Abstract
Objective
To test 3 proposed models for adaptive thermogenesis in compartments of energy expenditure following different degrees of weight loss. Specifically, 1.) There is no adaptive thermogenesis (constant relationship of energy expenditure (EE) to metabolic mass). 2.) There is a fixed degree of adaptive thermogenesis once fat stores are below a “threshold”. 3.) The degree of adaptive thermogenesis is proportional to weight loss.
Methods
The relationship between weight loss and EE was examined in seventeen weight stable in-patient subjects with obesity studied at usual weight and again following a 10% and a 20% weight loss.
Results
Following initial weight loss (10%), resting (REE) and non-resting (NREE) EE were significantly below those predicted on the basis of the amount and composition of weight lost. Further reductions below predicted values of NREE but not REE occurred following an additional 10% weight loss. Changes in body weight, composition, and/or energy stores were significantly correlated with changes in EE.
Conclusion
All models are applicable to the decline in EE following weight loss. The disproportionate decline in REE is consistent with a threshold model (no change with further weight loss) while the disproportionate decline in NREE is largely reflective of the degree of weight loss.
Keywords: Obesity, Thermogenesis, Modeling, Leptin, Weight Regain
Introduction
In adults, the constancy of body weight and composition over long periods of time (the average American gains 0.5–2kg/year or about 4000 kcal of stored energy)1–4, despite ingestion of ~900,000–1,000,000 kcal/year2, 3, suggests that there are compensatory changes in energy intake and/or output that favor weight (fat mass) homeostasis5. It has been proposed that declines in energy expenditure (EE) following weight loss are simply proportional changes in body mass or composition (mechanical model); includes reductions in EE once a minimum threshold for fat mass is crossed (threshold model), or invoked continuously, with increasing strength, in proportion to the reduction in fat mass (spring-loading model) (Figure 1)6, 7.
Figure 1.
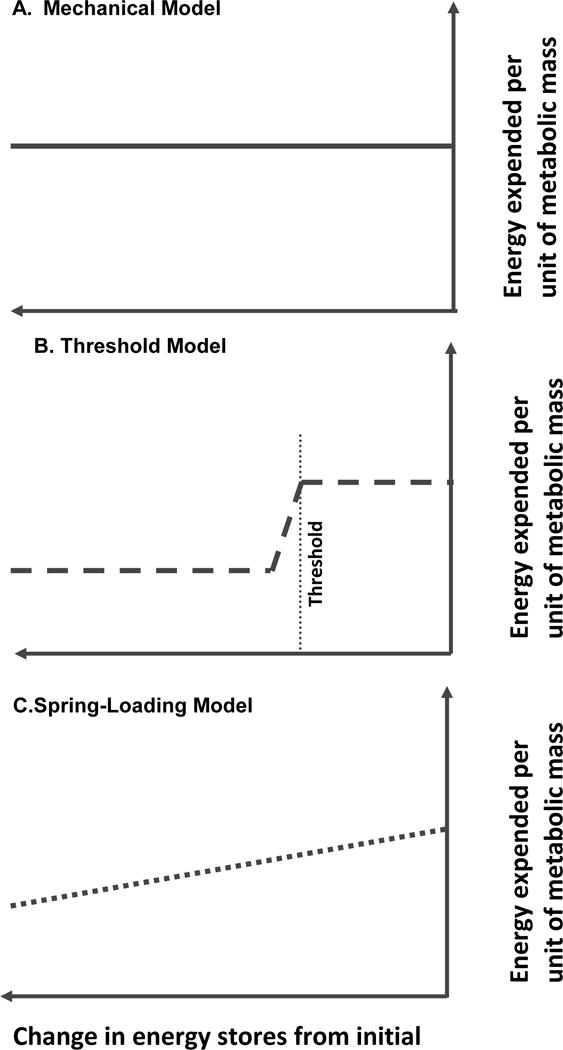
Models of changes in EE during maintenance of a reduced body weight.
A. Mechanical (solid line): In a mechanical model the reduction in EE following weight loss is directly proportional to the loss of energy stores (predominantly fat).
B. Threshold (dashed line: In a threshold model, reduction of body energy stores below a threshold induces adaptive thermogenesis resulting in a decline in EE but there is no further increase in adaptive thermogenesis following more weight loss below the threshold.
C. Spring loading (dotted line): In the stretching model the degree of adaptive thermogenesis is directly proportional to the amount of weight reduction being maintained.
The mechanical model is similar to that originally proposed by Wirtshafter and Davis8 as a body weight “settling point” which is dictated primarily by the environment. This model was analogized by Speakman et al7 to a lake. If rain (energy input) is increased or decreased there will be a change in the depth of the water (energy stores) and in drainage velocity (energy output) in the same direction, resulting passively in a new equilibrium between intake, storage, and output without changing the relationship of these variables.
In the lipostatic threshold model, maintenance of energy stores below an individual’s minimum limit (determined by genetics, development, and possibly environmentally-induced changes in the brain) provokes decreases in EE to below those attributable solely to changes in body mass or composition9–11. This model predicts that maximal adaptive thermogenesis will be invoked once energy stores are brought below this threshold5, 12.
In the spring-loading model, the relative strength of adaptive thermogenesis is proportional to the decline in energy stores in a manner that can be analogized to Hooke’s law13 which stipulates that the tension (T) on a spring is equal to the product of a constant (k) multiplied by the change in length of the spring (x). “T” in our body weight regulation experiments would be adaptive thermogenesis, “k” would vary between individuals but not be affected by the amount of weight lost, and “x” would be the amount of weight lost.
As shown in Figure 1, in the mechanical model, the relationship between EE and body composition is not affected by weight loss. In the threshold model, there is a new relationship between EE and body composition that is constant at all weights below the threshold. In the spring-loading model, the degree of adaptive thermogenesis is proportional to the decline in energy stores. Calculation of “residuals”, i.e changes in energy expenditure beyond those predicted solely on the basis of changes in body weight and composition following weight loss14 are a good means to test these models. The mechanical model predicts a zero residual at all degrees of weight loss since there is a constant relationship between EE and body composition. The threshold model predicts a significant residual once fat stores have fallen below a threshold but then no additional residual following further weight loss. Finally, the spring-loading model predicts that there is a significant negative residual that increases in proportion to the degree of weight loss.
We examined these models using data collected during in-patient studies of individuals who were obese and maintained on a controlled liquid formula diet at their usual weight, again at 10% reduced body weight; and again at 20% below usual15. We found that no model fully accounts for the decline in 24 hour EE following weight loss, and that adaptive thermogenesis – which does occur – was differentially accounted for by combined elements of the several models depending upon the specific component of EE being examined.
