Abstract
Introduction
Escherichia coli O157:H7, an important foodborne pathogen, can cause serious renal damage, which can also lead to mortality. Since a rapid and sensitive method is needed to identify this pathogenic agent, we evaluated Loop-Mediated Isothermal Amplification Assay (LAMP) to detect Escherichia coli O157:H7.
Methods
We used six primers that specifically identified the rfbE gene. To examine the sensitivity of the method, different dilutions were subjected to the LAMP reaction. Other bacterial strains also were investigated to determine the specificity of the test. The turbidity of the amplified products was assayed by visual detection. The amplified products were detected by addition of SYBR Green II to the reaction tubes.
Results
Amplification products were observed as a ladder-like pattern on the agarose gel. A white turbidity emerged in the positive tubes. Under UV light, the positive samples were green, whereas the negative samples were orange. The detection limit of the LAMP was 78 pg/tube, and this indicated that it was 100 times more sensitive than PCR for the detection of EHEC. No LAMP products were detected when template DNA of non-EHEC strains were used, suggesting high specificity of the LAMP assay.
Conclusion
The results indicated that the LAMP assay is a valuable diagnostic assay to identify EHEC O157:H7. In addition, the simplicity, sensitivity, specificity, and rapidity of this assay make it a useful method to diagnose pathogens in primary labs without any need for expensive equipment or specialized techniques.
Keywords: Enterohemorrhagic Escherichia coli O157:H7, rfbE gene, Loop-Mediated Isothermal Amplification, SYBR Green II
1. Introduction
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 initially was identified as an important human pathogen in the United States, and this pathogen recently has become the most prevalent type of enterohemorrhagic E. coli (EHEC). EHEC is a cause of infection worldwide in both human and animals (1–7). E. coli O157:H7 is a food-borne pathogen and a significant gastrointestinal agent, and it can cause serious enteric diseases in people, with the complications of the infection potentially leading to renal damage and death, particularly in young children and the elderly (8–11). E. coli O157:H7 has been shown to be one of the most frequently isolated bacterial pathogens from meat and fresh products after Campylobacter, Salmonella, and Shigella spp. (12). Diarrhea and hemorrhagic colitis are characteristic clinical symptoms of E. coli O157:H7 infections, and their progression may cause life-threatening hemolytic uremic syndrome (HUS) and lead to serious renal damage and even death (13–16). The universal threats of this organism for public health are demonstrated by the high morbidity and mortality rate of diseases due to EHEC O157:H7in several recent large-scale epidemics (17). E. coli O157:H7 has a very low infection threshold, such that ingestion of 10 microorganisms may be sufficient to cause severe gastrointestinal disease (18). The above points indicate that the development of quick, specific, and sensitive assays to detect EHEC O157:H7 is now a major global concern (19). Conventional culture methods based on biochemical features are tiresome and typically require up to 72 h; therefore, the development of rapid techniques for detection based on immunological and genetic targets has become the focus of attention (20). Immunological methods recently have been introduced for detecting Shiga toxins. Nevertheless, such methods have failed to differentiate between O157:H7 and other less-virulent EHEC, and several Shiga toxins may not be detected at all (14). The techniques based on the detection of O157 somatic and H7 flagellar antigens also are inadequate due to their lack of specificity (8). Enzyme immunoassay, a rapid detection method, has shown a high requirement for the population of pathogens (21). However, other methods, such as immuno-magnetic beads and colloidal gold immunochromatography assay, have shortcomings in reproducibility despite their ease and rapidness (22–26). Molecular methods, such as PCR and real-time PCR assay, have been used during the past decade to detect food-borne pathogens (such as E. coli O157:H7) by amplifying a number of relevant genes. Nonetheless, the need for trained staff, operating space, equipment, and reagents has impeded its usefulness (27, 28). Hence, there has been an increasing demand for simple and cost-effective molecular tests. Loop-mediated Isothermal Amplification (LAMP), a novel nucleic acid amplification method that relies on an auto-cycling strand displacement DNA synthesis, is performed by Bst DNA polymerase (29–31). Four or six primers that identify six or eight distinct regions are used in this method. It is operated under a constant temperature (60 to 65 °C), and it eliminates the need for specialized thermal cycler equipment (32). This technique was first used to detect bacteria, and it is being used increasingly for the rapid detection of other bacteria, such as E. coli (33–36). An important advantage of LAMP is its ability to amplify specific sequences of DNA under isothermal conditions. In addition, a positive reaction with LAMP easily can be investigated by the naked eye, without the need for electrophoresis (37). One of the most tempting features of LAMP is the fact that results can be observed by addition of SYBR Green II. Thus, it can be appropriate in primary clinics or field labs. The purpose of this study was to establish a rapid, sensitive, specific, and easy method to detect Enterohemorrhagic E. coli O157:H7 using a visualized detection system with SYBR Green II.
2. Material and Methods
2.1. Research design and setting
The standard strains used in this research included E. coli 0157:H7 (ATCC 12900), Shigella dysentriae, Shigella flexneri (ATCC 12022), Shigella sonnei ATCC 9290 (NCTC 12698), Shigella boydii, Salmonella typhi, Salmonella enteritidis, Salmonella infantis, Salmonella murium, Vibrio cholera (ATCC 14035), and Staphylococcus aureus. The bacteria were cultured overnight in Luria-Bertani broth with constant shaking (180 rpm) at 37 °C and then prepared for DNA extraction. These strains were tested in the LAMP and PCR assays. Bacterial DNA belonging to standard strains was extracted using standard purification methods. For gram negative bacteria, genomic DNA was extracted from cultivated strains using the phenol-chloroform method as previously published (38), and DNA extraction was performed for gram positive bacteria according to the procedures published earlier (39). The DNA extracted from the strains was dissolved in TE buffer and quantified using a spectrophotometer at a wavelength of 260 nm after which it was used as a template for LAMP and PCR reactions.
