Abstract
Bioelectrical impedance analysis (BIA) is a common method for assessing body composition in research and clinical trials. BIA is convenient but when compared with other reference methods, the results have been inconclusive. The level of obesity degree in subjects is considered to be an important factor affecting the accuracy of the measurements. A total of 711 participants were recruited in Taiwan and were sub-grouped by gender and levels of adiposity. Regression analysis and Bland-Altman analysis were used to evaluate the agreement of the measured body fat percentage (BF%) between BIA and DXA. The BF% measured by the DXA and BIA methods (Tanita BC-418) were expressed as BF%DXA and BF%BIA8, respectively. A one-way ANOVA was used to test the differences in BF% measurements by gender and levels of adiposity. The estimated BF%BIA8 and BF%DXA in the all subjects, male and female groups were all highly correlated (r = 0.934, 0.901, 0.916, all P< 0.001). The average estimated BF%BIA8 (22.54 ± 9.48%) was significantly lower than the average BF%DXA (26.26 ± 11.18%). The BF%BIA8 was overestimated in the male subgroup (BF%DXA< 15%), compared to BF%DXA by 0.45%, respectively. In the other subgroups, the BF%BIA8 values were all underestimated. Standing BIA estimating body fat percentage in Chinese participants have a high correlation, but underestimated on normal and high obesity degree in both male and female subjects.
Background
The prevalence of overweight and obesity has increased tremendously in the global population [1]. Obesity is defined as the over accumulation of body fat and correlates to a risk of high blood pressure, heart problems and diabetes [2]. Thus, periodic assessments of percentage body fat (BF%) may provide valuable information to monitor public health that is quick, low cost, non-invasive and accurate. Many assessment methods can be used to determine BF%, such as the underwater weighing method [3], air-displacement plethysmography and dual energy X-ray absorptiometry (DXA) [4, 5]. However, the application of these methods is limited by their cost and complexity. Therefore, more convenient methods, such as bioelectrical impedance analysis (BIA) and the skinfold method are widely used to assess large populations [6].
In recent years, the measurement protocol of BIA has changed from the traditional supine position with disposable contact electrodes to the standing up position with reusable stainless steel plates as electrodes [7, 8]. Most standing BIA systems operate on a digital scale, and while assessing impedance, the system simultaneously measures the subject's weight through the weight transducer. Measuring body weight during the BIA obtained more accurate estimates than using a self-reported body weight to assess body composition.
Several studies have compared the BF% results as assessed by BIA with other referenced methods [7–11], and the results have been inconclusive. Some concluded that BIA overestimates BF% and some concluded that it underestimates [12, 13]; some concluded that BIA lacks precision while others concluded that it measures with accuracy [10, 14–18]. Questions arise regarding the degree of measurement bias in BF% compared to the reference DXA methods in a healthy population. Furthermore, there is a need for gathering mass quantitative BF% data as limited validation studies exist in the Asian or Chinese population. In the study, we hypothesized that the standing BIA is an accurate method for evaluating body fat percentage in Chinese healthy adult population.
We further compared the differences between the sexes and among different adiposity level subgroups to verify whether the BIA measurement is biased by the levels of adiposity of the Chinese subjects.
Materials and Methods
Subjects
Test subjects were selected by a non-random purposive sampling method. The 711 subjects were recruited voluntarily from different locations in Taiwan through advertisements. The subjects were asked to complete health history questionnaires, including personal information, physical characteristics and health conditions. The subjects were further asked to refrain from alcoholic drinks 48 hours prior to the test, from diuretics 7 days prior, and from strenuous physical activities 24 hours prior. Subjects were to void urinary bladder and after a fast > 1.5 h to the experiment. Health questionnaires were distributed to all participants and no test subjects reported any endocrine disorder, nutritional or growth disorders or major chronic conditions, such as diabetes, cancer, kidney dysfunction, asthma and electronic implants, such as an artificial heart or electrodes. Female participants were excluded from measurements if pregnant or during menstruation cycle. The measurements were carried out in the Taichung County Dali Jen Ai Hospital, radiology department. The experimental procedure and research plan were approved by the board of clinical trials of the Jen Ai Hospital (IRB 97–01). All subjects were recruited and signed an informed consent before participating in the study.
Anthropometry
Each subject was weighed using a Tanita BC-418 (Tanita Co., Tokyo, Japan, BIA8 denoted in text) to the nearest 0.1 kg. Subjects’ height was measured without shoes by a Stadiometer to the nearest 0.5 cm and body mass index (BMI) was calculated was calculated as weight divided by height squared (kg/m2). The intraexaminer coefficient of variation was 3.6%.
