Abstract
Background
Many patients with chronic myeloid leukemia in chronic phase (CML-CP) experience chronic treatment-related adverse events (AEs) on imatinib therapy. These AEs can impair quality of life (QOL) and lead to reduced treatment adherence, which is associated with poor clinical outcomes.
Patients and Methods
In the phase 2 Exploring Nilotinib to Reduce Imatinib Related Chronic Adverse Events (ENRICH) study (N = 52), the impact of switching patients with imatinib-related chronic low-grade nonhematologic AEs from imatinib to nilotinib was evaluated.
Results
Three months after switching to nilotinib, 84.6% of patients had overall improvement in imatinib-related AEs (primary endpoint). Of 210 imatinib-related AEs identified at baseline, 62.9% resolved within 3 months of switching to nilotinib. Among evaluable patients, most had improvements in overall QOL after switching to nilotinib. At screening, 65.4% of evaluable patients had a major molecular response (MMR; BCR-ABL1 ≤ 0.1% on the International Scale). After switching to nilotinib, the rate of MMR was 76.1% at 3 months and 87.8% at 12 months. Treatment-emergent AEs reported on nilotinib were typically grade 1/2; however, some patients developed more serious AEs, and 8 patients discontinued nilotinib due to new or worsening AEs.
Conclusions
Overall, results from ENRICH demonstrated that switching to nilotinib can mitigate imatinib-related chronic low-grade nonhematologic AEs in patients with CML-CP in conjunction with acceptable safety and achievement of molecular responses. This trial was registered at www.clinicaltrials.gov as NCT00980018.
Keywords: Chronic myeloid leukemia, Clinical trials
Introduction
In patients with newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP), chronic mild to moderate imatinib-related adverse events (AEs) can negatively affect quality of life (QOL) and can lead to reduced adherence to therapy,1-3 which is associated with poor responses and poor long-term outcomes.4-7 Therefore, proper management of AEs is critical for ensuring optimal outcomes.1,3,6,8 When treatment interruptions and reduced adherence are due to imatinib-related toxicities, switching patients to another tyrosine kinase inhibitor (TKI) may improve tolerability and treatment adherence and thereby optimize responses.
Nilotinib is more potent and selective than imatinib,9 has demonstrated superior efficacy versus imatinib,10-14 and is associated with a distinct safety profile from that of imatinib.10-14 Compared with imatinib, nausea, vomiting, diarrhea, muscle spasms, and edema are less common with nilotinib, whereas rash, headache, and pruritus are more common with nilotinib.12 In a subset analysis of 95 patients with CML-CP who discontinued imatinib due to intolerance (> 75% for grade 3/4 AEs) and switched to nilotinib, cross-intolerance (defined as occurrence of the same AE with nilotinib that was associated with intolerance to imatinib) was uncommon.15 In that study, no patient required nilotinib dose reduction or discontinued nilotinib treatment due to the same AE that led to imatinib discontinuation.15 Cardiovascular AEs have been reported to varying degrees with all TKIs approved for treatment of CML14,16-23 and were reported more frequently with nilotinib than with imatinib in the pivotal trial of frontline nilotinib versus imatinib (ENESTnd).13,14
The phase 2 ENRICH (Exploring Nilotinib to Reduce Imatinib Related Chronic Adverse Events) study was conducted to evaluate whether imatinib-related chronic low-grade nonhematologic AEs could be improved and responses optimized by switching patients from imatinib to nilotinib.
Patients and Methods
ENRICH was a phase 2, single-arm, open-label, multicenter, exploratory study to determine the effect of switching to nilotinib on the AE profile of patients with low-grade toxicities associated with imatinib therapy (ClinicalTrials.gov identifier: NCT00980018).
Study design and treatments
Adults (aged ≥ 18 years) with CML-CP and an ECOG performance status ≤ 2 were eligible. Patients had been treated with imatinib (any dose) for ≥ 3 months prior to screening and experienced a Common Terminology Criteria for Adverse Events (CTCAE) grade 1 or 2 nonhematologic AE on imatinib that persisted for > 2 months or recurred > 3 times despite best supportive care. Patients were required to have achieved the following efficacy milestones on imatinib: after 3 months, complete hematologic response (CHR); after 6 months, Ph+ < 95% (≥ 20 metaphases required for standard bone marrow cytogenetics); after 12 months, Ph+ < 35%; and after 18 months, Ph+ 0% or BCR-ABL1 ≤ 0.1% on the International Scale (BCR-ABL1IS; documented within 3 months). Patients meeting any of the following criteria were excluded: any grade ≥ 3 nonhematologic AE within 30 days of screening; prior accelerated or blast phase; loss of CHR or cytogenetic response (CyR); previously documented T315I mutation; prior treatment with any TKI other than imatinib; impaired cardiac function (including congenital long QT syndrome or a known family history of long QT syndrome, history or presence of clinically significant ventricular or atrial tachyarrhythmias, clinically significant resting bradycardia, inability to monitor the QT interval by electrocardiogram, Fridericia-corrected QT (QTcF) > 450 ms on baseline electrocardiogram, myocardial infarction within 1 year of starting study drug, or other clinically significant heart disease); impaired gastrointestinal function or gastrointestinal disease that could significantly alter absorption of nilotinib; acute or chronic liver, pancreatic, or renal disease; history of significant bleeding disorder; pregnancy or nursing; treatment with a cytochrome P450 3A4 inhibitor; or medication with the potential to prolong the QT interval.
Enrolled patients received nilotinib 300 mg twice daily for 12 cycles (1 cycle = 28 days) on study. No washout period was required between imatinib and nilotinib treatment. Patients were followed up for safety for 28 days after the last dose of study drug.
Endpoints and assessments
Imatinib-related chronic low-grade (grade 1 or 2) nonhematologic AEs, hereafter referred to as imatinib-related AEs, were assessed on days 1 and 15 of cycle 1 and at the end of cycles (EOC) 1, 2, 3, 6, 9, and 12. The primary endpoint of the study was the percentage of patients with an overall improvement in imatinib-related AEs at EOC 3 following the switch to nilotinib; overall improvement was defined as either a decrease in CTCAE grade or resolution of ≥ 50% of a patient's imatinib-related AEs. Secondary endpoints included rate of complete CyR (CCyR; defined as negative fluorescence in situ hybridization [FISH] or 0% Ph+ cells) among patients without CCyR at baseline; rate of major molecular response (MMR) at EOC 1, 2, 3, 6, 9, and 12; BCR-ABL1IS log changes following switch to nilotinib; time to and duration of CCyR and MMR on study; time to first documented and optimal improvement in imatinib-related AEs; and safety.
