Abstract
Institution of a low-NaCl diet beginning at embryonic day 3 and continued throughout pre- and postnatal development has widespread effects on the neuroanatomical organization of the first gustatory relay in the nucleus of the solitary tract. To determine when these effects are expressed postnatally, the terminal field of the chorda tympani nerve was compared between sodium-restricted and sodium-replete rats at postnatal days 15–17, postnatal days 25–27, postnatal days 35–37, and adults. Total terminal fields were significantly larger in postnatal days 35–37 and adult sodium-restricted rats compared with aged-matched controls. The group-related differences appear related more to a remodeling of the terminal field in the dorsal zone of the terminal field in controls. Specifically, the terminal field volume in the dorsal zone in controls decreased dramatically from postnatal days 25–27 to postnatal days 35–37 and then again from postnatal days 35–37 to adulthood. In contrast, the fields did not change during development in sodium-restricted rats. These findings suggest that remodeling of the chorda tympani field occurs in controls at about the developmental period of taste response maturation. The lack of remodeling in sodium-restricted rats may be explained by a corresponding lack of functional response development to sodium salts. These results also illustrate the specificity and extent of how early dietary manipulations shape the developing brainstem.
Keywords: gustatory, sodium, plasticity, activity-dependent, chorda tympani nerve
An extensive literature examines the consequences of sensory restriction during development and the reversal of such effects in the auditory, olfactory, visual, and somatosensory systems (Hubel and Wiesel, 1970; Renehan et al., 1989, 1994; Henderson et al., 1992; Cummings and Brunjes, 1997; Cummings et al., 1997; Buonomano and Merzenich, 1998; Catalano and Shatz, 1998; Rauschecker, 1999; Fox et al., 2002; Katz and Crowley, 2002; Yan, 2003). Findings from these studies have been important in not only elucidating the responses of the restricted sensory system to abnormal environments, but also in understanding the processes required for normal development.
The developing gustatory system is no exception. Restriction of maternal dietary sodium (0.03% NaCl) beginning on or before embryonic day 8 (E8) and continuing throughout development results in profound peripheral and central functional alterations. The early dietary restriction results in a 50%–70% reduction of chorda tympani nerve responses to sodium salts when compared with rats maintained on sodium-replete chow (0.5% NaCl) (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). These reductions are specific to sodium salts, since responses to non-sodium salts are unaffected. Widespread functional alterations also occur at the first central relay. Early dietary sodium restriction reduces sodium salt response frequencies of neurons in the nucleus of the solitary tract (NTS) by as much as 50%, and severely decreases the proportion of cells that respond best to NaCl (Vogt and Hill, 1993). Similar to the periphery, only responses to sodium salts are affected in NTS neurons. Therefore, there are similar and specific functional taste response alterations in peripheral and central gustatory neurons following early dietary sodium restriction.
Restriction of dietary NaCl at early stages of development also alters the morphology of the NTS (King and Hill, 1991). Specifically, the dorsal-most region of the chorda tympani terminal field in the NTS is significantly increased in size in sodium-restricted rats, whereas more ventral zones are unaffected. Moreover, the terminal zone of the lingual-tonsillar branch of the glossopharyngeal nerve and the terminal zone of the greater superficial petrosal nerve are unaffected by the dietary restriction paradigm (King and Hill, 1991; Sollars and Hill, 2000). Further morphological alterations occur in the structure of neurons located within the NTS. The dendritic organization of NTS neurons in developmentally sodium restricted rats and in restricted rats fed the sodium replete diet after weaning are much different than that in control rats (King and Hill, 1993). The dendrites of putative relay neurons, multipolar and fusiform neurons, are longer and/or have more dendritic processes in sodium restricted rats compared with controls. Thus, the expanded terminal field of the chorda tympani nerve co-exists with increased dendritic branching of neurons in the NTS.
In all of the studies noted above, dietary sodium restriction was performed early in prenatal development and the consequences of the manipulation were observed at adulthood only. Therefore, it is possible that selective restriction-induced alterations to the adult terminal field pattern could be in place at or near the time of birth and, therefore, be present at early postnatal ages. Alternately, the abnormal afferent terminal field may be expressed only at later postnatal times. These two outcomes imply much different mechanisms.
During normal development, significant stimulus-elicited responses are not seen until about the second post-natal week (Hill and Almli, 1980; Yamada, 1980; Ferrell et al., 1981). However, sodium-restricted rats fail to develop normal functional responses to sodium (Hill et al., 1986; Hill, 1987; Hill and Przekop, 1988). If terminal field morphology is similar between sodium-restricted and control rats during early development, this finding would imply that the diet-related differences in taste activity expressed later in development are responsible for the morphological alterations at adulthood. Conversely, if terminal field morphology is abnormal in sodium-restricted rats early in development, this would suggest that factors established prior to the onset of mature taste function are responsible for the alterations in terminal field morphology.