Measurements of percentage body fat
The subjects wore light cotton robes and removed all metallic objects from their bodies. Body composition parameters, such as total body fat, fat-free soft tissue and bone mineral content, were measured by DXA (Lunar prodigy; GE medical System, Madison, WI). BF% was calculated as fat mass / (fat mass + fat free mass) × 100%. The fat-free mass (FFM) is the sum of the measured total fat-free tissue mass and the bone mineral content. DXA was completed by the Encore 2003 Version 7.0 analytical software. DXA measurements were taken at 2:00 pm each day, and once the DXA measurements were completed, BIA was conducted immediately afterwards. All DXA and BIA8 examinations were performed by the same investigator. The intraexamination coefficient of variation for DXA, BIA8 was 2% and 2.5%.
We used the BIA8 to measure the impedances of the body and of each limb in the standing position. Subjects were asked to hold an electrode shaped like a hand grip in each hand and to stand on base plate electrodes. The electrodes allowed a current to pass through the subject's body and impedance was further measured. The impedances were measured via the pathway from the left foot to the left hand. These impedance measurements were used to estimate the BF%, adjusting for other physical parameters, such as height, weight, age and sex. The BF%, as measured by the DXA and BIA methods, was expressed as BF%DXA and BF%BIA8, respectively.
Statistical analysis
In this study, values are expressed as the mean ± SDs. The paired t-test was used to compare the difference in BF%BIA8 and BF%DXA, Pearson’s correlation, Lin’s concordance correlation coefficient (ρc) [19] and ordinary least products regression analysis was used to examine the relationship between BF%BIA8 and BF%DXA [20]. Statistical significance was set at P < 0.05. The Bland-Altman analysis was used to test the agreement between BF%BIA8 and BF%DXA [21]. Additionally, one-way ANOVA was used to compare the differences between BF%BIA8 and BF%DXA within the different BF% subgroups. We categorised subjects according to the measured adiposity level into: lean, normal and obese categories [22]. Scatter plots for the total, male, and female participants were produced using the BF%DXA as the x-axis and FFMBIA8 –FFMDXA as the y-axis, and regression analysis was conducted. All statistical analyses were conducted using SPSS for Windows (Version 17.0; SPSS Inc, Chicago) and Medcalc (Version 11.5; Medcalc Software, Mariakerke, Belgium).
Results
Physical characteristics of the subjects
The physical characteristics of the subjects are listed in Table 1. A total of 711 subjects were tested, 412 males and 299 females. The subjects' ages ranged from 18 to 82 years old, with matched age distribution in both sexes. The average male weight exceeded that of females by 14.6 kg, and the height of males exceeded that of females by 13.1 cm. The BMI reported from the subjects ranged from 15.8 to 42.7 kg/m2.
Table 1. Physical characteristics of the subjects1.
| All subjects (n = 711) | Male (n = 412) | Female (n = 299) | |
|---|---|---|---|
| Age (y) | 34.99 ± 16.64 (18, 82) | 33.18 ± 16.89 (18, 82) | 37.49 ± 15.98 (18, 78)2 |
| Weight (kg) | 68.53 ± 14.60 (38, 133) | 74.65 ± 13.07 (42, 133) | 60.09 ± 12.22 (38, 108)3 |
| Height (cm) | 167.27 ± 9.73 (145, 200) | 172.76 ± 7.61 (152,200) | 159.70 ± 6.83 (143, 181)3 |
| BMI (kg/m2) | 24.38 ± 4.12 (15.8, 42.7) | 24.96 ± 3.71 (16.8,41.8) | 23.57 ± 4.51 (15.8, 42.7)2 |
1 All values are mean ± SDs; minimum and maximum in parentheses.
2,3 Significantly different from male (one-factor ANOVA); 2P < 0.05, 3P < 0.001.
Comparison of BF% measured by BIA and DXA
The subjects' BF% measured by BIA8 and DXA are listed in Table 2. The average BF%BIA8 and BF%DXA were 22.54 ± 9.48% and 26.26 ± 11.18% respectively. The measured BF%BIA8 was significantly lower than BF%DXA in both male and female subjects. The correlation coefficient between BF%BIA and BF%DXA for all subjects was calculated as 0.93, while for the male and female subgroups, they were 0.90 and 0.92, respectively. (Fig 1)
Table 2. Percentage body fat measured by standing-posture bioelectrical impedance analysis (BIA) and by dual-energy X-ray absorptiometry (DXA)1.
| Method | All subjects (n = 711) | Male (n = 412) | Female (n = 299) |
|---|---|---|---|
| BF%BIA8 | 22.54 ± 9.48 (5.5, 48.7)2 | 17.24 ± 6.53(5.5, 36.9)2 | 29.85 ± 7.93(11.7, 48.7)2 |
| BF%DXA | 26.26 ± 11.18 (5.1, 56.6) | 20.89 ± 9.05 (5.1,41.0) | 33.66 ± 9.49(10.6, 56.6) |
1 All values are mean ± SDs; minimum and maximum in parentheses.
2 Significantly different from DXA, P < 0.001 (paired t-test).
Fig 1.
