Abstract
Chorda tympani nerve transection (CTX) results in morphological changes to fungiform papillae and associated taste buds. When transection occurs during neonatal development in the rat, the effects on fungiform taste bud and papillae structure are markedly more severe than observed following a comparable surgery in the adult rat. The present study examined the potential “sensitive period” for morphological modifications to tongue epithelium following CTX. Rats received unilateral transection at 65, 30, 25, 20, 15, 10, or 5 days of age. With each descending age at the time of transection, the effects on the structural integrity of fungiform papillae were more severe. Significant losses in total number of taste buds and filiform-like papillae were observed when transection occurred 5–30 days of age. Significant reduction in the number of taste pores was indicated at every age of transection. Another group of rats received chorda tympani transection at 10, 25, or 65 days of age to determine if the time course of taste bud degeneration differed depending on the age of the rat at the time of transection. Taste bud volumes differed significantly from intact sides of the tongue at 2, 8, and 50 days posttransection after CTX at 65 days of age. Volume measurements did not differ 2 days posttransection after CTX at 10 or 25 days of age, but were significantly reduced at the other time points. Findings demonstrate a transitional period throughout development wherein fungiform papillae are highly dependent upon the chorda tympani for maintenance of morphological integrity.
Keywords: gustatory, tongue epithelium, regeneration, geniculate ganglion
In many sensory systems, developmentally focused experimental manipulations have led to important discoveries of neurobiological mechanisms that differentiate stages of neural dependency and plasticity (Hashisaki and Rubel, 1989; Renehan et al., 1994; Fiske and Brunjes, 2001; Kanold et al., 2003). In the gustatory system of the rat, sensitive periods have been noted for the effects of developmental dietary manipulation (Hill and Przekop, 1988; Krimm and Hill, 1997) and also for immunological responses in the tongue following manipulation of the chorda tympani nerve (CT) that innervates fungiform and foliate papillae (McCluskey and Hill, 2002). In addition, transection of the glossopharyngeal nerve (Hosley et al., 1987) or chorda tympani nerve (Sollars and Bernstein, 2000; Sollars et al., 2002) early in the development of the rat results in greater loss of taste buds and/or associated papillae structure than the same manipulation imposed in adulthood.
Fungiform papillae can be characterized morphologically by their circular structure that protrudes slightly above the tongue surface. Toward the center of the papilla is a circular indentation that surrounds a hillock containing the taste pore (Miller and Reedy, 1990; Parks and Whitehead, 1998; Sollars et al., 2002). The pore opens to expose the microvilli of the taste receptor cells. The presence of a pore is indicative of the presence of a taste bud, whereas the absence of a pore usually indicates that no taste bud is present (Parks and Whitehead, 1998). In the adult rat, transection of the CT (CTX) results in degeneration of taste buds within fungiform papillae. Additionally, the epithelium of fungiform papillae is structurally modified during the time that taste buds are in a state of degeneration. The indentation, hillock, and pore are generally no longer visible, and the epithelial surface appears flattened (Parks and Whitehead, 1998). In some instances, papillae become heavily keratinized and form a spike-shaped protrusion that resembles large filiform papillae and thus are termed “filiform-like” (Hård af Segerstad et al., 1989; Oakley et al., 1990; Nagato et al., 1995; Sollars et al., 2002). However, when transection is performed in the adult rat, the majority of taste buds regenerate upon reinnervation by the CT, approximately 40 days following transection (St. John et al., 1995; Kopka et al., 2000). Reinnervated fungiform papillae structurally modify to resemble the predenervated morphology (Montavon et al., 1996).
In contrast to events after CTX in the adult rat, dramatic alterations in papillae morphology occur when the CT is transected at 10 days of age. After neonatal CTX, nearly 65% of fungiform papillae are structurally changed to such a degree that their appearance precludes their identification as fungiform in origin (Sollars and Bernstein, 2000). A large percentage of the remainder (80%) is morphologically altered; although they can be identified as fungiform, they are strikingly different in appearance from papillae innervated by the CT. Thus, there is a large developmental gradient in the degree of morphological alteration in taste papillae: manipulation at 10 days of age produces markedly more severe alterations than transection in adulthood.
The present study examines the “sensitive period” for the effects of CTX on taste bud and fungiform papillae morphology. Rats received transection of the CT as early as 5 days of age and at several time points throughout development and into adulthood. Fungiform papillae morphology was examined at 50 days posttransection. In addition, taste bud volume was measured at 2, 8, and 50 days posttransection to determine whether the time course of degeneration of taste receptor cells is dependent upon the age of the rat at the time of transection. These results provide important information regarding the developmental role of the CT in the maintenance of gustatory and nongustatory epithelium. Insight into the sensitive period for maintenance of fungiform papillae provides a foundation for studies to uncover the mechanisms whereby fungiform papillae become increasingly less dependent on trophic support from the CT and/or taste buds for morphological integrity.
MATERIALS AND METHODS
Animals
Litters were obtained from Sprague-Dawley rats bred at the University of Virginia or University of Nebraska Omaha. The date that pups were born was designated as day 0. Litters were culled on day 1 to a maximum of 10 pups per litter. Pups were weaned between day 22 and day 25, and maintained on Teklad rat chow and water. All procedures were carried out under the approval of the Institutional Animal Care and Use Committee and in full accordance with NIH guidelines.
