Abstract
During natural parasitization, Asobara japonica wasps introduce lateral oviduct (LO) components into their Drosophila hosts soon after the venom injection to neutralize its strong toxicity; otherwise, the host will die. Although the orchestrated relationship between the venom and LO components necessary for successful parasitism has attracted the attention of many researchers in this field, the molecular natures of both factors remain ambiguous. We here showed that precipitation of the venom components by ultracentrifugation yielded a toxic fraction that was inactivated by ultraviolet light irradiation, boiling, and sonication, suggesting that it is a virus-like entity. Morphological observation of the precipitate after ultracentrifugation showed small spherical heterogeneous virus-like particles 20–40 nm in diameter. The venom’s detrimental effect on D. melanogaster larvae was not directly neutralized by the LO components but blocked by a hemolymphal neutralizing factor activated by the LO factor. Furthermore, we found that A. japonica venom and LO components acted similarly on the larvae of the common cutworm Spodoptera litura: the venom injection caused mortality but coinjection of the LO factor protected S. litura larvae from the venom’s toxicity. In contrast, D. ficusphila and D. bipectinata, which are closely related to D. melanogaster but non-habitual host species of A. japonica, were not negatively affected by A. japonica venom due to an intrinsic neutralizing activity in their hemolymph, indicating that these species must have acquired a neutralizer of A. japonica venom during evolution. These results give new insights into the characteristics of both the venom and LO components: A. japonica females have utilized the virus-like toxic venom factor to exploit a wider range of host species after the evolutionary process enabled them to use the LO factor for activation of the host hemolymph neutralizer precursor, although the non-habitual host Drosophila species possess an active intrinsic neutralizer in their hemolymph.
Introduction
During evolution, endoparasitoid wasps have developed a variety of strategies for successful parasitism[1–3]. The innate immune system of host insects serves as a defense not only against microbial infection but also against parasitoids; therefore, habitual parasitoid wasps must manipulate the host immune system. In other words, only parasitoids that acquired the strategies to overcome the host defense systems have survived the long battle against host insects[4–8]. It is well known that many endoparasitoids harbor viruses or virus-like particles in their reproductive apparatus and that these particles are introduced into the host at parasitization[9–15]. Polydnaviruses (PDVs) are among the best-known examples of endoparasitoid symbiotic effectors[16–18]. PDVs manipulate the host defense system by using a variety of strategies, often categorized as passive or active[19–21]. As a passive strategy, PDV particles possess surface features that prevent the host from recognizing the parasitoid as non-self. For example, parasitoid Cotesia kariyai eggs and larvae are covered by many molecules of immunoevasive protein (IEP), one component of the C. kariyai PDV particles, which is not recognized as non-self by hemocytes of the wasp’s host[22–25]. An active strategy is suppression of the host immune system due to expression of various virulent factors coded on PDV genomes. Ankyrin-repeat motif proteins[26–28] and protein tyrosine phosphatases[29–31], which are widely distributed in many PDVs, have been reported as virulence factors that are responsible for disrupting the function of immune cells. Although the detailed mechanism of PDV-induced suppression of the host immunity is not fully understood, PDV-carrying parasitoid wasps are generally thought to owe successful parasitism to their PDVs.
Parasitoid wasps A. japonica are generalist parasitoids that successfully parasitize many Drosophila species but lack PDVs. Instead of PDVs, A. japonica wasps utilize a venom component for successful parasitization. We previously showed that the host Drosophila larvae are killed by envenomization at a dose that is naturally injected by A. japonica female wasps at parasitization[32, 33]. Such a toxic venom was demonstrated to be essential for the wasp to prevent host cellular immune defenses from killing the wasp’s progeny[33]. During natural parasitism, this toxicity is neutralized by subsequent injection of the lateral oviduct (LO) components; otherwise, the wasps cannot survive. Therefore, both the highly toxic venom and its neutralizer, LO, are indispensable for the successful parasitism of A. japonica. However, the functional consequences as well as the molecular natures of both factors remain ambiguous. Our present examination first revealed that the venom toxicity is due to a virus-ike entity because it was nullified by certain physical treatments such as UV irradiation and sonication, and precipitated by ultracentrifugation. Functional characterization of the LO components was then performed to elucidate how the LO factor neutralizes the venom toxicity. A series of characterizations demonstrated that the LO factor does not directly neutralize the venom toxicity but activates a neutralizer precursor that is present in the hemolymph of D. melanogaster larvae. Finally, a similar precursor of the venom neutralizer was found in the non-Drosophila insect Spodoptera litura larvae, which are also sensitive to A. japonica venom, while non-habitual host Drosophila species larvae retain the active form of the neutralizer that protects them from A. japonica parasitism.
Materials and Methods
Insects
Drosophila melanogaster have been maintained in our laboratory. D. ficusphila and D. bipectinata were collected in Iriomote-jima, Japan (Dr. Masahito Kimura (Hokkaido University) issued permission for collection) [32]. All fly strains were reared on cornmeal-malt-glucose-yeast medium at 23±1°C with a photoperiod of 15 h light: 9 h dark. Asobara japonica was collected in Tokyo, Japan, and reared in D. simulans or D. melanogaster as hosts. Parasitoids were maintained under the same temperature and light as the host strains[34].
Spodoptera litura was used for this study as an insect species example that is phylogenetically far from Drosophila. They were reared on an artificial diet (10% kidney beans, 10% wheat bran, 4.2% dried yeast, 0.5% ascorbic acid, 0.3% antiseptic reagents, and 1.3% agar (all w/w)) at 25°C with a photoperiod of 16 h light:8 h dark[35].
Microinjection of venom with/without lateral oviduct fluid into test larvae
Venom reservoirs and lateral oviducts were dissected from Asobara wasp females and were separately placed into a drop of chilled phosphate-buffered saline (PBS: 8 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl, pH 7.2). Venom reservoirs were lightly homogenized, lateral oviducts were squeezed with fine forceps, and centrifuged at 20,000 g for 15 min at 4°C to collect the supernatants[33]. In some experiments, the supernatant was further centrifuged at 120,000 g or 450,000 g for 30 min at 4°C.
