Abstract
A large correlational study took a latent-variable approach to the generality of executive control by testing the individual-differences structure of executive-attention capabilities and assessing their prediction of schizotypy, a multidimensional construct (with negative, positive, disorganized, and paranoid factors) conveying risk for schizophrenia. Although schizophrenia is convincingly linked to executive deficits, the schizotypy literature is equivocal. Subjects completed tasks of working memory capacity (WMC), attention restraint (inhibiting prepotent responses), and attention constraint (focusing visual attention amid distractors), the latter two in an effort to fractionate the “inhibition” construct. We also assessed mind-wandering propensity (via in-task thought probes) and coefficient of variation in response times (RT CoV) from several tasks as more novel indices of executive attention. WMC, attention restraint, attention constraint, mind wandering, and RT CoV were correlated but separable constructs, indicating some distinctions among “attention control” abilities; WMC correlated more strongly with attentional restraint than constraint, and mind wandering correlated more strongly with attentional restraint, attentional constraint, and RT CoV than with WMC. Across structural models, no executive construct predicted negative schizotypy and only mind wandering and RT CoV consistently (but modestly) predicted positive, disorganized, and paranoid schizotypy; stalwart executive constructs in the schizophrenia literature — WMC and attention restraint — showed little to no predictive power, beyond restraint’s prediction of paranoia. Either executive deficits are consequences rather than risk factors for schizophrenia, or executive failures barely precede or precipitate diagnosable schizophrenia symptoms.
Keywords: working memory capacity, executive attention, inhibition, mind wandering, schizotypy, individual differences
People endeavor to regulate their mental processes — their attentional focus, their reactions to alluring distractions, their thought content — with varying success. That is, some people seem to have better cognitive control than others: showing minimal distraction from environmental events, persisting in goal-directed activities despite tempting diversions, and staying focused on tasks without their thoughts being derailed by personal concerns. One might wonder who these people are, and what makes them successful at self-control. However, we should first determine whether there truly is a class of “these people” to identify. That is, are adults who are less distractible also more successful at withholding impulsive comments? Does a person’s distractibility from environmental events also predict distractibility from their own thoughts? These questions are fundamentally about whether individual differences in cognitive, or “executive,” control are domain general and stable across different threats to control.
Given that most intellectual abilities share individual-differences variance (e.g., Carroll, 1993; Gustafsson, 1984; Horn, 1968), we expect some generality of control capabilities. Indeed, empirical research by Miyake, Friedman, and colleagues (e.g., Friedman et al., 2008, 2011; Miyake, Friedman, Emerson, Witzi, & Howerter, 2000; see Miyake & Friedman, 2012, for a review) suggests both domain generality (“unity”) and domain specificity (“diversity”) of executive control. Confirmatory factor analyses of task batteries including response inhibition, memory updating, and task-set switching measures indicate that these three executive factors are distinguishable. That is, one’s response-inhibition capabilities are not identical to one’s memory-updating or task-switching capabilities. At the same time, the three factors correlate substantially (≈.40 – .60), indicating some domain generality. It therefore seems that there is a group of “these people,” who are effective cognitive regulators across domains.
At the same time, purely cognitive approaches to executive individual differences fail to capture all the ways in which control abilities, and control failures, may manifest in both laboratory and everyday settings (e.g., Altamirano, Miyake, & Whitmer, 2010; Unsworth et al., 2009; Young et al., 2009). In order to expand the field’s consideration of executive-control variation, the present study assesses its association with a personality construct — schizotypy — that has been linked to control deficits (e.g., Gooding, 1999; Kerns, 2006; Tallent & Gooding, 1999). Schizotypy refers to a spectrum of unusual experiential, emotional, behavioral, and interpersonal traits, with psychosis and schizophrenia at its extreme (e.g., Meehl, 1990). Our present goal, then, is to explore the individual-differences structure of executive control — focusing on fractionating the response-inhibition construct and exploring additional executive attention factors, such as working memory capacity, mind-wandering propensity, and reaction-time variability — and testing their associations to a personality construct (schizotypy) that is associated with executive-control deficits and with strange subjective experiences suggesting attentional differences.
Executive Attention and Schizotypy
Etiological models of schizophrenia (Andreasen, 1999; Gottesman, 1991; Meehl, 1990) assume that an interaction of genetic, neurodevelopmental, and psychosocial factors underlie vulnerability for schizophrenia and spectrum disorders, which is expressed across a continuum known as “schizotypy.” Most people high in schizotypy will not decompensate into schizophrenia, but many will experience attenuated or transient symptoms, ranging from sub-clinical deviance, to spectrum personality disorders, to psychosis (e.g., Kwapil, Barrantes-Vidal, & Silvia, 2008). Schizotypy is a multidimensional construct comprising latent factors that mirror those of schizophrenia: negative, positive, disorganized, and paranoid (e.g., Arndt, Alliger, & Andreasen, 1991; Bilder, Mukherjee, Rieder, & Pandurangi, 1985; Horton, Barantes-Vidal, Silvia, & Kwapil, 2014; Liddle, 1987). Negative schizotypy involves functional and experiential deficits, such as social withdrawal, avolition, anhedonia, and diminished affect, whereas positive schizotypy involves experiential excesses, such as unusual beliefs (magical and referential thinking; delusions) and perceptual experiences (illusions; hallucinations). Like positive schizotypy, and also reflecting prototypical features of psychosis, both paranoid and disorganized schizotypy exhibit abundant but abnormal thought: Paranoid schizotypy features suspiciousness and expectation of mistreatment or persecution, whereas disorganized schizotypy reflects confused, disordered speech, thought, and behavior.
Questionnaire measures, such as the Schizotypal Personality Questionnaire (SPQ; Raine, 1991) and the Wisconsin Schizotypy scales (WSS; e.g., Chapman, Chapman, & Raulin, 1976, 1978; Eckblad & Chapman, 1983), validly assess schizotypic traits (Kwapil & Chun, 2015). Psychometrically assessed schizotypy is associated with psychotic-like, prodromal, schizophrenia-spectrum, and subjective cognitive symptoms (e.g., Barrantes-Vidal, Chun, Myin-Germeys, & Kwapil, 2013; Blanchard, Collins, Aghevli, Leung, & Cohen, 2011; Kwapil et al., 2008; Yon, Loas, & Monestès, 2009). Longitudinally, positive schizotypy predicts development of psychotic disorders and negative schizotypy predicts schizophrenia-spectrum disorders (Chapman et al., 1994; Gooding, Tallent, & Matts, 2005; Kwapil, 1998; Kwapil, Gross, Silvia, & Barrantes-Vidal, 2013). Cross-sectionally, schizotypy predicts schizophrenic-like patterns of neuro- and social-cognitive impairment, neurological soft signs, and neuroimaging signatures (e.g., Coleman, Levy, Lenzenweger & Holzman, 1996; Fuggetta, Bennett, Duke, & Young, 2014; Gooding, Matts, & Rollmann, 2006; Kaczorowski, Barrantes-Vidal, & Kwapil, 2009; Modinos et al., 2010). Daily-life experience sampling further indicates that positive schizotypy predicts momentary psychotic-like symptoms, negative affect, suspiciousness, and stress-reactivity, whereas negative schizotypy predicts decreased positive affect and social interest, and diminished thoughts and emotions (e.g., Kwapil et al., 2009, 2012; Barrantes-Vidal et al., 2013).
Considerable research has explored cognitive and, specifically, executive-control correlates of schizophrenia (see Barch, 2005; Barch & Ceasar, 2012; Heinrichs & Zakzanis, 1998; Park & Gooding, 2014). Studying schizotypy in currently healthy adults, however, has advantages regarding questions about risk versus resilience for psychopathology. From a cognitive perspective, a further advantage is that mental processes associated with schizotypy can be studied unconfounded by the severe behavioral, social, and medical consequences of schizophrenia, which may obfuscate disease-specific effects. Indeed, even in first-episode, medication-naïve schizophrenia patients, who are free of such chronic influences (e.g., Barch et al., 2001, 2003), acute symptoms in the moment may impair motivation or ability to perform cognitive tasks. Any observed executive deficits in schizophrenia are thus ambiguous regarding cognitive versus motivational influences and whether cognitive deficits confer liability for, or follow from, the disorder.
Unfortunately, only a small literature has addressed the association between schizotypy and executive control. This limited work, moreover, presents mixed findings that are difficult to reconcile. Different studies use different schizotypy measures — some average across multiple schizotypy factors and others on a particular dimension (e.g., social anhedonia). Some studies assess schizotypy continuously and others dichotomize schizotypy and control groups arbitrarily. Some studies test university students, others draw from the broader community, and most do so with underpowered samples. Most studies also use only a single instrument to assess schizotypy, but even those that use multiple measures tend not to combine them using latent-variable techniques. Similarly, they assess particular cognitive constructs with widely different tasks and almost always with only a single, multiply determined task per construct (and when multiple tasks are used, they are usually treated individually).
Schizotypy and Working Memory Capacity
Schizotypy studies typically measure working memory capacity (WMC), the ability to maintain information in the service of ongoing activities, with digit- or letter-number span tests, n-back tasks, or delayed match-t0-sample tasks. The findings are incoherent. Matheson and Langdon (2008) found that some schizotypy factors, but not others, correlated modestly with letter-number span, but most studies have found no differences between higher and lower schizotypy subjects in letter-number, digit, or other span tasks (Avons, Nunn, Chan, & Armstrong, 2003; Chan, Wang, et al., 2011; Chun, Minor, & Cohen, 2013; Daly, Afroz, & Walder, 2012; Iati, 2012; Lenzenweger & Gold, 2000; M. Peters, Smeets, Giesbrecht, Jelici, & Merckelbach, 2007; Tervo, 2004; Unsworth et al., 2009; Wang et al., 2008). Regarding n-back and delayed-match-to-sample studies, they are each about evenly split between those finding null schizotypy effects (Chan, Wang et al., 2011; Smyrnis et al., 2007; Park & McTigue, 1997; N. Smith & Lenzenweger, 2013; Wang et al., 2008) and those that show limited and inconsistent schizotypy effects, with schizotypy-related differences arising in some dependent measures but not others, or for some schizotypy dimensions but not others (Gooding & Tallent, 2003; Kerns & Becker, 2008; Koychev, El-Deredy, Haenschel, & Deakin, 2010; Koychev et al., 2012; Park, Holzman & Lenzenweger, 1995; Schmidt-Hansen & Honey, 2009; Tallent & Gooding, 1999). On balance, there may be some limited signal amid this noise, but unlike the schizophrenia literature, it is not clear whether all or any schizotypy dimensions are associated with WMC impairment.
Schizotypy and Executive Attention
In terms of other, relevant executive dimensions, enough studies have assessed the relation of schizotypy to sustained attention and inhibitory control to review here. Sustained attention has been most frequently measured with the continuous performance identical-pairs test (CPT-IP); subjects respond only when two consecutive stimuli (e.g., 4-digit numbers) in a sequence are identical. Several studies found either a negative correlation between schizotypy scores and CPT-IP accuracy (Bergida & Lenzenweger, 2006; Chen, Hsiao & Lin, 1997; Rawlings & Goldberg, 2001) or a mean deficit in CPT-IP for high compared to low schizotypy groups (Gooding et al., 2006; Lenzenweger, 2001; Lenzenweger, Cornblatt, & Putnick, 1991; Obiols, García-Domingo, de Trinchería, & Doménech, 1993). Many of these effects were small, however, and either relied on 1-tailed tests, or arose only in some outcome measures, or arose inconsistently for some schizotypy dimensions but not others. Moreover, other CPT-IP studies have found only null effects of schizotypy (Smyrnis et al., 2007; Tervo, 2004), as have studies using different sustained attention measures, the Sustained Attention to Response Task (SART; Chan, Wang et al., 2011; Chan, Yan et al., 2011), and the COGLAB sustained attention test (Otteson, 1995; Spaulding, Garbin, & Dras, 1989).
Inhibiton-control results are also mixed. The SART, mentioned with the null studies above, is a go/no-go task that demands response inhibition in addition to sustained attention. The venerable Stroop task also yields primarily null results: most studies find no deficits associated with schizotypy (Beech, Baylis, Smithson & Claridge, 1989; Cimino & Haywood, 2008; Dinn, Harris, Aycicegi, Greene, & Andover, 2002; Höfer, Della Casa, & Feldon, 1999; Kerns, 2006; Lipp, Siddle, & Arnold, 1994; Martin & Kerns, 2010; E. Peters, Pickering & Hemsley, 1994; Steel, Hemsley, & Jones, 1996). Only three studies have reported significant Stroop results, but inconsistently across different schizotypy dimensions (Moritz et al., 1999; Surh, 1997; Swerdlow, Filion, Geyer, & Braff, 1995). Finally, regarding inhibition, two other measures show limited sensitivity to schizotypy. In the antisaccade task and the Preparation for Overcoming a Prepotent Response task, which require subjects to respond in opposition to strong visual cues, people who are high in some schizotypy dimensions (or in some measures), but not in others, show worse performance (Gooding, 1999; Kerns, 2006; O’Driscoll, Lenzenweger, & Holzman, 1998; Unsworth et al., 2009).
In short, our review indicates that the schizotypy literature — which features many studies lacking in statistical power, in optimal construct measurement, or both — currently lacks clear evidence that either WMC, sustained attention, or inhibitory control are deficient in people who are high in schizotypy. A more comprehensive and sophisticated approach to measuring schizotypy and executive control abilities, at the level of constructs, is needed to make significant theoretical progress.
The Structure of WMC and Executive Attention
Individual-differences research on executive attention has two historical roots. One, currently focused on the constructs of inhibition, memory updating, and switching, grew from questions regarding neuropsychological tests of ostensible frontal-lobe functions and whether these “executive functions” were unitary or distinguishable (see Miyake & Friedman, 2012). The other arose from testing theoretical claims about working memory’s “central executive” component (Baddeley, 1986) and the generality of its predictive power. That is, individual differences in WMC clearly predicted important and diverse intellectual abilities (e.g., Daneman & Carpenter, 1980; Engle, Tuholski, Laughlin, & Conway, 1999; Kyllonen & Christal, 1990; Shute, 1991) and a candidate mechanism was a domain-general set of “executive attention” capabilities. Engle and colleagues tested this idea and discovered that attention-demanding components of memory retrieval, such as controlling interference, discriminated higher- from lower-WMC adults (e.g., Conway & Engle, 1994; Rosen & Engle, 1997, 1998). Moreover, relatively “simple” attention tasks also varied with WMC (e.g., Conway, Cowan, & Bunting, 2001; Kane, Bleckley, Conway, & Engle, 2001; Kane & Engle, 2003). Such findings suggested that variation in domain-general attention-control processes contributed to WMC variation and its covariation with complex cognition (e.g., Braver, Gray, & Burgess, 2007; Hasher, Lustig, & Zacks, 2007; Kane, Conway, Hambrick, & Engle, 2007).
More recent, large-scale studies of WMC and attention control have assumed that a variety of tasks tap into a single “executive attention” factor, whether they require focusing on a target stimulus amid distractors, over-riding a prepotent response to a stimulus, or sustaining optimal response readiness over long tasks. Most of these studies mix 2–4 such tasks and take their shared variance to reflect a latent executive construct via structural equation modeling. These models fit the data, indicating generality, but these studies have not used enough tasks of each type to test for dissociable forms of control. What these studies do show clearly is that WMC and executive attention are strongly linked, with latent-variable correlations in the .50–.70 range (reported correlations in brackets: Chuderski, 2014 [.61]; Chuderski, Taraday, Nęcka, & Smoleń, 2012 [.63, Study 1; .60, Study 2]; Colom et al., 2008 [.52]; Dang, Braeken, Colom, Ferrer, & Liu 2014 [.61 with spatial WMC; .45 with verbal WMC]; McVay & Kane, 2012b [.73]; Schweizer & Moosbrugger, 2004 [.50]; Shipstead, Harrison, & Engle, 2015 [.74, data set 2]; Shipstead, Lindsey, Marshall, & Engle, 2014 [.68]; Unsworth, Brewer, & Spillers, 2012 [.64]; Unsworth, Fukuda, Awh, & Vogel, 2014 [.54]; Unsworth & Spillers, 2010 [.58]; Unsworth, Spillers, & Brewer, 2009 [.41]; but for outlying null correlations, see Keye, Wilhelm, Oberauer, & Ravenzwaaij, 2009 [.07 and .16] and Keye, Wilhelm, Oberauer, & Stürmer, 2013 [.06]). We therefore argue that WMC and attention-control abilities share 25–50% of their variance.
These strong WMC-attention correlations suggest generality, but two large-scale studies have attempted to fractionate the executive-attention construct further. Chuderski et al. (2012, Study 1) tested whether goal-maintenance, response-competition, and response-inhibition abilities showed unity and diversity, and whether they correlated with WMC. The attention constructs did not correlate with each other and they differentially correlated with WMC, both indicating diversity of executive attention. Unfortunately, the study tested poorly operationalized constructs and used too few tasks and subjects. Friedman and Miyake (2004) asked a more tractable question: whether response inhibition tasks (overriding dominant responses; e.g., stop-signal and Stroop tasks) tap the same construct as distractor interference tasks (ignoring distractor stimuli; e.g., flanker tasks). Their structural model indicated a strong (.68) correlation between response-inhibition and distractor-interference factors. In fact, a single “inhibition-distraction” factor fit the data, indicating that response and distractor control were strongly related, if not isomorphic. We have more confidence in Friedman and Miyake’s conclusions — that executive attention constructs are reasonably well correlated — given their study’s larger sample, their more adequate task battery, and their non-zero correlations among attention tasks matching those from other studies (e.g., Chuderski, 2014; McVay & Kane, 2012b; Shipstead et al., 2014; Unsworth & Spillers, 2010).
At the same time, with only two contradictory studies, we must withhold strong judgment about the unity versus diversity of executive attention. This is unfortunate because important theoretical questions can hinge on whether particular tasks are good indicators of a general executive construct. For example, Paap and Greenberg’s (2013; see also Paap, Johnson, & Sawi, 2014; Paap & Sawi, 2014) arguments against a bilingual advantage in executive control are based in part on weak correlations among different putative inhibition tasks (antisaccade, flanker, and Simon tasks): If bilingual advantages are seen on one or another of these tasks, but the tasks don’t correlate, then the evidence cannot support a task- or domain-general bilingual benefit. Similarly, executive-attention theories of WMC may be considered either falsified or specified in light of null WMC effects in particular attention tasks, such as Simon, visual search, or task switching (e.g., Draheim, Hicks, & Engle, 2016; Keye et al., 2009; Meier & Kane, 2015; Oberauer, Süß, Wilhelm, & Wittmann, 2003; Poole & Kane, 2009). To advance our understanding of executive attention constructs, the present study rigorously tests the generality versus specificity of response inhibition and distractor interference constructs, with a large participant and task sample (we use the labels attention restraint and attention constraint, respectively, as neutral descriptions for these tasks’ demands).
Mind Wandering Propensity as Another Marker of Executive Attention
People’s thoughts often drift from their ongoing task and immediate environment, a phenomenon described as “daydreaming,” “mind wandering,” or “task-unrelated thought” (TUT; e.g., Giambra, 1989; Klinger, 1999; Singer, 1966; Smallwood & Schooler, 2006). Scientific studies typically assess TUTs by interrupting subjects’ ongoing tasks with unpredictable thought probes that ask them whether their immediately preceding thoughts were on-task or off-task. To the extent that someone intends to stay task-focused, a TUT experience may reflect executive-control failure, much like distraction by irrelevant environmental stimuli (McVay & Kane, 2010). Mind wandering isn’t always unintentional or problematic, however, and so executive processes cannot completely account for individual differences in TUTs (e.g., Seli, Cheyne, Xu, Purdon, & Smilek, 2015). Indeed, a theoretical consensus is emerging that executive control does not simply prevent mind wandering by actively maintaining task-oriented cognition: Executive processes may also support mind wandering by maintaining internally focused cognition when situations allow it (Christoff, Gordon, Smallwood, Smith & Schooler, 2009; Smallwood, 2013; Smallwood & Andrews-Hanna, 2013; see also Thomson, Besner, & Smilek, 2015); they may also dynamically shift focus between on- and off-task thought based on task demands (Rummel & Boywitt, 2014).
The current study presented demanding contexts where TUTs impair performance and thus better (but imperfectly) indicate control failures. Our primary question was whether executive-control variation, such as in WMC, attention restraint, and constraint, would predict TUT rates, with people of higher control reporting fewer TUTs. We further asked whether executive constructs differentially predicted mind-wandering. Limited evidence suggests that attention restraint correlates more strongly with TUT rates than does WMC (McVay & Kane, 2012b; Unsworth & McMillan, 2014) and that constraint abilities do not correlate at all with questionnaire measures of daydreaming, which contradicts executive-attention accounts of TUT vulnerability (Forster & Lavie, 2014). Should researchers continue to use WMC tasks to explore executive contributions to mind wandering? Are constraint abilities uniquely independent of TUTs?
WMC correlates negatively with TUTs during demanding tasks (for reviews, see Kane & McVay, 2012; Randall, Oswald, & Beier, 2014). In a week-long, daily-life study, Kane, Brown et al. (2007) provided WMC-screened subjects with a digital device that probed their thoughts and asked about their context. Lower WMC subjects reported more TUTs than did higher WMC subjects only during activities they rated as requiring more concentration and as more challenging and effortful. In lab tasks that assess attention restraint (McVay & Kane, 2009, 2012a), memory updating (Rummel & Boywitt, 2014), or reading (McVay & Kane, 2012b; Unsworth & McMillan, 2013), WMC also negatively predicts TUTs. But in relatively easy but tedious tasks, such as vigilance or “pop-out” visual search (Levinson, Smallwood, & Davidson, 2012; McVay & Kane, 2012a), WMC is uncorrelated with TUTs; indeed, trivially demanding tasks may even elicit more mind wandering in higher than in lower WMC subjects (Levinson et al., 2012; Rummel & Boywitt, 2014).
