated for each subsequent visit.
Figure 1.
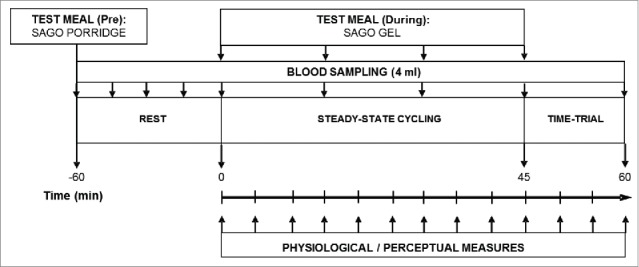
A schematic overview of the experimental protocol.
Preliminary testing and familiarization
Following body weight and height measurements, this session was conducted in a moderate laboratory environment (18-22°C) with a fan located in front of the participants with an airflow of 20 km·h−1. A submaximal test required the participant to cycle for 6 min at each of 4 consecutive submaximal power outputs, which were 100 W, 150 W, 200 W, and 250 W. Following 10 min rest, a ramp protocol (45 W·min−1 beginning at 100 W) until volitional fatigue was used to determine VO2peak. Expiratory gases were collected continuously for the determination of VO2, and heart rate recorded every minute. Following this, a linear relationship between the mean rate of VO2 during the last 2 min of each submaximal stage and power output was determined and used to calculate a power output which would elicit 55% (steady-state) and 75% (time-trial) of VO2peak for each participant for the remaining 3 trials.
The familiarization trial was undertaken to ensure participants were accustomed to the procedures employed during the investigation and to minimise any potential learning effects during the experimental trials. These trials replicated entirely the experimental trials outlined below.
Dietary and exercise control
The 24 hours prior to any experimental trial was marked by abstinence from alcohol and only habitual caffeine use (as abstinence would in itself confound from withdrawal effects). On the day before any experimental trial, participants' only exercise was when they attended the laboratory to complete a standardized training ride 60 min in duration at a fixed power output that elicited ∼65% of their maximum heart rate in moderate environment (18–22°C). Following this, they were provided with a standardized snack (1x Sanitarium UP & GO, New Zealand: 823 kJ providing 30.3 g carbohydrate, 8.3 g protein and 3.8 g fat) to be consumed immediately, dinner (2x Watties Snack Meals, New Zealand: 2100 kJ providing 42.0 g carbohydrate, 31.6 g protein and 22 g fat, and 1x One Square Meal, New Zealand: 1450 kJ providing 45.1 g carbohydrate, 8.4 g protein and 11.7 g fat) and breakfast (at least 2 but not more than 4 h prior to visiting the laboratory) for the day of the trial (1x Sanitarium UP & GO, New Zealand: 823 g providing 30.3 g carbohydrate, 8.3 g protein and 3.8 g fat, and 1x One Square Meal, New Zealand: 1450 kJ providing 45.1 g carbohydrate, 8.4 g protein and 11.7 g fat). This dietary and exercise control minimised any variation in pre-trial metabolic state and skeletal muscle glycogen level. Fluid was encouraged and available ad libitum to ensure adequate hydration. A euhydrated state was further ensured by instructing the participants to drink a pre-measured bolus of water (5 ml·kg−1 bodyweight) 2 hours prior to each trial.
Experimental procedure
These sessions were conducted in a thermally stressful environment at a dry-bulb temperature of 30 ± 2°C and relative humidity of 78 ± 3 °C with a fan located in front of the participants with an airflow of 20 km·h−1.
On arrival to the laboratory participants voided and they then self-inserted a rectal thermistor 10 cm beyond the anal sphincter. A cannula was inserted into a forearm vein and a 4 ml baseline (−60 min) venous blood sample collected. Following measurement of body weight participants were given their sago-porridge (Pre-Sago) or nothing (Dur-Sago and Control) and rested seated for a further hour, during which time blood samples were collected at −45, −30, and −15 minutes for the Pre-Sago and Control trials. Then participants entered the environmental chamber wearing only cycling shorts, shoes and socks. Once seated on the ergometer, the heart rate monitor was positioned across the chest and 4 skin surface thermistors were attached to the chest, arm, thigh, and calf on the right side of the body and connected to a USB-based Temperature Measurement Device. Resting values for all measurements were recorded.
