ABSTRACT
Rodent models are frequently used to improve our understanding of the molecular mechanisms of pain and to develop novel analgesics. Robust behavioral assays that quantify nociceptive responses to different sensory modalities, such has heat, are therefore needed. Here, we describe a novel behavioral assay to quantify thermal paw withdrawal thresholds in mice, called the thermal probe test, and compared it with other methods commonly used to measure heat thresholds, namely the Hargreaves test and the dynamic and conventional hot plate tests. In the thermal probe test, a slightly rounded 2.5 mm diameter metal probe that heats on contact at a rate of 2.5°C/sec, is applied to the plantar surface of the hind paw in mice at a starting temperature of ∼37°C, and the temperature at which a withdrawal response occurs, designated as the paw withdrawal temperature, is automatically recorded. The thermal probe test is effective at quantifying thermal allodynia in carrageenan-induced inflammation (paw withdrawal temperature 3 h: contralateral, 50.3 ± 0.6°C; ipsilateral, 43.1 ± 1.0°C), burns injury (paw withdrawal temperature 3 d: contralateral, 50.8 ± 0.5°C; ipsilateral, 43.2 ± 0.6°C) and after topical capsaicin (paw withdrawal temperature: vehicle control, 49.7 ± 0.6°C; capsaicin, 44.8 ± 1.2°C), giving comparable results to the Hargreaves test. In addition, the thermal probe test can detect opioid mediated analgesia in carrageenan-induced inflammation. Therefore the thermal probe test is a novel behavioral assay effective for quantifying thermal allodynia and analgesia in mouse models of pain.
KEYWORDS: behavioral, Heat allodynia, heat thresholds, mouse model, nociception, thermal probe test
Introduction
Rodent models of pain are commonly used to improve our understanding of the molecular mechanisms underlying nociception and to test and develop novel analgesics. Therefore, robust behavioral assays that quantify nociceptive responses to different sensory modalities (mechanical, heat and cold) in these models are essential. Heat allodynia, which is pain provoked by a heat stimulus that is not normally painful, is present in many inflammatory and neuropathic pain states,1,2 and thus several behavioral assays have been developed to quantify responses to heat stimuli in mice and rats.
The most commonly used behavioral assays to measure withdrawal responses to heat stimuli in rodents are the tail flick test, hot plate test and Hargreaves test.3 In the tail flick test, the tail of an animal is exposed to either a radiant heat source or immersed in hot water, and the time taken to elicit a withdrawal response or ‘tail flick’ is measured.4,5 For the conventional hot plate test, a mouse or rat is placed on a metal surface, maintained at a constant temperature between 50–55°C, and the time taken to elicit a nociceptive response, such as paw lick, hind paw withdrawal or jump, is recorded.5 Alternatively, the conventional hot plate test can be modified to record the sum of nociceptive responses that occur at a single temperature over a short period of time.6 In the dynamic hot plate test, rather than exposing a mouse or rat to a constant temperature, the temperature of the plate is increased until a nociceptive response is observed, with the temperature that this occurs at dependent on the rate of heating.6,7 In the Hargreaves method, mice or rats are placed on heated glass and the plantar surface of one hind paw is exposed to a radiant heat source, with the time taken to elicit a withdrawal response measured.8 This permits measurement of ipsilateral (treated hind paw) and contralateral (non-treated hind paw) heat thresholds, with each animal serving as its own internal control, unlike in the hot plate tests where all 4 paws and the tail are in contact with the heated metal surface.
In this study, we describe a novel method to quantify thermal paw withdrawal thresholds in mice, named the thermal probe test. In the thermal probe test, a slightly rounded 2.5 mm diameter metal probe that heats at a rate of 2.5°C/sec is applied to the plantar surface of the hind paw in mice at a starting temperature of ∼37°C, and the temperature at which a withdrawal response occurs is recorded.
