Abstract
A d-allulose 3-epimerase from Flavonifractor plautii was cloned and expressed in Escherichia coli and Corynebacterium glutamicum. The maximum activity of the enzyme purified from recombinant E. coli cells was observed at pH 7.0, 65°C, and 1 mM Co2+ with a half-life of 40 min at 65°C, Km of 162 mM, and kcat of 25280 1/s. For increased d-allulose production, recombinant C. glutamicum cells were permeabilized via combined treatments with 20 mg/L penicillin and 10% (v/v) toluene. Under optimized conditions, 10 g/L permeabilized cells produced 235 g/L d-allulose from 750 g/L d-fructose after 40 min, with a conversion rate of 31% (w/w) and volumetric productivity of 353 g/L/h, which were 1.4- and 2.1-fold higher than those obtained for nonpermeabilized cells, respectively.
Introduction
The International Society of Rare Sugars (ISRS) stated at the 2014 Rare Sugar Symposium that ‘d-psicose’ should be referred to as ‘d-allulose’ and use of the ‘d-psicose’ term should be discontinued because d-allulose is an isomerized product of d-allose. d-Allulose (d-psicose, d-ribo-2hexulose) is a rare sugar that is present in small amounts as a non-fermentable component of commercial carbohydrates [1] and as a free sugar in agricultural products [2]. This sugar has attracted a great deal of attention in the field of functional foods owing to its health benefits. d-Allulose is used as a low-calorie sweetener and as a functional sugar for diabetes because it does not contribute calories and exhibits hypoglycemic, hypolipidemic, and antioxidant activities [3–6].
d-Allulose has been produced from d-fructose by the reactions of biocatalysts, including d-tagatose 3-epimerases (DTEases) from Pseudomonas cichorii [7] and Rhodobacter sphaeroides [8]; d-allulose 3-epimerases (DAEases) from Agrobacterium tumefaciens [9], Clostridium sp. [10], Clostridium bolteae [11], Clostridium cellulolyticum [12], Clostridium scindens [13], Desmospora sp. [14], Dorea sp. [15], Ruminococcus sp. [16], and Treponema primitia [17]; whole cells of R. sphaeroides [18] and Sinorhizobium sp. [19]; and whole recombinant cells of Escherichia coli expressing DAEases from C. bolteae [11], C. cellulolyticum [12], and A. tumefaciens [20]. Whole cells show greater stability and resistance to environmental perturbations than enzymes. Moreover, cells eliminate the need for purification steps, such as cell lysis, precipitation, and dialysis, and therefore the reactions are more commercially feasible [20]. Recombinant E. coli cells are suitable for d-allulose production because the specific productivity of these cells is significantly higher than that of wild-type cells. However, d-allulose produced by E. coli is limited in its use as a food additive because E. coli is not a generally recognized as safe (GRAS) host [21]. This problem can be solved by transferring the DAEase gene to a GRAS host such as Corynebacterium glutamicum. The organism is typically used for the industrial production of fine chemicals, owing to its ease of gene manipulation, rapid growth, and ability to grow to high density on cheap growth media [22]. In addition, its cell wall is structurally stable due to a lipid-rich outer layer mostly consisting of mycolic acid [23, 24]. However, the production of d-allulose using a GRAS host has not yet been attempted.
Permeabilized cells treated with detergents such as cetyl trimethylammonium bromide (CTAB), Tween 20, Tween 80, and Triton X-100 [18, 25]; solvents such as acetone, chloroform, ethanol, methanol, and toluene [19, 26, 27]; salts such as NaCl and MgCl2 [28]; and chemicals such as dimercaptosuccinic acid, EDTA, and polyethylenimine [28] have been used in whole-cell bioprocesses because they increase the production of metabolites by enhancing the transfer of reaction substrates and products across the cell membrane [26, 29]. Although penicillin has been used in glutamic acid fermentation to permeabilize C. glutamicum [30, 31], cell permeabilization with antibiotics has not yet been applied to whole-cell bioprocesses.
In the present study, a putative DAEase gene from F. plautii was cloned and expressed in E. coli and C. glutamicum. The biochemical properties, including metal ions, pH, temperature, and kinetic parameters, of the enzyme purified from recombinant E. coli cells were investigated. To increase the production of d-allulose from d-fructose, recombinant C. glutamicum cells expressing DAEase from F. plautii were permeabilized using several types of substances, including antibiotics, detergents, and solvents; and the most effective antibiotic, detergent, and solvent for d-allulose production were selected. The most effective combined permeabilizers were determined by treatment with the selected permeabilizers in combination. The reaction conditions, including pH, temperature, metal ions, and the concentrations of cells and substrate, were optimized for the permeabilized cells. Under the optimized conditions, the increased production of d-allulose from d-fructose was achieved.
Materials and Methods
Materials
d-Allulose, d-fructose, penicillin, ethambutol, ethionamide, and isoniazid standards were purchased from Sigma (St. Louis, MO, USA). Bio-LC grade sodium hydroxide solution was purchased from Fisher Scientific (Hanover Park, IL, USA). All of the restriction enzymes were purchased from New England Biolabs (Hertfordshire, UK, USA). Solvents and detergents were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cloning and gene expression
The genomic DNA from F. plautii ATCC 29863 (ATCC, Manassas, USA), E. coli ER2566 (New Englands Biolab, Hertfordshire, UK), and pET15b plasmid (Novagen, Madison, WI) were used as the sources of the DAEase gene, host cells, and expression vector. The gene encoding the putative DAEase was amplified by PCR using F. plautii genomic DNA as a template. The primer sequences used for gene cloning were based on the DNA sequence of the putative DAEase from F. plautii (GenBank accession number EHM40452.1). Forward (5′- CATATGAACCCGATTGGAATGCACTAC-3′) and reverse primers (5′- CTCGAGTTACGCGGTCAGCTCCTTGAGG-3′) were designed to introduce the underlined NdeI and XhoI restriction sites, respectively, and were synthesized by Bioneer (Daejon, Korea). The DNA fragment amplified by PCR was purified using a PCR purification kit (Promega, Madison, WI, USA) and inserted into the pET15b vector digested with the same restriction enzymes. The resulting plasmid was transformed into E. coli strain ER2566 using an electroporator (MicroPulser, Bio-Rad, Hercules, CA, USA). The transformed E. coli was plated on Luria-Bertani (LB) agar containing 25 μg/mL ampicillin. An ampicillin-resistant colony was selected, and the plasmid DNA from the transformant was isolated with a plasmid purification kit (Promega). DNA sequencing was carried out at the Macrogen facility (Seoul, Korea). Gene expression was estimated by both SDS-PAGE and enzyme activity assay.
C. glutamicum ATCC 13032 (ATCC, Manassas, USA), and E. coli−C. glutamicum shuttle expression vector pEKEx2 (Juelich Research Centre, Juelich, Germany) were used as the sources of host cells and expression vector, respectively. The DAEase gene from F. plautii was ligated into the expression vector pEKEx2. A ribosomal binding site (rbs) was encoded upstream of the DAEase gene, which was amplified by PCR using the template vector pET15b from E. coli. The primer sequences used for gene cloning were based on the DNA sequence of F. plautii DAEase. Forward (5′-CTGCAGAAAGGAGATATAGATGAACCCGATTGGAATGCACTACGGC-3′) and reverse primers (5′-GTCGACTTACGCGGTCAGCTCCTTGAG-3′) were designed to introduce the underlined PstI and SalI restriction sites, respectively, and were synthesized by Bioneer. The amplified DNA fragment obtained by PCR was purified and inserted into the pEKEx2 vector digested with the same restriction enzymes. The resulting plasmid was transformed into C. glutamicum strain ATCC 13032 using an electroporator. The transformed C. glutamicum cells were plated on brain-heart infusion (BHI) agar containing 15 μg/mL kanamycin. A kanamycin-resistant colony was selected, and the plasmid DNA from the transformant was isolated with a plasmid purification kit (Promega). DNA sequencing was carried out at the Macrogen facility.
Culture conditions
Recombinant E. coli containing the DAEase/pET15b gene from F. plautii was cultivated in a 2 L flask containing 500 mL of LB medium and 25 μg/mL ampicillin at 37°C with shaking at 200 rpm. When the optical density of the bacterial culture at 600 nm reached 0.6, 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as a final concentration was added to the culture to induce DAEase expression, and the culture was then incubated with shaking at 150 rpm at 16°C for 16 h to express the enzyme.
Recombinant C. glutamicum containing the DAEase/pEKEx2 gene from F. plautii was cultivated in a 2 L flask containing 500 mL of BHI medium and 15 μg/mL kanamycin at 30°C with shaking at 200 rpm. When the optical density of bacteria culture at 600 nm reached 0.6, 1.0 mM IPTG and 20 mg/L penicillin as final concentrations were added to the culture to induce DAEase expression and to injure the peptidoglycan layers of C. glutamicum cell wall, respectively. For enzyme expression, the culture was further incubated with shaking at 200 rpm at 30°C for 20 h.
Enzyme preparation
Recombinant E. coli expressing F. plautii DAEase was harvested from the culture broth by centrifugation at 8,000 × g for 30 min at 4°C, and then washed twice with 0.85% NaCl. Washed recombinant cells were resuspended in 50 mM phosphate buffer (pH 7.0) containing 300 mM NaCl and 1 mg/mL lysozyme. Resuspended cells were disrupted using a sonicator on ice for 2 min. Unbroken cells and cell debris were removed by centrifugation at 13000g for 20 min at 4°C, and the supernatant was filtered through a 0.45 μm pore size filter. All purification steps were carried out at 4°C with a Profinia protein purification system (Bio-Rad). The filtrate was loaded onto an immobilized metal ion affinity chromatography (IMAC) cartridge (Bio-Rad, Hercules, CA, USA), which was previously equilibrated with 50 mM phosphate buffer (pH 8.0). The bound protein was eluted with a linear gradient between 10 to 500 mM imidazole at a flow rate of 1 mL/min. The eluent was collected and loaded immediately onto a Bio-Gel P-6 desalting cartridge (Bio-Rad), previously equilibrated with 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) buffer (PIPES) (pH 7.0). The loaded protein was eluted with 50 mM PIPES buffer (pH 7.0) at a flow rate of 1 mL/min. The active fractions were collected and dialyzed against 50 mM PIPES buffer (pH 7.0) for 16 h. The solution resulting from dialysis was used as the purified enzyme.