BF%BIA8 and BF%DXA scatter plot and regression line (a) all participants (b) male subjects (C) female subjects; Bold line represents regression line, dotted line represents identical line.
BF%BIA8 bias on the basis of BF%DXA
The Bland-Altman analysis was used to test the agreement between BF%DXA and BF%BIA8 by dividing all male and female subjects in two different ways: (i) uncategorised, including all subjects, (ii) categorised into lean, normal and obese subgroups. The results are shown in Fig 2(A), 2(B) and 2(C), Fig 3(A), 3(B) and 3(C) respectively.
Fig 2. Bland-Altman plot of the difference between BF%BIA8 and BF%DXA in mean difference expressed as bias, 95% confidence interval expressed as bias ± 2 SD.
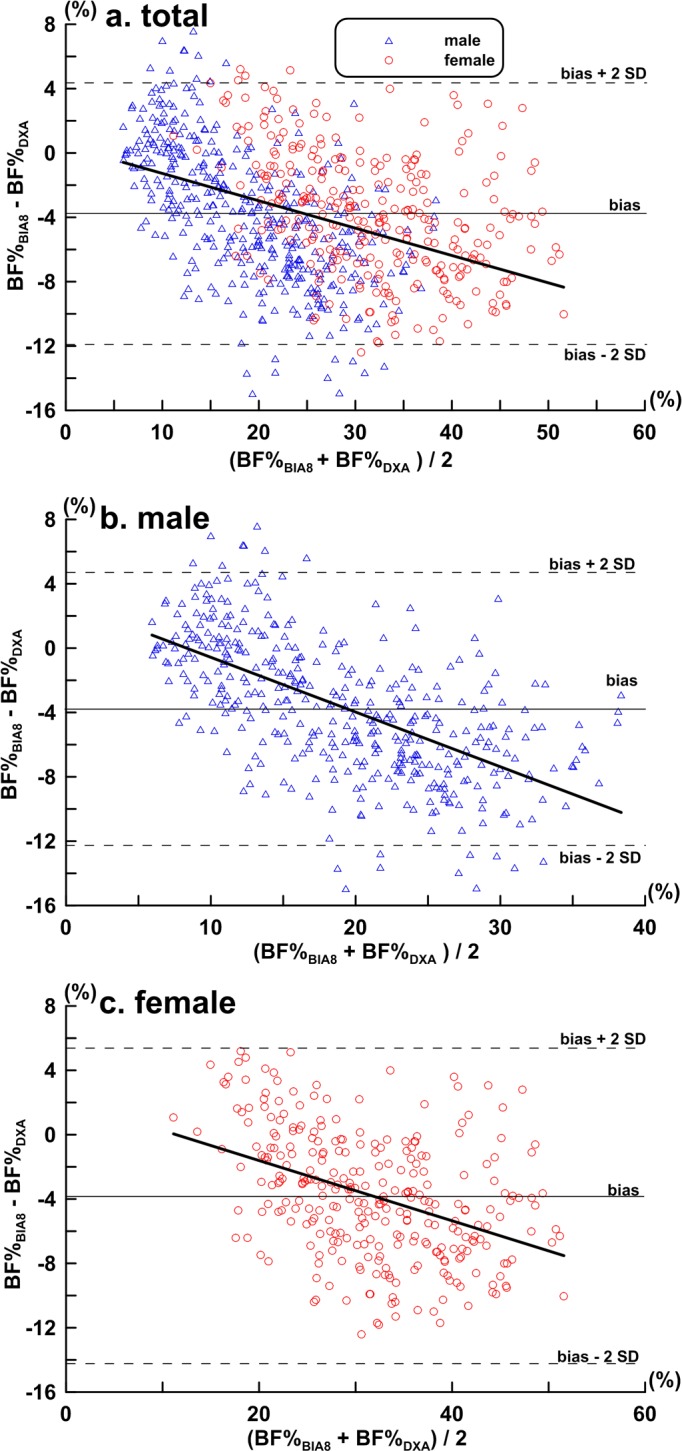
(a) Total subjects (n = 711); bias ± SD: -3.72 ± 4.09%, bias– 2SD: -11.90%, bias + 2 SD: 4.46%, regression equation y = - 0.170 x + 0.430 (r = 0.42, P < 0.01); (b) Male (n = 412); bias ± SD: -3.66 ± 4.24%, bias– 2SD: -12.14%, bias + 2 SD: 4.83%, regression equation y = - 0.340 x + 2.830(r = 0.61, P < 0.01); (c) Female (n = 299); bias ± SD: -3.81 ± 3.87%, bias– 2SD: -11.56%, bias + 2 SD: 3.94%, regression equation y = - 0.187 x +2.134 (r = 0.41, P < 0.01).
Fig 3.