Chorda Tympani Nerve Transection
Unilateral CTX was performed in rats at 5, 10, 15, 20, 25, 30, or 65 days of age. Pups were anesthetized with Brevital® Sodium (Methohexital Sodium; 50 mg/kg, i.p.). An incision was placed on the ventromedial surface of the neck. Using microfine forceps, the digastric and masseter muscles were bluntly dissected on the right side. The lingual nerve was visualized and traced to its point of bifurcation with the CT. The CT was crushed and evulsed, resulting in removal of the CT to its point of entrance at the tympanic bulla. In every case, the CT was visualized after its removal to provide an additional source of verification of the completeness of the surgery. We have previously demonstrated that this surgical procedure does not damage the lingual nerve (Sollars et al., 2002). Sham operations do not noticeably alter papillae morphology (Sollars and Bernstein, 2000), so surgery was not performed on the contralateral side of each rat. Thus, the left side of each rat served as the control (intact) side. The surgical site was sutured and the rats were allowed to recover on a warm heating pad. Rats were away from their dams for a total of 30–60 min, and were often observed suckling within an hour of their return to the dam.
Fungiform Papillae Histology
Morphology of fungiform papillae was analyzed 50 days after CTX. Five rats were used at each surgical age except 20 and 65 days, wherein 6 rats were included in the analysis. Rats were given an overdose of sodium pentobarbital and perfused with modified KREBS solution. The tongues were removed and post-fixed in 8% paraformaldehyde for a minimum of 2 days. Tongues were dissected directly posterior to the intermolar eminence and the anterior portion was prepared for histology. Ventral muscle layers of the tongue were removed with scissors and the remaining muscle tissue was scraped away with a scalpel, leaving the dorsal epithelium intact. In order to achieve consistency across samples, none of the ventral surface of the tongue was histologically analyzed. Approximately 80% of fungiform taste buds are on the dorsal epithelium of the anterior tongue (Miller and Preslar, 1975)—thus, the prepared tongue tissue resulted in a large region available for analysis. The dorsal epithelium was dipped into a 5% solution of methylene blue, dried slightly, and placed between two microscope slides. The tongue was flattened by securing the slides together with tape. Using a combination of dark-field and phase-contrast microscopy, counts were made of fungiform papillae with a pore (Pore), fungiform papillae without a pore (No Pore), and filiform-like fungiform papillae. The number of fungiform papillae in each category was recorded along with topography of papillae using a computerized program for histological reconstruction (Neurolucida; MicroBrightField, Inc., Colchester, VT). The technician recording the morphological features was not informed of the surgical condition of the rat. Using this method, fungiform papillae within each morphological category were easily differentiated.
Fungiform Papillae with Pores
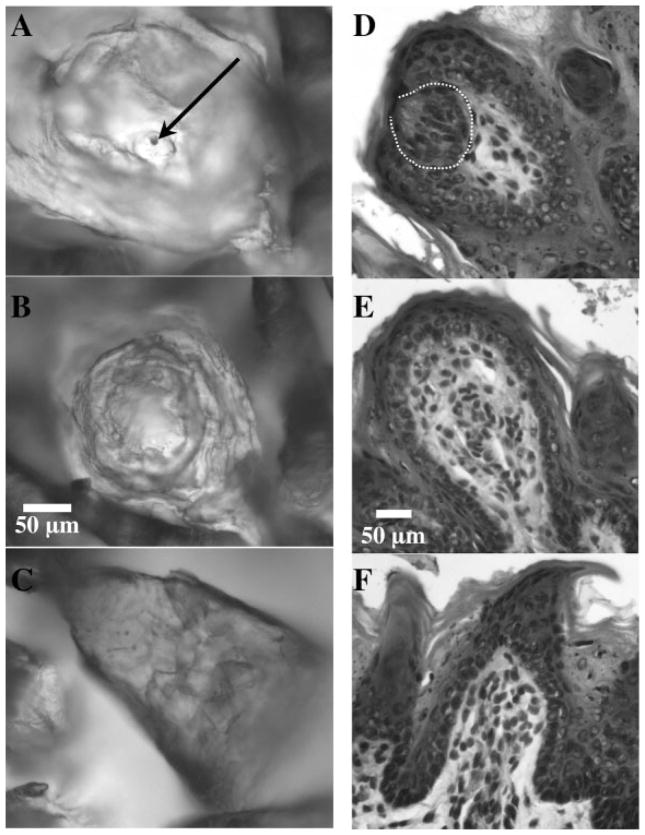
The method used to describe taste papillae structure is fully described in Sollars et al. (2002; see also Parks and Whitehead, 1998). Briefly, fungiform papillae usually appear as circular structures that protrude slightly upward above the surface of the tongue. In order for papillae structures to be classified as “Pore” papillae, three morphological features needed to be apparent. Each Pore papilla had a rim on the inside of the structure that separated the external and internal portions of the papilla, an indentation that sloped downward from the rim, and a hillock that protruded upward from the indentation and contained the pore [see Fig. 1(A)].
Figure 1.
(A–C) Examples of the epithelial surface of fungiform papillae stained with methylene blue. A: Pore papilla (the arrow indicates the pore); B: No Pore papilla; C: Filiform-like papilla. The scale bar (50 μm) in B applies to each section in this series. (D–F) Ten-micrometer sections of fungiform papillae stained with hematoxylin. D: Fungiform papilla with a taste bud (the taste bud region is indicated with the dashed line); E: “empty” papilla; F: filiform-like papilla. The scale bar in E applies to this series of figures.
Fungiform Papillae without a Pore
“No Pore” papillae had an epithelial structure that was flattened in appearance, although similar to Pore papillae in that they generally appeared as circular structures on the tongue surface. The surface was smooth in comparison to Pore papillae, without apparent rim and indentation features [see Fig. 1(B)].