Determination of molecular mass
The subunit molecular mass of F. plautii DAEase was determined by SDS-PAGE under denaturing conditions, using a ladder of pre-stained proteins (MBI fermentas, Hanover, MD, USA) as references. All protein bands were stained with Coomassie Blue for visualization. The molecular mass of the native enzyme was investigated by gel-filtration chromatography using a Sephacryl S-300 preparative-grade column HR 16/60 (GE Healthcare, Piscataway, NJ, USA). The purified enzyme was loaded onto the column and eluted with 50 mM Tris-HCl (pH 7.5) buffer containing 150 mM NaCl at a flow rate of 1mL/min. The column was calibrated with ferritin (440 kDa), catalase (206 kDa), aldolase (158 kDa), and conalbumin (75 kDa) as reference proteins (GE Healthcare). The molecular mass of the native enzyme was calculated by comparing with the migration length with that of the reference proteins.
Determination of specific activity and kinetic parameters
One unit (U) of enzyme activity for d-fructose was defined as the amount of enzyme required to liberate 1 μmol of d-allulose per min at pH 7.0 and 65°C. The specific activity (U/mg) was defined as the amount of monosaccharide produced per enzyme amount per unit reaction time. Various concentrations of d-fructose and d-allulose (5−80 mM) were used to decide the kinetic parameters for the enzyme. Km (mM) and kcat (1/s) were determined by the Lineweaver-Burk plot derived from the Michaelis-Menten equation. The catalytic constant, kcat, was calculated by dividing the subunit molecular mass by the DAEase concentration.
Preparation of permeabilized cells
To prepare permeabilized C. glutamicum cells, cells were harvested from the culture broth by centrifugation at 13,000 × g for 20 min at 4°C and then washed twice with 0.85% sodium chloride solution. The washed cells were resuspended in solutions containing 2 and 5 mg/L penicillin, and 2, 5, 50, and 100 mg/L ethambutol, ethionamide, and isoniazid as antibiotics; 0.2% and 0.5% (w/v) CTAB and Triton X-100, and 2% and 5% (w/v) Span 20, Span 80, Tween 20, Tween 40, and Tween 80 as detergents; and 10% and 20% (v/v) acetone, DMSO, ethanol, hexane, methanol, 1-propanol, iso-propanol, and toluene as solvents. The cell suspension was incubated at 4°C for 15 min and washed twice with 50 mM PIPES buffer (pH 7.0). The treated cells were used as permeabilized cells for the production of d-allulose from d-fructose. Unless otherwise stated, the enzyme and cell reactions were performed at 65°C for 10 min in 50 mM PIPES buffer (pH 7.0) containing 50 mM d-fructose in the presence of 1 mM Co2+ with 0.5 U/mL enzyme and 7.5 g/L cells, respectively, as standard conditions.
Effects of temperature, pH, and metal ions on DAEase activity
The effects of temperature and pH on the activity of DAEase, were investigated by varying the temperature from 30 to 70°C at a constant pH of 7.0 and by varying the pH from 6.0 to 8.5 at a constant temperature of 65°C under the standard conditions. To evaluate the effect of metal ions on enzyme activity, the enzyme assay was conducted after treatment with 1 mM ethylenediaminetetraacetic aicd (EDTA) at 4°C for 1 h or after the individual additions of 1 mM MnSO4, MnCl2, NiSO4, CoCl2, MgSO4, and FeCl3.
Effects of temperature, pH, and metal ions on the activity of recombinant cells
The effects of temperature and pH on the activity of permeabilized cells were examined by varying the temperature from 50 to 75°C in 50 mM PIPES buffer at a constant pH of 7.5 and by varying the pH from 6.0 to 8.5 at a constant temperature of 65°C, respectively. The effect of metal ions on the production of d-allulose from d-fructose was evaluated by nonpermeabilized and permeabilized C. glutamicum cells expressing DAEase from F. plautii.
Thermal inactivation for the enzyme in permeabilized C. glutamicum cells and the purified enzyme
The influence of temperature on the stability of the enzyme in permeabilized cells was investigated as a function of the incubation time by placing the cell solution at five different temperatures (45, 50, 55, 60, and 65°C) in 50 mM PIPES buffer (pH 7.5). The influence of temperature on enzyme stability was a function of incubation time by placing the enzyme solution at five different temperatures (45, 50, 55, 60, and 65°C) in 50 mM PIPES buffer (pH 7.0). Samples were withdrawn at specific time intervals and the activities were measured under standard conditions. The half-life of the enzyme was calculated using Sigma Plot 9.0 software (Systat Software, San Jose, CA).
Optimization of cell and substrate concentrations, and d-allulose production under the optimized conditions
The optimal concentrations of cells and substrate for increased production of d-allulose from d-fructose by whole permeabilized cells were determined by varying the cell concentration from 1 to 15 g/L at a constant d-fructose concentration of 750 g/L, and by varying the substrate concentration from 50 to 750 g/L at a constant cell concentration of 10 g/L. The production of d-allulose from d-fructose by whole permeabilized cells or nonpermeabilized cells was performed in 50 mM PIPES buffer (pH 7.5) containing 10 g/L cells, 750 g/L d-fructose, and 1 mM Co2+ at 65°C for 1 h.
Analytical methods
Cell mass was determined using a linear calibration curve of optical density at 600 nm verse dry cell weight. The OD600 values of culture broth were measured and then converted to the dry cell weight (1 OD600 = 0.42 g/L dry cell weight). The concentrations of monosaccharides, including d-fructose, d-allulose, d-sorbose, d-tagatose, d-xylulose, and d-ribulose, were determined using a Bio-LC system (Dionex ICS-3000, Sunnyvale, CA, USA) with an electrochemical detector and a CarboPac PA10 column. The column was eluted at 30°C with 200 mM sodium hydroxide at a flow rate of 1 mL/min.
Results and Discussion
Gene cloning, purification, and molecular mass determination of DAEase from F. plautii
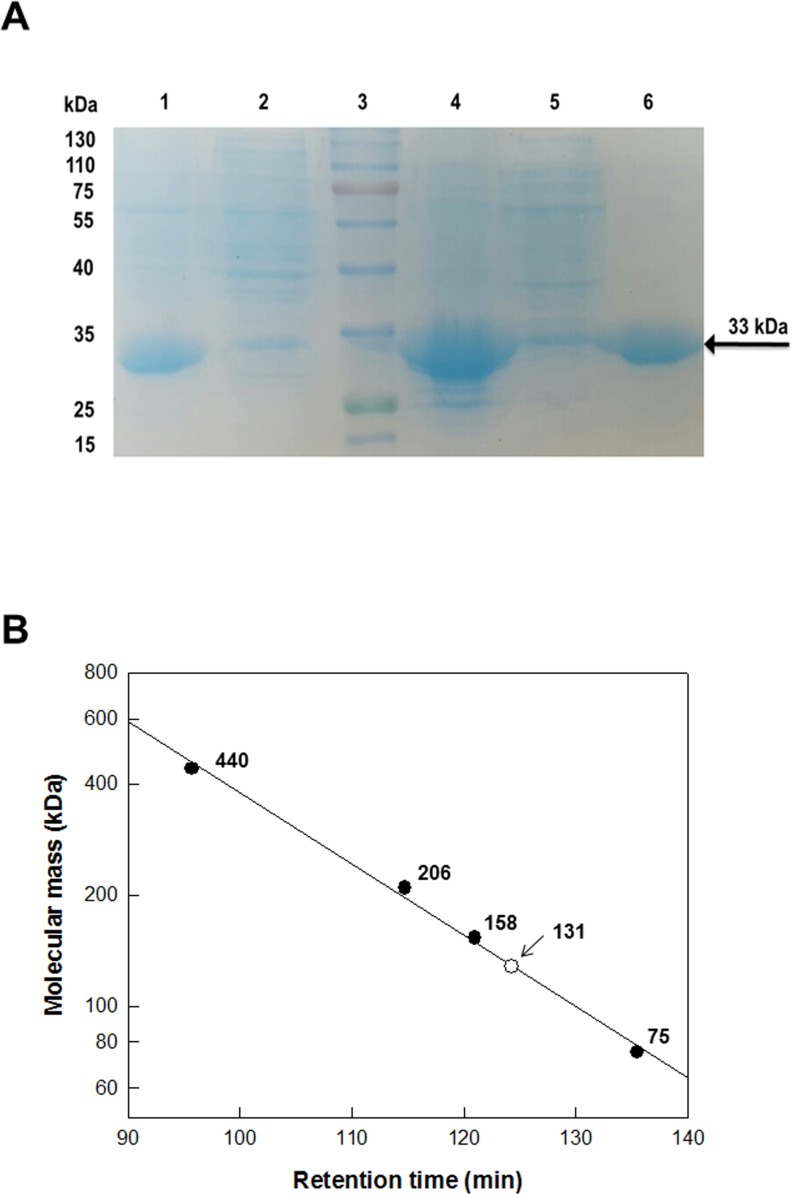
The gene (885 bp) encoding F. plautii DAEase (GenBank Accession No. EHM40452.1) was cloned and expressed in E. coli. The expressed enzyme was purified as a soluble protein from a crude cell extract by His-trap affinity chromatography. The specific activity of the purified enzyme for d-allulose was 20 U/mg. The expressed protein analyzed by SDS-PAGE showed a single band with a molecular mass of approximately 33 kDa, consistent with the calculated value of 32,987 Da based on the 295 amino acids plus the hexa-histidine tag. The expression level of the protein in C. glutamicum was lower than that of E. coli (Lanes 1 and 4 of Fig 1A). However, the enzyme was expressed as a soluble protein with a similar level between two host cells in crude extracts (Lanes 2 and 5 of Fig 1A). The native enzyme existed as a tetramer with a molecular mass of 131 kDa as determined by gel filtration chromatography (Fig 1B and S1 Fig). The total molecular masses and association forms of DAEases and DTEases were 125–132 kDa and tetramers, respectively.