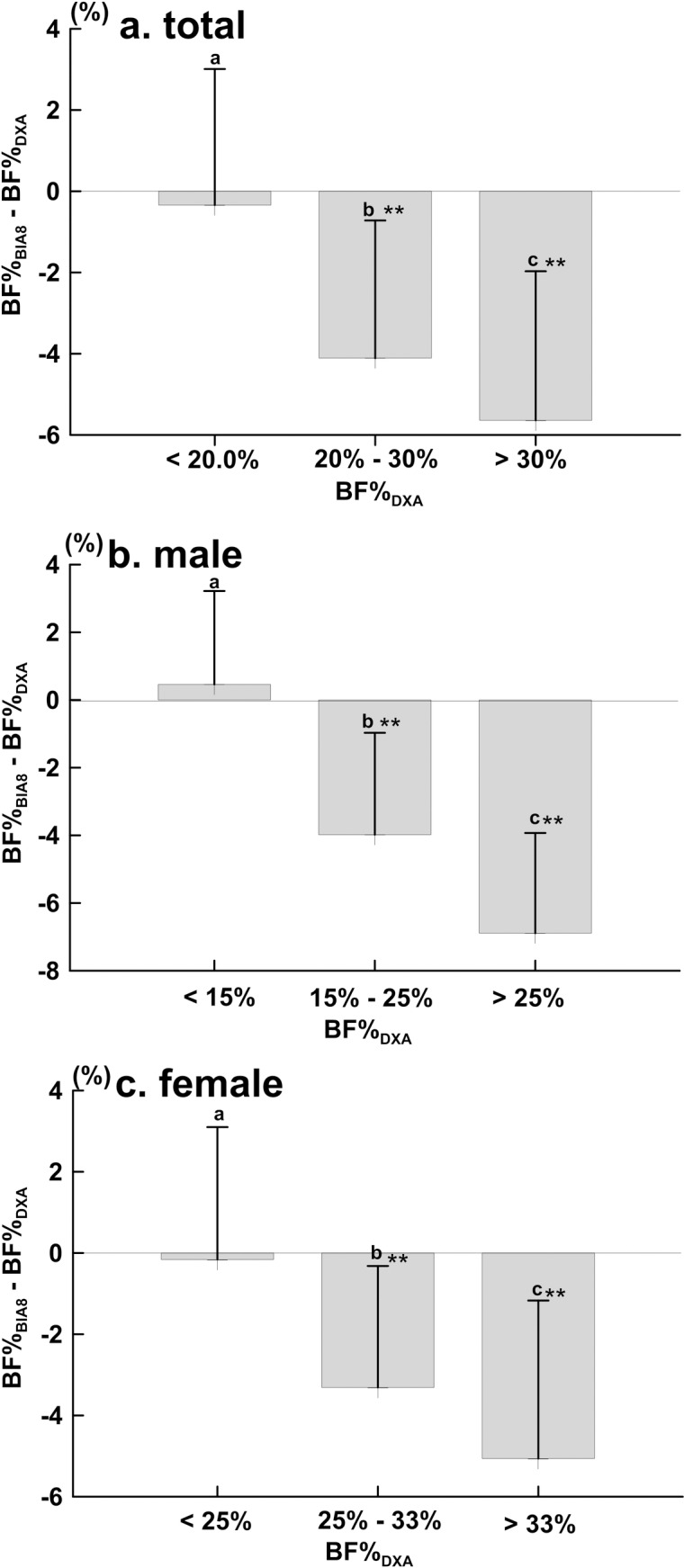
BF% dependent bias of BIA8 compared with DXA in (a) total (n = 711), (b) male (n = 412), and (c) female (n = 299). Data are presented as the mean difference ± SD. Means with symbol are significantly different, P< 0.001 (**).
Fig 2(A) presents the results of the Bland-Altman analysis between the BF%DXA and BF%BIA8 across all subjects, and the bias ± SD between the two results was -3.72 ± 4.09%. Fig 3(A) presents the results when subjects were categorised into lean—BF%DXA: <20% (n = 212), normal—BF%DXA: 20%-30% (n = 229) and obese—BF%DXA: >30% (n = 270) subgroups. BF%BIA8 underestimated BF % compared to BF%DXA in the lean, normal and obese subgroups by -0.40± 3.31%, -4.13 ± 3.42% and -5.98 ± 3.42%, respectively.
Fig 2(B) presents the results of the Bland-Altman analysis between BF%DXA and BF%BIA8 across all male (n = 412) subjects, and the bias ± SD between the two results was -3.66 ± 4.24%. Fig 3(B) presents the results when the male subjects were divided into lean- BF%DXA:< 15% (n = 135), normal- BF%DXA: 15%-25% (n = 129) and obese- BF%DXA:>25% (n = 148) subgroups. BF%BIA8 overestimated BF% compared to BF% DXA in the lean subgroups by 0.45± 2.76%, but it underestimated BF% in the normal and obese subgroup by -4.01 ± 2.77% and -7.01 ± 3.11%, respectively.