Therefore, the age at which the terminal fields of the chorda tympani nerve differs between control and sodium restricted rats may provide insights about mechanisms of synaptic plasticity in the central gustatory system. To explore the potential mechanisms of plasticity, the current study focused on the time course of the expression of the terminal field development at four postnatal ages in control and sodium-restricted rats.
EXPERIMENTAL PROCEDURES
Animals
The topography of the chorda tympani nerve terminal field in the NTS was studied via anterograde transport of 3 kD biotinylated dextran amine in two groups of Sprague–Dawley rats at four developmental ages. Both sodium-restricted and control rats were studied at 15–17 days postnatal (P15–17), 25–27 days postnatal (P25–27), 35–37 days postnatal, and 43–200 days postnatal (adult). These ages were selected to correspond to important periods during normal development: during normal increased sensitivity to Na+ salts in the NTS (P15–17) (Hill et al., 1983); during the end of the period when the chorda tympani nerve terminal field is adult-like in size within the NTS and when peripheral responses to salts are nearly mature (P25–27) (Hill and Almli, 1980; Yamada, 1980; Ferrell et al., 1981; Lasiter et al., 1989; Lasiter, 1992); before taste responses in NTS neurons mature (P35–37) (Hill et al., 1983), and at adulthood (adult; >50 days) when adult morphology and function occur (Hill and Almli, 1980; Yamada, 1980; Ferrell et al., 1981; Lasiter et al., 1989; Lasiter, 1992). The adult period also corresponds with the age used in our prior studies of dietary-induced changes in chorda tympani nerve terminal field (King and Hill, 1991). The number of rats per group was as follows: restricted P15–17, n=5; control P15–17, n=5; restricted P25–27, n=6; control P25–27, n=5; restricted P35–37, n=5; P35–37, n=6; restricted adult, n=5; control adult, n=5.
Sodium restriction during early development was accomplished by feeding pregnant rats (Harlan Sprague–Dawley; Dublin, VA, USA) a sodium-deficient diet consisting of 0.03% NaCl (I.C.N-Nutritional Biochemicals; Aurora, OH, USA) from E3 until the time of weaning (21 days postnatal). Pups born to mothers fed the NaCl-deficient diet were weaned on the NaCl-deficient diet. Control rats were born to mothers that were always maintained on normal 0.5% NaCl rat chow. Distilled water was freely available to all rats throughout the study.
Surgical procedures
All experiments were performed according to procedures approved by the University of Virginia Animal Care and Use Committee and National Institutes of Health guidelines. All efforts were made to minimize the number of animals used and their suffering. Rats were anesthetized with Methohexital sodium (Brevital®, 50 mg/kg, i.p.; King Pharmaceuticals; Bristol, TN, USA). A small incision was made on the ventromedial portion of the neck. The ventral aspect of the tympanic bulla was exposed and a hole was placed in its surface using microfine forceps. The chorda tympani nerve was sectioned distal to the geniculate ganglion and dimethylsulfoxide was applied to the cut end for approximately 15 s. Crystals of biotinylated dextran amine (Molecular Probes; Eugene, OR, USA) were placed on the nerve and the animal remained stabilized in a supine position for approximately 30 min. Thermal regulation was maintained through the use of a standard heating pad. Upon recovery from the anesthetic, rats were returned to their home cage.
Optimal time of transport for the dextran was determined to be a minimum of 8 h. Rats were killed with sodium pentobarbital (80 mg/kg, i.p.) 8–24 h after the application of the anterograde tracer. No differences were noted as a result of the length of time that the tracer was allowed to transport. Rats were perfused transcardially with modified Krebs solution (pH=7.3) containing 0.5% glucose (Lasiter, 1992) followed by 8% paraformaldehyde. Medullae were then removed and placed in 8% paraformaldehyde overnight. Brainstems were sectioned in the horizontal plane at 50 μm with a vibratome to allow the visualization of the entire extent of the terminal fields in both the rostral–caudal and medial–lateral planes (King and Hill, 1991; Krimm and Hill, 1997). Tissue was then processed using standard diaminobenzidine (DAB) procedures.
Quantification
Terminal fields of the chorda tympani nerve were observed under brightfield microscopy. Serial reconstruction of the labeled chorda tympani afferent field was accomplished using a computer microscope system (Neurolucida; MicroBrightField, Inc.; Burlington, VT, USA). Companion software calculated the area of each outlined section and the area was multiplied by the thickness of the section. All sections were summed to give an estimate of the total volume of the chorda tympani nerve terminal field (Lasiter et al., 1989; King and Hill, 1991; Lasiter, 1992). The data were coded prior to measurement so that terminal field measurements were obtained blind relative to the experimental group.
Statistical tests
Planned, a priori comparisons were used to examine differences in mean terminal field volumes. Separate analyses were done for each dietary group to determine age-related differences. A separate analysis was done to compare group-related differences at each age. Accordingly, the α-level of 0.05 was divided by the number of comparisons in each analysis (0.05/3 for each age-related analysis and 0.05/4 for diet-related differences).