Fig 1.
(A) SDS-PAGE of DAEase from F. plautii. Lanes: 1, pellet of C. glutamicum expressing DAEase; 2, crude extract of C. glutamicum expressing DAEase; 3, protein marker (130, 100, 75, 55, 40, 35, 25, and 17 kDa); 4, pellet of E. coli expressing DAEase; 5, crude extract of E. coli expressing DAEase; and 6, purified enzyme of E. coli expressing DAEase. (B) Determination of total molecular mass for DAEase from F. plautii by gel filtration chromatography with reference proteins. Ferritin (440 kDa), catalase (206 kDa), aldolase (158 kDa), and conalbumin (75 kDa); and DAEase from F. plautii.
Identification of the active-site residues of DAEase from F. plautii
The amino acid sequence of F. plautii DAEase was aligned with those of DTEase from P. cichorii [32] and DAEases from A. tumefaciens [33] and C. cellulolyticum [34], having the determined crystal structures (S2 Fig). The active site residues of A. tumefaciens DAEase were the same as those of C. cellulolyticum DAEase. The metal-binding residues were coordinated by Glu152, Asp185, His211, and Glu246 in P. cichorii DTEase; and Glu150, Asp183, His209, and Glu244 in A. tumefaciens DAEase. The metal-binding residues in F. plautii DAEase were absolutely conserved with Glu155, Asp188, His214, and Glu 250. The substrate-binding residues were Phe7, Trp15, Cys66, Leu108, Trp113, Glu158, His188, Arg217, and Phe248 in P. cichorii DTEase; and Tyr6, Trp14, Gly65, Ala107, Trp112, Glu156, His186, Arg215, and Phe248 in A. tumefaciens DAEase. Among the predicted substrate-binding residues of F. plautii DAEase, Trp15, Trp115, Glu161, His191, Arg220, and Phe252, were absolutely conserved across P. cichorii DTEase and A. tumefaciens DAEase. However, the other substrate-binding residues, including His7, Ser68, and Val110 in F. plautii DAEase, were different for the three enzymes.
Biochemical properties of DAEase from F. plautii
The biochemical properties of F. plautii DAEase are compared with other reported DTEases and DAEases in Table 1. The maximum enzyme activity of the conversion of d-fructose to d-allulose by F. plautii DAEase was observed at 65°C (S3A Fig) and pH 7.0 (S3B Fig). The activities of DAEase from Dorea sp. [15] and DTEase from R. sphaeroides [8], were maximal at pH 6.0 and pH 9.0, respectively. The pH values of other DAEases and DTEases were maximal in the range of 7.0 to 8.0. The lowest and highest temperatures for maximal activity were 40°C for DTEase from R. sphaeroides [8] and 70°C for DAEases from Dorea sp. [15] and T. primitia. [17] The effect of metal ions such as MnSO4, MnCl2, NiSO4, CoCl2, MgSO4, and FeCl3 on the activity of F. plautii DAEase was evaluated (S3C Fig). The activity was highest in the presence of Co2+, and DAEase form F. plautii was not activated without divalent cations, indicating that the enzyme is metal-dependent. Most other DAEases [10–12, 14, 15, 17] also showed the highest activity in presence of Co2+. The half-life of DAEase from C. cellulolyticum at 60°C was 408 min [12], which was the highest reported thermal stability among the DAEases and DTEases that was 3.1-fold higher than that of DAEase from F. plautii. DAEase from F. plautii produced 239 g/L d-allulose from 750 g/L d-fructose at pH 7.0 and 65°C after 60 min, with a conversion rate of 32% and a productivity of 239 g/L/h (S4 Fig). This is the highest production of d-allulose ever reported. The conversion rates of d-fructose to d-allulose by DAEases were higher than those by DTEases.
Table 1. Biochemical properties of DAEases and DTEases.
| Enzyme | pH | Temp. (°C) | Metal ions | Half-life at 60°C (min) | kcat (1/s) | Km (mM) | kcat/K m (1/mM/min) | d-Allulose (g/L)[d-Fructose, g/L] | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d-Allulose | d-Fructose | d-Allulose | d-Fructose | d-Allulose | d-Fructose | |||||||
| A. tumefaciens DAEase | 8.0 | 50 | Mn2+ | 4b | 40 | 35 | 12 | 24 | 205 | 85 | 230 [700] | [9] |
| Clostridium sp. DAEase | 8.0 | 65 | Co2+ | 10c | 537 | 273 | 228 | 279 | 141 | 59 | 120 [500] | [10] |
| C. bolteae DAEase | 7.0 | 55 | Co2+ | 45b | 49 | 59 | 27 | 60 | 107 | 59 | 216 [750] | [11] |
| C. cellulolyticum DAEase | 8.0 | 55 | Co2+ | 408c | 54 | 56 | 17 | 54 | 186 | 63 | 218 [750] | [12] |
| C. scindens DAEase | 7.5 | 55 | Mn2+ | 50b | 31 | 9 | 28 | 40 | 65 | 9 | NR | [13] |
| Desmospora sp. DAEase | 7.5 | 60 | Co2+ | NRa | 86 | 1,060 | 81 | 549 | 327 | 116 | 143 [500] | [14] |
| Dorea sp. DAEase | 6.0 | 70 | Co2+ | 60c, 30b | 1,311 | 508 | 191 | 153 | 412 | 199 | 115 [500] | [15] |
| P. cichorii DTEase | 7.5 | 60 | None | NR | NR | NR | NR | NR | NR | NR | 150 [780] | [7] |
| R. sphaeroides DTEase | 9.0 | 40 | Mn2+ | NR | NR | NR | NR | NR | NR | NR | 118 [700] | [8] |
| Ruminococcus sp. DAEase | 7.5–8.0 | 60 | Mn2+ | 96b | 41 | 59 | 48 | 216 | 51 | 16 | 125 [500] | [16] |
| T. primita DAEase | 8.0 | 70 | Co2+ | < 30b | 503 | 292 | 209 | 279 | 144 | 63 | 138 [500] | [17] |
| F. plautii DAEase | 7.0 | 65 | Co2+ | 130b | 421 | 342 | 162 | 323 | 156 | 64 | 239 [750] | This study |
aNR, not reported.
b and c represent that the half-life of each enzyme was measured without and with metal ions, respectively.
DAEases from Desmospora sp. [14] and Dorea sp. [15] exhibited the highest turnover number (kcat) for d-fructose and d-allulose, respectively. The catalytic efficiencies of DAEases for d-allulose were 2−3-fold higher than those for d-fructose except for C. scindens DAEase [13], which showed 7.2-fold higher catalytic efficiency for d-allulose than for d-fructose. Among the hexoketose substrates, the specific activity of F. plautii DAEase followed the order d-allulose > d-fructose > d-ribulose > d-xylulose > d-sorbose > d-tagatose (Table 2). The substrate specificity of A. tumefaciens DAEase followed the order d-allulose > d-fructose > d-tagatose > d-ribulose > d-xylulose > d-sorbose [9].
Table 2. Substrate specificity of F. plautii DAEase for hexoketose.
| Ketose | Relative activity (%) |
|---|---|
| d-Allulose | 100.0 ± 1.82 |
| d-Fructose | 41.1 ± 1.71 |
| d-Tagatose | 2.6 ± 0.07 |
| d-Sorbose | 6.9 ± 0.04 |
| d-Xylulose | 8.3 ± 0.16 |
| d-Ribulose | 10.5 ± 1.45 |
Data expressed as the mean of three separate experiments ± standard deviation.