Participants cycled for 45 min at the pre-determined power output that was estimated to elicit 55 %VO2peak with the ergometer set in the cadence-independent mode. Every 15-min a venous blood sample was obtained, every 10-min an expiratory gas sample was collected for 3 min, every 5 min heart rate and every 15 min Borg's rating of perceived exertion were recorded. Rectal and skin temperature readings were taken every 5 min throughout the trial. Tap water was provided to drink ad libitum in aliquots of 3 ml·kg−1 bodyweight either at 15-min intervals or when requested to minimise dehydration. Immediately on completion of the 45-min steady-state period, the ergometer was set to linear mode, based on the formula of Jeukendrup et al.,22 and participants were asked to complete as much work as possible in the 15 min with the only information received being when every 3 min had elapsed. Following completion of the time-trial, participants performed a low-intensity cool-down for at least 5 min where recovery was monitored.
Sago supplementation
For Pre-Sago 0.8g/kg bodyweight Sago was consumed as a bolus an hour before exercise whereas for Dur-Sago this same amount (0.8g/kg bodyweight) was divided into 4 equal amounts and consumed at 0, 15, 30 and 45 min during exercise. A dose of 0.8 g/kg bodyweight was chosen because it fits nicely within what is suggested by the ACSM Position Stand for CHO ingestion during exercise.23 For example, sago is ∼86% CHO w/w, therefore ingestion of sago at a rate of 0.8 g/kg bodyweight for a 75 kg person, equates to 52 grams per hour of CHO. Preparation of sago followed that by Ahmad et al.20 The sago pearls (Marsanta Foods Ltd. New Zealand) were prepared fresh by soaking in 790 ml distilled water and being left to stand for 10 min. Thereafter, the mixture was steam cooked for 25 min. Stirring of this mixture was done for about 2 min after 20 min of cooking. At the end of cooking, 7 ml artificial flavour and 0.8 g natural sweetener (Hansells Food Group Ltd, New Zealand) were added and left to cool to room temperature. This sago formulation was used for Pre-Sago while for Dur-Sago the sago was first ground before soaking (as above) – this provided a more malleable gel that easily fit into tubes for feeding directly into the mouth (squeezing) while on the bike. Proximate analysis was performed on a sample of 100 g cooked sago, the results of which can be seen in Table 1.
Table 1.
Basic nutrient composition in 100 g of cooked sago.
| % CHO | % Starch | % Fat | % Protein | Calculated Energy (kJ)/ 100 g |
|---|---|---|---|---|
| 11.3 |
10.5 |
0.1 |
0.2 |
199 |
|
% Ash |
% Moisture |
GE kJ/g |
% TDF |
Sugars g/100 g |
| < 0.1 | 88.3 | 2.0 | 0.1 | 0.03 |
GE: Gross Energy; TDF: total dietary fiber
Measurements
The subject's height and weight were measured using a stadiometer (Seca, Germany; accurate to 0.1 cm) and scale (Jadever, Taiwan; accurate to 0.01 kg). The calibrated skin thermistors (Grant Instruments Ltd., Cambridgeshire, UK; accurate to 0.2°C) were secured in place with Transpore Surgical Tape (3 M Healthcare, St. Paul, Minnesota, USA). The skin and calibrated rectal (Covidien Mon-a-Therm, USA; accurate to 0.1°C) thermistors were then connected to a USB-based Temperature Measurement Device and displayed using TracerDAQ® software. Weighted mean skin temperature was calculated according to the equation of Ramanathan.24 Expiratory gases were collected and recorded via Turbofit (VacuMed Vista Turbofit, USA) metabolic software for determination of minute ventilation, oxygen uptake and carbon dioxide production and hence the respiratory exchange ratio (RER); all values as STPD. Prior to each experimental trial, the instrument received a 2-point calibration using a zero and a known gas mixture (β-standard: O2 15.01%, CO2 5.02%) and volume (VacuMed 3L Calibration Syringe, USA). Substrate oxidation rates (g·min−1) were calculated from indirect calorimetry measurements using the stoichiometric equation proposed by Jeukendrup and Wallis,25 assuming a nonprotein contribution. Borg's rating of perceived exertion scale was used;26 the scale ranges from 6 (no exertion) to 20 (maximal exertion). Participants were familiarised with the scale during the submaximal and peak exercise tests. For venous blood sampling the cannula (BD Venflon I.V Cannula, Sweden) was kept patent by regular flushing with 3 ml of sterile saline (sodium chloride 0.9% IV-IM; Multichem NZ Ltd., New Zealand). At each time-point, the initial 2 ml drawn was discarded and then 4 ml blood was collected into a lithium heparin containing vacutainer (Becton-Dickinson, UK). Whole blood was used to measured hemoglobin concentration (HemoCue® Hb+ 201 System, Sweden) and a capillary tube (Heparinized Capillary, USA) was filled (in duplicate) for determination of haematocrit by micro-centrifugation. From the changes in haematocrit and hemoglobin concentrations from rest to the end of exercise, percentage change in plasma volume was estimated using the formula described by Dill and Costill.27 The remaining whole blood was then centrifuged at 4°C and 805 g for 15 min. Following this, aliquots of plasma were transferred into Eppendorf tubes (Genuine Axygen Quality, USA) and stored at −80°C until further analysis. Plasma glucose, lactate, sodium and potassium concentrations were measured using an automated analyzer (ABL FLEX, Radiometer, Denmark) with a repeatability of ≤ 0.1 mmol/L.