Venom supernatant (after centrifugation at 20,000 g) (V) or a mixture of venom and lateral oviduct supernatants (V+LO) were diluted to 15 μl per a female with PBS. Six-day-old (third instar) Drosophila larvae were injected with 0.15 μl of each sample unless otherwise stated, put on the diet medium, and their survival rates were measured after injection. All test larva groups with survival rates higher than 60% at 24 h after injection normally produced puparia, and those with survival rates lower than 20% died within a few days. The fates of test larvae with survival rates between 20% and 60% at 24 h after injection varied in each case, and their subsequent survival rates are indicated in the figure legends.
When the above samples were injected into third instar larvae (body weight: 1.5 ± 0.1 mg) of S. litura, the amount of the sample was increased in proportion of S. litura larva weight to Drosophila larva weight.
Preparation of Drosophila larval hemolymph
After washing Drosophila larvae well with PBS, the body was partly dipped in 50 μl of chilled PBS containing 0.1% N -phenylthiourea (PBS-PTU), and the hemolymph was collected by slightly tearing the cuticle using fine forceps on ice. The collected hemolymph was immediately centrifuged at 2,000 rpm for 3 min at 4°C, the protein concentration of the supernatant was measured by the method of Bradford using a Bio-Rad Protein assay reagent with bovine serum albumin as standard[36], and used for the the following experiments. Twenty larvae were usually necessary to collect about 10 μl hemolymph.
Enzymatic treatment of venom components
After ultracentrifugation at 450,000 g for 30 min at 4°C, virus components (equivalent to two female) were treated by 0.01 μg/μl trypsin (Sigma-Aldrich, USA) at 25°C for 12 h, 0.2 units/μl DNase I (Takara, Japan) at 37°C for 2 h, 0.2 units/μl Benzonase (Merck, USA) at 37°C for 2 h, or 0.1 units/μl RNase A (Wako, Japan) at 37°C for 2 h. After each reaction, the reaction mixture was diluted 32 times and injected into test Drosophila larvae. Each ernzyme reaction mixture without virus components was used as a control sample.
Measurement of protease activity using synthetic substrate-MCA
The release of 7-amino-4-methylcoumarin from synthetic peptide-MCA substrate (Boc-Ile-Glu-Gly-Arg-MCA (Peptide Institute, Inc., Japan)) during the hydrolase reaction was detected using a multimode detector DTX800 (Beckman Coulter) as described previously[33]; the hydrolase activity was regarded as a protease activity of each sample. Hemolymph collected from test Drosophila larvae was placed into a 50 μl drop of chilled PBS-PTU, immediately centrifuged at 300 g for 3 min at 4°C, and the supernatant was collected. Five μl of each hemolymph sample was incubated in 100 μl of 10 mM Tris-HCl buffer solution (pH 7.8) containing 20 μM peptide-MCA at 30°C for 1 h, and the increased fluorescence of the mixture was measured to calculate protease activities in the samples. One unit (U) of the protease activity was defined as that which hydrolyzed 1 nmol of the peptide-MCA substrate within 1 min.
Production of anti-venom antibody
The pellet fraction after centrifugation at 450,000 g for 30 min was solubilized in 0.5% SDS and subcutaneously injected into rabbits with Freund’s complete adjuvant (TiterMax Gold, CytRx Corporation). Anti-venom IgG was precipitated by adding ammonium sulfate to 40% saturation and further purified by a protein A-Sepharose 4B column as described previously [37].
Microscopic observation
For negative-staining electron microscopic analysis, carbon-coated 300-mesh copper grids were exposed to glow discharge in air for 20 s. Venom pellet suspensions (20 μl) were placed on grids and incubated for 3 min. Negative-staining was performed with 2% phosphotungstic acid (PTA, pH 7.2) for 30 sec[38]. The specimens were observed using a JEM-1400 transmission electron microscope (JEOL, Japan).