The WMC-TUT association is thus moderated by task demands and is only modest, even in demanding contexts. Most studies have found correlations between individual WMC and TUT measures in the −.10 to −.20 range (e.g., McVay & Kane, 2009; Rummel & Boywitt, 2014; Unsworth & McMillan, 2014) and between WMC and TUT latent variables in the −.20 to −.30 range (McVay & Kane, 2012a, 2012b; Unsworth & McMillan, 2012, 2014). Along with reported null associations (e.g., Krawietz, Tamplin, & Radvansky, 2012; Smeekens & Kane, in press), the meta-analytic estimate for the correlation between broad cognitive ability measures (including WMC) and laboratory TUT rates is weak, at only ρ= −.14 [−.09 – −.19] (Randall et al., 2014). The field has reported far fewer tests of TUTs’ association with attention-control or restraint measures, but two latent variable studies indicate correlations in the range of .40 – .50 (McVay & Kane, 2012b; Unsworth & McMillan, 2014). If such findings are replicable, it would suggest that researchers interested in mind-wandering variation would be best served examining cognitive-ability influences with lower-level attention tasks rather than WMC tasks.
Response Time Variability as Another Marker of Executive Attention
People with good cognitive control should show stable performance within a task despite distractions. Indeed, the “worst performance rule” (Larson & Alderton, 1990) describes that people of higher and lower intelligence don’t differ much in their best performance on tasks (e.g., in their shortest RTs in attention tasks) but they differ greatly in their worst performance (e.g., in their longest RTs; for a review, see Coyle, 2003). Lower WMC subjects similarly produce more very-slow responses than do higher WMC subjects, so their RT distributions are more positively skewed and yield a larger τ parameter in formal ex-Gaussian models (e.g., Schmiedek, Oberauer, Wilhelm, Süß, & Wittmann, 2007; Unsworth, Redick, Lakey, & Young, 2010; Unsworth, Redick, Spillers, & Brewer, 2012). TUT rates during challenging tasks, another marker of executive control, also predict RT variability (Bastian & Sakur, 2013; Seli, Cheyne, & Smilek, 2013) and partially mediate WMC’s association with RT variability (McVay & Kane, 2009; 2012b).
Unsworth (2015) re-analyzed three studies to explore RT variability’s association to other executive-attention indices. All three assessed coefficient of variation (CoV; i.e., SD / M) in RT in multiple attention tasks (e.g., Stroop, flanker); two also measured CoV from lexical-decision tasks. CoV from attention and lexical-decision tasks correlated modestly, and models separating these constructs fit better. Moreover, CoV from only the attention tasks correlated with WMC, TUT rates, and other measures. CoV in attention-control tasks may thus be a novel, useful indicator of executive capabilities. We did not design the present study to explore CoV’s nomological net, but we addressed a question about CoV assessment. Unsworth calculated CoV from tasks that either required executive control on all trials (antisaccade, psychomotor vigilance) or included both control-demanding and non-demanding trials (Stroop, flanker); for the latter, CoV was calculated across both trial types. CoV measures may thus have been confounded with the basic experimental effect — and executive ability — of interest. That is, someone who is very slow on Stroop incongruent trials versus congruent trials will not only show a larger Stroop effect, but also more variability across both trial types. We reasoned that, in tasks where subjects attempt to bring attention control to bear, good control should be evident not only on trials eliciting conflict, but also on non-conflict trials (cf., McVay & Kane, 2009, 2012). We therefore took a more conservative approach to the question of how RT variability relates to other executive-control constructs by measuring CoV from only non-conflict trials.
Goals and Hypotheses
Schizophrenia is convincingly linked to executive-control deficits, but psychometrically assessed schizotypy is not. If some schizotypy dimensions have cognitive correlates, the field must more rigorously assess both schizotypy and executive control to confirm this. In addition to measuring mind wandering, which has barely been considered in light of schizophrenia’s positive symptoms (D. Shin et al., 2015), the present study measures multiple factors of executive control and schizotypy, with multiple indicators each, and uses latent-variable analyses to assess their associations in a large sample. Our theoretical questions concern the associations among executive constructs — WMC, attention restraint, attention constraint, mind wandering, and intra-individual variability — and their associations to dimensions of schizotypy.
We predicted that attention restraint and constraint would be distinguishable but correlated, that CoV would reflect a distinct but correlated factor of executive control, and that our attention constructs would more strongly predict TUT rate than would WMC. Also, TUTs should predict schizotypy dimensions associated with cognitive and experiential excess — positive, disorganized, and paranoid — but not negative schizotypy, which is characterized by a paucity of inner experience. Although the schizophrenia literature suggests executive deficits, our review of the schizotypy literature left open whether WMC, attention restraint, constraint, or CoV should predict particular (or any) schizotypy dimensions.
METHOD
Across Method and Results sections, we report how we determined our sample size and all data exclusions, manipulations, and measures in the study (Simmons, Nelson, & Simonsohn, 2012).
Subjects
Our data-collection stopping rule was to test subjects for 4 – 5 complete semesters, until we had at least 400 subjects with 3 sessions of laboratory data and at least 200 of these subjects with usable data from a subsequent daily-life experience sampling study (not reported here). Subjects could sign up for that daily-life study after completing the second or third laboratory session.
We recruited some subjects from “mass screening” sessions each semester to allow oversampling of high schizotypy. Subjects completed short forms (Winterstein et al., 2011) of the WSS used in this study: Magical Ideation (Eckblad & Chapman, 1983), Perceptual Aberration (Chapman, Chapman, & Raulin, 1978), Physical Anhedonia (Chapman et al., 1976), and Revised Social Anhedonia (Eckblad, Chapman, Chapman, & Mishlove, 1982) Scales. The short forms yield two factors, positive and negative schizotypy, accounting for 75% of their variance (Gross, Silvia, Barrantes-Vidal, & Kwapil, 2012). Based on short-form scores, all mass screening subjects earned a positive and negative score, based on factor loadings from 6,137 prior students. Mass screening subjects were not required to complete the present study, but we sent email invitations to anyone scoring at least 1.5 SD above the positive or negative schizotypy dimension mean. Participation in the study was open to students regardless of whether they completed mass screening.
Five hundred forty-five undergraduates, aged 18–35, provided informed consent to begin the study between January, 2012 and April, 2014. All were students at the University of North Carolina at Greensboro, a comprehensive and Minority-Serving state university (M first-year student SAT scores = 1032 – 1041 for cohorts entering Fall 2011 – Fall 2013; 27% African American undergraduates in Fall 2015), who participated as partial fulfillment of an introductory course requirement. Of the 545 subjects who completed the first session, 492 completed two sessions, and 472 completed all three.
Apparatus and Materials
We programmed all measures in E-Prime 1.2 or 2.0. Dell (Windows XP) computers with QWERTY keyboards presented all stimuli on 17″ CRT monitors (a few individual-subject sessions used LCD monitors).
Measures
We provide more detailed descriptions of some of the measures below in supplemental materials.
Schizotypy Questionnaires
All subjects completed a battery of questionnaires (including three exploratory measures not analyzed here), regardless of whether they had completed short forms of some of these measures in mass screening sessions. Computer administration of the schizotypy questionnaires was split between the first two sessions, each with items from different scales mixed with one another.
Wisconsin Schizotypy Scales (WSS)
The WSS, including the Perceptual Aberration (PERCABER), Magical Ideation (MAGCIDEA), Physical Anhedonia (PHY-ANHD), and Revised Social Anhedonia (SOC-ANHD) Scales, were administered in the first session. The WSS contain 166 true-false items that were intermixed with a 13-item infrequency scale (Chapman & Chapman, 1983) to rule out invalid protocols (e.g., I find that I often walk with a limp, which is the result of a skydiving accident; I believe that most light bulbs are powered by electricity); the WSS administration also included one of the unanalyzed exploratory measures mentioned above. Subjects saw one item at a time on-screen, and responded by mouse-clicking either the “True” or “False” box below each item. The WSS scales have good internal consistency in college student samples, with coefficient alphas of .84 – .88 in 6,137 participants (Gross, Silvia, Barrantes-Vidal, & Kwapil, 2012). Confirmatory factor analytic studies support that positive and negative schizotypy factors underlie the WSS measures (e.g., Kwapil et al., 2008), with positive reflecting primarily Perceptual Aberration and Magical Ideation measures and negative reflecting primarily Physical Anhendonia and Social Anhedonia scales. Moreover, these two factors predict different patterns of symptoms and impairment in cross-sectional (e.g., Barrantes-Vidal et al., 2013) and longitudinal (e.g., Kwapil et al., 2013) studies. The remaining schizotypy questionnaires were administered in the second session, all intermixed and including six infrequency items (see Chapman & Chapman, 1983), two of the unanalyzed questionnaires, and, in order to reduce the overall level of deviance implied by the questions in the second session, 9 extraversion and 9 agreeableness items from the Hexaco Personality Inventory-Revised (Lee & Ashton, 2004).
Schizotypal Personality Questionnaire (SPQ) subscales and additional schizotypy measures
The odd speech (ODSPEECH) and odd behavior (ODBEHAVR) subscales assessed disorganization, the referential thinking (REFTHINK) subscale assessed positive schizotypy, and the suspiciousness (SUSPICIO) subscale assessed paranoia. The SPQ is widely used in college samples and the subscales have adequate reliability (Raine, 1991). Although administered, we did not analyze the “no close friends” or “constricted affect” subscales because they may better tap neuroticism than negative schizotypy (Gross, Mellin, Silvia, Barrantes-Vidal, & Kwapil, 2014). Participants completed the Paranoia Checklist (PARACHEK; Freeman et al., 2005), an 18-item scale measuring a range of clinical and non-clinical paranoia that correlates with other paranoia measures has good internal consistency (Horton et al., 2014). The 34-item Cognitive Slippage Scale (COGSLIPG; Miers & Raulin, 1987) taps disruptions in thought and speech, and thus disorganization. The scale is associated with other questionnaire measures of schizotypy and has good internal consistency in college samples (Gooding, Tallent, & Hegyi, 2001). Six items from the Cognitive Dysregulation subscale of the Dimensional Assessment of Personality Pathology - Basic Questionnaire (COGDYSRG; Livesley & Jackson, 2009) assessed thought disturbance characteristic of disorganization.
WMC Tasks
We measured WMC with six tasks that required maintaining target items in the face of additional processing. Of these, four automated “complex span” tasks (operation, reading, symmetry, and rotation span) required subjects to memorize short sequences of either verbal-numerical or visuo-spatial items (Redick et al. 2012; Unsworth, Heitz, Schrock, & Engle, 2005; Unsworth, Redick, Heitz, Broadway, & Engle, 2009). Each item appeared after an unrelated processing task that required a true-false decision under a response deadline, made by mouse-clicking a YES or NO box on-screen. At the end of each trial sequence of unpredictable length, subjects recalled the memory items in order by using the mouse to select them from the complete pool of 12–16 possible items. Subjects began each complex span with practice: (1) memorizing small sets (with no processing task); (2) the processing task alone, then; (3) both sub-tasks combined. Processing-only practice trials recorded decision RTs; during the real task, if any processing-task decision was not made within 2.5 standard deviations of the processing-only practice RT mean, the program skipped the subsequent memory stimulus and the trial was counted as a processing error.
Operation Span (OPERSPAN)
Subjects memorized sequences of 3–7 capital letters, each presented in alternation with a compound arithmetic equation to verify [e.g., (3 × 2) – 1 = 4; half were true], and randomly selected without replacement from a set of 12. At recall, all 12 letters appeared in a grid. Subjects selected each letter from the most recent memory set in its serial position by clicking on its corresponding check box. Each set length of 3–7 occurred three times in a random order generated for each subject. The dependent measure was the total number of letters recalled in correct serial position (of 75).
Reading Span (READSPAN)
Subjects memorized sequences of 2–6 four-letter words, each presented in alternation with a sentence to verify as either sensible or nonsensical (e.g., “During winter you can get a room at the beach for a very low rate”; half were sensible), and randomly selected without replacement from a set of 15. The recall phase was identical to operation span, but with 15 words presented in a grid. Each set length of 2–6 occurred three times in a random order generated for each subject. The dependent measure was the total number of words recalled in correct serial position (of 60).
Symmetry Span (SYMMSPAN)
Subjects memorized sequences of 2–5 red squares appearing within a 4 × 4 matrix. Each red square appeared in alternation with a black-and-white pattern made from an 8 × 8 grid to verify as either symmetrical or asymmetrical along its vertical axis (half were symmetrical), and randomly selected without replacement from the 16 possible squares in the matrix. For the recall phase, subjects saw an empty 4 × 4 matrix and mouse-clicked the red square locations in serial order. Each set length of 2–5 occurred three times in a random order generated for each subject. The dependent measure was the total number of red-square locations recalled in correct serial position (of 42).
Rotation Span (ROTASPAN)
Subjects memorized sequences of 2–5 large and small arrows, radiating from the center of the screen in one of 8 directions. Each arrow appeared alternation with a rotated capitalized letter (F, G, J, R) to verify as either normal or mirror-reversed (half were normal), and randomly selected without replacement from 16 possible size-orientation arrow combinations. For the recall phase, subjects saw a centered array of 8 small and 8 large arrows, and clicked on the arrowheads in serial order. Each set length of 2–5 occurred three times in a random order generated for each subject. The dependent measure was the total number of arrows recalled in correct serial position (of 42).
Running Span (RUNNSPAN)
This task (see Broadway & Engle, 2010) did not present a secondary processing task. Instead, each trial presented a sequence of to-be-memorized letters (drawn without replacement from a set of 12) and only the final 3 – 7 letters were to be recalled. Each trial began with a digit to indicate the set size, or the number of letters to remember from the end of the list. For each set size, the entire trial length was unpredictably 2, 1, or 0 items longer than set size (one trial of each length for each set size, for 15 trials). Set sizes were blocked, with block order randomized for each subject. At recall, all 12 letters appeared in a grid, along with the set size. Subjects selected each letter from the memory set in its serial position by clicking on its check box The dependent measure was the total number of letters recalled in their correct serial position (of 75).
Updating Counters (COUNTERS)
Subjects recalled the numerical values of boxes, some of which updated their original values (see Lewandowsky, Oberauer, Yang, & Ecker, 2010). Each trial presented 3–5 boxes horizontally, and consisted of 3 phases: learning, updating, recall. At learning, a digit (1–9) appeared in each box in random order. During updating, 2–6 box values were changed by presenting a digit with a plus or minus sign (e.g., +2; −5); each update ranged from −7 to +7. During updating, some boxes might change multiple times while others not at all. Subjects retained only the current value for each box, which always ranged from 1–9. At recall, each box outline turned red (in random order) to prompt the subject to enter its final value. Each set size of 3–5 boxes was crossed with number of updates (2–6) to generate 15 trials. The dependent measure was the proportion of 60 final box values recalled correctly.
Attention Restraint Tasks
Attention restraint tasks required subjects to override a prepotent response with a novel, goal-directed one. We used five tasks to represent this construct.
Antisaccade Letters (ANTI-LET)
Subjects identified a letter on one side of the screen that was cued by a flash on the opposite side (see Kane et al., 2001). Each of 90 trials first presented a central-fixation array of three asterisks for 200–1800 ms, followed by a flashing cue (“=”) 8.6 cm to the left or right of fixation, followed by a to-be-identified target letter (B, P, or R) in the opposite screen location from the cue (8.6 cm from fixation). The target letter was pattern-masked after 100 ms. Subjects responded via keys on the number keypad labeled B, P, and R. The dependent measure was proportion of errors on 90 test trials.
Antisaccade Arrows (ANTI-ARO)
Subjects identified an arrow on one side of the screen that was cued by a flash on the opposite side (see McVay & Kane, 2012b). Each of 72 trials first presented a central-fixation array for 250–2250 ms, followed by a flashing cue (“=”) 11.4 cm to the left or right of fixation, followed by a to-be-identified arrow (pointing up, down, left, or right) in the opposite screen location from the cue (11.4 cm from fixation). Subjects responded with the 2, 4, 8, and 6 keys on the number keypad for down, left, up, and right arrows, respectively. The dependent measure was the proportion of errors on 72 test trials. (During the first semester of data collection, we presented cues and targets for longer durations than in the final task; error scores were positively skewed and clustered near floor, and so we adjusted the task for all remaining subjects and retained task data from only these latter subjects).
Semantic Sustained Attention To Response Task (SEM-SART)
This go/no-go task required subjects to press the space bar for words from one category (animals; 89% of trials) while withholding response to another (vegetables; 11% of trials; see McVay & Kane, 2012b). Each of 675 trials presented a word for 300 ms, then a mask for 1500 ms. Trials were divided into five seamless blocks, each comprising 3 mini-blocks of 45 trials that presented 40 unique animal names and 5 unique vegetable names. The dependent measures were d′ (i.e., hit rate to animals minus false alarm rate to vegetables) and SD of RTs to “go” (animals) trials.
Number Stroop (N-STROOP)
Subjects reported the number of digits presented on each trial while ignoring the identity of the digits (see McVay & Kane, 2012b). Each trial presented a row of 2–4 digits and subjects pressed one of three labeled keys to indicate the number of digits on-screen. The 300 test trials were divided into two seamless blocks of 150 trials; 80% of trials were congruent and presented matching digits and counts (e.g., 4444) and 20% were incongruent and presented mismatching stimuli (e.g., 2222). Dependent measures were RTs and error rates for congruent and incongruent trials from the first test block only (the second block was used to independently assess mind wandering, as described below).
Spatial Stroop (S-STROOP)
Subjects reported the relative position of a word to an asterisk, with the word and asterisk both presented to the left or right, or above or below, fixation; subjects ignored both the identity of the word (“LEFT,” “RIGHT,” “ABOVE,” “BELOW”) and the absolute location of the word and asterisk on-screen (after Palef, 1978). Subjects responded to the relative position of the word via the numeric keypad arrow keys. Each of 120 trials presented stimuli until response. Forty trials presented words that were congruent with both absolute location and relative position (e.g., “LEFT” presented to the left of the asterisk and both presented to the left of fixation), 40 presented words that were congruent for absolute location but incongruent for relative position (e.g., “LEFT” presented right of the asterisk and both presented left of fixation), and 40 presented words that were incongruent for both absolute location and relative position (e.g., “LEFT” presented right of the asterisk and both presented right of fixation). Dependent measures were RTs and error rates for trials where both absolute location and relative position were congruent and where both were incongruent.
Attention Constraint Tasks
Constraint tasks required subjects to identify targets amid visual distractors. Sometimes distractors evoked stimulus-response (S-R) conflict by cuing an erroneous response (e.g., in a task with central H or S targets: SSHSS; HHSHH) and sometimes they evoked only stimulus-stimulus (S-S) conflict because they were not associated with an allowable response (e.g., BBHBB; Kornblum, Hasbroucq, & Osman, 1990). We used six flanker and cued-search tasks to represent this construct; four presented both S-R and S-S conditions, one presented S-R conflict only, and one presented S-S conflict only.
Arrow Flanker (ARROFLNK)
Subjects reported the direction that a centrally presented arrow (“<” vs. “>”) via key-press, with the arrow flanked horizontally by 4 distractors. Each trial presented a fixation cross just below the upcoming target, followed by the target-distractor array (and fixation symbol). In each of two blocks of 96 trials, 24 neutral trials presented the target arrow amid dots (“•”), 24 congruent trials presented arrays with target and distractor arrows all pointing in the same direction, 24 S-R incongruent trials presented the target pointing in the opposite direction as distractors, and 24 S-S incongruent trials presented the target amid upward pointing arrows. Dependent measures for S-R conflict were RTs for congruent and S-R incongruent trials, and for S-S conflict were RTs for neutral and S-S incongruent trials.
Letter Flanker (LETTFLNK)
Subjects reported the direction that a centrally presented letter “F” (normal vs. backward) via key-press, with that letter flanked horizontally by 6 distractors. Each of 144 trials presented a fixation cross presented in the location of the upcoming target, followed by seven underline symbols (“_”) that cued the locations of the stimuli in the upcoming target-distractor array. Twenty-four neutral trials presented the target F or backward-F amid dots, 48 congruent trials presented arrays with the target and distractor Fs all facing the same direction, 24 S-R incongruent trials presented the target facing the opposite direction as distractors, and 24 S-S incongruent trials presented the target amid right- and left-facing Es and tilted Ts at 90° and 270°. Dependent measures for S-R conflict were RTs for S-R incongruent trials and 24 of the congruent trials (selected randomly for each subject), and for S-S conflict were RTs for neutral and S-S incongruent trials. (During the first semester of data collection, target letter location was varied and cued on each trial, but the data indicated that subjects did not use the cues; we thus adjusted the task for all remaining subjects, as above, and retained only their task data).
Conditional Accuracy Flanker (ACCYFLNK)
Following Heitz and Engle (2007), subjects reported whether a centrally presented letter was an H or S via key-press, with the central letter flanked horizontally by 4 distractors; each of two blocks presented a response deadline — 600 ms for block 1 and 500 ms for block 2 — with instructions to respond as quickly as possible, before the deadline, by sacrificing accuracy if necessary. Every missed deadline was followed immediately by, “Deadline Missed. Faster!” on-screen for 1000 ms; the program checked the proportion of met deadlines every 15 trials and, if 10 or more were missed, subjects saw: “You are missing too many deadlines. You MUST respond faster, even if it means making errors.” Each trial presented a fixation dot presented just above the upcoming target stimulus, followed by a warning tone (subjects wore headphones) and then the stimulus array for 100 ms. Each block presented 64 trials: 32 congruent trials presenting arrays of all one letter (SSSSS, HHHHH), 16 S-R conflict trials presenting a target flanked by the opposite letter (SSHSS, HHSHH), and 16 S-S conflict trials presenting a target flanked by Bs (BBSBB, BBHBB). Dependent measures for S-R conflict were error rates for S-R incongruent trials and 32 congruent trials (randomly selected for each subject), and for S-S conflict were error rates for S-S incongruent trials and 32 congruent trials (randomly selected for each subject).