Time to first documented improvement of imatinib-related AEs was defined as the time from first dose of study drug until first documented decrease in CTCAE grade, and time to optimal improvement of imatinib-related AEs was defined as the time from first dose of study drug until maximum decrease in the sum of the CTCAE grades of the events. AEs with an onset date on or after the date of study drug initiation or that worsened or recurred during study treatment were included in the analysis of AEs occurring during nilotinib treatment. Serious AEs (SAEs) occurring at any time from the initiation of study drug until 28 days after stopping study participation were also analyzed. Nilotinib dose reductions were required for patients with grade 3/4 AEs concerning white blood cells and platelets as well as grade 2 to 4 nonhematologic AEs. Discontinuation from the study was required if any toxicity did not resolve after 28 days. AEs were assessed using CTCAE version 4.0.
Times to CCyR and MMR were defined as the time from first dose of study drug to first documented CCyR or MMR, respectively. The duration of CCyR was defined as the time from first documented CCyR to the date of first documented loss of CCyR or study termination, whichever was earlier; duration of MMR was defined similarly. Bone marrow cytogenetic assessment was required at screening if there was no documentation that the patient achieved efficacy milestones on imatinib that were required for study eligibility. For patients who had < 18 months of prior imatinib therapy and did not have CCyR at screening, additional bone marrow cytogenetic assessments were required on study. Once CCyR was documented by cytogenetics, no additional bone marrow assessments were required unless there was a suspected loss of response or early discontinuation before EOC 12. Peripheral blood FISH was performed at EOC 1, 2, 3, 6, and 9 until achievement of CCyR and at EOC 12 in all patients.
Exploratory endpoints included change in overall QOL (assessed at baseline and at EOC 1, 3, 6, 9, and 12) and change in MD Anderson Symptom Inventory Chronic Myeloid Leukemia Module (MDASI-CML) score (assessed at baseline and at EOC 1, 3, 6, 9, and 12). For evaluation of overall QOL, patients rated their QOL within the prior 24 hours and within the prior 7 days on an 11-point scale by responding to the following: “rate your quality of life within the last 24 hours on a scale of 0 to 10” and “rate your quality of life within the last 7 days on a scale of 0 to 10,” with an increasing QOL score indicating improvement. The MDASI-CML module is a patient-reported outcome measure for evaluation of symptom burden in patients with CML and comprises 20 core and CML-specific symptom items (vomiting, nausea, diarrhea, dry mouth, pain, drowsiness, shortness of breath, sadness, difficulty remembering, disturbed sleep, distress, fatigue, numbness, muscle soreness, swelling, malaise, rash or skin change, bruising easily or bleeding, lack of appetite, and headache)3 and 6 interference items (general activity, work, walking, enjoyment of life, mood, and relations with other people). Following completion of this study, the MDASI-CML module was validated, and headache was added as a CML-specific item.3 Patients scored each symptom or interference item on an 11-point scale, with a decreasing MDASI-CML score indicating improvement. For MDASI-CML symptom items, a rating of 0 indicates “not present” and 10 indicates severity “as bad as you can imagine.” For MDASI-CML interference items, a rating of 0 indicates “did not interfere” and 10 indicates “interfered completely.”
Statistical analyses
Efficacy analyses included all patients who received ≥ 1 dose of study drug. Safety analyses included all patients who received ≥ 1 dose of study drug and had ≥ 1 evaluable post-baseline safety assessment. The primary endpoint was assessed employing a 95% CI using the normal approximation to the binomial. The proportion of imatinib-related AEs with improvement was assessed using a quasi-likelihood method24,25 that accommodated the unknown covariance associated with measuring the overall effect of AEs for individual patients. Time to CCyR, time to MMR, time to first documented improvement in any imatinib-related AE, and time to optimal improvement in imatinib-related AEs were analyzed using the Kaplan-Meier product limit method. The planned sample size was 50 patients to assess the primary endpoint with a 2-sided 95% CI within 14% of the true percentage. All statistical analyses were performed using SAS software (version 9.1.3).
Ethics statements
The study was conducted in accordance with the Declaration of Helsinki and local applicable laws and regulations. The protocol was approved by the institutional review board or independent ethics committee at each participating study center. Written informed consent was obtained from each patient prior to study participation.
Results
Patients and treatments
ENRICH was conducted across 15 centers in the United States and 4 centers in Canada; 52 patients were enrolled from December 10, 2009, to August 15, 2012 (study completion date: December 27, 2012). Baseline demographics and characteristics of all enrolled patients are shown in Table 1. All evaluable patients had CHR at baseline; most patients had CCyR and MMR at baseline (86.5% and 65.4%, respectively). Forty patients (76.9%) completed the study per protocol and 12 discontinued early, 8 due to AEs (15.4%), 3 due to withdrawal of consent (5.8%), and 1 due to loss to follow-up (1.9%). The median duration of nilotinib exposure on study was 336 days (range, 6-617 days).
Table 1. Patient Demographic and Baseline Characteristics.
| Nilotinib 300 mg BID (N = 52) | |
|---|---|
| Mean age (range), years | 51.7 (34-82) |
| Male, % | 50.0 |
| Caucasian, % | 86.5 |
| ECOG performance status, % | |
| 0 | 44.2 |
| 1 | 48.1 |
| 2 | 7.7 |
| Median time since diagnosis (range), months | 31.4 (3.0-179.3) |
| Median prior imatinib dose (range), mg/d | 400 (300-800) |
| Median duration of prior imatinib treatment (range), months | 31.1 (2-145)a |
| Patients with complete cytogenetic response, %b | 86.5 |
| Patients with major molecular response, % | 65.4 |
| Median number of imatinib-related AEs at baseline (range), n | 3 (1-11) |
| Imatinib-related AEs per patient at baseline, % | |
| 1 | 5.8 |
| 2 | 21.2 |
| 3 | 30.8 |
| ≥ 4 | 42.3 |
AE, adverse event; BID, twice daily; ECOG, Eastern Cooperative Oncology Group.