2.2. Primers
The E. coli-specific rfbE gene, one of the major virulent genes in E. coli O157:H7, coding O-antigen synthesis gene, was selected to distinguish between strains. The rfbE gene is specific to the O157:H7 serotype, and it is particularly used because all strains expressing this antigen are associated with severe clinical symptoms (40–42). A basic set of four primers is needed, including FIP (Forward Inner Primer), BIP (Backward Inner Primer), F3 (Forward Outer Primer), and B3 (Backward Outer Primer) to identify six distinct regions on the target DNA. To accelerate the LAMP reaction, two additional primers, i.e., LF (Loop Forward Primer) and LB (Loop Backward Primer), also were used. FIP consisted of the complementary sequence of F1, a T-T-T-T linker, and F2. BIP consisted of B1c, a T-T-T-T linker, and the complementary sequence of B2c. Primers F3 and B3 were located outside of the F2 and B2 regions, while loop primers LF and LB were located between F2 and F1 and between B1 and B2, respectively. The primers used in this study were selected from the literature (43). Primers F3 and B3 also were used in the PCR reactions as forward and backward primers.
2.3. LAMP amplification and detection of products
LAMP was performed in a 25-μl total reaction mixture containing the inner primers FIP and BIP at 0.8 mM, the outer primers F3 and B3 at 0.2 mM, loop primers LF and LB at 0.8 mM, 2.5 μl of 10X ThermoPol Reaction Buffer, each deoxynucleotide at 5.6 mM, 0.42 mM MgSO4, 0.5 M Beanie, 4 U of large fragment Bst DNA polymerase (New England Biolab), and 1 μl of template DNA. The mixture was incubated at 63 °C for 30 min and then heated at 80 °C for 2 min in order to terminate the reaction. LAMP products were subjected to electrophoresis on a 2% agarose gel, stained by ethidium bromide, and then examined under UV light. Visual detection based on turbidity of the by-product of DNA amplification was performed in real time. In addition, a positive amplification was indicated by a color change after adding 1 μl of SYBR Green II to the reaction mixture and observing under UV light. To confirm the accuracy of the structures, the amplified products were analyzed by sequencing.
2.4. PCR reaction and detection of products
PCR amplification was conducted in a 25-μl reaction mixture that contained two forward and backward outer primers, F3 and B3. The thermal profile for PCR was initiated at 95 °C for 3 min, followed by 30 cycles at 95 °C for 1 min, 54 °C for 1 min, and 72 °C for 1 min, with a final extension cycle at 72 °C for 5 min. The amplified products were analyzed by gel electrophoresis in 2% agarose gels, stained with ethidium bromide, and then observed under UV light.
2.5. Confirmation of PCR products
To confirm the accuracy of the amplicon fragments, the amplified products were digested with the appropriate restriction enzyme (Eco47I, Fermentase co.) in 20 μl of reaction mixture and then electrophoresed on 1% agarose gel. Restricted products were stained with ethidium bromide and observed under UV light.
