ABSTRACT
We tested the hypotheses that thermoregulatory behavior is initiated before changes in blood pressure and that skin blood flow upon the initiation of behavior is reflex mediated. Ten healthy young subjects moved between 40°C and 17°C rooms when they felt ‘too warm’ (W→C) or ‘too cool’ (C→W). Blood pressure, cardiac output, skin and rectal temperatures were measured. Changes in skin blood flow between locations were not different at 2 forearm locations. One was clamped at 34°C ensuring responses were reflex controlled. The temperature of the other was not clamped ensuring responses were potentially local and/or reflex controlled. Relative to pre-test Baseline, skin temperature was not different at C→W (33.5 ± 0.7°C, P = 0.24), but was higher at W→C (36.1 ± 0.5°C, P < 0.01). Rectal temperature was different from Baseline at C→W (−0.2 ± 0.1°C, P < 0.01) and W→C (−0.2 ± 0.1°C, P < 0.01). Blood pressure was different from Baseline at C→W (+7 ± 4 mmHg, P < 0.01) and W→C (−5 ± 5 mmHg, P < 0.01). Cardiac output was not different from Baseline at C→W (−0.1 ± 0.4 L/min, P = 0.56), but higher at W→C (0.4 ± 0.4 L/min, P < 0.01). Skin blood flow between locations was not different from Baseline at C→W (clamped: −6 ± 15 PU, not clamped: −3 ± 6 PU, P = 0.46) or W→C (clamped: +21 ± 23 PU, not clamped: +29 ± 15 PU, P = 0.26). These data indicate that the initiation of thermoregulatory behavior is preceded by moderate changes in blood pressure and that skin blood flow upon the initiation of this behavior is under reflex control.
KEYWORDS: blood pressure, body temperature, cardiovascular strain, skin blood flow
Introduction
Over the next 50 years the frequency and severity of heat waves is very likely to increase, while periodic cold spells will persist.1 Epidemiological and etiological data indicate that cardiovascular health is particularly susceptible to heat and cold.2 Heat or cold exposure induces a hypo- or hyper- tensive challenge that is stimulated by changes in vascular resistance, which are driven by increases or decreases in skin and/or internal temperatures.3 The corresponding increases in cardiac afterload in the cold and increases in cardiac output in the heat can acutely increase cardiovascular risk,4 particularly in populations with an already compromised cardiovascular system (e.g., older adults, hypertensive and heart failure patients, etc.).5,6 Given that this increased cardiovascular risk occurs secondary to changes in body temperature, there is a need to understand interactions between temperature regulation and cardiovascular responses.
Body temperature is regulated via a combination of autonomic (e.g., sweating, shivering) and behavioral (e.g., adding/removing clothing) responses.7 Behavior plays a comparatively larger role in human temperature regulation,8 which is likely due to behavior being the most efficient and effective thermoregulatory response.9 When given the opportunity to behave, thermal behavior is elicited primarily by changes in skin temperature.10-12 This arrangement prevents changes in internal temperature 11,12 and minimizes the reliance upon sweating or shivering to regulate body temperatures.11,13 Thus, thermal behavior prevents large changes in body temperature, which may avert subsequent changes in blood pressure. However, this has yet to be experimentally determined.
Estimates of skin blood flow indicate that the initiation of thermal behavior is accompanied by cutaneous vasoconstriction in the cold and vasodilation in the heat.11 Notably, skin blood flow within a range of thermoneutral skin temperatures (33–35°C) is under reflex control.14 However, it currently remains unknown whether the skin blood flow responses leading up to and upon the initiation of thermal behavior are reflex mediated and/or whether local changes in skin temperature also play a role. Given the contribution of skin blood flow to blood pressure regulation,15 such knowledge may provide insights regarding understanding interactions between blood pressure regulation and thermal behavior.
The purpose of this study was to test 2 hypotheses: i) Thermoregulatory behavior is initiated prior to thermal induced changes in blood pressure, and ii) skin blood flow responses leading up to and upon the decision to initiate thermoregulatory behavior are reflex mediated.
Methods
Subjects
Ten healthy young subjects participated in this study. The subject characteristics are presented in Table 1. All subjects were physically active, normotensive, non-smokers, not taking medications, and were free from any known cardiovascular, metabolic, neurological, or psychological diseases. The female subjects were not pregnant, which was confirmed via a urine pregnancy test. Each subject was fully informed of the experimental procedures and possible risks before giving informed, written consent. The study was approved by the Institutional Review Board at the University at Buffalo, and performed in accordance with the standards set by the latest revision of the Declaration of Helsinki. Subjects visited the laboratory on 2 occasions. Visit one was a screening and familiarization visit, while visit 2 was the experimental trial.
Table 1.
Subject characteristics.
| Sex (M/F) | 6/4 |
|---|---|
| Age (y) | 26 ± 4 |
| Height (cm) | 173 ± 13 |
| Weight (kg) | 73.4 ± 16.0 |
| Body surface area (m2) | 1.9 ± 0.3 |
| Body fat (%) | 22 ± 11 |
| Resting heart rate (bpm) | 58 ± 7 |
| Resting systolic blood pressure (mmHg) | 114 ± 12 |
| Resting diastolic blood pressure (mmHg) | 66 ± 7 |
| Resting mean arterial pressure (mmHg) | 82 ± 9 |
| Physical activity (high/moderate/low) a | 5/5/0 |
Mean ± SD
stratified according to Craig et al. 69
Instrumentation, measurements, and calculations
Height and weight were measured with a stadiometer and scale (Sartorius Corp. Bohemia, NY, USA), and body surface area was calculated accordingly.16 Seven site skinfold thickness was measured in triplicate at the chest, axilla, triceps, subscapula, abdomen, suprailliac, and thigh (Harpenden, Baty International, UK). Percent body fat was estimated from body density,17 which was calculated from the sum of 7 skinfolds for males 18 and females.19 Urine specific gravity was measured in duplicate using a refractometer (Atago USA, Inc., Bellevue, WA, USA).
Rectal temperature was measured at a depth of 10 cm past the anal sphincter using a general purpose thermocouple (Mon-a-therm, Mallinckrodt Medical, Inc., St. Louis, MO, USA). Mean skin temperature was measured as the weighted average of 6 thermocouples (Omega Engineering, Inc. Stamford, CT, USA) attached to the following locations: abdomen (14%), calf (11%), chest (22%), lower back (19%), thigh (14%), and upper back (19%).20
Heart rate was measured continually from a 3 lead electrocardiogram (DA100C, Biopac Systems, Inc. Goleta, CA, USA). Beat-to-beat blood pressure was measured continually via the Penaz method (Finometer Pro, FMS, Amsterdam, The Netherlands). Prior to data collection, Finometer derived blood pressure data were confirmed via a manual blood pressure taken during the screening by an experienced member of the research team. Finometer derived blood pressure waveforms were maintained throughout all experimental testing. Stroke volume was estimated from the blood pressure waveform using Modelflow.21 Cardiac output was calculated as the product of stroke volume and heart rate, while total peripheral resistance was calculated as the quotient of cardiac output and mean arterial pressure. Stroke work (stroke volume x mean arterial pressure), rate pressure product (systolic pressure x heart rate), and cardiac power output [mean arterial pressure x cardiac output x (2.22 × 10−3)] were calculated as indices of left ventricular work,22 myocardial oxygen demand,23 and left ventricular power.24
Skin blood flow was measured via integrated laser Doppler flowmetry (Periflux System 5010, Perimed, Stockholm, Sweden) at 3 locations. Two of these locations were on the dorsal surface of the left forearm, while the other was on the pad of the fingertip on the index finger of the left hand. At one of the forearm locations, the laser Doppler probe was inserted into a local heating device (Periflux System 5020, Perimed, Stockholm, Sweden), the temperature of which was clamped at 34°C throughout the protocol. This ensured that at this location any changes in skin blood flow were reflex mediated.14 At the other location, the laser Doppler probe was inserted into a thin plastic holder and the local temperature was allowed to drift with changes in skin temperature. Changes in skin blood flow at this location were potentially mediated by both reflex and local mechanisms.14 Given that only cutaneous vasoconstrictor nerves innervate glabrous skin,25 the skin blood flow measured on the fingertip provided an index of reflex cutaneous vasoconstrictor activity. At all locations, the accuracy of the skin blood flow measurement was ensured based upon the observation of a clear pulsatile signal that coincided with the pulse wave. Forearm skin temperature was measured with a thermocouple (Omega Engineering, Inc. Stamford, CT, USA) secured next to the plastic holder at the non-thermally controlled skin blood flow location, providing an index of local skin temperature at this location. Temperature on the pad of the fingertip of the ring finger was also measured, with the temperature gradient between the forearm and fingertip providing an indirect estimate of skin blood flow.26,27
Affect (11 point scale: −5 = feeling bad, +5 = feeling good),28 thermal sensation (7 point scale: 1=cold, 4 = neutral, 7=hot),29 thermal discomfort (4 point scale: 1 = comfortable, 4 = very uncomfortable),29 and perceptions of sweating and shivering (11 point scale: 0=none, 10 most ever) [adapted from 11] were measured on standard subjective scales upon the initiation of thermoregulatory behavior.
