Abstract
Understanding the mechanisms by which climate variation can drive population changes requires information linking climate, local conditions, trophic resources, behaviour and demography. Climate change alters the seasonal pattern of emergence and abundance of invertebrate populations, which may have important consequences for the breeding performance and population change of insectivorous birds. In this study, we examine the role of food availability in driving behavioural changes in an insectivorous migratory songbird; the Eurasian reed warbler Acrocephalus scirpaceus. We use a feeding experiment to examine the effect of increased food supply on different components of breeding behaviour and first-brood productivity, over three breeding seasons (2012–2014). Reed warblers respond to food-supplementation by advancing their laying date by up to 5.6 days. Incubation periods are shorter in supplemented groups during the warmest mean spring temperatures. Nestling growth rates are increased in nests provisioned by supplemented parents. In addition, nest predation is reduced, possibly because supplemented adults spend more time at the nest and faster nestling growth reduces the period of vulnerability of eggs and nestlings to predators (and brood parasites). The net effect of these changes is to advance the fledging completion date and to increase the overall productivity of the first brood for supplemented birds. European populations of reed warblers are currently increasing; our results suggest that advancing spring phenology, leading to increased food availability early in the breeding season, could account for this change by facilitating higher productivity. Furthermore, the earlier brood completion potentially allows multiple breeding attempts. This study identifies the likely trophic and behavioural mechanisms by which climate-driven changes in invertebrate phenology and abundance may lead to changes in breeding phenology, nest survival and net reproductive performance of insectivorous birds.
Introduction
A central task for ecologists is to understand the drivers of population change and community structure. Food availability is a central consideration and understanding food limitation in natural populations helps to inform the impacts of environmental change on animal populations. In this study, we investigate the mechanisms linking changes in food availability with changes in the breeding behaviour and productivity of a bird population. Migratory insectivorous songbirds are well-studied and respond to short-term changes in the environment [1]. For example, in temperate regions, climate change is advancing the emergence dates of invertebrates in spring, so that food for many breeding insectivorous songbirds peaks earlier in the breeding season [2–5]. As income breeders, whose breeding productivity depends primarily on current resources [6], songbirds require large quantities of food to support the energetically costly activities of egg production, incubation and nestling provisioning. The breeding season is therefore a period when birds are strongly affected by changes in food abundance, and in the timing of food availability.
Many songbird species have shown advances in mean laying date in response to warming spring temperatures, which are likely to be linked to phenological advances in their prey [7–11]. Failure to adapt to this shift due to phenological mismatch has resulted in population declines in some long-distance migrant species reliant on short-lived peaks in invertebrate abundance early in the breeding season[12–14]. The influence of phenological mismatch, however, may have less impact on population sizes than widely believed [15]. Instead, the abundance of prey, rather than timing of prey peaks has been suggested as a more important consideration for breeding [16,17]. In wetlands, where the peaks of invertebrate abundance are more prolonged, the effects on breeding birds of climate-driven advancement of prey phenology may be less marked or even beneficial [11].
In many songbird species, increases in food availability are associated with larger clutch sizes [18,19] and with earlier laying dates [20–25]. In multi-brooding species, earlier initiation and completion of the first brood can result in a greater likelihood of successful completion of the second brood, and higher overall productivity [26, 27]. However, while larger clutches potentially increase first brood productivity by producing more offspring, the larger clutches take longer to lay and incubate [28,29]. As well as delaying the completion of the first brood, additional eggs prolong the period of high vulnerability to nest predation and brood parasitism [21]. In order to maximise overall productivity, birds could prioritise early completion of the first brood by maintaining typical clutch sizes. Increases in food availability earlier in the breeding season may result in higher breeding success and population growth by enabling earlier breeding and faster nestling growth, particularly if birds do not increase their clutch size. In the present study, we use a food supplementation experiment to measure the extent to which changes in food availability (as may occur in future climate scenarios) may drive changes in the different aspects of breeding behaviour that can affect overall demography.
Eurasian reed warblers, Acrocephalus scirpaceus (hereafter ‘reed warblers’) have an increasing West-European population and a northwest-advancing geographic distribution [30, 31]. This trend, linked with increasing spring temperatures suggests that, despite inhabiting a habitat with plentiful resources throughout the breeding season, reed warblers are limited during the very early stages, either by phenology of their prey, growth of nesting habitat or by weather conditions.
In this study, we test this limitation by experimentally increasing food availability (i.e. through the supply of more food) for a sample of breeding reed warblers, during the early stages of three breeding seasons, and compared the behaviour and breeding performance of this sample with that of a control group with natural food availability. Individual breeding pairs were supplemented with an unlimited food supply; they could not be provisioned with the additional amount of food predicted to occur under a particular climate projection. This was because multiple birds (and species) used the same supplementary feeders and so precisely regulating the amount of food for each pair was not feasible. The study should not, therefore, be viewed as an attempt to simulate future food availability. Rather, by ad libitum food supplementation during the early stages of the breeding season, at a time when the natural levels of food availability are gradually increasing, the food peak can be artificially and immediately advanced. This approach allows focussed investigation of the aspects of reed warbler behaviour and breeding performance that are currently limited by food availability during the early stages of the breeding season.