Intraplantar injection of carrageenan leads to the rapid development of inflammation, which is accompanied by mechanical and thermal allodynia, making it a useful model to study the anti-inflammatory and/or antinociceptive effects of novel compounds.9 We therefore evaluated the ability of the thermal probe test to detect thermal allodynia and analgesia in the carrageenan model, and compared it with the Hargreaves test and the dynamic and conventional hot plate tests. The thermal probe test was found to be effective for quantifying thermal allodynia and analgesia in the carrageen-induced model of inflammation, giving comparable results to the Hargreaves test, as well as being effective in quantifying heat allodynia in a mouse model of burns injury and after application of topical capsaicin.
Materials and methods
Animals
For behavioral assessment we used adult male C57BL/6J mice aged 6–8 weeks. Animals were housed in groups of 3 or 4 per cage under 12 h light-dark cycles, had standard rodent chow and water ad libitum, and were supplied with a red polycarbonate Mouse House (Tecniplast, Italy) and shredded paper nesting material for enrichment. Animals were acclimatized to the housing room (ambient temperature of 21–23°C) for at least one week prior to testing and the behavioral room (ambient temperature of 21–23°C) for at least one hour prior to testing.
Ethical approval for in vivo experiments in animals was obtained from the University of Queensland animal ethics committee. Experiments involving animals were conducted in accordance with the Animal Care and Protection Regulation Qld (2012), the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th edition (2013) and the International Association for the Study of Pain Guidelines for the Use of Animals in Research.
Thermal probe test
Mice (naïve, carrageneen-treated, burns-injured, or capsaicin-treated) were habituated in individual mouse runs for at least 5 min prior to testing. The test utilises a novel automated thermal probe device (MouseMet Thermal, Topcat Metrology Ltd, United Kingdom) consisting of a 2.5 mm diameter, slightly rounded, lead-free solder/brass probe that is mounted on the measurement arm of a MouseMet electronic von Frey transducer (Fig. 1). Heating of the probe is triggered when the device handle is rotated while the probe is lightly placed against the plantar surface of the mouse paw. The resultant force (∼1 g) depresses the measurement arm and initiates heating of the probe while in contact with the mouse's paw, ensuring consistent thermal transfer. The probe is preheated to ∼37°C before coming in contact with the paw, then once in contact heats at a rate of 2.5°C/sec, with a cut out set at 60°C to prevent tissue damage. Removal of the paw and/or the probe by the investigator terminates heating, and triggers display of the withdrawal temperature on the readout, without the need for the investigator to manually stop the heating or record the temperature, until the reset button is pressed. The temperature that elicited a paw withdrawal, known as the paw withdrawal temperature (PWT), was determined by a single test.
Figure 1.
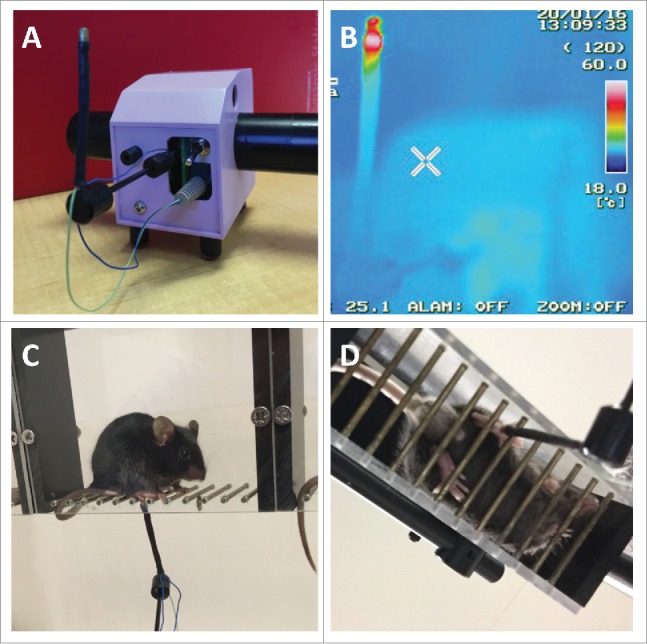
The thermal probe test apparatus. (A) Image of the thermal probe test apparatus (MouseMet Thermal). (B) Thermal image taken with an infrared camera (InfRec) of the thermal probe with heating triggered. (C) Side view and (D) bottom view of the thermal probe being applied to the plantar surface of a C57BL/6J mouse.