Filiform-like Fungiform Papillae
Fungiform papillae occasionally undergo major structural transformation following denervation of the CT and/or lingual nerve. They can develop a conical protrusion on the epithelial surface that has been described as “filiform-like” because of the resemblance to filiform papillae. Filiform-like papillae are easily distinguished from actual filiform papillae because the cornified epithelial protrusion is larger than that of filiform papillae and the “cone” is typically angled in a direction atypical of the orientation of the filiform papillae [Fig. 1(C); Ganchrow and Ganchrow, 1989; Hård af Segerstad et al., 1989; Oakley et al., 1990, 1993; Nagato et al., 1995; Iwasaki et al., 1997; Sollars, et al., 2002].
Taste Bud Volume
Assessment of changes in taste bud morphology of fungiform papillae was examined following CT denervation at 10, 25, or 65 days of age. For the current study, taste buds were examined at 2, 8, and 50 days posttransection in rats that received the surgery at either 10, 25, or 50 days of age [to avoid unnecessary duplication, tissue from a previous study (Sollars et al., 2002) was used to assess animals CT transected at 10 days of age and analyzed 8 days later]. These ages were selected to determine whether the age of the rat at the time of transection influenced the time course of morphological changes to the taste buds. CTX procedures were the same as those described above. Tongues were removed at the specified intervals after providing an overdose of sodium pentobarbital and perfusing the rats with modified KREBS solution followed by 8% paraformaldehyde. Tongues were stored in 8% paraformaldehyde for a minimum of one week and cryoprotected in 30% sucrose. The anterior-most 2 mm of the tongue was removed and discarded. Using a cryostat, serial sections (10 μm) were obtained through the subsequent 2 mm of tissue and sections were stained with hematoxylin and eosin. Preparation of material in this manner provided a consistent sample size and included both denervated and intact taste buds. Using Neurolucida software, volume measurements across the entire 2 mm of tongue tissue were made for every fungiform papilla that contained a taste bud. The entire volume of each taste bud was reconstructed by drawing around the perimeter of each serial section and multiplying the area measurement by 10 (the section thickness). Denervation results in taste buds that lose their typical orientation and structure (Oakley et al., 1993), so remnant taste buds were defined as darkly stained regions directly below the apical surface of fungiform papillae. To be consistent with earlier reports, border cells were included in the volume measurements (Krimm and Hill, 1998; Sollars et al., 2002). “Empty” papillae had a heavily keratinized apical surface and no darkly stained cells in the region underneath the surface layer.
Papillae were classified into one of three categories [(Fig. 1D, E, and F)]: (1) with a taste bud, (2) empty with no taste bud, and (3) filiform-like. As compared to analysis of papillae surface structure, it is presumed that “taste bud” papillae could be either Pore or No Pore papillae, “empty” papillae are strictly No Pore papillae, and “filiform-like” papillae are the same in both types of analyses. These classifications are consistent with our earlier reports (Sollars et al., 2002).
Data Analysis
Analysis of variance was used to examine papillae count and taste bud volumes across of each of the categories. Bonferroni tests were used to examine statistical differences between papillae types or taste bud volumes at individual ages and times posttransection.
RESULTS
Alteration in Papillae Structure
Papillae structure was affected 50 days after CTX, but the severity of effect varied dependent upon the age of the rat when the transection occurred.
Total Number of Papillae
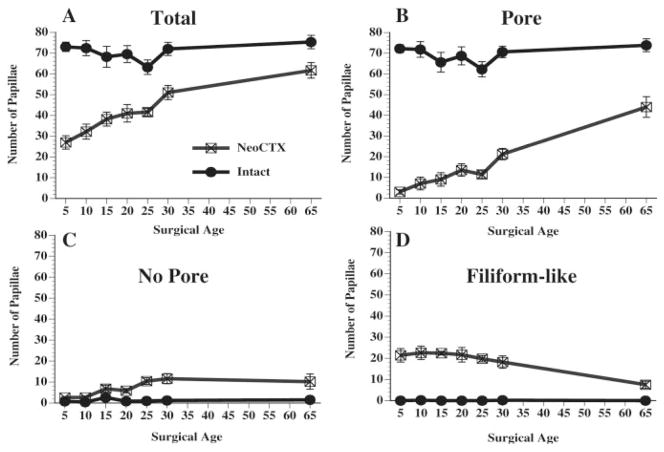
Fungiform papillae structure was altered significantly on the CTX side of the tongues as compared to the intact sides [F(1, 72) = 118.35, p < 0.00001]. As seen in Figure 2A, the total number of observable fungiform papillae on the CTX side tended to increase as the age of the rat at the time of transection increased. While a loss in total number of fungiform papillae occurred across all age groups, the largest reduction in the total number of papillae occurred when the CT was transected at 5 days of age (62% reduction as compared to the intact side). Numbers of papillae differed significantly (p < 0.05) between intact and CTX sides when surgery was performed at 5, 10, 15, 20, 25, and 30 days of age. In contrast, the reduction in total numbers was only 18% when transection occurred at 65 days of age. No significant differences were found in total number of papillae when surgery was performed at 65 days of age. The total number of papillae on the intact side remained relatively consistent across age.
Figure 2.
Average number (±SEM) of fungiform papillae observed 50 days posttransection following CTX at various developmental ages. Counts were made on the denervated side of the tongue (NeoCTX) and the intact sides of the tongue (Intact). A: The total number of papillae across all categories (B: Pore papillae; C: No Pore papillae; D: Filiform-like papillae).
Pore Papillae
The largest effect on papillae structure was noted in the number of Pore papillae observed after transection [see Fig. 2(B)]. Nearly all fungiform papillae on the intact side at each age had a pore on the epithelial surface. Overall, CTX surgery had the effect of reducing the number of Pore papillae on the surgical side [F(1, 72) = 348.46, p < 0.00001]. After CTX at 5 days of age, of the 27.0 ± 3.2 identifiable fungiform papillae on the surgical side, only 3.0 ± 1.0 papillae had an identifiable pore. The number of Pore papillae observed on the surgical side increased slightly as surgical age increased, but the number of Pore papillae was always significantly different (p < 0.05) from the number of Pore papillae on the intact side of the tongue. However, when surgery was performed at 65 days of age, the majority (71.3%) of papillae identifiable as fungiform contained a pore when examined 50 days posttransection.