Methods
Subjects
Fourteen female and 3 male subjects with obesity (BMI>30.0 kg/m2) were studied at usual weight (Wtinitial), while maintaining a 10% weight loss (Wt−10%), and again while maintaining a 20% reduced body weight (Wt−20%). All subjects were healthy and at their maximal lifetime weight and had maintained this weight within a range of 2 kg for at least six months prior to enrollment. None were taking medications or eating special diets. These studies were IRB-approved and are consistent with guiding principles for research involving humans16. Written informed consent was obtained from all subjects 9 of whom have been described previously15. Screening and exclusion criteria have been described previously15.
Study Design
Subjects were in-patients on a Clinical Research Center and fed a liquid formula (40 percent fat [corn oil], 45 percent carbohydrate [glucose polymer], and 15 percent protein [casein hydrolysate]) supplemented daily with 5.0 g of iodized NaCl, 1.9 g of potassium ions as potassium salt, and 2.5 g of calcium carbonate, bi-weekly with1 mg of folic acid, and t36 mg of ferrous iron every other day throughout the study. The formula caloric content was 1.36 kcal/gm (bomb calorimetry) and 1.25 kcal/gm corrected for standard digestibility quotients17. Caloric intake was adjusted until weight stability, defined as mean weight variation of <10 gm/day over 14 days, was achieved15.
All subjects then underwent studies of body composition (Dual-energy X-ray Absorptiometry)18 and EE during an approximately 14 day. Twenty-four hour EE (TEE) defined as caloric intake required to maintain weight as described above, which correlates well (r2=0.88) with studies in the same subjects using the doubly labeled water method19. Resting EE (REE) and the thermic effect of feeding (TEF) were determined by indirect calorimetry with the use of a Beckman MMC Horizon Metabolic Cart (Beckman Instruments, Fullerton, Calif.). REE was measured in the post-absoprtive state at 8 AM. At 9 AM subjects were given 60 percent of the 24-hour REE measured that morning. TEF was calculated as the area of the polygon whose base is the prefeeding value of REE and whose other vertexes are EE at 9 AM,11 AM, and 1 PM. Non-resting EE (NREE, energy expended above resting not related to diet-induced thermogenesis) was calculated as:
Once subjects had completed studies at Wtinitial, all were fed 800 kcal/day of the liquid formula diet (range 7–13 weeks) until they had lost approximately 10% of Wtinitial. Energy intake was then titrated to maintain weight stability at a reduced weight as described above. Once stabilized, subjects underwent the same studies at Wt−10% as they had at Wtinitial. Following completion of studies at Wt−10%, all 17 subjects were again fed 800 kcal/day (range 8–14 weeks) until they had lost an additional 10% of their initial body weight (Wt−20%). Caloric intake was then titrated to maintain them at this reduced body weight and studies were repeated as described above.
Calculations and Statistical Analyses
All data are expressed as mean (S.D.). TEE and REE are expressed as kcal and as kcal/kg of fat-free mass (FFM). NREE is expressed as kcal kcal/kg of body weight20. For calculation of energy stores, chemical energy content was assigned as 9.4 kcal/g wet weight of fat mass and 0.91kcal/g wet weight of fat-free mass excluding bone4.
Initial statistical analyses were conducted using a forward stepwise regression analysis containing (F to enter = 1.0, F to remove = 0, Tolerance = 0.0010) in which EE (TEE, REE, or NREE) was the dependent variable and weight, fat mass, and fat-free mass, were the independent variables. Analyses presented include only variables that were included in the stepwise regression. Similar stepwise regressions were then run to see if the fractional change in body weight was a significant covariate of the relationship between EE and body composition or mass. When there was a significant weight plateau effect, similar analyses were conducted comparing each plateau. Comparisons between plateau values were made by ANOVA with repeated measures.
Regression lines relating EE to body composition and body mass do not have zero intercepts (Table 2A) and thus ratios of energy to mass may be different following weight loss even if values remain on the same regression line14. To enable the increased sensitivity of paired testing, and account for the non-zero intercepts described above, regression equations at Wtinitial were used to predict EE at Wt−10% and Wt−20%. The observed-minus-predicted values (residuals) were then tested against the null hypothesis that they equaled zero to determine there were significant plateau effects on the relationship of EE to body mass or composition14. Residual values between Wt−10% and Wt−20% were compared by ANOVA with repeated measures to determine whether additional weight loss significantly altered the magnitude of the residual. Regression equations relating changes from baseline in weight, FFM, and FM to residual changes in EE across plateaus were also calculated to determine if the magnitude of the residual was correlated with the absolute change in body composition (as predicted by the spring-loading model).
Table 2. Regressions and Correlations.
A. Regression equations relating different components of EE to body composition/weight without addition of fractional weight loss as a covariate.
B. Regression equations relating EE to body composition in all weight reduced subjects and in subjects at each weight plateau period. Residuals are calculated. Independent variables in italics are significant covariates of the dependent variable or, in the case of the intercept, significantly different from zero.