Masked Flanker (MASKFLNK)
Subjects reported the identity of a centrally positioned letter (D, F, G, H, J, K) by pressing its corresponding key; the target was flanked above, below, to the left, and right by four distractors, yielding a cross-shaped array (see Styles & Allport, 1986). On each of the 192 trials, the entire array appeared above or below fixation, cued by a dot in the location of the upcoming target. A variable-duration blank screen (1100–2300 ms) preceded the stimulus array that appeared for 50 or 70 ms (determined randomly) and then was pattern masked. Forty-eight neutral trials presented distractor colons (“:”), 36 congruent trials presented distractor letters that matched the target, 36 S-R conflict trials presented distractors from the target letter set, and 36 S-S conflict trials presented distractor letters that were not allowable targets. Dependent measures for S-R conflict were error rates for S-R incongruent trials and congruent trials, and for S-S conflict were error rates for S-S incongruent trials and neutral trials.
Cued Search (CUEDSRCH)
Subjects reported the direction that a target letter “F” (or backward-F) faced, via key-press; the letter appeared equally often in one of 8 locations along the inner 3 × 3 square within a 5 × 5 matrix, with different eligible locations cued on each trial (Poole & Kane, 2009). The possible target locations on each trial were pre-cued by a 2- or 4-headed arrow at fixation, indicating the allowable two or four target locations to search on each trial (50% of each). We instructed subjects to use these cues to maintain focus on the cued locations. Non-target locations were populated randomly by right- and left-facing Es and tilted Ts at 90° or 270°, plus one “lure” (an F or backward-F in a non-cued location along the internal 3 × 3 square or in the central location). The presence of the lure required subjects to focus on the cued locations only. Each of 160 trials began with a 2- or 4-location cue, then a fixation grid of dots appearing in each of the upcoming 5 × 5 locations for 1500 ms, and then the stimulus array. Because each trial presented a lure, the dependent measure — mean RT across all trials — reflected S-R conflict.
Circle Flanker (CIRCFLNK)
Subjects reported whether a target letter was an X or N, via key-press, with the target flanked by two different distractors (from the set H, K, M, V, Y, Z). Targets appeared in one of 8 equidistant locations in a circular arrangement, with distractors appearing one position clockwise and counterclockwise from the target; the other positions were occupied by colons. Each of 160 trials presented a fixation cross followed by an underline cue appearing just beneath the upcoming target location; the target-distractor array then appeared after a variable-length blank screen. Eighty neutral trials presented the target letter surrounded by colons and 80 S-S conflict trials presented the target flanked by two letter distractors. The dependent measures were RTs for S-S incongruent trials and neutral trials.1
Thought Probes
In five tasks (and one practice task), subjects reported their immediately preceding thoughts by responding to unpredictably appearing probes. Each probe asked “What are you thinking about?” and had subjects “Please press a number on the keyboard” that most closely matched their thought content in the instant before the probe (see McVay & Kane, 2009, 2012a, 2012b). The on-screen choices (italicized below) were re-explained for each probed task: 1. The task, on-task thoughts about the stimuli or response; 2. Task experience/performance, evaluative thoughts about one’s task performance; 3. Everyday things, thoughts about routine things that have happened or may happen; 4. Current state of being, thoughts about one’s current physical or emotional state, such as being sleepy, hungry, or cheerful; 5. Personal worries, thoughts about one’s concerns or worries; 6. Daydreams, fantastic thoughts disconnected from reality; 7. External environment, thoughts about something task-unrelated in the immediate environment; 8. Other, only those thoughts that do not fit the other categories. The TUT dependent measure for each task (aside from the unanalyzed Probe Practice task) was the proportion of probe responses 3–8.
Probe Practice
As the first (unanalyzed) task of the study, subjects practiced responding to probes. Ninety trials presented X’s in a warm (red, yellow, pink) or cool (blue, dark blue, purple) color for 3000 ms; subjects judged warm versus cold via key-press. Probes followed 12 (13.3%) trials
Semantic SART (SART-TUT)
Probes followed 45 no-go target trials (i.e., 7% of all SART trials).
Number Stroop (NUMS-TUT)
Two unanalyzed probes appeared in the first block of the task and 20 to-be-analyzed probes appeared in the second block (13% of block-2 trials), always after incongruent trials.
Arrow Flanker (ARRO-TUT)
Four probes appeared in the first trial block (4.2% of block-1 trials) and 16 appeared in the second block (16.6% of block-2 trials); we analyzed all 20 probe responses.
Letter Flanker (LETT-TUT)
Of the 12 probes presented during the task (following 8.3% of all trials), 4 appeared following congruent trials, 2 following neutral trials, 2 following S-R incongruent trials, 2 following S-S incongruent trials, and 2 following trials of the exploratory (unanalyzed) trial type.
2-Back (2BAC-TUT)
Fifteen probes (6.3% of trials) appeared during an independent, non-analyzed task (McVay, Meier, Touron, & Kane, 2013). Subjects decided whether each word matched the one presented two trials ago; 25% of trials were 2-back matching targets, and 21% were 1- and 3-back lures.
General Procedure
We tested subjects in groups of 1–4, each at their own workstation. Each of 3 sessions lasted approximately 120 min. Subjects scheduled all sessions within one academic semester; the M duration between sessions 1 and 2 was 18.4 days (SD = 15.8) and between sessions 2 and 3 was 17.4 days (SD = 14.1). Table 1 presents the session and order of task completion for all subjects. All subjects also completed a demographics questionnaire at the beginning of session 1 about age, gender, race, and ethnicity.
Table 1.
Task order across three laboratory sessions, fixed for all subjects.
| Session 1 | Session 2 | Session 3 |
|---|---|---|
|
|
|
Note. “(P)” = task including thought probes to assess mind wandering; “SART” = Sustained Attention to Response Task.
An experimenter read aloud all on-screen instructions and remained to answer questions and monitor subjects (and record problems). Experimenters initiated a task only when all subjects in a session finished the prior task, and subjects left the session only after the last subject completed the last task.
RESULTS
We calculated descriptive statistics after each of the first several semesters of data collection in order to check for floor and ceiling effects, and thus modified two problematic tasks after the first semester (see Methods). Inferential statistics were not conducted until completion of the entire project.
Data Analysis Exclusions
As noted above, 472 of the 545 consented subjects completed all 3 sessions. We analyzed data from all 541 subjects who completed the first session and did not have their data excluded casewise (see below). Missing observations were handled via full-information maximum likelihood (ML) estimation. Given certain assumptions, simulation studies show that the ML approach provides unbiased parameter estimates (but slightly higher standard errors) when observations are missing (Enders, 2010; McKnight, McKnight, Sidani, & Figueredo, 2007). The models were estimated with Mplus 7.0 (Muthén & Muthén, 2012), using maximum likelihood with robust standard errors.
Experimenter Notes
All data-exclusion decisions, based on session notes recorded by experimenters, were made jointly by the first three authors at the completion of the project, while blind to the subjects’ task or questionnaire data. Our conservative approach dropped subjects casewise (from all analyses) or taskwise (from one or more tasks) only with clear evidence and specification of a significant problem that likely compromised the subject’s data. We excluded all data from four subjects, two who fell asleep in multiple tasks across sessions, one with poor English comprehension who did not understand task instructions, and one with self-declared dyslexia and difficulty with all letter stimuli. We excluded all performance data — retaining questionnaire data — from one subject who fell asleep during many of the performance tasks but not the schizotypy questionnaires. We excluded subjects’ data from individual tasks, typically for falling asleep, not following instructions, or stopping a task due to illness. In all, 20 subjects had data excluded from one or more tasks. For these subjects, we deleted data from M = 1.4 tasks (Mdn = 1; range = 1 – 3).
Complex Span Task Processing Accuracy
Complex span tasks required subjects to engage an unrelated processing task between items; if subjects do not comply with this demand, their memory data may be contaminated by rehearsal strategies. As is conventional, we excluded subjects’ data from operation (N = 57), reading (N = 53), symmetry (N = 66), or rotation (N = 74) span for processing accuracy < 85% (e.g., Conway et al., 2005; Redick et al., 2012).
Outliers
The first two authors jointly based all taskwise data-exclusion decisions about outlying scores on the individual task conditions from which the analyzed dependent measures (i.e., difference scores) would be derived, while blind to subjects’ other task scores. Our conservative strategy was to base exclusions exclusively on neutral and congruent conditions, rather than the theoretically critical incongruent conditions, and to define outliers via boxplots, as any observations falling more than three times the interquartile range (IQR) away from the upper or lower hinges of the plot. For tasks using error rates as the dependent measure, we did not drop data based on outlying RTs; for tasks using RTs as the dependent measure, we dropped data based on outlying neutral or congruent RTs or below-chance accuracy in neutral or congruent trials. Altogether, we excluded data from 3 subjects in Semantic SART, 10 subjects in Number Stroop, 7 subjects in Spatial Stroop, 2 subjects in Arrow Flanker, 5 subjects in Letter Flanker, 10 subjects in Conditional Accuracy flanker, 13 subjects in Cued Search, and 12 subjects in Circle Flanker.
Infrequency Responses in Questionnaire Measures
We excluded the questionnaire data from 7 subjects due to elevated infrequency scores (total infrequency score of 5 or higher across both sessions’ schizotypy scales).
Other Missing Data
All other missing data were due to subjects not completing particular tasks or sessions, or to lost data due to computer or experimenter error (or, as noted above, due to revisions to the antisaccade arrows and letter flanker tasks following the first semester of data collection).
Difference-Score Calculations
For Stroop and flanker tasks, the dependent measures reflected the difference in RT or error rate between incongruent trials and congruent or neutral trials. We evaluated four possible indicators by examining their correlations with only the tasks designed for that same construct (i.e., Number and Spatial Stroop difference scores only with each other and the other restraint tasks; all the flanker measures of constraint only with each other). We thus determined which difference score provided the best indicators of the intended constructs without being influenced by how these difference scores might affect between-construct associations. All four difference-score assessments correlated strongly (almost all rs > .95), but a “residual” measure most consistently provided the best within-construct correlations. So, for all relevant tasks and analyses, we expressed difference scores for each subject as the residual of the incongruent trials regressed on their congruent or neutral trials. Our only exception was for Number Stroop, which did not correlate with the other restraint measures regardless of difference-score method; we instead used the mean incongruent RT from each subject, which did generally correlate with the other restraint measures (for similar results and resolution, see McVay & Kane, 2012b). For Spatial Stroop, we used the residual difference score for error rates because it correlated better with other restraint tasks than did RTs.
Final Sample Demographics
Sixty-six percent of our 541 analyzed subjects self-identified as female and 34% as male (5 missing cases), with a mean age of 19 years (sd = 2; 2 missing cases). Also by self-report, the racial composition of the sample was 49% White (European/Middle Eastern descent); 34% Black (African/Caribbean descent); 7% Multiracial; 4% Asian; <1% Native American/Alaskan Native; 0% Native Hawaiian/Pacific Islander; 4% Other (4 missing cases). Finally, self-reported ethnicity, asked separately, was 7% Latino/Hispanic (1 missing case).
Descriptive Statistics and Bivariate Correlations
Tables 2 and 3 provide descriptive statistics for the questionnaire and cognitive-task measures, respectively. All variables showed reasonably normal distributions; although three variables were leptokurtic, none were both skewed and leptokurtic enough to require transformation. Table 2 shows that endorsement rates on the schizotypy questionnaires were low, as expected given their implied deviance (e.g., Horton et al., 2014; Kwapil et al., 2008), but we still obtained substantial variability: Scores on each scale ranged from 0 to near maximum. Table 3 indicates mean TUT rates (proportions of thought reports indicated as off-task) of about .45–.60 across tasks, and minimum and maximum rates from .00–1.00.
Table 2.
Descriptive statistics for all questionnaire measures used in subsequent analyses.
| Measure | Mean [95% CI] | SD | Min | Max | Skew | Kurtosis | N |
|---|---|---|---|---|---|---|---|
| PERCABER | 6.394 [5.967, 6.821] | 5.015 | 0.000 | 31.000 | 1.597 | 3.607 | 533 |
| MAGCIDEA | 11.392 [10.920, 11.864] | 5.545 | 0.000 | 28.000 | 0.260 | −0.493 | 533 |
| REFTHINK | 3.340 [3.156, 3.525] | 2.051 | 0.000 | 7.000 | 0.106 | −1.014 | 479 |
| SOC-ANHD | 11.462 [10.915, 12.008] | 6.421 | 0.000 | 38.000 | 0.989 | 1.335 | 533 |
| PHY-ANHD | 4.966 [14.393, 15.540] | 6.740 | 1.000 | 38.000 | 0.643 | 0.442 | 533 |
| PARACHEK | 3.616 [3.299, 3.933] | 3.533 | 0.000 | 17.000 | 1.335 | 1.479 | 479 |
| SUSPICIO | 3.297 [3.091, 3.501] | 2.286 | 0.000 | 8.000 | 0.316 | −0.893 | 479 |
| COGSLIPG | 12.497 [11.867, 13.127] | 7.022 | 0.000 | 32.000 | 0.324 | −0.695 | 479 |
| COGDYSRG | 2.729 [2.580, 2.878] | 1.660 | 0.000 | 6.000 | 0.082 | −0.764 | 479 |
| ODSPEECH | 4.142 [3.928, 4.356] | 2.387 | 0.000 | 9.000 | 0.157 | −0.818 | 479 |
| ODBEHAVR | 2.977 [2.773, 3.181] | 2.271 | 0.000 | 7.000 | 0.206 | −1.211 | 479 |
Table 3.
Descriptive statistics for cognitive-performance and thought-probe measures.
| Measure | Mean [95% CI] | SD | Min | Max | Skew | Kurtosis | N |
|---|---|---|---|---|---|---|---|
| OPERSPAN | 50.667 [49.377, 51.958] | 14.313 | 0.000 | 75.000 | −0.743 | 0.293 | 475 |
| READSPAN | 33.820 [32.758, 34.882] | 11.114 | 3.000 | 59.000 | −0.225 | −0.451 | 423 |
| SYMMSPAN | 26.657 [25.962, 27.353] | 7.651 | 2.000 | 42.000 | −0.390 | −0.164 | 467 |
| ROTASPAN | 25.336 [24.543, 26.129] | 7.934 | 0.000 | 42.000 | −0.552 | 0.018 | 387 |
| RUNNSPAN | 35.444 [34.523, 36.365] | 10.074 | 8.000 | 64.000 | 0.231 | −0.103 | 462 |
| COUNTERS | 0.398 [0.384, 0.413] | 0.161 | 0.070 | 0.920 | 0.552 | 0.146 | 480 |
| ANTI-LET | 0.475 [0.462, 0.488] | 0.146 | 0.080 | 0.800 | −0.401 | −0.535 | 470 |
| ANTI-ARO | 0.363 [0.345, 0.381] | 0.185 | 0.010 | 0.790 | 0.399 | −0.695 | 405 |
| SEM-SART d′ | 1.644 [1.559, 1.728] | 0.987 | −0.170 | 4.540 | 0.398 | −0.508 | 526 |
| SEM-SART rtsd | 214.99 [207.15, 222.83] | 91.516 | 87.600 | 570.460 | 1.255 | 1.301 | 526 |
| N-STROOP | 666.71 [658.04, 675.38] | 95.737 | 422.05 | 1045.450 | 0.697 | 1.267 | 468 |
| S-STROOP | 0.001 [−0.008, 0.010] | 0.102 | −0.140 | 0.940 | 3.610 | 21.441 | 458 |
| ARROFLNK-SR | 0.000 [−5.920, 5.921] | 66.111 | −201.190 | 268.240 | 0.485 | 1.290 | 479 |
| ARROFLNK-SS | 0.000 [−3.355, 3.356] | 37.468 | −134.960 | 132.160 | 0.031 | 0.254 | 479 |
| LETTFLNK-SR | 0.000 [−7.707, 7.707] | 84.514 | −320.940 | 702.190 | 2.231 | 14.620 | 462 |
| LETTFLNK-SS | 0.000 [−7.202, 7.202] | 78.977 | −248.640 | 703.420 | 1.922 | 13.626 | 462 |
| ACCYFLNK-SR | −0.001 [−0.011, 0.010] | 0.114 | −0.280 | 0.520 | 0.612 | 0.812 | 481 |
| ACCYFLNK-SS | −0.000 [−0.008, 0.007] | 0.085 | −0.260 | 0.370 | 0.580 | 0.795 | 481 |
| MASKFLNK-SR | 0.000 [−0.012, 0.012] | 0.132 | −0.480 | 0.280 | −0.731 | 0.908 | 458 |
| MASKFLNK-SS | −0.000 [−0.012, 0.0123] | 0.129 | −0.320 | 0.330 | −0.043 | −0.455 | 458 |
| CUEDSRCH | 1184.75 [1161.92, 1207.57] | 247.90 | 442.08 | 2058.31 | 0.405 | 0.657 | 453 |
| CIRCFLNK | 0.050 [−4.003, 4.102] | 44.618 | −167.880 | 265.550 | 1.242 | 6.370 | 468 |
| SART-TUT | 0.510 [0.489, 0.531] | 0.244 | 0.000 | 1.000 | −0.042 | −0.803 | 526 |
| NUMS-TUT | 0.451 [0.423, 0.479] | 0.310 | 0.000 | 1.000 | 0.298 | −1.066 | 478 |
| ARRO-TUT | 0.484 [0.456, 0.512] | 0.297 | 0.000 | 1.000 | 0.120 | −1.009 | 424 |
| LETT-TUT | 0.585 [0.561, 0.609] | 0.263 | 0.000 | 1.000 | −0.489 | −0.523 | 460 |
| 2BAC-TUT | 0.424 [0.395, 0.452] | 0.313 | 0.000 | 1.000 | 0.293 | −1.116 | 461 |
Note. We express millisecond response time (RT) values for means and standard deviations to only two decimal places.
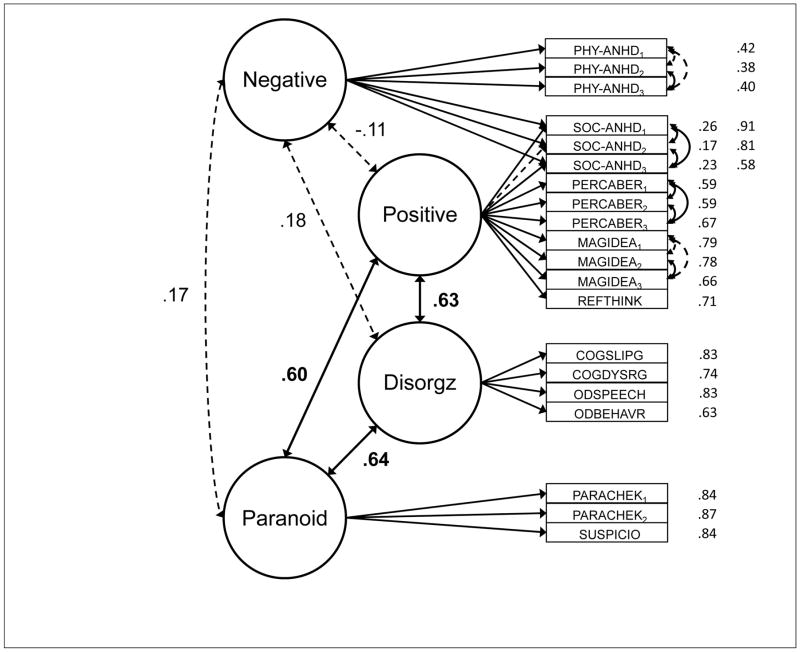
Before conducting multivariate analyses, we censored any scores that were ≥4 SDs from the mean and replaced them with a value of 3.999 SDs (affecting 0.2% of scores). Table 4 presents the correlation matrix (reliabilities along the diagonal) that provides evidence for convergent and discriminant validity. Indicators of a given construct correlated well with each other and more strongly than with indicators of other constructs. The schizotypy constructs were particularly well captured, with Perceptual Aberration, Magical Ideation, and Referential Thinking scores correlating as indices of positive schizotypy, with Social and Physical Anhedonia scores correlating as indices of negative schizotypy, with Paranoid Checklist and Suspiciousness scores correlating strongly as indices of paranoid schizotypy, and with Cognitive Slippage, Cognitive Dysfunction, Odd Speech, and Odd Behavior scores correlated strongly as indices of disorganized schizotypy. At the same time, and consistent with prior research (Cicero & Kerns, 2010; Horton et al., 2014; Stefanis et al., 2002), measures of positive, paranoid, and disorganized schizotypy correlated more strongly with each other (Mdn r = .44) than with indicators of negative schizotypy (Mdn r = .11).
Table 4.