2.6. Optimization of LAMP assay
The initial optimization of the assay was conducted using a set of four basic primers, and optimal concentrations were determined. The reaction mixture was incubated at temperatures ranging from60 to 65 °C. The optimal temperature was found to be 63 °C for 45 min. We tried two additional loop primers with optimized values in order to accelerate the reaction and reduce the time of amplification to 20 min. Values and concentrations of other components of the reaction were optimized.
3. Results
Amplification products were observed as a ladder-like pattern on the agarose gel (Figure 1, lane 2).
Figure 1.
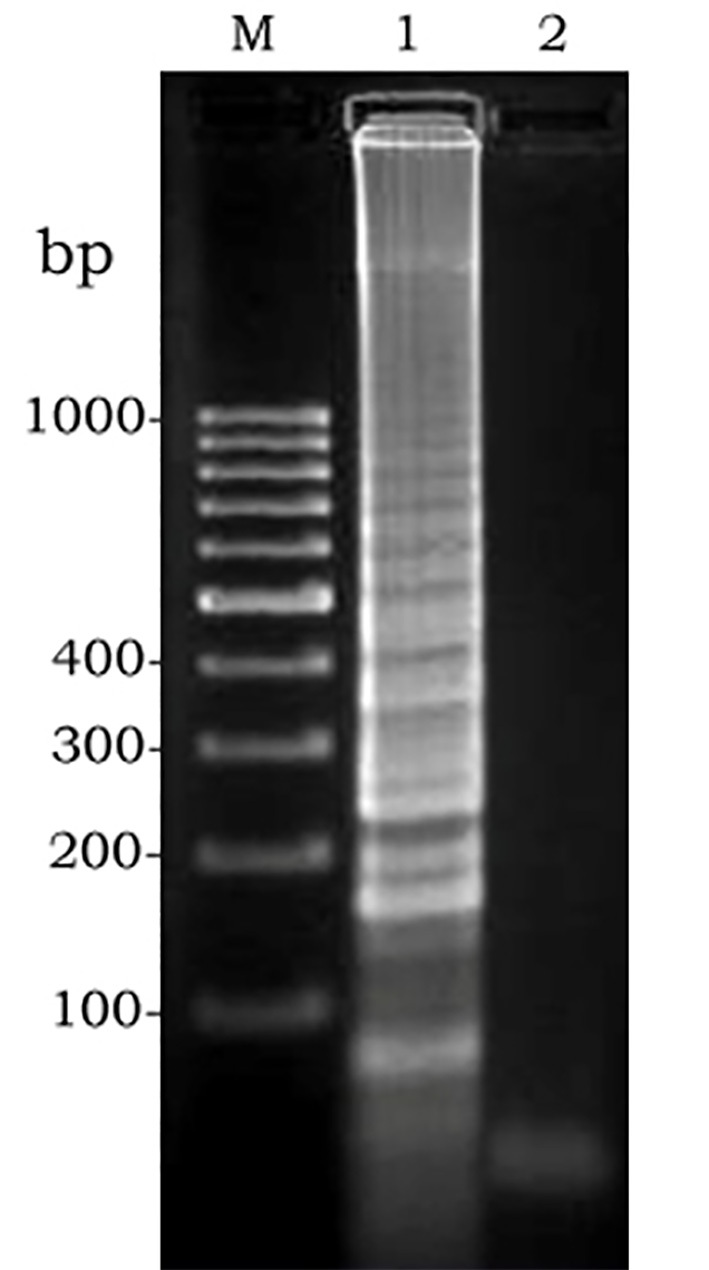
Gel electrophoresis of amplified LAMP products: M, molecular weight. 1, positive control. 2, negative control
Since amplified LAMP reaction mixtures contained magnesium pyrophosphate (as a by-product), a white turbidity, which could be observed by the naked eye, emerged in the positive tubes. There was no turbidity in the negative tubes (Figure 2). Naked-eye detection was performed by SYBR Green II that was added to the reaction mixture, and the color change was observed. The positive samples appeared to be green under UV light, whereas the negative samples were orange (Figure 3). Confirmation of amplified LAMP products was conducted by sequencing. Classic PCR showed a 193 bp amplicon under UV light (Figure 4). Enzymatic digestion of this amplicon produced two 119 bp and 74 bp bands on gel, indicating the accuracy of the amplification (Figure 5). The specificity of the rfbE gene primers was initially proved using BLASTN (www.ncbi.nlm.nih.gov). To evaluate the specificity of the LAMP assay for detecting EHEC, thereafter, template DNA of EHEC and various non-EHEC bacteria were applied to the LAMP reactions. No LAMP products were detected when template DNA of non-EHEC strains was used, suggesting high specificity of the LAMP assay. The result of the specificity test of the LAMP assay is shown in Figure 6. To evaluate the detection limit of the LAMP assay for the detection of EHEC, 10-fold serial dilutions of a known concentration of extracted DNA were applied, and the same DNA template DNA was compared to PCR product. The reaction mixtures with more than 78 pg/tube of template DNA showed a ladder-like pattern on the gel (Figure 7). Thus, the detection limit of the LAMP assay was determined to be 78 pg. However, the PCR assay amplified the expected fragment in the concentrations that exceeded 7.8 ng/tube of DNA (Figure 8). Under the conditions established in this study, the comparative sensitivity of LAMP and PCR indicated that LAMP is 100-fold more sensitive than PCR for the detection of EHEC.
Figure 2.
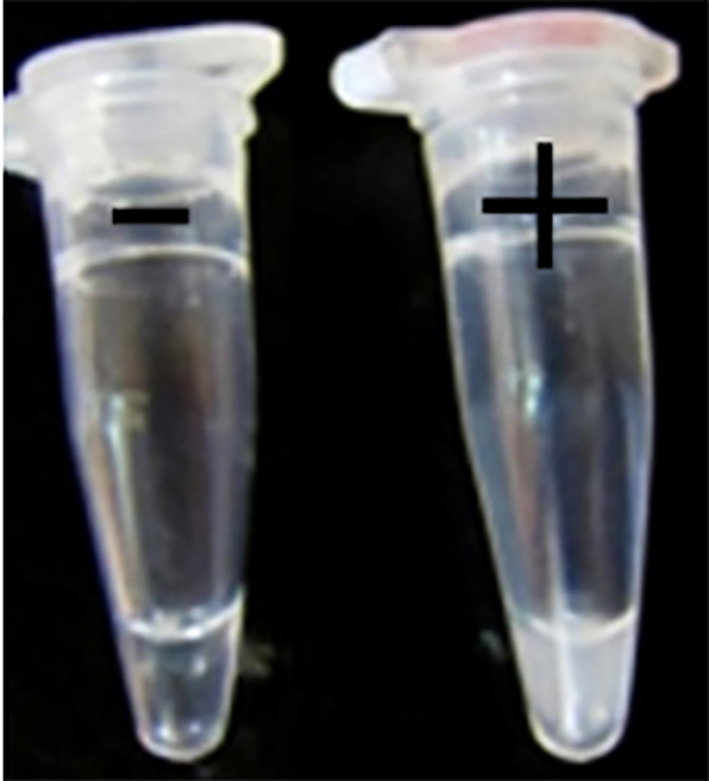
White turbidity of LAMP reaction. Left: negative control; right: positive control
Figure 3.