Fig 2(C) presents the results of the Bland-Altman analysis between BF%DXA and BF%BIA8 across all female subjects (n = 299), and the bias ± SD between the two results was -3.81 ± 3.87%. Fig 3(C) presents the results when all female subjects were categorised into lean -BF%DXA: < 25% (n = 64), normal—BF%DXA: 25%–33% (n = 78) and obese -BF%DXA > 33% (n = 157) subgroups. BF%BIA8 underestimated BF% compared to BF%DXA in lean, normal and obese subgroups by -0.15± 3.23%, -3.35 ± 2.91% and -5.53 ± 3.42%, respectively.
When focus on the different levels of adiposity subgroups in male and female subjects and applying linear regression analysis to the data, the result showed no fixed and proportional bias from the slope and intercept 95%CI between BF%BIA8 and BF%DXA in male and female subject. Other subgroup all showed proportional and/or fixed bias (Table 3). Lin’s concordance correlation coefficients (ρc) are shown in Table 3. McBride [23] suggests the following descriptive scale for values of the ρc: Value of ρc < 0.90 is poor and 0.90 to 0.95 is moderate. In Table 3, the concordance between the these two methods was poor for all of the ρc value were less than 0.09.
Table 3. Body fat percentage outcome of analyses by ordinary least products regression.
| Proportional1 | r | a | 95%CI | b | 95%CI | Proportional bias | Fixed bias | ρc |
|---|---|---|---|---|---|---|---|---|
| (a) total | 0.933 | 1.609 | 0.829, 2.389 | 1.083 | 1.051, 1.115 | Yes | Yes | 0.874 |
| (b) male | 0.902 | -0.710 | -1.802, 0.382 | 1.243 | 1.183, 1.303 | Yes | No | 0.782 |
| (c) female | 0.910 | 1.916 | 0.144, 3.688 | 1.053 | 0.996, 1.110 | No | Yes | 0.837 |
| (c) totallean | 0.654 | 4.482 | 3.072, 5.892 | 0.674 | 0.568, 0.780 | Yes | Yes | 0.850 |
| (d) totalnormal | 0.479 | 17.778 | 15.935, 19.620 | 0.346 | 0.259, 0.433 | Yes | Yes | 0.676 |
| (e) totalobese | 0.857 | 14.263 | 12.426, 16.111 | 0.732 | 0.676, 0.788 | Yes | Yes | 0.687 |
| (f) malelean | 0.488 | 5.636 | 4.092, 7.178 | 0.442 | 0.305, 0.579 | Yes | Yes | 0.480 |
| (g) malenormal | 0.581 | 12.343 | 10.265, 14.421 | 0.492 | 0.369, 0.615 | Yes | Yes | 0.327 |
| (h) maleobese | 0.777 | 14.183 | 11.794, 16.571 | 0.695 | 0.597, 0.793 | Yes | Yes | 0.344 |
| (i) femalelean | 0.491 | 9.580 | 4.527, 14.632 | 0.539 | 0.295, 0.784 | Yes | Yes | 0.489 |
| (j) femalenormal | 0.408 | 21.277 | 16.995, 25.559 | 0.306 | 0.141, 0.470 | Yes | Yes | 0.223 |
| (k) femaleobese | 0.791 | 16.117 | 12.835, 19.399 | 0.694 | 0.604, 0.783 | Yes | Yes | 0.572 |
1 Relationship between different subgroups in BF%DXA and BF%BIA8, lean, normal, obese subscripts represents DXA measured results; r, product-moment correlation coefficient; a, b, coefficients in ordinary least products regression model E(A) = a + b(B); a, A (y axis) intercept; b, slope; proportional bias, if 95% confidence interval (CI) for b does not include 1; fixed bias, if 95% CI for a does not include; Lin’s concordance correlation coefficient (ρc).