We assess how different aspects of breeding behaviour including laying date, clutch size, incubation period, nestling growth and first brood productivity responded to increasing food availability. We predict that: i) reed warblers will respond to increases in food availability by advancing their laying date and increasing nestling growth rates, in order to prioritise advancement of first brood completion; ii) clutch size will be unchanged in favour of maintaining a short period of incubation; and iii) the net effect of increased food availability will result in an increase in first brood productivity as well as earlier fledging of the first brood. Late completion of first broods may preclude the successful completion of second broods [32]. These mechanisms, linking protracted climate-driven increases in food supply across the spring in wetland habitats, to reed warbler breeding behaviour and productivity, may explain the current growth of reed warbler populations.
Materials and Methods
Ethics statement
The guidelines promoted by the Association for the Study of Animal Behaviour for the ethical use of animals in research are followed. The species caught as part of this study are common and not registered as an endangered or protected species in any country. All fieldwork was conducted after ethical approval from the British Trust for Ornithology ringing unit (Vafidis licence no; 5475; Facey licence no: 5411; Thomas licence no: 4127). Special approval from the animal ethics committee was not required for these activities as they are included on the authors’ bird ringing licences.
Study sites
Two wetland locations in South Wales, UK, were used for the study. Cardiff Bay Wetland Reserve (CBWR; 51° 27’ 32” N, 3° 10’ 11” W) is a four-hectare wetland consisting of mixed scrub habitat, open pools and large areas of Phragmites australis reedbed. Cosmeston Lakes Country Park (CLCP, 51° 24′ 53″ N 3° 6′ 0″ W) supports two adjacent small reedbed sites (with a total area of approximately 1.5 hectares) separated by 200 m of freshwater lake habitat. Both sites are publicly owned and access was arranged through the local authority.
Bird ringing / banding
Breeding adult reed warblers were monitored as part of an established monitoring project at the sites. All monitored pairs were caught during regular mist-netting sessions and fitted with a unique combination of three plastic colour rings and a numbered metal ring, to enable individual identification in the field.
Food supplementation
Prey availability for reed warblers was experimentally increased across the two study sites over three breeding seasons by providing live and dried mealworms, Tenebrio molitor larvae (Coleoptera), in containers (2 litre-capacity) resting on small tables at 1.5 m height, from 1st of April onwards. Each study site supports three feeding stations, which were supplied with at least 200 mealworms (mean weight ± SD of mealworms = 112.00 ± 2.60 g, n = 100) and refilled every day (when required), until the end of the breeding season (September). Feeding bowls were enclosed in a wire mesh cage (measuring 300 x 200 x 200 mm of 10mm mesh) to prevent the mealworms being taken by carrion crows (Corvus corvus) and black-billed magpies (Pica pica), but permitting access to reed warblers. Each feeding station was regularly visited by between three and five pairs of colour-ringed breeding reed warblers each season (CBWR 2013–2014: 22 supplemented pairs out of 56 monitored territories; CLCP 2012–2013: 19 supplemented pairs out of 46 monitored territories). Other small passerines including European robins (Erithacus rubecula), common reed buntings (Emberiza schoeniclus) and Eurasian blue tits (Cyanistes caeruleus) occasionally gained access to the mealworms, but mealworms were always available for reed warblers using the feeders. All adults nesting within 50 m of the feeding stations discovered the mealworms within two to five days, and fed daily on the supplemented food from the laying period until the end of the breeding season in late July. Fed and control sites were located at least 150 m apart, to minimise the likelihood of accidental supplementary feeding of control birds visiting fed areas (no such incidences were observed). This was confirmed by individually identifying the adult reed warblers using the feeding stations and by remotely monitoring the stations with small video cameras (Sony Handycam DCR-SR32, Sony Corporation, http://sony.co.uk) and infrared-triggered trail cameras (Bushnell HD. http://Bushnell.co.uk). Activity around the feeding stations was recorded between 06:00 and 18:00 BST every two days throughout the breeding cycle. The positions of feeding stations were moved between successive breeding seasons to allow the treatment state of site-faithful individuals to change between years.
Nest monitoring
Active nests (n = 102) were located by systematic searching of suitable nesting habitat (dense, tall stands of Phragmites) and by visually tracking adults back to the nest. The status of each nest was checked every two days until the first eggs were laid (in April and May), then they were checked twice a day (at ~10:00 and ~16:00 BST) until fledging (in June and July). In order to minimise disturbance to breeding birds and damage to Phragmites habitat, only first broods were monitored in this study.
The number of days since 1 April of that year was used as a measure of laying date earliness. The incubation period was defined as the time between the laying day of the last egg and the day of hatching of the first egg. Nestlings (at day four and day six after hatching) were weighed to 0.1 g using an electronic balance (Satrue SA-500). Since the nestlings were ringed on day six, it is not possible to assign growth rates to individual birds between day four and day six. Instead nestling weights were modelled using age and nest identity as independent variables to account for non-independence of measurements from the same age groups and nests. The effect of food supplementation on total first brood productivity was measured by comparing the mean number of chicks produced for each nest (including depredated nests and failures) in control and fed treatments. All nests in both treatment groups were filmed on at least three occasions during the nestling period, for up to two hours on each occasion, to confirm whether or not nestlings were being provisioned with mealworms as well as with other invertebrate taxa.