No Pore Papillae
Numbers of No Pore papillae differed significantly between intact and CTX sides of the tongue [F(1, 72) = 41.36, p < 0.00001]. The number of No Pore papillae on the intact side of the tongues ranged from 0.4 ± 0.2 to 2.6 ± 0.9. When CTX surgery was performed in postweanling rats, it resulted in an increase in the number of No Pore papillae observed on the CTX side. The first significant difference (p < 0.05) between CTX and intact sides of the tongue occurred when surgery was performed at 25 days of age [Fig. 2(C)]. The increased number of observable No Pore papillae remained significantly different between sides of the tongues when surgery was performed at 30 and 65 days of age. These results reflect the observation that the structural integrity of the epithelial surface of fungiform papillae was altered to a greater degree when surgery was performed at preweanling ages in the rat; there were fewer remaining fungiform papillae that maintained the flattened epithelial appearance characteristic of papillae innervated by the CT.
Filiform-like Papillae
Significantly greater numbers of filiform-like papillae were observed on the CTX side of the tongues [F(1, 72) = 227.82, p < 0.00001; see Fig. 2(D)]. No filiform-like papillae were noted on the intact side of the tongues at any age. The average number of filiform-like papillae was similar when surgery was performed at the earlier ages and significantly different (p < 0.05) from the intact side at each time. While filiform-like papillae were noted when surgery was performed at 65 days of age, there were not enough to produce a significantly different finding at this age. Interestingly, although the absolute numbers of filiform-like papillae were similar across the early surgical time points, the percentage of total fungiform papillae that were filiform-like varied, dependent upon the age at transection. When surgery was performed at 5 days of age, 79.2% of the total number of observable fungiform papillae were filiform-like. In contrast, when surgery was performed at 30 days of age, 35.7% of total papillae were filiform-like. And when transection occurred at 65 days of age, only 12% were filiform-like.
Taste Bud Volume
Taste bud volume was reduced as a result of CTX, independent of the age of the rat at the time of transection. Significant differences were apparent across surgical age in the time course of taste bud volume degeneration [F(17, 650) = 40.97, p < 0.0001].
CTX at 10 Days of Age
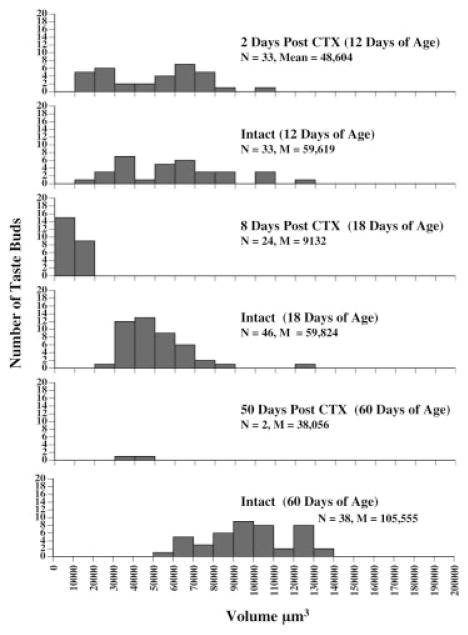
Transection of the CT at 10 days of age produced marked changes to taste bud volume [F(5, 175) = 20.00, p < 0.0001; Fig. 3]. When measurements of taste buds were taken 2 days after transection, no significant changes in volume were noted. Additionally, no filiform-like or empty papillae were observed at this time point. Similar to that observed from the analysis of epithelial surface (see above), the first filiform-like (N = 9) and empty (N = 16) papillae were seen on the cut side of the tongue 8 days post-CTX. Although the total number of papillae counted was not smaller than the count on the intact side, the number of papillae with taste buds accounted for only 50% of the total. Additionally, there was a significant reduction in taste bud volume compared to the intact side (p < 0.05). When transection was performed at 10 days of age and taste buds examined at 50 days posttransection, major changes in structure were observed. There were 22 filiform-like papillae and 3 empty papillae at this age, accounting for 92.6% of the total papillae on the cut side of the tongue. The failure to find a statistically significant reduction in taste bud volume on the cut side is likely due to the small number of taste buds (N = 2) found on that side. Clearly, there were striking changes in fungiform taste bud maintenance given the finding that only 2 taste buds were observable across the entire 4 mm of tongue epithelium examined (2 mm per rat). Figure 4 shows the percent difference in number of taste buds as compared to the intact side of the tongue at each age examined.
Figure 3.
Volume measurements of taste buds 2, 8, or 50 days after CTX at 10 days of age. Histograms represent the number of taste buds within each volume range for the denervated (CTX) and Intact sides of the tongue. The age of the animals at the time of sacrifice is included in parentheses at each time point. Two animals were included at each time point and 2 mm of tongue tissue per rat is represented. The total number (N) and average volume (M) of taste buds is included at each age.
Figure 4.