One patient with < 3 months of prior imatinib treatment was enrolled and treated (protocol deviation).
Based on pre-study results, fluorescence in situ hybridization assessment at screening, and bone marrow aspirate at screening.
Impact of switching to nilotinib on imatinib-related AEs
Among the 52 patients, 210 imatinib-related AEs were identified at baseline, including 154 grade 1 AEs and 56 grade 2 AEs. The most common imatinib-related AE at baseline was fatigue (n = 29; 13.8%), followed by diarrhea and nausea, each occurring in 20 patients (9.5%; Table 2).
Table 2. Change in Most Frequently Reported (≥ 3 patients) Imatinib-Related Chronic Low-Grade (grade 1 or 2) Nonhematologic AEs at Baseline.
| Imatinib-Related AE | Patients With AE at Baseline, na | Change in Imatinib-Related AEs After Switching to Nilotinib | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| EOC 1, n (%) | EOC 3, n (%) | EOC 12, n (%) | |||||||||
|
| |||||||||||
| Resolved | Resolved | Improved | Unchanged | Worsened | Resolved | Improved | Unchanged | Worsened | Resolved and Reappeared | ||
| Fatigue | 29 | 5 (17.2) | 10 (34.5) | 6 (20.7) | 10 (34.5) | 2 (6.9) | 11 (37.9) | 6 (20.7) | 10 (34.5) | 1 (3.4) | 3 (10.3) |
| Diarrhea | 20 | 14 (70.0) | 19 (95.0) | 0 | 1 (5.0) | 0 | 19 (95.0) | 0 | 1 (5.0) | 0 | 4 (20.0) |
| Nausea | 20 | 14 (70.0) | 16 (80.0) | 0 | 3 (15.0) | 0 | 16 (80.0) | 0 | 3 (15.0) | 0 | 4 (20.0) |
| Muscle spasms | 14 | 8 (57.1) | 12 (85.7) | 0 | 2 (14.3) | 0 | 12 (85.7) | 0 | 2 (14.3) | 0 | 5 (35.7) |
| Peripheral edema | 13 | 6 (46.2) | 8 (61.5) | 2 (15.4) | 3 (23.1) | 0 | 8 (61.5) | 2 (15.4) | 3 (23.1) | 0 | 0 |
| Periorbital edema | 13 | 5 (38.5) | 9 (69.2) | 0 | 3 (23.1) | 0 | 9 (69.2) | 0 | 3 (23.1) | 0 | 0 |
| Arthralgia | 10 | 4 (40.0) | 6 (60.0) | 1 (10.0) | 3 (30.0) | 0 | 6 (60.0) | 1 (10.0) | 3 (30.0) | 0 | 2 (20.0) |
| Myalgia | 9 | 5 (55.6) | 6 (66.7) | 0 | 3 (33.3) | 0 | 6 (66.7) | 0 | 3 (33.3) | 0 | 2 (22.2) |
| Headache | 8 | 1 (12.5) | 4 (50.0) | 0 | 3 (37.5) | 1 (12.5) | 4 (50.0) | 0 | 2 (25.0) | 2 (25.0) | 2 (25.0) |
| Dyspepsia | 4 | 2 (50.0) | 2 (50.0) | 0 | 2 (50.0) | 0 | 3 (75.0) | 0 | 1 (25.0) | 0 | 1 (25.0) |
| Rash | 4 | 1 (25.0) | 2 (50.0) | 0 | 2 (50.0) | 0 | 3 (75.0) | 0 | 1 (25.0) | 0 | 2 (50.0) |
| Face edema | 4 | 3 (75.0) | 3 (75.0) | 0 | 1 (25.0) | 0 | 3 (75.0) | 0 | 1 (25.0) | 0 | 1 (25.0) |
| Weight increased | 4 | 1 (25.0) | 3 (75.0) | 0 | 1 (25.0) | 0 | 4 (100.0) | 0 | 0 | 0 | 0 |
| Insomnia | 4 | 1 (25.0) | 1 (25.0) | 0 | 3 (75.0) | 0 | 1 (25.0) | 0 | 3 (75.0) | 0 | 0 |
| Pruritus | 3 | 2 (66.7) | 2 (66.7) | 0 | 0 | 1 (33.3) | 2 (66.7) | 0 | 0 | 1 (33.3) | 2 (66.7) |
| Vomiting | 3 | 3 (100.0) | 3 (100.0) | 0 | 0 | 0 | 3 (100.0) | 0 | 0 | 0 | 0 |
| Abdominal pain | 3 | 1 (33.3) | 1 (33.3) | 0 | 1 (33.3) | 0 | 1 (33.3) | 0 | 1 (33.3) | 0 | 0 |
| Bone pain | 3 | 2 (66.7) | 3 (100.0) | 0 | 0 | 0 | 3 (100.0) | 0 | 0 | 0 | 1 (33.3) |
| Amnesia | 3 | 3 (100.0) | 3 (100.0) | 0 | 0 | 0 | 3 (100.0) | 0 | 0 | 0 | 0 |
AE, adverse event; EOC, end of cycle.
For each AE type, each event occurred in a distinct patient (i.e., the number of events was equal to the number of patients with that event type).