Correlations among all measures used in subsequent analyses (reliabilities presented on diagonal in parentheses).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 OPERSPAN | (.81) | |||||||||||||||||||
| 2 READSPAN | .58 | (.76) | ||||||||||||||||||
| 3 SYMMSPAN | .40 | .38 | (.68) | |||||||||||||||||
| 4 ROTASPAN | .45 | .32 | .54 | (.76) | ||||||||||||||||
| 5 RUNNSPAN | .45 | .37 | .27 | .20 | (.54) | |||||||||||||||
| 6 COUNTERS | .36 | .23 | .37 | .29 | .39 | (.85) | ||||||||||||||
| 7 ANTI-LET | −.21 | −.18 | −.34 | −.21 | −.25 | −.35 | (.89) | |||||||||||||
| 8 ANTI-ARO | −.25 | −.19 | −.30 | −.36 | −.27 | −.33 | .59 | (.92) | ||||||||||||
| 9 SEM-SART d′ | .15 | .20 | .19 | .14 | .21 | .17 | −.36 | −.27 | (.96) | |||||||||||
| 10SEM-SART rtsd | −.15 | −.19 | −.21 | −.11 | −.23 | −.21 | .36 | .28 | −.63 | (.98) | ||||||||||
| 11 N-STROOP | −.17 | −.03 | −.19 | −.18 | −.10 | −.21 | .22 | .26 | −.12 | .21 | (.95) | |||||||||
| 12 S-STROOP | −.04 | −.05 | −.08 | −.18 | −.09 | −.07 | .19 | .21 | −.17 | .16 | .08 | (.80) | ||||||||
| 13 ARROFLNK-SR | −.13 | −.01 | −.07 | −.11 | −.03 | −.09 | .05 | .00 | .05 | −.07 | .12 | −.06 | (.51) | |||||||
| 14 ARROFLNK-SS | −.06 | −.05 | −.11 | −.09 | −.08 | −.09 | .10 | .10 | .05 | −.03 | .10 | .09 | .39 | (.48) | ||||||
| 15 LETTFLNK-SR | −.06 | −.07 | −.01 | −.10 | −.09 | −.14 | .15 | .19 | −.06 | .07 | .02 | .06 | .17 | .16 | (.59) | |||||
| 16 LETTFLNK-SS | −.07 | −.05 | −.02 | −.02 | −.14 | −.14 | .12 | .15 | −.05 | .08 | .04 | .03 | .17 | .15 | .39 | (.56) | ||||
| 17 ACCYFLNK-SR | −.09 | −.07 | −.09 | −.06 | −.13 | −.08 | .26 | .19 | −.22 | .17 | .06 | .02 | .08 | .14 | .20 | .12 | (.47) | |||
| 18 ACCYFLNK-SS | −.02 | −.00 | −.10 | −.09 | −.03 | −.07 | .23 | .15 | −.15 | .07 | .08 | .14 | .01 | .07 | .12 | .10 | .26 | (.34) | ||
| 19 MASKFLNK-SR | −.04 | −.07 | −.17 | −.09 | −.15 | −.09 | .19 | .24 | −.12 | .10 | .08 | .14 | .12 | .14 | .04 | .10 | .21 | .14 | (.69) | |
| 20 MASKFLNK-SS | −.10 | −.05 | −.15 | −.12 | −.09 | −.15 | .10 | .19 | −.07 | .02 | .08 | .05 | .11 | .09 | .10 | .15 | .17 | .15 | .54 | (.59) |
| 21 CUEDSRCH | −.21 | −.10 | −.27 | −.16 | −.18 | −.26 | .30 | .28 | −.15 | .19 | .44 | .03 | .20 | .11 | .06 | .12 | .11 | .01 | .28 | .23 |
| 22 CIRCFLNK | −.00 | .02 | −.13 | .02 | −.15 | −.10 | .12 | .09 | −.06 | .04 | .03 | .04 | .03 | .08 | .15 | .12 | .12 | −.09 | .06 | .06 |
| 23 SART-TUT | −.01 | −.14 | −.08 | .00 | −.05 | −.06 | .13 | .04 | −.26 | .30 | .10 | .09 | .07 | .06 | .08 | .10 | .04 | −.02 | .12 | .06 |
| 24 NUMS-TUT | −.03 | −.11 | −.03 | −.03 | −.12 | −.02 | .13 | .12 | −.21 | .19 | .15 | .06 | .06 | .04 | .04 | .04 | .08 | .04 | .12 | .08 |
| 25 ARRO-TUT | .01 | −.09 | −.01 | .01 | −.11 | −.04 | .19 | .11 | −.20 | .13 | .15 | .03 | .03 | .05 | .06 | .04 | .09 | .04 | .15 | .09 |
| 26 LETT-TUT | .09 | .00 | −.09 | −.01 | .04 | −.03 | .11 | .08 | −.19 | .15 | .09 | .16 | .02 | .08 | −.04 | −.02 | .06 | .14 | .16 | .05 |
| 27 2BAC-TUT | −.05 | −.09 | −.06 | −.14 | −.20 | −.13 | .19 | .21 | −.29 | .27 | .13 | .26 | .04 | .07 | .03 | .04 | .07 | .08 | .22 | .10 |
| 28 PERCABER | −.00 | −.00 | .07 | .07 | −.12 | −.05 | .05 | .02 | −.10 | .08 | .03 | .04 | −.10 | .01 | −.04 | .02 | .06 | .07 | .02 | .03 |
| 29 MAGCIDEA | .01 | −.04 | .10 | .08 | −.09 | −.06 | .05 | −.03 | −.14 | .06 | .03 | .07 | −.15 | −.09 | −.06 | −.05 | −.01 | .00 | −.05 | −.07 |
| 30 REFTHINK | −.05 | −.09 | −.01 | .04 | −.13 | −.12 | .09 | .06 | −.15 | .13 | .10 | .03 | −.12 | −.00 | −.03 | −.04 | .02 | −.02 | −.01 | −.02 |
| 31 SOC-ANHD | −.07 | .01 | .00 | .01 | −.08 | −.00 | .09 | .05 | −.06 | .08 | −.04 | −.02 | −.05 | −.04 | .07 | .00 | −.02 | .06 | −.07 | −.01 |
| 32 PHY-ANHD | −.10 | −.08 | −.13 | −.01 | −.18 | −.08 | .08 | .12 | −.12 | .12 | −.06 | .01 | −.06 | −.03 | .11 | .06 | .04 | .10 | .05 | .05 |
| 33 PARACHEK | −.03 | −.01 | −.02 | .04 | −.18 | −.12 | .20 | .13 | −.17 | .17 | .09 | .09 | −.10 | −.01 | −.01 | −.02 | .07 | .06 | .04 | .01 |
| 34 SUSPICIO | −.03 | −.00 | .01 | .02 | −.14 | −.07 | .16 | .02 | −.13 | .13 | .05 | .06 | −.10 | −.01 | −.02 | −.05 | .05 | .01 | −.02 | −.08 |
| 35 COGSLIPG | −.07 | −.06 | .01 | .06 | −.17 | −.08 | .14 | .05 | −.13 | .09 | .06 | .08 | −.11 | −.03 | −.01 | .02 | .05 | −.01 | .05 | .04 |
| 36 COGDYSRG | −.08 | −.10 | −.02 | .02 | −.16 | −.13 | .14 | .02 | −.14 | .09 | .06 | .06 | −.04 | .01 | −.02 | −.06 | .02 | .05 | .01 | .03 |
| 37 ODSPEECH | −.03 | −.03 | .01 | .01 | −.10 | −.03 | .05 | −.04 | −.06 | .00 | .01 | .06 | −.04 | .01 | .02 | −.03 | .04 | −.00 | .04 | .00 |
| 38 ODBEHAVR | .01 | .01 | .01 | −.05 | −.07 | −.03 | .09 | .01 | −.03 | .00 | .00 | .07 | −.09 | −.05 | −.02 | −.04 | .05 | .06 | −.02 | −.03 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 CUEDSRCH | (.97) | |||||||||||||||||
| 22 CIRCFLNK | .09 | (.25) | ||||||||||||||||
| 23 SART-TUT | .11 | −.00 | (.93) | |||||||||||||||
| 24 NUMS-TUT | .10 | .09 | .45 | (.90) | ||||||||||||||
| 25 ARRO-TUT | .10 | .03 | .41 | .68 | (.91) | |||||||||||||
| 26 LETT-TUT | .12 | −.09 | .52 | .32 | .37 | (.78) | ||||||||||||
| 27 2BAC-TUT | .12 | .04 | .39 | .43 | .39 | .33 | (.90) | |||||||||||
| 28 PERCABER | .07 | .14 | .05 | .12 | .16 | .13 | .12 | (.85) | ||||||||||
| 29 MAGCIDEA | .02 | .08 | .05 | .10 | .16 | .11 | .10 | .64 | (.81) | |||||||||
| 30 REFTHINK | .09 | .01 | .12 | .15 | .20 | .09 | .09 | .41 | .61 | (.70) | ||||||||
| 31 SOC-ANHD | −.01 | .08 | .03 | −.00 | .02 | .02 | .02 | .20 | .12 | .04 | (.85) | |||||||
| 32 PHY-ANHD | .04 | .07 | .02 | .04 | −.00 | −.02 | .07 | .08 | −.08 | −.06 | .40 | (.81) | ||||||
| 33 PARACHEK | .03 | .14 | .10 | .14 | .17 | .09 | .10 | .37 | .42 | .50 | .25 | .11 | (.85) | |||||
| 34 SUSPICIO | .04 | .08 | .08 | .14 | .16 | .01 | .05 | .31 | .39 | .48 | .30 | .11 | .76 | (.77) | ||||
| 35 COGSLIPG | .12 | .05 | .15 | .12 | .18 | .16 | .08 | .42 | .44 | .39 | .28 | .08 | .45 | .46 | (.88) | |||
| 36 COGDYSRG | .14 | .11 | .10 | .11 | .17 | .09 | .05 | .34 | .39 | .40 | .21 | .09 | .46 | .48 | .60 | (.64) | ||
| 37 ODSPEECH | .11 | .07 | .09 | .08 | .11 | .09 | .06 | .34 | .35 | .38 | .23 | .02 | .44 | .41 | .70 | .61 | (.74) | |
| 38 ODBEHAVR | .03 | .10 | .03 | .07 | .14 | .11 | .06 | .32 | .32 | .27 | .27 | −.01 | .41 | .36 | .47 | .43 | .56 | (.82) |
The cognitive tasks were more variable in capturing their intended constructs. Working memory tasks correlated well with one another, with somewhat stronger correlations for tasks of the same content domain (verbal versus spatial), but substantial correlations across domains (see Kane et al., 2004). TUT rate correlations similarly indicated a stable, trait-like construct, but they also suggested state-like influences, with strongest correlations within the same experimental session (Mdn r = .60) versus across different sessions (Mdn r = .39). For attention restraint tasks, whereas antisaccade tasks and the SART measures correlated well, the Stroop measures correlated more weakly with them and with each other. Attention constraint tasks performed more poorly, overall. Although the S-R and S-S interference measures taken from the same task correlated well, the S-R measures and the S-S measures correlated weakly across tasks.
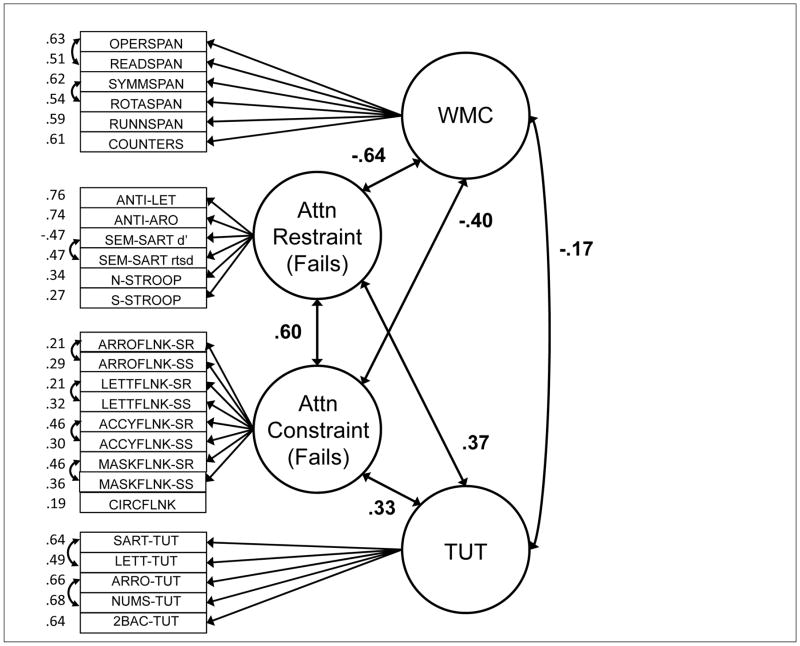
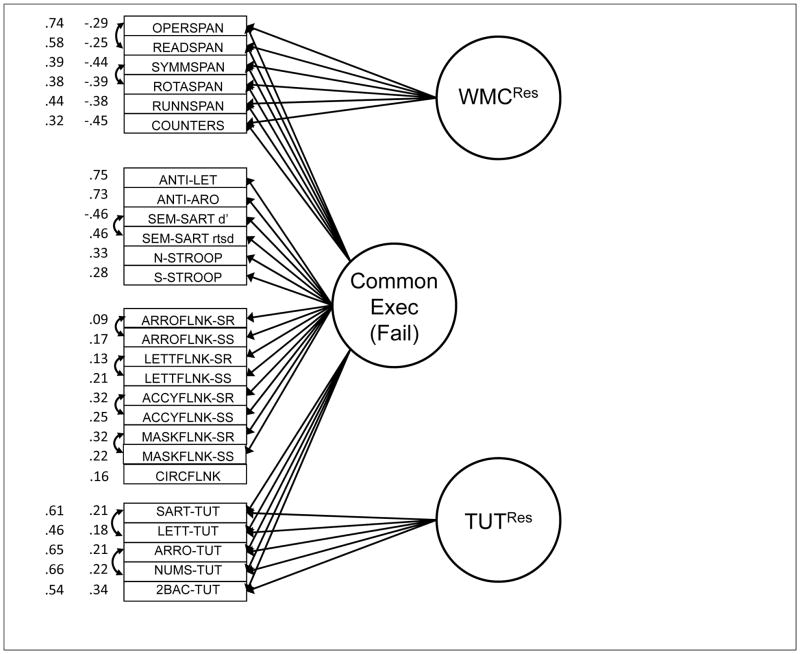
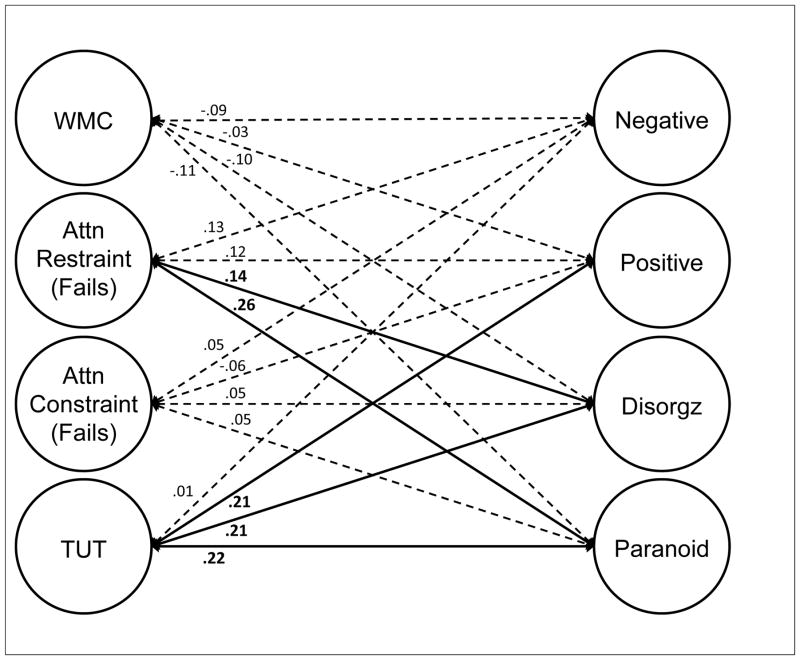
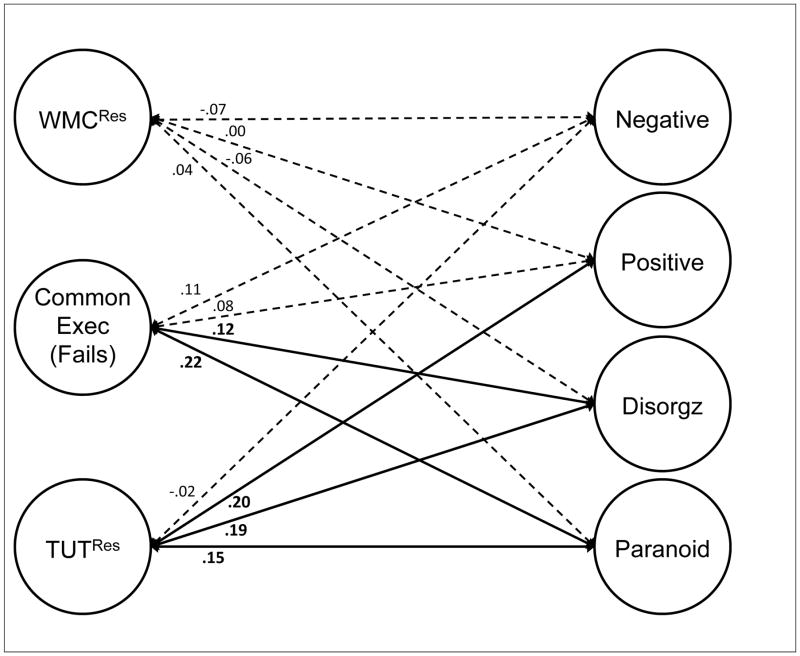
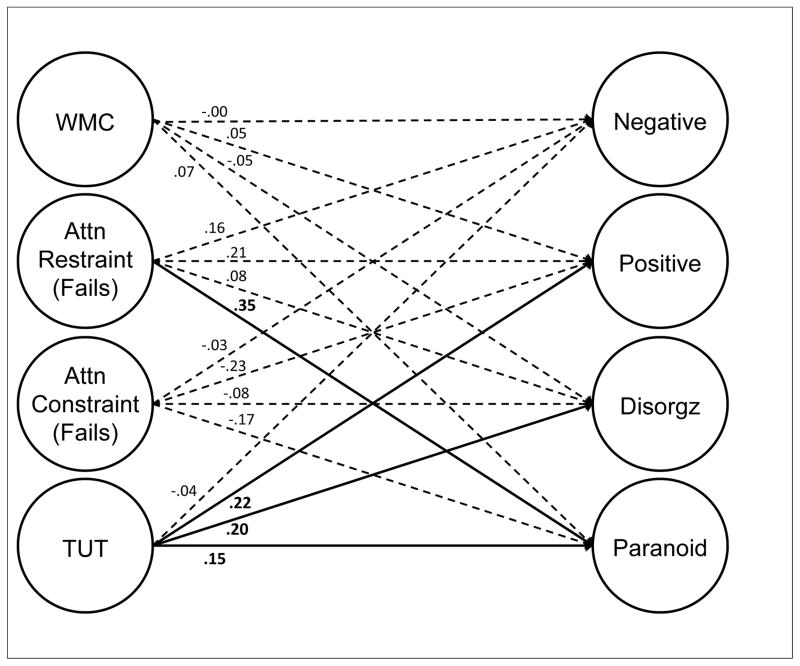
Although latent-variable models will be critical to assessing the associations between constructs, the correlation matrix suggests that between-construct correlations varied substantially across domains. WMC tasks correlated more strongly with attention restraint than with constraint tasks and generally weakly with TUTs. Restraint tasks correlated reasonably well with both WMC and attention constraint tasks, as well as with TUT rates. Constraint tasks correlated more weakly with TUT rates than did restraint tasks. Finally, our cognitive measures did not strongly predict schizotypy measures, with most correlations weaker than r = .10; however, the cognition-schizotypy correlations that were stronger than .10 tended to cluster between attention restraint and both paranoid and disorganized schizotypy measures, and between TUTs and positive, paranoid, and disorganized schizotypy measures.
Latent Variable Models
Latent-variable models allowed us to evaluate associations at the construct level, relatively free of method- and task-specific variance. We designed our questionnaire (criterion) measures to tap four constructs: negative, positive, paranoid, and disorganized schizotypy. We intended our cognitive (predictor) tasks to measure WMC, TUT rate, attention restraint, and attention constraint. Here, we first test measurement models (via confirmatory factor analysis) separately for our criterion and predictor constructs. Note that higher scores on WMC factors indicate better performance, whereas higher scores for TUT rate, attention restraint, and attention constraint factors indicate worse performance.
For all model testing (using Mplus 7.0), we report several fit statistics. Non-significant chi-square tests indicate adequate model fit; with large samples like ours, however, they are nearly always significant and so we also report χ2/df, for which values ≤2 indicate adequate fit. Comparative fit indices (CFI) and Tucker-Lewis indices (TLI) of ≥.90 indicate adequate fit, whereas the Root Mean Square Error of Approximation (RMSEA; with its 90% CI) and Standardized Root Mean Square Residual (SRMR) values of ≤.08 indicate adequate fit (e.g., Schermelleh-Engel, Moosbrugger, & Müller, 2003).
Measurement Model: Questionnaire Measures
We tested a 4-factor schizotypy model, consistent with prior research using these and related self-report instruments (e.g., Cicero & Kerns, 2010; Horton et al., 2014; Stefanis et al., 2002). The model included a positive schizotypy factor loaded by perceptual aberration, magical ideation, and referential ideas scales (also with cross-loadings from social anhedonia; Kwapil et al., 2008; E. Smith et al. 2016), a paranoid factor loaded by paranoia and suspiciousness measures, a disorganized factor loaded by cognitive slippage-dysfunction and odd behavior-speech questionnaires, and a negative factor loaded by physical and social anhedonia measures. Following published recommendations (Coffman & MacCallum, 2005; Little, Cunningham, Shahar, & Widaman, 2002) and consistent with our previous work (e.g., Kwapil et al., 2008), we divided each of the four WSS scales into three parcels, and the Paranoia Checklist into two parcels, in order to produce more robust estimates. We did not compute parcels for the other scales because they had fewer items. To create parcels, we distributed groups of items to the parcels in sequential order to ensure that each parcel contained a comparable proportion of items from the beginning, middle, and end of each scale. We allowed residual correlations among parcels from the same measure.