Experimental protocol
Subjects arrived at the laboratory euhydrated, confirmed via urine specific gravity (1.007 ± 0.004), and having refrained from strenuous exercise, alcohol and caffeine for 12 h, and food for 2 h. To control for menstrual cycle hormones, females were tested during the first 10 days following self-identified menstruation.30 Experimental testing was conducted during the summer months in Buffalo, NY, USA. This climate induces minimal autonomic heat acclimatization.31 Time of day was not controlled. Subjects wore a cotton t-shirt, athletic shorts, and light sandals (i.e., flip-flops).
Following instrumentation, subjects rested quietly, seated on a mesh chair in a 23 ± 1°C (58 ± 8% relative humidity) environment for 20 min. Following this period the 90 min thermal behavior assessment commenced when the temperature of the room in which subjects were seated decreased to 17 ± 1°C (49 ± 3% relative humidity, Fig. 1). At any time during room cooling, and throughout the thermal behavior assessment, subjects could move between the cool room to the adjacent warm room, which was maintained at 40 ± 0°C (20 ± 0% relative humidity, Fig. 1). The decision to behaviorally thermoregulate was defined as the decision to move from cool to warm (C→W) or from warm to cool (W→C).11,12 Subjects passively moved between the 2 rooms by pressing a button on a remote control (Fig. 2). This shuttled them between the 2 rooms without exertion and allowed for continual data collection. The amount of time spent in each environment prior to behaving was recorded. Subjects read literature of their choice and maintained the same seated posture throughout the assessment. During the baseline period, subjects were read an instructional script that reviewed the experimental procedures and instructed them to exit the warm room when they became ‘too warm’ and exit the cool room when they became ‘too cool’.11,12 This ‘shuttle box’ thermal behavioral model provides a reliable index of thermoregulatory behavior in humans,12 and is considered better than other models [e.g., thermal gradient, self-paced exercise 32] because it allows for the determination of the exact moment at which a decision to behaviorally thermoregulate is made.33
Figure 1.
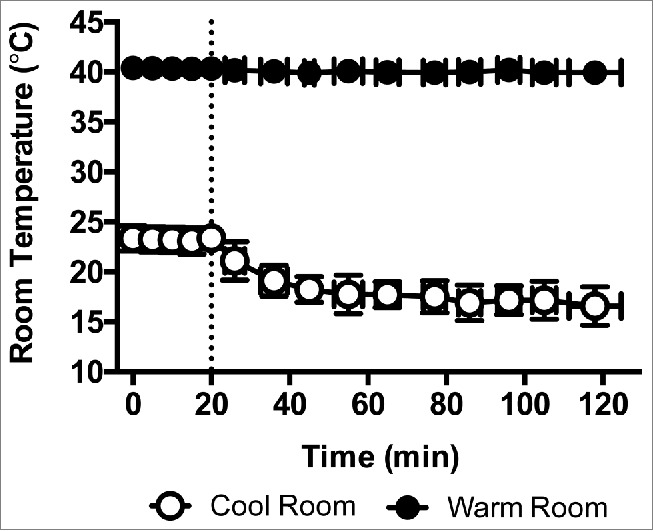
Temperature dynamics of the cool and warm rooms. Baseline measurements were taken from 0–20 min in the cool room, which was maintained at 23 ± 1°C (58 ± 8% relative humidity) during this time period. After the Baseline period, the cool room was set to 17 ± 1°C (49 ± 3% relative humidity) and the 90 min behavioral assessment commenced (indicated by a dashed line) in the midst of this progressive room cooling. The warm room was maintained at 40 ± 0°C (20 ± 0% relative humidity) throughout. Mean ± SD, n = 10.
Figure 2.

Photo of experimental set-up.
Data and statistical analyses
Thermal and hemodynamic data were sampled continuously at 100 Hz via a data acquisition system (Biopac MP150, Goleta, CA, USA). These data were analyzed at Baseline, which was a 60 s average at the end of the 20 min pre-protocol resting period, and 90 s, 60 s, and 30 s immediately prior to C→W and W→C (all 30 s averages). Each subject behaved a different number of times during the thermal behavior protocol, and thus, data were averaged across behaviors for a given subject, as we have done previously.11,12 Skin blood flow data are reported as absolute values and were also normalized to mean arterial pressure, providing an index of cutaneous vascular conductance (CVC). Skin blood flow data are also reported as the absolute change from Baseline, which allowed for comparison between the responses at the 2 forearm skin blood flow locations. Given the differences in Baseline between these locations, absolute changes in skin blood flow were considered the more physiologically meaningful approach to normalizing the skin blood flow data when compared to percent changes. Perceptual data were collected at Baseline, and upon C→W and W→C. To examine any effects of time, data collected during the first and final behaviors at C→W and W→C were also analyzed.
Thermal, hemodynamic, and the change over time data were analyzed using 2-way repeated measures ANOVA [behavior × time, 2 (C→W, W→C) × 4 (Baseline, 90 s, 60 s, 30 s)]. Perceptual data were analyzed using one-way repeated measures ANOVA (3 levels: Baseline, C→W, W→C). Data were assessed for approximation to a normal distribution and sphericity, and no corrections were necessary. Where appropriate, post hoc Sidak adjusted pair-wise comparisons were made. Data were analyzed using Prism software (Version 6, GraphPad Software Inc. La Jolla, CA, USA). A priori statistical significance was set at P ≤ 0.05 and actual p-values are reported where possible. All data are reported as mean ± SD.
Results
Subjects moved from C→W 5 ± 1 times and from W→C 4 ± 1 times. Time prior to C→W (9.1 ± 3.5 min) was shorter than W→C (14.5 ± 4.5 min, P < 0.01).
Thermal responses
Rectal temperature upon C→W and W→C was 0.2 ± 0.2°C lower than at Baseline (P < 0.01, Fig. 3). Rectal temperature was 0.1 ± 0.0°C higher at C→W compared to W→C (P < 0.01, Fig. 3). Mean skin temperature at C→W was not different from Baseline (P = 0.24), but was 2.8 ± 0.6°C higher than Baseline at W→C (P < 0.01), which was also different from C→W (P < 0.01, Fig. 3). Forearm skin temperature followed a similar profile to mean skin temperature, while fingertip temperature was lower than Baseline at C→W (P < 0.01) and higher than Baseline at W→C (P < 0.01, Table 2).
Figure 3.