Invertebrate monitoring
A measure of weekly invertebrate prey availability was determined at each site using double-sided yellow (dry-stick) invertebrate traps (measuring 50 mm2; Oecos Ltd, UK) set in eight locations across the reedbed habitat at a height of 50–120 cm (following Vafidis et al. 2014 [33]). Total weekly captures of Diptera, Aranea, Hymenoptera and Hemiptera, as well as other less-frequently encountered taxa (<1%) such as Coleoptera and Lepidoptera are recorded for each trap. The number of invertebrates captured by these traps reflects both their abundance and activity [34,35], so the mean weekly captures are used as an invertebrate “activity-density” to reflect the availability of naturally occurring prey for foraging reed warblers.
Phragmites height
The mean total height of all Phragmites stems (from ground level to the top of the uppermost panicle) supporting each nest when the first egg was laid was measured using a fixed measuring rod. In order to obtain a measure of the status of Phragmites growth at the time of egg-laying, the mean total height is transformed into positive or negative differences of the overall mean total height of all measured stems (i.e. ‘Phragmites height index’).
Weather variables
Mean air temperature, mean wind speed and total rainfall data for each day were summarised from measurements collected by an automated weather station (Davis Instruments Vantage Pro 2, Hayward, CA) located at 51° 27’ 17” N, 3° 9’ 45” W, 0.75 km and 4.5 km from CBWR and CLCP, respectively. Temperature measurements were summarised for the analysis to include mean temperature for all April measurements, the mean temperature during the egg laying period (the mean of air temperature measurements taken in the five days leading up to the production of the first egg for each nest), mean temperature during the incubation period (the mean of air temperature measurements during the incubation period for each nest) and mean temperature during the nestling period (the mean of air temperature measurements during the six days after hatching for each nest). Total rainfall (in mm) was the total measure of all the rainfall measurements taken during the laying, incubation or nestling periods (as specified above for mean temperature), for each nest. Likewise, wind speed (m/sec-1) was the mean wind speed value for the laying, incubation or nestling periods for each nest.
Statistical analysis
This study tests the effects of food treatment on reed warbler laying date, clutch size, incubation duration, hatching success, rates of nestling growth, and overall first brood productivity (of all nesting attempts). As well as the availability of food, these reproductive parameters may also be affected by factors such as local weather conditions, and by other unmeasured constraints which may vary by year and site (e.g. competitive pressure, predator density).
These effects are investigated using the R statistical software, version 3.2.2 [36] fitting generalised linear models (GLMs) and generalised linear mixed-effects models (GLMMs) using the R package ‘lme4’ [37] (Table 1). Where appropriate, the identity number for each nest is used as a random effect in mixed-effects models to account for repeated measures (e.g. nestling measurements) from the same nest. The final models are selected using backward deletion of non-significant (P> 0.05) terms. The rates of predation are compared between fed and unfed treatments using a Fisher’s exact test due to the low instances of predation in the treatment group. Data exploration and model validation procedures follow the protocol described in Zuur et al. 2007 and Thomas et al. 2015 [38–39]. Outliers are identified using Cleveland plots, and collinearity is assessed using pair plots and variance inflation factor values. Homogeneity of variance is assessed by plotting the model residuals against the fitted values, and normality of residuals is assessed using histogram and QQ plots. Finally, influential observations are assessed using Cooks’ distance values.
Table 1. Starting models of GLM and GLMMs relating breeding performance parameters of reed warblers monitored in Cardiff Bay Wetland Reserve and Cosmeston Lakes Country Park in South Wales, UK, between 2012 and 2014.
| Model | Sampling unit | Error family/link | Variables |
|---|---|---|---|
| Laying date | Nest | Guassian/identity | ✓ Treatment (fed/control ✓Site ✓Invertebrate availability ✓Mean temperature ✓Mean April temperature ✓Mean wind speed ✓Total rainfall ✓Phragmites height ✓All two-way interactions |
| Clutch size | Nest | Poisson/log | ✓ Treatment ✓Site ✓Laying date ✓Invertebrate availability ✓Mean temperature ✓Mean April temperature ✓Mean wind speed ✓Total rainfall ✓All two-way interactions |
| Incubation duration | Nest | Guassian/identity | ✓ Treatment ✓Site ✓Clutch size ✓Incubation date ✓Invertebrate availability ✓Mean temperature ✓Mean April temperature ✓Mean wind speed ✓Total rainfall ✓All two-way interactions |
| Hatching success | Nest | Binomial/ logit | ✓ Treatment ✓Clutch size ✓Laying date ✓Site ✓Invertebrate availability ✓Mean temperature ✓Mean April temperature ✓Mean wind speed ✓Total rainfall ✓All two-way interactions |
| Nestling growth | Individual nestling | Guassian/identity | ✓ Treatment ✓Nestling age ✓Time of day ✓Brood size ✓Site ✓Incubation duration ✓Hatch date ✓Invertebrate availability ✓Mean temperature ✓Mean wind speed ✓Total rainfall ✓All two-way interactions |
| Total 1st brood productivity | Nest | Poisson/log | ✓ Treatment ✓Site ✓Invertebrate availability ✓Mean temperature ✓Mean wind speed ✓Total rainfall |
Results
Between 2012 and 2014, 200 breeding adult reed warblers, 102 nests, 416 eggs and 324 nestlings were monitored in CBWR and CLCP. With these data it is possible to examine how increases in food availability during the early stage of the breeding season affect different reproductive parameters of breeding reed warblers.