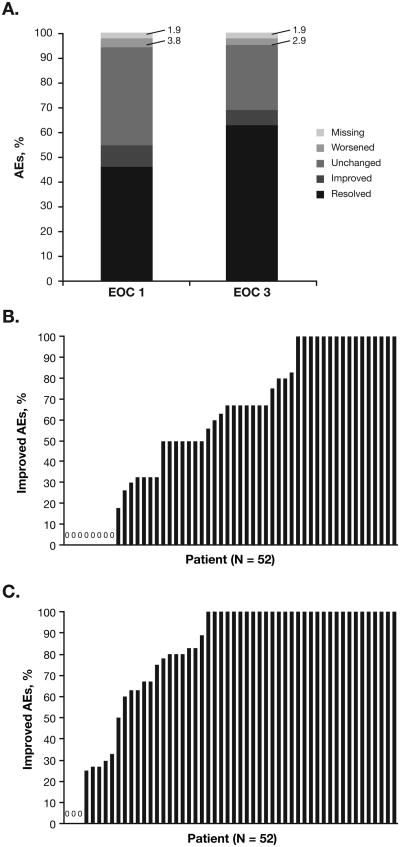
Of 210 imatinib-related AEs at baseline, 132 (62.9%) had resolved, 13 (6.2%) had improved, 55 (26.2%) were unchanged, and 6 (2.9%) had worsened at EOC 3; information was missing for 4 (1.9%; Figure 1A). Of imatinib-related fatigue AEs, 16 of 29 (55.2%) had improved or resolved at EOC 3, as had 19 of 20 imatinib-related diarrhea AEs (95.0%) and 16 of 20 imatinib-related nausea AEs (80.0%); similarly, ≥ 50% of imatinib-related muscle spasms, peripheral edema, periorbital edema, arthralgia, myalgia, headache, dyspepsia, rash, face edema, weight increase, pruritus, vomiting, bone pain, and amnesia AEs improved or resolved by EOC 3. Of the 6 imatinib-related AEs that worsened by EOC 3, 5 worsened from grade 1 to grade 2 (fatigue [n = 2], pruritus [n = 1], headache [n = 1], and memory impairment [n = 1]) and 1 worsened from grade 2 to grade 3 (generalized pain). At EOC 12, 151 of 210 imatinib-related AEs (71.9%) had either improved or resolved; 34 imatinib-related AEs (16.2%) had resolved and reappeared. The AE type that most frequently resolved and reappeared was muscle spasm (resolved and reappeared in 5 of 14 patients [35.7%]).
Figure 1. Effects on Imatinib-Related Chronic Low-Grade Nonhematologic AEs.
A) Change in status of imatinib-related AEs at EOC 1 and EOC 3 relative to baseline (percentages were derived from the 210 total imatinib-related AEs reported at baseline). B and C) Proportion of imatinib-related AEs that were improved at (B) EOC 1 and (C) EOC 12 for each patient.
AE, adverse event; EOC, end of cycle.
An overall improvement (resolution of or reduction in CTCAE grade for ≥ 50% of a patient's imatinib-related AEs) was observed in 37 of 52 patients (71.2%) at EOC 1 (Figure 1B). By EOC 3, 7 additional patients achieved an overall improvement; thus, the total number of patients with an overall improvement at EOC 3 (primary endpoint) was 44 of 52 (84.6%; 95% CI, 72.5%-92.0%). No additional patients achieved an overall improvement after EOC 3, and all 44 patients with an overall improvement at EOC 3 maintained the improvement through EOC 12; thus, the proportion of patients with an overall improvement was 84.6% at all time points beyond EOC 3. The estimated median time to first improvement of any imatinib-related AE was 1 month (95% CI, 0.3-1.0 month), and the estimated median time to optimal (i.e., maximum) improvement of imatinib-related AEs was 1.9 months (95% CI, 1.0-2.1 months). No patient had an overall worsening (defined as an increase in CTCAE grade for ≥ 50% of imatinib-related AEs) at any time point. In 30 patients (57.7%), all imatinib-related AEs had improved at EOC 12, while 3 patients (5.8%) did not have improvement in any imatinib-related AEs at EOC 12 (Figure 1C). As expected, there was a negative correlation at all time points (at EOC 3: r = −0.42) between the number of imatinib-related AEs and the proportion of AEs that improved after switching to nilotinib (i.e., patients with a higher number of imatinib-related AEs were less likely to achieve an overall improvement).
AEs during nilotinib treatment
After switching to nilotinib, 51 patients (98.1%) developed new or worsening AEs, including AEs with suspected relationship to study treatment in 44 patients (84.6%). The most common new or worsening nonhematologic AEs (regardless of relationship to study drug) of any grade were fatigue (19 of 52 patients; 36.5%), rash (18 of 52 patients; 34.6%), headache (17 of 52 patients; 32.7%), constipation (14 of 52 patients; 26.9%), arthralgia (13 of 52 patients; 25.0%), nausea (12 of 52 patients; 23.1%), and pruritus (12 of 52 patients; 23.1%). The most common AEs suspected to be related to study treatment were headache (14 of 52 patients; 26.9%), rash (13 of 52 patients; 25.0%), fatigue (12 of 52 patients; 23.1%), and pruritus (11 of 52 patients; 21.2%). Although most AEs reported during nilotinib treatment were grade 1 or 2, 20 patients (38.5%) had grade 3 AEs on nilotinib (Table 3) and 1 patient had a grade 4 AE of cardiac arrest. This patient had a history of hypertension prior to study entry and no other known cardiovascular risk factors. Grade 1 QT prolongation (450-480 ms) was observed in 1 patient; QT or QTcF > 500 ms was not observed in any patient.
Table 3. Patients With Grade 3 AEs Reported During Treatment With Nilotinib (N = 52)a.
| Grade 3 Events, n (%) | Total Frequency (any grade), n (%) | |||
|---|---|---|---|---|
|
| ||||
| Any Cause | Suspected Relationship to Study Drug | Any Cause | Suspected Relationship to Study Drug | |
| Any AE | 20 (38.5) | 16 (30.8) | 51 (98.1) | 44 (84.6) |
|
| ||||
| Nonhematologic AEs | ||||
| Rash | 3 (5.8) | 2 (3.8) | 18 (34.6) | 13 (25.0) |
| Arthralgia | 2 (3.8) | 2 (3.8) | 13 (25.0) | 6 (11.5) |
| Hypotension | 2 (3.8) | 1 (1.9) | 3 (5.8) | 1 (1.9) |
| Pruritus | 1 (1.9) | 1 (1.9) | 12 (23.1) | 11 (21.2) |
| Myalgia | 1 (1.9) | 0 | 4 (7.7) | 2 (3.8) |
| Bronchitis | 1 (1.9) | 1 (1.9) | 2 (3.8) | 1 (1.9) |
| Gastroenteritis | 1 (1.9) | 1 (1.9) | 2 (3.8) | 1 (1.9) |
| Pain | 1 (1.9) | 0 | 2 (3.8) | 1 (1.9) |
| Pneumonia | 1 (1.9) | 0 | 2 (3.8) | 1 (1.9) |
| Rash erythematous | 1 (1.9) | 1 (1.9) | 2 (3.8) | 2 (3.8) |
| Rash exfoliative | 1 (1.9) | 1 (1.9) | 2 (3.8) | 2 (3.8) |
| Rash papular | 1 (1.9) | 1 (1.9) | 2 (3.8) | 2 (3.8) |
| Arteriosclerosis | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Cartilage injury | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Cholecystitis acute | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Dehydration | 1 (1.9) | 1 (1.9) | 1 (1.9) | 1 (1.9) |
| Intervertebral disc protrusion | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Malignant mesothelioma | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Menorrhagia | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Pancreatitis | 1 (1.9) | 1 (1.9) | 1 (1.9) | 1 (1.9) |
| Pancreatitis acute | 1 (1.9) | 1 (1.9) | 1 (1.9) | 1 (1.9) |
| Pleural effusion | 1 (1.9) | 1 (1.9) | 1 (1.9) | 1 (1.9) |
| Pleural fibrosis | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Rheumatoid arthritis | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Scapula fracture | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Tendon rupture | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Wound infection bacterial | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Wound infection staphylococcal | 1 (1.9) | 0 | 1 (1.9) | 0 |
| Laboratory abnormalities | ||||
| Hyperglycemia | 2 (3.8) | 2 (3.8) | 4 (7.7) | 4 (7.7) |
| Hypokalemia | 1 (1.9) | 1 (1.9) | 3 (5.8) | 3 (5.8) |
| Hypophosphatemia | 1 (1.9) | 1 (1.9) | 3 (5.8) | 2 (3.8) |
| Lipase increase | 1 (1.9) | 1 (1.9) | 3 (5.8) | 3 (5.8) |
| Blood bilirubin increase | 1 (1.9) | 1 (1.9) | 2 (3.8) | 2 (3.8) |
| Hyperuricemia | 1 (1.9) | 0 | 2 (3.8) | 0 |
AE, adverse event.