Data and statistical analyses
All statistical analyses were performed with SPSS software for windows (IBM SPSS Statistics 20, NY, USA). Descriptive values were obtained and reported as means and standard deviation (SD) unless stated otherwise. A Shapiro-Wilk test was used to ensure data did not differ substantially from a normal distribution. Time-trial performance (work completed), changes in body weight (sweat loss) and plasma volume were analyzed by one-way (trial) ANOVA whereas all other measures were analyzed by 2-way (trial x time) ANOVA for repeated measures with post-hoc pairwise analyses performed using a paired samples t-test (Bonferroni correction if appropriate) where main or interaction effects occurred, with statistical significance set at P < 0.05. Sphericity was assessed and where the assumption of sphericity could not be assumed, adjustments to the degrees of freedom were made (ϵ > 0.75 = Huynh-Feldt; ϵ < 0.75 = Greenhouse-Geisser).
Results
All eight participants were able to complete all experimental trials in ambient temperature and relative humidity (RH) as follows: Control (29.9 ± 1.7°C and 77.9 ± 3.1% RH), Pre-Sago (30.0 ± 1.4°C and 76.4 ± 4.3% RH), and Dur-Sago (29.9 ± 1.6°C and 78.3 ± 2.8% RH) trials.
Time trial performance
The average work completed in the 15-min time trial for the Control, Pre-Sago and Dur-Sago trials was 221 ± 33 kJ, 222 ± 31 kJ and 219 ± 32 kJ, respectively (Fig. 2). This equated to a non-significant (P > 0.05) improvement of 0.5 ± 4.0% (Pre-Sago) and decrement of 0.9 ± 2.3% (Dur-Sago) when compared to the Control, respectively. Similarly, there were no differences in pacing strategies between conditions (P > 0.05).
Figure 2.
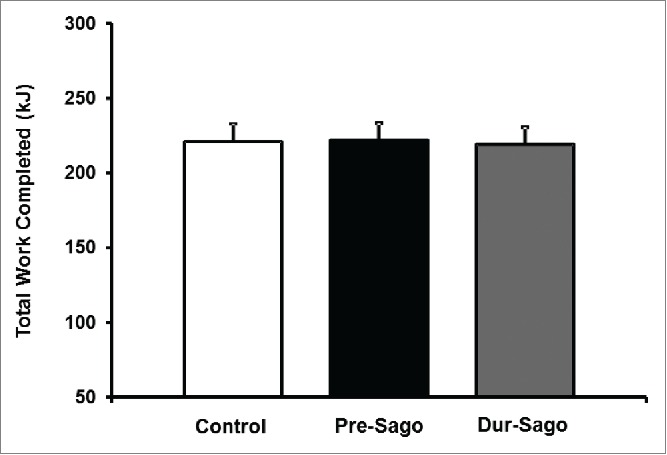
Total work completed (kJ) during the 15-min time-trial for Control, Pre-Sago and Dur-Sago Trial. Data are expressed as mean ± SE.
Metabolic responses
Plasma glucose and lactate concentration responses for all trials are presented in Figure 3. There was no difference in baseline values for plasma glucose between trials, however an interaction effect (time*treatment) was observed (P < 0.05). During rest (−60 to 0 min), glucose concentrations remained stable in Control, whereas they increased during the first 30 min in Pre-Sago such that concentrations were higher than Control from −45 until −15 min. During exercise (0 to 60 min), glucose concentrations increased above basal levels from 30 (Control), 45 (Dur-Sago) and at 60 min (Pre-Sago), such that concentrations were higher than Control at 45 min (Dur-Sago). Similarly, there was no difference in baseline values for plasma lactate between trials, however an effect of time was observed (P < 0.05) such that for all 3 conditions lactate concentrations were only elevated above resting following the time-trial (60 min).