Permeabilization of recombinant C. glutamicum cells expressing DAEase from F. plautii for increased production of d-allulose
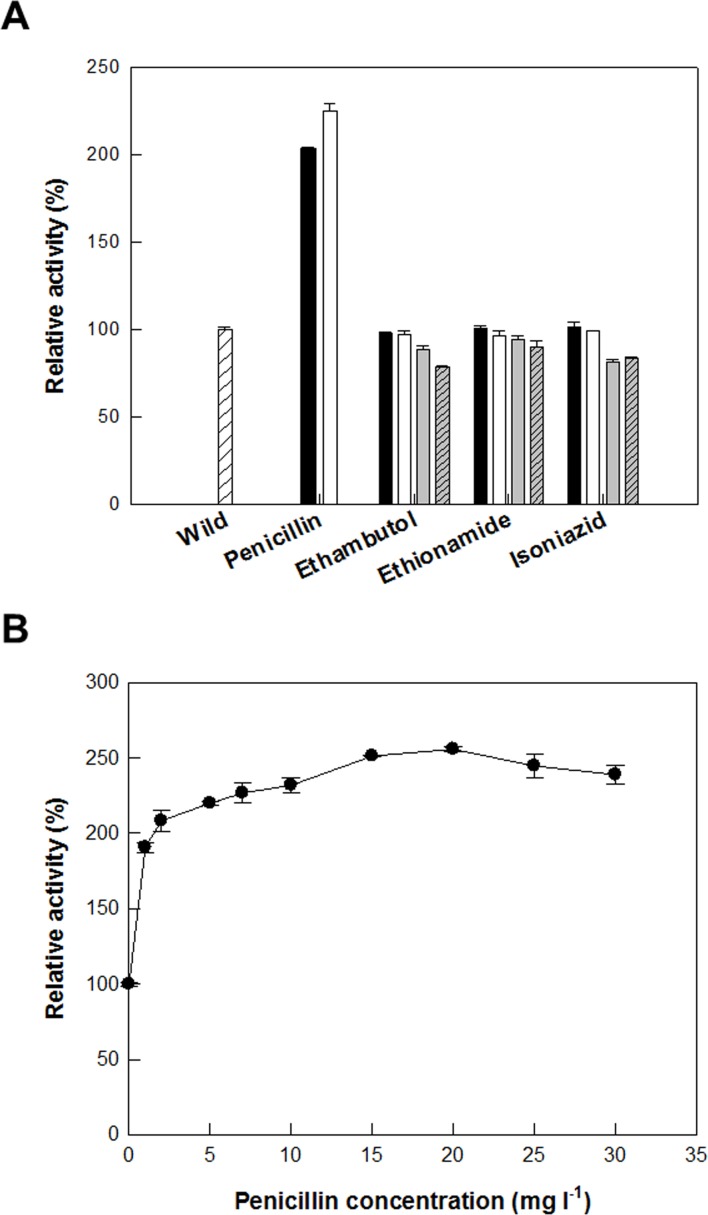
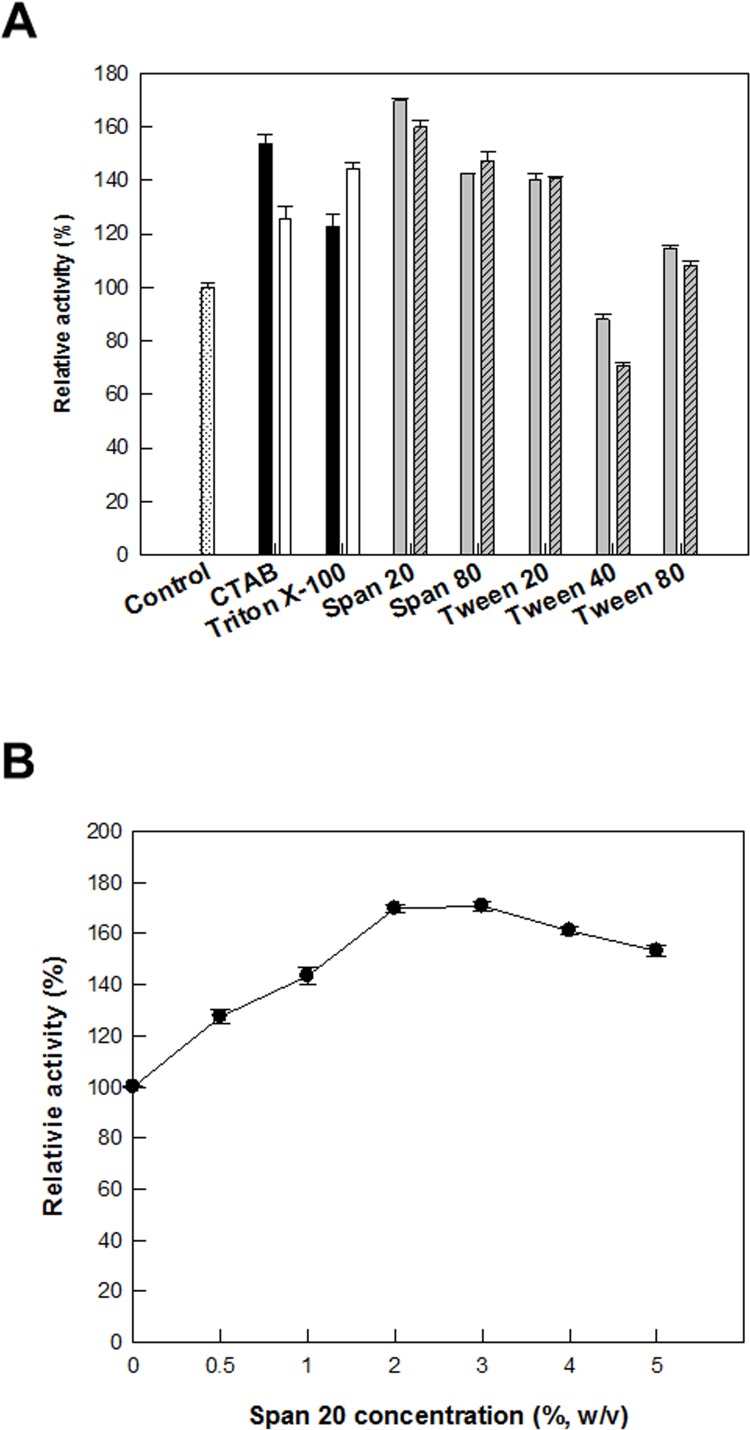
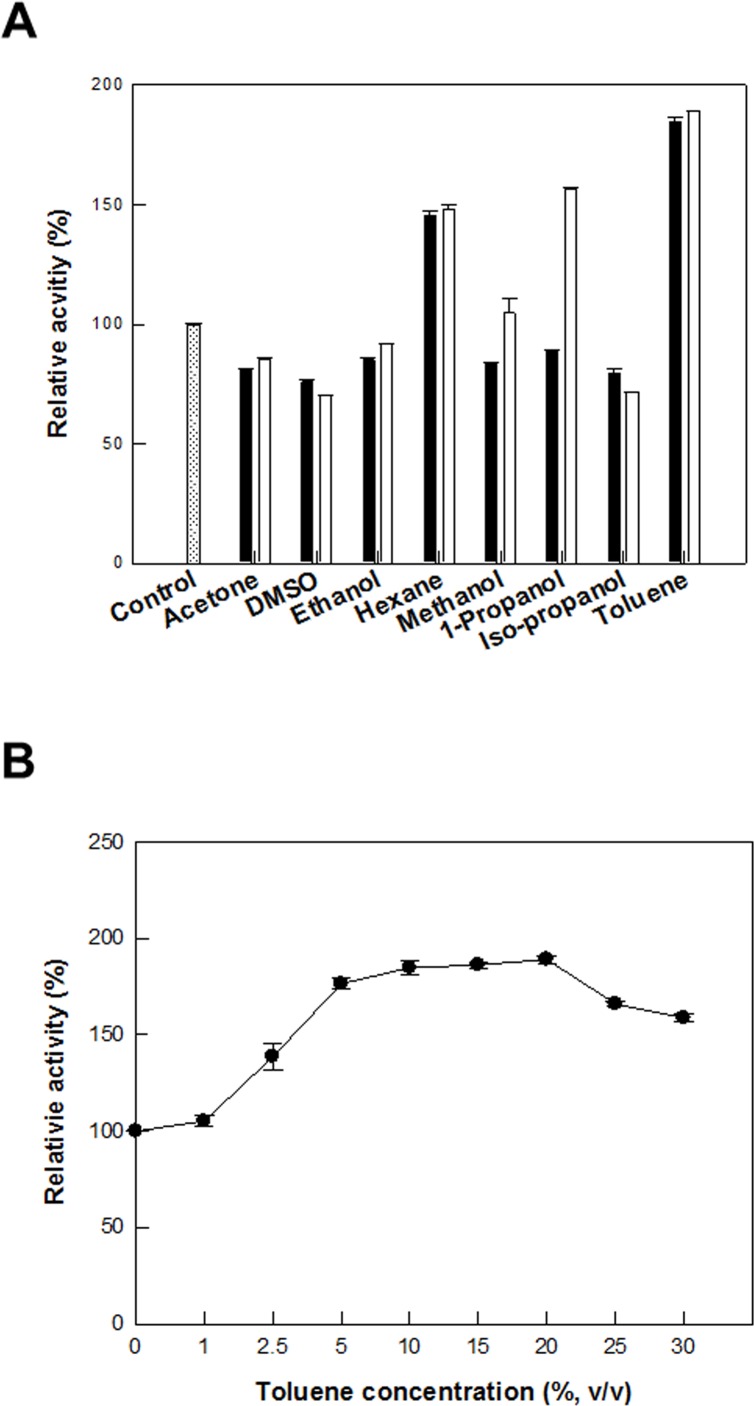
To increase d-allulose production, recombinant C. glutamicum cells expressing DAEase from F. plautii were permeabilized by treatment with antibiotics, including ethambutol, ethionamide, isoniazid, and penicillin; detergents, including CTAB, Span 20, Span 80, Triton X-100, Tween 20, Tween 40, and Tween 80; and solvents, including acetone, DMSO, ethanol, hexane, methanol, 1-propanol, isopropanol, and toluene. Permeabilized cells treated with 5 mg/L penicillin, 2% (w/v) span 20, and 20% (v/v) toluene showed the highest activity among the antibiotics, detergents, or solvents, respectively, and showed 2.2-, 1.6-, and 1.7-fold higher activity than that obtained by non-treated cells, respectively (Figs 2A, 3A and 4A). The effects of the concentrations of penicillin, span 20, and toluene on the production of d-allulose from d-fructose were investigated. The production of d-allulose was maximal in permeabilized cells treated with 20 mg/L penicillin, 3% (w/v) span 20, and 20% (v/v) toluene (Figs 2B, 3B and 4B), and was 2.5-, 1.7-, and 1.8-fold higher than that by non-treated cells, respectively. Penicillin was used to permeabilize C. glutamicum during glutamic acid fermentation [31] and toluene was an efficient permeabilizer to G. suboxydans for the production of l-sorbose [35].
Fig 2. Effect of antibiotic treatment on the permeabilization of C. glutamicum expressing DAEase from F. plautii for the production of d-allulose from d-fructose.
(A) Effect of antibiotic treatment. 0 (white bar with diagonal line), 2 (black bar), 5 (white bar), 50 (gray bar), and 100 mg/L (gray bar with diagonal line). (B) Effect of penicillin concentration. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L cells and 50 mM d-fructose at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
Fig 3. Effect of detergent treatment on the permeabilization of C. glutamicum expressing DAEase from F. plautii for the production of d-allulose from d-fructose.