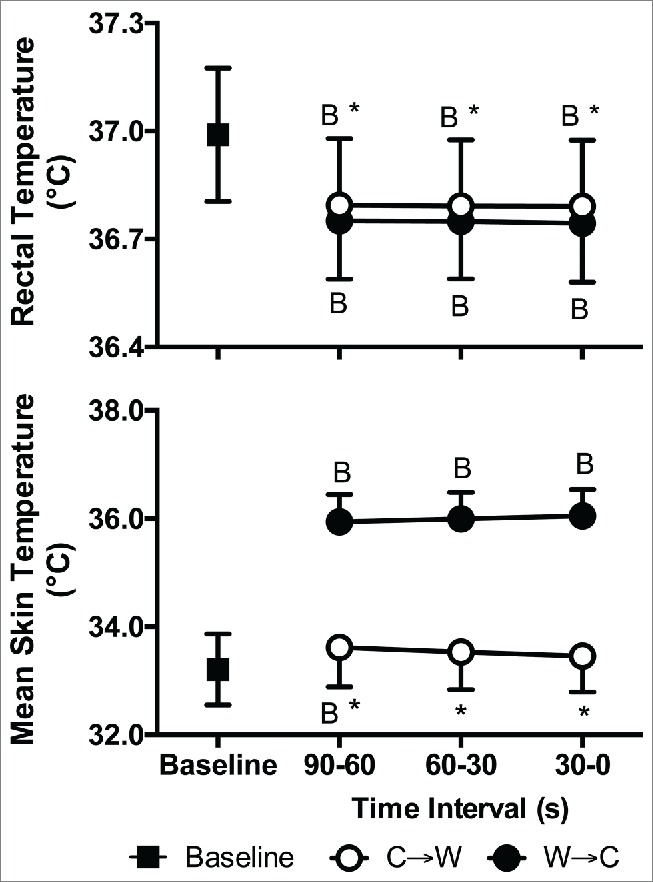
Rectal (top) and mean skin (bottom) temperatures at Baseline and 90 s, 60 s, and 30 s immediately prior to moving from cool to warm (C→W) and from warm to cool (W→C). Mean ± SD, n = 10. * Different from W→C (P < 0.01), BDifferent from Baseline (P < 0.01).
Table 2.
Skin blood flow, local skin temperature, and cutaneous vascular conductance at Baseline, leading up to, and upon the initiation of thermoregulatory behavior.
| C→W | W→C | ||||||
|---|---|---|---|---|---|---|---|
| Time Interval (s) |
Time Interval (s) |
||||||
| Measurement Location | Baseline | 90–60 | 60–30 | 30–0 | 90–60 | 60–30 | 30–0 |
| Clamped @ 34°C | |||||||
| Skin blood flow (PU) | 44 ± 25 | 37 ± 17 *,B | 37 ± 16 *,B | 38 ± 18 *,B | 63 ± 20B | 63 ± 18B | 65 ± 20B |
| CVC (PU/mmHg) | 0.46 ± 0.23 | 0.37 ± 0.15 *,B | 0.37 ± 0.15 *,B | 0.38 ± 0.16 *,B | 0.71 ± 0.18B | 0.71 ± 0.16B | 0.73 ± 0.18B |
| Not Clamped | |||||||
| Skin blood flow (PU) | 17 ± 9 | 14 ± 5 * | 14 ± 5 * | 14 ± 5 * | 43 ± 19B | 43 ± 19B | 45 ± 19B |
| CVC (PU/mmHg) | 0.18 ± 0.10 | 0.14 ± 0.05 * | 0.14 ± 0.05 * | 0.14 ± 0.06 * | 0.50 ± 0.22 | 0.49 ± 0.22 | 0.51 ± 0.21 |
| Fingertip | |||||||
| Skin blood flow (PU) | 204 ± 102 | 86 ± 53 *,B | 82 ± 48*,B | 81 ± 51 *,B | 400 ± 133B | 406 ± 131B | 362 ± 122B |
| CVC (PU/mmHg) | 2.20 ± 1.11 | 0.89 ± 0.57 *,B | 0.83 ± 0.50 *,B | 0.81 ± 0.53 *,B | 4.62 ± 1.54B | 4.68 ± 1.52B | 4.20 ± 1.55B |
| Local Skin Temperatures | |||||||
| Forearm temperature (°C) | 31.5 ± 1.5 | 31.8 ± 0.7 * | 31.7 ± 0.6 * | 31.5 ± 0.6 * | 35.6 ± 0.6B | 35.7 ± 0.6B | 35.8 ± 0.6B |
| Fingertip temperature (°C) | 30.5 ± 4.1 | 26.9 ± 3.7 *,B | 26.6 ± 3.7 *,B | 26.3 ± 3.7 *,B | 36.0 ± 1.2 B | 36.1 ± 1.2 B | 36.2 ± 1.3 B |
| Forearm to finger gradient (°C) | 0.9 ± 2.8 | 4.9 ± 3.7 *,B | 5.1 ± 3.7 *,B | 5.2 ± 3.6 *,B | −0.4 ± 1.1 | −0.5 ± 1.2 | −0.5 ± 1.2 |
Mean ± SD, CVC: Cutaneous vascular conductance, C→W: the decision to move from cool to warm, W→C: the decision to move from warm to cool
Different from W→C (P < 0.01)
Different from Baseline (P ≤ 0.02).
Cardiovascular responses
Mean arterial pressure at C→W was greater than Baseline by 7 ± 4 mmHg (Systolic: +8 ± 8 mmHg, Diastolic: +4 ± 3 mmHg, P < 0.01) and was lower than Baseline at W→C by 5 ± 5 mmHg (Systolic: −11 ± 5 mmHg, Diastolic: −4 ± 5 mmHg, P < 0.01, Fig. 4). Cardiac output was 0.5 ± 0.5 L/min lower at C→W compared to W→C (P < 0.01, Fig. 5). Stroke volume was not different between Baseline, C→W, and W→C (P = 0.39). Heart rate was lower at C→W compared to W→C (by 8 ± 4 bpm, P < 0.01) and both were different from Baseline (P < 0.01, Fig. 4). Total peripheral resistance was higher at C→W compared to W→C (by 4.3 ± 2 mmHg/L/min, P < 0.01) and both were different from Baseline (P < 0.01, Fig. 5). Stroke work was higher at C→W compared to W→C (by 1035 ± 767 mL/beat × mmHg, P < 0.01) and both were different from Baseline (P ≤ 0.05, Fig. 6), but rate pressure product and cardiac power output were not different between Baseline, C→W, and W→C (P = 0.13, Fig. 6).
Figure 4.

Systolic (top), diastolic (middle) and mean (bottom) arterial pressures at Baseline and 90 s, 60 s, and 30 s immediately prior to moving from cool to warm (C→W) and from warm to cool (W→C). Mean ± SD, n = 10. * Different from W→C (P < 0.01), BDifferent from Baseline (P < 0.01).
Figure 5.
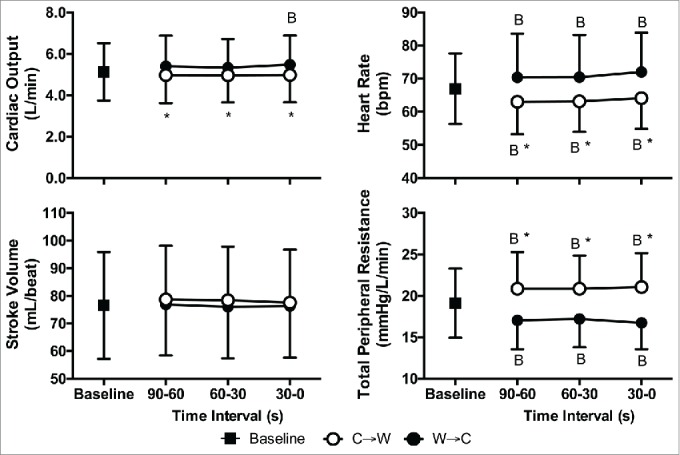
Cardiac output (top-left), heart rate (top-right), stroke volume (bottom-left), and total peripheral resistance (bottom-right) at Baseline and 90 s, 60 s, and 30 s immediately prior to moving from cool to warm (C→W) and from warm to cool (W→C). Mean ± SD, n = 10. * Different from W→C (P < 0.01), BDifferent from Baseline (P < 0.01).
Figure 6.