Statistical analyses
For comparison of survival rates, body weights, and enzyme activities of test animals, Tukey’s HSD tests were carried out. A normality test, the Shapiro-Wilk test, showed that data sets do not deviate from the normality. All statistical analyses were performed using JMP 9.0.2 (SAS Institute).
Results
Functions of venom components
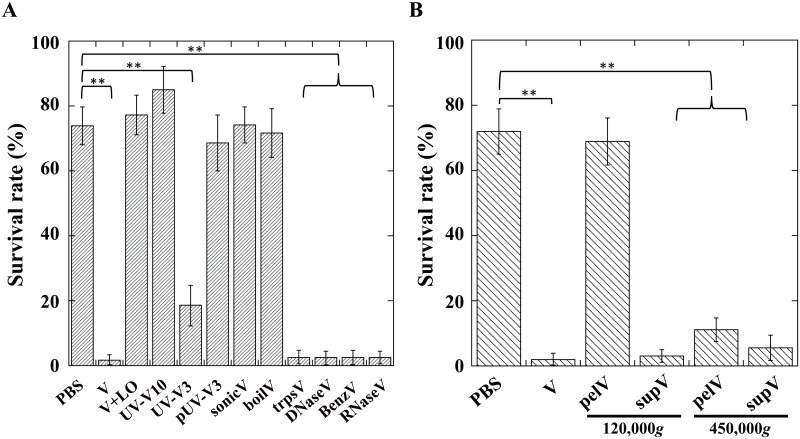
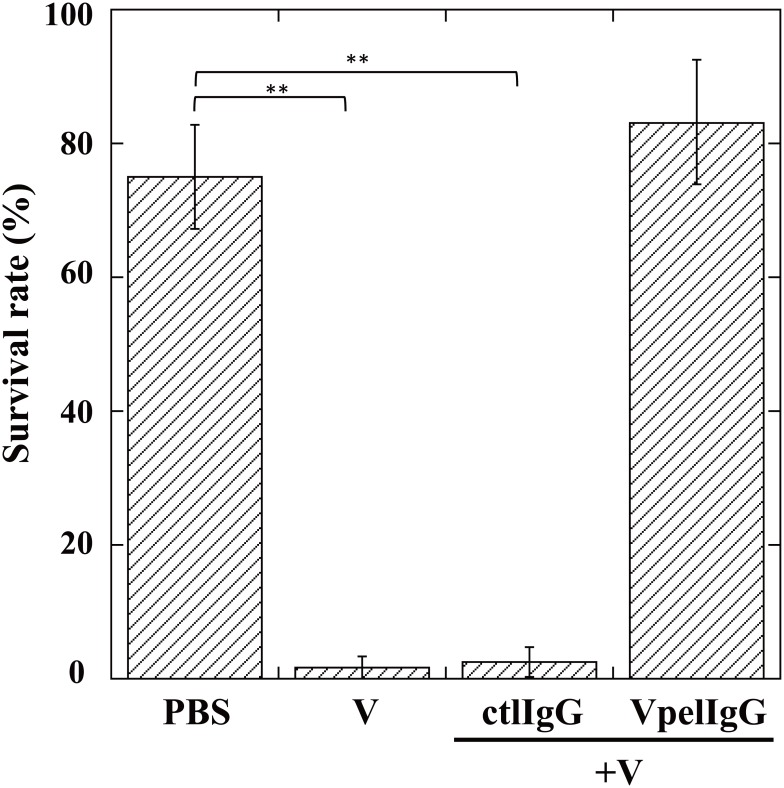
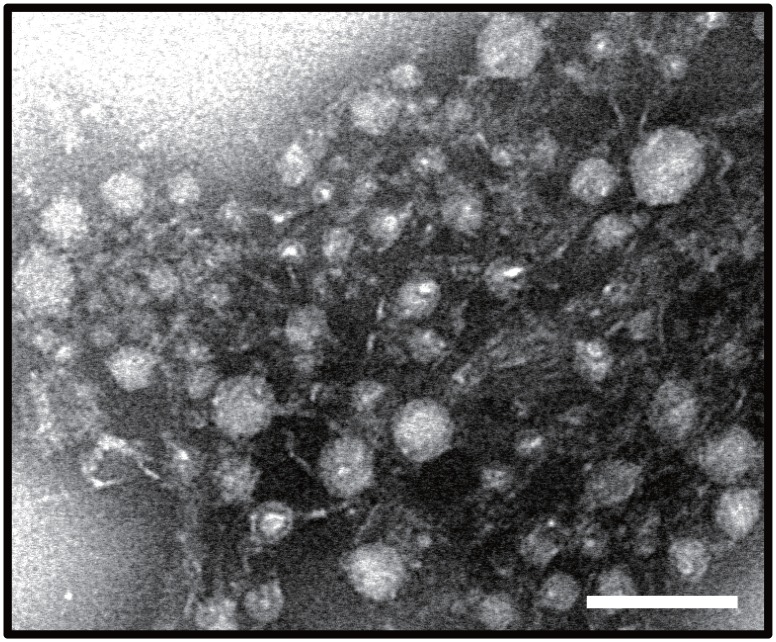
Introduction of only A. japonica venom components without subsequent injection of the oviduct components caused mortality of the host Drosophila larvae. To characterize the molecular nature of the venom toxic factor(s), we examined the effects of several physical or chemical treatments on the activity of the venom factor. Ultraviolet light (UV) irradiation, sonication, and boiling completely nullified the insecticidal effect of the venom factor. Although treatments with trypsin, DNase, Benzonase, or RNase did not affect its toxicity at all, addition of psoralen significantly shortened the period of UV irradiation required for inactivation of the venom toxicity, indicating the possibility that this toxicity is due to a virus-like particle with certain polynucleotides (Fig 1A), indicating the possibility that this toxicity is due to a virus-like particle containing certain polynucleotides. To assess this interpretation, venom components were centrifuged at 120,000 g or 450,000 g for 1 h. The toxic activity was not detected in the pellet after centrifugation at 120,000 g but was clearly detected in the pellet after centrifugation at 450,000 g (Fig 1B). These observations indicate that the venom toxic factor is not water-soluble but has a specific gravity high enough to be precipitated by ultracentrifugation at 450,000. Furthermore, the antibody against the pellet fraction after centrifugation at 450,000 g for 1 h neutralized the venom toxicity (Fig 2). To demonstrate the presence of certain virus particles in venom, the pellet fraction of the venom after centrifugation at 450,000 g was analyzed by electron microscopy. We found that the venom pellet fraction contained heterogeneous spherical virus-like particles whose diameters are approximately 20 to 40 nm (Fig 3), implying that virulent virus-like agent(s) could be one or some of these particles.
Fig 1. Survival rates of Drosophila melanogaster larvae one day after injection of indicated samples.
(A) PBS, venom (V), venom plus lateral oviduct (V+LO), venom irradiated with UV for 10 min (UV-V10), venom irradiated with UV for 3 min (UV-V3), venom plus 10 μg/ml psoralen irradiated with UV for 3 min (pUV-V3), venom sonicated for 5 min (sonicV), venom boiled for 5 min (boilV), trypsinized venom (trpsV), DNase-treated venom (DNaseV), Benzonase-treated venom (BenzV), or RNase-treated venom (RNaseV) was injected. Each value represents the mean ± S.D. for six independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (**P<0.01). (B) PBS, venom (V), pellet (pelV) or supernatant (supV) after centrifugation of venom at 120,000 g or 450,000 g was injected. Other explanations are as in (A).
Fig 2. Survival rates of Drosophila melanogaster larvae one day after injection of PBS, venom (V), venom plus non-immunized IgG (V+ctlIgG), or venom plus anti-venom pellet (after centrifugation) IgG (V+VpelIgG).
Venom components were treated with IgG for 3 h before injection into test D. melanogaster larvae. Each value represents the mean ± S.D. for five independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (**P<0.01).
Fig 3. Transmission electron micrographs of ultracentrifuged precipitate of venom.
A. japonica venom was centrifuged at 450,000 g for 1 h, and the pellet fraction was observed by transmission electron microscopy after negative-staining with aqueous phosphotungstic acid. Scale bar indicates 100 nm.