(A) Effects of detergent treatment. 0 (white bar with dotted line), 0.2 (black bar), 0.5 (white bar), 2 (gray bar), and 5% (w/v) (gray bar with diagonal line). (B) Effect of span 20 concentration. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L cells and 50 mM d-fructose at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
Fig 4. Effect of solvent treatment on the permeabilization of C. glutamicum expressing the DAEase from F. plautii for the production of d-allulose from d-fructose.
(A) Effect of solvent treatment. 0 (white bar with dotted line), 10 (black bar), and 20% (v/v) (white bar). (B) Effect of toluene concentration. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L cells and 50 mM d-fructose at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
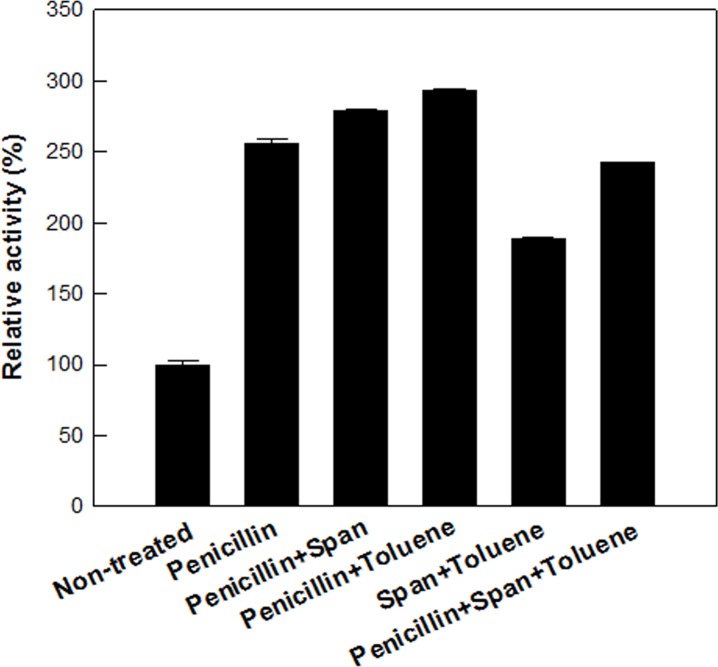
To determine the synergistic effect in d-allulose production, cells were treated with 20 mg/L penicillin, 3% (w/v) Span 20, and 20% (v/v) toluene in combination. Permeabilized cells treated with 20 mg/L penicillin and 20% (v/v) toluene showed the highest activity among cells permeabilized by the combined treatment, and exhibited 3-fold higher d-allulose production than that by non-treated cells (Fig 5). The increases in d-allulose production by permeabilized cells treated with efficient permeabilizers such as penicillin, Span 20, and toluene were not critical above 2 mg/L, 1% (w/v), and 5% (v/v), respectively, which were all lower concentrations than those used for the maximal production of d-allulose, respectively. Cells were treated with a combination of the permeabilizers using these concentrations (S5 Fig). Permeabilized cells treated with 2 mg/L penicillin and 5% (v/v) toluene also showed the highest activity among cells permeabilized with the combined treatment and lower d-allulose production than that by permeabilized cells treated with 20 mg/L penicillin and 20% (v/v) toluene. Thus, permeabilized cells treated with 20 mg/L penicillin and 20% (v/v) toluene were used for d-allulose production.
Fig 5. Effect of the combined treatment of permeabilizers on the permeabilization of C. glutamicum expressing DAEase from F. plautii for the production of d-allulose from d-fructose.
The concentrations of penicillin, span 20, and toluene used were 20 mg/L, 2% (w/v), and 10% (v/v), respectively. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L cells and 50 mM d-fructose at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
Effects of temperature, pH, and metal ions on the production of d-allulose from d-fructose by permeabilized recombinant C. glutamicum cells
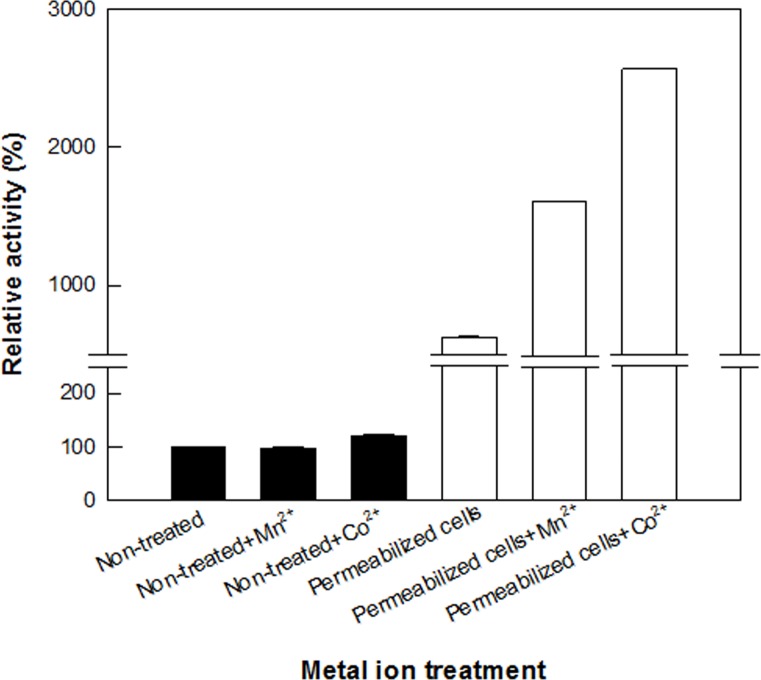
The maximum activity of permeabilized C. glutamicum cells expressing DAEase from F. plautii for d-allulose production was observed at 65°C (S6A Fig) and pH 7.5 (S6B Fig). The reactions of whole recombinant E. coli cells expressing DAEase from C. bolteae [11], C. cellulolyticum [12], and A. tumerfaciens [20] were performed at pH 6.5 and 55°C, pH 8.0 and 55°C, and pH 8.5 and 60°C, respectively. Mn2+ and Co2+ strongly enhanced d-fructose epimerization by DAEases and DTEases. Thus, the effect of metal ions such as Mn2+ and Co2+ on the activity of d-allulose production was evaluated in nonpermeabilized and permeabilized C. glutamicum cells expressing DAEase from F. plautii (Fig 6). The activities of both recombinant nonpermeabilized and permeabilized C. glutamicum cells in the presence of Co2+ were higher than those in the presence of Mn2+. d-Allulose production by permeabilized cells in the presence of Co2+ was 25-fold higher than that by nonpermeabilized cells. The significantly increased production of d-allulose may be due to the improved contact of metal ions and the enzyme caused by cell permeabilization.
Fig 6. Effect of metal ions on the production of d-fructose to d-allulose by nonpermeabilized and permeabilized cells of C. glutamicum expressing DAEase from F. plautii.
Production of d-allulose from d-fructose by nonpermeabilized cells (black bar) and permeabilized cells (white bar). The reactions were conducted in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L cells and 50 mM d-fructose in the presence of 1 mM metal ions at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
Thermal inactivation for DAEase from F. plautii in permeabilized recombinant C. glutamicum cells and the purified DAEase
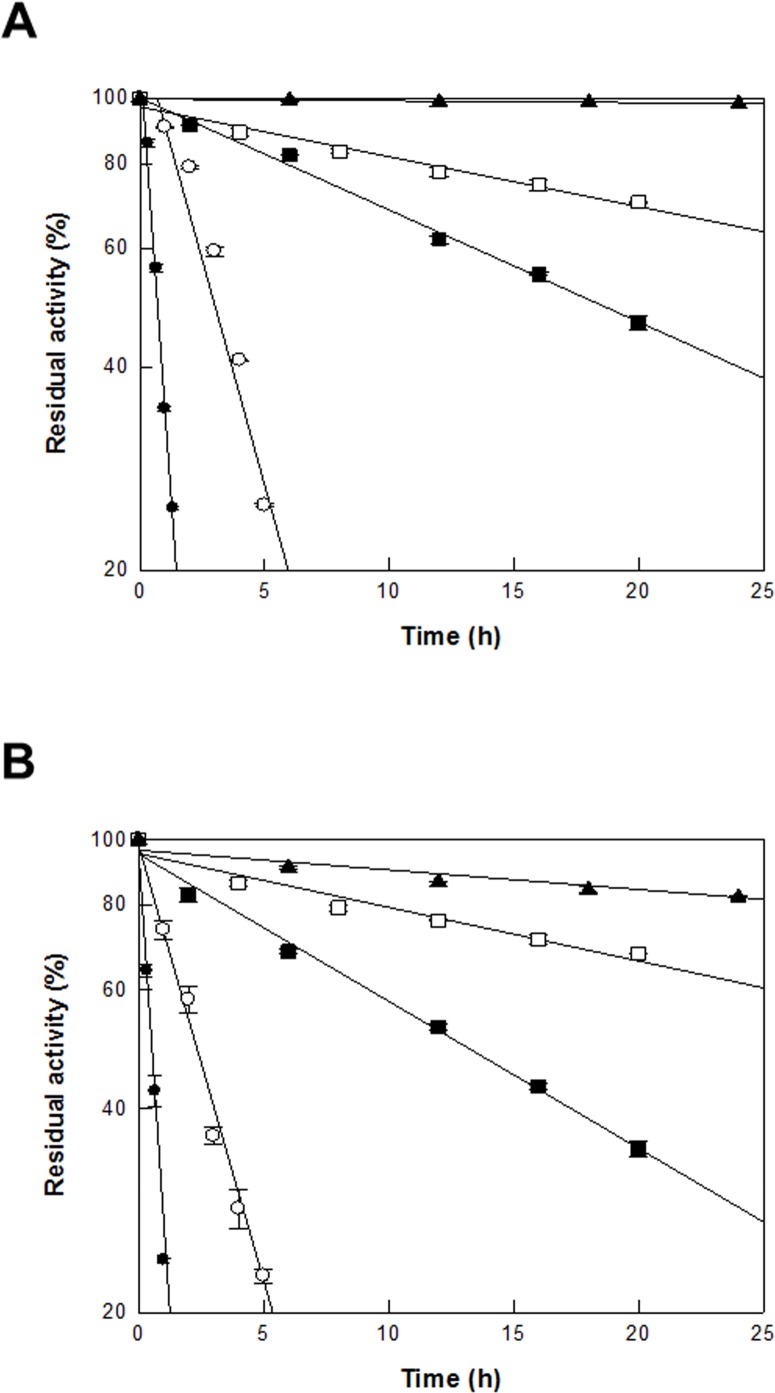
The thermal stabilities of DAEase from F. plautii in recombinant permeabilized C. glutamicum cells and the purified enzyme were examined by measuring the activities after incubation at temperatures ranging from 45 to 65°C. Thermal inactivation of the enzyme in cells and the purified enzyme followed first-order kinetics. The half-lives of the enzyme in cells at 45, 50, 55, 60, and 65°C were 9000, 2340, 1098, 211, and 48 min, respectively (Fig 7A), which were 1.8-, 1.0-, 2.3-, 1.4-, and 1.1-fold higher than those of the double-site variant DAEase from A. tumefaciens [20] in recombinant E. coli cells, respectively. The half-lives of the purified enzyme at 45, 50, 55, 60, and 65°C were 5760, 2010, 762, 130, and 40 min (Fig 7B), respectively, which were 1.5-, 1.1-, 1.4-, 1.6-, and 1.2-fold lower than those of the enzyme in recombinant C. glutamicum cells, respectively. Thus, DAEase in cells is more thermally stable than the purified DAEase.