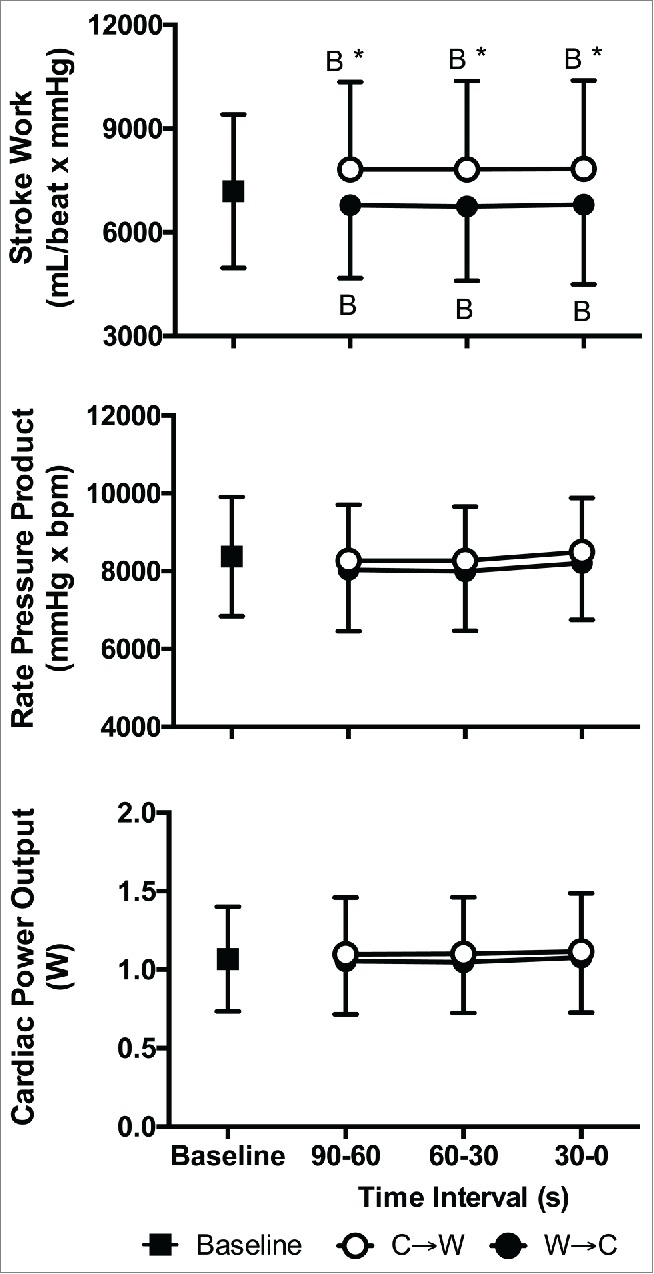
Stroke work (top), rate pressure product (middle), and cardiac power output (bottom) at Baseline and 90 s, 60 s, and 30 s immediately prior to moving from cool to warm (C→W) and from warm to cool (W→C). Mean ± SD, n = 10. * Different from W→C (P < 0.01), BDifferent from Baseline (P ≤ 0.05).
Skin blood flow responses
At the fingertip and thermally clamped locations, skin blood flow and CVC were lower than Baseline at C→W (P ≤ 0.02) and were greater than Baseline at W→C (P < 0.01, Table 2). At the not clamped location, skin blood flow and CVC were not different from Baseline at C→W (P ≥ 0.79), but were higher than Baseline at W→C (P < 0.01, Table 2). The forearm to fingertip temperature gradient was lower than Baseline at C→W (P < 0.01) and not different from Baseline at W→C (P ≥ 0.29, Table 2). Skin blood flow was higher at Baseline at the thermally clamped location compared to the not clamped location, which was maintained leading up to and upon C→W and W→C (P < 0.01, Fig. 7). The differences between locations were due to a Baseline shift, presumably due to the removal of locally mediated cutaneous vasoconstrictor tone, as the change from Baseline skin blood flow responses were not different between the thermally clamped and not clamped locations at either C→W (P ≥ 0.35) or W→C (P ≥ 0.26, Fig. 7). Given that the perfusion pressure was the same between the 2 locations, CVC responses were the same as the skin blood flow responses (CVC data not shown).
Figure 7.

Skin blood flow (top) and the absolute change from baseline (Δ, bottom) 90 s, 60 s, and 30 s immediately prior to moving from cool to warm (C→W, right) and from warm to cool (W→C, left) at a forearm location that was clamped at 34°C (Clamped @ 34°C) and a forearm location that was not clamped (Not Clamped). Mean ± SD, n = 10. B Different from Baseline (P < 0.01), † Different from Not Clamped (P < 0.01). For the absolute change data there were no differences between locations (P ≥ 0.26) or over time (P > 0.05).
Perceptual responses
Affect was not different between C→W (+1.6 ± 1.9 a.u.) and W→C (+1.6 ± 1.8 a.u., P = 0.73), both of which were also not different from Baseline (+3.2 ± 1.9 a.u., P ≥ 0.08). Subjects felt cooler at C→W (2.5 ± 0.4 a.u.) and warmer at W→C (5.5 ± 0.5 a.u., P < 0.01), and both were different from Baseline (3.8 ± 0.4 a.u., P < 0.01). Subjects felt thermally comfortable at Baseline (1.1 ± 0.2 a.u.), but slightly uncomfortable at both C→W (1.9 ± 0.3 a.u., P < 0.01) and W→C (1.9 ± 0.5 a.u., P < 0.01), which were not different (P = 0.87). At C→W, subjects perceived to be slightly shivering (1.7 ± 0.2 a.u.) and at W→C they perceived to be slightly sweating (0.8 ± 0.6 a.u.), while perceptions of both sweating and shivering were absent at Baseline (both 0.0 ± 0.0 a.u., P < 0.01). The magnitude of sweating and shivering perception at C→W and W→C were not different (P = 0.15).
First vs. final behavior
The time before initiating thermal behavior was not different between the first and final behavior at C→W and W→C (P ≥ 0.86, Table 3). Rectal temperature was lower upon the final behavior at C→W and W→C compared to Baseline (P < 0.01) and the first behavior (P ≤ 0.03). Upon the final behavior rectal temperature was not different between C→W and W→C (P = 0.99, Table 3). Upon the first behavior, mean skin temperature was lower than Baseline at C→W (by 0.6 ± 0.4°C, P = 0.03) and higher than Baseline at W→C (by 2.2 ± 0.9°C, P < 0.01), while upon the final behavior mean skin temperature was warmer than Baseline at both C→W (by 0.6 ± 0.8°C, P = 0.02) and W→C (by 3.2 ± 0.8°C, P < 0.01, Table 3). Mean skin temperature upon the final behavior was also warmer than the first behavior at both C→W (by 1.3 ± 0.8°C, P < 0.01) and W→C (by 1.0 ± 1.0°C, P < 0.01, Table 3). Forearm temperature followed mean skin temperature profile, while fingertip temperature was lower than Baseline at C→W and higher than Baseline at W→C (P < 0.01), and were not different between the first and final behavior (P ≥ 0.06). The forearm to finger temperature gradient was higher upon the first behavior compared to W→C (P = 0.02). With the exception of affect at C→W (P = 0.02), there were no differences between the first and final behaviors (P ≥ 0.08, Table 3) for all cardiovascular, skin blood flow, and perceptual measures.
Table 3.
Ambient conditions, and thermal, cardiovascular, skin blood flow and perceptual responses at Baseline and upon the decision to initiate the first and the final thermal behavior.