1 grade 4 event (cardiac arrest, with suspected relationship to study drug) was reported during treatment with nilotinib.
A total of 9 patients (17.3%) had SAEs. These included 3 patients with infection (2 with suspected study drug relationship [1 also had ovarian torsion]; the third patient had acute cholecystitis and hypotension); 2 patients with pancreatitis (both with suspected study drug relationship); 1 patient noted above with cardiac arrest (suspected study drug relationship); 1 patient with injury (scapula fracture/cartilage injury); 1 patient with facial palsy and coronary artery disease; and 1 patient with arthralgia (suspected study drug relationship), pain (suspected study drug relationship), pleural effusion (suspected study drug relationship), arteriosclerosis, malignant mesothelioma, and pleural fibrosis. None of the 3 patients with SAEs of infection had grade 3/4 neutropenia at the time of the infections.
Twenty-three patients (44.2%) had AEs that led to dose interruption or reduction. Of the 8 patients who discontinued due to AEs (15.4%), 2 discontinued due to hyperglycemia (neither had a known history of diabetes); 1 discontinued due to cardiac arrest (SAE), hypercholesterolemia, and hypotension; 1 discontinued due to pleural effusion (SAE), malignant mesothelioma (SAE), and pleural fibrosis (SAE); 1 discontinued due to headache, sore mouth, and stomach pain; and 1 each discontinued due to cough, vertigo, and myasthenia gravis. Among the AEs leading to discontinuation of study treatment, all except malignant mesothelioma, pleural fibrosis, and myasthenia gravis were suspected to be related to the study drug.
Response
Forty-two patients had bone marrow cytogenetic assessments at screening, and there were 7 patients without CCyR at baseline (13.5%; median prior imatinib treatment duration, 4.8 months). All 7 patients achieved CCyR by EOC 6 (as assessed by FISH); among these 7 patients, the estimated median time to achieve CCyR was 1.9 cycles and the median duration of CCyR was 282 days.
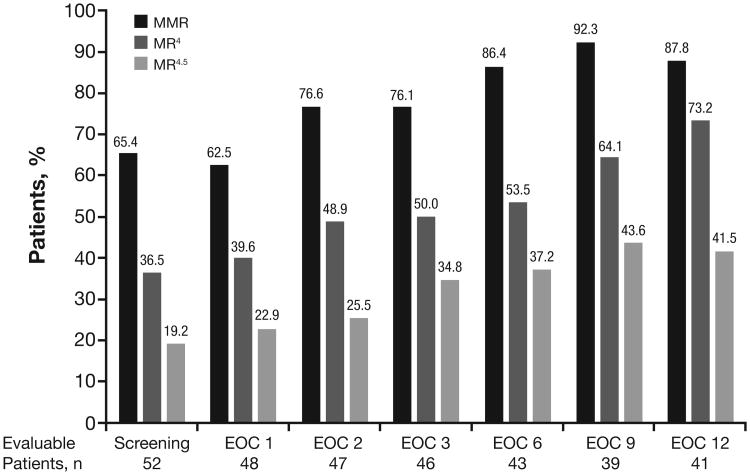
At screening, 34 patients (65.4%) had MMR; regarding deeper MR, 19 (36.5%) had MR4 (BCR-ABL1IS ≤ 0.01%), and 10 (19.2%) had MR4.5 (BCR-ABL1IS ≤ 0.0032%; Figure 2). Of the 18 patients without MMR at screening (median prior imatinib treatment duration, 9.5 months), 11 (61.1%) achieved MMR by EOC 3 and 15 (83.3%) achieved MMR at any time on study. The estimated median time to achieve MMR was 2.8 cycles. The median duration of MMR among patients who achieved MMR on study was 253 days. Four patients who achieved MMR on study later lost MMR; no patient with MMR at screening lost MMR on study. Among 41 patients evaluable for MR at EOC 12, 36 (87.8%) had MMR, 30 (73.2%) had MR4, and 17 (41.5%) had MR4.5.
Figure 2. Molecular Response After Switch to Nilotinib (N = 52).
Rates of MMR, MR4, and MR4.5 were calculated among evaluable patients at each time point.
EOC, end of cycle; MMR, BCR-ABL1IS ≤ 0.1%; MR4, BCR-ABL1IS ≤ 0.01%; MR4.5, BCR-ABL1IS ≤ 0.0032%.
Of the 52 patients, 15 did not have results for ≥ 1 time point shown.
At screening, the median BCR-ABL1IS level reduction was 3.375 (range, 5.13 to 0.64 reduction). Among 46 evaluable patients at EOC 3, the median BCR-ABL1IS level reduction was 4.010 (range, 5.04 to 1.45 reduction), representing a median log reduction from study baseline of 0.413 (range, 2.21 reduction to 0.39 increase). Among 41 evaluable patients at EOC 12, the median BCR-ABL1IS level reduction was 4.379 (range, 5.32 to 2.36 reduction), representing a median log reduction from study baseline of 0.527 (range, 3.08 reduction to 0.45 increase).