Figure 3.
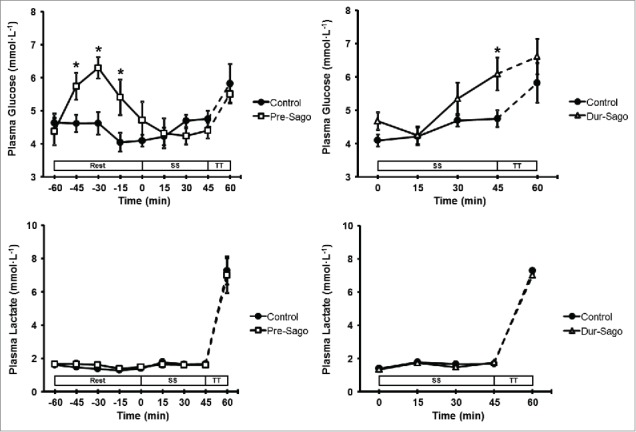
Plasma glucose and lactate concentration during rest, steady-state cycling (SS) and 15-min time trial (TT) for Control, Pre-Sago and Dur-Sago trials. Data are expressed as mean ± SE. * indicates significantly different to Control at that time-point (p < 0.05).
The RER and substrate oxidation rates can be seen in Table 2. None of RER, CHO or fat oxidation during the 45-min steady-state exercise showed any main effects of time, trial or an interaction (P > 0.05).
Table 2.
Carbohydrate (CHO) and fat oxidation rates and RER during steady-state exercise.
| Time (min) | |||||
|---|---|---|---|---|---|
| 5 | 15 | 25 | 35 | 45 | |
| CHO Oxidation (g·min−1) | |||||
| Control | 2.8 ± 0.3 | 2.6 ± 0.3 | 2.5 ± 0.4 | 2.6 ± 0.3 | 2.7 ± 0.3 |
| Pre-Sago | 2.8 ± 0.4 | 2.6 ± 0.3 | 2.8 ± 0.4 | 2.9 ± 0.4 | 2.8 ± 0.3 |
| Dur-Sago | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.6 ± 0.5 |
| Fat Oxidation (g·min−1) | |||||
| Control | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Pre-Sago | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 |
| Dur-Sago | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| RER | |||||
| Control | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.92 ± 0.01 | 0.93 ± 0.02 | 0.91 ± 0.02 |
| Pre-Sago | 0.94 ± 0.01 | 0.95 ± 0.02 | 0.94 ± 0.01 | 0.94 ± 0.02 | 0.94 ± 0.02 |
| Dur-Sago | 0.92 ± 0.01 | 0.93 ± 0.02 | 0.92 ± 0.02 | 0.92 ± 0.01 | 0.92 ± 0.03 |
Data are presented as mean ± SE; N = 8
Thermoregulatory responses
Thermoregulatory measures of rectal and mean skin temperatures are depicted in Figure 4. There was no difference in baseline values for rectal temperature between trials, however a time*treatment interaction effect was observed (P < 0.05). During exercise rectal temperature increased above basal levels from 5 (Control) and 15 (Dur-Sago and Pre-Sago) minutes and continued to rise, such that rectal temperature was higher than Control between 5 and 25 min (Pre-Sago), with changes of 2.1 ± 0.5, 1.8 ± 0.4 and 2.0 ± 0.6°C for Control, Pre-Sago and Dur-Sago, respectively. Similarly, there was no difference in baseline values for mean skin temperatures between trials, however an effect of time was observed (P < 0.05) such that for all 3 conditions mean skin temperature increased until 15 min and plateaued thereafter.
Figure 4.
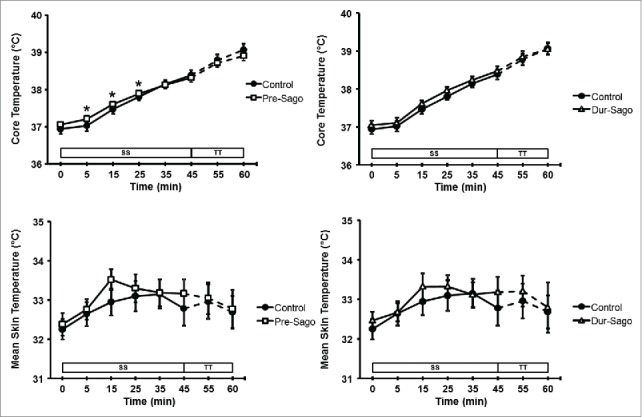
Core and mean skin temperature at rest, during steady-state cycling (SS) and 15-min time trial (TT) for Control, Pre-Sago and Dur-Sago trials. Data are expressed as mean ± SE. * indicates significantly different to Control at that time-point (p < 0.05).