RESULTS
Quality of terminal field label
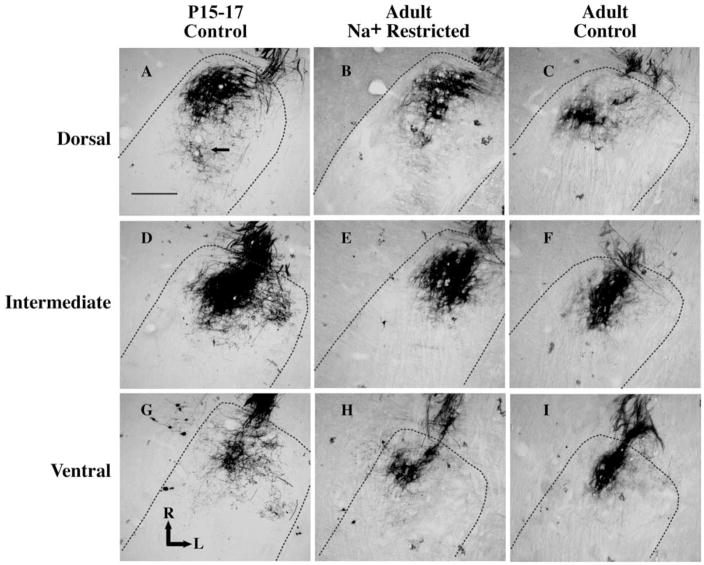
As seen in Fig. 1, terminal fields were densely labeled in control and sodium-restricted rats in all age groups, and systematic differences in labeling efficiency were not noted among groups. In many sections, clear anterograde labeling was evident beyond the densest portion of the terminal field and was included in the volume measurement (e.g. Fig. 1A). Terminal endings and varicosities could be seen at the margins of the label (see Fig. 1A). Therefore, terminal field volume measurements included the entire region occupied by all labeled axons with terminals. Data were not included from animals in which robust and distinct labeling was not evident.
Fig. 1.
Photomicrographs of horizontal sections through the rostral NTS in a P15–17 day control rat (A, D, G), an adult sodium-restricted rat (B, E, H) and an adult control rat (C, F, I). The dorsal (A, B, C), intermediate (D, E, F) and ventral (G, H, I) zones of the chorda tympani nerve terminal field are shown for each rat. The chorda tympani terminal field is the dark reaction product within the rostral pole of the NTS. Dashed lines outline the borders of the NTS. The scale bar in panel A denotes 200 μm and the solid arrow points to examples of axons with visible varicosities. L, lateral; R, rostral.
Total terminal field volumes
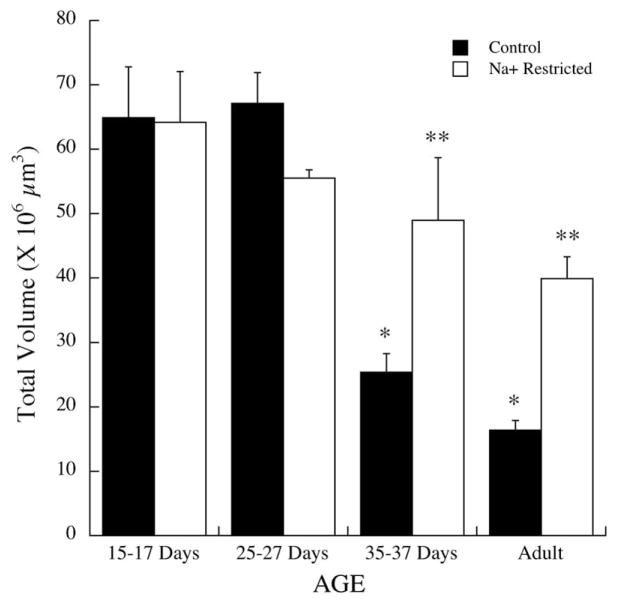
Diet-related differences in the chorda tympani terminal field initially occurred in the P35–37 group and were maintained at adulthood (Fig. 2). At P15–17 days, the mean (±S.E.M.) terminal field volume in the NTS of controls was 64.9±7.9×106 μm3 compared with 64.2±8.0×106 μm3 in sodium-restricted rats (P>0.10). Similarly, there were no significant differences between controls and sodium-restricted rats at P25–27 (controls: 67.2±4.8×106 μm3; sodium restricted: 55.5±1.3×106 μm3; P>0.10). However, at P35–37 the terminal field volume in controls was approximately 50% smaller than in sodium-restricted rats (controls: 25.4±2.9×106 μm3; sodium restricted: 49.0±9.7×106 μm3; P<0.01), and the terminal field in controls was approximately 60% smaller than in sodium-restricted rats at adulthood (controls: 16.3±1.5×106 μm3; sodium restricted: 39.9±3.4; P< 0.0001).
Fig. 2.
Total chorda tympani nerve terminal field volumes in the NTS in control (solid bars) and sodium-restricted rats (open bars) at P15–17, P25–27, postnatal days 35–37 and at adulthood. Standard errors (S.E.M.) are shown above the respective bar. Single asterisks denote mean volumes significantly different than the preceding age group of controls. Double asterisks denote significantly different means between same-aged control and sodium-restricted rats.