C. Correlations of changes in body weight, composition, and energy stores with changes and residuals in Twenty-four Hour EE (TEE), Resting EE (REE), the Thermic Effect of Feeding (TEF), and non-Resting EE (NREE) following a 10% weight loss from Wtinitial to Wt−10% and from Wt−20% to Wt−10%. N.S. – Not Significant. Significant correlations are in bold type
| A. | Overall Regression | Regression including % Weight Loss |
|---|---|---|
| TEE (kcal/day) |
211 + 26.5 (FFM) + 16.7 (FM) Overall adjusted R2=0.77, p<0.0001 Semipartial rFFM =0.56, p<0.0001 Semipartial rFM=0.40, p<0.0001 Intercept not significantly different from zero |
692 + 27.1 (FFM) + 10.8 (FM) + 26.1 (% Weight Loss) Overall adjusted R2=0.85, p<0.0001 Semipartial rFFM =0.57, p=0.0001 Semipartial rFM=0.25, p<0.0001 Semipartial rweight plateau =0.27, p<0.0001 Intercept p=0.0007 |
| REE (kcal/day) |
204 + 20.6 (FFM) + 4.4 (FM) Overall adjusted R2=0.75, p<0.0001 Semipartial rFFM =0.71, p<0.0001 Semipartial rFM=0.18, p=0.011 Intercept not significantly different from zero |
402 + 20.8 (FFM) + 2.4 (FM) +10.7 (% Weight Loss) Overall adjusted R2=0.78, p<0.0001 Semipartial rFFM =0.72, p<0.0001 Semipartial rFM=0.09, not significant Semipartial rweight plateau = 0.18, p=0.017 Intercept p=0.0042 |
| NREE (kcal/day) |
−94 + 8.5 (Weight) Overall R2 = 0.43, p<0.0001 Intercept not significantly different from zero |
247 + 7.0 (Weight) + 16.4 (% Weight Loss) Overall adjusted R2=0.52, p<0.0001 Semipartial rWeight =0.50, p<0.0001 Semipartial rweight plateau=0.32, p=0.0022 Intercept not significantly different from zero |
| B. | Regression Including Wt−10% and Wt−20% | Regressions at each plateau | Residuals vs. Wtinitial |
|---|---|---|---|
| TEE (kcal/day) |
597 + 27.8 (FFM) + 8.4 (FM) + 16.5 (% Weight Loss) Overall adjusted R2=0.81, P<0.0001 Semipartial rFFM =0.67, p<0.0001 Semipartial rFM = 0.21, p=0.024 Semipartial rweight plateau = 0.13, p=0.097 Intercept p=0.029 |
Wtinitial: 588 + 26.6(FFM) + 13.6(FM) Overall adjusted R2=0.79, P<0.0001 Wt−10% : 482 + 27.4(FFM) + 7.8(FM) Overall adjusted R2=0.76, P<0.0001 Wt−20% : 215 + 28.3(FFM) + 9.0(FM) Overall adjusted R2=0.80, P<0.0001 |
Wt−10%: −356 (292) P=0.0002 vs. 0 Wt−20%: −471 (248) P<0.0001 vs. 0 P=0.015 vs Wt−10% |
| REE (kcal/day) |
300 + 20.8 (FFM) + 1.5 (FM) + 2.3 (% Weight Loss) Overall adjusted R2=0.72, p<0.0001 Semipartial rFFM =0.75, P<0.0001 Semipartial rFM = 0.03, Not Significant Semipartial rweight plateau = 0.06, Not Significant Intercept not significantly different from zero |
Wtinitial: 356 + 21.1(FFM) + 3.3(FM) Overall adjusted R2=0.84, P<0.0001 Wt−10% : 174 + 23.1(FFM) + 0.8(FM) Overall adjusted R2=0.75, P<0.0001 Wt−20% : 211 + 22.9 (FFM) + 0.8 (FM) Overall adjusted R2=0.60, P=0.0003 |
Wt−10%: −190 (208) P=0.0002 vs. 0 Wt−20%: −186 (235) P<0.0001 vs. 0 P not significant vs Wt−10% |
| NREE (kcal/day) |
311 + 6.4 (Weight) + 16.8 (% Weight Loss) Overall adjusted R2=0.34, P=0.0006 Semipartial rWeight =0.50, p=0.0014 Semipartial rweight plateau=0.23, not significant Intercept not significantly different from zero |
Wtinitial: 142 + 7.8(Weight) R2=0.48, P=0.0019 Wt−10%: 213 + 5.8(Weight) R2=0.27, P=0.034 Wt−20%: −99 + 7.2 (Weight) R2=0.31, P=0.022 |
Wt−10%: −166 (288) P=0.035 vs. 0 Wt−20%: −285 (247) P=0.0006 vs. 0 P = 0.083 vs Wt−10% |
| C. | Delta Weight | Delta FFM | Delta FM | Delta Energy Stores | ||||
|---|---|---|---|---|---|---|---|---|
| Wt−10% vs. Wtinitial |
Wt−20% vs. Wt−10% |
Wt−10% vs. Wtinitial |
Wt−20% vs. Wt−10% |
Wt−10% vs. Wtinitial |
Wt−20% vs. Wt−10% |
Wt−10% vs. Wtinitial |
Wt−20% vs. Wt−10% |
|
| Delta TEE | R=0.44, p=0.085 | R=0.70, P=0.002 | N.S. | N.S. | N.S. | R=0.63, P=0.007 | N.S. | R=0.64, P=0.005 |
| TEE Residual | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Delta REE | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| REE Residual | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Delta NREE | R=0.47 P=0.059 | R=0.53, P=0.029 | N.S. | N.S. | N.S. | R=0.54, P=0.026 | R=0.43, p=0.088 | R=0.54, P=0.025 |
| NREE Residual | R=−0.41, P=0.091 | N.S. | R=0.48 P=0.051 | N.S. | N.S. | N.S. | N.S. | N.S. |
To ascertain whether data analyses of the effects of 10% weight loss on EE and the correlations between changes in EE and body composition were similar in this relatively small group of subjects with obesity to a larger more somatotypically diverse population, similar analyses were performed on an additional 50 subjects (30 obese, 20 never-obese; 20 males, 30 females) who were studied by us at Wtinitial and Wt−10% but not at Wt−20%15, 21–23. To compare effects of weight maintenance of increased and decreased body weight, we also included similar analyses of 12 subjects studied at Wtinitial, during maintenance of a 10% weight gain (Wt+10%) at Wt−10%.
Following weight loss, the changes in EE predicted based on Wtinitial data are predicted by the mechanical model. The residual at Wt−10%, minus any additional residual at Wt−20%, are accounted for by the threshold model. Any additional residual at Wt−20% vs. Wt−10% reflects the spring-loading model.
All statistical tests were two-tailed and statistical significance was prospectively defined as pα<0.05.
Results
Composition of Weight Loss of 10% and 20%
Changes in somatic energy stores were similar during both weight loss periods [Table 1, Mean (SD) % of weight loss as fat was 78.6 (22.7)% from Wtinitial to Wt−10% and 77.5 (24.7) % from Wt−10% to Wt−20%].
Table 1.
Mean (S.D.) description of subjects and EE at each weight plateau. EE is compartmentalized into Twenty-four Hour EE (TEE), Resting EE (REE), the Thermic Effect of Feeding (TEF), and non-Resting EE (NREE).