Changes in QOL and MDASI-CML scores
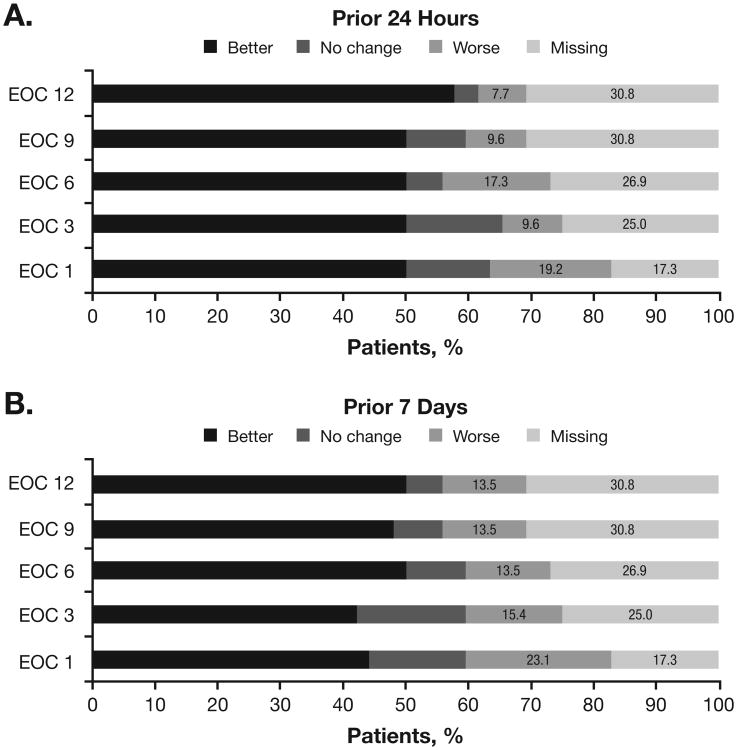
Throughout the study, QOL improvements relative to baseline were observed in most evaluable patients, whereas few patients had worsening QOL relative to baseline (Figure 3). Among 43 patients evaluable for change in QOL at EOC 1 relative to baseline, mean QOL scores for the prior 24 hours and the prior 7 days were 7.0 (SD, 2.37) and 6.8 (SD, 2.06), respectively, at EOC 1, compared with 6.3 (SD, 2.11) and 6.1 (SD, 2.19), respectively, at baseline. Considering all patients, 50.0% and 44.2% had improved QOL scores for the prior 24 hours and the prior 7 days, respectively, at EOC 1 relative to baseline; 13.5% and 15.4% had unchanged QOL scores, respectively, and 19.2% and 23.1% had worse QOL scores, respectively (17.3% of patients were not evaluable for change in QOL from baseline to EOC 1). Among 36 patients evaluable for change in QOL at EOC 12, the mean overall QOL score for the prior 24 hours was 8.0 (SD, 2.00) and for the prior 7 days was 7.8 (SD, 2.04). Considering all patients, 57.7% and 50.0% had improved QOL scores for the prior 24 hours and the prior 7 days, respectively, at EOC 12 relative to baseline; 3.8% and 5.8% had unchanged QOL scores, respectively, and 7.7% and 13.5% had worse QOL scores, respectively (30.8% of patients were not evaluable for change in QOL from baseline to EOC 12).
Figure 3. Change in Overall QOL.
For each time point, the proportion of patients reporting better, unchanged, or worse QOL relative to baseline was calculated based on the total patient population (N = 52). QOL was evaluated at each time point for (A) the prior 24 hours and (B) the prior 7 days.
EOC, end of cycle; QOL, quality of life.
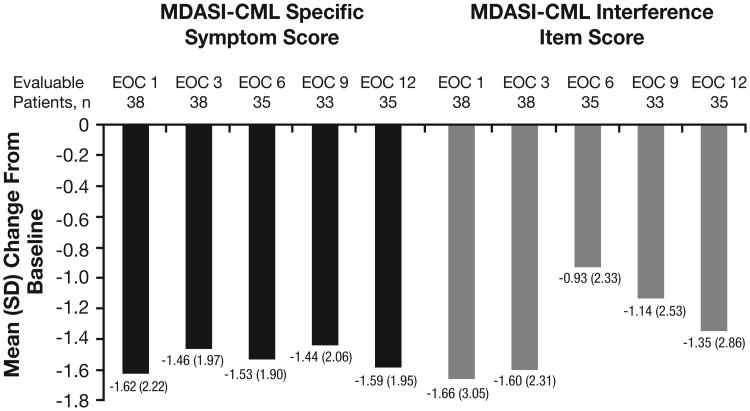
At baseline, the mean MDASI-CML specific symptom score and mean MDASI-CML interference item score were 3.07 (SD, 2.03) and 3.92 (SD, 2.78), respectively. Improvements in both MDASI-CML scores relative to baseline were observed throughout the study (Figure 4). Among 38 patients evaluable for change in MDASI-CML scores at EOC 1, specific symptom scores decreased by a mean of 1.62 (SD, 2.22) relative to baseline and interference item scores decreased by a mean of 1.66 (SD, 3.05) relative to baseline. Among 35 patients evaluable for change in MDASI-CML scores at EOC 12, specific symptom and interference item scores decreased by a mean of 1.59 (SD, 1.95) and 1.35 (SD, 2.86), respectively, relative to baseline.
Figure 4. Mean Change in MDASI-CML Scores.
For each time point, the mean change in MDASI-CML specific symptom and MDASI-CML interference item scores relative to baseline were calculated among evaluable patients. A decrease in MDASI-CML score indicates improvement.
EOC, end of cycle; MDASI-CML, MD Anderson Symptom Inventory-Chronic Myeloid Leukemia.
Discussion
In ENRICH, switching from imatinib to nilotinib led to improvements in imatinib-related AEs in most patients. Within 1 month after switching to nilotinib, most imatinib-related AEs either resolved or improved, and by 3 months, the frequency of AEs that improved increased further. New AEs were reported during nilotinib therapy, including SAEs; however, overall QOL and MDASI-CML scores generally improved throughout the study. Nilotinib therapy also resulted in effective disease control, as evidenced by the maintaining and/or achieving of CyR and MR in most patients.