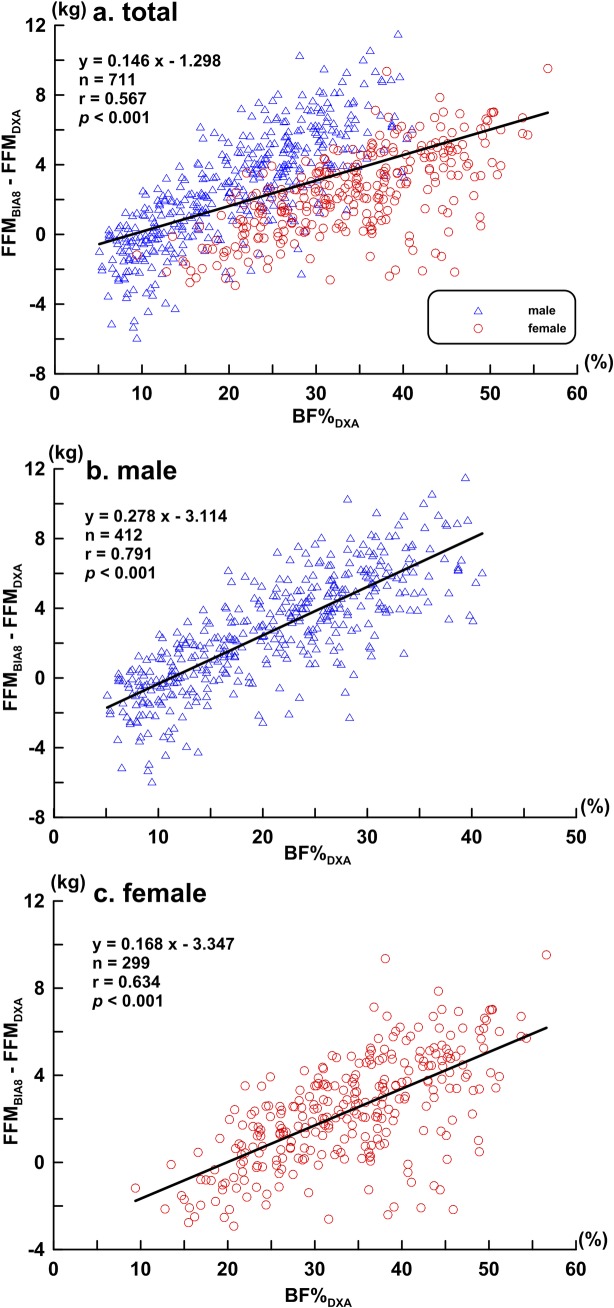
Fig 4 shows the scatter plot and regression analyses of the BF%DXA according to FFMBIA8 and FFMDXA differences.
Fig 4. Scatter and regression plots of BF%DXA according to FFMBIA8 and FFMDXA differences.
(a) total subjects (n = 711), (b) male subjects (n = 412), and (c) female subjects (n = 299). The bold line represents the regression line.
Discussion
The use of Tanita BC-418 for estimating and validating body composition has been reported in numerous studies [13, 16, 18, 24–26], but studies have only utilized a relatively smaller sample size to validate the accuracy of BIA. Hence, the present study compared the measured BF% by both the BIA8 and DXA methods in a large sample of a healthy Chinese population in Taiwan. The results of the study showed that using BIA8 to estimate BF% yields results that are similar to those of the DXA method in both male and female subjects. Sun et al. compared the BF%DXA with an estimated BF% in a supine posture BIA with multiple frequencies in a large healthy Canadian population and attained similar results. However, according to the results of our study, BF%BIA8 had a higher correlation with BF%DXA than BF% did when estimated by a supine posture BIA [9].
Body composition information can be applied in clinical trials and other medicine-related fields [27, 28]. Some assessment methods, such as DXA, air displacement plethysmography and underwater weighing, can provide accurate estimates, but these methods are often expensive and cannot be widely applied to the general public. BIA is a feasible alternative for practical use in assessing a large-scale sample. Existing BIA studies have shown conflicted and inconsistent results regarding the accuracy of BF% estimation by BIA. To clarify these results, our study used four different approaches: 1. the sample size was 711, larger than that of previous BIA-related research; 2. the sample had a wider age-range with even age distribution in male and female subjects; 3. we grouped subjects into different BF% categories, compared the results of the measured BF% by BIA8 against that by DXA and examined the trend line between the two measurements; and 4. Use different genders, obesity level, analysing the DXA proportional bias and fixed bias results.
The manufacturers of the currently available BIA measuring equipment have not released their built-in prediction equations or identified a suitable testing population, which limits the reference and application value of BIA [14]. This work used the raw data obtained from the Tanita BC-418 model instead of using the built-in prediction equations for estimating BF%.
This research shows that a high degree of correlation exists between BF%BIA8 and BF%DXA, which is consistent with other research [13, 17]. A high correlation is shown in both the male and the female subgroups; however, testing of the correlation alone may not guarantee the equivalence of the two measuring methods [20, 21]. After comparison of the differences between BF%BIA8 and BF%DXA by the Bland-Altman analysis, the results show that for mixed, male and female sample sets, BF%BIA8 underestimates BF% compared to BF%DXA, but the differences do not deviate with statistical significance (male: -3.66 ± 4.24%, female: -3.81 ± 3.87%).
The Bland-Altman analysis is usually used to explore variables without categorising or splitting the sample so that the agreement between two measurement methods may be analysed. However, this research examined the distributions and trends of the measured differences not only by splitting the sample into male and female subgroups but also by categorising them according to their measured BF%DXA. Sun et al. used the Bland-Altman analysis to investigate the differences between supine posture BIA and DXA measurements of BF% according to sex and adiposity, and they also categorised subjects into total, male and female, as well as lean, normal and obese [9]. Although research has tested the differences between BIA and DXA by lean, normal, obese criteria, further comparison of the two methods by other criteria may be necessary to fully understand the differences in the measurements. As for the lean, normal, obese subgroups in the male, female and combined subgroups, BF% was still underestimated by BIA8 compared to DXA, and the degree of the bias also increased as the BF%DXA increased. These results are consistent with the BF% of obese women measured by the Tanita BC-418, as reported by Neovius et al. [16] Studied male and female participants’ obesity level using Tanita BC-418 and comparing with DXA measured results and showed that BF% has been underestimated. Further, the present study also showed consistent result as reported by Hemmingsson, Mally, Lee et al. used different race, age and obesity level to estimate BF% between Tanita BC-418 and DXA measurements. At the end, the authors reported that BC-418 have underestimated BF% than of DXA measured results [24–26].