Table 5 indicates that the measurement model, depicted in Figure 1, provided a good fit to the data, with only one non-significant factor loading (one Social Anhedonia parcel on positive schizotypy). As predicted, positive schizotypy correlated strongly with the paranoid and disorganized factors, which also correlated strongly with each other. Also as expected, negative schizotypy correlated non-significantly with the positive, paranoid, and disorganized factors.2
Table 5.
Fit statistics for latent variable models.
| χ2 (df) | χ2/df | CFI | TLI | RMSEA [90% CI] | SRMR | |
|---|---|---|---|---|---|---|
| Questionnaire Measurement Model | ||||||
| 4-factors | 304.16 (149) | 2.04 | .969 | .961 | .044 [.037, .051] | .045 |
| Cognitive Task Measurement Models | ||||||
| 4-factors | 509.46 (284) | 1.79 | .914 | .901 | .038 [.033, .044] | .052 |
| Bifactor | 508.78 (279) | 1.82 | .912 | .897 | .039 [.034, .045] | .053 |
| Confirmatory Factor Analysis Models | ||||||
| 4-factors for cognitive tasks | 1406.00 (937) | 1.50 | .941 | .935 | .030 [.027, .034] | .053 |
| Bifactor for cognitive tasks | 1678.45 (940) | 1.79 | .907 | .898 | .038 [.035, .041] | .057 |
| CoV model | 1490.21 (932) | 1.60 | .929 | .921 | .033 [.030, .036] | .053 |
Figure 1.
Four-factor measurement model of the schizotypy questionnaires. All solid paths are statistically significant at p < .05; all dotted paths are non-significant. The circles represent the latent variables for negative schizotypy (Negative), positive schizotypy (Positive), disorganized schizotypy (Disorgz), and paranoid schizotypy (Paranoid). The boxes represent the observed variables loaded onto each latent factor. The arrows represent the modeled direction of the pathway between variables (double-headed arrows indicate correlation). Numbers next to boxes indicate task factor loadings (leftmost column indicates loadings onto positive, disorganized, or paranoid factors; rightmost column indicates loadings onto the negative factor), numbers along double-headed arrows indicate correlations between constructs. For the observed variables, PHY-ANHD1 = physical anhedonia scale (item parcel 1), PHY-ANHD2 = physical anhedonia scale (parcel 2), PHY-ANHD3 = physical anhedonia scale (parcel 3), SOC-ANHD1 = social anhedonia scale (parcel 1), SOC-ANHD2 = social anhedonia scale (parcel 2), SOC-ANHD3 = social anhedonia scale (parcel 3), PERCEABER1 = perceptual aberration scale (parcel 1), PERCEABER2 = perceptual aberration scale (parcel 2), PERCEABER3 = perceptual aberration scale (parcel 3), MAGIDEA1 = magical ideation scale (parcel 1), MAGIDEA2 = magical ideation scale (parcel 2), MAGIDEA3 = magical ideation scale (parcel 3), REFTHINK = referential thinking subscale from the Schizotypal Personality Questionnaire (SPQ), COGSLIPG = cognitive slippage scale, COGDYSRG = cognitive dysregulation subscale of the Dimensional Assessment of Personality Pathology – Basic Questionnaire, ODSPEECH = SPQ odd speech subscale, ODBEHAVR = SPQ odd behavior subscale, PARACHEK1 = paranoia checklist (item parcel 1), PARACHEK2 = paranoia checklist (parcel 2), SUSPICIO = SPQ suspiciousness subscale.
Measurement Models: Cognitive Measures
For models of the cognitive tasks, we allowed residual correlations among a limited number of manifest variables, a priori: operation and reading span to account for shared method variance as complex span tasks with verbal memoranda, symmetry and rotation span to account for shared method variance as complex span tasks with visuo-spatial memoranda, and the SART d′ and SART RT standard deviation measures. We also made three post hoc modeling decisions after considering the bivariate correlations in Table 3. First, because TUT measures from the same session correlated more strongly than they did across sessions, indicating both state- and trait-based variation, we allowed residual correlations for the within-session pairs. Second, the S-R and S-S effects within each flanker task were much more strongly correlated than we anticipated, so we let them correlate.3 Finally, we dropped Cued Search from all analyses because it seemed to correlate more strongly with the attention restraint than constraint tasks, so it was neither a good constraint measure (as we designed it to be) nor an a priori restraint measure.
We tested two kinds of models with our cognitive tasks: (1) a 4-factor model with separate but correlated constructs reflecting WMC, TUT rate, attention restraint, and attention constraint; (2) a nested “bifactor” model with a common “executive” factor reflecting the variance common to all the cognitive measures and two residual “WMC-r” and “TUT-r” factors reflecting the variance shared among the indicators of these constructs that was not shared with the other measures.
4-Factor Model
The 4-factor model presented in Figure 2 provided an adequate fit to the data (see Table 5). Although some attention-task loadings were weak, all were statistically significant, and the model suggested four correlated latent variables. Propensity for mind wandering during ongoing tasks was a stable trait across multiple tasks and occasions (see also McVay & Kane, 2012; Mrazek et al., 2012; Unsworth & McMillan, 2014). Inconsistent with Friedman and Miyake (2004), however, we could not fix the restraint-constraint correlation to equal 1.0 and still fit the data. That model would not converge here, and so along with the fact that the 95% confidence interval around the correlation [.46, .74] did not include 1.0, our findings indicate that restraint and constraint capabilities are distinguishable constructs.
Figure 2.
4-factor measurement model of the cognitive variables. All paths are statistically significant at p < .05. The circles represent the latent variables for working memory capacity (WMC), attention restraint failure [Attn Restraint (Fails)], attention constraint failure [Attn Constraint (Fails)], and mind wandering rate (TUTs). The boxes represent the observed variables loaded onto each latent factor. The arrows represent the modeled direction of the pathway between variables (double-headed arrows indicate correlation). Numbers next to boxes indicate task factor loadings, numbers along double-headed arrows indicate correlations between constructs. For the observed variables, OPERSPAN = operation span, READSPAN = reading span, SYMMSPAN = symmetry span, ROTASPAN = rotation span, RUNNSPAN = running span, COUNTERS = updating counters, ANTI-LET = antisaccade with letters, ANTI-ARO = antisaccade with arrows, SEM-SART d′ = d′ score from semantic SART, SEM-SART rtsd = intrasubject standard deviation in RT from semantic SART, N-Stroop = number Stroop, S-Stroop = spatial Stroop, ARROFLNK-SR = Stimulus-response (S-R) conflict effect in arrow flanker, ARROFLNK-SO = Stimulus-stimulus (S-S) conflict effect in arrow flanker, LETTFLNK-SR = S-R conflict effect in letter flanker, LETTFLNK-SO = S-S conflict effect in letter flanker, ACCYFLNK-SR = S-R conflict effect in conditional accuracy flanker, ACCYFLNK-SO = S-S conflict effect in conditional accuracy flanker, MASKFLNK-SR = S-R conflict effect in masked flanker, MASKFLNK-SS = S-S conflict effect in masked flanker, CIRCFLNK = circle flanker, SART-TUT = proportion of TUTs reported in the semantic SART, LETT-TUT = proportion of TUTs reported in letter flanker, ARRO-TUT = proportion of TUTS reported in arrow flanker, NUMS-TUT = proportion of TUTs reported in number Stroop, 2BAC-TUT = proportion of TUTs reported in two-back task.
WMC was strongly associated with attention restraint (higher WMC scores predicted less restraint failure) and less strongly, but substantially, with constraint (higher WMC scores predicted less constraint failure). Among the attention-related constructs, TUT rate was least strongly associated with WMC, with a similarly modest correlation to other latent-variable studies from our laboratory (≈ −.20; McVay & Kane, 2012a, 2012b). TUT rate was more strongly correlated with both restraint failures and constraint failures.
Bifactor Model
Using the same variables as in the 4-factor model above, the bifactor model presented in Figure 3 provided an adequate fit (see Table 5). All tasks loaded significantly onto the general “Executive Attention” factor, indicating common variance across these diverse measures, although many tasks’ loadings (particularly for TUT rates and flanker tasks) were less than .30. The residual WMC and TUT factors both had substantial task loadings, indicating ample WMC-specific and TUT-specific variance to account for beyond that shared with the other measures in the battery.
Figure 3.
Bifactor measurement model of the cognitive variables. All paths are statistically significant at p < .05. The circles represent the latent variables for Common Executive Failures [Common Exec (Fail)], the “residual” variance shared only among the WMC measures (WMCRes), and the “residual” variance shared only among the mind wandering measures (TUTRes). The boxes represent the observed variables loaded onto each latent factor. The arrows represent the modeled direction of the pathway between variables (double-headed arrows indicate correlation). The rightmost column of numbers next to boxes indicates factor loadings onto the Common Executive factor and the leftmost column of numbers next to the boxes indicates factor loadings on the WMC-specific or TUT-specific factors. For the observed variables, OPERSPAN = operation span, READSPAN = reading span, SYMMSPAN = symmetry span, ROTASPAN = rotation span, RUNNSPAN = running span, COUNTERS = updating counters, ANTI-LET = antisaccade with letters, ANTI-ARO = antisaccade with arrows, SEM-SART d′ = d′ score from semantic SART, SEM-SART rtsd = intrasubject standard deviation in RT from semantic SART, N-Stroop = number Stroop, S-Stroop = spatial Stroop, ARROFLNK-SR = Stimulus-response (S-R) conflict effect in arrow flanker, ARROFLNK-SO = Stimulus-stimulus (S-S) conflict effect in arrow flanker, LETTFLNK-SR = S-R conflict effect in letter flanker, LETTFLNK-SO = S-S conflict effect in letter flanker, ACCYFLNK-SR = S-R conflict effect in conditional accuracy flanker, ACCYFLNK-SO = S-S conflict effect in conditional accuracy flanker, MASKFLNK-SR = S-R conflict effect in masked flanker, MASKFLNK-SS = S-S conflict effect in masked flanker, CIRCFLNK = circle flanker, SART-TUT = proportion of TUTs reported in the semantic SART, LETT-TUT = proportion of TUTs reported in letter flanker, ARRO-TUT = proportion of TUTS reported in arrow flanker, NUMS-TUT = proportion of TUTs reported in number Stroop, 2BAC-TUT = proportion of TUTs reported in two-back task.
Confirmatory Factor Analyses of the Cognitive and Questionnaire Measures
The following confirmatory factor analyses (CFAs) assessed the correlations between the cognitive predictor constructs and the schizotypy outcome constructs. Across all models, the outcomes reflected the four schizotypy factors from the measurement model (positive, paranoid, disorganized, negative).
Four-Factor Predictor Model
As expected from the individual measurement models, the full model with all predictor and criterion constructs provided an adequate fit to the data (see Table 5 for fit statistics; see Table 6 for all factor loadings). Figure 4 shows that none of the cognitive constructs correlated significantly with negative schizotypy. Positive, disorganized, and paranoid schizotypy factors, in contrast, shared significant variance with both TUT rate and attention restraint failure (with the exception of positive schizotypy × restraint; p = .052). Neither WMC, nor attention constraint failure, predicted individual differences in any of the schizotypy constructs.
Table 6.
Standardized factor loadings (with standard errors) for confirmatory factor analyses and structural equation models.
| Construct/Measure | Figure 4 Model | Figure 5 Model | CoV Model |
|---|---|---|---|
| WMC/WMCres | |||
| OPERSPAN | .63 (.05) | .76 (.09) | .63 (.05) |
| READSPAN | .51 (.05) | .60 (.09) | .50 (.05) |
| SYMMSPAN | .62 (.05) | .38 (.06) | .61 (.05) |
| ROTASPAN | .54 (.06) | .38 (.06) | .54 (.06) |
| RUNNSPAN | .59 (.05) | .43 (.07) | .60 (.05) |
| COUNTERS | .61 (.04) | .31 (.08) | .62 (.04) |
| ATTN RESTRAINT | |||
| ANTI-LET | .77 (.03) | .77 (.03) | |
| ANTI-ARRO | .73 (.04) | .77 (.04) | |
| SEM-SART d′ | −.47 (.05) | ||
| SEM-SART rtsd | .48 (.04) | ||
| N-STROOP | .33 (.05) | ||
| S-STROOP | .26 (.05) | .27 (.05) | |
| ATTN CONSTRAINT | |||
| ARROFLNK-SR | .24 (.08) | ||
| ARROFLNK-SS | .30 (.07) | ||
| LETTFLNK-SR | .21 (.08) | ||
| LETTFLNK-SS | .32 (.07) | ||
| ACCYFLNK-SR | .45 (.06) | .31 (.05) | |
| ACCYFLNK-SS | .29 (.07) | .24 (.06) | |
| MASKFLNK-SR | .47 (.07) | .79 (.05) | |
| MASKFLNK-SS | .37 (.07) | .66 (.04) | |
| CIRCFLNK | .17 (.06) | .09 (.05) | |
| TUT/TUTres | |||
| SART-TUT | .64 (.04) | .60 (.05) | .63 (.05) |
| LETT-TUT | .50 (.06) | .47 (.07) | .52 (.06) |
| ARRO-TUT | .67 (.05) | .67 (.06) | .65 (.05) |
| NUMS-TUT | .68 (.05) | .67 (.06) | .67 (.05) |
| 2BAC-TUT | .64 (.05) | .53 (.05) | .65 (.05) |
| COMMON EXEC | |||
| OPERSPAN | −.29 (.05) | ||
| READSPAN | −.24 (.05) | ||
| SYMMSPAN | −.44 (.05) | ||
| ROTASPAN | −.39 (.05) | ||
| RUNNSPAN | −.39 (.05) | ||
| COUNTERS | −.46 (.05) | ||
| ANTI-LET | .73 (.04) | ||
| ANTI-ARRO | .72 (.04) | ||
| SEM-SART d′ | −.46 (.05) | ||
| SEM-SART rtsd | .45 (.04) | ||
| N-STROOP | .33 (.05) | ||
| S-STROOP | .28 (.05) | ||
| ARROFLNK-SR | .12 (.06) | ||
| ARROFLNK-SS | .19 (.06) | ||
| LETTFLNK-SR | .15 (.06) | ||
| LETTFLNK-SS | .23 (.06) | ||
| ACCYFLNK-SR | .34 (.05) | ||
| ACCYFLNK-SS | .28 (.05) | ||
| MASKFLNK-SR | .36 (.06) | ||
| MASKFLNK-SS | .28 (.06) | ||
| SART-TUT | .22 (.06) | ||
| LETT-TUT | .17 (.06) | ||
| ARRO-TUT | .20 (.06) | ||
| NUMS-TUT | .22 (.05) | ||
| 2BAC-TUT | .34 (.05) | ||
| CoV | |||
| SEM-SART go | .36 (.07) | ||
| N-STROOP congruent | .42 (.07) | ||
| S-STROOP neutral | .50 (.07) | ||
| ARROFLANK neutral | .41 (.07) | ||
| ARROFLANK congruent | .41 (.06) | ||
| LETTFLANK neutral | .41 (.06) | ||
| LETTFLANK congruent | .42 (.07) | ||
| NEGATIVE SCHIZ | |||
| PHY-ANHD1 | .43 (.10) | .46 (.09) | .43 (.11) |
| PHY-ANHD2 | .39 (.10) | .41 (.10) | .39 (.11) |
| PHY-ANHD3 | .41 (.10) | .44 (.09) | .41 (.11) |
| SOC-ANHD1 | .88 (.19) | .83 (.16) | .88 (.22) |
| SOC-ANHD2 | .78 (.18) | .74 (.15) | .78 (.19) |
| SOC-ANHD3 | .55 (.15) | .52 (.12) | .55 (.16) |
| POSITIVE SCHIZ | |||
| SOC-ANHD1 | .26 (.10) | .25 (.09) | .26 (.10) |
| SOC-ANHD2 | .17 (.09) | .16 (.08) | .16 (.09) |
| SOC-ANHD3 | .23 (.07) | .22 (.07) | .22 (.07) |
| PERCABER1 | .59 (.04) | .59 (.04) | .59 (.04) |
| PERCABER2 | .58 (.04) | .59 (.04) | .59 (.04) |
| PERCABER3 | .67 (.03) | .67 (.03) | .66 (.03) |
| MAGIDEA1 | .79 (.03) | .79 (.03) | .79 (.03) |
| MAGIDEA2 | .78 (.03) | .78 (.03) | .77 (.03) |
| MAGIDEA3 | .66 (.04) | .66 (.04) | .66 (.04) |
| REFTHINK | .71 (.03) | .71 (.03) | .71 (.03) |
| DISORGANIZED SCHIZ | |||
| COGSLIPG | .83 (.02) | .83 (.02) | .83 (.02) |
| COGDYSRG | .74 (.03) | .74 (.03) | .74 (.03) |
| ODSPEECH | .83 (.02) | .83 (.02) | .83 (.02) |
| ODBEHAVR | .63 (.03) | .62 (.03) | .62 (.03) |
| PARANOID SCHIZ | |||
| PARACHEK1 | .84 (.02) | .84 (.02) | .84 (.02) |
| PARACHEK2 | .87 (.02) | .87 (.02) | .87 (.02) |
| SUSPICIO | .83 (.02) | .83 (.02) | .83 (.02) |
Figure 4.
Confirmatory factor analysis depicting the relations between the four-factor cognitive model and the four-factor schizotypy model. The circles represent the latent variables for working memory capacity (WMC), attention restraint failure [Attn Restraint (Fails)], attention constraint failure [Attn Constraint (Fails)], mind wandering rate (TUTs), negative schizotypy (Negative), positive schizotypy (Positive), disorganized schizotypy (Disorgz), and paranoid schizotypy (Paranoid). Double-headed arrows represent correlations between constructs. All solid paths are statistically significant at p < .05; all dotted paths are non-significant. For ease of interpretation, factor loadings for manifest variables are not depicted (see Table 6).
Bifactor Predictor Model
This model also provided adequate fit (see Tables 5 and 6; Figure 5). Again, negative schizotypy did not correlate with the cognitive predictors. In contrast to the 4-factor model, however, only the residual-TUT factor correlated with positive schizotypy. Paranoia and disorganization also correlated with residual-TUT, and they additionally correlated with the general executive factor, reflecting failures of executive control.
Figure 5.
Confirmatory factor analysis depicting the relations between the bifactor cognitive model and the four-factor schizotypy model. The circles represent the latent variables for Common Executive Failures [Common Exec (Fail)], the “residual” variance shared only among the WMC measures (WMCRes), and the “residual” variance shared only among the mind wandering measures (TUTRes), negative schizotypy (Negative), positive schizotypy (Positive), disorganized schizotypy (Disorgz), and paranoid schizotypy (Paranoid). Double-headed arrows represent correlations between constructs. All solid paths are statistically significant at p < .05; all dotted paths are non-significant. For ease of interpretation, factor loadings for manifest variables are not depicted (see Table 6).
Structural-Equation Models
We used a structural equation model (SEM) to assess the unique predictive power of the four-factor model’s cognitive constructs (because the bifactor model requires orthogonal predictors, its SEM results are identical to the CFA results presented above). SEMs are analogous to simultaneous regression, with path coefficients reflecting the unique variance accounted for by each predictor (and so interpreted like standardized beta weights in regression). Note that the fit statistics and the factor loadings for the SEM model are identical to those from the corresponding CFA model presented in Tables 5 and 6.
Figure 6 shows that none of the cognitive factors predicted significant variance in negative schizotypy (see Table 6 for factor loadings). Although both TUT rate and restraint failures had correlated with positive schizotypy and disorganization in the CFA, only TUT rate predicted unique variance in each. For paranoia, in contrast, both TUT rate and restraint failure predicted significant variance.
Figure 6.
Structural equation model depicting the prediction of the four-factor schizotypy model by the four-factor cognitive model. The circles represent the latent variables for working memory capacity (WMC), attention restraint failure [Attn Restraint (Fails)], attention constraint failure [Attn Constraint (Fails)], mind wandering rate (TUTs), negative schizotypy (Negative), positive schizotypy (Positive), disorganized schizotypy (Disorgz), and paranoid schizotypy (Paranoid). Arrows represent the modeled direction of pathway between constructs. All solid paths are statistically significant at p < .05; all dotted paths are non-significant. For ease of interpretation, factor loadings for manifest variables are not depicted (see Table 6).
Secondary Latent Variable Analyses with CoV Measures
We added a CoV factor, indicated by manifest variables representing the CoV values for non-conflict trials from five tasks (SART, number Stroop, spatial Stroop, arrow flanker, and letter flanker, with separate variables for neutral and congruent trials for arrow and letter flanker tasks). We changed the Constraint factor to eliminate the arrow and letter flanker tasks, and the Restraint factor to eliminate SART and number Stroop, because these contributed to the CoV construct. This ensured that any construct correlations with CoV were not due to shared task variance (a priori, we attempted to retain SART d′ as an accuracy measure of restraint, but it was so strongly correlated with SART CoV that it drove the correlation between Restraint and CoV factors to > 1.0; we therefore dropped SART d′ from the model).
Table 7 presents the descriptive statistics for the CoV variables, and Table 8 presents bivariate correlations among the CoV measures and between the CoV and other variables in the latent variable models. The CoV variables correlated modestly with one another, with strongest correlations between indicators from the same task. Not surprisingly, then, they did not correlate strongly with other cognitive measures. Finally, CoV indices did not strongly predict schizotypy, with most rs < .10. The CoV-schizotypy correlations that were stronger than .10 clustered in positive schizotypy.
Table 7.