| C→W |
W→C |
||||
|---|---|---|---|---|---|
| Baseline | First Behavior | Final Behavior | First Behavior | Final Behavior | |
| Ambient conditions | |||||
| Cool room temperature (°C) | 23 ± 1 | 21 ± 2B | 18 ± 1B,1 | — | — |
| Cool room humidity (%) | 58 ± 8 | 49 ± 5B | 51 ± 5B | — | — |
| Warm room temperature (°C) | 40 ± 0 | — | — | 40 ± 1 | 40 ± 0 |
| Warm room relative humidity (%) | 22 ± 2 | — | — | 21± 1 | 22 ± 0 |
| Time before behaving (min) | — | 10.4 ± 5.9 | 9.2 ± 3.6 | 13.4 ± 7.8 | 13.8 ± 5.2 |
| Body temperatures | |||||
| Rectal temperature (°C) | 37.0 ± 0.2 | 36.9 ± 0.2 * | 36.7 ± 0.2B,1 | 36.8 ± 0.2B | 36.7 ± 0.2B,1 |
| Mean skin temperature (°C) | 33.2 ± 0.7 | 32.6 ± 0.8 *,B | 33.9 ± 0.6 *,B,1 | 35.4 ± 0.9B | 36.4 ± 0.5B,1 |
| Forearm temperature (°C) | 31.5 ± 1.5 | 30.3 ± 1.3 *,B | 32.2 ± 0.7 *,B | 34.5 ± 1.4B | 36.4 ± 0.9B,1 |
| Fingertip temperature (°C) | 30.5 ± 4.1 | 25.6 ± 3.9 *,B | 26.5 ± 4.3 *,B | 35.8 ± 1.2 B | 36.5 ± 1.5 B |
| Cardiovascular measures | |||||
| Mean arterial pressure (mmHg) | 93 ± 5 | 99 ± 10 *,B | 100 ± 7 *,B | 88 ± 10B | 89 ± 8B |
| Cardiac output (L/min) | 5.1 ± 1.4 | 5.2 ± 1.4 * | 4.9 ± 1.2 * | 5.7 ± 1.5B | 5.5 ± 1.5B |
| Stroke volume (mL/beat) | 77 ± 19 | 78 ± 19 * | 78 ± 19 * | 77 ± 19 | 76 ± 20 |
| Heart rate (bpm) | 67 ± 11 | 66 ± 11 * | 63 ±10 * | 73 ± 13B | 72 ± 11B |
| Skin blood flow | |||||
| Skin blood flow (clamped, PU) | 44 ± 25 | 42 ± 8 * | 37 ± 16 * | 63 ± 24B | 70 ± 25B |
| Skin blood flow (not clamped, PU) | 17 ± 9 | 11 ± 5 * | 18 ± 8 * | 36 ± 23 | 59 ± 41B |
| Skin blood flow (fingertip, PU) | 204 ± 102 | 84 ± 64 *,B | 84 ± 70 *,B | 340 ± 105B | 341 ± 156B |
| Forearm to finger gradient (°C) | 0.9 ± 2.8 | 4.7 ± 3.2 *,B | 5.7 ± 4.3 *,B | −1.3 ± 2.0 B | −0.1 ± 1.0 1 |
| Perceptual measures | |||||
| Affect (a.u.) | 3.2 ± 1.9 | 2.3 ± 1.7B | 1.5 ± 1.9B,1 | 2.1 ± 1.7B | 1.7 ± 1.9B |
| Thermal sensation (a.u.) | 3.8 ± 0.4 | 2.7 ± 0.4 *,B | 2.5 ± 0.7 *,B | 5.5 ± 0.4 B | 5.6 ± 0.5 B |
| Thermal discomfort (a.u.) | 1.1 ± 0.2 | 1.7 ± 0.4 *,B | 1.8 ± 0.4 *,B | 2.1 ± 0.4 B | 2.2 ± 0.6 B |
| Sweating perception (a.u.) | 0.0 ± 0.0 | 0.0 ± 0.0 * | 0.0 ± 0.0 * | 0.6 ± 0.6 B | 0.9 ± 0.7 B |
| Shivering perception (a.u.) | 0.0 ± 0.0 | 1.1 ± 1.0 *,B | 1.3 ± 1.7 *,B | 0.0 ± 0.0 | 0.0 ± 0.0 |
Mean ± SD, C→W: the decision to move from cool to warm, W→C: the decision to move from warm to cool
Different from W→C (P ≤ 0.04)
Different from Baseline (P ≤ 0.03)
Different from First Behavior (P ≤ 0.03).
Discussion
The present study demonstrates that, despite modest differences in body temperature (Fig. 3), the initiation of thermoregulatory behavior coincides with reductions in blood pressure at W→C and increases in blood pressure at C→W (Fig. 4). The present study has also identified that cutaneous vasoconstriction and vasodilation leading up to and upon C→W and W→C (Table 2) are largely reflex mediated (Fig. 7). These data uniquely demonstrate the cardiovascular and cutaneous vascular adjustments that occur upon the initiation of thermoregulatory behavior in young healthy adults.
Cardiovascular responses
It is well established that heat and cold present hypo- and hyper- tensive challenges that occur secondary to reductions or increases in vascular resistance, which promote heat loss or conservation.3 In young healthy adults exposed to heat and subsequent increases in skin and/or internal temperature, blood pressure is typically well maintained owing to appropriate increases in cardiac output that are mediated by elevations in heart rate and the maintenance of stroke volume.34 It is typically only after relatively large increases in internal temperature (+1.0–1.5°C) that hypotension is observed.35,36 Given that rectal temperature was lower and mean skin temperature was moderately higher than Baseline (Fig. 3), reductions in blood pressure leading up to and upon W→C were unexpected. The observed reductions in blood pressure at W→C (Fig. 3) were likely the result of decreases in total peripheral resistance that were not completely offset by increases in cardiac output (Fig. 5). Importantly, the moderate increases in heart rate and cardiac output leading up to and upon W→C (Fig. 5) did not alter indices of stroke work, rate pressure product, or cardiac power output (Fig. 6).
The mechanism for the hypotensive response at W→C is not inherently clear from the present study. For instance, it could be that the observed hypotension at W→C was a byproduct of the shuttle box methodology. A dynamic change in skin temperature, as would occur when moving from the cool to the warm room, could result in a greater reduction in vascular resistance compared to more gradual increases in skin temperature due to the initial hyper-responsiveness of thermoreceptors during dynamic temperature changes.37 Notably however, during the 90 s immediately leading up to W→C skin temperatures were stable (Fig. 3). This suggests that the hypotension at W→C was not an artifact of the shuttle box experimental paradigm and corresponding dynamic changes in skin temperature. Rather, the hypotensive response at W→C is likely due to the temporal cardiovascular changes induced by acute heat exposure. For instance, increases in skin temperature, independent of a rise in internal temperature, have been shown to induce a transient hypotension (by ˜5 mmHg) that is largely abolished as internal temperature begins to rise and cardiac output increases.38,39 This is likely due to the activation of baroreflex mechanisms.40 Our data corroborate these findings such that cardiac output was greater than Baseline immediately prior to W→C (Fig. 5), suggesting that the hypotension would have been reversed had the heat exposure been sustained. These findings highlight the importance of understanding the mechanisms of continuous cardiovascular adjustments during thermal transients, particularly as these situations are regularly encountered in many real world situations.
Reducing mean skin temperature without changing internal temperature is well known to elicit mild hypertension in young healthy adults.41-43 This pressor response is largely due to increases in vascular resistance, as cardiac output does not change under such circumstances.41,44 In the present study, skin temperatures at C→W were 33.5 ± 0.7°C (Fig. 3), temperatures that are well above the 29–31°C skin temperatures that are known to elicit cold induced pressor responses.41-43 Thus, the observation that blood pressure was elevated leading up to and upon C→W (Fig. 4) was unexpected. It is likely that the hypertensive responses at C→W were due to increases in total peripheral resistance that were not accompanied by reductions in cardiac output (Fig. 5). Notably, the modest pressor response at C→W increased the amount of work completed by the left ventricle on a per beat basis (i.e., stroke work, Fig. 5). This observation is presumably due to cold induced increases in cardiac afterload.44 However, rate pressure product and cardiac power output, per minute indices of myocardial oxygen demand and left ventricular power, were unaffected at C→W (Fig. 6). This observation is likely due to the cold induced decreases in heart rate (Fig. 5). Thus, cardiovascular strain per unit time leading up to and upon C→W was minimal despite moderate increases in blood pressure.
The reason for the hypertensive response at C→W, despite modestly cool skin temperatures, is not inherently clear, but may be explained by a combination of 4 factors. First, rectal temperatures were lower at C→W compared to Baseline, which may have promoted further increases in vascular resistance for a given skin temperature. Second, the lower temperature of the extremities at C→W compared to Baseline, independent of mean skin temperature, may have been a sufficient hypertensive stimulus. Third, as alluded to above, the shuttle box methodology may have exaggerated blood pressure responses when moving from the warm into the cool room owing to thermoreceptor characteristics during dynamic temperature changes.37 Notably however, during the 90 s leading up to C→W skin temperatures were stable (Fig. 3), suggesting that such effect was minimal. Fourth, this study was conducted in the summer months, and despite that heat acclimatization is minimal during the summer months in a humid continental climate,31 our subjects may have exhibited heightened autonomic sensitivity (i.e., increases in vascular resistance) to cool temperatures in the warmer months.45 This is supported by data, which were collected during spring months, that indicate that thermal behavior in the cold was initiated when skin temperatures were much cooler (˜29°C).11,12 It should be noted however, that this comparison should be approached cautiously as: i) the cool temperatures utilized in the shuttle box methodology in these studies were more extreme than those used in the present study,11,12 and ii) thermal preference is not different between the summer and winter months in healthy young adults.46 Thus, further research is required to elucidate the mechanisms by which modest cold induces moderate hypertension during thermal transients.