Oshima et al. stated that a standing hand-to-foot BIA is a stable and suitable method for assessing whole body BF%, and the variation of within-day impedance measurements determined by standing hand-to-foot BIA was smaller than the variation of those determined by standing hand-to-hand or foot-to-foot BIAs [29]. Additionally, BIA methods generated more accurate results, compared to foot-to-foot BIA [30].
A distinct measuring difference was found when it comes to the DXA apparatus designed by different manufactories. The evaluated BF% data measured from the Hologic QDR series were always higher than that of Lunar DPX series [31]. In lower adiposity level subjects, the evaluated BF% measured from Lunar Expert was lower than Hologic QDR4500, but in high adiposity level subjects, Lunar Expert had a higher BF% than Hologic QDR4500 [32]. Furthermore, based on the DXA BF% measuring results, we conclude that with different DXA apparatus and adiposity levels will affect the measuring results of the study.
This study used parallel measurements of BF% by hand-to-foot BIA8 and DXA to examine the measurement differences with in various BF% subgroups. In both male and female subjects except for subjects with adiposity lower than normal levels, BIA8 has the tendency to underestimate BF%. These differences increase with the level of adiposity; BIA8 overestimated BF% in the low BF% group and underestimated BF% in the high BF% group. These findings are similar to the trend reported by previous supine posture BIA study [9]. In the present study, a high correlation was found for evaluating BF% between standing BIA and DXA. The standing BIA estimated results was significantly underestimated, therefore we reject the hypothesis for the present study.
Conclusions
In summary, BIA8 may generate inaccurate measurements when subjects with high adiposity and when applying measurements to the Chinese population, as participants’ adiposity levels increases, BF% will increase its underestimation correspondingly.
Abbreviations
- BF%
Body fat percentage
- BIA
Bioelectrical impedance analysis
- BMI
Body mass index
- CI
Confidence interval
- DXA
dual-energy X-ray absorptiometry
- FFM
Fat free mass
- SD
standard deviations
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
This work was supported by grants from the ministry of Health and Welfare of the Republic of China (PG-10417) and the National Science Council of the republic of China (NSC 100-2410 H-H-028-MY3). Additionally, Co-author Kuen-Chang Hsieh received salary from the commercial company Charder Electronic Co., Ltd during this study. Charder Electronic Co., Ltd did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of co-author KCH are articulated in the 'author contributions' section.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011; 377: 557–67. 10.1016/S0140-6736(10)62037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014; 7: 75–88. 10.2147/IJNRD.S39739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katch FI. Practice curves and errors of measurement in estimating underwater weight by hydrostatic weighing. Med Sci Sports 1969;1: 212–6. [Google Scholar]
- 4.Johansson AG, Forslund A, Sjödin A, Mallmin H, Hambraeus L, Ljunghall S. Determination of body composition–a comparison of dual-energy x-ray absorptiometry and hydrodensitometry. Am J Clin Nutr 1993; 57: 323–6. . [DOI] [PubMed] [Google Scholar]
- 5.Temple D, Denis R, Walsh MC, Dicker P, Byrne AT. Comparison of anthropometric-based equations for estimation of body fat percentage is a normal-weight and overweight female cohort: validation via air-displacement plethysmography. Public Health Nutr 2015; 18: 446–52. 10.1017/S1368980014000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974; 32: 77–97. . [DOI] [PubMed] [Google Scholar]
- 7.Tan YX, Nuñez C, Sun Y, Zhang K, Wang Z, Heymsfield SB. New electrode system for rapid whole-body and segmental bioimpedance assessment. Med Sci Sports Exerc 1997; 29: 1269–73. . [DOI] [PubMed] [Google Scholar]
- 8.Bosaeus M, Karlsson T, Holmäng A, Ellegård L. Accuracy of quantitative magnetic resonance and eight-electrode bioelectrical impedance analysis in normal weight and obese women. Clin Nutr 2014; 33: 417–7. 10.1016/j.clnu.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 9.Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in large, healthy population. Am J Clin Nutr 2005; 81: 74–8. . [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Kim CH, Kim DW, Park M, Park HS, Min SS, et al. External cross-validation of bioelectrical impedance analysis for the assessment of body composition in Korean adults. Ntr Res Pract 2011; 5: 246–52. 10.4162/nrp.2011.5.3.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böhm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr 2013; 67 Suppl 1: S79–S85. 10.1038/ejcn.2012.168 [DOI] [PubMed] [Google Scholar]