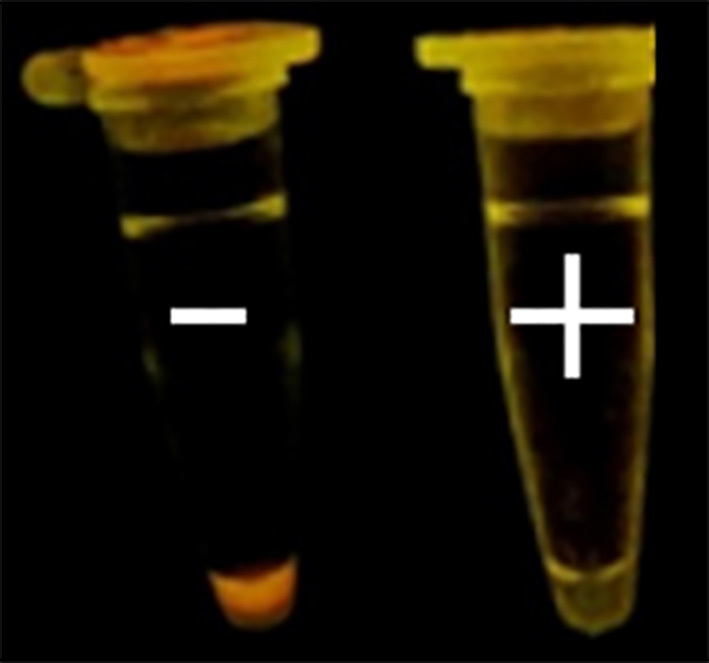
Detection system based on SYBR Green II. Left: negative control; right: positive control
Figure 4.
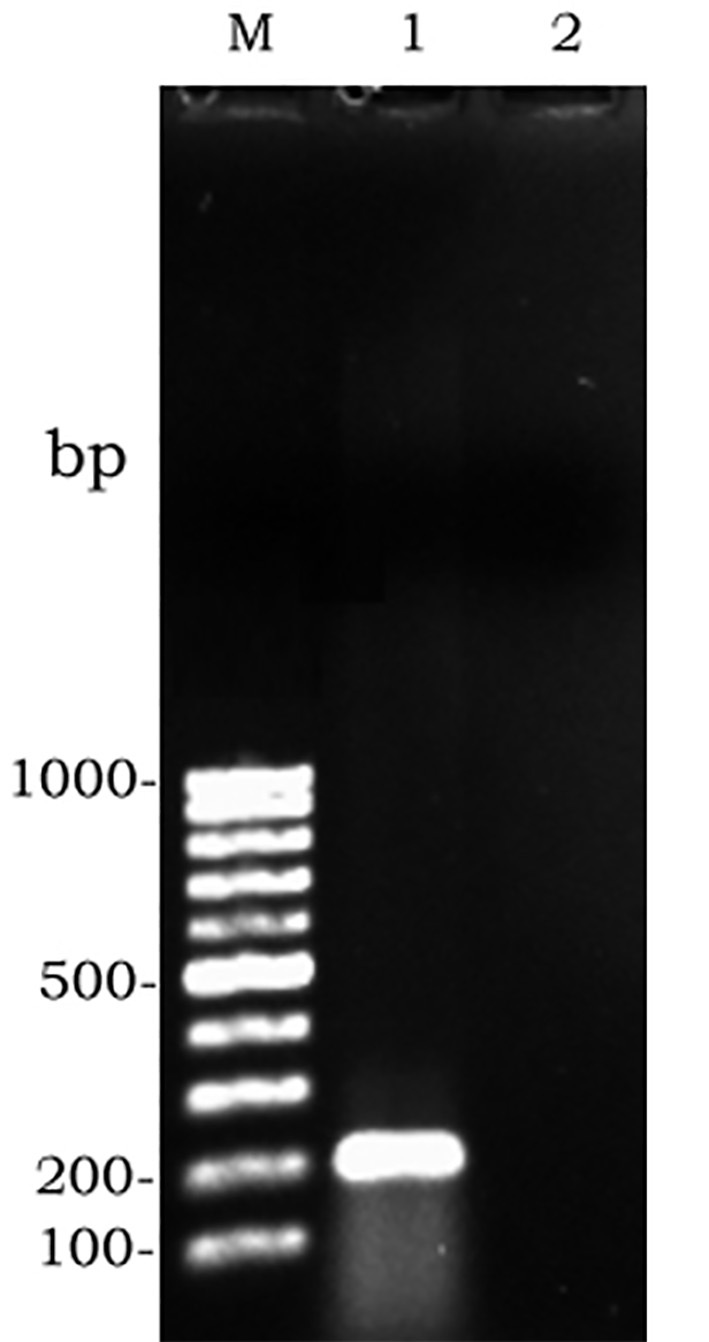
Gel electrophoresis of amplified PCR products: M, molecular weight. 1, positive control. 2, negative control
Figure 5.
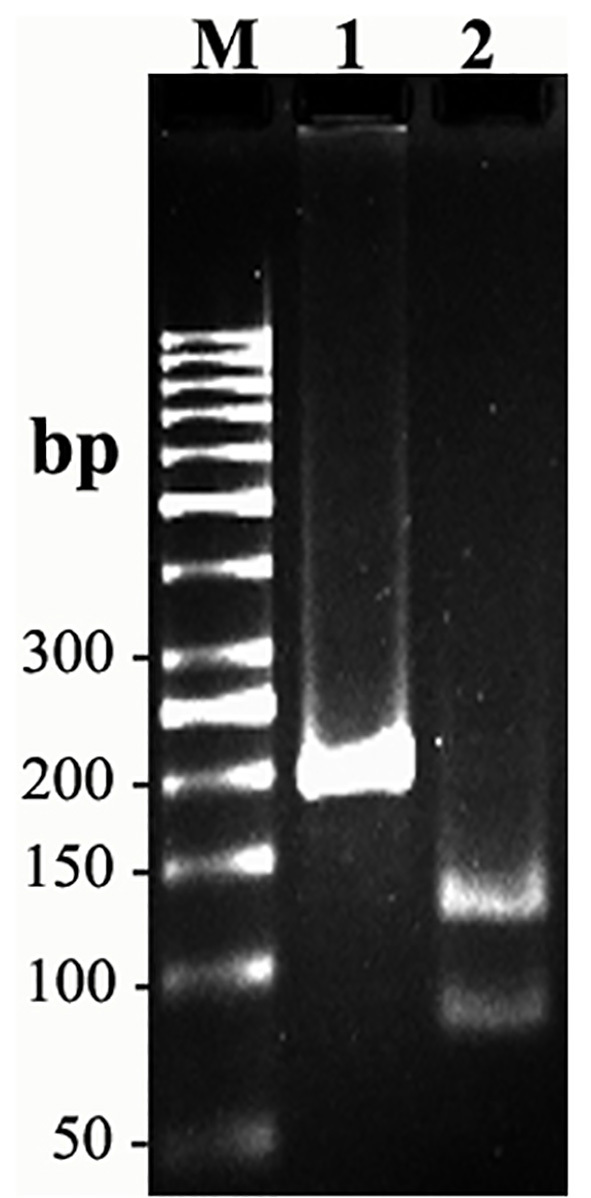
Enzymatic digestion: M, molecular weight. 1, PCR fragment. 2, digested fragments
Figure 6.