Water consumption was not different between trials (P > 0.05) at 752 ± 267, 656 ± 306 and 622 ± 302 ml for Control, Pre-Sago and Dur-Sago, respectively. However, whole-body sweat loss was smaller (P < 0.05) for Dur-Sago (1236 ± 398 g) than Control (1704 ± 583 g) with neither different to Pre-Sago (1486 ± 331 g), which led to a smaller decrease in plasma volume (P < 0.05) following Dur-Sago (−4 ± 8%) than Control (−15 ± 11%) but neither was different to Pre-Sago (−9 ± 18%).
Plasma concentrations of sodium showed an effect of trial (P < 0.05), such that values for sodium were higher for Dur-Sago (140 ± 5 mmol·l−1) than Control (138 ± 4 mmol·l−1) and Pre-Sago (137 ± 6 mmol·l−1), while plasma concentrations of potassium showed an effect of time (P < 0.05) with values increasing above resting values (3.8 ± 0.4 mmol·l−1) from 15 min onwards, reaching 4.6 ± 0.5 mmol·l−1 by 60 min.
Cardiorespiratory and perceptual responses
There was no difference in baseline values for heart rate between trials, however a trial*time interaction effect was observed (P < 0.05). During exercise heart rate increased progressively although to a greater extent in Pre-Sago (5 min: 119 ± 11 beats·min−1, 45 min: 139 ± 16 beats·min−1, 60 min: 172 ± 6 beats·min−1) and Dur-Sago (5 min: 118 ± 11 beats·min−1, 45 min: 141 ± 17 beats·min−1, 60 min: 171 ± 9 beats·min−1) compared to Control (5 min: 116 ± 13 beats·min−1, 45 min: 132 ± 18 beats·min−1, 60 min: 169 ± 10 beats·min−1), such that heart rate was lower during Control from 15-25 min and at 45 min compared to both sago treatments. Similarly, there was no difference in 5-min values for ventilation between trials (61 ± 8 l·min−1), however an effect of time was observed (P < 0.05) such that for all 3 conditions ventilation increased until 25 min and plateaued thereafter (69 ± 8 l·min−1). Finally, perceived exertion during steady-state exercise remained constant (11.3 ± 1.3 units) but increased (P < 0.05) following the time-trial (16.7 ± 1.4 units), with no difference between trials or any interaction (both P > 0.05).
Discussion
This is the first study to determine whether sago, a starch staple found across Southeast Asia and prepared through boiling pearls into porridge/gel, influences cycling performance under conditions that simulate a tropical environment i.e. warm-humid. The main finding is that in a post-prandial state feeding sago before or during such exercise does not confer any performance benefit (or detriment) when compared to a control. However, there was a smaller reduction in plasma volume found when consuming sago during steady-state exercise through reduced whole-body sweating, with a concomitant higher plasma sodium concentration. Heart rate was also higher when sago was ingested either before or during exercise compared to control. Lastly, core temperature was greatest at the beginning of exercise when sago was ingested prior, but then in this trial the rise in core temperature was attenuated compared to the control condition.