Neutralizing effect of lateral oviduct components
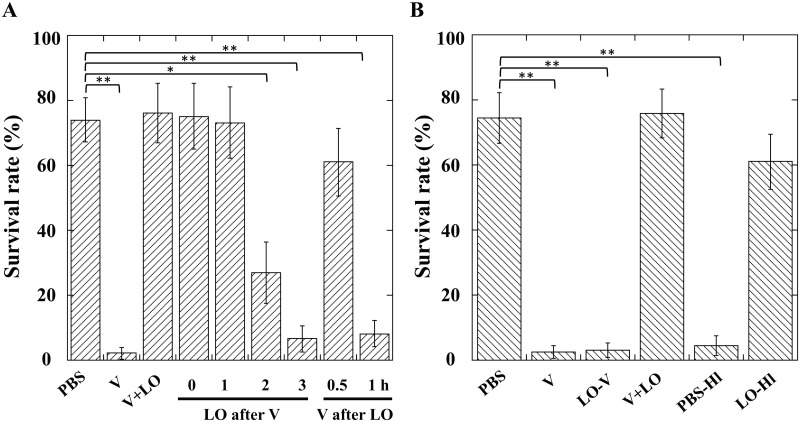
Although we previously showed that simultaneous injection of the LO components neutralizes the venom toxicity[32], we had not tested different timings of the LO component injection. We examined the effects of the LO injection at certain intervals before and after the venom injection. Injection of the LO components 1 h to 2 h prior to the venom injection showed a significant neutralizing effect on the venom toxin, although injection of the venom 3 h before did not. Injection of the LO components 0.5 h after the venom injection also neutralized the venom, while injection 1 h later did not (Fig 4A). Furthermore, direct pretreatment of venom factors with the LO components before injection did not neutralize the venom toxicity when the LO factor was removed by ultracentrifugation (Fig 4B). The hemolymph itself prepared from larvae preinjected with the LO components showed a neutralizing effect similar to the direct injection of the LO components (Fig 4B), suggesting that the LO components did not inactivate the toxic venom directly but activated a precursor form of the hemolymph neutralizer.
Fig 4. Survival rates of Drosophila melanogaster larvae one day after injection of indicated samples.
(A) PBS, venom (V), or venom plus lateral oviduct (V+LO) was injected at indicated intervals before or after injection of venom. Survival rates of test larvae injected the LO factor 2 h after the venom injection: 12.6 ± 9.5% at 48 h, 8.5 ± 5.4% at 72 h, 0% at 96 h. Each value represents the mean ± S.D. for seven independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (*P<0.05, **P<0.01). (B) PBS, venom (V), venom pretreated with lateral oviduct (LO-V), venom plus lateral oviduct (V+LO), or hemolymph of D. melanogaster larvae that had been pretreated with PBS (PBS-Hl) or lateral oviduct (LO-Hl) was injected. Each value represents the mean ± S.D. for six independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (**P<0.01).
Effects of A. japonica venom and oviduct components on Spodoptera litura
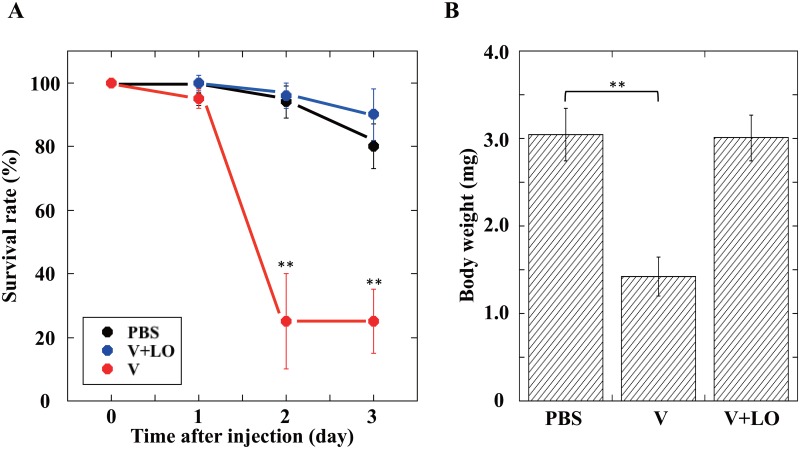
The biological effects of A. japonica venom and oviduct components have been examined mainly using D. melanogaster larvae as the habitual host but not using the non-habitual hosts or non-Drosophila insect species[33]. We examined the effects of these two components on larvae of the common cutworm Spodoptera litura. Injection of only A. japonica venom without the LO components caused approximately 75% mortality in S. litura larvae (Fig 5A). Moreover, significant growth retardation of surviving S. litura larvae was observed one day after the venom injection (Fig 5B). However, A. japonica venom did not have any detrimental effects on S. litura larvae when it was co-injected with the LO components, indicating that the effects of both components, venom and LO factors, affected a non-Drosophila insect species in a way similar to the habitual host species, D. melanogaster.
Fig 5. Effects of A. japonica venom and LO components on survival rates and growth of Spodoptera litura larvae.
(A) Life span curves of S. litura larvae after injection of PBS, venom (V), or venom plus lateral oviduct (V+LO). The amount injected was increased with the increasing ratio of S. litura larva weight to Drosophila larva weight. Third instar larvae of S. litura (body weight: 1.50 ± 0.10 mg) were used for this experiment. Each value represents the mean ± S.D. for ten independent experiments performed separately. Significant differences compared to the value at 0 day are indicated by Tukey’s HSD (**P<0.01). (B) Body weights of surviving S. litura larvae one day after injection of PBS, venom (V), or venom plus lateral oviduct (V+LO). Other explanations are as in (A).