Descriptive statistics for RT coefficient of variation measures.
| Measure | Mean [95% CI] | SD | Min | Max | Skew | Kurtosis | N |
|---|---|---|---|---|---|---|---|
| SEM-SART go | 0.795 [0.766, 0.823] | 0.330 | 0.225 | 2.083 | 1.227 | 1.427 | 456 |
| N-STROOP cong | 0.233 [0.2200, 0.247] | 0.147 | 0.102 | 2.107 | 7.065 | 72.263 | 468 |
| S-STROOP cong | 0.304 [0.285, 0.322] | 0.203 | 0.088 | 1.753 | 3.366 | 15.441 | 458 |
| ARROFLANK neut | 0.196 [0.189, 0.203] | 0.075 | 0.073 | 0.505 | 0.934 | 0.650 | 479 |
| ARROFLANK cong | 0.209 [0.202, 0.216] | 0.078 | 0.072 | 0.811 | 1.935 | 8.883 | 479 |
| LETTFLANK neut | 0.235 [0.225, 0.245] | 0.113 | 0.065 | 0.799 | 1.821 | 4.111 | 462 |
| LETTFLANK cong | 0.236 [0.227, 0.245] | 0.098 | 0.090 | 0.720 | 1.437 | 2.805 | 462 |
Table 8.
Correlations with RT coefficient of variation (CoV) scores for variables used in structural models.
| Measure | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|---|
| 1. SEM-SART go CoV | -- | ||||||
| 2. N-STROOP cong CoV | .17 | -- | |||||
| 3. S-STROOP cong CoV | .12 | .25 | -- | ||||
| 4. ARROFLANK neut CoV | .10 | .18 | .30 | -- | |||
| 5. ARROFLANK cong CoV | .11 | .13 | .17 | .50 | -- | ||
| 6. LETTFLANK neut CoV | .11 | .13 | .24 | .10 | .21 | -- | |
| 7. LETTFLANK cong CoV | .03 | .18 | .23 | .20 | .21 | .55 | -- |
| 8. OPERSPAN | −.13 | −.03 | .01 | −.06 | −.07 | .03 | .03 |
| 9. READSPAN | −.16 | .08 | −.00 | −.00 | −.14 | −.08 | −.04 |
| 10. SYMMSPAN | −.13 | −.14 | .04 | .02 | −.12 | −.11 | −.06 |
| 11. ROTASPAN | −.11 | −.05 | −.04 | −.05 | −.13 | −.06 | −.04 |
| 12. RUNNSPAN | −.16 | −.07 | −.08 | −.12 | −.19 | −.06 | −.06 |
| 13. COUNTERS | −.11 | −.11 | −.11 | −.16 | −.22 | −.10 | −.07 |
| 14. ANTI-LET | .28 | .11 | .08 | .12 | .23 | .22 | .17 |
| 15. ANTI-ARO | .20 | .09 | .10 | .14 | .17 | .17 | .15 |
| 16. S-STROOP | .09 | .13 | .22 | .07 | .09 | .14 | .12 |
| 17. ACCYFLNK-SR | .22 | .06 | .02 | .00 | .03 | −.03 | −.01 |
| 18. ACCYFLNK-SS | .12 | .07 | −.01 | .05 | .03 | .02 | .03 |
| 19. MASKFLNK-SR | .05 | .02 | .10 | .05 | .10 | .19 | .16 |
| 20. MASKFLNK-SS | .07 | .04 | .01 | .04 | .08 | −.01 | .06 |
| 21. CIRCFLNK | .03 | .15 | .09 | .07 | .06 | .08 | .05 |
| 22. SART-TUT | .16 | .14 | .13 | −.00 | .03 | .16 | .17 |
| 23. NUMS-TUT | .17 | .17 | .16 | .16 | .14 | .11 | .10 |
| 24. ARRO-TUT | .13 | .13 | .09 | .11 | .12 | .05 | .05 |
| 25. LETT-TUT | .15 | .21 | .13 | .01 | .03 | .22 | .25 |
| 26. 2BAC-TUT | .21 | .15 | .28 | .09 | .11 | .16 | .16 |
| 27. PERCABER | .07 | .09 | .07 | .10 | .11 | .02 | .09 |
| 28. MAGCIDEA | .10 | .10 | .11 | .08 | .05 | .07 | .14 |
| 29. REFTHINK | .16 | .14 | .13 | .07 | .07 | .10 | .06 |
| 30. SOC-ANHD | .08 | .01 | −.00 | .02 | .03 | .00 | −.01 |
| 31. PHY-ANHD | .04 | .00 | .14 | .05 | .10 | .08 | −.03 |
| 32. PARACHEK | .12 | .11 | .08 | .06 | .04 | .08 | .06 |
| 33. SUSCPICIO | .11 | .07 | .13 | .06 | .02 | .07 | −.02 |
| 34. COGSLIPG | .15 | .08 | .05 | .08 | .05 | .02 | .05 |
| 35. COGDYSRG | .13 | .08 | .08 | .05 | .04 | .04 | .02 |
| 36. ODSPEECH | .10 | .02 | .06 | .01 | −.03 | .06 | −.02 |
| 37. ODBEHAVR | .04 | .08 | .07 | .02 | −.00 | −.04 | −.02 |
A CFA tested whether the CoV variables reflected a common factor and, if so, how strongly it correlated with executive and schizotypy constructs. In modeling the CoV data, we allowed residual correlations between the two measures from arrow flanker and from letter flanker. As shown in Table 5, the model fit the data (see Table 6 for factor loadings). First, a CoV factor emerged across tasks, indicating a coherent construct (factor loadings = .36 – .50). Second, the CoV factor correlated moderately-to-strongly with the cognitive constructs: WMC (−.32), restraint (.48), constraint (.24), and TUTs (.54). Like the other executive constructs in the previous models, CoV did not correlate with negative schizotypy (.04). It did, however, correlate modestly with positive (.28), paranoid (.20), and disorganized schizotypy (.16).
GENERAL DISCUSSION
Latent variable analyses of our large-N correlational dataset indicated both “unity and diversity” (Miyake & Friedman, 2012) of executive attention constructs, in both their associations to one another and their prediction of schizotypy. Our primary findings regarding the individual-difference structure of executive control were that: 1) WMC, TUTs, attention restraint, and attention constraint were correlated but separable; 2) WMC correlated more strongly with attention restraint than constraint, and TUTs more strongly with restraint and constraint than with WMC; 3) the cognitive constructs were strongly enough associated that a common executive factor fit the data, along with residual factors for WMC- and TUT-specific variation; 4) CoV measured from the “control” trials of attention tasks shared enough variance to derive a latent variable that correlated strongly with restraint and TUTs and moderately with WMC.
Executive attention factors predicted variation in schizotypy in our undergraduate sample, but only modestly and selectively. Our primary findings regarding the cognitive correlates of schizotypy were that: 1) None of the executive factors predicted negative schizotypy; 2) TUT propensity consistently predicted positive, disorganized, and paranoid schizotypy, above and beyond the variance it shared with other executive constructs; 3) attention restraint correlated most strongly with paranoia, predicting variance above and beyond TUTs and other executive factors; although restraint failure also predicted positive and disorganized schizotypy, it did not do so over and above TUT rate; 4) neither WMC nor attention constraint correlated with schizotypy factors; 5) RT variability (CoV), like TUTs and attention restraint, correlated with positive, disorganized, and paranoid schizotypy, but CoV correlated most strongly with positive (and was positive schizotypy’s strongest predictor in any model).
Executive Attention Deficits in Schizotypy?
Our review of the schizotypy literature suggested inconsistent cognitive findings and widespread measurement problems. We sought to correct many of the literature’s weaknesses with a large-N study, distinguishing four dimensions of schizotypy via multiple measures, and measuring several executive constructs with multiple, well-motivated tasks. Of course, no one study provides conclusive answers to any question, but our findings were clear: None of the executive abilities we assessed predicted normal variation among undergraduates in negative schizotypy, and we found few cognitive correlates of positive, disorganized, or paranoid schizotypy. In short, the stalwart executive constructs in both the schizotypy and schizophrenia literatures — WMC and attention restraint — were generally unimpressive as predictors of schizotypy. In contrast, our more novel measures — TUT rate and RT CoV — showed promise.
The lack of associations with negative schizotypy was surprising, given that negative symptoms in schizophrenia often predict disrupted cognition (e.g., Addington, Addington, & Maticka-Tyndale, 1991; Harvey, Koren, Reichenberg, & Bowie, 2006; Heinrichs & Zakzanis, 1998). Our lack of correlations may reflect that we measured negative schizotypy with questionnaires that primarily tapped anhedonia and social disinterest, but not alogia, avolition, or anergia. Nevertheless, some smaller-scale studies show that these same negative schizotypy measures predict neurological soft signs (Kaczorowski et al., 2009), and deficits in sustained attention (Gooding et al., 2006) and episodic memory (Sahakyan & Kwapil, 2015).
Mind Wandering, Schizotypy, and Current Concerns
Only mind-wandering rate consistently predicted positive, disorganized, and paranoid schizotypy, and it did so above and beyond any contributions of WMC and performance-based attention measures. People having more TUTs during attention and memory tasks also reported more strange perceptual experiences and beliefs (positive features), more confusion and cognitive difficulties (disorganized features), and more suspiciousness and persecution (paranoid features). These correlations were not large, but their consistency across statistical models is encouraging, especially for this mixed literature. Moreover, findings of increased TUTs corroborate the characterization of positive, disorganized, and paranoid schizotypy as reflecting experiential excesses, versus negative schizotypy’s experiential deficits.
TUTs are multiply determined, so we cannot know whether the higher TUT rates in positive, disorganized, and paranoid schizotypy reflected uncontrolled or unwanted thought intrusions. However, TUTs predicted schizotypy over and above the influences of other executive measures, suggesting that the (residual) TUT-schizotypy associations were not due to control failures. Perhaps the TUT-schizotypy links reflect that, other things being equal, people scoring high on measures of positive, disorganized, and paranoid schizotypy are more vulnerable to the influence of current personal concerns and affective dysregulation (e.g., Klinger, 2013; McVay & Kane, 2010; Smallwood et al., 2009) on their stream of thought. To the extent that positive, disorganized, and paranoid schizotypy produce relatively loose conceptual associations and abundant spreading activation along semantic networks (e.g., Kreher, Holdcomb, Goff. & Kuperberg, 2008; Moritz, Woodward, Küppers, Lausen, & Schickel, 2002), and relatively low thresholds for perceptual, conceptual, or motivational salience (e.g., Kapur, 2003; Kapur, Mizrahi, & Li, 2005), people high on these dimensions should find more cues to trigger concern-relevant thoughts during ongoing activities. Thus, positive, disorganized, and paranoid schizotypy may elicit an overabundance of interfering material for consciousness that must be contended with by executive-control mechanisms; paranoid thinking, in particular, should be reactive to a task context in which one is repeatedly probed by a computer program to report one’s thoughts, triggering ongoing concerns about being observed and judged by others. We conclude, then, in accord with the perspective that executive abilities and concern-related interference jointly influence mind wandering (McVay & Kane, 2010), that increased mind-wandering in schizotypy may be more tied to the excessive activation of personal concerns than to a deficit in cognitive control.
Schizotypy and Intra-individual Variation in RT
At the same time, our executive measure that was most closely associated with mind wandering (see also McVay & Kane, 2012a; Unsworth 2015) — intra-individual variation in RT — also predicted positive, disorganized, and paranoid schizotypy. Were these correlations redundant? Did RT CoV predict schizotypy simply as an alternative measure of mind-wandering propensity, with tuning in and out of tasks creating RT variability? Our prior work suggests that RT variability and TUTs are not redundant, correlating significantly but imperfectly (McVay & Kane, 2012a), as in the present study. RT variability may thus capture more subtle vacillation in attention than do TUTs; that is, some attentional fluctuations may have behavioral consequences without producing conscious dissociations from ongoing activities, experienced as TUTs, and they may be somewhat less influenced by the cuing of one’s personal concerns.
Here, RT CoV correlated numerically more strongly with positive schizotypy than did TUT rate (they correlated similarly with disorganization and paranoia). We tested the relative contributions of CoV and TUTs to positive symptoms via a SEM version of the CFA reported above, in which CoV and TUT rate competed (with the other cognitive constructs) to explain unique variance in positive schizotypy. CoV predicted positive schizotypy (β= .28, p = .009) but TUT rate did not (β = .11, p = .200), and so CoV tells us something about positive schizotypy beyond its shared contribution with TUT rate. Although several studies have found increased RT variability in schizophrenia (e.g., Rentrop et al., 2010; Vinogradov, Poole, Willis-Shore, Ober & Shenaut, 1998) and in high risk samples (Cole, Weinberger & Dickinson, 2011; Y. Shin et al., 2013), we are aware of only one other study that assessed schizotypy-related variance in RT variability. Schmidt-Hansen and Honey (2009) found that RT variability in a 2-back WMC task predicted positive schizotypy more strongly than either disorganized or negative dimensions. Together, these findings suggest that positive symptoms reflect a general dysregulation of cognitive and affective functioning (e.g., Barrantes-Vidal et al., 2013; Kwapil & Barrantes-Vidal, 2014; Kerns, 2005; Cicero & Kerns, 2010). Our CoV findings suggest that future research on executive control and schizotypy should take a latent-variable approach to intra-individual variability and assessing its links to different symptoms.
WMC, Attention Restraint, and Schizotypy: Why So Little Covariation?
Contrary to our expectations, but in line with the inconclusive literature on schizotypy and executive control, neither WMC nor restraint constructs broadly predicted schizotypy. Attention restraint (and the common variance across executive constructs in our bifactor model, where restraint tasks had the strongest loadings) correlated modestly with only paranoid schizotypy. Paranoia has been neglected by researchers investigating cognitive or neuropsychological correlates of schizotypy, perhaps because paranoid schizophrenia tends to not be associated with cognitive deficits (see Zalewski, Johnson-Selfridge, Ohriner, Zarrella, & Seltzer, 1998). Our finding that restraint failures predict paranoid thinking seems consistent with claims that delusions follow from failures to selectively filter preconscious thoughts from consciousness (e.g., Frith, 1979) or from the assignment of significance (e.g., Kapur, 2003), but we did not predict the restraint-paranoia association to be especially robust, and so we should probably not speculate too deeply about this intriguing finding until it is independently replicated.
But what should we make of the failures of WMC, attention restraint, or common executive-attention variance to predict schizotypy more comprehensively? Given the strengths of the present study in terms of sample size, statistical power, construct coverage, and construct measurement, we suggest that subclinical manifestations of schizotypy are not generally associated with significant executive-control disruptions. If we are correct, it should also follow that, either: (1) the executive deficits typically seen in schizophrenia represent a consequence of the disease rather than a cause or risk factor, or; (2) declines in executive capabilities appear only far along the schizotypy spectrum, and so they scarcely precede — 0r may precipitate — the transition to diagnosable psychosis. In the former case, we would not expect to generally find correlations between schizotypy and executive performance; in the latter case, we would only expect executive-schizotypy correlations to arise in “ultra high-risk” groups (e.g., McGorry, Yung, & Phillips, 2003; Yung et al., 1998) and not from most community or university samples.
Indeed, a clear limitation of our empirical approach is that our subjects were university students, healthy and cognitively intact enough to begin pursuit of a bachelor’s degree. They thus provided us with a somewhat conservative test of cognition-schizotypy associations. Subjects recruited from the community may have shown more extreme schizotypy scores and more (and more strongly associated) cognitive dysfunction. At the same time, college-aged adults are just entering the window of greatest risk for developing schizophrenia, many published studies on executive functions and schizotypy have tested university samples, and ours came from a comprehensive state university with a diverse student body (UNCG is a Minority-Serving Institution for African Americans) and modest admissions criteria (60% of first-year applicants were accepted for Fall 2012; UNCG Fact Book 2012–13, downloaded July, 2015 from http://ire.uncg.edu/pages/factbook/default.asp?T=2012-13), making it more like a community sample than are many university populations.
Another limitation, shared with other studies, is in the psychometric assessment of schizotypy. Despite our questionnaires’ well-established validity (e.g., Chapman et al., 1994; Kwapil, 1998), they do not tap broadly or deeply into schizotypy’s more cognitive features. Indeed, those that do ask about cognitive symptoms may not capture them effectively. Negative symptoms of schizophrenia, for example, such as diminished speech, language, and thought, tend not to appear on negative schizotypy scales, which instead emphasize social, emotional, and motivational functioning. And even if they did, it is not clear that someone suffering from those cognitive symptoms would have the insight to report them accurately.
We were not surprised by the strong correlation between positive and paranoid schizotypy, given that clinical and subclinical paranoia share unrealistic thoughts and beliefs with positive schizotypy, but their strong correlations with disorganized schizotypy raises concerns about its psychometric assessment. For example, measures purporting to assess disorganization may, in fact, be tapping odd speech and behavior that is secondary to positive schizotypy, rather than disruptions in the form and regulation of thought (Gross et al., 2014). Furthermore, self-report measures of disorganization correlate strongly with neuroticism (as do other cognitive-failure questionnaires; e.g., Broadbent, Cooper, Fitzgerald & Parkes, 1982; Matthews, Coyle, & Craig, 1990; Wilhelm, Witthöft, & Schipolowski, 2010), which is problematic because the nomological network around disorganization does not include affective dysregulation and distress (Gross et al., 2014). In relatively healthy young adults, then, self-reported difficulties in attention, memory, speech, and thought may tell us as much about people’s overall psychological adjustment and sense of well-being as they do about significant or specific cognitive impairments.
We suggest, then, that future investigations into the cognitive correlates of schizotypy should not only use large samples of subjects and measures, as we did. They should also consider alternative means by which to assess schizotypy’s more cognitive dimensions, perhaps via reports from close others or through corroboration from performance records from school or work settings. In the face of our largely null results — aside from TUT and CoV findings — we suggest that the burden of proof is on those claiming significant cognitive components to normal variation in schizotypy.
The Structure of Executive Control: Unity and Diversity
The variance shared, versus not shared, by our cognitive measures clarifies the nomological network for the frequently invoked — but gravely underspecified — construct of executive control. Here we focus our discussion on: 1) WMC’s associations with attention restraint, constraint, and TUTs, and; 2) executive contributions to mind-wandering propensity and intra-individual variability in task performance.
Attention Restraint and Constraint, and Their Links to WMC
Consistent with Friedman and Miyake (2004), we found a robust association between attention restraint and constraint abilities — as well as both with WMC. Our estimate of the restraint-constraint correlation (.60) was similar to theirs (.68), but their study was underpowered to statistically distinguish them. We found that, although people who were better at overriding dominant-but-wrong responses in antisaccade, SART, and Stroop tasks were also generally better at focusing on target stimuli presented amid distractors in flanker tasks, these skills were distinguishable and not identical. Moreover, restraint failures were considerably more strongly correlated with WMC than were constraint failures.
Why? Perhaps WMC correlated more weakly with constraint than restraint due to measurement problems: We assessed constraint ability exclusively with flanker tasks. Even though we varied their stimuli, array properties, responses, and dependent measures, the constraint factor had a narrower psychometric bandwidth (Cronbach, 1990) than did restraint. That is, the restraint tasks (antisaccade, SART, number and spatial Stroop) were diverse in structural and surface characteristics and so shared less method variance than did the flanker tasks. Shared method variance should drive down the constraint factor’s association with other constructs that do not share that method. To address this possibility, post hoc, we modeled restraint with only the antisaccade tasks (letter and arrow), thus narrowing its bandwidth to nearly identical measures (but those with the highest restraint loadings). We modeled constraint with two more dissimilar (but highest loading) tasks, conditional accuracy flanker (S-R and S-S conditions) and masked flanker (S-R and S-S), thus providing a wider bandwidth for constraint than restraint. In a good-fitting CFA that included WMC and TUTs (CFI and TLI > .93; SRMR and RMSEA < .05), the 2-indicator restraint factor and 4-indicator constraint factor correlated strongly, and similarly to that in the full-construct model (.63). Again, however, restraint was more closely associated with WMC (−.62) than was constraint (−.35), suggesting that method variance was not responsible for constraint’s weaker association with WMC.
Provisionally, then, we propose that WMC has more in common with attention restraint than constraint because restraint tasks tap more strongly into maintenance mechanisms than do constraint tasks, particularly those that keep current task goals accessible. Restraint tasks are especially challenging because they ask subjects to engage a task goal that conflicts strongly with habit, while providing little contextual support for that unusual goal (Kane et al. 2001; Kane & Engle, 2003). In the Stroop task, particularly one that presents mostly congruent trials, subjects must endogenously regulate their cognition and behavior by maintaining access to the novel task goal, which biases responding in the demanded direction. If subjects momentarily forget that, in this context, they should ignore what a word says or look away from a salient visual cue, they will make overt errors or very slow “just-in-time” responses on conflict trials (e.g., Kane & Engle, 2003; McVay & Kane, 2012; Unsworth et al., 2004, 2010, 2012). So, in addition to presenting subjects with conflict between target and non-target stimuli, restraint tasks also challenge subjects to maintain enough accessibility to a non-dominant task set to allow quick reactions to that conflict (for related arguments about “inhibition” tasks, see Miyake & Friedman, 2012).
Constraint tasks like the flanker, in contrast, do not particularly test subjects’ ability to maintain task goals, as they present little conflict with prepotent behaviors. There is nothing in a flanker display that draws one’s attention away from the target array, or that otherwise derails one from the goal of classifying the target while ignoring distractors. Although attention may be initially captured by the full extent of the flanker-dominating array (e.g., Eriksen & St. James, 1986; Gratton, Coles, Sirevaag, Eriksen & Donchin, 1988; Heitz & Engle, 2007), the main challenge is not in maintaining the correct task set, but rather in combatting the competition from flankers in selecting the target or the response. Constraint and restraint tasks thus have in common the requirement to overcome acute conflict in encoding or responding to stimuli. Restraint tasks, however, also primarily tax goal-maintenance mechanisms that likely overlap with those involved in working-memory maintenance more generally.