Laying date
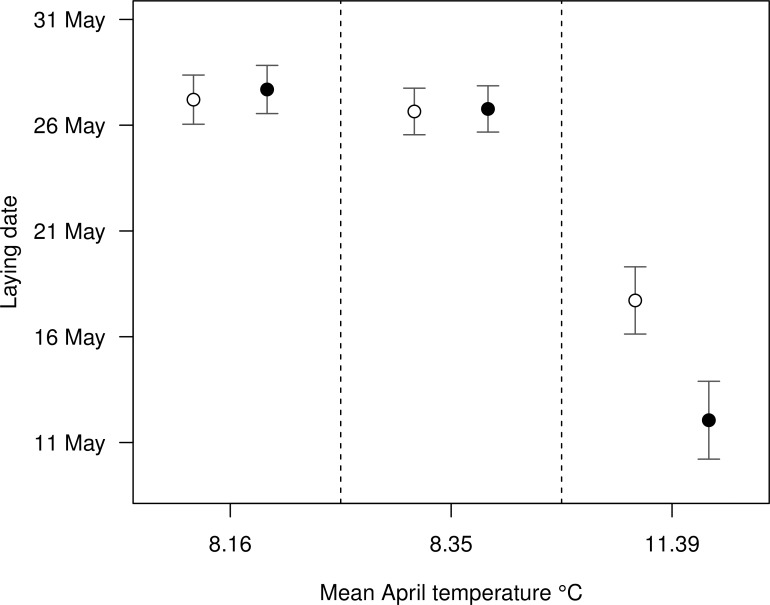
Mean April temperatures, rain, invertebrate availability and reed growth are significant predictors of the date of laying (adj R2 = 0.63, F6,70 = 22.88, P<0.0001). Earlier laying dates are associated with warmer mean April temperatures (-2.94 ± 0.60; F1,75 = 75.03; P<0.0001) and higher rainfall (-0.04 ± 0.01; F1,74 = 40.56; P<0.0001), while later breeding dates are associated with greater invertebrate availability (0.27 ± 0.11; F1,73 = 5.85; P = 0.018; Table 2). The effect of fed treatment was dependant on April temperatures, with each degree increase representing an advance by 1.90 days (± 0.88; F1,70 = 4.71, P = 0.0333; Fig 1). The mean difference in laying date between fed and control groups on the warmest recorded mean April temperature of 11.39°C is 5.66 days (± 0.30).
Table 2. GLM predicted effects of supplementary feeding treatment and environmental variables on laying date in reed warblers.
| Parameter | Estimate ± SE | F | P |
|---|---|---|---|
| April temp | -2.94 ± 0.60 | 75.03 | <0.0001 |
| Rain | -0.04 ± 0.01 | 40.56 | <0.0001 |
| Invert avail. | 0.27 ± 0.11 | 5.85 | 0.0181 |
| Reed height | 0.11 ± 0.05 | 10.35 | 0.0020 |
| Treatment | 16.00 ± 7.97 | 0.78 | 0.3796 |
| Treatment × April temp | -1.90 ± 0.88 | 4.71 | 0.0333 |
Fig 1. The effect of treatment and mean April temperature on reed warbler laying date (± SE).
Control (unfed) nests are represented by unfilled circles and fed nests are represented by filled circles.
Clutch size, incubation duration and hatching success
Of the 102 nests monitored, only 80 completed their clutch, with the other 20 completely depredated, abandoned or destroyed by bad weather before clutch completion. Most completed clutches (69) contained a total of four eggs, ten clutches contained five eggs and one clutch contained three eggs. Clutch size is uncorrelated with treatment (z = -0.03; P = 0.1990), laying date (z = -0.05; P = 0.9780) or any environmental variables (P>0.8). The incubation period varies between nine and 13 days and is strongly influenced by clutch size, invertebrate availability, and total rainfall (Adj R2 = 0.34, F6,70 = 7.46; P<0.0001; Table 3). Each additional egg in the clutch increases the incubation duration by 1.01 days (± 0.07; F1,71 = 18.06; P<0.0001). Increases in incubation duration are correlated with total rainfall (0.04 days ± 0.01; F1,72 = 10.91; P = 0.0015) and invertebrate availability (0.01 days ± 0.00; F1,74 = 5.41; P = 0.0229). There is a significant interaction between treatment and mean temperature, with increasing temperatures corresponding with shorter incubation durations in the treatment group (-0.17 days ± 0.07; F1,70 = 6.47; P = 0.0132). Of the 102 nests monitored across the three breeding seasons, 80 nests resulted in successful hatching of at least one egg. Of these successful nests, 14 contained unhatched eggs; overall 313 eggs hatched out of 329. Hatching success is uncorrelated with treatment (LRT = 0.07; P = 0.7891), laying date (LRT = 1.03; P = 0.3089) and any environmental variables (P> 0.4).