| Wtinitial | Wt−10% | Wt−20% | |
|---|---|---|---|
| Age (years) | 28.4 (8.8) | ||
| Gender | 3 Males, 14 Females | ||
| Weight (kg) | 126.9 (32.5) | 113.5 (29.4)* | 100.1 (26.5) |
| Body Mass Index (BMI,kg/m2) | 44.6 (11.2) | 39.5 (10.2)* | 34.4 (8.7) |
| Fat-free Mass (FFM, kg) | 63.4 (18.3) | 61.2 (16.5)* | 57.8 (16.0) |
| Fat Mass (FM, kg) | 63.4 (21.2) | 52.3 (17.9)* | 42.3 (16.0) |
| % Body Fat | 49.3 (9.3) | 45.3 (8.8)* | 41.4 (9.8) |
| FFM compared to Wtinitial (kg) | −2.3 (4.6) | −5.6 (6.0) | |
| FM compared to Wtinitial (kg) | −11.1 (4.2) | −21.2 (6.7) | |
| Change in Energy Stores compared to Wtinitial (kcal) | −106,773 (37,258) | −210,986 (73,635) | |
| TEE (kcal/day) | 3151 (724) | 2575 (600)* | 2232 (575) |
| TEE/FFM (kcal/kg/day) | 50.6 (7.6) | 42.2 (5.7)†‡ | 39.0 (5.1) |
| REE (kcal/day) | 1904 (429) | 1630 (438)* | 1522 (370) |
| REE/ FFM (kcal/kg/day) | 30.5 (3.7) | 26.9 (3.6)† | 26.8 (4.0) |
| TEF (kcal/day) | 114 (74) | 74 (47) | 82 (39) |
| NREE (kcal/day) | 1133 (364) | 871 (330)* | 618 (341) |
| NREE/body weight (kcal/kg/day) | 9.0 (2.0) | 7.5 (2.6)†# | 6.1 (3.0) |
P<0.001 vs., Wtinitial and Wt−20%;
P<0.001 vs. Wtinitial;
P<0.005 vs. Wt−20%;
p<0.05 vs. Wt−20%.
Regression Equations
At Wtinitial, and in analyses entering weight plateau as a covariate, TEE and REE were significantly correlated with both FFM and FM; therefore, both components of body composition were utilized in the calculation of residual values for TEE and REE following weight loss. NREE was significantly correlated with body weight and entry of FFM or FM into the regression did not improve the correlation. Therefore, calculations using NREE data included only body weight as a covariate (Table 2A).
Weight Plateau Effects
Fractional body weight change was a significant covariate of all measures of EE over all study plateaus (Table 2A) but in regressions containing data only at Wt−10% and Wt−20% was only significant for TEE and NREE (Table 2B). Maintenance of a reduced body weight was associated with significant declines in both the intercept and the regression coefficient relating TEE and REE to FM but not FFM. Similarly, there was a significant negative residual for all measures of EE at both reduced weight plateaus compared to Wtinitial. Compared to Wt−10%, residuals at Wt−20% were significantly lower for TEE, nearly significantly lower for NREE, and essentially unchanged for REE. The relative contributions of REE and NREE to adaptive thermogenesis following 10% weight loss, and the magnitude of the additional decline in the NREE residual from Wt−10% to Wt−20%, are similar to those in an earlier report on 9 of these subjects studied at 10% and 20% below usual weight15. Apportioning these changes to initial weight loss (Wt−10% − Wtinitial) and subsequent weight loss (Wt−20% − Wt−10%), declines in TEE during initial weight loss reflect approximately equal net contributions of REE and NREE while declines during subsequent weight loss reflect about 70% NREE and 30% REE (Table 1).
Overall, there were significant correlations of TEE and NREE residuals with changes in body weight across all three study periods (Figure 2). Further analyses of changes in the three components of EE between weight plateaus found no significant correlations between changes in EE and changes in body composition or body weight, between Wtinitial and Wt−10%. However, changes in TEE and NREE, but not REE, were significantly correlated with changes in weight, energy stores and FM, but not FFM between Wt−10% and Wt−20% (Table 2B).
Figure 2.
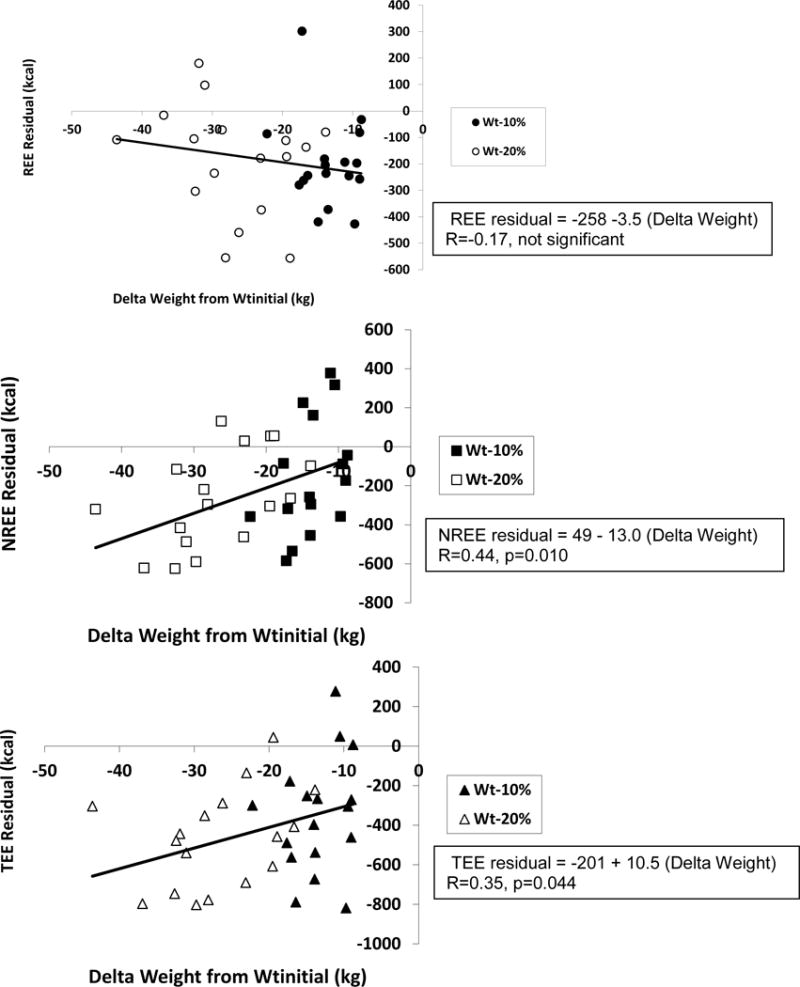
Additional Data Analyses
It is not clear from this study whether the correlations between EE and body weight/compensation noted above would be evident in a larger more diverse population and whether they would be evident following weight gain. We conducted similar analyses from our previous data sets15 of a separate group of 50 subjects (20 male, 30 female; 20 never-obese, 30 obese) who were studied at Wtinitial and Wt−10% and 12 subjects (8 obese, 4 never-obese; 7 female, 5 male) who were studied before and after a 10% weight gain (Wt+10%) accomplished by overfeeding with solid food (5000–7000 kcal/day) and again at Wt−10% following weight reduction on 800 kcal/day of the liquid formula diet using a similar study design (schematized in Figure 3). As shown in Table 3, EE normalized to FFM or weight, and EE residuals following weight loss, were not significantly different from the 17 subjects described in Table 1.