We have previously demonstrated that using the same experimental standardization in trained, familiarized males a protocol of 45-min steady-state at 55% VO2peak followed by a 15-min time trial at ∼75% of VO2peak in warm-humid conditions is a highly reliable protocol, with a test-retest coefficient of variation of 2-4% for physiological and performance variables.21 Therefore, it can be said with confidence that the observed similarities in time-trial performance in this study are real. The careful exercise and dietary control applied not only likely isolated the effects of our dietary intervention but also mimicked typical pre-competition behavior i.e., reduced physical activity and a CHO-rich diet to ensure skeletal muscle and hepatic glycogen stores are filled. While ecologically-valid however, the consumption of a low-GI breakfast 2-3 hours prior to their experimental trial could well have overwhelmed the effects of a more subtle sago intervention, at least when comparing timing of ingestion (pre- vs during). Importantly, many previous studies (see refs. 11-13) that have investigated CHO supplementation during exercise heat stress had participants complete their exercise following an overnight fast. This may go some way in explaining any performance discrepancy as the maintenance of glycaemia becomes more challenging in overnight fasted exercise,28 while at the same time the contracting muscle's CHO requirement increases in the heat.7
The (resting) glycaemic response to (pre-exercise) sago ingestion is similar to that found previously with ingestion of other high GI foods, and confirms that sago is quickly absorbed and metabolised to glucose (i.e. has a high GI, see ref. 20). That plasma glucose was then slightly depressed as exercise progressed with pre-ingestion compared to the control condition again replicates previous work29 and likely reflects the combined effects of insulin- and contraction-mediated glucose uptake. That being the case, we expected RER to be greater in Pre-Sago (reflecting increased CHO uptake and oxidation by muscle), but this was not found. It may be that a whole-body measure such as RER does not have the sensitivity to resolve the small increase in muscle CHO uptake and utilization that a single meal would bring, or that insulin-induced inhibition of hepatic glucose release explains the early exercise transient hypoglycaemia. In contrast, when ingested only during exercise (Dur-Sago), plasma glucose was higher than control; again this has been observed elsewhere with ingestion of a CHO supplement with similar physical properties.13 It is less surprising, in this trial, that RER was not significantly different from control, as insulin release is blunted during exercise.30
Perhaps the most interesting observation was that when sago was supplemented during exercise (Dur-Sago), fluid regulation was altered, with a higher plasma sodium concentration, attenuated reduction in plasma volume and lower whole-body sweat rate. Carbohydrate consumption could enhance fluid retention through one of 2 potential mechanisms31: (1) gastric contents with a higher energy density and osmolality decrease the rate of emptying and absorption,32,33 that in turn could cause a slower movement of fluid into the bloodstream, sustain a higher plasma osmolality and attenuate urine production, and/or (2) the insulinaemic response invoked by carbohydrate ingestion has been shown to increase urinary sodium reabsorption.34 The latter is less likely, because as previously noted insulin release is reduced with CHO ingestion once exercise begins.
That heart rate was increased during both types of sago feeding can be explained by the additional digestive load (see ref. 35). Additionally, that rectal temperature was higher at the start of exercise with Pre-Sago, one hour following consumption, can be explained by dietary-induced thermogenesis (see ref. 36). However, our observation of a subsequent attenuated rise in rectal temperature when sago was consumed prior to exercise (Pre-Sago) was unexpected and contrary to previous reports (see ref. 37). Our measures most closely related to metabolic heat production and loss (i.e., work completed, mean skin temperature and sweat loss) for Pre-Sago were not different to Control, and while it has previously been demonstrated that hyperosmolality (see previous paragraph: sago and fluid regulation) elevates the threshold for sweating38 this would result in the opposite effect. Therefore, it remains to be determined whether this effect can be explained by more specific measures not taken within this study (i.e. local sweat rate, skin blood flow) or as a consequence of non-thermal factors.
Although the primary aim with the current study was to identify whether there was any difference between supplementing sago at different time-points i.e., before versus during exercise, we used a control condition where nothing was consumed to see if sago ingestion at either time was beneficial or detrimental. Had we observed (more) significant differences, especially ones pertaining to performance, the next logical step would be to assess this against a suitable and known CHO source (e.g., Pre-Sago vs. pasta, Dur-Sago versus glucose) to determine efficacy. However, the resting glycaemic response to sago ingestion (Fig. 3) showing that sago is quickly digested to glucose and absorbed (high GI), indicates that supplementing sago following exercise may be beneficial when recovering for subsequent exercise bouts.
In summary, the present study has shown that while consuming equal total volumes of sago porridge an hour before and sago gel during exercise in warm-humid conditions does confer some physiological benefit, these are not ergogenic above a control condition when in a post-prandial state.