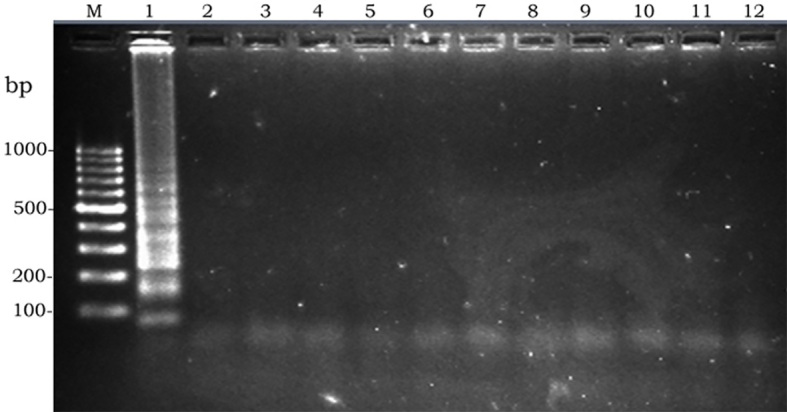
Specificity of LAMP; left to write: M, molecular weight. 1, Escherichia coli 0157:H7. 2, Shigella dysentriae. 3, Shigella flexneri. 4, Shigella sonnei. 5, Shigella boydii. 6, Salmonella typhi. 7, Salmonella enteritidis. 8, Salmonella infantis. 9. Salmonella murium. 10, Vibrio cholera. 11, Staphylococcus aureus. 12, Negative control
Figure 7.
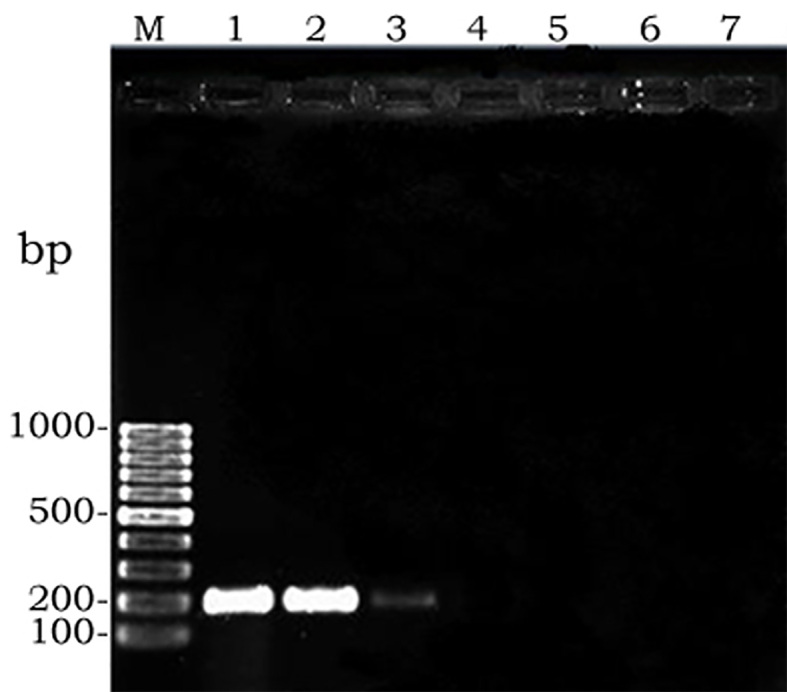
Sensitivity of PCR; left to right: M, molecular weight. 1, 10-1. 2, 10-2. 3, 10-3. 4, 10-4. 5, 10-5. 6, 10-6. 7, Negative Control
Figure 8.