Hargreaves test
Mice (carrageenan-treated or burns-injured) were habituated individually in polyvinyl boxes (10 × 10 × 10 cm) placed on glass heated to 25°C for at least 30 min prior to behavioral assessment using the Hargreaves Test (Plantar Analgesia Meter, IITC, CA, USA). The radiant heat light source (intensity 15%) was focused on the plantar surface of each hind paw and the time taken for the mouse to withdraw the paw was recorded, with a cut off of 20 s to prevent tissue damage. The mean time to withdrawal was determined from the average of 3 tests, separated by at least 1 min.
Dynamic hot plate test
Mice (naïve or carrageenan-treated) were placed on a temperature-controlled Peltier plate (Hot/Cold Plate, Ugo Basile, Comerio, Italy) set at 42°C, with an increasing ramp of 3.3°C/min initiated as soon as the animal came in contact with the plate. The temperature at which a nociceptive response (hind paw lick, flinch or jump) was observed was recorded as the PWT and was determined from a single test.
Conventional hot plate test
Mice (naïve or carrageenan-treated) were placed on a temperature-controlled Peltier plate (Hot/Cold Plate, Ugo Basile, Comerio, Italy) set at 50°C, and the time taken to observe a nociceptive response (hind paw lick, flinch or jump) was recorded. The mean time to withdrawal was determined from the average of 3 tests, separated by at least 5 min.
Electronic von frey
Mechanical allodynia was assessed using an electronic von Frey apparatus (MouseMet Electronic von Frey, TopCat Metrology Ltd, United Kingdom). Mice (carrageenan-treated, burns-injured or capsaicin treated) were habituated in individual mouse runs for at least 5 min prior to testing. A soft-tipped 0.3 mm diameter flat ended polypropylene probe was applied to the plantar surface of each hind paw with pressure applied at a force rise rate of ∼1 g/s. The force that elicited paw withdrawal was calculated using the MouseMet Software. The paw withdrawal force (PWF) was determined from the average of 3 tests, separated by at least 2 min.
Carrageenan model
λ-Carrageenan (Sigma Aldrich, Castle Hill, NSW) was dissolved in phosphate buffered saline to a 1% w/v solution (prepared 24 h prior) and administered by intraplantar injection to the left hind paw of mice in a volume of 40 μL under light isoflurane (3%) anesthesia. Behavioral assessment was performed at the time points indicated. Different animals were used for each of the behavioral tests, except for the electronic von Frey and the thermal probe test, which were performed on the same animal.
The antinociceptive effects of oxycodone were assessed 5 h after injection of carrageenan. Animals were randomized to receive either oxycodone (3 mg/kg) (Mundipharma Pty Ltd, Sydney, NSW) or vehicle control (saline) administered by intraperitoneal (i.p.) injection in a volume of 10 μL/g 30 min prior to behavioral assessment, which was performed by a blinded investigator unaware of the treatment each individual animal received. The same animals were used for each of the behavioral tests.
Burn injury model
To induce a mild burn injury, the plantar skin of the left hind paw of mice was applied with firm pressure to a Peltier plate (Hot/Cold Plate, Ugo Basile, Comerio, Italy) set at 52.5°C for 25 s (modified protocol from rats)10 under light isoflurane (3%) anesthesia. Behavioral assessment was performed at the time points indicated. The same animals were used for each of the behavioral tests.
Capsaicin-induced thermal sensitivity
Capsaicin (Sigma Aldrich, Castle Hill, NSW) was dissolved in ethanol to a 1 mM solution and applied topically to the plantar skin of the left hind paw in mice with a cotton-tipped applicator under light isoflurane (3%) anesthesia. Animals were randomized to receive either capsaicin or vehicle control (ethanol). Behavioral assessment was performed 15 min post application by a blinded investigator unaware of the treatment each individual animal received. The same animals were used for each of the behavioral tests.