Skin blood flow responses
The present study confirms previous indirect evidence 11 that the initiation of thermoregulatory behavior is accompanied by cutaneous vasoconstriction and vasodilation leading up to and upon C→W and W→C (Table 2). By comparing skin blood flow responses at a forearm location clamped at 34°C to that in which local temperature was allowed to naturally change, the present study also demonstrates that skin blood flow leading up to and upon C→W and W→C is mostly under reflex control with minimal influence of control mechanisms associated with local changes in skin temperature (Fig. 7). Such findings were expected given previous data indicating that skin blood flow at skin temperatures between 33–35°C is largely under reflex control.14 Independent of changes in internal temperature, moderately cool skin temperatures elicit cutaneous vasoconstriction that is mediated via increases in sympathetic cutaneous vasoconstrictor tone,47 and moderately warm skin temperatures elicit cutaneous vasodilation via the withdrawal of cutaneous vasoconstrictor tone and an increase in sympathetic vasodilator activity.48 Based on the skin blood flow and CVC responses at the finger, a region innervated by only cutaneous vasoconstrictor nerves,25 it is likely that cutaneous vasoconstrictor activity was increased at C→W and decreased W→C (Table 2). Unfortunately, the present study is unable to discern the relative contribution of cutaneous vasodilator activity. Previous data would suggest that the skin blood flow responses leading up to and upon W→C are due to both cutaneous vasoconstrictor withdrawal and an increase in vasodilator activity,48 but this remains to be experimentally determined leading up to and upon the initiation of thermal behavior.
First vs. final behavior
To obtain Baseline data, the thermal behavior assessment commenced during room cooling (Fig. 1). This necessity encouraged analysis of any changes that may be occurring over time. Thus, data collected upon the first and final behavior at C→W and W→C were compared. Interestingly, throughout the shuttle box paradigm rectal temperature fell and the skin temperatures upon which behavior was initiated increased (Table 3). Although novel given that previous studies utilizing this methodology did not conduct such an analysis,11,12 these findings were not unlikely given the influence of posture on rectal temperature.49,50 Whether these progressive reductions in rectal temperature meaningfully influence thermal behavior remains uncertain. However, given that the mean skin temperatures upon which behavior was initiated were higher upon the final behavior (Table 3), suggests an effect is likely.
That thermal behavior was initiated despite differences in mean skin and rectal temperature between the first and final behavior seemingly supports previous studies that suggested thermoregulatory behavior is not mediated by threshold changes in body temperature.12,33 However, given that fingertip temperatures were not different between the first and final behavior at both C→W and W→C, these data highlight the potential role of extremity temperature in modulating thermal behavior (Table 3). This is supported by the findings that thermal discomfort, thermal sensation, and perceptions of shivering and sweating were not different upon C→W and W→C between the first and final behaviors (Table 3). The temperature of the extremities strongly influences thermal perception in the cold.51,52 The present data indicate that these changes in thermal perception are translated to initiate thermal behavior even when mean skin temperatures are only modestly cool (Table 3). These findings support data indicating that thermal perception ultimately drives thermoregulatory behavior.53 A role for the temperature of the extremities as a mediator of thermal perception in the heat appears unlikely, as the temperatures of the face and neck have a stronger influence on thermal perception.54,55 Thus, because fingertip temperatures were not different between the first and final behavior is likely a function of the withdrawal of cutaneous vasoconstrictor tone that, based on the fingertip skin blood flow data, occurred similarly upon the first and final behavior (Table 3). Unfortunately, we did not measure the temperature of the face and neck, so we cannot speculate regarding the potential role of these locations in the initiation of thermal behavior. However, such an influence appears likely.11
Surprisingly, despite differences in mean skin and rectal temperature, the cardiovascular and skin blood flow responses at C→W and W→C were not different between the first and final behaviors (Table 3). Such findings might suggest a potential role of the temperature of the extremities in modulating cardiovascular and skin blood flow responses or that thermal/cardiovascular efferent signals play a role in the decision to behaviorally thermoregulate. However, any relationships are unknown.
Considerations
There are a number of methodological considerations that warrant attention. First, Modelflow underestimates stroke volume during whole-body passive heat stress sufficient to raise internal temperature ˜1.2°C 56. Thus, our estimates of stroke volume may be inaccurate. However, the use of Modelflow in the present study was deemed acceptable for 2 reasons: i) To our knowledge there are no other methods that can provide an online measure of stroke volume, and ii) It is unlikely that moderate changes in skin temperature modify aortic impedance and compliance. These factors are inherent to the Modelflow estimate of stroke volume and have been proposed as the reason why Modelflow is inaccurate during passive heat stress.56 Second, given that rectal temperature is slow to change to dynamic alterations in body heat loss or gain,57 our measurement of rectal temperature may not have been sensitive to small changes in body heat content. Nevertheless, we deem the measurement of rectal temperature acceptable for 2 reasons: i) Neither esophageal or rectal temperature meaningfully change prior to the initiation of thermoregulatory behavior using a similar experimental paradigm and subject population.11 This is supported by the present study, whereby rectal temperature was slightly higher at C→W compared to W→C (Fig. 3). And ii) changes in internal temperature were not the focus of the present study. Third, given that the accuracy of mean skin temperature measurements is generally improved as the number of measurement locations increases,58,59 our 6 site mean skin temperature measurement may not have accurately portrayed the average temperature of the skin in our study. This is perhaps best highlighted in our fingertip temperature data in which the temperature of this extremity did not follow that of mean skin temperature (Table 2 and 3). Thus, based on current recommendations,58 future studies should utilize 10 or more skin measurement sites. Fourth, our baseline thermal conditions may have confounded our skin blood flow conclusions at C→W, owing to a potential basement effect associated with prevailing vasoconstrictor tone, as demonstrated in the relatively low skin blood flow values at the not clamped location (Table 2 and 3, Fig. 7). A potential influence of baseline vasoconstrictor tone cannot be discounted seeing as the cutaneous vasculature is tonically under vasoconstrictor control in humans.60 However, these baseline conditions likely did not elicit maximal vasoconstriction and thus, were deemed acceptable given that: i) subjects deemed the environment as thermally comfortable (Table 3), ii) the forearm to finger temperature gradient was minimal (Table 2 and 3), and iii) fingertip skin blood flow demonstrated a profile that was neither vasoconstricted nor vasodilated (Table 2 and 3). However, future studies aiming to evaluate relationships between skin blood flow and thermal behavior should take into consideration the baseline thermal conditions and whether such conditions influence their conclusions. Fifth, behavioral thermoregulation exhibits circadian variations,61 but we did not control for time of day. This was considered acceptable because all comparisons were within subject. However, it should be noted that the effects of time of day on the cardiovascular and skin blood flow responses leading up to and upon the decision to behaviorally thermoregulate remains unknown. Finally, the current study utilized both male and female subjects, and controlled for the menstrual cycle. We are underpowered to make formal comparisons between males and females. However, differences appear likely given that sex modulates autonomic temperature regulation 30 and thermal perception.62,63 Formal research is required to discern the effect of sex on behavioral temperature regulation and the cardiovascular and autonomic responses leading up to and upon the initiation of this behavior.
Perspectives
Cardiovascular health is particularly susceptible to hot and cold ambient temperatures.2 Such deleterious health outcomes have been suggested to be driven by the cardiovascular adjustments that occur secondary to changes in body temperature.5,6 These adjustments can acutely increase cardiovascular risk,4 particularly in populations with an already compromised cardiovascular system. The current study demonstrates the presence of meaningful cardiovascular and cutaneous vascular adjustments leading up to and upon the initiation of thermoregulatory behavior, despite that this behavior was elicited by relatively modest changes in skin temperature. Such findings further highlight the role that skin temperature plays in both autonomic and behavioral temperature regulation,64-67 and invites the study of behavioral thermoregulation in those populations at risk of cardiovascular morbidity and mortality during heat and cold events (e.g., older adults, heart failure patients, diabetics, etc. 68). Such studies should be considered of vital importance given the predicted increases in the frequency and severity of heat events and the persistence of periodic cold events in the coming decades.1
Conclusions
The present study demonstrates that the initiation of thermal behavior in young healthy adults is preceded by reductions in blood pressure in a warm environment and increases in blood pressure in a cool environment, despite that this behavior was initiated following relatively modest changes in skin temperature. These hypo- and hyper- tensive responses are likely driven by changes in vascular resistance that were not offset by changes in cardiac output. As a result, the decision to behaviorally thermoregulate in a cool environment was accompanied by an increase in stroke work, while in a warm environment there was minimal evidence of cardiovascular strain upon the initiation of this behavior. The present study has also identified that cutaneous vasomotor responses leading up to and upon the decision to initiate thermal behavior are largely reflex mediated. These data highlight the role of skin temperature in modulating both autonomic and behavioral temperature regulation in young healthy adults, and demonstrate the importance of understanding interactions between skin temperature and cardiovascular adjustments in healthy, diseased, and at risk populations.