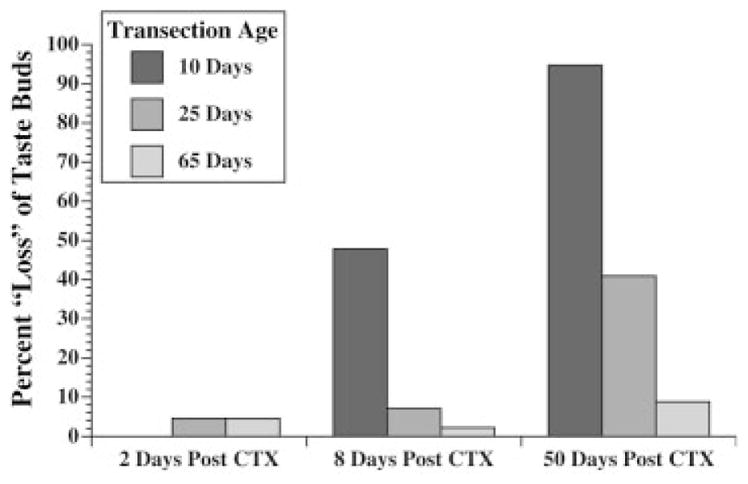
The percent difference in numbers of taste buds on intact and denervated sides of the tongue following transection at 10, 25, or 65 days of age. Since sides of the tongue normally have relatively equal numbers of taste buds, the percent difference may be interpreted as a “loss” of taste buds on the denervated (CTX) side of the tongue. Percent differences in total numbers are shown 2, 8, or 50 days after CTX.
CTX at 25 Days of Age
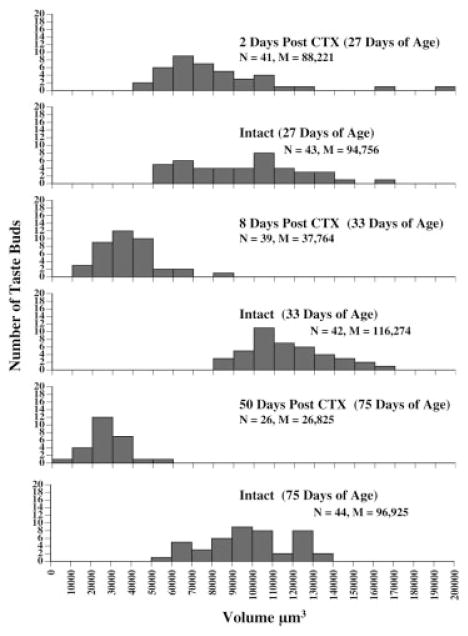
Across all time points examined, there was a significant reduction in taste bud volume on the transected side of the tongue when CTX surgery occurred at 25 days of age [F(5,234) = 53.56, p < 0.0001; see Fig. 5]. However, at 2 days posttransection, no significant changes were noted in taste bud volume. Additionally, when tongues were examined 2 days after surgery, there were no observed filiform-like or empty papillae. By 8 days posttransection, there was a marked reduction in the volume of taste buds (p < 0.05), but the total number of taste buds was not greatly reduced (N = 42 on intact side, N = 39 on CTX side). There was only one filiform-like papilla and one empty papilla observed at this age. A further decrement in taste bud volume occurred by 50 days posttransection. The average taste bud volume was significantly reduced as compared to the intact side (p < 0.05). In addition, 10 filiform-like papillae and 9 empty papillae were noted, indicating structural modification of papillae similar to that observed from the surface analysis of the epithelium.
Figure 5.
Histograms representing volume measurements of taste buds at 2, 8, or 50 days after CTX at 25 days of age. The age of the animals at the time of sacrifice is presented in parentheses. Denervated (CTX) and intact sides of the tongue are presented as separate graphs at each time point. Histological analysis of a total of 4 mm of tongue tissue (2 rats per group, 2 mm tissue per rat) is shown along with the total number (N) of taste buds and the average (M) taste bud volume per group.
CTX at 65 Days of Age
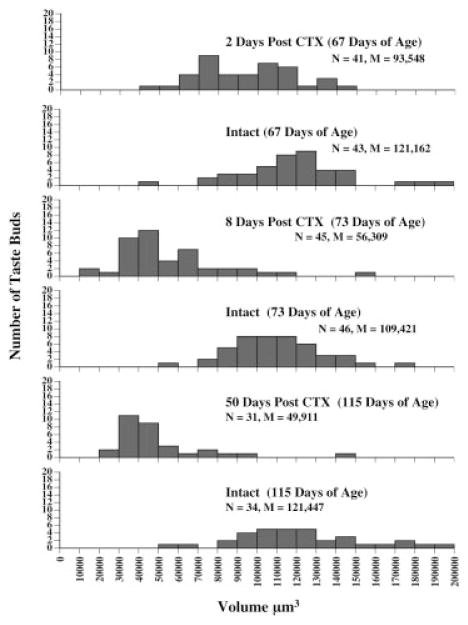
CTX resulted in an overall reduction in taste bud volume when surgery was performed at 65 days of age [F(5, 239) = 53.30, p < 0.0001; Fig. 6]. In this group of rats, taste bud volume was significantly decreased by 2 days posttransection (p < .05). Notably, rats that received transection at 10 or 25 days of age did not show a significant reduction in taste bud volume until the 8-day posttransection time point. Further reduction in taste bud volume occurred by 8 days posttransection (p < 0.05), resulting in an average taste bud volume that was nearly half the volume of taste buds on the intact side. Normal numbers of taste buds were observed at both 2 and 8 days after surgery, and in neither case were filiform-like or empty papillae observed in the sections of tissue analyzed. However, by 50 days posttransection, both filiform-like (N = 6) and empty (N = 5) papillae were noted. Volume of taste buds on the CTX side was significantly different than taste bud volume on the intact side (p < 0.05). The number of taste buds was similar on intact and CTX sides (numbers of taste buds were slightly lower in this group on both sides of the tongue, perhaps indicative of an overall increase in surface area of the tongue at this age). These results closely parallel the surface analysis of papillae in that this age was the only group that did not experience a significant decrease in total papillae numbers.
Figure 6.