Fig 7. Thermal stability of the activities of DAEase from F. plautii and DAEase from F. plautii in recombinant C. glutamicum cells.
(A) Thermal stability of the activity of recombinant cells. The permeabilized cells were incubated at 45°C (filled triangle), 50°C (empty square), 55°C (filled square), 60°C (empty circle), and 65°C (filled circle) for various incubation times. (B) Thermal stability of enzyme activity. The enzymes were incubated at 45°C (filled triangle), 50°C (empty square), 55°C (filled square), 60°C (empty circle), and 65°C (filled circle) for various incubation times. A sample was withdrawn at each time point and the relative activity was measured. Data represent the means of three separate experiments and error bars represent the standard deviation.
Effects of cell and substrate concentrations on d-allulose production by permeabilized recombinant C. glutamicum cells
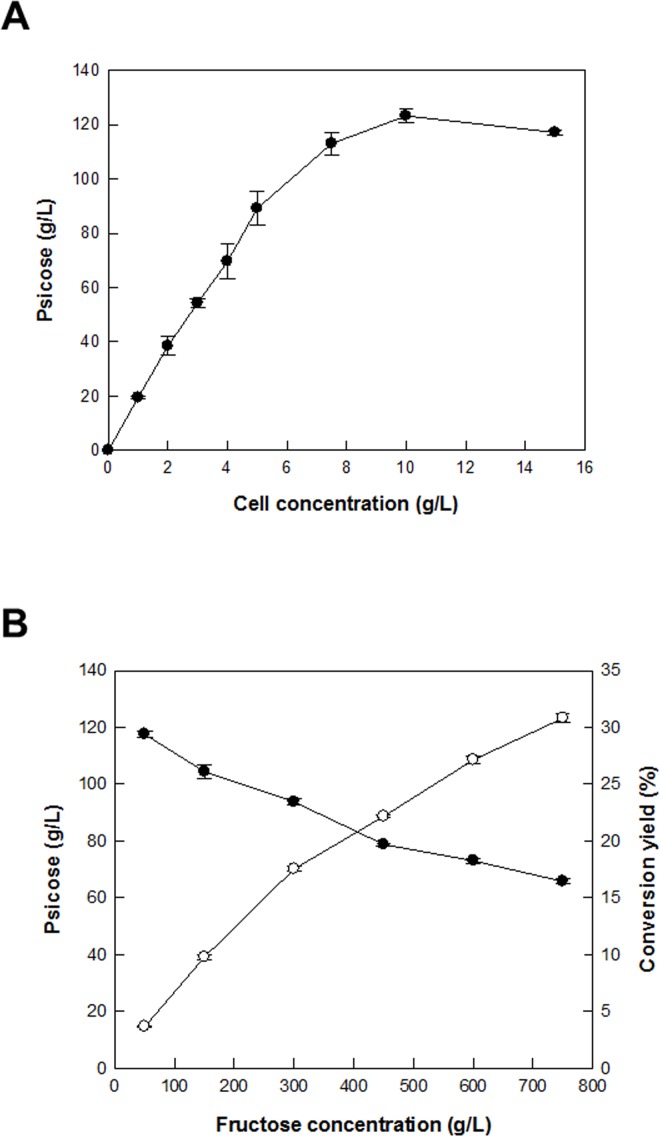
The optimal concentration of recombinant permeabilized cells for d-allulose production was determined by varying the concentration of permeabilized cells from 0.5 to 15 g/L with 750 g/L d-fructose as a substrate for 10 min (Fig 8A). Below 10 g/L cells, d-allulose production increased with increasing cell concentration up to 10 g/L cells. However, above 10 g/L cells, the production reached a plateau. Therefore, the cell concentration for the maximal production of d-allulose was 10 g/L. The production of d-allulose from d-fructose was investigated by varying the substrate concentration from 50 to 750 g/L with 10 g/L cells (Fig 8B). As the d-fructose concentration increased, d-allulose production increased, but the conversion rate decreased. To obtain the highest concentration of d-allulose, the concentration of d-fructose as a substrate was determined to be 750 g/L.
Fig 8. Effects of cell and substrate concentrations on d-allulose production from d-fructose by permeabilized C. glutamicum cells expressing DAEase from F. plautii.
(A) Effect of cell concentration. The reactions were performed by varying the cell concentration from 1 to 15 g/L in 50 mM PIPES buffer (pH 7.5) containing 750 g/L d-fructose in the presence of 1 mM Co2+ at 65°C for 10 min. (B) Effect of substrate concentration. The reactions were performed by varying the d-fructose concentration from 50 to 750 g/L in 50 mM PIPES buffer (pH 7.5) containing 10 g/L permeabilized cells in the presence of 1 mM Co2+ at 65°C for 10 min. Data represent the means of three separate experiments and error bars represent the standard deviation.
Production of d-allulose from d-fructose by nonpermeabilized and permeabilized recombinant C. glutamicum cells under the optimized conditions
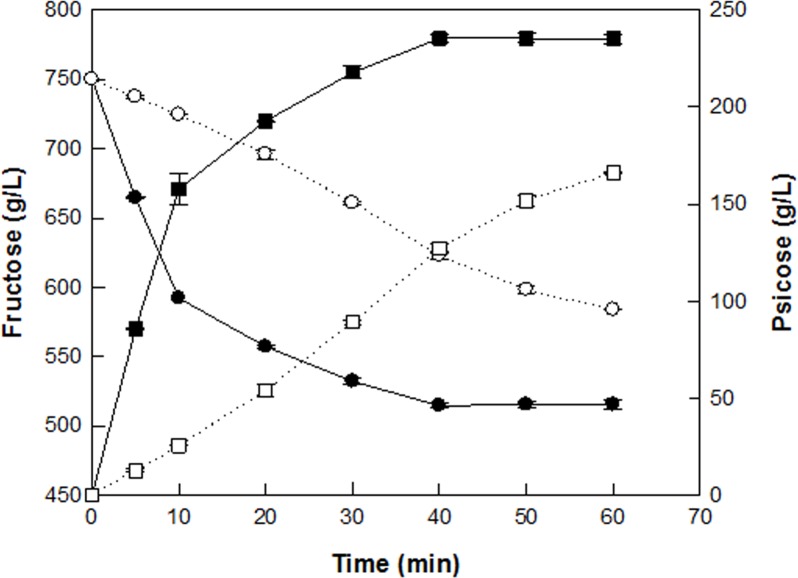
The optimal reaction conditions for the production of d-allulose from d-fructose by permeabilized recombinant C. glutamicum cells expressing DAEase from F. plautii were pH 7.5, 65°C, 1 mM Co2+, 10 g/L cells, and 750 g/L d-fructose. Under the optimized conditions, the time-course reactions for d-allulose production were performed using nonpermeabilized and permeabilized cells (Fig 9). The initial production rate within 10 min of d-allulose by permeabilized cells was significantly higher than that by nonpermeabilized cells. Nonpermeabilized cells produced 166 g/L d-allulose after 60 min, with a conversion rate of 22% (w/w), a specific productivity of 16.6 g/g/h, and a volumetric productivity of 166 g/L/h, whereas permeabilized cells produced 235 g/L d-allulose after 40 min, with a conversion rate of 31% (w/w), a specific productivity of 35.3 g/g/h, and a volumetric productivity of 353 g/L/h, which were 1.4-, 2.1- and 2.1-fold higher than those of nonpermeabilized cells, respectively, and the volumetric productivity of permeabilized cells was 1.5-fold higher than that of purified enzyme. Thus, cell permeabilization was an effective method for increasing d-allulose production.
Fig 9. Time-course reactions for the production of d-allulose from d-fructose by permeabilized and nonpermeabilized cells of C. glutamicum expressing DAEase from F. plautii.
The production of d-allulose (filled square) from d-fructose (filled circle) by permeabilized cells and the production of d-allulose (empty square) from d-fructose (empty circle) by nonpermeabilized cells. The reactions were performed in 50 mM PIPES buffer (pH 7.5) containing 10 g/L cells and 750 g/L d-fructose in the presence of 1 mM Co2+ at 65°C for 1 h. Data represent the means of three separate experiments and error bars represent the standard deviation.