Abbreviations
- C→W
The decision to move from the cool to the warm room
- CVC
Cutaneous vascular conductance
- PU
Perfusion units
- W→C
The decision to move from the warm to the cool room
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the subjects for participating in our study. We would also like to thank Lindsey Russo, MS, for her technical assistance and Dr. David Hostler for his assistance with participant screening.
References
- [1].IPCC Climate Change 2013 - The Physical Science Basis. New York, NY, USA: Cambridge University Press, 2013. [Google Scholar]
- [2].Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiol 2009; 20:205; http://dx.doi.org/ 10.1097/EDE.0b013e318190ee08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilson TE, Klabunde RE, Monahan KD. Using thermal stress to model aspects of disease states. J Therm Biol 2014; 43:24-32; PMID:24956954; http://dx.doi.org/ 10.1016/j.jtherbio.2014.03.003 [DOI] [PubMed] [Google Scholar]
- [4].Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation 2006; 114:1863-72; PMID:17060396; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.105.596189 [DOI] [PubMed] [Google Scholar]
- [5].Kenney WL, Craighead DH, Alexander LM. Heat waves, aging and human cardiovascular health. Med Sci Sport Exer 2014; 46:1891-9; http://dx.doi.org/ 10.1249/MSS.0000000000000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De Blois J, Kjellstrom T, Agewall S, Ezekowitz J, Armstrong P, Atar D. The effects of climate change on cardiac health. Cardiology 2015; 131:209-17; PMID:25997478; http://dx.doi.org/ 10.1159/000398787 [DOI] [PubMed] [Google Scholar]
- [7].Schlader ZJ, Stannard SR, Mundel T. Human thermoregulatory behavior during rest and exercise - a prospective review. Physiol Behav 2010; 99:269-75; PMID:20006632; http://dx.doi.org/ 10.1016/j.physbeh.2009.12.003 [DOI] [PubMed] [Google Scholar]
- [8].Hardy JD. Thermal comfort and health. Ashrae Journal 1971; 13:43-51. [Google Scholar]
- [9].Parsons KC. Human thermal environments: The effects of hot, moderate, and cold environments on human health, comfort, and performance. Boca Raton, FL: CRC Press, 2002. [Google Scholar]
- [10].Cabanac M, Bleichert R, Massonne B.. Preferred skin temperature as a function of internal and mean skin temperature. J Appl Physiol 1972; 33:699-703; PMID:4643844 [DOI] [PubMed] [Google Scholar]
- [11].Schlader ZJ, Perry BG, Che Jusoh MR, Hodges LD, Stannard SR, Mundel T. Human temperature regulation when given the opportunity to behave. Eur J Appl Physiol 2013; 113:1291-301; PMID:23179204; http://dx.doi.org/ 10.1007/s00421-012-2544-0 [DOI] [PubMed] [Google Scholar]
- [12].Schlader ZJ, Prange HD, Mickleborough TD, Stager JM. Characteristics of the control of human thermoregulatory behavior. Physiol Behav 2009; 98:557-62; PMID:19748517; http://dx.doi.org/ 10.1016/j.physbeh.2009.09.002 [DOI] [PubMed] [Google Scholar]
- [13].Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 2007; 292:R37-46; PMID:17008453; http://dx.doi.org/ 10.1152/ajpregu.00668.2006 [DOI] [PubMed] [Google Scholar]
- [14].Savage MV, Brengelmann GL. Control of skin blood flow in the neutral zone of human body temperature regulation. J Appl Physiol 1996; 80:1249-57; PMID:8926253 [DOI] [PubMed] [Google Scholar]
- [15].Johnson J. Nonthermoregulatory control of human skin blood flow. J Appl Physiol 1986; 61:1613-22; PMID:3536836 [DOI] [PubMed] [Google Scholar]
- [16].Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 73:863-71; http://dx.doi.org/ 10.1001/archinte.1916.00080130010002 [DOI] [Google Scholar]
- [17].Siri WE. Body composition from fluid spaces and density: analysis of methods In: Henschel A J. B, eds. Techniques for measuring body composition. Washington DC: National Academy of Sciences, National Research Coucil, 1961:223-43. [Google Scholar]
- [18].Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978; 40:497-504; PMID:718832; http://dx.doi.org/ 10.1079/BJN19780152 [DOI] [PubMed] [Google Scholar]
- [19].Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc 1980; 12:175-81; PMID:7402053 [PubMed] [Google Scholar]
- [20].Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 1989; 66:1586-92; PMID:2732150 [DOI] [PubMed] [Google Scholar]
- [21].Wesseling K, Jansen J, Settels J, Schreuder J. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 1993; 74:2566-73; PMID:8335593 [DOI] [PubMed] [Google Scholar]
- [22].Wilson T, Brothers R, Tollund C, Dawson E, Nissen P, Yoshiga C, Jons C, Secher NH, Crandall C. Effect of thermal stress on Frank–Starling relations in humans. The Journal of physiology 2009; 587:3383-92; PMID:19417092; http://dx.doi.org/ 10.1113/jphysiol.2009.170381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gobel FL, Norstrom L, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 1978; 57:549-56; PMID:624164; http://dx.doi.org/ 10.1161/01.CIR.57.3.549 [DOI] [PubMed] [Google Scholar]
- [24].Schlader ZJ, Mundel T, Barnes MJ, Hodges LD. Peak cardiac power output in healthy, trained males. Clin Physiol Funct Imaging 2010; 30:480 - 4; PMID:20718806; http://dx.doi.org/ 10.1111/j.1475-097X.2010.00959.x [DOI] [PubMed] [Google Scholar]
- [25].Johnson J, Pergola P, Liao F, Kellogg D, Crandall C. Skin of the dorsal aspect of human hands and fingers possesses an active vasodilator system. Journal of Applied Physiology 1995; 78:948-54; PMID:7775340 [DOI] [PubMed] [Google Scholar]
- [26].House JR, Tipton MJ. Using skin temperature gradients or skin heat flux measurements to determin thresholds of vasoconstriction and vasodilatation. Eur J Appl Physiol 2002; 88:141-5; PMID:12436282; http://dx.doi.org/ 10.1007/s00421-002-0692-3 [DOI] [PubMed] [Google Scholar]
- [27].Rubinstein EH, Sessler DI. Skin-Surface Temperature-Gradients Correlate with Fingertip Blood-Flow in Humans. Anesthesiology 1990; 73:541-5; PMID:2393139; http://dx.doi.org/ 10.1097/00000542-199009000-00027 [DOI] [PubMed] [Google Scholar]
- [28].Hardy CJ, Rejeski WJ. Not what, but how one feels: The measurement of affect during exercise. J Sport Exerc Psychol 1989; 11:304-17. [Google Scholar]
- [29].Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1967; 1:1-20; PMID:5614624; http://dx.doi.org/ 10.1016/0013-9351(67)90002-3 [DOI] [PubMed] [Google Scholar]
- [30].Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol 2012; 590:5963-73; PMID:23045336; http://dx.doi.org/ 10.1113/jphysiol.2012.240739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bain AR, Jay O. Does summer in a humid continental climate elicit an acclimatization of human thermoregulatory responses? Eur J Appl Physiol 2011; 111:1197-205; PMID:21127900; http://dx.doi.org/ 10.1007/s00421-010-1743-9 [DOI] [PubMed] [Google Scholar]
- [32].Schlader ZJ, Stannard SR, Mundel T. Evidence for thermoregulatory behavior during self-paced exercise in the heat. J Therm Biol 2011; 36:390-6; http://dx.doi.org/ 10.1016/j.jtherbio.2011.07.002 [DOI] [Google Scholar]
- [33].Barber BJ, Crawford EC. Dual threshold control of peripheral temperature in the lizard dipsosaurus-dorsalis. Physiol Zool 1979; 52:250-63; http://dx.doi.org/ 10.1086/physzool.52.2.30152568 [DOI] [Google Scholar]