Data analysis
Data were plotted and analyzed by GraphPad Prism, version 6.0. Statistical significance was defined as P < 0.05 and was determined by unpaired t-test assuming equal variance. Data is expressed as the mean ± standard error of the mean (SEM).
Results
The thermal probe test is a novel behavioral assay that can quantify thermal paw withdrawal thresholds in mice
In naïve mice, application of the thermal probe test (Fig. 1) to the plantar surface of the hind paw lead to a consistent withdrawal response at approximately 50°C, with minimal variability between mice (PWT: 49.7 ± 0.4°C, 95 % confidence intervals 48.7–50.7°C; n = 8). The withdrawal response observed was similar to that seen in the Hargreaves test, where the paw is rapidly withdrawn from the heat stimulus, often accompanied by toe spreading, flinching or shaking. Application of the probe at the force required to trigger heating (∼1 g) for an equivalent amount of time (∼5 s) with the heat turned off causes no withdrawal response (data not shown).
The thermal probe test can quantify thermal allodynia in the carrageenan model
Following intraplantar injection of carrageenan, the thermal probe test detected a significant decrease in the temperature that elicited a withdrawal response in the carrageenan treated paw, evident 2 h after injection and peaking at 3 h post-injection (PWT 3 h: contralateral, 50.3 ± 0.6°C; ipsilateral, 43.1 ± 1.0°C; P < 0.05; Fig. 2A). Interestingly, the development of thermal allodynia did not coincide with the development of mechanical allodynia, which developed earlier and became evident at 0.5 h post-injection of carrageenan (PWF 0.5 h: contralateral, 3.4 ± 0.2 g; ipsilateral, 1.4 ± 0.1 g; P < 0.05; Fig. 2B). The development of thermal allodynia was similar in the Hargreaves test, with a significant reduction in paw withdrawal times evident at 2 h post-injection of carrageenan, and peaking at 3 h post-injection, consistent with time courses reported previously8,11 (time to withdrawal 3 h: contralateral, 12.2 ± 0.8 s; ipsilateral, 2.6 ± 0.4 s; P < 0.05; Fig. 2C). No significant differences between vehicle control and carrageenan mice were detected on the hot plate using either the dynamic hot plate test (PWT 5 h: vehicle control, 47.1 ± 0.1°C; carrageenan, 47.0 ± 0.4°C; P > 0.05; Fig. 2D) or the conventional hot plate test (time to withdrawal 5 h: vehicle control, 30.8 ± 1.5 s; carrageenan, 30.7 ± 2.1 s; P > 0.05; Fig. 2E).
Figure 2.
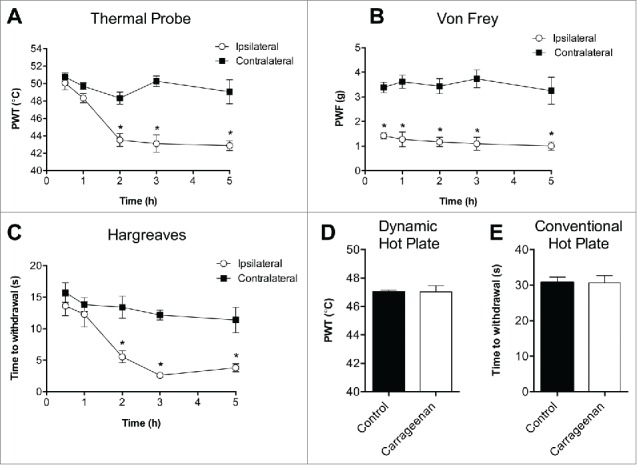
Behavioral assessment of carrageenan-induced thermal allodynia. (A) Time course of development of heat allodynia measured using the novel thermal probe test (n = 8). (B) Time course of development of mechanical allodynia measured using electronic von Frey (n = 8). (C) Time course of development of heat allodynia measured using the Hargreaves test (n = 8). No significant differences (5 h post injection of carrageenan) between vehicle control and carrageenan mice were detected on the hot plate using either the (D) dynamic hot plate or (E) conventional hot plate test at 50°C (n = 4 per group). Statistical significance was determined using t-test, *P < 0.05 compared to contralateral paw or vehicle control as indicated. Data are presented as mean ± SEM.
