Abstract
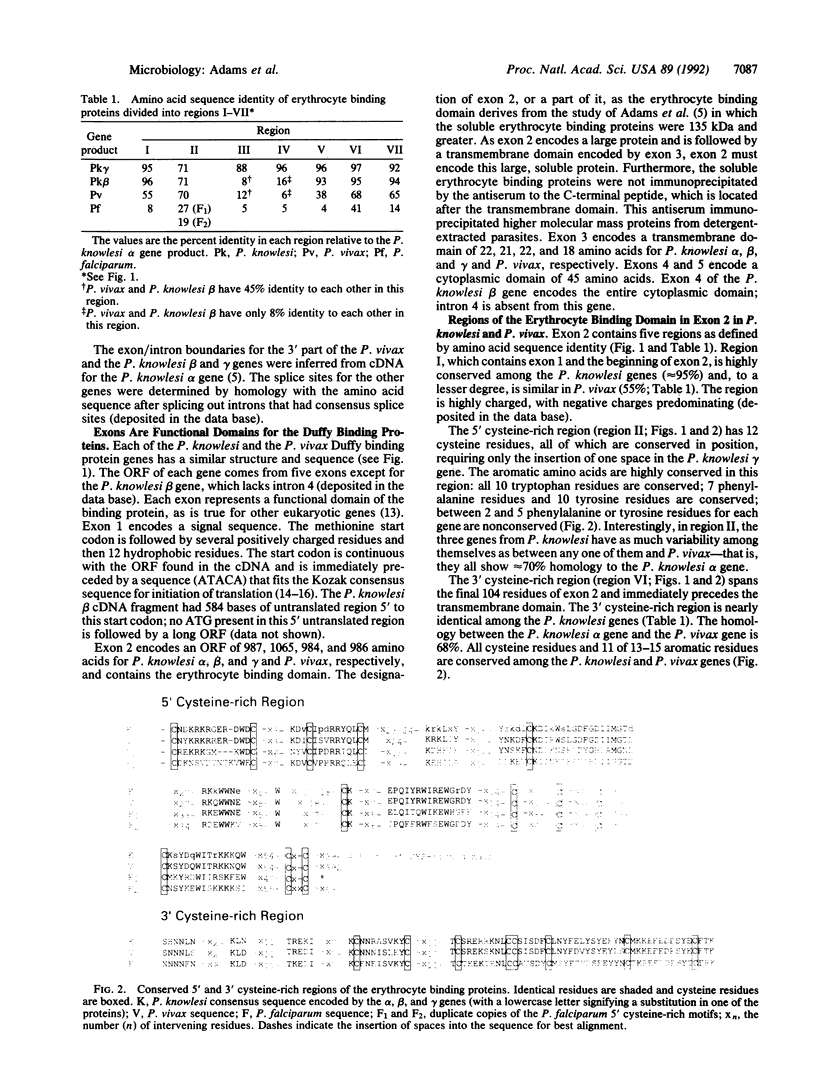
Malaria erythrocyte binding proteins use the Duffy blood group antigen (Plasmodium vivax and Plasmodium knowlesi) and sialic acid (Plasmodium falciparum) on the erythrocyte surface as receptors. We had previously cloned the one P. vivax gene, the one P. falciparum gene, and part of one of the three P. knowlesi genes encoding these erythrocyte binding proteins and described the homology between the P. knowlesi and P. vivax genes. We have completed the cloning and sequencing of the three P. knowlesi genes and identified introns in the P. vivax and P. falciparum genes that correct the previously published deduced amino acid sequences. All have similar structures, with one or two exons encoding the signal sequence and the erythrocyte binding domain, an exon encoding the transmembrane domain, and two exons encoding the cytoplasmic domain with the exception of the P. knowlesi beta gene. The regions of amino acid sequence homology among all the genes are the 5' and 3' cysteine-rich regions of the erythrocyte binding domain. On the basis of gene structure and amino acid homology, we propose that the Duffy binding proteins and the sialic acid binding protein are members of a gene family. The level of conservation (approximately 70%) of the deduced amino acid sequences in the 5' cysteine-rich region between the P. vivax protein and the three P. knowlesi proteins is as great as between the three P. knowlesi proteins themselves; the P. knowlesi beta protein just 3' to this cysteine-rich region is homologous to the P. vivax protein but not to the other P. knowlesi proteins. Conservation of amino acid sequences among these organisms, separated in evolution, may indicate the regions where the adhesin function resides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Hudson D. E., Torii M., Ward G. E., Wellems T. E., Aikawa M., Miller L. H. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell. 1990 Oct 5;63(1):141–153. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- Aley S. B., Bates M. D., Tam J. P., Hollingdale M. R. Synthetic peptides from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium knowlesi recognize the human hepatoma cell line HepG2-A16 in vitro. J Exp Med. 1986 Dec 1;164(6):1915–1922. doi: 10.1084/jem.164.6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. J., Coppel R. L. Primary structure of a Plasmodium falciparum rhoptry antigen. Mol Biochem Parasitol. 1991 Nov;49(1):99–110. doi: 10.1016/0166-6851(91)90133-q. [DOI] [PubMed] [Google Scholar]
- Camus D., Hadley T. J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985 Nov 1;230(4725):553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Dolan S. A., Miller L. H., Wellems T. E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990 Aug;86(2):618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. D., Kaslow D. C., Adams J. H., Miller L. H. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991 Jan;44(1):125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Marchionni M., McKnight G. On the antiquity of introns. Cell. 1986 Jul 18;46(2):151–153. doi: 10.1016/0092-8674(86)90730-0. [DOI] [PubMed] [Google Scholar]
- Goundis D., Reid K. B. Properdin, the terminal complement components, thrombospondin and the circumsporozoite protein of malaria parasites contain similar sequence motifs. Nature. 1988 Sep 1;335(6185):82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- Hadley T. J. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu Rev Microbiol. 1986;40:451–477. doi: 10.1146/annurev.mi.40.100186.002315. [DOI] [PubMed] [Google Scholar]