- [34].Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Comp Physiol 2015; 5:17-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ganio MS, Brothers RM, Lucas RA, Hastings JL, Crandall CG. Validity of auscultatory and Penaz blood pressure measurements during profound heat stress alone and with an orthostatic challenge. Am J Physiol Regul Integr Comp Physiol 2011; 301:R1510-6; PMID:21832209; http://dx.doi.org/ 10.1152/ajpregu.00247.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schlader ZJ, Gagnon D, Lucas RA, Pearson J, Crandall CG. Baroreceptor unloading does not limit forearm sweat rate during severe passive heat stress. J Appl Physiol 2015; 118:449-54; PMID:25525210; http://dx.doi.org/ 10.1152/japplphysiol.00800.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dostrovsky JO, Hellon RF. The representation of facial temperature in the caudal trigeminal nucleus of the cat. J Physiol 1978; 277:29-47; PMID:650531; http://dx.doi.org/ 10.1113/jphysiol.1978.sp012258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 1998; 84:1323-32; PMID:9516200 [DOI] [PubMed] [Google Scholar]
- [39].Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 1969; 27:673-80; PMID:5360442 [DOI] [PubMed] [Google Scholar]
- [40].Crandall CG. Heat stress and baroreflex regulation of blood pressure. Med Sci Sports Exer 2008; 40:2063; PMID:18981943; http://dx.doi.org/ 10.1249/MSS.0b013e3-18180bc98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 2007; 103:1257-62; PMID:17673561; http://dx.doi.org/ 10.1152/japplphysiol.00401.2007 [DOI] [PubMed] [Google Scholar]
- [42].Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol-Heart Circulat Physiol 2005; 289:H2429-H33; PMID:16024573; http://dx.doi.org/ 10.1152/ajpheart.00383.2005 [DOI] [PubMed] [Google Scholar]
- [43].Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol 2014; 117:648-57; PMID:25103970; http://dx.doi.org/ 10.1152/japplphysiol.00516.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1627-R33; PMID:20375268; http://dx.doi.org/ 10.1152/ajpregu.00099.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Werner J. Beneficial and detrimental effects of thermal adaptation Thermal Balance in Health and Disease: Springer, 1994:141-54. [Google Scholar]
- [46].Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K. Preferred ambient temperature for old and young men in summer and winter. Int J Biometeorol 1992; 36:1-4; PMID:1582717; http://dx.doi.org/ 10.1007/BF01208726 [DOI] [PubMed] [Google Scholar]
- [47].Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003; 78:603-12; PMID:12744548; http://dx.doi.org/ 10.4065/78.5.603 [DOI] [PubMed] [Google Scholar]
- [48].Kamijo Y-I, Lee K, Mack GW. Active cutaneous vasodilation in resting humans during mild heat stress. J Appl Physiol 2005; 98:829-37; PMID:15489258; http://dx.doi.org/ 10.1152/japplphysiol.00235.2004 [DOI] [PubMed] [Google Scholar]
- [49].Cranston W, Gerbrandy J, Snell E. Oral, rectal and oesophageal temperatures and some factors affecting them in man. J Physiol 1954; 126:347; PMID:13222289; http://dx.doi.org/ 10.1113/jphysiol.1954.sp005214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nielsen M, Herrington LP, Winslow C-E. The effect of posture upon peripheral circulation. Am J Physiol Legacy Cont 1939; 127:573-80. [Google Scholar]
- [51].Arens E, Zhang H, Huizenga C. Partial- and whole-body thermal sensation and comfort - Part 1: Uniform environmental conditions. J Therm Biol 2006; 31:53-9; http://dx.doi.org/ 10.1016/j.jtherbio.2005.11.028 [DOI] [Google Scholar]
- [52].Arens E, Zhang H, Huizenga C. Partial- and whole-body thermal sensation and comfort - Part II: Non-uniform environmental conditions. J Therm Biol 2006; 31:60-6; http://dx.doi.org/ 10.1016/j.jtherbio.2005.11.027 [DOI] [Google Scholar]
- [53].Schlader ZJ, Simmons SE, Stannard SR, Mundel T. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav 2011; 103:217-24; PMID:21315099; http://dx.doi.org/ 10.1016/j.physbeh.2011.02.002 [DOI] [PubMed] [Google Scholar]
- [54].Cotter JD, Taylor NA. The distribution of cutaneous sudomotor and alliesthesial thermosensitivity in mildly heat-stressed humans: an open-loop approach. J Physiol 2005; 565:335-45; PMID:15760945; http://dx.doi.org/ 10.1113/jphysiol.2004.081562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nakamura M, Yoda T, Crawshaw LI, Yasuhara S, Saito Y, Kasuga M, Nagashima K, Kanosue K. Regional differences in temperature sensation and thermal comfort in humans. J Appl Physiol 2008; 105:1897-906; PMID:18845785; http://dx.doi.org/ 10.1152/japplphysiol.90466.2008 [DOI] [PubMed] [Google Scholar]
- [56].Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol 2011; 300:R486-R91; PMID:21084673; http://dx.doi.org/ 10.1152/ajpregu.00505.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mündel T, Carter JM, Wilkinson DM, Jones DA. A comparison of rectal, oesophageal and gastro‐intestinal tract temperatures during moderate‐intensity cycling in temperate and hot conditions. Clin Physiol Funct Imaging 2014; PMID:25178454 [DOI] [PubMed] [Google Scholar]
- [58].Liu W, Lian Z, Deng Q, Liu Y. Evaluation of calculation methods of mean skin temperature for use in thermal comfort study. Build Environ 2011; 46:478-88; http://dx.doi.org/ 10.1016/j.buildenv.2010.08.011 [DOI] [Google Scholar]
- [59].Mitchell D, Wyndham CH. Comparison of weighting formulas for calculating mean skin temperature. J Appl Physiol 1969; 26:616-22; PMID:5781615 [DOI] [PubMed] [Google Scholar]
- [60].Pergola PE, Kellogg DL, Johnson JM, Kosiba WA. Reflex control of active cutaneous vasodilation by skin temperature in humans. Am J Physiol 1994; 266:H1979-84; PMID:8203597 [DOI] [PubMed] [Google Scholar]
- [61].Cabanac M, Hildebrandt G, Massonnet B, Strempel H. A study of the nycthemeral cycle of behavioural temperature regulation in man. J Physiol 1976; 257:275-91; PMID:985881; http://dx.doi.org/ 10.1113/jphysiol.1976.sp011368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gerrett N, Ouzzahra Y, Redortier B, Voelcker T, Havenith G. Female thermal sensitivity to hot and cold during rest and exercise. Physiol Behav 2015; 152:11-9; PMID:26343771; http://dx.doi.org/ 10.1016/j.physbeh.2015.08.032 [DOI] [PubMed] [Google Scholar]
- [63].Gerrett N, Ouzzahra Y, Coleby S, Hobbs S, Redortier B, Voelcker T, Havenith G. Thermal sensitivity to warmth during rest and exercise. A sex comparison. Eur J Appl Physiol 2014; 114:1451-62. [DOI] [PubMed] [Google Scholar]
- [64].Romanovsky A. Skin temperature: its role in thermoregulation. Acta Physiol 2014; 210:498-507; http://dx.doi.org/ 10.1111/apha.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bratincsak A, Palkovits M. Evidence that peripheral rather than intracranial thermal signals induce thermoregulation. Neuroscience 2005; 135:525-32; PMID:16125855; http://dx.doi.org/ 10.1016/j.neuroscience.2005.06.028 [DOI] [PubMed] [Google Scholar]
- [66].Flouris AD, Cheung SS. Thermal basis of finger blood flow adaptations during abrupt perturbations in thermal homeostasis. Microcirculation 2011; 18:56-62; PMID:21166926; http://dx.doi.org/ 10.1111/j.1549-8719.2010.00068.x [DOI] [PubMed] [Google Scholar]
- [67].Almeida M, Vizin R, Carrettiero D. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature 2015; 2:483-90; http://dx.doi.org/ 10.1080/23328940.2015.1095270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ 2010; 182:1053-60; PMID:19703915; http://dx.doi.org/ 10.1503/cmaj.081050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al.. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35:1381-95; PMID:12900694; http://dx.doi.org/ 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]