- 12.Niqam P, Misra A, Colles SL. Comparison of DEXA-derived body fat measurement to two race-specific bioelectrical impedance equations in healthy Indians. Diabetes Metab Syndr 2013; 7: 72–7. 10.1016/j.dsx.2013.02.031 [DOI] [PubMed] [Google Scholar]
- 13.Wang JG, Zhang Y, Chen HE, Li Y, Cheng XG, Xu L, et al. Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J Strength Cond Res 2013; 27: 236–43. 10.1519/JSC.0b013e31824f2040 [DOI] [PubMed] [Google Scholar]
- 14.Lukaski HC, Siders WA. Validity and accuracy of regional bioelectrical impedance devices to determine whole-body fatness. Nutrition 2003; 19: 851–7. . [DOI] [PubMed] [Google Scholar]
- 15.Wing L, Hui SS, Wong SH. Validity of bioelectrical impedance measurement in predicting fat-free mass of Chinese children and adolescents. Med Sci Monit 2014; 20:2298–310. 10.12659/MSM.890696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neovius M, Hemmingsson E, Freyschuss B, Uddén J. Bioelectrical Impedance Underestimates total and truncal fatness in abdominally obese women. Obesity (Silver Spring) 2006; 14: 1731–8. . [DOI] [PubMed] [Google Scholar]
- 17.Erceg DN, Dieli-Conwright CM, Rossuello AE, Jensky NE, Sun S, Schroeder ET. The Stay healthy bioelectrical impedance analyzer predicts body fat in children and adults. Nutr Res 2010; 30: 297–304. 10.1016/j.nutres.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr 2004; 58: 1479–84. . [DOI] [PubMed] [Google Scholar]
- 19.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989; 45: 255–68. . [PubMed] [Google Scholar]
- 20.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol 2002; 29: 527–36. . [DOI] [PubMed] [Google Scholar]
- 21.Bland JM. Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10. . [PubMed] [Google Scholar]
- 22.Bary GA. Contemporary diagnosis & management of obesity Newtown, PA: Publishers in Health Care, 1998. [Google Scholar]
- 23.McBride G. A proposal for strength-of-agreement criteria for Lin’s concordance correlation coefficient. NIWA Client Report: HAM2005-062 Report to Ministry of Health; 2005.
- 24.Hemmingsson E, Udden J, Neovius M. No apparent progress in bioelectrical impedance accuracy: validation against metabolic risk and DXA. Obesity 2009; 17: 183–7. 10.1038/oby.2008.474 [DOI] [PubMed] [Google Scholar]
- 25.Mally K, Trentmann, Heller M, Dittmar M. Reliability and accuracy of segmental bioelectrical impedance analysis for assessing muscle and fat mass in older Europeans: a comparison with dual-energy X-ray absorptiometry. Eur J Appl Physiol 2011; 111: 1879–87. 10.1007/s00421-010-1795-x [DOI] [PubMed] [Google Scholar]
- 26.Lee LC, Hsieh KC, Wu CS, Chen YJ, Chiang J, Chen YY. Validity of standing posture eight-electrode bioelectrical impedance to estimate body composition in Taiwanese elderly. Int J Gerontology 2014; 8: 137–42. 10.1016/j.ijge.2013.08.010. [DOI] [Google Scholar]
- 27.Sentongo TA, Semeao EJ, Piccoli DA, Stallings VA, Zemel BS. Growth, body composition, and nutritional status in children and adolescents with Crohn's disease. J Pediatr Gastroenterol Nutr 2000; 31: 33–40. . [DOI] [PubMed] [Google Scholar]
- 28.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med 1995; 123: 673–5. . [DOI] [PubMed] [Google Scholar]
- 29.Oshima Y, Shiga T. Within-day variability of whole-body and segmental bioelectrical impedance in a standing position. Eur J Clin Nutr 2006; 60: 938–41. . [DOI] [PubMed] [Google Scholar]
- 30.Gupta N, Balasekaran G, Victor Govindaswamy V, Hwa CY, Shun LM. Comparison of body composition with bioelectric impedance (BIA) and dual energy X-ray absorptiometry (DEXA) among Singapore Chinese. J Sci Med Sport 2011; 14: 33–5. 10.1016/j.jsams.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Tothill P, Hannan WJ, Wilkinson S. Comparisons between a pencil beam and two fan beam dual energy X-ray absorptiometers used for measuring total body bone and soft tissue. Br J Radiol 2001; 74: 166–76. . [DOI] [PubMed] [Google Scholar]
- 32.Tothill P, Hannan WJ. Comparisons between Hologic QDR 1000W, QDR 4500 A, and Lunar Expert dual energy X-ray absorptiometry scanners used for measuring total body bone and soft tissue. Ann N Y Acad Sci 2002; 904: 63–71. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files