Abstract
Objectives
We sought to determine the rate of response to hepatitis B (HBV) vaccination among HIV-infected adults in Vietnam.
Methods
We retrospectively abstracted data from a cohort of HIV-infected adults who had received HBV vaccine at an HIV clinic in Hanoi. We examined demographic, clinical and laboratory factors for associations with development of a protective antibody (Ab) response following vaccination (defined as ‘responders’ with anti-HBs >10 IU/L).
Results
Out of 302 HIV-infected patients who completed the vaccine series and follow-up serology testing, 189 (62.6%) had a positive protective Ab response. Female patients had a higher response rate compared to male patients (71.4% vs 56.8%, P=0.01). Among responders, mean CD4 T cell count was 309 cells/μL as compared to 204 cells/μL in non-responders (P<0.0001). On multivariable analysis, CD4 T cell count prior to vaccination was the only factor independently associated with a positive Ab response. Compared to patients with a count less than 100 cells/μL, those with a CD4 T cell count between 100 and 200 cells/μL were 20% more likely to be responders (relative risk [RR] 1.20, 95% confidence interval [CI] 0.77–1.87), those with a CD4 T cell count between 200 and 300 cells/μL were 61% more likely to be responders (RR 1.61, 95% CI 1.05–2.45), and those with a CD4 T cell count greater than 300 cells/μL were 89% more likely to be responders (RR 1.89, 95% CI 1.26–2.83).
Conclusions
We found that the CD4 T cell count at the time of vaccination to be the sole predictor of response to HBV vaccination among HIV-infected Vietnamese adults. Our findings highlight the importance of vaccinating HIV-infected adults prior to advanced immunosuppression.
Keywords: HIV, human immunodeficiency virus, Vietnam, hepatitis B, vaccination, CD4
Introduction
Chronic hepatitis B virus (HBV) infection is the most common cause of chronic liver disease and liver-related deaths worldwide [1]. Globally, more than 240 million people have chronic liver infection and there are more than 780,000 HBV-related deaths per year [2]. Due to shared routes of transmission, HBV and HIV co-infection is common. Worldwide, approximately 10% of HIV-infected individuals are co-infected with chronic HBV [1,3]. Compared with HBV-infected patients without HIV infection, patients with HIV-HBV co-infection have accelerated liver disease, higher rates of cirrhosis and liver cancer, and increased liver-related mortality [4]. Liver disease is a leading cause of death among HIV-infected patients in the United States [5].
The majority people with HIV-HBV co-infection live in low- and middle-income countries in Africa and Asia where the prevalence of both HIV and chronic HBV is disproportionally high [1,3]. Vietnam, a country of more than 90 million people, has one of the highest burdens of chronic HBV in the world. HBV is highly endemic in Vietnam, with a prevalence of chronic HBV of 10–20%, making it one of the leading causes of morbidity and mortality among the Vietnamese population [6]. Although vaccination against HBV has recently been included in Vietnam's national immunisation schedule, most HIV-infected adults have never been vaccinated. Of the estimated 280,000 people living with HIV in Vietnam, approximately 15% have chronic HBV infection [7].
Prevention of HBV infection through the administration of vaccine to non-immune individuals is an important component of the care of HIV-infected individuals. Vaccination against HBV is highly effective in healthy adults with response rates of greater than 90% [8], but HIV-infected individuals have significantly lower rates of vaccine response (range 18–71%) [9]. Clinical factors associated with a lower vaccine response rate have included older age [10–13], male gender [14,15], HCV-positivity [16,17], lack of ART use [14,15,18], low nadir CD4 T cell counts [13,19] and high HIV RNA levels [13,15,19–22]. In addition, some studies have found CD4 T cell count at the time of vaccination to be associated with vaccine response [10,12,15,18,19,21], but this has not been a consistent finding across all studies.