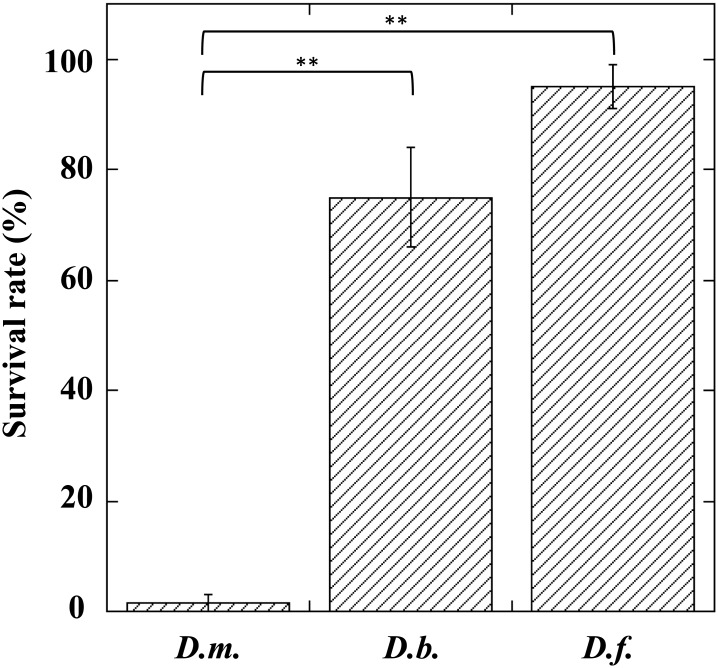
Effects of A. japonica venom on D. bipectinata and D. ficusphila
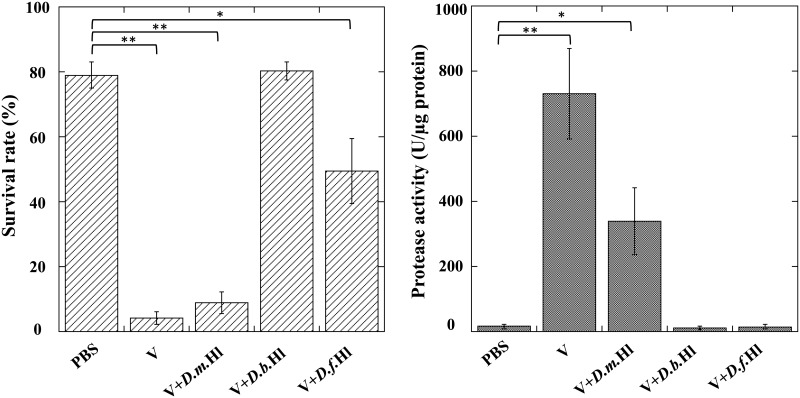
We previously reported that the injection of A. japonica venom did not have a toxic effect on the non-habitual host species of Drosophila, D. ficusphila[33]. To confirm and expand this observation, we performed the same experiments using another non-habitual Drosophila host species, D. bipectinata. As we expected, A. japonica venom did not have any toxic effect on D. bipectinata (Fig 6), thus implying that these non-habitual Drosophila host larvae possess an active neutralizer in the hemolymph. To assess this interpretation, we injected A. japonica venom together with hemolymph prepared from D. bipectinata or D. ficusphila larvae into D. melanogaster larvae. Although the hemolymph of D. melanogaster larvae did not show any neutralizing activity at all, both hemolymphs prepared from D. bipectinata and D. ficusphila larvae significantly neutralized A. japonica venom toxicity (Fig 7A).
Fig 6. Survival rates of Drosophila melanogaster (D.m.), Drosophila bipectinata (D.b.), and Drosophila ficusphila (D.f.) larvae one day after injection of A. japonica venom.
Each value represents the mean ± S.D. for eight independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (**P<0.01).
Fig 7. Survival rates (A) and hemolymph protease activities (B) of Drosophila melanogaster one day after injection of indicated samples. PBS, venom (V), or venom plus larval hemolymph of D. melanogaster (V+D.m.Hl), D. bipectinata (V+D.b.Hl), or D. ficusphila (V+D.f.Hl).
Survival rates of test larvae injected the venom factor together with D. ficusphila hemolymph: 49.3 ± 10.6% at 48 h, 48.6 ± 11.1% at 72 h, 46.9 ± 11.5% at 96 h. Each value represents the mean ± S.D. for eight independent experiments performed separately. Significant differences are indicated by Tukey’s HSD (*P<0.05, **P<0.01).
We previously found that the proteolytic activity in the plasma (void of hemocytes) was drastically elevated following the venom injection, but its elevation was blocked by coinjection of the LO components[33]. Because this enzymatic elevation is closely related to the successful parasitism, measurement of this enzyme activity is useful to evaluate the effectiveness of the venom activity as well as the neutralizer activity. In order to examine the effects of D. bipectinata and D. ficusphila larval hemolymph on the venom-induced proteolytic activity, each plasma fraction prepared from these larvae was coinjected with the venom components. D. melanogaster larval hemolymph did not affect the proteolytic activity at all, while the plasma of both D. bipectinata and D. ficusphila larvae significantly blocked the venom-induced elevation of proteolytic activity (Fig 7B). These results indicate that these two non-habitual Drosophila host species, D. ficusphila and D. bipectinata, possess the hemolymph factor(s) that neutralize the toxic venom factor of A. japonica to repress the venom-induced elevation of the proteolytic activity.
Discussion
The parasitic success of many parasitoid wasps inside their hosts is attributed in large part to symbiotic viruses or virus-like particles (VLPs). In the absence of these factors, the eggs are recognized as a dangerous foreign substance and destroyed by the host defense system. The most studied symbionts are the polydnaviruses (PDVs), which have been investigated for more than 40 years[18]. In this study, we focused on A. japonica to analyze the parasitism strategy of a parasitoid wasp lacking PDVs. Instead of PDVs, A. japonica females use their toxic venom to prevent the host defense system from killing their progeny. Prior studies demonstrated that A. japonica venom especially diminished the host cellular defense reactions, spreading and phagocytosis, by host D. melanogaster hemocytes[33]. Moreover, the venom significantly induced cell death in D. melanogaster hemocytes by coincubation for one hour. Although a highly toxic venom is essential for successful parasitization by A. japonica wasps, the venom toxicity must be neutralized soon after the manipulation of the host defense system; otherwise, the wasps would lose their hosts by killing them. For this neutralization, A. japonica females use the proteinaceous factor(s) in the lateral oviduct (LO) that are introduced with the eggs a few seconds after venomization. The present study showed that the LO factor can neutralize the venom toxin by its injection into the host hemocoel pre- or post-venomization: LO-factor injection can function from two hours before venomization to half an hour after venomization. Furthermore, the hemolymph itself prepared from D. melanogaster larvae preinjected with the LO factor can neutralize the venom toxin, suggesting that the LO factor does not directly affect the venom components but activates a precursor of the neutralizer in D. melanogaster hemolymph. This interpretation was supported by the fact that the precipitate after the ultracentrifugation of the venom pretreated with the LO components retained high toxicity.