The observed improvements in QOL and MDASI-CML scores in this study were consistent with prior reports demonstrating the impact of AEs on QOL. Low-grade AEs, including gastrointestinal disorders (e.g., nausea and diarrhea), blood and lymphatic system disorders (e.g., thrombocytopenia and neutropenia), musculoskeletal disorders (e.g., muscle spasms and arthralgia), psychiatric disorders (e.g., insomnia and anxiety), and general disorders and administration-site conditions (e.g., fatigue and peripheral edema), have been shown to negatively impact health-related QOL in patients with CML-CP receiving long-term TKI therapy.26 Specifically, a survey identified fatigue, muscle cramps, and swelling among the factors that most impacted QOL.8 In this study, fatigue and muscle cramps were among the most frequently reported imatinib-related AEs, and fatigue was also the most frequently observed AE during nilotinib therapy. Due to the potential impact of AEs on QOL and treatment adherence,1-3,8,26 adequate management of chronic low-grade AEs is crucial for optimizing outcomes of patients with CML-CP. In this study, most imatinib-related AEs resolved after switching to nilotinib, while few imatinib-related AEs worsened or recurred on nilotinib; these results are consistent with previous data15 showing minimal cross-intolerance between imatinib and nilotinib and suggesting that switching to nilotinib may be an effective option for managing such events for most patients.
Most patients developed new AEs after switching to nilotinib. Most treatment-emergent AEs were grade 1/2; however, some patients developed grade 3/4 AEs, SAEs, or AEs leading to discontinuation of study treatment. Because the patients in this study had only low-grade AEs prior to switching to nilotinib, the benefits of nilotinib must be considered together with the potential risk of developing new AEs. The treatment-emergent AEs observed in this study were consistent with the known safety profile of nilotinib.10-12,14 Similar to the pattern of AEs observed in this study, the most frequently reported AEs in a previous trial of second-line nilotinib for patients with imatinib resistance or intolerance were grade 1/2 rash, pruritus, nausea, fatigue, headache, and constipation.11 Although in this study the general trend was favorable, further evaluation is needed to determine the impact of new AEs on QOL for patients switching from imatinib to nilotinib, particularly considering that almost one-third of patients were not evaluable for change in QOL at 12 months. In addition to these more common AE types,10-12,14,17 a higher incidence of cardiovascular AEs has been shown to result from long-term nilotinib compared with long-term imatinib.13,14,17 In the current study, cardiovascular SAEs of cardiac arrest, arteriosclerosis, and coronary artery disease were observed in 1 patient each; because this study was completed after a median of ≈ 12 months of nilotinib therapy, the long-term incidence of cardiovascular AEs and SAEs in this patient population remains unknown. Of note, some biochemical abnormalities that have been reported to occur with nilotinib therapy, namely lipid and glucose elevations,27 are modifiable cardiovascular risk factors.28 Thus, it is important that patients with CML receive proper monitoring and management of cardiovascular risk factors and comorbidities while being treated with any TKI.16 While this trial was not designed to monitor lipid levels, glucose levels were recorded over the course of the study.
Switching to nilotinib also resulted in CyR and MR in this patient population. Importantly, the ENRICH study eligibility criteria excluded patients with treatment failure, as defined by CML management recommendations in place at the time (European LeukemiaNet 2009 recommendations29); thus, all enrolled patients were responding (optimally or suboptimally29) to frontline imatinib therapy at the time of switch to nilotinib. Although it is not possible to evaluate whether switching to nilotinib led to improvements in patients' response levels compared with what they would have achieved with continued imatinib therapy, the high rates of MR and CyR observed following switch indicate that nilotinib therapy was effective in this patient population. Most patients without MMR at baseline achieved MMR on study, and no patient who entered the study with MMR lost MMR on nilotinib. Among evaluable patients at EOC 12, most had MR4, and the median BCR-ABL1IS level was reduced by more than a half log from study baseline. Additionally, all 7 patients without CCyR at baseline achieved CCyR after switching to nilotinib (although considering the short duration of prior imatinib therapy among these 7 patients, some of them may have eventually achieved CCyR on imatinib with continued treatment). These findings are consistent with several studies in which patients with suboptimal MR, resistance, or intolerance to imatinib achieved improved responses after switching to nilotinib; furthermore, in prior studies, the higher response rates on nilotinib were associated with improved long-term clinical outcomes.11,30-32 Overall, results from ENRICH demonstrated the positive impact of switching to nilotinib in a patient population not previously studied.
Conclusion
Overall, results from ENRICH supported a positive impact of switching to nilotinib for some patients with chronic low-grade nonhematologic AEs on imatinib. The optimal course of therapy for each patient with CML-CP must be determined through consideration of several factors, including imatinib-related AEs, potential nilotinib-related AEs, the relative efficacy of nilotinib versus imatinib, and the relative impact of each drug on overall QOL and treatment adherence.
Clinical Practice Points.
It is well known that many patients with CML-CP treated with imatinib experience chronic low-grade AEs. Prior to the approval of second-generation TKIs, there was no option for patients experiencing such AEs on imatinib therapy. In the registrational study for nilotinib (NCT00109707) in patients with resistance and/or intolerance to imatinib, only patients with recurring grade 3/4 AEs, or with intolerance to imatinib doses of 600-800 mg/day were included. That study was conducted at a time when second-generation TKIs were not yet approved and imatinib dose escalation was the only option for patients with resistance, outside of a clinical trial. Thus, although several approved TKI options are now available for patients with resistance or intolerance to imatinib, no clinical trial has ever evaluated switch to nilotinib for patients with chronic low-grade AEs on imatinib. The ENRICH study was conducted specifically to address this question. For patients with these chronic low-grade treatment-related AEs on imatinib therapy, switching to nilotinib provided improvement of many of these AEs, improved QOL, and led to achievement of CyR and MR. With many TKI options now available to physicians who treat patients with CML, these results indicate that nilotinib may be a tolerable and effective treatment option for those patients on imatinib who suffer from chronic, low-grade AEs.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. The authors thank Staci Heise, PhD, and Karen Kaluza, PhD (Articulate Science), for medical editorial assistance with this manuscript. This study and the work presented here were sponsored and funded by Novartis Pharmaceuticals Corporation.