The thermal probe test can detect opioid-mediated analgesia
We next evaluated if the thermal probe test can detect an increase in the paw withdrawal temperature following administration of an analgesic in the carrageenan model. Administration of the opioid agonist oxycodone led to a significant increase in the temperature that elicited a withdrawal response in the carrageenan treated paw following application of the thermal probe (PWT 5 h: vehicle control, 42.9 ± 0.6°C; oxycodone, 50.0 ± 0.4°C; P < 0.05; Fig. 3A). This analgesic effect on carrageenan-induced thermal sensitivity was consistent with the Hargreaves test (time to withdrawal 5 h: vehicle control, 3.4 ± 0.4 s; oxycodone, 9.6 ± 2.8 s; P < 0.05; Fig. 3B). Interestingly, at a dose that abolished thermal allodynia, oxycodone had no significant effect on mechanical allodynia (PWF 5 h: vehicle control, 1.0 ± 0.2 g; oxycodone, 1.4 ± 0.2 g; P > 0.05; Fig. 3C).
Figure 3.
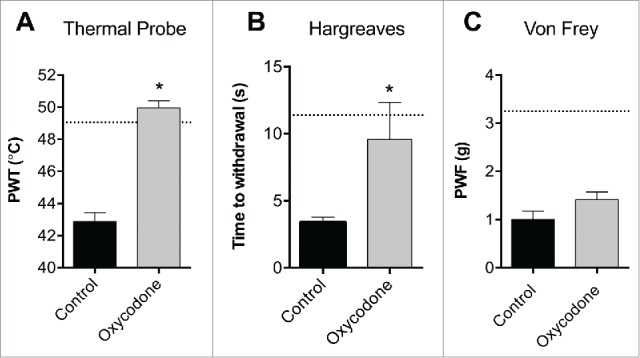
Opioid-mediated analgesia in the carrageenan model. Oxycodone (3 mg/kg) significantly reversed carrageenan-induced heat allodynia (5 h post injection of carrageenan) as measured by (A) the thermal probe test and (B) the Hargreaves test. (C) Oxycodone (3 mg/kg) had no significant effect on mechanical thresholds as measured by electronic von Frey. Statistical significance was determined using t-test, *P < 0.05 compared vehicle control. Data are presented as mean ± SEM, n = 4 per group.
The thermal probe test can quantify thermal allodynia in a mouse model of burns injury
To ensure that the use of the thermal probe test is not restricted to the carrageenan model, we next assessed its ability to detect thermal allodynia in a mouse model of burns injury. Following burns injury, the thermal probe test found a significant decrease in the temperature that elicited a withdrawal response in the burns-injured paw, evident at day 1 and peaking at day 3 after burns injury (PWT 3 d: contralateral, 50.8 ± 0.5°C; ipsilateral, 43.2 ± 0.6°C; P < 0.05; Fig. 4A). The time to development of thermal allodynia was similar in the Hargreaves test, although the degree of decrease in withdrawal latency was greater at day 1 and 2 after burns injury compared to the thermal probe test (time to withdrawal 3 d: contralateral, 11.8 ± 1.5 s; ipsilateral, 4.2 ± 0.8 s; P < 0.05; Fig. 4B). Mechanical allodynia in the burns-injured paw was evident at day 1, remaining relatively constant to day 3 (PWF 3 d: contralateral, 3.9 ± 0.7 g; ipsilateral, 1.1 ± 0.2 g; P < 0.05; Fig. 4C).
Figure 4.
Behavioral assessment of burns-induced thermal allodynia using the thermal probe test. Time course of development of heat allodynia measured using the (A) thermal probe test and (B) Hargreaves test. (C) Time course of development of mechanical allodynia measured using electronic von Frey. Statistical significance was determined using t-test, * P < 0.05 compared to contralateral paw. Data are presented as mean ± SEM, n = 8.