Mind-Wandering Variation and Covariation
Despite our assessing TUT rates across different kinds of tasks, they formed a coherent latent variable, indicating a trait-like vulnerability to off-task thought (see also Grodsky & Giambra, 1990–91; McVay & Kane, 2012; Mrazek et al., 2012; Unsworth, 2015; Unsworth & McMillan, 2014). We also found, however, that correlations between TUT rates were highest for assessments within the same experimental session (separated by 15 – 60 min) than for those across sessions (separated by days or weeks). This same-session increase in correlations suggests state-like, in addition to trait-like, variation in mind-wandering propensity, perhaps reflecting the influence of current goals, concerns, and mood in providing fodder for TUTs (e.g., Franklin et al., 2013; Klinger, 2013; McVay & Kane, 2013; Smallwood, Fitzgerald, Miles & Phillips, 2009).
TUT rate correlated well with other executive constructs (including constraint, contradicting Forster & Lavie, 2014), so we argue that it reflects — in part — control processes that operate to keep thoughts on-task (McVay & Kane, 2010; Kane & McVay, 2012). At the same time, TUTs correlated more strongly with attention restraint and constraint than with WMC. An uninteresting explanation for this is that we measured TUTs during some of the restraint and constraint tasks, so they shared method and error variance that drove their correlations higher. We can rule out this possibility through a post-hoc model that eliminates restraint and constraint performance measures that included thought probes. Here, we modeled attention restraint with only the two antisaccade tasks plus spatial Stroop (dropping the probed SART and number Stroop tasks), and modeled constraint with only the S-R and S-S measures from conditional accuracy and masked flanker tasks (dropping the probed arrow and letter flanker tasks); the model fit with CFI and TLI > .92 and RMSEA and SRMR < .05. TUT correlations were similar to those in our original model, and the WMC-TUT correlation of −.18 was again numerically smaller than those for restraint-TUT (.28) and constraint-TUT (.35). A better explanation for our findings, then, is that memory-related variance shared by WMC tasks is less associated with mind-wandering vulnerability than is the attention-control-related variance that is tapped more fully by “simpler” attention tasks of restraint and constraint.
Pragmatically, then, we recommend that investigators aiming to explore executive-task covariation with mind wandering use tasks of attention restraint or constraint rather than (or in addition to) WMC. Moreover, given the weak correlations among individual measures of executive control and TUTs (see also McVay & Kane, 2012b; Randall et al., 2014), we suggest that researchers use multiple assessments of control and TUTs to allow latent-variable methods. TUT vulnerability is only affected in part, however, by executive control abilities: Our bifactor models indicated that TUT rates share substantial variance that is not accounted for by other executive abilities (and this residual TUT variance predicted positive, disorganized, and paranoid schizotypy). We think it most likely that non-cognitive influences related to personality, motivation, mood, or current concerns also drive variation in mind-wandering (e.g. Klinger, 2013; Seli et al., in press; Smallwood, O’Connor, Sudberry & Obonsawin, 2007, Zhiyan & Singer, 1996).
RT Variability as an Executive Construct
Several literatures converge on the idea that intra-individual variation in performance tells us important things about people (for a review, see MacDonald, Nyberg & Bäckman, 2006), such as their intelligence (e.g., Larson & Alderton, 1990; Ratcliff, Schmiedek, & McKoon, 2008), age (e.g., Li et al., 2004; Rabbitt, Osman, Moore & Stollery, 2001), and risk or diagnostic status for attention-deficit/hyperactivity disorder or dementia (e.g., Bidwell, Willcutt, DeFries, & Pennington, 2007; Castellanos, Sonuga-Barke, Milham, & Tannock, 2006; Christensen et al., 2005; Murtha, Cismaru, Waechter, & Chertkow, 2002; Tamm et al., 2012). Such findings indicate that performance instability reflects a stable, trait-like individual difference, often indicated by RT variability, as in the current study.
We aimed to extend our prior findings that relied on individual tasks to assess TUTs and RT variability (McVay & Kane, 2009, 2012a), as well as Unsworth’s (2015) findings that CoV measured across numerous executive tasks yielded a latent variable that correlated strongly with WMC and TUTs. Because Unsworth measured CoV across trials from tasks that presented many conflict-inducing trials, we wanted to be sure that his findings didn’t result from CoV measures being contaminated by attention restraint and constraint processes. If large CoV scores reflect a general executive failure to consistently maintain optimal task focus (or implementation of task sets), then those momentary failures should be detectable in RTs for trials that do not elicit conflict. In fact, consistent with the McVay-Kane findings from non-conflict “go” trials in the SART, CoV measured from non-conflict trials reflected a single factor associated with other executive abilities. Despite the measurement differences, our key findings complemented Unsworth (2015): RT CoV correlated with WMC (see also Schmeidek et al., 2007; Unsworth et al., 2010, 2012), with TUT rate (see also Bastian & Sakur, 2013; Seli et al., 2013), and with attention restraint failure.
However, we question Unsworth’s (2015) conclusion that CoV in attention-control tasks is fundamentally different from CoV in other tasks. His Study 1 and 2 clearly showed a distinction between CoV from attention-control and lexical-decision tasks, but intra-individual variation in lexical decision seems likely to be driven as much by vocabulary knowledge and the particular stimuli presented on each trial as by fluctuations in task-focused thought or implementation of task set. Thus, we suspect that lexical-decision tasks reflect more of a special case than do attention-control tasks. We suggest that CoV measured across simple RT tasks, or choice RT tasks that don’t draw heavily on crystallized knowledge, will produce results similar to ours from neutral and congruent trials from executive-control tasks. Indeed, Schmiedek et al. (2007) assessed RT variability across a large number of relatively simple choice-RT tasks and it correlated strongly with WMC; moreover, ADHD-related increases in RT variability are evident over a wide range of task types (for a review, see Kuntsi & Klein, 2012). Our view, then, is that intra-individual fluctuations in RT within simple tasks are partly a reflection of attention-control mechanisms, and that individual differences in these fluctuations provide a useful and underused marker of executive control. We further recommend that RT CoV and mind wandering vulnerability, both indicative of intraindividual variation in attentional focus, be explored further in testing theories about the cognitive components of schizotypal traits.
Supplementary Material
Acknowledgments
For assistance in data collection, we thank Jasmyn Bobbs, Assma Boucteb, Brian Bulla, Cindy Dezern, Alec Delgado, Ali Faraz, Paul Grey, Caitlin Grindall, Amy Harrill, Melanie Hayes, Shaquinta Hedgepeth, Kevin Hine, Sara Howe, Kevin Kane, Eric Kaplan, Edward Kim, Laura Lerebours, James McClain, Laura Michael, Brent Nara, Jacqueline Palmer, Joshua Perkins, Leah Rash, Wyatt Smith, Parker Steel, Robert Steigerwald, Paul Thomas, and Helen Wilson. We also thank Paul Seli for his helpful comments on an earlier draft of this article.
This research was supported by award number R15MH093771 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Subjects completed two additional cognitive tasks that we do not analyze here. These divergent-thinking tests of creativity were conducted to address separate questions, under a different order of authorship, and so will be reported in a subsequent article.
Given the high correlations among positive, disorganized, and paranoid schizotypy factors, we computed a post hoc, two-factor CFA in which the positive, disorganized, and paranoid scales loaded on a single “positive” factor (and the original negative schizotypy factor was again modeled). This model had inadequate fit, and descriptively poorer fit than the original four-factor model [χ2(154)=861.74, χ2/df = 5.60, CFI = .860, TLI = .827, SRMR = .076, RMSEA = .093 (90% CI = .087, .099)]. Furthermore, we examined the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to formally compare the two- and four-factor models (note that these indices penalize more complex models with smaller values indicating better fit). Both indicated that the original four-factor model (AIC = 41181.38, BIC = 41527.94) had better fit than the two-factor model (AIC = 41744.14, BIC = 42069.31).
Because our constraint tasks were designed to elicit S-R conflict, S-S conflict, or both, we also tested a 5-factor model that distinguished S-R constraint from S-S constraint. However, this model did not fit the data [χ2(284)=646.57, χ2/df = 2.28, CFI = .861, TLI = .841, SRMR = .058, RMSEA = .049 (90% CI = .044, .053)] and it yielded a > 1.0 correlation between S-R and S-S factors.
Contributor Information
Michael J. Kane, University of North Carolina at Greensboro
Matt E. Meier, Western Carolina University
Bridget A. Smeekens, University of North Carolina at Greensboro
Georgina M. Gross, University of North Carolina at Greensboro
Charlotte A. Chun, University of North Carolina at Greensboro
Paul J. Silvia, University of North Carolina at Greensboro
Thomas R. Kwapil, University of North Carolina at Greensboro
References
- Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophrenia Research. 1991;5:123–134. doi: 10.1016/0920-9964(91)90039-t. [DOI] [PubMed] [Google Scholar]
- Altamirano LJ, Miyake A, Whitmer AJ. When mental inflexibility facilitates executive control: Beneficial side effects of ruminative tendencies on goal maintenance. Psychological Science. 2010;21:1377–1382. doi: 10.1177/0956797610381505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S, Alliger RJ, Andreasen NC. The distinction of positive and negative symptoms. The failure of a two-dimensional model. The British Journal of Psychiatry. 1991;158:317–322. doi: 10.1192/bjp.158.3.317. [DOI] [PubMed] [Google Scholar]
- Avons SE, Nunn JA, Chan L, Armstrong H. Executive function assessed by memory updating and random generation in schizotypal individuals. Psychiatry Research. 2003;120:145–154. doi: 10.1016/s0165-1781(03)00174-4. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. London/New York: Oxford University Press; 1986. [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barch D, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naïve patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch D, Carter CS, MacDonald AW, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: Diagnostic specificity, 4-week course, and relationships to clinical symptoms. Journal of Abnormal Psychology. 2003;112:132–143. [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Chun C, Myin-Germeys I, Kwapil TR. Psychometric schizotypy predicts the experience of psychotic-like, paranoid, and negative symptom experiences in daily life. Journal of Abnormal Psychology. 2013;122:1077–87. doi: 10.1037/a0034793. [DOI] [PubMed] [Google Scholar]
- Bastian M, Sackur J. Mind-wandering at the tips of the fingers: Automatic parsing of subjective states based on response time variability. Frontiers in Psychology. 2013;4:573. doi: 10.3389/fpsyg.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech A, Baylis GC, Smithson P, Claridge G. Individual differences in schizotypy as reflected in measures of cognitive inhibition. British Journal of Clinical Psychology. 1989;28:117–129. doi: 10.1111/j.2044-8260.1989.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Bergida H, Lenzenweger M. Schizotypy and sustained attention: Confirming evidence from an adult community sample. Journal of Abnormal Psychology. 2006;115:545–551. doi: 10.1037/0021-843X.115.3.545. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK. Symptomatic and neuropsychological components of defect states. Schizophrenia Bulletin. 1985;11:409–419. doi: 10.1093/schbul/11.3.409. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Collins LM, Aghevli M, Leung WW, Cohen AS. Social anhedonia in a community sample: The Maryland Longitudinal Study of Schizotypy. Schizophrenia Bulletin. 2011;37:587–602. doi: 10.1093/schbul/sbp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Broadbent DE, Cooper PF, Fitzgerald P, Parkes LR. The cognitive failures questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Broadway JM, Engle RW. Validating running memory span: Measurement of working memory capacity and links with fluid intelligence. Behavior Research Methods. 2010;42:563–570. doi: 10.3758/BRM.42.2.563. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York: Cambridge University Press; 1993. [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Wang Y, Yan C, Song L-L, Wang Y-N, Shi Y-F, Gong Q-Y, Cheung EFC. Contribution of specific cognitive dysfunction to people with schizotypal personality. Psychiatry Research. 2011;186:71–75. doi: 10.1016/j.psychres.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Yan C, Qing YH, Wang Y, Wang YN, Ma Z, Hong XH, Li ZJ, Gong QY, Yu X. Subjective awareness of everyday dysexecutive behavior precedes ‘objective’ executive problems in schizotypy: A replication and extension study. Psychiatry Research. 2011;185:340–346. doi: 10.1016/j.psychres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency Scale. 1983. Unpublished test (copies available from T.R. Kwapil, Department of Psychology, University of North Carolina at Greensboro, P.O. Box 26170, Greensboro, NC, 27402–6170) [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body image aberration in schizophrenia. Journal of Abnormal Psychology. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Hsiao CK, Lin CCH. Schizotypy in community samples: The three-factor structure and correlation with sustained attention. Journal of Abnormal Psychology. 1997;106:649–654. doi: 10.1037//0021-843x.106.4.649. [DOI] [PubMed] [Google Scholar]
- Christensen H, Dear KBG, Anstey KJ, Parslow RA, Sachdev P, Jorm AF. Within-occasion intraindividual variability and preclinical diagnostic status: Is intraindividual variability an indicator of mild cognitive impairment? Neuropsychology. 2005;19:309–317. doi: 10.1037/0894-4105.19.3.309. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuderski A. How well can storage capacity, executive control, and fluid reasoning explain insight problem solving. Intelligence. 2014;46:258–270. [Google Scholar]
- Chuderski A, Taraday M, Nęcka E, Smoleń T. Storage capacity explains fluid intelligence but executive control does not. Intelligence. 2012;40:278–295. [Google Scholar]
- Chun CA, Minor KS, Cohen AS. Neurocognition in psychometrically defined college schizotypy samples: We are NOT measuring the “right stuff. Journal of the International Neuropsychological Society. 2013;19:324–337. doi: 10.1017/S135561771200152X. [DOI] [PubMed] [Google Scholar]
- Cicero DC, Kerns JG. Can disorganized and positive schizotypy be discriminated from dissociation? Journal of Personality. 2010;78:1239–1270. doi: 10.1111/j.1467-6494.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- Cimino M, Haywood M. Inhibition and facilitation in schizotypy. Journal of Clinical and Experimental Neuropsychology. 2008;30:187–198. doi: 10.1080/13803390701336866. [DOI] [PubMed] [Google Scholar]
- Coffman DL, MacCallum RC. Using parcels to convert path analysis models into latent variable models. Multivariate Behavioral Research. 2005;40:235–259. doi: 10.1207/s15327906mbr4002_4. [DOI] [PubMed] [Google Scholar]
- Cole VT, Weinberger DR, Dickinson D. Intra-individual variability across neuropsychological tasks in schizophrenia: A comparison of patients, their siblings, and healthy controls. Schizophrenia Research. 2011;129:91–93. doi: 10.1016/j.schres.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Levy DL, Lenzenweger MF, Holzman PS. Thought disorder, perceptual aberrations, and schizotypy. Journal of Abnormal Psychology. 1996;105:469–473. doi: 10.1037//0021-843x.105.3.469. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin and Review. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Engle RW. Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Coyle TR. A review of the worst performance rule: Evidence, theory, and alternative hypotheses. Intelligence. 2003;31:567–587. [Google Scholar]
- Cronbach LJ. Essentials of psychological testing. 5. New York: Harper & Row; 1990. [Google Scholar]
- Daly MP, Afroz S, Walder DJ. Schizotypal traits and neurocognitive functioning among nonclinical young adults. Psychiatry Research. 2012;200:635–640. doi: 10.1016/j.psychres.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Dang CP, Braeken J, Colom R, Ferrer E, Liu C. Why is working memory related to intelligence? Different contributions from storage and processing. Memory. 2014;22:426–441. doi: 10.1080/09658211.2013.797471. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Aycicegi A, Greene P, Andover MS. Positive and negative schizotypy in a student sample: Neurocognitive and clinical correlates. Schizophrenia Research. 2002;56:171–185. doi: 10.1016/s0920-9964(01)00230-4. [DOI] [PubMed] [Google Scholar]
- Draheim C, Hicks KL, Engle RW. Combining reaction time and accuracy: The relationship between working memory capacity and task switching as a case example. Perspectives on Psychological Science. 2016;11:133–155. doi: 10.1177/1745691615596990. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. 1982. Unpublished test (copies available from T.R. Kwapil, Department of Psychology, University of North Carolina at Greensboro, P.O. Box 26170, Greensboro, NC, 27402–6170) [Google Scholar]
- Enders CK. Applied missing data analysis. New York, NY: Guilford; 2010. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, St James JD. Visual attention within and around the field of focal attention: A zoom lens model. Perception and Psychophysics. 1986;25:249–263. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- Forster S, Lavie N. Distracted by your mind? Individual differences in distractibility predict mind wandering. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40:251–260. doi: 10.1037/a0034108. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Mrazek MD, Anderson CL, Smallwood J, Kingstone A, Schooler JW. The silver lining of a mind in the clouds: Interesting musings are associated with positive mood while mind-wandering. Frontiers in Psychology. 2013;4(583) doi: 10.3389/fpsyg.2013.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Garety PA, Bebbington PE, Smith B, Rollinson R, Fowler D, Kuipers E, Ray K, Dunn G. Psychological investigation of the structure of paranoia in a non-clinical population. British Journal of Psychiatry. 2005;186:427–435. doi: 10.1192/bjp.186.5.427. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Consciousness, information processing, and schizophrenia. British Journal of Psychiatry. 1979;134:225–235. doi: 10.1192/bjp.134.3.225. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Bennett MA, Duke PA, Young MA. Quantitative electroencephalography as a biomarker for proneness toward developing psychosis. Schizophrenia Research. 2014;153:68–77. doi: 10.1016/j.schres.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Giambra LM. Task-unrelated thought frequency as a function of age: A laboratory study. Psychology and Aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Gooding DC. Antisaccade task performance in questionnaire-identified schizotypes. Schizophrenia Research. 1999;35:157–166. doi: 10.1016/s0920-9964(98)00120-0. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Matts CW, Rollman EA. Sustained attention deficits in relation to psychometrically identified schizotypy: Evaluating a potential endophenotypic marker. Schizophrenia Research. 2006;82:27–37. doi: 10.1016/j.schres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. Spatial, object, and affective working memory in social anhedonia: An exploratory study. Schizophrenia Research. 2003;63:247–260. doi: 10.1016/s0920-9964(02)00326-2. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Hegyi JV. Cognitive slippage in schizotypic individuals. Journal of Nervous and Mental Disease. 2001;189:750–756. doi: 10.1097/00005053-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: Further validation of the psychometric high-risk strategy. Journal of Abnormal Psychology. 2005;114:170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gottesman . Schizophrenia genesis: The origins of madness. San Francisco: Freeman; 1991. [Google Scholar]
- Gratton G, Coles MGH, Sirvaag EJ, Eriksen CW, Donchin E. Pre-and poststimulus activation of response channels: A psychophyiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Grodsky A, Giambra LM. The consistency across vigilance and reading tasks of individual differences in the occurrence of task-unrelated and task-related images and thoughts. Imagination, Cognition and Personality. 1990–91;10:39–52. [Google Scholar]
- Gross GM, Mellin J, Silvia PJ, Barrantes-Vidal N, Kwapil TR. Comparing the factor structure of the Wisconsin Schizotypy Scales and the Schizotypal Personality Questionnaire. Personality Disorders: Theory, Research, and Treatment. 2014;5:397–405. doi: 10.1037/per0000090. [DOI] [PubMed] [Google Scholar]
- Gross GM, Silvia PJ, Barrantes-Vidal N, Kwapil TR. Psychometric properties and validity of short forms of the Wisconsin Schizotypy Scales in two large samples. Schizophrenia Research. 2012;134:267–272. doi: 10.1016/j.schres.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Gustafsson JE. A unifying model for the structure of intellectual abilities. Intelligence. 1984;8:179–203. [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophrenia Bulletin. 2006;32:250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford: Oxford University Press; 2007. pp. 227–249. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Engle RW. Focusing the spotlight: Individual differences in visual attention control. Journal of Experimental Psychology: General. 2007;136:217–240. doi: 10.1037/0096-3445.136.2.217. [DOI] [PubMed] [Google Scholar]
- Höfer I, Della Casa V, Feldon J. The interaction between schizotypy and latent inhibition: Modulation by experimental parameters. Personality and Individual Differences. 1999;26:1075–1088. [Google Scholar]
- Horn JL. Organization of abilities and the development of intelligence. Psychological Review. 1968;75:242–259. doi: 10.1037/h0025662. [DOI] [PubMed] [Google Scholar]
- Horton LE, Barrantes-Vidal N, Silvia PJ, Kwapil TR. Worries about being judged versus being harmed: Disentangling the association of social anxiety and paranoia with schizotypy. PLoS ONE. 2014;9:e96269. doi: 10.1371/journal.pone.0096269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski J, Barrantes-Vidal N, Kwapil TR. Neurological soft signs in psychometrically identified schizotypy. Schizophrenia Research. 2009;115:293–302. doi: 10.1016/j.schres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LE, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Hambrick DZ, Engle RW. Variation in working memory as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. New York: Oxford University Press; 2007. pp. 21–48. [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kane MJ, McVay JC. What mind wandering reveals about executive-control abilities and failures. Current Directions in Psychological Science. 2012;21:348–354. [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis – Linking biology, pharmacology and phenomenology of psychosis. Schizophrenia Research. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Positive schizotypy and emotion processing. Journal of Abnormal Psychology. 2005;114:392–401. doi: 10.1037/0021-843X.114.3.392. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Schizotypy facets, cognitive control, and emotion. Journal of Abnormal Psychology. 2006;115:418–427. doi: 10.1037/0021-843X.115.3.418. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Becker TM. Communication disturbances, working memory, and emotion in people with elevated disorganized schizotypy. Schizophrenia Research. 2008;100:172–180. doi: 10.1016/j.schres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keye D, Wilhelm O, Oberauer K, van Ravenszwaaij D. Individual differences in conflict-monitoring: Testing means and covariance hypothesis about the Simon and the Eriksen flanker task. Psychological Research. 2009;73:762–776. doi: 10.1007/s00426-008-0188-9. [DOI] [PubMed] [Google Scholar]