As seen in Fig. 2, the diet-related changes that occurred in P35–37 and adult rats were due to changes in the terminal field volume in controls and not to changes in sodium-restricted rats. That is, the volume decreased significantly in control rats from P25–27 to P35–37 days (P<0.0001) and then again from P35–37 to adulthood (P<0.001). In contrast, there were no age-related changes in terminal field volume in sodium-restricted rats (Fig. 2; P>0.10).
Terminal field analysis by dorsal/ventral zones
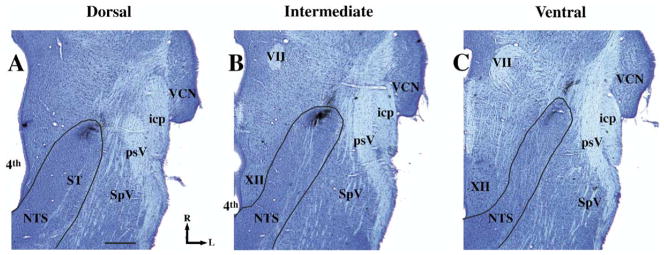
To be consistent with our previous studies of the chorda tympani terminal field in the NTS (King and Hill, 1991; Krimm and Hill, 1997), the terminal field was divided into three contiguous horizontal zones designated as dorsal, intermediate and ventral. However, due to smaller NTS sizes for the youngest age in the current study and difficulty in visualizing all anatomical landmarks at this age or developmental differences in the location of the structure (e.g. the salivatory nucleus extended much more dorsally in young rats), the dorsal–ventral zone classifications were revised from those used previously (King and Hill, 1991; Krimm and Hill, 1997). The dorsal zone extended dorsally from the ventral-most extent of the solitary tract and included sections in which the fourth ventricle occupied the largest medial–lateral extent (Fig. 3A). The dorsal zone was further characterized by the spinal trigeminal tract extending to approximately the rostral-most extent of the NTS and by the lack of the hypoglossal nucleus and the facial nucleus (Fig. 3). The intermediate zone was defined by sections in which the hypoglossal nucleus was present, where the spinal trigeminal tract extended rostrally beyond the inferior cerebellar peduncle, and by the presence of the dorsal extent of the facial nucleus (Fig. 3B). The ventral zone of the field had an expanded hypoglossal nucleus and facial nucleus compared with the intermediate sections (Fig. 3B and 3C). In all animals, regardless of age- or diet-related group, the terminal field in the intermediate zone was most easily identified and most consistent across animals. Axons entered the NTS from a rostral–lateral direction and projected into the NTS to form a dense and compact terminal field. The trajectory of the entering axons was characteristic in that they were parallel to the orientation of the field (Figs. 2B, 2E, 2H, 3B). Dorsal zones were characterized by a less compact terminal field organization and axons from a rostral–lateral direction, while the terminal field was oriented primarily medial to lateral (Figs. 2A, 2D, 2G, 3A). Ventral zones lacked the topography of the terminal field seen in the intermediate zone in that it was narrower than the intermediate zone and less oval in shape (Figs. 2C, 2F, 2I, 3C). Therefore, both landmarks near the terminal field and the overall shape of the terminal field were used to categorize sections into dorsal, intermediate and ventral sections. It must be pointed out that we use the terms “dorsal,” “intermediate,” and “ventral” zones here to be consistent with earlier reports (King and Hill, 1991; Krimm and Hill, 1997); however, the orientation of the NTS within the brainstem is such that the dorsal-most portion of the chorda tympani nerve terminal field label in the NTS is caudal to the majority of terminal field label in the intermediate and ventral zones. Therefore, the “dorsal” zone more accurately represents the dorsal–caudal portion of the field in the NTS and the intermediate and ventral sections represent more ventral–rostral portion of the terminal field in the NTS.
Fig. 3.
Photomicrographs of Nissl-stained horizontal sections in a P35 day control rat illustrating sections typical of the dorsal (A), intermediate (B), and ventral (C) zones of the chorda tympani nerve terminal field and anatomical landmarks in the brainstem. The solid line denotes the outline of the NTS. The terminal fields of the chorda tympani nerve are shown in the rostral pole of the NTS. This tissue is used for illustration purposes only and data were not obtained from Nissl-stained sections. The scale bar=500 μm in A. icp, inferior cerebellar peduncle; L, lateral; psV, spinal trigeminal tract; R, rostral; SpV, interpolar division of spinal trigeminal nucleus; ST, solitary tract; VCN, ventral cochlear nucleus; VII, facial nucleus; XII, hypoglossal nucleus; 4th, fourth ventricle.