Sensitivity of LAMP; left to right: M, molecular weight. 1, 10-1. 2, 10-2. 3, 10-3. 4, 10-4. 5, 10-5. 6, 10-6. 7, Negative Control
4. Discussion
It is very important to keep E. coli O157:H7 under control in related infections, so effective detection strategies must be evaluated and used. During different monitoring procedures against EHEC O157:H7 infection, various methods have been utilized to detect this pathogen, including culturing and PCR-based amplification methods (44). These techniques are specific and accurate, but most of them require extensive technical expertise, and they are time-consuming (45). PCR has not been generally established in clinical laboratories because it is complex and requires a high-precision thermal cycler (46). Therefore, an easy and inexpensive diagnostic method is needed, especially in developing countries with insufficient facilities and health resources. Thus, the application of LAMP is rapidly expanding and currently covers different areas, including infectious diseases. In the present study, we focused on an advantageous visual-based LAMP method to detect EHEC O157:H7 because of its simplicity, rapidity, and ease to detect, which can appropriately meet the demands in primary clinical settings in developing countries or for field use. In the LAMP assay, positive reactions are indicated by the turbidity of the reaction solution, but, recently, some new detection systems using DNA intercalating dyes have been developed (32, 47). This is the first report to describe the application of SYBR Green II-based detection system in the LAMP assay to detect EHEC O157:H7. In this simple and helpful LAMP assay, the color of positive samples is supposed to change to green. In previous studies, other dyes were applied for better visibility of the reaction result, such as propidium iodide or Picogreen (32, 48, 49). In several other developed colorimetric LAMP assays fluorescence calcein, Hydroxy Naphthol Blue, and Gene Finder were used as well (50–52). In the current study, we evaluated the LAMP and PCR assays for detection of EHEC O157:H7. The results showed that, under the conditions established in this study, the sensitivity of LAMP and PCR assay was 78 pg and 7.8 ng per test tube, respectively. The sensitivity of the LAMP process was demonstrated to be 100 times higher than that of PCR, which is due to higher speed and greater yield achieved in LAMP as the result of target gene amplification compared to other assays (53). Our results were not consistent with previous reports with a detection limit of 100 fg and 10 pg in LAMP and PCR assays targeting rfbE gene, respectively, indicating that LAMP was at least 10 times more sensitive than PCR (43). Moreover, strains of non-EHEC O157:H7 were not detected by the LAMP assay. Based on these results, LAMP had high specificity, which was concluded because the target sequence was recognized by six independent sequences in the initial stage and by four independent sequences during the later stages of LAMP reaction. When the reaction was performed in the absence of each of four primers, no amplification was seen. This demonstrated a strict requirement for recognition of six distinct sequences in the target DNA (29). We could detect EHEC O157:H7 in less than 30 min using the LAMP method with loop primers, which was similarly mentioned in preceding studies (43). Overall time in PCR method reached near 2 hr. The results showed that the LAMP assay is more rapid than PCR in EHEC O157:H7 detection.
The LAMP assay is a highly sensitive and specific approach to detect EHEC O157:H7 in which facilitated laboratories are not needed to conduct the test. Unlike PCR, it just requires a simple water bath or heat block that provides a constant temperature of60 to 65 °C. This property emphasizes the potential application for the detection of EHEC O157:H7 in field laboratories. It is easy to perform. Furthermore, LAMP is a rapid diagnostic method, because no time is lost as a result of changes in temperature, and this rapidity is provided by the short reaction time and the real-time turbidimetric detection of magnesium pyrophosphate precipitates with the naked eye without using gel electrophoresis (37). The existence of a color-based detection system can be more useful and makes it even simpler to detect color change in an amplified reaction. Designing the primer is the principle step of the LAMP technique, which directly influences the specificity and sensitivity of detection. It is a complex aspect of this approach that should be given more attention to develop effective primers. Generally, it is easy to bring non-specific amplification beyond 60 min. Therefore, the amplification time should be controlled properly (54). In addition, this assay is suited for qualitative, but not quantitative, information (32). This property is considered to be a limitation of the LAMP assay.
5. Conclusions
We demonstrated that the LAMP assay was time-efficient, sensitive, and easy-to-perform compared to the PCR assay in detecting EHEC O157:H7. Using SYBR Green II detection system facilitated the diagnosis of this bacterium and made the LAMP assay more practical to use. Alternatively, we can utilize this assay to detect other pathogenic agents in order to reduce detection time. However, further improvements are required to establish this approach. We suggest that this colorimetric detection system be applied in small clinical laboratories where early detection of infectious agents is required.
Acknowledgments
This research was supported financially by the Department of Agriculture and Plant Breeding, Faculty of Agriculture, Zanjan University, Zanjan, Iran. We also are grateful for Dr. Ali Haghnazari’s scientific support and helpful guidance in this study.
Footnotes
iThenticate screening: October 26, 2015, English editing: February 23, 2016, Quality control: June 11, 2016
Conflict of Interest: There is no conflict of interest to be declared.