Histograms of the number of taste buds per volume range on the denervated (CTX) and intact sides of the tongue following CTX at 65 days of age. Animals were sacrificed 2, 8, or 50 days after CT transection (age of animal at time of sacrifice listed within parentheses). The total number (N) of taste buds and the average (M) volume of taste buds is shown. Histological analysis included 2 mm of tongue tissue per rat with 2 rats per age group.
DISCUSSION
The data reported here demonstrate a dynamic role of the CT in maintaining the morphological integrity of fungiform papillae. Typical fungiform papillae morphology is altered to a greater degree when innervation by the CT is disrupted at early postnatal ages than at adult ages in the rat. Although previous studies have demonstrated the effects of CTX at early postnatal ages in the rat (Sollars and Bernstein, 2000; Sollars et al., 2002) and adult (Hård af Segerstad et al., 1989; Oakley et al., 1990), the present study sought to systematically examine the effect of CTX at various ages throughout development and into adulthood. The present data suggest a transitional period for the role of the CT in papillae maintenance; with each ascending age group, transection of the CT had less of an effect than observed in the previous age group. The greatest difference between early postnatal CTX and adult CTX is the degree to which fungiform papillae, not simply the taste buds, are dependent upon innervation by the CT. Thus, trophic maintenance of fungiform papillae is likely due to circulating factors available during development that diminish or change in function as the animal matures. This finding is particularly intriguing because it points to an important role of gustatory system control of nongustatory epithelium and highlights developmental regulation of this dependence.
The trophic dependence of fungiform taste buds on innervation by the CT is well documented (Hård af Segerstad et al., 1989; Oakley et al., 1990, 1993). In the adult rat, CTX results in degeneration of taste buds, but taste buds re-form upon reinnervation by the CT. The morphology of fungiform papillae undergoes a stage after denervation wherein the dorsal epithelium of the papillae become more heavily keratinized, making the pore, indentation, and hillock indistinguishable from the rim of the papillae (Parks and Whitehead, 1998). Once reinnervation occurs, there is up to 90% recovery of the typical structural features characteristic of papillae with a taste bud (St. John et al., 1995; Kopka et al., 2000). The reinnervation process starts to occur as quickly as 2–3 weeks following transection and continues for at least another 3–4 weeks. Importantly, when transection occurs in the adult rat, over 70% of papillae retain a typical morphology even when the CT is not allowed to reinnervate the tissue (Hård af Segerstad et al., 1989). This is in sharp contrast to the degree of morphological disruption of fungiform papillae that occurs when CTX is imposed throughout early postnatal and juvenile ages in the rat, a time when taste bud maturation is not yet complete (Krimm and Hill, 1998).
The current study focused on the early effects of CT transection at various postnatal ages to determine age-related CT dependency of taste buds and fungiform papillae. Previously, we showed a marked and permanent reduction in the number of taste buds and the number of fungiform papillae when the CT was transected in the 10-day-old rat (Sollars and Bernstein, 2000; Sollars et al., 2002). In the present report, we show variation in the degree of morphological change to papillae, dependent upon the age of the rat at the time of transection. The highest degree of structural damage to papillary epithelium occurred when CTX was given at 5 days of age. With each subsequent increase in age at the time of surgery past 5 days of age, there was a corresponding increase in structural integrity of fungiform papillae. When tissue was examined 50 days posttransection, the total number of papillae remaining after CTX at 5 days of age was 58% fewer than the number of papillae remaining after CTX at 65 days of age. Of the remaining papillae, 79% were filiform-like after CTX at 5 days of age, whereas only 12% of the remaining papillae were filiform-like after CTX at 65 days of age. In addition, the formation of filiform-like papillae occurred more rapidly and in greater numbers when surgery was instituted during early developmental time periods.
The CT innervates taste buds within the fungiform papillae and the lingual branch of the trigeminal nerve innervates the papillae. CT fibers terminate within the intragemmal region, but not in perigemmal regions of the papillae (Miller, 1974). Thus, these results demonstrate the dependence of nongustatory epithelium (the fungiform papillae) upon innervation of a gustatory nerve that forms synaptic contact with taste buds within the papilla, but not the papilla itself. Since lingual nerve fibers are not directly affected by CT transection (Sollars et al., 2002), it appears that the CT or taste buds provide a source of trophic support for the maintenance of normal papillae morphology. Such findings have been noted in studies of brain-derived neurotrophic factor (BDNF) and neurotrophin-4/5 (NT4/5) null mutant mice; in the absence of these neurotrophic factors, geniculate ganglion volume is reduced, but the trigeminal ganglion is minimally affected. Importantly, the number of fungiform papillae is also reduced in these animals (Liebl et al., 1999; Mistretta et al., 1999). Given the apparent availability of lingual nerve fibers in BDNF and NT3/4 null mutant mice and the finding that lingual nerve fiber counts are not disrupted after neonatal CTX (Sollars et al., 2002), the studies indicate that the lingual nerve and/or trophic factors within the papillae itself are not sufficient to support papillary epithelium. Rather, circulating factors from taste buds or CT fibers appear necessary, especially during development. As the animal matures, the structural integrity of the papillary epithelium becomes less dependent on these factors. Since a characteristic feature of CT-denervated fungiform papillae is a morphological reconstruction of the upper epithelial surface (Parks and Whitehead, 1998), it should be noted that CT denervation affects the papillae regardless of the age at the time of transection. The difference noted in the current report is the degree of morphological alteration observed when transection occurred at different time points throughout development.