Dorsal zone
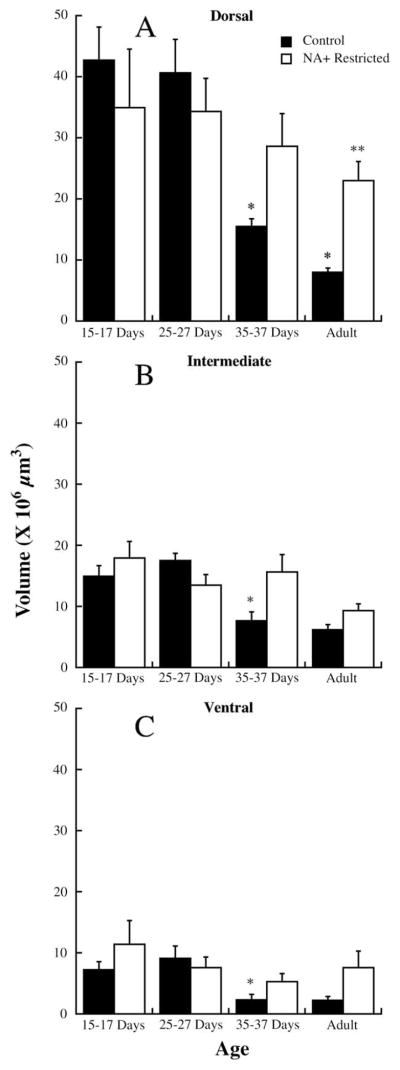
The pattern of group-related and age-related differences in the dorsal zone of the chorda tympani field was generally the same as that seen for the total terminal field. The only exception was that diet-related differences did not occur until after P35–37 days in the dorsal region (Fig. 4A), as compared with before P35–37 days in the total terminal field (Fig. 2).
Fig. 4.
Chorda tympani nerve terminal field volumes for control (solid bars) and sodium-restricted rats (open bars) in the dorsal (A), intermediate (B) and ventral (C) zones of the terminal field in rats aged P15–17, P25–27, postnatal days 35–37, and adults. S.E.M.s are shown above the respective bar. Single asterisks denote mean volumes significantly different than the preceding age group of controls. Double asterisks denote significantly different means between same-aged control and sodium-restricted rats.
There were no differences in the mean (±S.E.M.) volumes between control and sodium-restricted rats at 15–17 days (control: 42.8±5.4×106 μm3; sodium restricted: 34.9± 9.6 106 μm3; P>0.10) at 25–27 days (control: 40.6±5.5×106 μm3; sodium restricted: 34.2±1.2×106 μm3; P>0.10) and at 35–37 days (control: 15.5±1.3×106 μm3; sodium-restricted: 28.6±6.5×106 μm3; P>0.10; Fig. 4A). However, the terminal field volume in the dorsal zone of adult control rats was approximately 65% smaller in controls compared with sodium-restricted rats (control: 8.0±0.8×106 μm3; sodium restricted: 23.0±3.1×106 μm3; P=0.002; Fig. 4A).
As noted for total terminal field measurements (Fig. 1), the dietary group-related effects appear due to an age-related decrease in terminal field size in controls, but not in sodium-restricted rats. There was approximately a 60% decrease in dorsal terminal field volume in controls from P25–27 to P35–37 (P=0.001) and an additional 50% decrease from P35–37 to adulthood (P=0.001). No significant age-related changes in dorsal terminal field volume occurred in sodium-restricted rats (Fig. 4A).
While there were obvious differences in the terminal field volumes in comparable sections across ages, the age-related differences for controls were also due to more sections in the dorsal zone of young rats containing terminal field label compared with rats aged 35–37 days and adults. Specifically, terminal field label was seen in an average (±S.E.M.) of 8.8 (±0.2), 7.6 (±0.8), 5.2 (±0.2), and 5.0 (±0.3) 50 μm sections in P15–17, P25–27, P35–37 and adult control rats, respectively. There was a significant decrease in the number of labeled sections between P25–27 and P35–37 (P=0.01) and between P35–37 and adults (P=0.01). Therefore, the label in P15–17 control rats extended approximately 190 μm dorsal to that seen in adult control rats. This is especially impressive given the large difference in brain size between the two groups. No significant differences in the number of sections containing label were found in sodium-restricted rats.
Intermediate zone
Unlike noted for the dorsal zone, there were no differences in terminal field volumes between control and sodium-restricted rats at any age (Fig. 4B). However, the means (±S.E.M.) at P35–37 days (control: 7.6±1.5×106 μm3; sodium-restricted: 15.6±2.9×106 μm3; P>0.05) and at adulthood (control: 6.2±0.8×106 μm3; sodium-restricted: 9.3±1.1×106 μm3; P>0.05) approached significance.
There was approximately a 56% decrease in terminal field volume in controls initially between P25–27 and P35–37 (P25–27: 17.4±1.2×106 μm3; P35–37: 7.6±1.5×106 μm3; P=0.001) and no further change in volume from P35–37 to adulthood (Fig. 4B). Therefore, the age-related decrease in terminal field volume of controls in the intermediate zone was similar to that seen in the dorsal zone.
There were no age-related or diet-related differences in the number of sections that contained terminal field label in the intermediate zone (P>0.10).