Table 3. GLM Predicted effects of supplementary feeding treatment and environmental variables on incubation duration in reed warblers.
| Parameter | Estimate ± SE | F | P |
|---|---|---|---|
| Clutch size | 1.01 ± 0.25 | 18.06 | <0.0001 |
| Rain | 0.04 ± 0.01 | 10.91 | 0.0015 |
| Invert avail. | 0.01 ± 0.00 | 5.41 | 0.0229 |
| Mean temperature | 0.03 ± 0.05 | 1.12 | 0.2944 |
| Treatment | 1.71 ± 0.82 | 2.78 | 0.0997 |
| Treatment × mean temp | -0.17 ± 0.07 | 6.47 | 0.0132 |
Nestling mass and predation
Treatment, brood size, nestling age, site and invertebrate availability are all significant predictors of nestling mass (Table 4). The mean difference in nestling mass between day four and day six is 2.90 g (± 0.10 g; F1,455 = 1520.74; P<0.0001). Supplemented nests are associated with heavier six-day-old nestlings (0.50 ± 0.16 g; F1,457 = 6.79; P = 0.0094). Brood size is also a highly significant predictor of nestling mass, with larger broods containing lighter nestlings (-0.52 ± 0.09; F1,456 = 35.93, P<0.0001). There is a significant and substantial effect of treatment on nest predation, with control nests suffering 21 predation events out of 61 nests (34%), and fed nests suffering one predation event out of 41 nests (2.5%; Fisher’s exact test; odds ratio = 0.07, P<0.0001). The 21 depredated control nests suffered complete predation of eggs, while the single depredated fed nest suffered the loss of two eggs (retaining three successfully hatched eggs). The study did not record any incidences of nestling predation from either treatment group.
Table 4. GLMM predicted effects of supplementary feeding treatment, age, brood size and environmental variables on nestling mass in reed warblers.
| Parameter | Estimate ± SE | F | P |
|---|---|---|---|
| Treatment (Fed) | 0.14 ± 0.13 | 41.21 | <0.0001 |
| Brood size | -0.52 ± 0.09 | 35.93 | <0.0001 |
| Nestling Age | 2.90 ± 0.10 | 1520.74 | <0.0001 |
| Site | -0.30 ± 0.08 | 42.46 | <0.0001 |
| Invertebrate availability | -0.004 ± 0.001 | 9.62 | 0.0028 |
| Treatment × Nestling age | 0.50 ± 0.16 | 6.79 | 0.0112 |
Total first brood productivity
The mean first brood productivity is 3.18 (± 0.15) chicks per pair. Treatment, site, total rain, and mean April temperature are all significant predictors of first brood productivity (adj R2 = 0.42; F4,95 = 19.28; P<0.0001; Table 5), with fed nests producing 1.14 (± 0.26; F1,98 = 29.18; P <0.0001) more chicks than control nests. Productivity was higher in warmer mean April temperature (0.42 chicks ± 0.11; F1,97 = 12.21; P<0.0001), while lower productivity was associated with higher rainfall (-0.04 chicks ± 0.01; F1,95 = 24.99; P<0.0001).
Table 5. GLM predicted effects of supplementary feeding treatment, site and environmental variables on total first brood productivity in reed warblers.
| Parameter | Estimate ± SE | F | P |
|---|---|---|---|
| Treatment | 1.14 ± 0.26 | 29.18 | <0.0001 |
| April temp | 0.42 ± 0.11 | 12.21 | 0.3716 |
| Site | -1.53 ± 0.30 | 10.74 | <0.0001 |
| Rain | -0.04 ± 0.01 | 24.99 | <0.0001 |
Discussion
Using supplementary feeding, this study investigates how changes in food availability affect different reproductive parameters that, together, influence the breeding success of reed warblers. Fed birds have earlier laying dates, higher nestling masses, and more fledged young. Incubation period was shorter in fed treatment groups when the mean April temperatures were warmest. In contrast, food supplementation does not measurably influence clutch size or hatching success.
Laying date
Supplemental food advances the laying date by up to 5.6 (± 0.3) days during the warmest mean April temperatures. These results are consistent with observational studies of reed warblers [31, 40], where earlier breeding was associated with warmer May to July temperatures. Continental European populations of reed warbler have been reported to be laying 18 days earlier over the last 33 years [31, 41], but it has been unclear whether the primary driver behind earlier laying is the faster growth of nesting vegetation, or the earlier rise in availability of invertebrate prey. The relationship identified between later laying dates and higher values of invertebrate availability and reed height is likely to be correlated with time rather than more invertebrate prey and higher reeds driving later breeding. The significant food supplementation effect on laying phenology in our study suggests that laying date is constrained by food availability, as birds in both treatments were exposed to the same Phragmites growth conditions. It is likely that reed warblers may only be able to advance their laying date in response to increased food availability if reed growth phenology (which is limited by spring temperatures [42]) has advanced sufficiently to provide habitat suitable for earlier nest building.
Consistent with our findings, experimental increases in food availability are associated with earlier breeding dates in other passerine birds, including Eurasian blue tit [43]; great tit, Parus major [44], common starling, Sturnus vulgaris [45]; and European blackbird, Turdus merula [46]. Many of these species rely on highly seasonal prey resources (e.g. caterpillars) and therefore risk mismatch between supply and demand if they nest too late. This is less likely for reed warblers, which feed their young on a diverse range of invertebrate prey populations which emerge sequentially throughout the summer, resulting in a prolonged period of high abundance without any clear peak [47–50]. Instead, reed warblers may be taking advantage of the higher abundance of invertebrates and earlier reed growth under warmer spring conditions to maximise fitness of their offspring by breeding earlier, enabling longer periods to acquire experience in foraging before autumn migration [21,31,51,52] or creating the opportunity to initiate a second brood earlier in the breeding season.
Clutch size
Clutch size in reed warblers appears to be less variable than in many other passerine species [53]. This may represent the outcome of a trade-off between current reproductive effort and future reproduction and survival [54]. Maintaining small clutch sizes reduces the period of egg-laying, as well as the duration required for incubation and nestling periods [55]. The advantages of this include reducing the period of vulnerability to predators and brood parasites [56], as well as leading to the earlier initiation of subsequent broods. Such benefits may outweigh the gains of increasing the clutch size of the first brood.