Interestingly, hemolymph of both D. bipectinata and D. ficusphila larvae, which are non-habitual Drosophila host species of A. japonica, was able to neutralize A. japonica venom without pretreatment by A. japonica LO factor, indicating that these species larvae possess an intrinsic hemolymph factor to neutralize the toxic venom. In contrast, the venom effect on the non-Drosophila insect species Spodoptera litura larvae was the same as that on habitual host D. melanogaster: S. litura larvae were detrimentally affected by injection of A. japonica venom but coinjection of the LO factor neutralized the venom. Based on these results, it is reasonable to presume that interaction between A. japonica (or the ancestral parasitoid species) and Drosophila species must have continued for a long time, and during that long period, some flies, such as D. bipectinata and D. ficusphila, acquired the active hemolymph neutralizer that inactivates A. japonica venom. This inference is partly supported by the fact that the generalist endoparasitoid A. japonica succeeded in parasitizing 12 of a total 14 tested Drosophila species, and that A. japonica failed to parasitize only two species, D. bipectinata and D. ficusphila[39]. In other words, because A. japonica females have acquired the highly toxic venom factor to overcome various immune reactions of Drosophila species, they have become generalist parasitoids in the evolutionary process, although a few species that have acquired the active neutralizer against the venom succeed in escaping from parasitism by A. japonica.
In the previous study[33], we also found that the proteolytic (peptide MCA substrate hydrolase) activity of the host D. melanogaster hemolymph was drastically elevated by injection of A. japonica venom when we used the MCA substrate (Boc-Ile-Glu-Gly-Arg-MCA) that is generally utilized as a Factor Xa substrate[40]. This elevation of proteolytic activity seems to be closely related to the insecticidal effect of the venom because coinjection of the venom with the LO components blocked elevation of the enzymatic activity. We demonstrated that both D. bipectinata and D. ficusphila larval hemolymphs also blocked the elevation of the venom-induced proteolytic activity without pre-injection of the A. japonica LO components. Therefore, it is reasonable to assume that D. bipectinata and D. ficusphila larval hemolymph intrinsically possess an active inhibitory factor that suppresses the venom-induced elevation of the proteolytic activity, although D. melanogaster larvae can induce the active inhibitory hemolymph factor only with the aid of the LO factor.
In this study, we provided chemical, physical, and morphological evidence that the A. japonica toxic venom factor could be a virus-like entity. The venom toxicity was significantly impaired by UV irradiation, sonication, and boiling but not by treatment with trypsin. The toxic venom compound was also precipitated by ultracentrifugation at 450,000 g for one hour. Furthermore, the precipitate was shown to contain spherical virus-like particles by electron microscopic analysis. Although the particles are heterogeneous in size, most of them are small spheres approximately 20 to 40 nm in diameter. Therefore, if these small sphere particles are the venom toxic factors, they are completely different from the larger size viruses reported in parasitoid wasps, which include PDVs[17, 18, 41], entomopoxviruses[42, 43], and ascoviruses[9, 44–47]. Nevertheless, if we postulate that the A. japonica venom factor belongs to an already known insect virus family, the candidate virus group must be limited to small RNA viruses such as picornavirus [48–50] or small DNA viruses such as parvovirus [51–53].
Because both small RNA and DNA viruses have been reported as pathogenic viruses in a broad range of insect species, these viruses could be regarded as candidates for the A. japonica toxic venom factor. We have tried several times to identify a virus genomic nucleotide sequence by using Next-generation sequencing technologies, but all trials failed, mostly due to significant contamination by a large amount of Wolbachia in the venom preparation after ultracentrifugation. Further purification of the venom virus preparation is necessary for successful sequencing.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Strand MR, Pech LL. Immunological basis for compatibility in parasitoid-host relationships. Annu Rev Entomol 1995;40: 31–56. 10.1146/annurev.en.40.010195.000335 . [DOI] [PubMed] [Google Scholar]
- 2.Vinson SB, Iwantsch GF. Host regulation by insect parasitoids. Quarterly Rev Biol 1980;55: 145–159.. [Google Scholar]
- 3.Vinson SB, Pennacchio F, Lanzrein B. Interactions between parasitoids and their hosts: an introduction and perspectives. J Insect Physiol 1998;44: 701–702. [Google Scholar]
- 4.Asgari S. Venom proteins from polydnavirus-producing endoparasitoids: their role in host-parasite interactions. Arch Insect Biochem Physiol 2006;61: 146–156. 10.1002/arch.20109 . [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa Y. Cellular immunosuppressive protein in the plasma of parasitized insect larvae. J Biol Chem 1994;269: 14536–14540. . [PubMed] [Google Scholar]