Abbreviations
- CHO
Carbohydrate
- CV
Coefficient of variation
- ICC
Intraclass correlation coefficient
- RER
Respiratory exchange ratio
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Burke LM, Hawley JA. Carbohydrate and exercise. Curr Opin Clin Nutr Metab Care 1999; 6:515-20; http://dx.doi.org/ 10.1097/00075197-199911000-00015 [DOI] [PubMed] [Google Scholar]
- [2].Vandenbogaerde TJ, Hopkins WG. Effects of acute carbohydrate supplementation on endurance performance a meta-analysis. Sports Med 2011; 41:773-92; PMID:21846165; http://dx.doi.org/ 10.2165/11590520-000000000-00000 [DOI] [PubMed] [Google Scholar]
- [3].Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American college of sports medicine position stand. exercise and fluid replacement. Med Sci Sports Exerc 2007; 39:377-90; PMID:17277604; http://dx.doi.org/ 10.1249/01.mss.0000272779.34140.3b [DOI] [PubMed] [Google Scholar]
- [4].Hawley JA, Burke LM, Angus DJ, Fallon KE, Martin DT, Febbraio MA. Effect of altering substrate availability on metabolism and performance during intense exercise. Br J Nutr 2000; 84:829-38; PMID:11177199; http://dx.doi.org/ 10.1017/S0007114500002440 [DOI] [PubMed] [Google Scholar]
- [5].Burke LM. Nutritional needs for exercise in the heat. Comp Biochem Physiol A Mol Integr Physiol 2001; 128:735-48; PMID:11282317; http://dx.doi.org/ 10.1016/S1095-6433(01)00279-3 [DOI] [PubMed] [Google Scholar]
- [6].Wendt D, van Loon LJ, Lichtenbelt WD. Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med 2007; 37:669-82; PMID:17645370; http://dx.doi.org/ 10.2165/00007256-200737080-00002 [DOI] [PubMed] [Google Scholar]
- [7].Febbraio MA. Alterations in energy metabolism during exercise and heat stress. Sports Med 2001; 31:47-59; PMID:11219501; http://dx.doi.org/ 10.2165/00007256-200131010-00004 [DOI] [PubMed] [Google Scholar]
- [8].Jeukendrup AE. Modulation of carbohydrate and fat utilization by diet, exercise and environment. Biochem Soc Trans 2003; 31:1270-3; PMID:14641041; http://dx.doi.org/ 10.1042/bst0311270 [DOI] [PubMed] [Google Scholar]
- [9].Yaspelkis BB, Ivy JL. Effect of carbohydrate supplements and water on exercise metabolism in the heat. J Appl Physiol 1991; 71:680-7; PMID:1938742 [DOI] [PubMed] [Google Scholar]
- [10].Jentjens RL, Wagenmakers AJ, Jeukendrup AE. Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol 2002; 92:1562-72; PMID:11896023; http://dx.doi.org/ 10.1152/japplphysiol.00482.2001 [DOI] [PubMed] [Google Scholar]
- [11].Below PR, Mora-Rodríguez R, González-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc 1995; 27:200-10; PMID:7723643; http://dx.doi.org/ 10.1249/00005768-199502000-00009 [DOI] [PubMed] [Google Scholar]
- [12].Carter J, Jeukendrup AE, Mundel T, Jones DA. Carbohydrate supplementation improves moderate and high-intensity exercise in the heat. Pflug Arch 2003; 446:211-9; PMID:12739159; http://dx.doi.org/ 10.1007/s00424-003-1020-4 [DOI] [PubMed] [Google Scholar]
- [13].Febbraio MA, Murton P, Selig SE, Clark SA, Lambert DL, Angus DJ, Carey MF. Effect of CHO ingestion on exercise metabolism and performance in different ambient temperatures. Med Sci Sports Exerc 1996; 28:1380-7; PMID:8933488; http://dx.doi.org/ 10.1097/00005768-199611000-00006 [DOI] [PubMed] [Google Scholar]
- [14].Abd-Aziz S. Sago starch and its utilisation. J Biosci Bioeng 2002; 94:526-9; PMID:16233345; http://dx.doi.org/ 10.1016/S1389-1723(02)80190-6 [DOI] [PubMed] [Google Scholar]
- [15].Singhal RS, Kennedy JF, Gopalakrishnan SM, Kaczmarek A. Industrial production, processing, and utilization of sago palm-derived products. Carbohyd Polym 2008; 72:1-20; http://dx.doi.org/ 10.1016/j.carbpol.2007.07.043 [DOI] [Google Scholar]
- [16].Goodpaster BH, Costill DL, Fink WJ, Trappe TA, Jozsi AC, Starling RD, Trappe SW. The effects of pre-exercise starch ingestion on endurance performance. Int J Sports Med 1996; 17:366-72; PMID:8858409; http://dx.doi.org/ 10.1055/s-2007-972862 [DOI] [PubMed] [Google Scholar]