Authors’ contributions: All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Keene WE, McAnulty JM, Hoesly FC, Williams LP, Jr, Hedberg K, Oxman GL, et al. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157: H7 and Shigella sonnei. N Engl J Med. 1994;331(9):579–84. doi: 10.1056/NEJM199409013310904. [DOI] [PubMed] [Google Scholar]
- 2.Kunisaki H, Tanji Y. Intercrossing of phage genomes in a phage cocktail and stable coexistence with Escherichia coli O157: H7 in anaerobic continuous culture. Appl Microbiol Biotechnol. 2010;85(5):1533–40. doi: 10.1007/s00253-009-2230-2. [DOI] [PubMed] [Google Scholar]
- 3.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308(12):681–5. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 4.Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol. 2005;295(6):405–18. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. The Lancet. 2005;365(9464):1073–86. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 6.Vanaja SK, Springman AC, Besser TE, Whittam TS, Manning SD. Differential expression of virulence and stress fitness genes between Escherichia coli O157: H7 strains with clinical or bovine-biased genotypes. Appl Environ Microbiol. 2010;76(1):60–8. doi: 10.1128/AEM.01666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farshad S, Ranjbar R, Japoni A, Hosseini M, Anvarinejad M, Mohammadzadegan R. Microbial susceptibility, virulence factors, and plasmid profiles of uropathogenic Escherichia coli strains isolated from children in Jahrom, Iran. Arch Iran Med. 2012;15(5):312–6. [PubMed] [Google Scholar]
- 8.Paton AW, Paton JC. Detection and Characterization of Shiga ToxigenicEscherichia coli by Using Multiplex PCR Assays forstx 1, stx 2, eaeA, Enterohemorrhagic E. coli hlyA, rfb O111, and rfb O157. J Clin Microbiol. 1998;36(2):598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nataro JP, Kaper JB. Diarrheagenic escherichia coli. Clin Microbiol Rev. 1998;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin X, Feng Y, Wheatcroft R, Chambers J, Gong J, Gyles CL. Adherence of Escherichia coli O157: H7 to epithelial cells in vitro and in pig gut loops is affected by bacterial culture conditions. Can J Vet Res. 2011;75(2):81. [PMC free article] [PubMed] [Google Scholar]
- 11.Momtaz H, Karimian A, Madani M, Safarpoor Dehkordi F, Ranjbar R, Sarshar M, et al. Uropathogenic Escherichia coli in Iran: serogroup distributions, virulence factors and antimicrobial resistance properties. Ann Clin Microbiol Antimicrob. 2013;12(8) doi: 10.1186/1476-0711-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter AO, Borczyk AA, Carlson JA, Harvey B, Hockin JC, Karmali M, et al. A severe outbreak of Escherichia coli O157: H7–associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987;317(24):1496–500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- 14.Karmali M, Petric M, Lim C, Fleming P, Steele B. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. The Lancet. 1983;322(8362):1299–300. doi: 10.1016/S0140-6736(83)91167-4. [DOI] [PubMed] [Google Scholar]
- 15.Baudouin V, Mariani-Kurkdjian P, Macher M, Ait-Ifrane S, Garnier A, Bingen E, et al. Pediatr Nephrol. springer; 233 spring st, New York, NY 10013 USA: 2008. Treatment of a relapsing HUS secondary to prolonged carriage of E. coli O157: H7. [Google Scholar]
- 16.Anvarinejad M, Farshad S, Ranjbar R, Giammanco GM, Alborzi A, Japoni A. Genotypic Analysis of E. coli Strains Isolated from Patients with Cystitis and Pyelonephritis. Iran Red Crescent Med J. 2012;14(7):408–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Liu F, Huo G, Ren D, Li Y. Development and evaluation of a loop - mediated isothermal amplification method for detecting escherichia coli o157 in raw milk. J Rapid Meth Aut Mic. 2009;17(1):55–66. doi: 10.1111/j.1745-4581.2008.00151.x. [DOI] [Google Scholar]
- 18.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13(1):60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 19.Fortin NY, Mulchandani A, Chen W. Use of real-time polymerase chain reaction and molecular beacons for the detection of Escherichia coli O157: H7. Anal Biochem. 2001;289(2):281–8. doi: 10.1006/abio.2000.4935. [DOI] [PubMed] [Google Scholar]
- 20.Vernozy - Rozand C. Detection of Escherichia coli O157: H7 and other verocytotoxin - producing E. coli (VTEC) in food. J Appl Microbiol. 1997;82(5):537–51. doi: 10.1111/j.1365-2672.1997.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 21.Chapman P, Ellin M, Ashton R. A comparison of immunomagnetic separation and culture, Reveal and VIP for the detection of E. coli O157 in enrichment cultures of naturally - contaminated raw beef, lamb and mixed meat products. Lett Appl Microbiol. 2001;32(3):171–5. doi: 10.1046/j.1472-765x.2001.00883.x. [DOI] [PubMed] [Google Scholar]
- 22.Crawford C, Wijey C, Fratamico P, Tu S, Brewster J, Immunomagnetic-Electrochemiluminescent Detection, Of E. Coli O157: H7 In Ground Beef. Journal of Rapid Methods & Automation in Microbiology. 2000;8(4):249–64. doi: 10.1111/j.1745-4581.2000.tb00327.x. [DOI] [Google Scholar]
- 23.Gehring AG, Tu S-I. Enzyme-linked immunomagnetic electrochemical detection of live Escherichia coli O157: H7 in apple juice. J FOOD PRO. 2005;68(1):146–9. doi: 10.4315/0362-028x-68.1.146. [DOI] [PubMed] [Google Scholar]
- 24.Shelton DR, Karns JS. Quantitative detection of Escherichia coli O157 in surface waters by using immunomagnetic electrochemiluminescence. Appl Environ Microbiol. 2001;67(7):2908–15. doi: 10.1128/AEM.67.7.2908-2915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TU SI, Golden M, FETT WF, Gehring A, Irwin P. Rapid detection of outbreak escherichia coli o157 and salmonella on alfalfa sprouts by immunomagnetic capture and time-resolved fluorescence. J Food Safety. 2003;23(2):75–89. doi: 10.1111/j.1745-4565.2003.tb00353.x. [DOI] [Google Scholar]
- 26.Jung BY, Jung SC, Kweon CH. Development of a rapid immunochromatographic strip for detection of Escherichia coli O157. J Food Prot. 2005;68(10):2140–3. doi: 10.4315/0362-028x-68.10.2140. [DOI] [PubMed] [Google Scholar]
- 27.Meng J, Zhao S, Doyle MP, Mitchell SE, Kresovich S. Polymerase chain reaction for detecting Escherichia coli O157: H7. Int J Food Microbiol. 1996;32(1):103–13. doi: 10.1016/0168-1605(96)01110-5. [DOI] [PubMed] [Google Scholar]