Ventral zone
The pattern of terminal field changes (or lack of changes) in the ventral zone was the same as described for the intermediate zone (Fig. 4C). Specifically, there were no differences in terminal field volumes between control and sodium-restricted rats at any age, although the means (±S.E.M.) at P35–37 days (control: 2.3±0.9×106 μm3; sodium-restricted: 5.3±1.3×106 μm3; P>0.05) and at adulthood (control: 2.2±0.6×106 μm3; sodium-restricted: 7.6±2.7×106 μm3; P>0.05) approached significance. Furthermore, there was approximately a 75% decrease in terminal field volume in controls initially between P25–27 and P35–37 (P25–27: 9.0±0.2×106 μm3; P35–37: 2.3±0.6×106 μm3; P=0.001) and no further change in volume from P35–37 to adulthood (Fig. 4C). There were no age-related or diet-related differences in the number of sections in the ventral zone that contained terminal field label (P>0.10).
DISCUSSION
The results of this study demonstrate that pre- and post-natal sodium restriction-induced alterations in the rat NTS occur between postnatal days 25 and 35. That is, the changes reported due to early dietary manipulations (King and Hill, 1991) are not expressed morphologically until after weaning. Therefore, even though the dietary manipulation must begin early in embryonic development to have profound central morphological influences (King and Hill, 1991), the anatomical expression of the effects does not occur until much later. The group-related differences appear related more to the lack of terminal field reorganization during development in sodium-restricted rats and not to an abnormally expanded field.
Findings from these experiments are the first to show that the chorda tympani field remodels with a four-fold decrease in terminal field volume from postnatal days 25–35 during normal development. Unlike controls, however, there was a lack of a developmental decrease in total volume and lack of terminal field reorganization in sodium-restricted rats. Once the field expanded normally by P15–17 in sodium-restricted rats, it was “frozen” at an immature state. That is, the overall size and the volume in the dorsal zone of the field did not change with age; the expanded terminal field in sodium-restricted rats in the dorsal zone was maintained well into adulthood (Fig. 4A and King and Hill, 1991).
Comparison with previous developmental studies
The data from control rats in the current study contrast with those reported by Lasiter (1992). They showed that there was a large increase in total terminal field volume of the facial nerve from P7 to approximately P25 and that the size was stable thereafter. The lack of observing a significant increase in terminal field volume by P15 followed by a reorganization after P25 may be partially due to differences in labeling techniques. Lasiter (1992) labeled the central stump of the facial nerve (combined chorda tympani and greater superficial petrosal nerves) with Lucifer Yellow in situ. Therefore, he killed the rats before applying the anterograde tracer and allowed the tracer to transport three to six hours. It is possible that these procedures are less sensitive for transport efficiency and for visualization of the terminal field compared with the procedures used here.
The difference in the labels and reaction used in our earlier studies (King and Hill, 1991; Krimm and Hill, 1997) compared with the current study may also explain the larger volumes seen here for both adult controls and sodium-restricted rats. Differences in how the dorsal–ventral zones were defined among studies also likely contribute to differences in absolute volumes. We chose to anchor our categorization of the dorsal–ventral zones with the intermediate zone (see Experimental Procedures), primarily because of the ability to reliably identify these sections in all animals. However, it is possible that differences in how the three zones were defined resulted in a larger dorsal zone here as compared with our previous work (King and Hill, 1991; Krimm and Hill, 1997). Nonetheless, the largest diet-related changes in all studies occurred in the dorsal zone.
Additionally, Pittman and Contreras (2002) provide an interesting contrast to our dietary-induced effects. By feeding rats a high NaCl diet (6.0% NaCl) throughout pre- and postnatal development, the chorda tympani terminal field was also abnormally large in the dorsal zone compared with controls. Thus, early low and high NaCl diets produced similar effects in terminal field development. Pittman and Contreras (2002) did not assess when the diet-related effects occurred; therefore, developmental comparisons cannot be made.
Neural activity and terminal field reorganization
Interestingly, the age when the total terminal field volume in sodium-restricted rats is noticeably different from that in control rats is at about the same age that functional sodium responses in the chorda tympani nerve diverge between groups (Hill, 1987). This is when stimulus-induced responses reach maturity in control rats (Hill and Almli, 1980; Yamada, 1980; Ferrell et al., 1981). Therefore, the afferent activity responsible for the reorganization of the terminal field in controls may not be present in sodium-restricted rats. It should be noted, however, that there is a nonsignificant decrease in terminal field volumes between P15–17 and adult sodium-restricted rats (Fig. 2), which is consistent with a corresponding, but small, age-related increase in sodium salt responses (Hill, 1987). Furthermore, rats given restricted taste stimulation early postnatally (Lasiter, 1995) failed to develop normal-sized terminal fields. However, the terminal fields of rats failing to receive adequate stimulation in Lasiter’s (1995) study were abnormally small, unlike the enlarged fields seen with developmental sodium restriction. Since Lasiter (1995) first manipulated the gustatory system after birth and rats in the current study had the diet manipulation initiated very early in development, it is likely that rats in the two studies do not share the same prenatal brain developmental programs and may explain the differences in experimentally induced outcomes.