The permeabilized cells of wild-type R. sphaeroides treated with 0.1% (w/v) CTAB produced 6.5 g/L d-allulose from 50 g/L d-fructose after 8 h, a specific productivity of 0.03 g/g/h, and a volumetric productivity of 0.81 g/L/h [18]. The permeabilized cells of wild-type Sinorhizobium sp. treated with 10% (v/v) toluene produced 37 g/L d-allulose from 700 g/L d-fructose after 15 h, a specific productivity of 0.04 g/g/h, and a volumetric productivity of 2.5 g/L/h [19]. Wild-type cells were not suitable for d-allulose production because of the markedly lower specific productivity than that of recombinant cells. The production of d-allulose from d-fructose by whole recombinant E. coli, Bacillus subtilis, or C. glutamicum cells expressing DAEase is presented in Table 3. Whole recombinant E. coli cells expressing the double-site variant (I33L-S213C) DAEase from A. tumefaciens produced 230 g/L d-allulose from 700 g/L d-fructose after 40 min, with a conversion rate of 33% (w/w), a specific productivity of 86.2 g/g/h, and a volumetric productivity of 345 g/L/h [20], which were previously the highest reported production values. The concentration, volumetric productivity, and thermal stability of recombinant permeabilized C. glutamicum cells expressing DAEase from F. plautii in d-allulose production were higher than those of recombinant E. coli cells expressing the double-site variant DAEase from A. tumefaciens, whereas the conversion rate and specific productivity were lower. DAEase from Clostridium scindens was expressed in B. subtilis as a food-grade host and the food-grade recombinant B. subtilis was used for the production of d-allulose [36]. The product concentration, conversion rate, and volumetric productivity of the recombinant C. glutamicum cells in the resent study were higher than those of the recombinant B. subtilis cells, but the specific productivity was lower.
Table 3. d-Allulose production from d-fructose by whole recombinant cells expressing DAEase.
| Host | Strain for enzyme source | Substrate (g l−1) | Product (g l−1) | Conversion rate (%, w/w) | Half-life at 60°C | Specific productivity (g/g/ h) | Volumetric productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|---|
| E. coli | Clostridium bolteae | 750 | 216 | 29 (55°C, pH 6.5) | NR | 40.5 | 81 | [11] |
| E. coli | Clostridium cellulolyticum | 750 | 218 | 29 (55°C, pH 8.0) | NR | 54.5 | 109 | [12] |
| E. coli | Agrobacterium tumefaciens | 700 | 230 | 33 (60°C, pH 8.5) | 150 | 86.2 | 345 | [20] |
| B. subtilis | Clostridium scindens | 700 | 196 | 28 (60°C, pH 7.5) | NR | 56.0 | 196 | [36] |
| C. glutamicum | Flavonifractor plautii | 750 | 235 | 31 (65°C, pH 7.5) | 211 | 35.3 | 353 | This study |
NR, not reported.
In conclusion, the putative DAEase from F. plautii was cloned and expressed in E. coli. The biochemical properties of the expressed enzyme for the epimerization of d-fructose to d-allulose were characterized. Recombinant C. glutamicum cells were permeabilized to increase the production of d-allulose. The reaction conditions, including pH, temperature, metal ions, and the concentrations of cells and substrate, were optimized for the permeabilized cells. Under the optimized conditions, the production of d-allulose from d-fructose by permeabilized C. glutamicum cells expressing DAEase from F. plautii treated with penicillin and toluene was significantly higher than that by nonpermeabilized cells. Whole permeabilized recombinant C. glutamicum cells produced d-allulose with the highest concentration and volumetric productivity reported to date.
Supporting Information
The X-axis and Y-axis represent retention time and UV absorbance at 280 nm, respectively. Violet peak represents DAEase, which was eluted at 123 min.
(TIF)
The GenBank accession numbers for DAEases and DTEases are as follows: F. plautii DAEase (Flpl-DAEase, EHM40452.1), Dorea sp. DAEase (Dosp-DAEase, WP_022318236.1), T. primitia DAEase (Trpr-DAEase, ZP_09717154.1), Desmospora sp. DAEase (Desp-DAEase, WP_009711885), C. cellulolyticum DAEase (Clce-DAEase, ACL75304), Clostridium sp. DAEase (Clsp-DAEase, WP_014314767.1), C. scindens DAEase (Clsc-DAEase, EDS06411.1), C. boltease DAEase (Clbo-DAEase, EDP19602), A. tumefaciens DAEase (Agtu-DAEase, AAK88700.1), Ruminococcus sp. DAEase (Rusp-DAEase, ZP_04858451.1), R. sphaeroides DTEase (Rhsp-DTEase, ACO59490), and P. cichorii DTEase (Psci-DTEase, BAA24429). The metal binding and catalytic residues are appear in blue boxes, and the substrate binding residues appear in red boxes.
(TIF)
(A) Effect of temperature. The reactions were conducted by varying the temperature from 30°C to 70°C for 10 min in 50 mM PIPES (pH 7.0) buffer containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM Co2+. (B) Effect of pH. The reactions were conducted by varying the pH from 6.0 to 8.5 at 65°C for 10 min in 50 mM MES buffer (filled circle, pH 6.0−6.5), 50 mM PIPES buffer (empty circle, pH 6.5−7.5), and EPPS buffer (filled square, pH 7.5−8.5) containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM Co2+. (C) Effect of metal ions. The reactions were conducted at 65°C for 10 min in 50 mM PIPES (pH 7.0) buffer containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM metal ions. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 8 U/mL enzyme and 750 g/L d-fructose at 65°C for 3 h. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
The concentrations of penicillin, span 20, and toluene were 2 mg/L, 1% (w/v), and 5% (v/v), respectively. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L permeabilized cells and 50 mM d-fructose at 65°C for 10 min. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
(A) Effect of temperature. The reactions were conducted by varying the temperature from 50°C to 75°C for 10 min in 50 mM PIPES (pH 7.5) buffer containing 50 mM d-fructose and 7.5 g/L permeabilized cells. (B) Effect of pH. The reactions were conducted by varying the pH from 6.0 to 8.5 at 65°C for 10 min in 50 mM MES buffer (filled circle, pH 6.0−6.5), 50 mM PIPES buffer (empty circle, pH 6.5−7.5), and EPPS buffer (filled square, pH 7.5−8.5) containing 7.5 g/L permeabilized cells and 50 mM d-fructose. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ01113701), Rural Development Administration, Republic of Korea.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ01113701), Rural Development Administration, Republic of Korea.
References
- 1.Binkley WW. The fate of cane juice simple sugars during molasses formation. IV. Probable conversion of D-fructose to D-psicose. Int Sugar J. 1963;65(1):105–106. [Google Scholar]
- 2.Miller BS, Swain T. Chromatographic analyses of the free amino acids, organic acids and sugars in wheat plant extracts. J Sci Food Agric. 1960;11(6):344–348. 10.1002/jsfa.2740110609 [DOI] [Google Scholar]
- 3.Levin GV, Zehner LR, Saunders JP, Beadle JR. Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr. 1995;62(5 Suppl):1161S–1168S . [DOI] [PubMed] [Google Scholar]
- 4.Matsuo T, Suzuki H, Hashiguchi M, Izumori K. D-psicose is a rare sugar that provides no energy to growing rats. J Nutr Sci Vitaminol (Tokyo). 2002;48(1):77–80 . [DOI] [PubMed] [Google Scholar]
- 5.Livesey G, Brown JC. D-Tagatose is a bulk sweetener with zero energy determined in rats. J Nutr. 1996;126(6):1601–1609 . [DOI] [PubMed] [Google Scholar]
- 6.Chung MY, Oh DK, Lee KW. Hypoglycemic health benefits of D-psicose. J Agric Food Chem. 2012;60(4):863–869. 10.1021/jf204050w . [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Yamada M, Nishitani T, Takada G, Izumori K, Kamitori S. Crystal structures of D-tagatose 3-epimerase from Pseudomonas cichorii and its complexes with D-tagatose and D-fructose. J Mol Biol. 2007;374(2):443–453. 10.1016/j.jmb.2007.09.033 . [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Mu W, Jiang B, Zhang T. Characterization of D-tagatose-3-epimerase from Rhodobacter sphaeroides that converts D-fructose into D-psicose. Biotechnol Lett. 2009;31(6):857–862. 10.1007/s10529-009-9942-3 . [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Hyun EK, Kim YS, Lee YJ, Oh DK. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl Environ Microbiol. 2006;72(2):981–985. 10.1128/AEM.72.2.981-985.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu W, Zhang W, Fang D, Zhou L, Jiang B, Zhang T. Characterization of a D-psicose-producing enzyme, D-psicose 3-epimerase, from Clostridium sp. Biotechnol Lett. 2013;35(9):1481–1486. 10.1007/s10529-013-1230-6 . [DOI] [PubMed] [Google Scholar]
- 11.Jia M, Mu W, Chu F, Zhang X, Jiang B, Zhou LL, et al. A D-psicose 3-epimerase with neutral pH optimum from Clostridium bolteae for D-psicose production: cloning, expression, purification, and characterization. Appl Microbiol Biotechnol. 2013;98(2):717–725. 10.1007/s00253-013-4924-8 . [DOI] [PubMed] [Google Scholar]