Disclosure of conflicts of interest: JEC acted as a consultant and received research funding from Novartis, Bristol-Myers Squibb, Ariad, Teva, and Pfizer. JHL acted as a consultant, received research funding and honoraria, and served on a board of directors or advisory committee for Novartis, Bristol-Myers Squibb, Pfizer, Ariad, and Teva. CBM acted as a consultant, received research funding and honoraria, and attended a speakers bureau for Novartis. LB received research funding from Novartis and acted as a consultant for Novartis, Bristol-Myers Squibb, and Pfizer. LPA acted as a consultant for Ariad, Bristol-Myers Squibb, Celgene, and Novartis; received research funding from Ariad, Bristol-Myers Squibb, Novartis, and Pfizer; and attended a speakers bureau for Ariad, Bristol-Myers Squibb, Celgene, Millennium, and Novartis. JP-I acted as a consultant and received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer; acted as a consultant for Ariad; received research funding from Novartis and Ariad; and served on a speakers bureau for Bristol-Myers Squibb. FPL is an employee of Novartis Pharmaceuticals Corporation. CK and GW are employees of and have equity ownership in Novartis Pharmaceuticals Corporation. MJM acted as a consultant for Novartis, Bristol-Myers Squibb, Ariad, and Pfizer and received research funding from Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey H. Lipton, Email: jeff.lipton@uhn.on.ca.
Carole B. Miller, Email: cmiller@stagnes.org.
Lambert Busque, Email: lbusque.hmr@ssss.gouv.qc.ca.
Luke P. Akard, Email: lakard@ibmtindy.com.
Javier Pinilla-Ibarz, Email: javier.pinilla@moffitt.org.
Christopher Keir, Email: christopher.keir@novartis.com.
Ghulam Warsi, Email: ghulam.warsi@novartis.com.
Felice P. Lin, Email: felice.lin@novartis.com.
Michael J. Mauro, Email: maurom@mskcc.org.
References
- 1.Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: the role of the midlevel practitioner. J Support Oncol. 2012;10(1):14–24. doi: 10.1016/j.suponc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–1519. doi: 10.1038/leu.2013.51. [DOI] [PubMed] [Google Scholar]
- 3.Williams LA, Gonzalez AGG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–647. doi: 10.1182/blood-2013-01-477687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117(14):3733–3736. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesan P, Sagar T Gnana, Dubashi Biswajit, et al. Non-adherence to imatinib adversely affects event free survival in chronic phase-chronic myeloid leukemia. Am J Hematol. 2011;86(6):471–474. doi: 10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]
- 8.Efficace F, Breccia M, Saussele S, et al. Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Ann Hematol. 2012;91(9):1371–1381. doi: 10.1007/s00277-012-1458-6. [DOI] [PubMed] [Google Scholar]
- 9.Blay JY, von Mehren M. Nilotinib: a novel, selective tyrosine kinase inhibitor. Semin Oncol. 2011;38(suppl 1):S3–S9. doi: 10.1053/j.seminoncol.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–112. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 12.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 13.Larson RA, Kim DW, Issaragrilsil S, et al. Efficacy and safety of nilotinib (NIL) vs imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): long-term follow-up (f/u) of ENESTnd. Blood. 2014;124(21) abstract 4541. [Google Scholar]
- 14.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016 Feb 3; doi: 10.1038/leu.2016.5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes JE, Hochhaus A, le Coutre PD, et al. Minimal cross-intolerance with nilotinib in patients with chronic myeloid leukemia in chronic or accelerated phase who are intolerant to imatinib. Blood. 2011;117(21):5600–5606. doi: 10.1182/blood-2010-11-318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 18.le Coutre PD, Hughes TP, Mahon FX, et al. Peripheral arterial occlusive disease (PAOD) in patients (pts) receiving dasatinib: experience across multiple clinical trials. Blood. 2013;122(21) abstract 1489. [Google Scholar]
- 19.Cortes JE, Kantarjian H, Khoury HJ, et al. Long-term evaluation of vascular toxicity in patients with ph+ leukemias treated with bosutinib. Haematologica. 2014;99(suppl 1) abstract P900. [Google Scholar]
- 20.Chuah C, Guerci-Bresler A, Rosti G, et al. EPIC: A phase 3 trial of ponatinib vs imatinib in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML) Haematologica. 2014;99(suppl 1) abstract S679. [Google Scholar]
- 21.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Long-term follow-up of ponatinib efficacy and safety in the phase 2 PACE trial. Blood. 2014;124(21) doi: 10.1182/blood-2016-09-739086. abstract 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saglio G, le Coutre P, Cortes J, et al. Safety and tolerability of dasatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome–positive acute lymphoblastic leukemia (ph+ ALL): pooled analysis of over 2400 patients. Haematologica. 2014;99 abstract P884. [Google Scholar]
- 23.Cortes JE, Saglio G, Baccarani M, et al. Final study results of the phase 3 dasatinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) trial (DASISION, CA180-056) Blood. 2014;124(21) abstract 152. [Google Scholar]
- 24.Williams DA. Extra-binomial variation in logistic linear models. Appl Statist. 1982;31(2):144–148. [Google Scholar]
- 25.Wedderburn RWM. Quasi-likelihood functions, generalized linear models, and the Gauss— Newton method. Biometrika. 1974;61(3):439–447. [Google Scholar]
- 26.Guérin A, Chen L, Ionescu-Ittu R, et al. Impact of low-grade averse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Curr Med Res Opin. 2014:1–12. doi: 10.1185/03007995.2014.944973. [DOI] [PubMed] [Google Scholar]
- 27.Tasigna [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; Nov, 2015. [Google Scholar]
- 28.Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 29.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes TP, Lipton JH, Spector N, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124(5):729–736. doi: 10.1182/blood-2013-12-544015. [DOI] [PubMed] [Google Scholar]
- 31.Hughes TP, Hochhaus A, Kantarjian HM, et al. Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on frontline imatinib or nilotinib 300 mg twice daily. Haematologica. 2014;99(7):1204–1211. doi: 10.3324/haematol.2013.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi SY, Lee SE, Oh YJ, et al. Nilotinib or high dose imatinib compared with standard-dose imatinib in early chronic phase CML patients who have suboptimal molecular responses to standard-dose imatinib: including updated data from RE-nice study. Blood. 2013;122(21) abstract 1499. [Google Scholar]