The role of afferent activity in shaping neuronal architecture during development has also been implicated in neurons presumed to be postsynaptic to chorda tympani neurons. Liu et al. (2000) showed that NTS neurons that are especially sensitive to salt taste stimuli decrease dendritic field size from P22–28 to adulthood in control rats; dendritic field size in neurons not sensitive to salt stimuli fails to change with age. This suggests that the postsynaptic targets follow an activity-dependent “pruning” similar to the decrease in terminal field size. Similar examples from other sensory systems demonstrate that neuronal activity shapes terminal fields (Cline, 1998; Zhang et al., 1998, 2000; Tao et al., 2000, 2001; Zhang and Poo, 2001) through “pruning” of axonal arbors (Nakamura and O’Leary, 1989; O’Leary et al., 1990; Weimann et al., 1999; Lichtman and Colman, 2000; Bagri et al., 2003; Kantor and Kolodkin, 2003; Watts et al., 2003).
Non-activity dependent reorganization of terminal fields
Dietary-induced alterations could also occur due to cellular/molecular mechanisms that act upon the incoming fibers and/or the target NTS. For example, differences in factors such as neurotrophins (Snider, 1994; Huang and Reichardt, 2001) and molecular gradients such as ephrins and their receptors (Goodhill and Richards, 1999; Prakash et al., 2000; Yates et al., 2001; Hansen et al., 2004; King et al., 2004; Person et al., 2004) may play a role in determining the diet-related differences in terminal fields at adulthood. As such, the effects seen here may not be entirely activity dependent, but may also include activity-independent mechanisms. It is possible that the processes that direct development of the chorda tympani nerve terminal field may be affected by dietary manipulations very early in development and only expressed much later.
Regardless of the mechanism(s) that underlie the current results, this study demonstrates that alterations in terminal field volumes of the chorda tympani nerve occur during normal development and points to a dramatic structural reorganization. Further, such age-related alterations are centered in the dorsal zone of the terminal field, suggesting that this region may be the focus of significant neuronal modifications that are coordinated with maturation of functional and/or behavioral development (current study and Lasiter and Kachele, 1990; King and Hill, 1991; Lasiter and Diaz, 1992; Lasiter, 1995; Krimm and Hill, 1997; Pittman and Contreras, 2002). Moreover, the lack of these alterations in sodium-restricted rats provides an excellent model to further examine the underlying mechanisms responsible for terminal field maturation and plasticity in the gustatory brainstem.
Acknowledgments
This work was supported by NIH grant DC-00407 to D.L.H. and DC-04846 to S.I.S.
Abbreviations
- E
embryonic day
- NTS
nucleus of the solitary tract
- P15–17
15–17 days postnatal
- P25–27
25–27 days postnatal
- S.E.M
standard error of the mean
References
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Cline HT. Topographic maps: developing roles of synaptic plasticity. Curr Biol. 1998;8:R836–R839. doi: 10.1016/s0960-9822(07)00525-8. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Brunjes PC. The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol. 1997;148:360–366. doi: 10.1006/exnr.1997.6660. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. J Neurosci. 1997;17:7433–7440. doi: 10.1523/JNEUROSCI.17-19-07433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell MF, Mistretta CM, Bradley RM. Development of chorda tympani taste responses in rat. J Comp Neurol. 1981;198:37–44. doi: 10.1002/cne.901980105. [DOI] [PubMed] [Google Scholar]
- Fox K, Wallace H, Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Philos Trans R Soc Lond B Biol Sci. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhill GJ, Richards LJ. Retinotectal maps: molecules, models and misplaced data. Trends Neurosci. 1999;22:529–534. doi: 10.1016/s0166-2236(99)01469-1. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Brain Res Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Hill DL. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol (Lond) 1987;393:413–424. doi: 10.1113/jphysiol.1987.sp016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Almli CR. Ontogeny of chorda tympani nerve responses to gustatory stimuli in the rat. Brain Res. 1980;197:27–38. doi: 10.1016/0006-8993(80)90432-1. [DOI] [PubMed] [Google Scholar]
- Hill DL, Bradley RM, Mistretta CM. Development of taste responses in rat nucleus of solitary tract. J Neurophysiol. 1983;50:879–895. doi: 10.1152/jn.1983.50.4.879. [DOI] [PubMed] [Google Scholar]
- Hill DL, Mistretta CM, Bradley RM. Effects of dietary NaCl deprivation during early development on behavioral and neurophysiological taste responses. Behav Neurosci. 1986;100:390–398. doi: 10.1037//0735-7044.100.3.390. [DOI] [PubMed] [Google Scholar]
- Hill DL, Przekop PR., Jr Influences of dietary sodium on functional taste receptor development: a sensitive period. Science. 1988;241:1826–1828. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor DB, Kolodkin AL. Curbing the excesses of youth: molecular insights into axonal pruning. Neuron. 2003;38:849–852. doi: 10.1016/s0896-6273(03)00364-7. [DOI] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- King C, Lacey R, Rodger J, Bartlett C, Dunlop S, Beazley L. Characterisation of tectal ephrin-A2 expression during optic nerve regeneration in goldfish: implications for restoration of topography. Exp Neurol. 2004;187:380–387. doi: 10.1016/j.expneurol.2004.02.006. [DOI] [PubMed] [Google Scholar]