Development of taste bud size continues through 40 days of age in the rat and is markedly immature at early postnatal ages (Krimm and Hill, 1998). Fungiform papillae are also immature until approximately 30 days of age (Iwasaki et al., 1997; Harada et al., 2000). Therefore, the dependence of fungiform papillae and taste bud maintenance on the CT throughout early development may be directly related to the immaturity of the papillae and taste buds. Growth-related factors that are less available during later stages of development may circulate at younger ages to promote normal development of these tissues at early postnatal ages. Disruption of CT or glossopharyngeal innervation has been shown to produce disruption in the molecular milieu of papillae (Miura et al., 2001; Ganchrow et al., 2003; Miura et al., 2004). BDNF and NT-3/4 are strongly implicated in maintenance of taste buds and papillae, and differences in expression occur within papillae during development (Nosrat et al., 1996). Ganchrow and colleagues (2003) found that CT denervation in adult hamsters resulted in a reduction, but not an elimination, of BDNF-like immunoreactivity. However, taste buds and papillae are more strongly maintained after CTX in the hamster as compared to the rat (Oliver and Whitehead, 1992).
Nongustatory systems show interesting parallels with the neonatal denervation effects observed in the present report. Sciatic nerve transection in the neonatal rat produces a reduction in motoneuron numbers not seen after the same transection in the adult rat, but cell sparing occurs when neonatal rats are given exogenous applications of BDNF or NT-3 (Yan et al., 1993). Changes in cortical innervation occur when the infraorbital nerve is transected neonatally, but not later in development. When either BDNF or NT-3 was applied to the whisker pads following neonatal denervation, barrel patterns developed in a manner more consistent with normal development (Calia et al., 1998). In another study, reinnervation of cerebellum occurred if climbing fibers were transected prior to postnatal day 7 in the rat, but reinnervation occurred only after the addition of exogenous BDNF or NT-3 if transection was given at later ages (Sherrard and Bower, 2001). Such studies not only lend further support for the role of neurotrophic regulation following denervation during developmental sensitive periods, but also provide impetus to determine if exogenous application of neurotrophins following neonatal CTX might result in maintenance of papillae and taste buds.
Taste bud volume was significantly reduced by 2 days posttransection in the oldest (65 days of age) group of rats in the present study, but volumes were not reduced until 8 days posttransection in 10-day and 25-day-old rats. These results might be explained by differing rates of taste cell proliferation in young and older animals. Although taste cell turnover is not changed across development, there is a significant differential in the number of taste cells produced at early postnatal ages vs. adulthood; the number of taste cells added to the taste bud is greater at 10 days of age than adulthood (Hendricks et al., 2004). Proliferation rates of taste receptor cells are not altered following denervation of the CT in the hamster (Oliver and Whitehead, 1992). Although the effects of CT transection appear to be less severe in the hamster than the rat (Whitehead et al., 1987), it is possible that higher rates of taste cell production continue for a longer period of time in the developing rat following CTX. Thus, CT transection in the adult rat, at a time of slower production of taste cells, could impose an environment where the overall volume of taste buds decreases at a faster rate.
Historically, the finding of developmentally sensitive periods has helped uncover an array of mechanisms involved in structural and functional regulation of neurobiology. These observations have played an important role in understanding normal developmental processes and finding the limits of biological plasticity (Wiesel, 1982; Hashisaki and Rubel, 1989; Rhoades et al., 1997; Fiske and Brunjes, 2001; Kral et al., 2002; Knudsen, 2004, for review). Condensing the developmental time frame in which major biological restructuring occurs after denervation (as in the present study) provides an important framework for further experimentation. For example, results obtained here show that the morphological integrity of fungiform papillae continue to be dramatically disrupted by denervation of the CT throughout early development until a time when the taste buds and papillae have matured (Iwasaki et al., 1997; Krimm and Hill, 1998; Harada et al., 2000). Had the results demonstrated an adult-like capacity for taste bud and papillae maintenance when denervation was performed any time subsequent to 10 days of age, the focus of future research would comprise a different scope. Instead, the time frame of denervation-induced changes to taste buds and papillae suggests that normal maturational processes involved in taste bud/papillae growth may be the key factors that are disrupted by denervation.
In summary, the present study found a developmentally related dependence on the CT of the nongustatory epithelium of fungiform papillae. Fungiform papillae become consistently more able to support normal morphological integrity in the absence of the CT as the rat matures. Previously, we observed a failure of the CT to regenerate fully (unpublished observations). The rapid degree of alteration to papillary epithelium when CTX occurs during early development may restrict the regeneration of the CT and thus result in the permanent loss of the ability of nerve fibers to support new taste bud proliferation. In fact, the loss of papillae and taste buds appears to be permanent (at least one year after CTX at 10 days of age; unpublished observations). It is not yet known whether the failure of the CT to regenerate completely is because of the rapid degeneration of papillae or whether degeneration of papillae occurs because the CT does not quickly regenerate as it does in the adult rat (St. John et al., 1995). Alternatively, local circulating factors within papillae may be necessary for both the growth of papillae during development and signaling for regenerating fibers of the CT. If these molecular cues are severely diminished after CTX in the neonatal rat, the CT, and thus the taste buds, may be markedly restricted in their ability to regenerate.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: NIDCD DC04846.