- 6.Kanost MR, Gorman MJ. Phenoloxidases in insect immunity In: Beckage NE, editor. Insect Immunology. San Diego: Academic Press; 2008. pp. 69–96. [Google Scholar]
- 7.Schmidt O. Insect immune recognition ans suppression In: Beckage NE, editor. Insect Immunology. San Diego: Academic Press; 2008. pp. 271–294. [Google Scholar]
- 8.Strand MR. Insect hemocytes and theri role in immunity In: Beckage NE, editor. Insect Immunology. San Diego: Academic Press; 2008. pp. 25–47. [Google Scholar]
- 9.Barratt BI, Evans AA, Stoltz DB, Vinson SB, Easingwood R. Virus-like particles in the ovaries of microctonus aethiopoides loan (Hymenoptera: braconidae), a parasitoid of adult weevils (Coleoptera: curculionidae). J Invertebr Pathol 1999;73: 182–188. 10.1006/jipa.1998.4826 . [DOI] [PubMed] [Google Scholar]
- 10.Bigot Y, Rabouille A, Doury G, Sizaret PY, Delbost F, Hamelin MH, et al. Biological and molecular features of the relationships between Diadromus pulchellus ascovirus, a parasitoid hymenopteran wasp (Diadromus pulchellus) and its lepidopteran host, Acrolepiopsis assectella. J Gen Virol 1997;78: 1149–1163. 10.1099/0022-1317-78-5-1149 . [DOI] [PubMed] [Google Scholar]
- 11.Edson KM, Barlin MR, Vinson SB. Venom apparatus of braconid wasps: comparative ultrastructure of reservoirs and gland filaments. Toxicon 1982;20: 553–562. . [DOI] [PubMed] [Google Scholar]
- 12.Krell PJ. Replication of long virus-like particles in the reproductive tract of the ichneumonid wasp. J Gen Virol 1987;68: 1477–1483. [Google Scholar]
- 13.Lawrence PO, Akin D. Virus-like particles from the poison glands of the parasitic wasp Biosteres longicaudatus (Hymenoptera: Braconidae). Can J Zool 1990;68: 539–546. [Google Scholar]
- 14.Morales J, Chiu H, Oo T, Plaza R, Hoskins S, Govind S. Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J Insect Physiol 2005;51: 181–195. 10.1016/j.jinsphys.2004.11.002 . [DOI] [PubMed] [Google Scholar]
- 15.Schmidt O, Schuchmann-Feddersen I. Role of virus-like particles in parasitoid-host interaction of insects. Subcell Biochem 1989;15: 91–119. . [DOI] [PubMed] [Google Scholar]
- 16.Fleming JA. Polydnaviruses: mutualists and pathogens. Annu Rev Entomol 1992;37: 401–425. 10.1146/annurev.en.37.010192.002153 . [DOI] [PubMed] [Google Scholar]
- 17.Stoltz DB, Krell P, Summers MD, Vinson SB. Polydnaviridae—a proposed family of insect viruses with segmented, double-stranded, circular DNA genomes. Intervirology 1984;21: 1–4. . [DOI] [PubMed] [Google Scholar]
- 18.Webb BA, Strand MR. The biology and genomics of polydnaviruses In: Gilbert LI, Iatrou I, Gill SS, editors. Comprehensive Molecular Insect Science. 6 San Diego, Calif: Elsevier; 2004. pp. 323–360. [Google Scholar]
- 19.Doremus T, Jouan V, Urbach S, Cousserans F, Wincker P, Ravallec M, et al. Hyposoter didymator uses a combination of passive and active strategies to escape from the Spodoptera frugiperda cellular immune response. J Insect Physiol 2013;59: 500–508. 10.1016/j.jinsphys.2013.02.010 . [DOI] [PubMed] [Google Scholar]
- 20.Moreau SJ, Eslin P, Giordanengo P, Doury G. Comparative study of the strategies evolved by two parasitoids of the genus Asobara to avoid the immune response of the host, Drosophila melanogaster. Dev Comp Immunol 2003;27: 273–282. . [DOI] [PubMed] [Google Scholar]
- 21.Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog 2007;3: e158 10.1371/journal.ppat.0030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furihata S, Tanaka K, Ryuda M, Ochiai M, Matsumoto H, Csikos G, et al. Immunoevasive protein (IEP)-containing surface layer covering polydnavirus particles is essential for viral infection. J Invertebr Pathol 2014;115: 26–32. 10.1016/j.jip.2013.10.013 . [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa Y, Yazaki K, Yamanaka A, Tanaka T. Expression of polydnavirus genes from the parasitoid wasp Cotesia kariyai in two noctuid hosts. Insect Mol Biol 1994;3: 97–103. . [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Matsumoto H, Hayakawa Y. Detailed characterization of polydnavirus immunoevasive proteins in an endoparasitoid wasp. Eur J Biochem 2002;269: 2557–2566. 10.1046/j.1432-1033.2002.02922.x [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Matsumoto H, Hayakawa Y. Analysis in the course of polydnavirus replication in ovarian calyx cells of the parasitoid wasp, Cotesia kariai (Hymenoptera: Braconidae). Appl Entomol Zool 2002;37: 323–328.. [Google Scholar]
- 26.Bitra K, Suderman RJ, Strand MR. Polydnavirus Ank proteins bind NF-kappaB homodimers and inhibit processing of Relish. PLoS Pathog 2012;8: e1002722 10.1371/journal.ppat.1002722 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fath-Goodin A, Kroemer JA, Webb BA. The Campoletis sonorensis ichnovirus vankyrin protein P-vank-1 inhibits apoptosis in insect Sf9 cells. Insect Mol Biol 2009;18: 497–506. 10.1111/j.1365-2583.2009.00892.x . [DOI] [PubMed] [Google Scholar]
- 28.Shi M, Chen YF, Huang F, Liu PC, Zhou XP, Chen XX. Characterization of a novel gene encoding ankyrin repeat domain from Cotesia vestalis polydnavirus (CvBV). Virology 2008;375: 374–382. 10.1016/j.virol.2008.02.027 . [DOI] [PubMed] [Google Scholar]
- 29.Chen YP, Taylor PB, Shapiro M, Gundersen-Rindal DE. Quantitative expression analysis of a Glyptapanteles indiensis polydnavirus protein tyrosine phosphatase gene in its natural lepidopteran host, Lymantria dispar. Insect Mol Biol 2003;12: 271–280. . [DOI] [PubMed] [Google Scholar]