- 28.Mullah B, Livak K, Andrus A, Kenney P. Efficient synthesis of double dye-labeled oligodeoxyribonucleotide probes and their application in a real time PCR assay. Nucleic Acids Res. 1998;26(4):1026–31. doi: 10.1093/nar/26.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagamine K, Watanabe K, Ohtsuka K, Hase T, Notomi T. Loop-mediated isothermal amplification reaction using a nondenatured template. Clin Chem. 2001;47(9):1742–3. [PubMed] [Google Scholar]
- 31.Nagamine K, Kuzuhara Y, Notomi T. Isolation of single-stranded DNA from loop-mediated isothermal amplification products. Biochem Biophys Res Commun. 2002;290(4):1195–8. doi: 10.1006/bbrc.2001.6334. [DOI] [PubMed] [Google Scholar]
- 32.Hill J, Beriwal S, Chandra I, Paul VK, Kapil A, Singh T, et al. Loop-mediated isothermal amplification assay for rapid detection of common strains of Escherichia coli. J Clin Microbiol. 2008;46(8):2800–4. doi: 10.1128/JCM.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Zhou F, Olman V, Li F, Xu Y. Prediction of pathogenicity islands in enterohemorrhagic Escherichia coli O157: H7 using genomic barcodes. FEBS Lett. 2010;584(1):194–8. doi: 10.1016/j.febslet.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 34.Song T, Toma C, Nakasone N, Iwanaga M. Sensitive and rapid detection of Shigella and enteroinvasive Escherichia coli by a loop-mediated isothermal amplification method. FEMS Microbiol Lett. 2005;243(1):259–63. doi: 10.1016/j.femsle.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M. Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Appl Environ Microbiol. 2003;69(8):5023–8. doi: 10.1128/AEM.69.8.5023-5028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safi S, Heidarnejhad O, Mosavari N, Sakha M, Afshar D, Moazami L, et al. Comparative evaluation of LAMP and Nested-PCR for the diagnosis of bovine paratuberculosis. Int J Mycobacteriol. 2015;4:98–9. doi: 10.1016/j.ijmyco.2014.09.015. [DOI] [Google Scholar]
- 37.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289(1):150–4. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Russell David W. Molecular cloning: a laboratory manual. Vol. 3. Cold spring harbor laboratory press; 1989. [Google Scholar]
- 39.Flamm R, Hinrichs D, Thomashow M. Introduction of pAM beta 1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect Immun. 1984;44(1):157–61. doi: 10.1128/iai.44.1.157-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertrand R, Roig B. Evaluation of enrichment-free PCR-based detection on the rfbE gene of Escherichia coli O157 application to municipal wastewater. WATER RES. 2007;41(6):1280–6. doi: 10.1016/j.watres.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Gordillo R, Córdoba JJ, Andrade MJ, Luque MI, Rodríguez M. Development of PCR assays for detection of Escherichia coli O157: H7 in meat products. Meat Sci. 2011;88(4):767–73. doi: 10.1016/j.meatsci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Desmarchelier PM, Bilge SS, Fegan N, Mills L, Vary JC, Tarr PI. A PCR specific for Escherichia coli O157 based on the rfb locus encoding O157 lipopolysaccharide. J Clin Microbiol. 1998;36(6):1801–4. doi: 10.1128/jcm.36.6.1801-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao X, Li Y, Wang L, You L, Xu Z, Li L, et al. Development and application of a loop-mediated isothermal amplification method on rapid detection Escherichia coli O157 strains from food samples. Mol Biol Rep. 2010;37(5):2183–8. doi: 10.1007/s11033-009-9700-6. [DOI] [PubMed] [Google Scholar]
- 44.Sanderson M, Gay J, Hancock D, Gay C, Fox L, Besser T. Sensitivity of bacteriologic culture for detection of Escherichia coli O157: H7 in bovine feces. J Clin Microbiol. 1995;33(10):2616–9. doi: 10.1128/jcm.33.10.2616-2619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16(3):223–9. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 46.Seki M, Yamashita Y, Torigoe H, Tsuda H, Sato S, Maeno M. Loop-mediated isothermal amplification method targeting the lytA gene for detection of Streptococcus pneumoniae. J Clin Microbiol. 2005;43(4):1581–6. doi: 10.1128/JCM.43.4.1581-1586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parida M, Horioke K, Ishida H, Dash PK, Saxena P, Jana AM, et al. Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol. 2005;43(6):2895–903. doi: 10.1128/JCM.43.6.2895-2903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dukes J, King D, Alexandersen S. Novel reverse transcription loop-mediated isothermal amplification for rapid detection of foot-and-mouth disease virus. Arch Virol. 2006;151(6):1093–106. doi: 10.1007/s00705-005-0708-5. [DOI] [PubMed] [Google Scholar]
- 49.Curtis KA, Rudolph DL, Owen SM. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) J Virol Methods. 2008;151(2):264–70. doi: 10.1016/j.jviromet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3(5):877–82. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 51.Ma X-j, Shu Y-l, Nie K, Qin M, Wang D-y, Gao R-b, et al. Visual detection of pandemic influenza A H1N1 Virus 2009 by reverse-transcription loop-mediated isothermal amplification with hydroxynaphthol blue dye. J Virol Methods. 2010;167(2):214–7. doi: 10.1016/j.jviromet.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Ren W, Renault T, Cai Y, Wang C. Development of a loop-mediated isothermal amplification assay for rapid and sensitive detection of ostreid herpesvirus 1 DNA. J Virol Methods. 2010;170(1):30–6. doi: 10.1016/j.jviromet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Hara-Kudo Y, Konishi N, Ohtsuka K, Hiramatsu R, Tanaka H, Konuma H, et al. Detection of verotoxigenic Escherichia coli O157 and O26 in food by plating methods and LAMP method: a collaborative study. Int J Food Microbiol. 2008;122(1):156–61. doi: 10.1016/j.ijfoodmicro.2007.11.078. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Zhang X, Wang D, Kuang Y, Xu Y. Simple And Rapid Method For Detecting Foodborne Shigella By A Loop - Mediated Isothermal Amplification. J Rapid Meth Aut Mic. 2009;17(4):465–75. doi: 10.1111/j.1745-4581.2009.00183.x. [DOI] [Google Scholar]