Nest predation
Egg predation occurs much less frequently in supplementally fed nests than in control nests. Furthermore, the depredated control nests were subject to complete predation of the whole clutch, while the one supplemented nest only lost two eggs to predation. This difference may be explained by different nest attendance rates between treatment groups, with supplemented parent reed warblers spending less time foraging, reducing the frequency and duration of eggs being undefended, and uncovered by the well-camouflaged parent [57,58]. Lower rates of nest predation have been found in species that incubate continuously, such as mourning doves, Zenaida macroura [59], common eiders, Somateria mollissima [60], and common pheasants, Phasianus colchicus [61], as a strategy to reduce the period of nesting to minimise vulnerability to predation and maximise opportunities to multi-brood. As no nestling predation was observed in this study, the egg-laying and incubation stage appears to be the period most vulnerable to nest predation. Having sufficient food supplies during this period may well represent a means to minimise substantial losses to predation.
Nestling growth
Supplementary feeding of parent reed warblers resulted in an increased rate of nestling growth. Nestlings of food-supplemented parents weighed 0.5 g (± 0.2) more at day six than nestlings of unsupplemented parents, which could be explained by more rapid growth or heavier stomach contents due to earlier feeding before being measured [62]. Increases in food abundance, parental investment and predation have all been shown to modify nestling growth rate in Acrocephalus warblers [63–65]. Likewise, the offspring of supplementally fed great tits grew faster than control nestlings [66]. Greater prey availability for parents is therefore likely to increase nestling growth rates, which could accelerate fledging and thus reduce the period of vulnerability to predation and adverse weather conditions. Nestling reed warblers normally fledge after 10–11 days but are able to jump from the nest to evade predators from day seven [67]. Given the high rates of predation in wetlands [68], adaptations to reduce the period of nestling vulnerability are likely to be strongly favoured by selection.
Conclusions
European wetlands currently provide abundant prey resources for breeding wetland migrants, but it is predicted that climate warming will further increase overall prey abundance [42], and that higher abundances of prey will become available earlier in the breeding season. To investigate the effects of such climate-driven increases and phenological advances in food availability, in the present study individual breeding pairs received either an unlimited food supply or natural food availability under current climate conditions. The results suggest that reed warblers advance the initiation of first broods in response to earlier onset of suitable foraging and nesting conditions, whereas clutch size, incubation duration and hatching success were not directly affected by additional food. First brood productivity was higher in food supplemented treatment groups, which is due to lower rates of predation. Further research is needed to test the possibility that offspring survival and recruitment may be increased through lower predation and earlier fledging of supplemented nests. Despite their access to extensive periods of abundant invertebrate food throughout the breeding period, the success rate of subsequent breeding attempts in reed warblers is low (≤30% [30, 31, 41, 52, 69]. Our ongoing research efforts will focus on whether earlier completion of the first brood actually leads to greater chance of success in subsequent breeding attempts.
Acknowledgments
We thank Cardiff Harbour Authority and Steve Pickering (Manager of CLCP, Vale of Glamorgan) for access to the study sites and for supply of equipment. Fieldwork help was provided by Mike Shewring, Wayne Morris, Liam Doyle and Todd Jenkins. We thank the Editor and reviewers for their comments in preparing this manuscript.
Data Availability
The data has been deposited in DRYAD with the DOI:10.5061/dryad.vm32c.
Funding Statement
The work was funded by the Welsh Government European Social Fund as part of a Knowledge Exchange Skills Scholarship (JOV). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pearce-Higgins JW, Green RE (2014) Birds and Climate Change: Impacts and Conservation Responses. Cambridge University Press. [Google Scholar]
- 2.Dell D, Sparks TH, Dennis RLH (2005) Climate change and the effect of increasing spring temperatures on emergence dates of the butterfly Apatura iris (Lepidoptera: Nymphalidae). European Journal of Entomology 102:161–167. [Google Scholar]
- 3.Studds CE, Marra PP (2005) Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory bird. Ecology 86:2380–2385. [Google Scholar]
- 4.Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. [DOI] [PubMed] [Google Scholar]
- 5.Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR et al. (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biology 16:3304–3313. [Google Scholar]
- 6.Perrins CM (1996) Eggs, egg formation and the timing of breeding. Ibis 138:2–15. [Google Scholar]
- 7.Crick HQ, Dudley C, Glue DE, Thomson DL (1997). UK birds are laying eggs earlier. Nature 388:526–526. [Google Scholar]
- 8.McCleery RH, Perrins CM (1993)…temperature and egg-laying trends. Nature 391:30–31. [Google Scholar]
- 9.Crick HQ, Sparks TH (1999) Climate change related to egg-laying trends. Nature 399:423–424. [Google Scholar]
- 10.Dunn PO (2004) Breeding dates and reproductive performance. Advances in Ecological Research 35:69–87. [Google Scholar]