The thermal probe test can detect thermal allodynia after topical application of capsaicin
Following topical application of capsaicin, the thermal probe test found a significant decrease in the temperature that elicited a withdrawal response in the capsaicin treated paw compared to vehicle control (PWT: vehicle control, 49.7 ± 0.6°C; capsaicin, 44.8 ± 1.1°C; P < 0.05; Fig. 5A). No significant mechanical allodynia was evident (PWF: vehicle control, 3.0 ± 0.2 g; capsaicin, 2.6 ± 0.7 g; P > 0.05; Fig. 5B).
Figure 5.
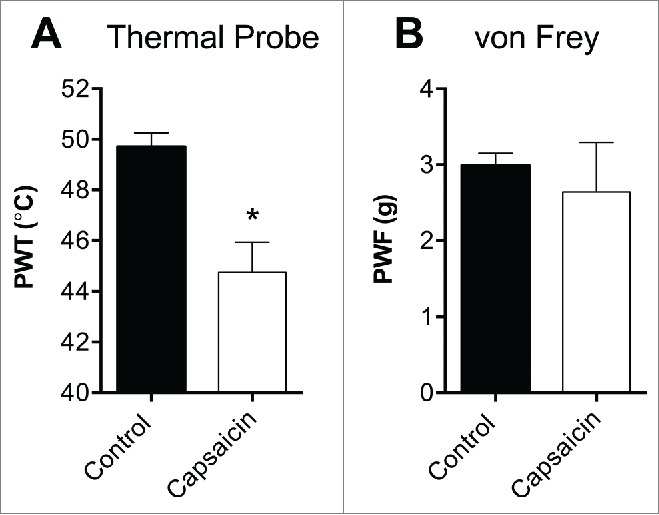
Behavioral assessment of thermal allodynia following topical application of capsaicin using the thermal probe test. (A) Topical capsaicin (1 mM) caused a significant reduction in the thermal paw withdrawal threshold measured using the thermal probe test, (B) but had no significant effect on mechanical thresholds as measured by electronic von Frey. Statistical significance was determined using t-test, * P < 0.05 compared to vehicle control. Data are presented as mean ± SEM, n = 4 per group.
Discussion
Behavioral assays that quantify nociceptive responses to different sensory modalities (mechanical, heat and cold) are essential for the use of rodent models of pain. Here, we describe for the first time the thermal probe test, a novel method to measure thermal paw withdrawal thresholds in mice.
Using the thermal probe test, naïve mice were found to withdraw the paw at a temperature of ∼50°C. While this value cannot directly be compared to other behavioral assays, as the withdrawal temperature is dependent on the method of heat exposure and rate of heating, it is similar to temperature withdrawal thresholds reported in humans using a similar method.7,12 It should be noted that the actual temperature at sensory nerve endings that elicits a withdrawal response is likely to be lower than the temperature readout of the probe, given the relatively fast rate of heating (2.5°C/s). In addition, it is possible that changes in skin temperature will influence the efficiency of probe-to-skin heat transfer, although similar studies in man found an effect of skin temperature on latency to first pain sensation only for a radiant but not contact heat stimulus.12 As with all tests assessing thermal withdrawal thresholds in rodents, skin temperature and skin blood flow at the start of heating may influence the detected withdrawal temperature. However, given that paw withdrawal temperatures detected using the thermal probe test were consistent both between days and between animals suggest that these factors have a minor influence on the behavioral read-out.
Like the Hargreaves test, the thermal probe test permits measurement of ipsilateral and contralateral paw withdrawal temperatures, which is advantageous in unilateral pain models, such as carrageenan-induced inflammation, burns injury and capsaicin-induced thermal hypersensitivity. In these models the thermal probe test measured decreased paw withdrawal temperatures of 43–45°C in the ipsilateral hind paw, demonstrating that the assay has a sufficient window to detect heat allodynia and partial or complete analgesia. In addition, the thermal probe test gave comparable results to the Hargreaves test, validating its use as a behavioral assay to assess thermal paw withdrawal thresholds in mice.