- King CT, Hill DL. Dietary sodium chloride deprivation throughout development selectively influences the terminal field organization of gustatory afferent fibers projecting to the rat nucleus of the solitary tract. J Comp Neurol. 1991;303:159–169. doi: 10.1002/cne.903030114. [DOI] [PubMed] [Google Scholar]
- King CT, Hill DL. Neuroanatomical alterations in the rat nucleus of the solitary tract following early maternal NaCl deprivation and subsequent NaCl repletion. J Comp Neurol. 1993;333:531–542. doi: 10.1002/cne.903330406. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Early prenatal critical period for chorda tympani nerve terminal field development. J Comp Neurol. 1997;378:254–264. [PubMed] [Google Scholar]
- Lasiter PS. Postnatal development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Bull. 1992;28:667–677. doi: 10.1016/0361-9230(92)90245-s. [DOI] [PubMed] [Google Scholar]
- Lasiter PS. Effects of orochemical stimulation on postnatal development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Bull. 1995;38:1–9. doi: 10.1016/0361-9230(95)00063-k. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Diaz J. Artificial rearing alters development of the nucleus of the solitary tract. Brain Res Bull. 1992;29:407–410. doi: 10.1016/0361-9230(92)90076-a. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Kachele DL. Effects of early postnatal receptor damage on development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Dev Brain Res. 1990;55:57–71. doi: 10.1016/0165-3806(90)90106-9. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Wong DM, Kachele DL. Postnatal development of the rostral solitary nucleus in rat: dendritic morphology and mitochondrial enzyme activity. Brain Res Bull. 1989;22:313–321. doi: 10.1016/0361-9230(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Liu YS, Schweitzer L, Renehan WE. Development of salt-responsive neurons in the nucleus of the solitary tract. J Comp Neurol. 2000;425:219–232. doi: 10.1002/1096-9861(20000918)425:2<219::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Nakamura H, O’Leary DD. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci. 1989;9:3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Bicknese AR, De Carlos JA, Heffner CD, Koester SE, Kutka LJ, Terashima T. Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harb Symp Quant Biol. 1990;55:453–468. doi: 10.1101/sqb.1990.055.01.045. [DOI] [PubMed] [Google Scholar]
- Person AL, Pat Cerretti D, Pasquale EB, Rubel EW, Cramer KS. Tonotopic gradients of Eph family proteins in the chick nucleus laminaris during synaptogenesis. J Neurobiol. 2004;60:28–39. doi: 10.1002/neu.10330. [DOI] [PubMed] [Google Scholar]
- Pittman DW, Contreras R. Dietary NaCl influences the organization of chorda tympani neurons projecting to the nucleus of the solitary tract in rats. Chem Senses. 2002;27:333–341. doi: 10.1093/chemse/27.4.333. [DOI] [PubMed] [Google Scholar]
- Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, Frostig RD. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20:5841–5847. doi: 10.1523/JNEUROSCI.20-15-05841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Crissman RS, Jacquin MF. Primary afferent plasticity following partial denervation of the trigeminal brainstem nuclear complex in the postnatal rat. J Neurosci. 1994;14:721–739. doi: 10.1523/JNEUROSCI.14-02-00721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan WE, Klein BG, Chiaia NL, Jacquin MF, Rhoades RW. Physiological and anatomical consequences of infraorbital nerve transection in the trigeminal ganglion and trigeminal spinal tract of the adult rat. J Neurosci. 1989;9:548–557. doi: 10.1523/JNEUROSCI.09-02-00548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Lack of functional and morphological susceptibility of the greater superficial petrosal nerve to developmental dietary sodium restriction. Chem Senses. 2000;25:719–727. doi: 10.1093/chemse/25.6.719. [DOI] [PubMed] [Google Scholar]
- Tao H, Zhang LI, Bi G, Poo M. Selective presynaptic propagation of long-term potentiation in defined neural networks. J Neurosci. 2000;20:3233–3243. doi: 10.1523/JNEUROSCI.20-09-03233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Zhang LI, Engert F, Poo M. Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron. 2001;31:569–580. doi: 10.1016/s0896-6273(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Hill DL. Enduring alterations in neurophysiological taste responses after early dietary sodium deprivation. J Neurophysiol. 1993;69:832–841. doi: 10.1152/jn.1993.69.3.832. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, McConnell SK. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Yamada T. Chorda tympani responses to gustatory stimuli in developing rats. Jpn J Physiol. 1980;30:631–643. doi: 10.2170/jjphysiol.30.631. [DOI] [PubMed] [Google Scholar]
- Yan J. Development and plasticity of the auditory cortex. Can J Neurol Sci. 2003;300:189–200. doi: 10.1017/s0317167100002572. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21:8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]