- 11.Dunn PO, Winkler DW (2010) Effects of climate change on timing of breeding and reproductive success in birds In Møller AP, Fielder W, Berthold P (eds). Effects of climate change on birds. pp 113–128. Oxford University Press, Oxford, UK. [Google Scholar]
- 12.Berthold P (1996) Control of Bird Migration. London UK: Chapman & Hall. [Google Scholar]
- 13.Sanz JJ (2003) Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography 26:45–50. [Google Scholar]
- 14.Both C, Van Turnhout CAM, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RPB (2010) Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B: Biological Sciences 277:1259–1266. 10.1098/rspb.2009.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn PO, Moller AP (2014) Changes in breeding phenology and population size of birds. J Anim Ecol 83: 729–739. 10.1111/1365-2656.12162 [DOI] [PubMed] [Google Scholar]
- 16.Durant JM, Hjermann DØ, Anker‐Nilssen T, Beaugrand G, Mysterud A, Pettorelli N, et al. (2005) Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecology Letters: 8: 952–958. [DOI] [PubMed] [Google Scholar]
- 17.Dunn PO, Winkler DW, Whittingham LA, Hannon SJ, Robertson RJ (2011) A test of the mismatch hypothesis: How is timing of reproduction related to food abundance in an aerial insectivore?. Ecology, 92:450–461. [DOI] [PubMed] [Google Scholar]
- 18.Arcese P, Smith JN (1988) Effects of population density and supplemental food on reproduction in song sparrows. The Journal of Animal Ecology 57:119–136. [Google Scholar]
- 19.Nager RG, Ruegger C, Van Noordwijk AJ (1997) Nutrient or energy limitation on egg formation: a feeding experiment in great tits. Journal of Animal Ecology 66:495–507. [Google Scholar]
- 20.Klomp H (1970) The determination of clutch-size in birds: a review. Ardea 58:1–124. [Google Scholar]
- 21.Daan S, Dijkstra C, Drent R, Meijer T (1988) Food supply and the annual timing of avian reproduction. Acta Congressus Internationalis Ornithologica 19:392–407. [Google Scholar]
- 22.Jarvinen A (1989) Patterns and causes of long-term variation in reproductive traits of the pied flycatcher Ficedula hypoleuca in Finnish Lapland. Ornis Fennica. 66:24–31. [Google Scholar]
- 23.Winkler DW, Allen PE (1996) The seasonal decline in Tree Swallow clutch size: physiological constraint or strategic adjustment? Ecology 77:922–932. [Google Scholar]
- 24.Winkel W, Hudde H (1997) Long-term trends in reproductive traits of tits (Parus major, P.caeruleus) and pied flycatchers Ficedula hypoleuca. Journal of Avian Biology 28:187–190. [Google Scholar]
- 25.Ruffino L, Salo P, Koivisto E, Banks PB, Korpimäki E (2014). Reproductive responses of birds to experimental food supplementation: a meta-analysis. Frontiers in zoology, 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verboven N, Verhulst S (1996). Seasonal variation in the incidence of double broods: the date hypothesis fits better than the quality hypothesis. Journal of Animal Ecology 65:264–273. [Google Scholar]
- 27.Seward AM, Beale CM, Gilbert L, Jones TH, Thomas RJ (2014) The impact of increased food availability on reproduction in a long-distance migratory songbird: implications for environmental change? PloS ONE 9:e111180 10.1371/journal.pone.0111180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HG (1989) Larger clutches take longer to incubate. Ornis Scandinavica 20:156–158. [Google Scholar]
- 29.Monaghan P, Nager RG (1997). Why don't birds lay more eggs?. Trends in Ecology & Evolution, 12: 270–274. [DOI] [PubMed] [Google Scholar]
- 30.Cramp S (1998) The Birds of the Western Palearctic (Cramp S. ed.) Oxford University Press. [Google Scholar]
- 31.Halupka L, Dyrcz A, Borowiec M (2008) Climate change affects breeding of reed warblers Acrocephalus scirpaceus. Journal of Avian Biology 35:95–100. [Google Scholar]
- 32.O’Brien EL, Dawson RD (2013) Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 172:689–699. 10.1007/s00442-012-2544-0 [DOI] [PubMed] [Google Scholar]
- 33.Vafidis JO, Vaughan IP, Jones TH, Facey RJ, Parry R, Thomas RJ (2014) Habitat use and body mass regulation among warblers in the Sahel Region during the non-breeding season. e113665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black W, Krafsur E (1985) Use of sticky traps to investigate seasonal trends in the spatial distribution of house flies and stable flies (Diptera: Muscidae). Journal of Medical Entomology 22(5):550–557. [Google Scholar]
- 35.Goulson D, Derwent LC, Hanley ME, Dunn DW, Abolins SR (2005) Predicting calyptrate fly populations from the weather, and probable consequences of climate change. Journal of Applied Ecology 42:795–804. [Google Scholar]
- 36.R Core Team (2015) R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing Vienna, Austria. [Google Scholar]
- 37.Bates D, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–6. Available: http://CRAN.R-project.org/package=lme4.
- 38.Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data, Springer, New York. [Google Scholar]
- 39.Thomas RJ, Lello J, Medieros R, Pollard A, Seward A, Smith J, et al. (2015) Data Analysis with R Statistical Software; A guidebook for scientists. Eco-Explore 2015. [Google Scholar]
- 40.Schaefer T, Ledebur G, Beier J, Leisler B (2006) Reproductive responses of two related coexisting songbird species to environmental changes: global warming, competition, and population sizes. Journal of Ornithology 147:47–56. [Google Scholar]
- 41.Halupka L, Wróblewski J (1998) Breeding ecology of the reed warbler (Acrocephalus scirpaceus) at Milicz fish-ponds in 1994. Birds of Silesia 12:5–15. [Google Scholar]
- 42.Vafidis JO (2014). The impacts of climate change on the ecology of a migrant wetland warbler (PhD Thesis, Cardiff University).