While having comparable performance to the Hargreaves test, the thermal probe test has several advantages. The first is that the mice are placed in individual runs standing on bars rather than glass (Figs. 1C, D), enabling access to the plantar surface to measure mechanical thresholds using the von Frey test. The second is that the time taken to habituate a mouse in the individual runs used for the thermal probe test is significantly shorter (∼5 mins) than the time taken to habituate a mouse in polyvinyl boxes on glass in the Hargreaves apparatus, which is often reported to be 30 mins or longer.13-15 While this length of habituation may be acceptable when testing compounds with favorable pharmacokinetics, it precludes assessment of compounds with very short half-lives, such as peptides.16 It should be noted that these habituation times might differ between mouse strains due to different behavioral traits.17 In addition, the Hargreaves apparatus, depending on the model, is generally quite an expensive purchase, while the thermal probe test is comparatively cheaper.
Typically, pain models that induce heat allodynia also cause mechanical allodynia,10,11,18 therefore a limitation of the thermal probe test is that a mechanical stimulus is applied in conjunction with a thermal stimulus, introducing a possible confounding factor. In both the carrageenan and burns injury models, the paw withdrawal threshold to application of a von Frey filament was ∼1 g, which is the same force required to trigger heating of the probe. However, application of the unheated probe did not cause withdrawal responses, likely because the surface area of the probe is 8 times larger and thus the actual pressure applied to paw is much less, and does not elicit a mechanical response. Our observation that development of heat allodynia was independent of the development of mechanical allodynia in the carrageenan, burns injury and capsaicin models further confirms that mechanical stimulation does not contribute to the paw withdrawal response elicited by the thermal probe test in these models, however use of the thermal probe test in other models that induce a more profound mechanical allodynia may be limited.
Interestingly, we were not able to detect a difference between carrageenan-treated mice and vehicle control mice using either the dynamic or conventional hot plate tests. This may be due to inconsistent and variable contact of the carrageenan-treated paw with the hot plate surface, as mice constantly move around the plate. This movement may be reduced by habituation with the apparatus before testing, however this is likely to be time consuming and impractical, as the hot plate can only house one animal at a time. In addition, the hot plate test is confounded by the fact that other parts of the mouse other than the carrageenan-treated paw are in contact with the metal surface, with nociceptive responses often observed in the contralateral hind paw. Therefore, although useful to assess the antinociceptive effects of systemically delivered drugs in naïve mice,3,19 in our hands, the conventional and dynamic hot plate tests are of limited value in unilateral pain models, at least in the C57BL/6J mouse strain. Notably, the dynamic and conventional hot plate tests, as well as certain models of the Hargreaves apparatus, require close observation of animal behavior followed by manual triggering by the investigator to record paw withdrawal latencies or temperatures. The need for reaction to an observed behavior may introduce additional error, thus, an additional advantage of the thermal probe test lies in its ability to automatically record paw withdrawal temperatures on paw withdrawal.
In conclusion, we have described the thermal probe test, a novel behavioral assay that is an efficient method to measure thermal allodynia and analgesia in mice. While comparable to the Hargreaves test, the thermal probe test has several advantages, including the assessment of mechanical thresholds with von Frey using the same enclosure.
Abbreviations
- PWF
paw withdrawal force
- PWT
paw withdrawal temperature
- SEM
standard error of the mean
Disclosure of potential conflicts of interest
No potential conflicts of interested were disclosed
Funding
This work was supported by an Australian Postgraduate Award (J.R.D.) and an Australian Future Fellowship (FT130101215) (I.V.).