- 30.Gundersen-Rindal DE, Pedroni MJ. Characterization and transcriptional analysis of protein tyrosine phosphatase genes and an ankyrin repeat gene of the parasitoid Glyptapanteles indiensis polydnavirus in the parasitized host. J Gen Virol 2006;87: 311–322. 10.1099/vir.0.81326-0 . [DOI] [PubMed] [Google Scholar]
- 31.Suderman RJ, Pruijssers AJ, Strand MR. Protein tyrosine phosphatase-H2 from a polydnavirus induces apoptosis of insect cells. J Gen Virol 2008;89: 1411–1420. 10.1099/vir.0.2008/000307-0 . [DOI] [PubMed] [Google Scholar]
- 32.Furihata SX, Kimura MT. Effects of Asobara japonica venom on larval survival of host and nonhost Drosophila species. Physiol Entomol 2009;34: 292–295. 10.1111/j.1365-3032.2009.00676.x [DOI] [Google Scholar]
- 33.Furihata SX, Matsumoto H, Kimura MT, Hayakawa Y. Venom components of Asobara japonica impair cellular immune responses of host drosophila melanogaster. Arch Insect Biochem Physiol 2013;83: 86–100. 10.1002/arch.21093 . [DOI] [PubMed] [Google Scholar]
- 34.Furihata S, Hirata M, Matsumoto H, Hayakawa Y. Bacteria endosymbiont, Wolbachia, promotes parasitism of parasitoid wasp Asobara japonica. PLoS One 2015;10: e0140914 10.1371/journal.pone.0140914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryuda M, Nakayama H, Hayakawa Y. A novel gene associated with intraspecific predation in Spodoptera litura larvae. Appl Entomol Zool 2008;43: 563–568. 10.1303/aez.2008.563 [DOI] [Google Scholar]
- 36.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72: 248–254. . [DOI] [PubMed] [Google Scholar]
- 37.Ohnishi A, Hayakawa Y, Matsuda Y, Kwon KW, Takahashi TA, Sekiguchi S. Growth-blocking peptide titer during larval development of parasitized and cold-stressed armyworm. Insect Bioch Mol Biol 1995;25: 1121–1127. . [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith CS, Miller SE. Modern uses of electron microscopy for detection of viruses. Clin Microbiol Rev 2009;22: 552–563. 10.1128/cmr.00027-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ideo S, Watada M, Mitsui H, Kimura MT. Host range of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of Drosophilid flies. Entomol Sci 2008;11: 1–6. 10.1111/j.1479-8298.2007.00244.x [DOI] [Google Scholar]
- 40.Morita T, Kato H, Iwanaga S, Takada K, Kimura T. New fluorogenic substrates for alpha-thrombin, factor Xa, kallikreins, and urokinase. J Biochem 1977;82: 1495–1498. . [DOI] [PubMed] [Google Scholar]
- 41.Burke GR, Strand MR. Polydnaviruses of parasitic wasps: Domestication of viruses to act as gene delivery vectors. Insects 2012;3: 91–119. 10.3390/insects3010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arif BM. Recent advances in the molecular biology of entomopoxviruses. J Gen Virol 1995;76 (Pt 1): 1–13. 10.1099/0022-1317-76-1-1 [DOI] [PubMed] [Google Scholar]
- 43.Lawrence PO. Morphogenesis and cytopathic effects of the Diachasmimorpha longicaudata entomopoxvirus in host haemocytes. J Insect Physiol 2005;51: 221–233. 10.1016/j.jinsphys.2004.12.003 . [DOI] [PubMed] [Google Scholar]
- 44.Bigot Y, Rabouille A, Sizaret PY, Hamelin MH, Periquet G. Particle and genomic characteristics of a new member of the Ascoviridae: Diadromus pulchellus ascovirus. J Gen Virol 1997;78: 1139–1147. 10.1099/0022-1317-78-5-1139 . [DOI] [PubMed] [Google Scholar]
- 45.Bigot Y, Stasiak K, Rouleux-Bonnin F, Federici BA. Characterization of repetitive DNA regions and methylated DNA in ascovirus genomes. J Gen Virol 2000;81: 3073–3082. 10.1099/0022-1317-81-12-3073 . [DOI] [PubMed] [Google Scholar]
- 46.Renault S, Bigot S, Lemesle M, Sizaret PY, Bigot Y. The cypovirus Diadromus pulchellus RV-2 is sporadically associated with the endoparasitoid wasp D. pulchellus and modulates the defence mechanisms of pupae of the parasitized leek-moth, Acrolepiopsis assectella. J Gen Virol 2003;84: 1799–1807. 10.1099/vir.0.19038-0 . [DOI] [PubMed] [Google Scholar]
- 47.Stasiak K, Renault S, Federici BA, Bigot Y. Characteristics of pathogenic and mutualistic relationships of ascoviruses in field populations of parasitoid wasps. J Insect Physiol 2005;51: 103–115. 10.1016/j.jinsphys.2004.07.004 . [DOI] [PubMed] [Google Scholar]
- 48.de Miranda JR, Cordoni G, Budge G. The acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex. J Invertebr Pathol 2010;103: S30–47. 10.1016/j.jip.2009.06.014 . [DOI] [PubMed] [Google Scholar]
- 49.Nakashima N, Uchiumi T. Functional analysis of structural motifs in dicistroviruses. Virus Res 2009;139: 137–147. 10.1016/j.virusres.2008.06.006 . [DOI] [PubMed] [Google Scholar]
- 50.Rossmann MG, Tao Y. Structural insight into insect viruses. Nat Struct Biol 1999;6: 717–719. 10.1038/11477 [DOI] [PubMed] [Google Scholar]
- 51.Bergoin M, Tijssen P. Molecular biology of Densovirinae. Contrib Microbiol 2000;4: 12–32. [DOI] [PubMed] [Google Scholar]
- 52.Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res 2006;68: 361–392. 10.1016/s0065-3527(06)68010-x . [DOI] [PubMed] [Google Scholar]
- 53.O'Neill SL, Kittayapong P, Braig HR, Andreadis TG, Gonzalez JP, Tesh RB. Insect densoviruses may be widespread in mosquito cell lines. J Gen Virol 1995;76: 2067–2074. 10.1099/0022-1317-76-8-2067 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.