- 43.Svensson E, Nilsson JÅ (1995) Food supply, territory quality, and reproductive timing in the blue tit (Parus caeruleus). Ecology 76:1804–1812. [Google Scholar]
- 44.Källander H (1974) Advancement of laying of great tits by the provision of food. Ibis 116:365–367. [Google Scholar]
- 45.Källander H, Karlsson J (1993) Supplemental food and laying date in the European starling. Condor 95:1031–1034. [Google Scholar]
- 46.Desrochers A (1992) Age-related differences in reproduction by European blackbirds: restraint or constraint? Ecology 73:1128–1131. [Google Scholar]
- 47.Dyrcz A (1979). Die Nestlingsnahrung bei Drosselrohrsänger Acrocephalus arundinaceus und Teichrohrsänger Acrocephalus scirpaceus an den Teichen bei Milicz in Polen und zwei Seen in der Westschweiz. Orn. Beob. 76:305–316. [Google Scholar]
- 48.Bibby CJ, Thomas DK (1985) Breeding and diets of the reed warbler at a rich and a poor site. Bird Study 32:19–31. [Google Scholar]
- 49.Schulze-Hagen K (1991) Acrocephalus paludicola (Vieillot 1817)—Seggenrohrsänger In: (Glutz Blotzheim UN von. Ed.) Handbuch der Vögel Mitteleuropas, vol 12 Aula, Wiesbaden. [Google Scholar]
- 50.Dyrcz A, Zdunek W (1996). Potential food resources and nestling food in the great reed warbler Acrocephalus arundinaceus and reed warbler Acrocephalus scirpaceus at Milicz fish- ponds. Birds of Silesia 11:123–132. [Google Scholar]
- 51.Price T, Kirkpatrick M, Arnold S (1988) Directional selection and the evolution of breeding date in birds. Science 4853:798–799. [DOI] [PubMed] [Google Scholar]
- 52.Møller AP (1994) Phenotype-dependent arrival time and its consequences in a migratory bird. Behavioral Ecology and Sociobiology 35:115–122. [Google Scholar]
- 53.Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biological Reviews 77:1–26. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson JA, Svensson E (1993) Energy constraints and ultimate decisions during egg-laying in the blue tit. Ecology 74:244–251. [Google Scholar]
- 55.Slagsvold T (1982). Clutch size variation in passerine birds: the nest predation hypothesis. Oecologia 54:159–169. [DOI] [PubMed] [Google Scholar]
- 56.Snow DW (1962) A field study of the Black and White Manakin, Manaeus manacus, in Trinidad. Zoologica 47:65–104. [Google Scholar]
- 57.Eikenaar C, Berg ML, Komdeur J (2003) Experimental evidence for the influence of food availability on incubation attendance and hatching asynchrony in the Australian reed warbler Acrocephalus australis. Journal of Avian Biology 34:419–427. [Google Scholar]
- 58.Skutch AF (1962) The constancy of incubation. Wilson Bulletin 74:115–152. [Google Scholar]
- 59.Westmoreland D, Best LB (1986) Incubation continuity and the advantage of cryptic egg coloration to Mourning Doves. The Wilson Bulletin 98:297–300. [Google Scholar]
- 60.Swennen C, Ursem JCH, Duiven P (1993) Determinate laying and egg attendance in common eiders. Ornis Scandinavica 24:48–52. [Google Scholar]
- 61.Persson I, Göransson G (1999) Nest attendance during egg laying in pheasants. Animal Behaviour, 58:159–164. [DOI] [PubMed] [Google Scholar]
- 62.Streby HM, Peterson SM, Lehman JA, Kramer GR, Vernasco BJ, Andersen DE (2014). Do digestive contents confound body mass as a measure of relative condition in nestling songbirds? Wildlife Society Bulletin.38:305–310 [Google Scholar]
- 63.Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithsonian Contributions to Zoology 9:1–48. [Google Scholar]
- 64.Duckworth JW (1991) Responses of breeding reed warblers Acrocephalus scirpaceus to mounts of sparrowhawk Accipiter nisus, cuckoo Cuculus canorus and jay Garrulus glandarius. Ibis 133:68–74. [Google Scholar]
- 65.Kleindorfer S, Hoi H, Ille R (1997) Nestling growth patterns and antipredator responses: a comparison between four Acrocephalus warblers. Biologia 52:677–685. [Google Scholar]
- 66.Bańbura J, Bańbura M, Glądalski M, Kaliński A, Markowski M, Michalski M, et al. (2011) Body condition parameters of nestling great tits Parus major in relation to experimental food supplementation. Acta Ornithologica 46:207–212. [Google Scholar]
- 67.Leisler B, Schulze-Hagen K, Quinn D, Hillcoat B, Martin MA (2011) The Reed Warblers: Diversity in a Uniform Bird Family. KNNV Publishing. [Google Scholar]
- 68.Honza M, Øien IJ, Moksnes A, Røskaft E (1998) Survival of Reed Warbler Acrocephalus scirpaceus clutches in relation to nest position. Bird Study 45:104–108. [Google Scholar]
- 69.Borowiec M (1994) Breeding ecology of the Reed Warbler Acrocephalus scirpaceus at Milicz fish-ponds. Birds Silesia 10:5–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data has been deposited in DRYAD with the DOI:10.5061/dryad.vm32c.