Figure 3.
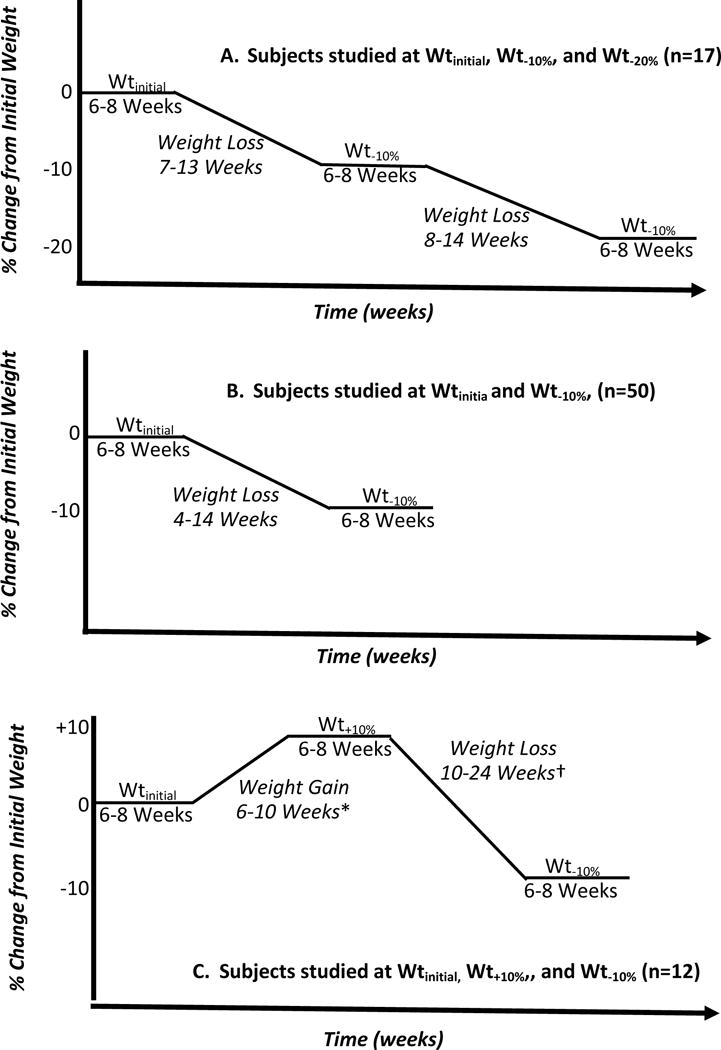
Schematic of study protocols and time range for each protocol phase for 17 subjects with obesity studied at Wtinitial, Wt−10%, and Wt−20% (Figure 3A), 50 subjects (20 never-obese, 30 obese) studied only at Wtinitial and Wt−10% (Figure 3B), and 12 subjects (8 never-obese, 4 obese) studied at Wtinitial,Wt+10%, and Wt−10% (Figure 3C). There was no subject overlap between groups and subject characteristics are presented in Table 3. All subjects underwent the same protocols during each period of weight stability. Analyses of these additional data were performed to examine the effects of population size and diversity and weight gain versus loss on the relationship of changes in EE to changes in body mass and composition. *Weight gain was accomplished by overfeeding subjects solid food with a daily intake of 5000–7000 kcal/day until they had gained 10% of Wtinitial at which time they were stabilized on the liquid formula diet15. † Weight loss was accomplished by underfeeding subjects 800 kcal/day of the liquid formula diet. Subjects with obesity suspended weight loss for 6–8 weeks when they had returned to their initial weight and were studied at that weight before reducing to Wt−10%15.
Table 3A.
Description of subjects and EE in a discrete group of 50 subjects studied at Wtinitial and Wt−10%. Twenty-four Hour EE (TEE) is divided into 3 components: Resting EE (REE), the Thermic Effect of Feeding (TEF), and non-Resting EE (NREE). Data are mean (S.D.).
| Wtinitial | Wt−10% | |
|---|---|---|
| Age (yrs) | 29.3 (7.9) | |
| Gender | 20 males, 30 females | |
| Somatotype | 20 never-obese, 30 obese | |
| Weight (kg) | 99.2 (34.2) | 87.3 (29.9)* |
| BMI (kg/m2) | 35.4 (12.2) | 31.0 (10.7)* |
| Fat-free Mass (kg) | 63.4 (18.3) | 61.2 (16.5)* |
| Fat Mass (kg) | 57.1 (13.4)# | 42.1 (25.9)*# |
| % Body Fat | 39.2 (14.3)# | 33.7 (15.4)*# |
| Delta Energy Stores (kcal) | −97393 (48497)† | |
| TEE (kcal/day) | 2913 (569) | 2301 (492)* |
| TEE/FFM (kcal/kg/day) | 51.8 (7.0) | 42.7 (7.3)8* |
| TEE Residual (kcal/day) | −434 (239)† | |
| REE (kcal/day) | 1708 (397) | 1572 (429)* |
| REE/ FFM (kcal/kg/day) | 30.3 (4.5) | 28.8 (4.9)† |
| REE Residual (kcal/day) | −85 (280)‡ | |
| TEF (kcal/day) | 84 (45) | 59 (51) |
| NREE (kcal/day) | 1120 (381) | 670 (352)* |
| NREE/ body weight (kcal/kg/day) | 11.7 (3.0) | 8.0 (3.39)† |
| NREE Residual (kcal/day) | −365 (338)† |
P<0.001 vs., Wtinitial;
P<0.001 vs. zero,
P<0.05 vs. zero.
Similar weight loss effects to the present study were noted in the larger weight-reduced group where changes in body weight, as well as body composition and energy stores, were correlated with changes in TEE and NREE. For both TEE and NREE, the correlation with body weight (representing ~ 23% of the variance in changes in TEE, and 10% of the variance in changes in NREE) was greater than that for FM, FFM, or energy stores. As shown in Table 3, there was significant adaptive thermogenesis following weight gain and weight loss in this population but, unlike weight loss, maintenance of an elevated body weight was not associated with any change in the slope function relating TEE to fat mass though there was a significant effect of both weight gain and weight loss on the y-intercept of the regression.