- [17].Saris WH, Goodpaster BH, Jeukendrup AE, Brouns F, Halliday D, Wagenmakers AJ. Exogenous carbohydrate oxidation from different carbohydrate sources during exercise. J Appl Physiol 1993; 75:2168-72; PMID:8307875 [DOI] [PubMed] [Google Scholar]
- [18].Mohamed A, Jamilah B, Abbas KA, RAR, Roselina K. A review on physicochemical and thermorheological properties of sago starch. Am J Agric Biol Sci 2008; 3:639-46; http://dx.doi.org/ 10.3844/ajabssp.2008.639.646 [DOI] [Google Scholar]
- [19].Sands AL, Leidy HJ, Hamaker BR, Maguire P, Campbell WW. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr Res 2009; 29:383-90; PMID:19628104; http://dx.doi.org/ 10.1016/j.nutres.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmad H, Singh R, Ghosh AK. Glycaemic & insulinaemic responses in men at rest following sago meal. Indian J Med Res 2009; 130:160-5; PMID:19797813 [PubMed] [Google Scholar]
- [21].Che Jusoh MR, Morton RH, Stannard SR, Mündel T. A reliable preloaded cycling time trial for use in conditions of significant thermal stress. Scand J Med Sci Sports 2015; 25 Suppl 1:296-301; PMID:25943681; http://dx.doi.org/ 10.1111/sms.12332 [DOI] [PubMed] [Google Scholar]
- [22].Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc 1996; 28:266-70; PMID:8775164; http://dx.doi.org/ 10.1097/00005768-199602000-00017 [DOI] [PubMed] [Google Scholar]
- [23].Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc 2009; 41:709-31; PMID:19225360; http://dx.doi.org/ 10.1249/MSS.0b013e31890eb86 [DOI] [PubMed] [Google Scholar]
- [24].Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 1964; 19:531-3; PMID:14173555 [DOI] [PubMed] [Google Scholar]
- [25].Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 2005; 26 (Suppl 1):S28-37; PMID:15702454; http://dx.doi.org/ 10.1055/s-2004-830512 [DOI] [PubMed] [Google Scholar]
- [26].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377-81; PMID:7154893 [PubMed] [Google Scholar]
- [27].Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974; 37:247-8; PMID:4850854 [DOI] [PubMed] [Google Scholar]
- [28].Thomas DE, Brotherhood JR, Brand JC. Carbohydrate Feeding before Exercise: Effect of Glycemic Index. Int J Sport Med 1991; 12:180-6; PMID:1860741; http://dx.doi.org/ 10.1055/s-2007-1024664 [DOI] [PubMed] [Google Scholar]
- [29].Stannard SR, Constantini NW, Miller JC. The effect of glycemic index on plasma glucose and lactate levels during incremental exercise. Int J Sport Nutr Exerc Metab 2000; 10:51-61; PMID:10722781 [DOI] [PubMed] [Google Scholar]
- [30].Galbo H. Hormonal and Metabolic Adaptations to Exercise. Stuttgart: Georg Thieme Verlag, 1983 [Google Scholar]
- [31].Osterberg KL, Pallardy SE, Johnson RJ, Horswill CA. Carbohydrate exerts a mild influence on fluid retention following exercise-induced dehydration. J Appl Physiol 2010; 108:245-50; PMID:19940093; http://dx.doi.org/ 10.1152/japplphysiol.91275.2008 [DOI] [PubMed] [Google Scholar]
- [32].Vist GE, Maughan RJ. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol 1995; 486:523-31; PMID:7473216; http://dx.doi.org/ 10.1113/jphysiol.1995.sp020831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gisolfi CV, Summers RW, Lambert GP, Xia T. Effect of beverage osmolality on intestinal fluid absorption during exercise. J Appl Physiol 1998; 85:1941-8; PMID:9804602 [DOI] [PubMed] [Google Scholar]
- [34].Quiñones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995; 268:E1-5; PMID: 78401652510692 [DOI] [PubMed] [Google Scholar]
- [35].Kelbaek H. Intrinsic heart function and food intake. Arch Mal Coeur Vaiss 1989; 82:45-8; PMID:2510692 [PubMed] [Google Scholar]
- [36].Glickman N, Mitchell HH. The total specific dynamic action of high-protein and high-carbohydrate diets on human subjects. J Nutr 1948; 36:41-57; PMID:18868796 [DOI] [PubMed] [Google Scholar]
- [37].Horswill CA, Stofan JR, Lovett SC, Hannasch C. Core temperature and metabolic responses after carbohydrate intake during exercise at 30 degrees C. J Athl Train 2008; 43:585-91; PMID:19030136; http://dx.doi.org/ 10.4085/1062-6050-43.6.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol Respir Environ Exerc Physiol 1984; 57:1688-1695; PMID:6511544 [DOI] [PubMed] [Google Scholar]