References
- Calia E, Persico AM, Baldi A, Keller F. BDNF and NT-3 applied in the whisker pad reverse cortical changes after peripheral deafferentation in neonatal rats. Eur J Neurosci. 1998;10:3194–3200. doi: 10.1046/j.1460-9568.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- Fiske BK, Brunjes PC. Cell death in the developing and sensory-deprived rat olfactory bulb. J Comp Neurol. 2001;431:311–319. [PubMed] [Google Scholar]
- Ganchrow JR, Ganchrow D. Long-term effects of gustatory neurectomy on fungiform papillae in the young rat. Anat Rec. 1989;225:224–231. doi: 10.1002/ar.1092250308. [DOI] [PubMed] [Google Scholar]
- Ganchrow D, Ganchrow JR, Verdin-Alcazar M, Whitehead MC. Brain-derived neurotrophic factor-, neurotrophin-3-, and tyrosine kinase receptor-like immunoreactivity in lingual taste bud fields of mature hamster after sensory denervation. J Comp Neurol. 2003;455:25–39. doi: 10.1002/cne.2164. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamaguchi K, Kanemaru N, Kasahara Y. Maturation of taste buds on the soft palate of the postnatal rat. Physiol Behav. 2000;68:333–339. doi: 10.1016/s0031-9384(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Hård af Segerstad C, Hellekant G, Farbman AI. Changes in number and morphology of fungiform taste buds after transection of the chorda tympani or chordalingual nerve. Chem Senses. 1989;14:335–348. [Google Scholar]
- Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol. 1989;283:465–473. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- Hendricks SJ, Brunjes PC, Hill DL. Taste bud cell dynamics during normal and sodium-restricted development. J Comp Neurol. 2004;472:173–182. doi: 10.1002/cne.20064. [DOI] [PubMed] [Google Scholar]
- Hill DL, Przekop PR., Jr Influences of dietary sodium on functional taste receptor development: a sensitive period. Science. 1988;241:1826–1828. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the rat. Anat Rec. 1997;247:528–541. doi: 10.1002/(SICI)1097-0185(199704)247:4<528::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of sub-plate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kopka SL, Geran LC, Spector AC. Functional status of the regenerated chorda tympani nerve as assessed in a salt taste discrimination task. Am J Physiol Regul Integr Comp Physiol. 2000;278:R720–731. doi: 10.1152/ajpregu.2000.278.3.R720. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Early prenatal critical period for chorda tympani nerve terminal field development. J Comp Neurol. 1997;378:254–264. [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- Liebl DJ, Mbiene J-P, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- McCluskey LP, Hill DL. Sensitive periods for the effect of dietary sodium restriction on intact and denervated taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1275–1284. doi: 10.1152/ajpregu.00282.2002. [DOI] [PubMed] [Google Scholar]
- Miller IJ., Jr Branched chorda tympani neurons and interaction among taste receptors. J Comp Neurol. 1974;158:155–166. doi: 10.1002/cne.901580204. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Preslar A. Spatial distribution of rat fungiform papillae. Anat Rec. 1975;181:679–684. doi: 10.1002/ar.1091810309. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Reedy FE., Jr Quantification of fungiform papillae and taste pores in living human subjects. Chem Senses. 1990;15:281–294. [Google Scholar]
- Mistretta CM, Goosens KIA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:1324. [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, Motoyama J, Hino A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001;106:143–145. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, Ninomiya Y, Hino A. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem Senses. 2004;29:823–831. doi: 10.1093/chemse/bjh248. [DOI] [PubMed] [Google Scholar]
- Montavon P, Hellekant G, Farbman A. Immunohistochemical, electrophysiological, and electron microscopical study of rat fungiform taste buds after regeneration of chorda tympani through the non-gustatory lingual nerve. J Comp Neurol. 1996;367:491–502. doi: 10.1002/(SICI)1096-9861(19960415)367:4<491::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H. Effect of denervation on morphogenesis of the rat fungiform papilla. Acta Anat. 1995;153:301–309. doi: 10.1159/000147739. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates a role in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Oakley B, Lawton A, Riddle DR, Wu L. Morphometric and immunocytochemical assessment of fungiform taste buds after interruption of the chordalingual nerve. Micros Res Tech. 1993;26:187–195. doi: 10.1002/jemt.1070260302. [DOI] [PubMed] [Google Scholar]
- Oakley B, Wu LH, Lawton A, DeSibour C. Neural control of ectopic filiform spines in adult tongue. Neurosci. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Oliver SD, Whitehead MC. Morphometry and cellular dynamics of denervated fungiform taste buds in the hamster. Chem Sens. 1992;17:529–542. [Google Scholar]
- Parks JD, Whitehead MC. Scanning electron microscopy of denervated taste buds in hamster: morphology of fungiform taste pores. Anat Rec. 1998;251:230–239. doi: 10.1002/(SICI)1097-0185(199806)251:2<230::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Crissman RS, Jacquin MF. Primary afferent plasticity following partial denervation of the trigeminal brainstem nuclear complex in the postnatal rat. J Neurosci. 1994;14:721–739. doi: 10.1523/JNEUROSCI.14-02-00721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Strang V, Bennett-Clarke CA, Killackey HP, Chiaia NL. Sensitive period for lesion-induced reorganization of intracortical projections within the vibrissae representation of rat’s primary somatosensory cortex. J Comp Neurol. 1997;389:185–192. doi: 10.1002/(sici)1096-9861(19971208)389:1<185::aid-cne14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sherrard RM, Bower AJ. BDNF and NT3 extend the critical period for developmental climbing fibre plasticity. Neuroreport. 2001;12:2871–2874. doi: 10.1097/00001756-200109170-00023. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Bernstein IL. Neonatal chorda tympani transection permanently disrupts fungiform taste bud and papilla structure in the rat. Physiol Behav. 2000;69:439–444. doi: 10.1016/s0031-9384(99)00259-0. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Smith PC, Hill DL. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol. 2002;51:223–236. doi: 10.1002/neu.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John SJ, Markison S, Spector AC. Salt discriminability is related to number of regenerated taste buds after chorda tympani nerve section in rats. Am J Physiol. 1995;269:R141–R153. doi: 10.1152/ajpregu.1995.269.1.R141. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME, Hettinger TP, Hou LT, Nah HD. Persistence of taste buds in denervated fungiform papillae. Brain Res. 1987;405:192–195. doi: 10.1016/0006-8993(87)91008-0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]