Discussion
Here we compare aspects of EE in seventeen subjects with obesity each studied at usual weight and following a 10% and 20% dietary weight loss. Diet macronutrient composition and physical activity were “clamped” throughout the study15. The major findings of these analyses are: 1.) There is a relationship between the amount of weight loss and the decline in TEE and REE adjusted for body composition, and NREE adjusted for body mass, during maintenance of that reduced weight. However, the declines in adjusted REE and NREE in response to the Wt−10% weight loss are greater than those following an additional 10% weight loss to Wt−20%; 2.) The contribution of FM but not FFM, to TEE and REE is similarly diminished following weight loss to Wt−10% or Wt−20% 3.) These findings most consistent with a multi-mechanism (mechanical, threshold, and spring-loading) model for metabolic responses to weight loss.
The mechanical model predicts that the slope and intercept of the line relating EE to body composition is constant at all weights and that there is no adaptive thermogenesis (zero residual). This model is supported by the significant correlation of the amount of weight lost and the decline in EE, but cannot account for the adaptive thermogenesis at Wt−10% and Wt−20%. In actuality, at both reduced weight plateaus there are quantitatively identical significant decreases in both the intercept and the regression coefficient for FM in equations relating REE and TEE, but not NREE, to body composition and weight which are most consistent with a threshold model in which the importance of adipose tissue in determining EE is maximal at ~Wt−10% and is equally diminished anywhere below a threshold. The threshold model is clearly applicable to REE, which declines disproportionately after the initial 10% weight loss without further disproportionate decline following weight loss to Wt−20%, but does not account for the additional decline in NREE following weight reduction from Wt−10% to Wt−20%. The spring loading model is consistent with the similar declines in NREE for each 10% of weight loss. Unlike REE, residuals of NREE, and as a result, TEE are significantly correlated with the degree of weight loss (Figure 2). These data suggest that all 3 models are relevant, but encompass different components of EE.
The contribution of FM to TEE (as reflected in the slope function of regression equations relating TEE to body composition) is not affected by weight gain, is significantly diminished following a10% weight loss, and is not further diminished by an additional 10% weight loss (Tables 2 and 3). These observations suggest that a “signal” from adipose tissue regulates EE asymmetrically with more potent effects to decrease EE after weight loss than to increase EE after weight gain. We have hypothesized that the “signal” is the adipocyte-derived hormone leptin24 based on its known pharmacology25. Teleologically, this early maximal response to reduction in fat mass preserves a “buffer” of energy stores critical to survival and reproductive5, 12, 26 as opposed to other metabolic signals, such as hypoglycemia, for which there is often a relatively narrow difference (<10 mg/dl) between the induction of counter-regulatory hormones and attainment of symptomatically critical low values5, 27–30. Leptin repletion during reduced weight maintenance reverses approximately 2/3 of the decline in TEE and NREE and increase in skeletal muscle work efficiency, and also partially reverses the increased expression of the more efficient myosin heavy chain 1 isoform but not the sarcoplasmic endoplasmic reticulum Ca++-dependent ATPase isoform in skeletal muscle22, 23, 31. Similarly, leptin repletion restores sympathetic nervous system (SNS), triiodothyronine, thyroxine, but not parasympathetic nervous system (PNS) tone or thyroid stimulating hormone to pre-weight loss levels32. The incomplete reversal of the weight-reduced phenotype following leptin repletion suggests that there are non-leptin dependent mechanisms producing adaptive thermogenesis following weight loss.
An alternative explanation for the additional adaptive thermogenesis noted after weight loss to Wt-20% is that some of the subjects did not descend below their “threshold weight” by virtue of the initial 10% weight loss. If this were true, then there should be a negative correlation of residuals between Wtinitial and Wt−10% vs. Wt−10% and Wt−20% since subjects who remained above threshold during the first weight loss period (smallest negative residual at Wt−10%) would have the largest negative residual below threshold at Wt−20%. As shown in Figure 4, the opposite is true. The correlation of residual EE after the first and second 10% of weight loss is most consistent with the spring loading model. As shown in Figure 5, it is only by invoking the 3 mechanisms of response that the changes in EE following increasing degrees of weight loss can be fully accounted for and the relative contribution of each mechanism to the decline in EE following weight loss (both adaptive and non-adaptive) is influenced by the degree of weight reduction. The diminished relative contribution of REE to adaptive thermogenesis with progressive weight reduction may account, in part, for the wide variation in reported REE-mediated adaptive thermogenesis following different degrees of weight reduction15, 33, 34.
Figure 4.
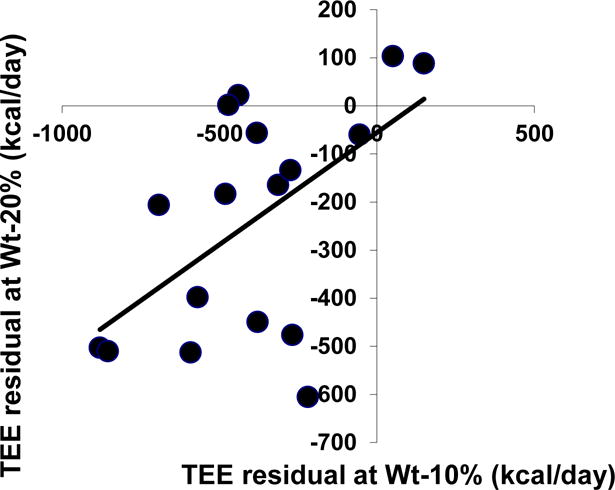
Figure 5.