- 12.Mu W, Chu F, Xing Q, Yu S, Zhou L, Jiang B. Cloning, expression, and characterization of a D-psicose 3-epimerase from Clostridium cellulolyticum H10. J Agric Food Chem. 2011;59(14):7785–7792. 10.1021/jf201356q . [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Fang D, Xing Q, Zhou L, Jiang B, Mu W. Characterization of a novel metal-dependent D-psicose 3-epimerase from Clostridium scindens 35704. PLoS One. 2013;8(4):e62987 10.1371/journal.pone.0062987 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Fang D, Zhang T, Zhou L, Jiang B, Mu W. Characterization of a metal-dependent D-psicose 3-epimerase from a novel strain, Desmospora sp. 8437. J Agric Food Chem. 2013;61(47):11468–11476. 10.1021/jf4035817 . [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Li H, Zhang T, Jiang B, Zhou L, Mu W. Characterization of a D-psicose 3-epimerase from Dorea sp. CAG317 with an acidic pH optimum and a high specific activity. J Mol Catal B Enzym. 2015;120:68–74. 10.1016/j.molcatb.2015.05.018 [DOI] [Google Scholar]
- 16.Zhu Y, Men Y, Bai W, Li X, Zhang L, Sun Y, et al. Overexpression of D-psicose 3-epimerase from Ruminococcus sp. in Escherichia coli and its potential application in D-psicose production. Biotechnol Lett. 2012;34(10):1901–1906. 10.1007/s10529-012-0986-4 . [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhang T, Jiang B, Mu W. Biochemical characterization of a D-psicose 3-epimerase from Treponema primitia ZAS-1 and its application on enzymatic production of D-psicose. J Sci Food Agric. 2015;96(1):49–56. 10.1002/jsfa.7187 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Jiang B, Mu W, Zhang T. Bioproduction of D-psicose using permeablilized cells of newly isolated Rhodobacter sphaeroides SK011. Font Chem Eng China. 2009;3(4):393–398. 10.1007/s11705-009-0252-z [DOI] [Google Scholar]
- 19.Oh DK, Kim NH, Kim HJ, Park CS, Kim SW, Ko M, et al. D-Psicose production from D-fructose using an isolated strain, Sinorhizobium sp. World J Microbiol Biotechnol. 2007;23(4):559–563. 10.1007/s11274-006-9265-7 . [DOI] [Google Scholar]
- 20.Park CS, Park CS, Shin KC, Oh DK. Production of D-psicose from D-fructose by whole recombinant cells with high-level expression of D-psicose 3-epimerase from Agrobacterium tumefaciens. J Biosci Bioeng. 2016;121(2):186–190. 10.1016/j.jbiosc.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 21.Burdock GA, Carabin IG. Generally recognized as safe (GRAS): history and description. Toxicol Lett 2004;150:3–15. 10.1016/j.toxlet.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Shyamkumar R, Moorthy IM, Ponmurugan K, Baskar R. Production of L-glutamic Acid with Corynebacterium glutamicum (NCIM 2168) and Pseudomonas reptilivora (NCIM 2598): A Study on Immobilization and Reusability. Avicenna J Med Biotechnol. 2014;6(3):163–168 . [PMC free article] [PubMed] [Google Scholar]
- 23.Bayan N, Houssin C, Chami M, Leblon G. Mycomembrane and S-layer: two important structures of Corynebacterium glutamicum cell envelope with promising biotechnology applications. J Biotechnol. 2003;104(1):55–67. 10.1016/S0168-1656(03)00163-9 . [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Yun JY, Kim SG, Seo JH, Park JB. Production of xylitol from D-xylose and glucose with recombinant Corynebacterium glutamicum. Enzyme Microb Technol. 2010;46(5):366–371. 10.1016/j.enzmictec.2009.12.012 [DOI] [Google Scholar]
- 25.An JU, Joo YC, Oh DK. New biotransformation process for production of the fragrant compound gamma-dodecalactone from 10-hydroxystearate by permeabilized Waltomyces lipofer cells. Appl Environ Microbiol. 2013;79(8):2636–2641. 10.1128/AEM.02602-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Leon A, Garcıa B, de la Rosa AB, Villasenor F, Estrada A, López-Revilla R. Periplasmic penicillin G acylase activity in recombinant Escherichia coli cells permeabilized with organic solvents. Process Biochem. 2003;39(3):301–305. 10.1016/S0032-9592(03)00079-7 [DOI] [Google Scholar]
- 27.Choi HY, Seo MJ, Shin KC, Oh DK. Production of 10-hydroxy-12,15(Z,Z)-octadecadienoic acid from alpha-linolenic acid by permeabilized Stenotrophomonas nitritireducens cells. Biotechnol Lett. 2015;37(11):2271–2277. 10.1007/s10529-015-1913-2 . [DOI] [PubMed] [Google Scholar]
- 28.Oh HJ, Shin KC, Oh DK. Production of 10-hydroxy-12,15(Z,Z)-octadecadienoic acid from alpha-linolenic acid by permeabilized cells of recombinant Escherichia coli expressing the oleate hydratase gene of Stenotrophomonas maltophilia. Biotechnol Lett. 2013;35(9):1487–1493. 10.1007/s10529-013-1231-5 . [DOI] [PubMed] [Google Scholar]
- 29.Chen RR. Permeability issues in whole-cell bioprocesses and cellular membrane engineering. Appl Microbiol Biotechnol. 2007;74(4):730–738. 10.1007/s00253-006-0811-x . [DOI] [PubMed] [Google Scholar]
- 30.Becker M, Borngen K, Nomura T, Battle AR, Marin K, Martinac B, et al. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828(4):1230–1240. 10.1016/j.bbamem.2013.01.001 . [DOI] [PubMed] [Google Scholar]
- 31.Ahmed YM, Khan JA, Abulnaja KA, Al-Malki AL. Production of glutamic acid by Corynebacterium glutamicum using dates syrup as carbon source. Afr J Microbiol Res. 2013;7:2071–2077. 10.5897/AJMR12.2223 [DOI] [Google Scholar]
- 32.Yoshida H, Yamada M, Nishitani T, Takada G, Izumori K, Kamitori S. Crystal structures of D-tagatose 3-epimerase from Pseudomonas cichorii and its complexes with D-tagatose and D-fructose. J Mol Biol. 2007;374(2):443–453. 10.1016/j.jmb.2007.09.033 . [DOI] [PubMed] [Google Scholar]
- 33.Kim K, Kim HJ, Oh DK, Cha SS, Rhee S. Crystal structure of D-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate D-fructose: a pivotal role of metal in catalysis, an active site for the non-phosphorylated substrate, and its conformational changes. J Mol Biol. 2006;361(5):920–931. 10.1016/j.jmb.2006.06.069 . [DOI] [PubMed] [Google Scholar]
- 34.Chan HC, Zhu Y, Hu Y, Ko TP, Huang CH, Ren F, et al. Crystal structures of D-psicose 3-epimerase from Clostridium cellulolyticum H10 and its complex with ketohexose sugars. Protein cell. 2012;3(2):123–131. 10.1007/s13238-012-2026-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park YM, Choi ES, Rhee SK. Effect of toluene-permeabilization on oxidation of D-sorbitol to L-sorbose by Gluconobacter suboxydans cells immobilized in calcium alginate. Biotechnol lett. 1994;16(4):345–348. 10.1007/BF00245048 [DOI] [Google Scholar]
- 36.He W, Mu W, Jiang B, Yan X, Zhang T. Construction of a food grade recombinant Bacillus subtilis based on replicative plasmids with an auxotrophic marker for biotransformation of D-fructose to D-allulose. J Agric Food Chem. 2016;64(16):3243–3250. 10.1021/acs.jafc.6b00278 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The X-axis and Y-axis represent retention time and UV absorbance at 280 nm, respectively. Violet peak represents DAEase, which was eluted at 123 min.
(TIF)
The GenBank accession numbers for DAEases and DTEases are as follows: F. plautii DAEase (Flpl-DAEase, EHM40452.1), Dorea sp. DAEase (Dosp-DAEase, WP_022318236.1), T. primitia DAEase (Trpr-DAEase, ZP_09717154.1), Desmospora sp. DAEase (Desp-DAEase, WP_009711885), C. cellulolyticum DAEase (Clce-DAEase, ACL75304), Clostridium sp. DAEase (Clsp-DAEase, WP_014314767.1), C. scindens DAEase (Clsc-DAEase, EDS06411.1), C. boltease DAEase (Clbo-DAEase, EDP19602), A. tumefaciens DAEase (Agtu-DAEase, AAK88700.1), Ruminococcus sp. DAEase (Rusp-DAEase, ZP_04858451.1), R. sphaeroides DTEase (Rhsp-DTEase, ACO59490), and P. cichorii DTEase (Psci-DTEase, BAA24429). The metal binding and catalytic residues are appear in blue boxes, and the substrate binding residues appear in red boxes.
(TIF)
(A) Effect of temperature. The reactions were conducted by varying the temperature from 30°C to 70°C for 10 min in 50 mM PIPES (pH 7.0) buffer containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM Co2+. (B) Effect of pH. The reactions were conducted by varying the pH from 6.0 to 8.5 at 65°C for 10 min in 50 mM MES buffer (filled circle, pH 6.0−6.5), 50 mM PIPES buffer (empty circle, pH 6.5−7.5), and EPPS buffer (filled square, pH 7.5−8.5) containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM Co2+. (C) Effect of metal ions. The reactions were conducted at 65°C for 10 min in 50 mM PIPES (pH 7.0) buffer containing 0.5 U/mL enzyme and 50 mM d-fructose in the presence of 1 mM metal ions. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 8 U/mL enzyme and 750 g/L d-fructose at 65°C for 3 h. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
The concentrations of penicillin, span 20, and toluene were 2 mg/L, 1% (w/v), and 5% (v/v), respectively. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 7.5 g/L permeabilized cells and 50 mM d-fructose at 65°C for 10 min. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
(A) Effect of temperature. The reactions were conducted by varying the temperature from 50°C to 75°C for 10 min in 50 mM PIPES (pH 7.5) buffer containing 50 mM d-fructose and 7.5 g/L permeabilized cells. (B) Effect of pH. The reactions were conducted by varying the pH from 6.0 to 8.5 at 65°C for 10 min in 50 mM MES buffer (filled circle, pH 6.0−6.5), 50 mM PIPES buffer (empty circle, pH 6.5−7.5), and EPPS buffer (filled square, pH 7.5−8.5) containing 7.5 g/L permeabilized cells and 50 mM d-fructose. Data are presented as the means of three separate experiments and error bars represent the standard deviation.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.