- Keye D, Wilhelm O, Oberauer K, Stürmer B. Individual differences in response conflict adaptations. Frontiers in Psychology. 2013;4:947. doi: 10.3389/fpsyg.2013.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E. Thought flow: Properties and mechanisms underlying shifts in content. In: Singer JA, Salovey P, editors. At play in the fields of consciousness: Essays in honor of Jerome L. Singer. Mahwah, NJ: Erlbaum; 1999. pp. 29–50. [Google Scholar]
- Klinger E. Goal commitments and the content of thoughts and dreams: Basic principles. Frontiers in Psychology. 2013;4(415) doi: 10.3389/fpsyg.2013.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: Cognitive basis for stimulus-response compatibility – A model and taxonomy. Psychological Review. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Koychev I, El-Deredy W, Haenschel C, Deakin JFW. Visual information processing deficits as biomarkers of vulnerability to schizophrenia: An event-related potential study in schizotypy. Neuropsychologia. 2010;48:2205–2214. doi: 10.1016/j.neuropsychologia.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Koychev I, McMullen K, Lees J, Dadhiwala R, Grayson L, Perry C, Schmechtig A, Walters J, Craig KJ, Dawson GR, Dourish CT, Ettinger U, Wilkinson L, Williams S, Deakin JFW, Barkus E. A validation of cognitive biomarkers for the early identification of cognitive enhancing agents in schizotypy: A three-center double-blind placebo-controlled study. European Neuropsychopharmacology. 2012;22:469–481. doi: 10.1016/j.euroneuro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Krawietz SA, Tamplin AK, Radvansky GA. Aging and mind wandering during text comprehension. Psychology and Aging. 2012;27:951–958. doi: 10.1037/a0028831. [DOI] [PubMed] [Google Scholar]
- Kreher DA, Holcomb PJ, Goff D, Kuperberg GR. Neural evidence for faster and further automatic spreading activation in schizophrenic thought disorder. Schizophrenia Bulletin. 2008;34:473–482. doi: 10.1093/schbul/sbm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. In: Stanford C, Tannock R, editors. Current Topics in Behavioral Neurosciences. Vol. 9. 2012. pp. 67–91. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. Journal of Abnormal Psychology. 1998;107:558–565. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N. Schizotypy: Looking back and moving forward. Schizophrenia Bulletin. 2015;41(supplement 2):S366–S373. doi: 10.1093/schbul/sbu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin Schizotypy Scales: Factor identification and construct validity. Schizophrenia Bulletin. 2008;34:444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Brown LH, Silvia PJ, Myin-Germeys I, Barrantes-Vidal N. The expression of positive and negative schizotypy in daily life: An experience sampling study. Psychological Medicine. 2012;42:2555–2566. doi: 10.1017/S0033291712000827. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Chun CA. The psychometric assessment of schizotypy. In: Mason O, Claridge G, editors. Schizotypy: New Dimensions. London: Routledge; 2015. pp. 7–32. [Google Scholar]
- Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. Journal of Abnormal Psychology. 2013;122:807–815. doi: 10.1037/a0033759. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Silvia PJ, Myin-Germeys I, Anderson AJ, Coates SA, Brown LH. The social world of the socially anhedonic: Exploring the daily ecology of asociality. Journal of Research in Personality. 2009;43:103–106. [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity? ! Intelligence. 1990;14:389–433. [Google Scholar]
- Larson GE, Alderton DL. Reaction time variability and intelligence: A “worst performance” analysis of individual differences. Intelligence. 1990;14:309–325. [Google Scholar]
- Lee K, Ashton MC. Psychometric properties of the HEXACO personality inventory. Multivariate Behavioral Research. 2004;39:329–358. doi: 10.1207/s15327906mbr3902_8. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Reaction time slowing during high-load, sustained-attention task performance in relation to psychometrically identified schizotypy. Journal of Abnormal Psychology. 2001;110:290–296. doi: 10.1037//0021-843x.110.2.290. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Cornblatt BA, Putnick M. Schizotypy and sustained attention. Journal of Abnormal Psychology. 1991;100:84–89. doi: 10.1037//0021-843x.100.1.84. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Gold JM. Auditory working memory and verbal recall memory in schizotypy. Schizophrenia Research. 2000;42:101–110. doi: 10.1016/s0920-9964(99)00121-8. [DOI] [PubMed] [Google Scholar]
- Levinson DB, Smallwood J, Davidson RJ. The persistence of thought: Evidence for a role of working memory in the maintenance of task-unrelated thinking. Psychological Science. 2012;23:375–380. doi: 10.1177/0956797611431465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowsky S, Oberauer K, Yang LX, Ecker UKH. A working memory test battery for MATLAB. Behavior Research Methods. 2010;42:571–585. doi: 10.3758/BRM.42.2.571. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lipp OV, Siddle DAT, Arnold SL. Psychosis proneness in a non-clinical sample II: A multi-experimental study of “attentional malfunctioning. Personality and Individual Differences. 1994;17:405–424. [Google Scholar]
- Little TD, Cunningham WA, Shahar G, Widaman KF. To parcel or not to parcel: Exploring the question, weighing the merits. Structural Equation Modeling. 2002;9:151–173. [Google Scholar]
- Livesley WJ, Jackson DN. Dimensional Assessment of Personality Pathology—Basic Questionnaire (DAPP-BQ): Technical Manual. Port Huron, MI: Sigma Assessment Systems, Inc; 2009. [Google Scholar]
- MacDonald SWS, Nyberg L, Bäckman L. Intraindividual variability in behavior: Links to brain structure, neurotransmission, and neuronal activity. Trends in Neurosciences. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Martin EA, Kerns JG. Social anhedonia associated with poor evaluative processing but not with poor cognitive control. Psychiatry Research. 2010;178:419–424. doi: 10.1016/j.psychres.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Matheson S, Langdon R. Schizotypal traits impact upon executive working memory and aspects of IQ. Psychiatry Research. 2008;159:207–214. doi: 10.1016/j.psychres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Matthews G, Coyle K, Craig A. Multiple factors of cognitive failure and their relationships with stress vulnerability. Journal of Psychopathology and Behavioral Assessment. 1990;12:49–65. [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: A safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophrenia Bulletin. 2003;29:771–790. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing data: A gentle introduction. New York, NY: Guilford; 2007. [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2005) and Watkins (2008) Psychological Bulletin. 2010;136:188–197. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Drifting from slow to “D’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012a;38:525–549. doi: 10.1037/a0025896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General. 2012b;141:302–320. doi: 10.1037/a0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Meier ME, Touron DR, Kane MJ. Aging ebbs the flow of thought: Adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica. 2013;142:136–147. doi: 10.1016/j.actpsy.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. Journal of Personality Disorders. 1990;4:1–99. [Google Scholar]
- Meier ME, Kane MJ. Carving executive control at its joints: Working memory capacity predicts stimulus-stimulus, but not stimulus-response, conflict. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015 doi: 10.1037/xlm0000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers TC, Raulin ML. Cognitive Slippage Scale. In: Corcoran K, Fischer J, editors. Measures for clinical practice: A sourcebook. New York: Free Press; 1987. pp. 125–127. [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Modinos G, Mechelli A, Ormel J, Groenewold NA, Aleman A, McGuire PK. Schizotypy and brain structure: A voxel-based morphometry study. Psychological Medicine. 2010;40:1423–1431. doi: 10.1017/S0033291709991875. [DOI] [PubMed] [Google Scholar]
- Moritz S, Andresen B, Naber D, Krausz &, Probsthein E. Neuropsychological correlates of schizotypal disorganisation. Cognitive Neuropsychiatry. 1999;4:343–349. [Google Scholar]
- Moritz S, Woodward TS, Küppers D, Lausen A, Schickel M. Increased automatic spreading activation in thought-disordered schizophrenic patients. Schizophrenia Research. 2002;59:181–186. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, Franklin MS, Chin JM, Baird B, Schooler JW. The role of mind-wandering in measurements of general aptitude. Journal of Experimental Psychology: General. 2012;141:788–798. doi: 10.1037/a0027968. [DOI] [PubMed] [Google Scholar]
- Murtha S, Cismaru R, Waechter R, Chertkow H. Increased variability accompanies frontal lobe damage in dementia. Journal of the International Neuropsychological Society. 2002;8:360–372. doi: 10.1017/s1355617702813170. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- O’Driscoll GA, Lenzenweger MF, Holzman PS. Antisaccades and smooth pursuit eye tracking and schizotypy. Archives of General Psychiatry. 1998;55:837–843. doi: 10.1001/archpsyc.55.9.837. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Süß HM, Wilhelm O, Wittmann WW. The multiple faces of working memory: Storage, processing, supervision, and coordination. Intelligence. 2003;31:167–193. [Google Scholar]
- Obiols JE, García-Domingo M, de Trinchería I, Doménech E. Psychometric schizotypy and sustained attention in young adult males. Personality and Individual Differences. 1993;14:381–384. [Google Scholar]
- Otteson JM. Unpublished doctoral dissertation. University of North Texas; Denton, TX: 1995. Attention and information processing variables in hypothetically psychosis-prone collect students. [Google Scholar]
- Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cognitive Psychology. 2013;66:232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Paap KR, Johnson HA, Sawi O. Are bilingual advantages dependent upon specific tasks or specific bilingual experiences? Journal of Cognitive Psychology. 2014;26:615–639. [Google Scholar]
- Paap KR, Sawi O. Bilingual advantages in executive functioning: Problems in convergent validity, discriminant validity, and the identification of theoretical constructs. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00962. article 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palef SR. Judging pictorial and linguistic aspects of space. Memory & Cognition. 1978;6:70–75. doi: 10.3758/bf03197430. [DOI] [PubMed] [Google Scholar]
- Park S, Gooding DC. Working memory impairment as an endophenotypic marker of a schizophrenia diathesis. Schizophrenia Research: Cognition. 2014;1:127–136. doi: 10.1016/j.scog.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, McTigue K. Working memory and the syndromes of schizotypal personality. Schizophrenia Research. 1997;26:213–220. doi: 10.1016/s0920-9964(97)00051-0. [DOI] [PubMed] [Google Scholar]
- Peters ER, Pickering AD, Hemsley DR. ‘Cognitive inhibition’ and positive symptomatology in schizotypy. British Journal of Clinical Psychology. 1994;33:33–48. doi: 10.1111/j.2044-8260.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Peters MJV, Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Confusing action and imagination: Action source monitoring in individual swith schizotypal traits. The Journal of Nervous and Mental Disease. 2007;195:752–757. doi: 10.1097/NMD.0b013e318142cc02. [DOI] [PubMed] [Google Scholar]
- Poole BJ, Kane MJ. Working memory capacity predicts the executive control of visual search among distractors: The influences of sustained and selective attention. Quarterly Journal of Experimental Psychology. 2009;62:1430–1454. doi: 10.1080/17470210802479329. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Osman P, Moore B, Stollery B. There are stable individual differences in performance variability, both from moment to moment and from day to day. Quarterly Journal of Experimental Psychology. 2001;54A:981–1003. doi: 10.1080/713756013. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ-A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia Bulletin. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Randall JG, Oswald FL, Beier ME. Mind-wandering, cognition, and performance: A theory-driven meta-analysis of attention regulation. Psychological Bulletin. 2014;140:1411–1431. doi: 10.1037/a0037428. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Schmiedek F, McKoon G. A diffusion model explanation of the worst performance rule for reaction time and IQ. Intelligence. 2008;36:10–17. doi: 10.1016/j.intell.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D, Goldberg M. Correlating a measure of sustained attention with a multidimensional measure of schizotypal traits. Personality and Individual Differences. 2001;31:421–431. [Google Scholar]
- Redick TS, Broadway JM, Meier ME, Kuriakose PS, Unsworth N, Kane MJ, Engle RW. Measuring working memory capacity with automated complex span tasks. European Journal of Psychological Assessment. 2012;28:164–171. [Google Scholar]
- Rentrop M, Rodewald K, Roth A, Simon J, Walther S, Fiedler P, Weisbrod M, Kaiser S. Intra-individual variability in high-functioning patients with schizophrenia. Psychiatry Research. 2010;178:27–32. doi: 10.1016/j.psychres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. Working memory capacity and suppression. Journal of Memory and Language. 1998;39:418–436. [Google Scholar]
- Rummel J, Boywitt CD. Controlling the stream of thought: Working memory capacity predicts adjustment of mind-wandering to situational demands. Psychonomic Bulletin & Review. 2014;21:1309–1315. doi: 10.3758/s13423-013-0580-3. [DOI] [PubMed] [Google Scholar]
- Sahakyan L, Kwapil TR. Positive and Negative Schizotypy are Associated with Differential Patterns of Episodic Memory Impairment. 2015 doi: 10.1016/j.scog.2016.07.001. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online. 2003;8:23–74. [Google Scholar]
- Schmidt-Hansen M, Honey RC. Working memory and multidimensional schizotypy: Dissociable influences of the different dimensions. Cognitive Neuropsychology. 2009;26:655–670. doi: 10.1080/02643291003644501. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süß HM, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General. 2007;136:414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Schweizer K, Moosbrugger H. Attention and working memory as predictors of intelligence. Intelligence. 2004;32:329–347. [Google Scholar]
- Seli P, Cheyne JA, Smilek D. Wandering minds and wavering rhythms: Linking mind wandering and behavioral variability. Journal of Experimental Psychology: Human Perception and Performance. 2013;39:1–15. doi: 10.1037/a0030954. [DOI] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Xu M, Purdon C, Smilek D. Motivation, intentionality, and mind wandering: Implications for assessments of task-unrelated thought. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2015;41:1417–1425. doi: 10.1037/xlm0000116. [DOI] [PubMed] [Google Scholar]
- Shin DJ, Lee TY, Jung WH, Kim SN, Jang JH, Kwon JS. Away from home: The brain of the wandering mind as a model for schizophrenia. Schizophrenia Research. 2015;165:83–89. doi: 10.1016/j.schres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Shin YS, Kim SN, Shin NY, Jun WH, Hur JW, Byun MS, Jan JH, An SK, Known JS. Increased intra-individual variability of cognitive processing in subjects at risk mental state and schizophrenia patients. PLoS ONE. 2013;8:e78354. doi: 10.1371/journal.pone.0078354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Harrison TL, Engle RW. Working memory capacity and the scope and control of attention. Attention, Perception & Psychophysics. 2015;77:1863–1880. doi: 10.3758/s13414-015-0899-0. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Lindsey DR, Marshall RL, Engle RW. The mechanisms of working memory capacity: Primary memory, secondary memory, and attention control. Journal of Memory and Language. 2014;72:116–141. [Google Scholar]
- Shute VJ. Who is likely to acquire programming skills? Journal of Educational Computing Research. 1991;7:1–24. [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. A 21 word solution. Dialogue: The Official Newsletter of the Society for Personality and Social Psychology. 2012;26:4–7. [Google Scholar]
- Singer JL. Daydreaming: An introduction to the experimental study of inner experience. New York: Random House; 1966. [Google Scholar]
- Smallwood J. Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychological Bulletin. 2013;139:519–535. doi: 10.1037/a0030010. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Andrews-Hanna J. Not all minds that wander are lost: The importance of a balanced perspective on the mind-wandering state. Frontiers in Psychology. 2013;4:441. doi: 10.3389/fpsyg.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Fitzgerald A, Miles LK, Phillips LH. Shifting moods, wandering minds: Negative moods lead the mind to wander. Emotion. 2009;9:271–276. doi: 10.1037/a0014855. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smeekens BA, Kane MJ. Working memory capacity, mind wandering, and creative cognition: An individual-differences investigation into the benefits of controlled versus spontaneous thought. Psychology of Aesthetics, Creativity, and the Arts. doi: 10.1037/aca0000046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Bolinskey PK, Guidi JP, Myers KR, Schuder KM, James AV, Hudak DV, Sheets V. Further examination of the factor structure of the Chapman Psychosis Proneness Scales (CPPS) Psychiatry Research. 2016;238:257–263. doi: 10.1016/j.psychres.2016.02.057. [DOI] [PubMed] [Google Scholar]
- Smith NT, Lenzenweger MF. Increased stress responsivity in schizotypy leads to diminished spatial working memory performance. Personality Disorders: Theory, Research, and Treatment. 2013;4:324–331. doi: 10.1037/per0000014. [DOI] [PubMed] [Google Scholar]
- Smyrnis N, Avramopoulos D, Evdokimidis I, Stefanis CN, Tsekou H, Stefanis NC. Effect of schizotypy on cognitive performance and its tuning by COMPT val158 MET genotype variations in a large population of young men. Biological Psychiatry. 2007;61:845–853. doi: 10.1016/j.biopsych.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Spaulding W, Garbin CP, Dras SR. Cognitive abnormalities in schizophrenic patients and schizotypal college students. The Journal of Nervous and Mental Disease. 1989;177:717–728. doi: 10.1097/00005053-198912000-00002. [DOI] [PubMed] [Google Scholar]
- Steel C, Hemsley DR, Jones S. ‘Cognitive inhibition’ and schizotypy as measured by the Oxford-Liverpool inventory of feelings and experiences. Personality and Individual Differences. 1996;20:769–773. [Google Scholar]
- Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, Verdoux H, Van Os J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychological Medicine. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Styles EA, Allport DA. Perceptual integration of identity, location, and colour. Psychological Research. 1986;48:189–200. doi: 10.1007/BF00309083. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA, Braff DL. “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biological Psychiatry. 1995;37:286–299. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- Tallent KA, Gooding DC. Working memory and Wisconsin Card Sorting Test performance in schizotypic individuals: A replication and extension. Psychiatry Research. 1999;89:161–170. doi: 10.1016/s0165-1781(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW, Jr, Epstein JN. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo KE. Unpublished doctoral dissertation. University of Maryland; College Park, MD: 2004. Neuropsychological characteristics of putative schizotypes: A comparative study. [Google Scholar]
- Thomson DR, Besner D, Smilek D. A resource-control account of sustained attention: Evidence form mind-wandering and vigilance paradigms. Perspectives on Psychological Science. 2015;10:82–96. doi: 10.1177/1745691614556681. [DOI] [PubMed] [Google Scholar]
- Unsworth N. Consistency of attentional control as an important cognitive trait: A latent-variable analysis. Intelligence. 2015;49:110–128. [Google Scholar]
- Unsworth N, Brewer GA, Spillers GJ. Variation in cognitive failures: An individual differences investigation of everyday attention and memory failures. Journal of Memory and Language. 2012;67:1–16. doi: 10.1037/a0028075. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognitive Psychology. 2014;71:1–26. doi: 10.1016/j.cogpsych.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Heitz RC, Schrock JC, Engle RW. An automated version of the operation span task. Behavior Research Methods. 2005;37:498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD. Mind wandering and reading comprehension: Examining the roles of working memory capacity, interest, motivation, and topic experience. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2013;39:832–842. doi: 10.1037/a0029669. [DOI] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD. Similarities and differences between mind-wandering and external distraction: A latent variable analysis of lapses of attention and their relation to cognitive abilities. Acta Psychologica. 2014;150:14–25. doi: 10.1016/j.actpsy.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Miller JD, Lakey CE, Young DL, Meeks JT, Campbell WK, Goodie AS. Exploring the relations among executive functions, fluid intelligence, and personality. Journal of Individual Differences. 2009;30:194–200. [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway J, Engle RW. Complex working memory span tasks and higher-order cognition: A latent variable analysis of the relationship between processing and storage. Memory. 2009;17:635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Lakey CE, Young DL. Lapses in sustained attention and their relation to executive control and fluid abilities: An individual differences investigation. Intelligence. 2010;38:111–122. [Google Scholar]
- Unsworth N, Redick TS, Spillers GJ, Brewer GA. Variation in working memory capacity and cognitive control: Goal maintenance and micoradjustments of control. The Quarterly Journal of Experimental Psychology. 2012;65:326–355. doi: 10.1080/17470218.2011.597865. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers G. Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. Journal of Memory and Language. 2010;62:392–406. [Google Scholar]
- Unsworth N, Spillers GJ, Brewer GA. Examining the relations among working memory capacity, attention control, and fluid intelligence from a dual-component framework. Psychology Science Quarterly. 2009;51:388–402. [Google Scholar]
- Vinogradov S, Poole JH, Willis-Shore J, Ober BA, Shenaut GK. Slower and more variable reaction times in schizophrenia. What do they signify? Schizophrenia Research. 1998;32:183–190. doi: 10.1016/s0920-9964(98)00043-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan RCK, Yu X, Shi C, Cui J, Deng Y. Prospective memory deficits in subjects with schizophrenia spectrum disorders: A comparison study with schizophrenic subjects, psychometrically defined schizotypal subjects, and healthy controls. Schizophrenia Research. 2008;106:70–80. doi: 10.1016/j.schres.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Wilhelm O, Witthöft M, Schipolowski S. Self-reported cognitive failures: Competing measurement models and self-report correlates. Journal of Individual Differences. 2010;31:1–14. [Google Scholar]
- Winterstein BP, Silvia PJ, Kwapil TR, Kaufman JC, Reiter-Palmon R, Wigert B. Brief assessment of schizotypy: Developing short forms of the Wisconsin Schizotypy Scales. Personality and Individual Differences. 2011;51:920–924. [Google Scholar]
- Yon V, Loas G, Monestès JL. Relationships between schizotypy and subjective experiences in a sample of 399 university students. Comparative Psychiatry. 2009;50:142–150. doi: 10.1016/j.comppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis: A step towards indicated prevention of schizophrenia. The British Journal of Psychiatry. 1998;172(sup 33):14–20. [PubMed] [Google Scholar]
- Zalewski C, Johns0n-Selfridge MT, Ohriner S, Zarrella K, Seltzer JC. A review of neuropsychological differences between paranoid and nonparanoid schizophrenia patients. Schizophrenia Bulletin. 1998;24:127–145. doi: 10.1093/oxfordjournals.schbul.a033305. [DOI] [PubMed] [Google Scholar]
- Zhiyan T, Singer JL. Daydreaming styles, emotionality and the big five personality dimensions. Imagination, Cognition and Personality. 1996;16:399–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.