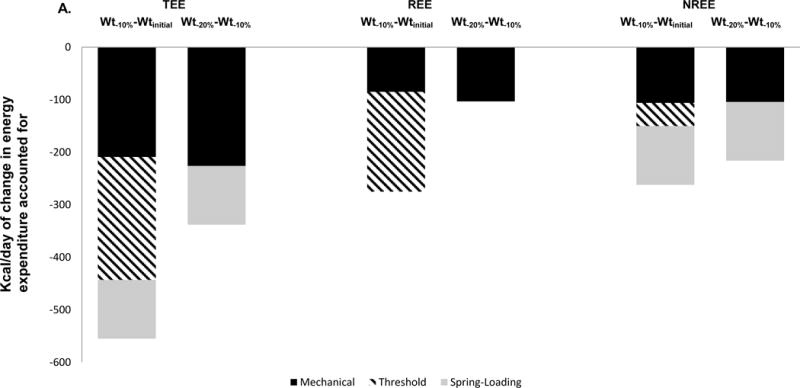
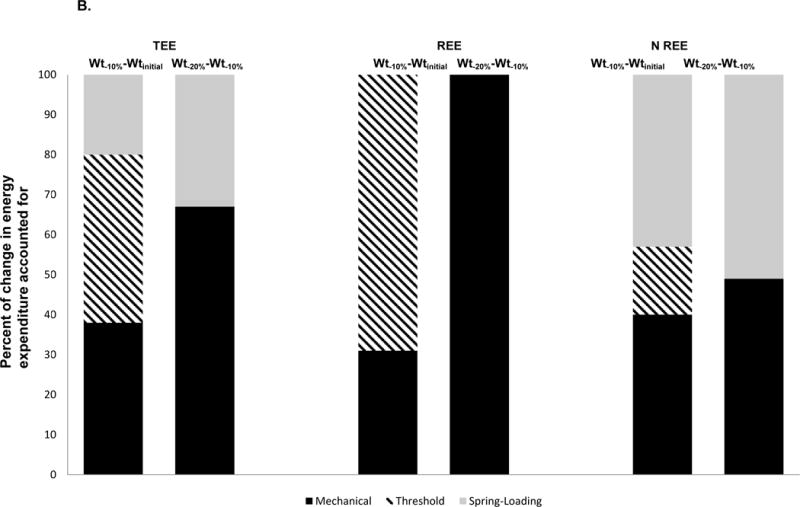
A. Partitioning of absolute changes (4A) and percentage of the total decline (4B) in EE during the 1st 10% weight loss (Wt−10%−Wtinitial) and 2nd 10% weight loss (Wt−20%−Wt−10%) plateaus attributable to each model. The mechanical effect is the mean number of calories predicted based on the regression equation relating EE to body composition (TEE and REE) or weight (NREE) at Wtinitial applied to both weight-reduced states. The threshold effect is whatever residual decline in EE occurs following the first 10% of weight loss but, by definition, does not increase once subjects have lost weight to a point below that threshold and cannot be attributed to the spring effect. The spring effect is whatever additional decline in EE residuals occurs between 10% and 20% weight loss (since the threshold model would predict no further decline in residuals after the first 10% weight reduction. As shown in Table 1, there is substantial inter-individual variation in these data.
Figure 4 also illustrates the substantial inter-individual variability in the degree of adaptive thermogenesis following weight loss. The significant within-individual correlation and between-individual variance in the degree of adaptive thermogenesis at different degrees of weight loss suggests that adaptive thermogenic responses to weight loss are individualized traits. These findings are consistent with Bouchard’s work documenting the substantial variation in metabolic responses to weight perturbation between fraternal twins versus identical twins35–37.
The highly controlled nature of this study was necessary to permit detailed assessment of energy expenditure without such confounding influences as lack of weight stability, adiposity-related co-morbidities, variations in dietary macronutrient content, and variations in physical activity. However, the applicability of these results to an environment outside of a Clinical Research Resource will require further investigation. This is also a small study population of predominantly women who lost 20% of their initial body weight. It is possible that a larger study population would reveal significant gender effects and that the models proposed might not be applicable in individuals maintaining a greater than 20% weight loss.
Adaptive thermogenesis is demonstrable following both short-term and long-term weight reduction9–11 and weight-reduced patients will have to eat less and/or exercise more than their never-obese peers of the same weight and body composition if they wish to sustain weight loss. This inference is consistent with observations of in-patients who have sustained weight loss which have demonstrated persistence of adaptive thermogenesis10 as well as endocrine changes (such as decreased thyroid hormones and alterations in circulating concentrations of various gut peptides) all of which favor weight regain38 and by out-patient studies via the National Weight Control Registry39, 40. Furthermore, the observation that the strength of adaptive thermogenesis increases with additional weight loss implies that the dichotomy between weight-reduced individuals and those of similar body composition but who have never lost weight will increase as more weight is lost, requiring further decreases in energy intake and/or increases in physical activity.
Table 3B.
Data from 12 subjects studied at usual weight (Wtinitial), during maintenance of a 10% weight gain (Wt+10%) and during maintenance of a 10% weight loss (Wt−10%).
| Wtinitial | Wt+10% | Wt-10% | |
|---|---|---|---|
| Gender/Somatotype | 5 males, 7 females; 8 obese, 4 never-obese | ||
| Weight (kg) | 112.3 (32.6) | 123.0 (34.6)* | 99.9 (29.1)* |
| Fat-free Mass (kg) | 61.2 (8.7) | 65.3 (18.9)* | 59.6 (8.7)† |
| Fat Mass (kg) | 51.1 (30.1) | 57.8 (31.5)* | 40.3 (25.0)* |
| TEE (kcal/day) | 3009 (629) | 3844 (717)* | 2463 (545)* |
| TEE/FFM (kcal/kg/day) | 49.0 (7.3) | 58.89 (7.6)* | 41.1 (5.0)* |
| TEE Residual (kcal/day) | 579 (472)‡ | -367 (252)‡ | |
| Regression Equation Relating TEE to body composition |
46.6(FFM) + 9.8(FM) −342 Adjusted R2=0.67, p=0.003 RFFM=0.64, p=0.005 RFM=0.47, p=0.025 |
51.2(FFM) + 9.7(FM) −96 Adjusted R2=0.71, p=0.002 RFFM=0.63, p=0.004 RFM=0.40, p=0.035 |
46.4(FFM) + 6.8(FM) −576 Adjusted R2=0.76, p<0.001 RFFM=0.70, p=0.002 RFM=0.29, p=0.076 |
P<0.005 vs. Wtinitial;
P<0.05 vs. Wtinitial;
P<0.005 vs. zero
What is known about this subject?
There is strong evidence that body weight is biologically regulated in individuals who are obese and never-obese.
Energy expenditure declines following weight loss to a greater degree than predicted solely on the basis of the amount of weight lost.
Mechanical, threshold, and spring-loading models for body weight regulation have been proposed each of which predicts different changes in energy expenditure following weight loss.
What does this study add?
This study examines different models for energy homeostasis in the context of different components of energy expenditure.
This study indicates that different components of energy expenditure are differentially regulated in concordance with various models of body weight regulation.
Acknowledgments
We gratefully acknowledge the invaluable assistance of all our volunteers and of the nursing staff and nutrition staffs of the Rockefeller University Hospital and the Irving Center for Clinical Research at The New York Presbyterian Hospital, Columbia University College of Physicians & Surgeons. We would also like to thank Dr. Ele Ferrannini at the University of Pisa School of Medicine for his critical review of this manuscript.
Grant Support: These studies were supported by NIH grants DK30583, DK64773, RR00645, UL 1 TR000040, and P30-DK26687.