References
- [1].Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014; 13:924-35; PMID:25142459; http://dx.doi.org/ 10.1016/S1474-4422(14)70102-4 [DOI] [PubMed] [Google Scholar]
- [2].Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron 2002; 35:383-93; PMID:12160755; http://dx.doi.org/ 10.1016/S0896-6273(02)00767-5 [DOI] [PubMed] [Google Scholar]
- [3].Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001; 53:597-652; PMID:11734620 [PubMed] [Google Scholar]
- [4].Luttinger D. Determination of antinociceptive efficacy of drugs in mice using different water temperatures in a tail-immersion test. J Pharmacol Methods 1985; 13:351-7; PMID:3927065; http://dx.doi.org/ 10.1016/0160-5402(85)90017-8 [DOI] [PubMed] [Google Scholar]
- [5].Gårdmark M, Höglund AU, Hammarlund-Udenaes M. Aspects on tail-flick, hot-plate and electrical stimulation tests for morphine antinociception. Pharmacol Toxicol 1998; 83:252-8; http://dx.doi.org/ 10.1111/j.1600-0773.1998.tb01478.x [DOI] [PubMed] [Google Scholar]
- [6].Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain 2009; 10:767-73; PMID:19409860; http://dx.doi.org/ 10.1016/j.jpain.2009.01.325 [DOI] [PubMed] [Google Scholar]
- [7].TjØlsen A, Rosland JH, Berge OG, Hole K. The increasing-temperature hot-plate test: an improved test of nociception in mice and rats. J Pharmacol Methods 1991; 25:241-50; PMID:2056753; http://dx.doi.org/ 10.1016/0160-5402(91)90014-V [DOI] [PubMed] [Google Scholar]
- [8].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32:77-88; PMID:3340425; http://dx.doi.org/ 10.1016/0304-3959(88)90026-7 [DOI] [PubMed] [Google Scholar]
- [9].McCarson KE. Models of inflammation: carrageenan- or complete Freund's adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol 2015; 70:5 4 1-9; PMID:26331888 [DOI] [PubMed] [Google Scholar]
- [10].Nozaki-Taguchi N, Yaksh TL. A novel model of primary and secondary hyperalgesia after mild thermal injury in the rat. Neurosci Lett 1998; 254:25-8; PMID:9780083; http://dx.doi.org/ 10.1016/S0304-3940(98)00648-X [DOI] [PubMed] [Google Scholar]
- [11].Gris G, Merlos M, Vela JM, Zamanillo D, Portillo-Salido E. S1RA, a selective sigma-1 receptor antagonist, inhibits inflammatory pain in the carrageenan and complete Freund's adjuvant models in mice. Behav Pharmacol 2014; 25:226-35; PMID:24776490; http://dx.doi.org/ 10.1097/FBP.0000000000000038 [DOI] [PubMed] [Google Scholar]
- [12].Pertovaara A, Kauppila T, Hämäläinen MM. Influence of skin temperature on heat pain threshold in humans. Exp Brain Res 1996; 107:497-503; PMID:8821389; http://dx.doi.org/ 10.1007/BF00230429 [DOI] [PubMed] [Google Scholar]
- [13].Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Experimental diabetes research 2011; 2011:848307; PMID:22144990; http://dx.doi.org/ 10.1155/2011/848307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Molecular Pain 2009; 5:18; PMID:19379487; http://dx.doi.org/ 10.1186/1744-8069-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O'Brien DE, Brenner DS, Gutmann DH, Gereau RWT. Assessment of pain and itch behavior in a mouse model of neurofibromatosis type 1. J Pain 2013; 14:628-37; PMID:23578956; http://dx.doi.org/ 10.1016/j.jpain.2013.01.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug discovery today 2015; 20:122-8; PMID:25450771; http://dx.doi.org/ 10.1016/j.drudis.2014.10.003 [DOI] [PubMed] [Google Scholar]
- [17].Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 2001; 72:271-81; PMID:11240006; http://dx.doi.org/ 10.1016/S0031-9384(00)00405-4 [DOI] [PubMed] [Google Scholar]
- [18].Chillingworth NL, Donaldson LF. Characterisation of a Freund's complete adjuvant-induced model of chronic arthritis in mice. J Neurosci Methods 2003; 128:45-52; PMID:12948547; http://dx.doi.org/ 10.1016/S0165-0270(03)00147-X [DOI] [PubMed] [Google Scholar]
- [19].Carbone ET, Lindstrom KE, Diep S, Carbone L. Duration of action of sustained-release buprenorphine in 2 strains of mice. Journal of the American Association for Laboratory Animal Science : JAALAS 2012; 51:815-9; PMID:23294889 [PMC free article] [PubMed] [Google Scholar]