- Haynes J. D., Dalton J. P., Klotz F. W., McGinniss M. H., Hadley T. J., Hudson D. E., Miller L. H. Receptor-like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med. 1988 Jun 1;167(6):1873–1881. doi: 10.1084/jem.167.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Isaacs S. N., Quakyi I. A., Gwadz R. W., Moss B., Keister D. B. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991 May 31;252(5010):1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- Klotz F. W., Orlandi P. A., Reuter G., Cohen S. J., Haynes J. D., Schauer R., Howard R. J., Palese P., Miller L. H. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol. 1992 Mar;51(1):49–54. doi: 10.1016/0166-6851(92)90199-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Edwards S. J., Smith G. L., Mitchell G. F., Moss B., Kemp D. J., Anders R. F. Anchoring a secreted plasmodium antigen on the surface of recombinant vaccinia virus-infected cells increases its immunogenicity. Mol Cell Biol. 1986 Sep;6(9):3191–3199. doi: 10.1128/mcb.6.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A., Klotz F. W., Haynes J. D. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal- sequences of glycophorin A. J Cell Biol. 1992 Feb;116(4):901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Marshall V. M., Smythe J. A., Crewther P. E., Lew A., Silva A., Anders R. F., Kemp D. J. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989 Jul;9(7):3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich K. A., George F. W., 4th, Law J. L., Martin W. J. Cell-adhesive motif in region II of malarial circumsporozoite protein. Science. 1990 Sep 28;249(4976):1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Jennings M. W. The structure of the calmodulin gene of Plasmodium falciparum. Mol Biochem Parasitol. 1991 May;46(1):19–34. doi: 10.1016/0166-6851(91)90195-c. [DOI] [PubMed] [Google Scholar]
- Saul A., Battistutta D. Analysis of the sequences flanking the translational start sites of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Aug;42(1):55–62. doi: 10.1016/0166-6851(90)90112-y. [DOI] [PubMed] [Google Scholar]
- Sim B. K., Orlandi P. A., Haynes J. D., Klotz F. W., Carter J. M., Camus D., Zegans M. E., Chulay J. D. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990 Nov;111(5 Pt 1):1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim B. K., Toyoshima T., Haynes J. D., Aikawa M. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1992 Mar;51(1):157–159. doi: 10.1016/0166-6851(92)90211-2. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Godson G. N., Nussenzweig V., Nussenzweig R. S., Barnwell J., Moss B. Plasmodium knowlesi sporozoite antigen: expression by infectious recombinant vaccinia virus. Science. 1984 Apr 27;224(4647):397–399. doi: 10.1126/science.6200932. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Higgins D. G., McCutchan T. F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. P., Thomas A. W., Deans J. A., Mitchell G. H., Hudson D. E., Miller L. H., McCutchan T. F., Cohen S. A merozoite receptor protein from Plasmodium knowlesi is highly conserved and distributed throughout Plasmodium. J Biol Chem. 1990 Oct 15;265(29):17974–17979. [PubMed] [Google Scholar]
- Wertheimer S. P., Barnwell J. W. Plasmodium vivax interaction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol. 1989 Nov;69(4):340–350. doi: 10.1016/0014-4894(89)90083-0. [DOI] [PubMed] [Google Scholar]