Footnotes
Disclosures: None
References
- 1.Lewis C, Jacobs D, McCreath H, Kiefe C, Schreiner P, Smith D, Williams O. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the cardia study. Coronary artery risk development in young adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 2.Du H, A Dvd, Ginder V, Jebb S, Forouhi N, Wareham N, Halkjaer T, Tjonneland A, Overvad K, Jakobsen M, Buijsse B, Steffen A, Palli D, Masala G, Saris W, Sorensen T, Feskens E. Dietary energy density in relation to subsequent changes of weight and waist circumference in european men and women. PLoS One. 2009;4:e5339. doi: 10.1371/journal.pone.0005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forouhi N, Sharp S, Du H, A Dvd, Halkjaer J, Schultze M, Tjonneland A, Overvad K, Jakobsen M, Boeing H, Buijsse B, Palli D, Masala G, Feskins E, Sorensen T, Wareham N. Dietary fat intake and subsequent weight change in adults: Results from the european prospective investigation in cancer and nutrition cohorts. Amer J Clin Nutr. 2009;90:1632–1641. doi: 10.3945/ajcn.2009.27828. [DOI] [PubMed] [Google Scholar]
- 4.Pietrobelli A, Allison D, Heshka S, Heo M, Wang Z, Berktay A, Laferrere B, Rosenbaum M, Aloia J, Pi-Sunyer F, Heymsfield S. Sexual dimorphism in energy content of weight change. Int J Obes Relat Metab Disord. 2002;26:1339–1348. doi: 10.1038/sj.ijo.0802065. [DOI] [PubMed] [Google Scholar]
- 5.Leibel R, Rosenbaum M. Metabolic response to weight perturbation. In: Clément K, editor. Novel insights into adipose cell functions, research and perspectives in endocrine interactions. Heidelberg: Springer-Verlag; 2010. pp. 121–133. [Google Scholar]
- 6.Leibel R. The physiology of body weight regulation in mice and humans. Int J Obes. 2008;32:S98–S108. doi: 10.1038/ijo.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman J, Levitsky D, Allison D, Bray M, de Castro J, Clegg D, Clapman J, Dulloo A, Gruer L, Haw S, Hebebrand J, Hetherington M, Higgs S, Jebb S, Loos R, Luckman S, Luke A, Mohammed-Ali V, O’Rahilly S, Pereira M, Perusse L, Robinson T, Rolls B, Symonds M, Westerterp-Plantenga M. Set points, settling points, and some alternative models: Theoretic options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirtshafter D, Davis J. Set points, settling points, and the control of body weight. Physiol Behav. 1977;19:75–78. doi: 10.1016/0031-9384(77)90162-7. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Leibel R. Adaptive thermogenesis in humans. Int J Obes. 2010;34:S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Hirsch J, Gallagher D, Leibel R. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Amer J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 11.Lowell B, Spiegelman B. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 12.Levin B. Central regulation of energy homeostasis intelligent design: How to build the perfect survivor. Obes. 2006;14(Suppl 5):192–196S. doi: 10.1038/oby.2006.307. [DOI] [PubMed] [Google Scholar]
- 13.Loiselle D, Crampin E, Niederer S, Smith N, Barclay C. Energetic consequence of mechanical loads. Prog Biophys Mol Biol. 2008;97:348–366. doi: 10.1016/j.pbiomolbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Ravussin E, Lillioja S, Anderson T, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Eng J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 16.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 17.Atwater W, Bryant A. The availability and fuel value of food materials. Conn (Storrs) Agr Expt Sta 12th Ann Rpt. 1990:73–110. [Google Scholar]
- 18.Jebbs S, Elia M. Techniques for the measurement of body composition: A practical guide. Int J Obes Relat Metab Disord. 1993;17:611–621. [PubMed] [Google Scholar]
- 19.Rosenbaum M, Ravussin E, Matthews D, Gilker C, Ferraro R, Heymsfield S, Hirsch J, Leibel R. A comparative study of different means of assessing long-term energy expenditure in humans. Amer J Physiol. 1996;270:R496–504. doi: 10.1152/ajpregu.1996.270.3.R496. [DOI] [PubMed] [Google Scholar]
- 20.Westerterp K. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front Physiol. 2013 doi: 10.3389/fphys.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Hirsch J, Murphy E, Leibel R. The effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Amer J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J, Heymsfield S, Joanisse D, Hirsch J, Murphy E, Matthews D, Segal K, Leibel R. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Endocrinol Metab. 2003;285:R183–192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith R, Joanisse D, Gallagher D, Pavlovich K, Shamoon E, Leibel R, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol. 2010;298:R79–88. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum M, Sy M, Pavlovich K, Leibel R, Hirsch J. Leptin reverses weight loss–induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Leibel R. The role of leptin in energy homeostasis in humans. J Endocrinol. 2015;223:T83–96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch R. Body fat, menarche, fitness, and fertility. Hum Reprod. 1987;2:521–533. doi: 10.1093/oxfordjournals.humrep.a136582. [DOI] [PubMed] [Google Scholar]
- 27.Johannsen D, Knuth N, Hulzenga R, Rood J, Ravussin E, Hall K. Metabolic slowing with masive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97:2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dulloo A, Jacquet J, Montani J, Schutz Y. Adaptive thermogenesis in human body weight regulation: More of a concept than a measurable entity. Obes Rev. 2012;13(Suppl 2):105–121. doi: 10.1111/j.1467-789X.2012.01041.x. [DOI] [PubMed] [Google Scholar]
- 29.Mitrakou A, Ryan C, Veneman T, Mokan M, Jensses T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cereberal dysfunction. Am J Phyiol. 1991;260:E67–74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 30.Bakatselos S. Hypoglycemia unawareness. Diab Res Clin Prac. 2011;93(Suppl):S92–96. doi: 10.1016/S0168-8227(11)70020-1. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin K, Joanisse D, Haddad F, Goldsmith R, Gallagher D, Pavlovich K, Shamoon E, Leibel R, Rosenbaum M. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Endocrinol Metab. 2011;301:R1259–1266. doi: 10.1152/ajpregu.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel R. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gemert W, Westerterp K, van Acker B, Wagenmakers A, Halliday D, Greve J, Soeters P. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 34.Amatruda J, Statt M, Welle S. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993;92:1236–1242. doi: 10.1172/JCI116695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 36.Bouchard C, Tremblay A, Despres J, Nadeau A, Lupien P, Moorjani S, Theriault G, Kim S. Overfeeding in identical twins: 5-year postoverfeeding results. Metabolism. 1996;45:1042–1050. doi: 10.1016/s0026-0495(96)90277-2. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127:943S–947S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 38.Sumithran P, Prendergast L, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistance of hormonal adaptations to weight loss. N Eng J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 39.Wing R, Hill J. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 40.McGuire M, Wing R, Klem M, Hill J. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res. 1999;7:334–341. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]