With few exceptions [12,18,23], the majority of data on the effectiveness of HBV vaccination in HIV-infected individuals comes from high-income countries with low HBV prevalence. Vietnam's HIV epidemic has been largely driven by injection drug use and, as a result, co-infection with chronic hepatitis C (HCV) is also common. None the less, vaccination against HBV is not routinely offered to non-immune HIV-infected adults. As a result, there are no data about the effectiveness of HBV vaccination in this population. In light of this, we conducted a retrospective cohort study among HIV-infected patients who had received HBV vaccination at a single site in Hanoi, Vietnam. We aimed to determine the percentage of Vietnamese HIV-infected patients who developed a protective Ab response (‘responder’) to a standard-dose HBV vaccination schedule and to determine the demographic, clinical and laboratory factors associated with the likelihood of the development of a protective Ab response.
Methods
We evaluated a pilot of HBV vaccination among a cohort of HIV-infected adult patients enrolled in the HIV outpatient clinic (OPC) at Bach Mai Hospital (BMH) in Hanoi, Vietnam. BMH is a large (1400 beds) national-level referral hospital providing the highest level of care for patients in the north of Vietnam. The OPC at BMH is a public clinic providing HIV care and treatment to more than 1000 HIV-positive adults. Standard of care at public OPCs across Vietnam includes testing of HBV surface antigen (HBsAg), Ab to HCV, haemoglobin, creatinine, and ALT during the initial evaluation. CD4 T cell count is performed at baseline and every 6 months. At the time of this study, patients were eligible for antiretroviral therapy (ART) if their CD4 T cell count was <350 cells/μL or at the WHO clinical stage III or IV.
According to the clinic vaccination protocol, patients who tested negative for HBsAg were tested for HBV surface Ab (anti-HBs). Patients testing negative for both HBsAg and anti-HBs were offered vaccination with HBV vaccine (Engerix-B, GlaxoSmithKline) at a dose of 20 μg. A subset of patients also received testing for HBV core Ab (anti-HBc). All patients, except those testing positive for anti-HBc, received a three-dose series at 0, 4 and 24 weeks. Patients who tested positive for anti-HBc received a single vaccine dose, followed by titres 4 weeks later. If the anti-HBs titre was ≥10 IU/L 4 weeks after the initial dose, no further vaccine was administered. Vaccination was offered regardless of CD4 cell count or ART use.
We retrospectively abstracted data from all patients enrolled in the BMH OPC who tested negative for HBsAg and anti-HBs at the time of the study. Only patients who completed the required number of vaccine doses according to the clinic protocol and had follow-up quantitative anti-HBs testing at least 2 weeks after their last vaccine dose were included in the final analysis. The study's primary outcome measure was a protective Ab response to HBV vaccination defined as an anti-HBs titre >10 IU/L at least 2 weeks after receipt of one or three vaccine doses according to the vaccine protocol [24]. We examined demographic, clinical and laboratory factors for associations with development of a protective Ab response. These factors included age, gender, risk factor for HIV infection (injection drug use, men who have sex with men (MSM), heterosexual transmission), WHO clinical stage, HCV Ab status, use of ART, ALT, nadir CD4 T cell count, the most recent CD4 T cell count, and HIV viral load measured prior to the first vaccine dose. Nadir CD4 T cell count was defined as the lowest count documented in the medical record prior to the date of the first vaccine dose. Patients were considered to be on ART if the documented ART start date was before the date of the first vaccine dose. HIV viral suppression was defined as having an undetectable HIV viral load or having a viral load <48 copies/mL.
We used Student's t test or nonparametric Wilcoxon to compare continuous variables and chi-square or Fisher's exact test for categorical variables. From these univariate analyses, variables that were statistically significant at 0.10 were selected for the multivariable model. Multivariable modelling using generalised linear model with log link and binary error was used to assess the association between demographic and clinical factors and the probability of the development of a protective Ab response. All statistical calculations were performed using Stata software, version 8.2 (Stata, College Station, TX, USA). The study was approved by the Institutional Review Boards (IRB) of Beth Israel Deaconess Medical Center (Boston, USA), Bach Mai Hospital (Hanoi, Vietnam), and the US Centers for Disease Control and Prevention (Atlanta, USA).
Results
Of the 880 patients registered at the OPC at the time of data collection, 124 (14.1%) tested positive for HBsAg and 756 (85.9%) tested HBsAg negative. Of the 756 HBsAg-negative patients, 589 were tested for anti-HBs and 349 (59.3%) tested negative. Of 318 patients who received the first dose of vaccine, 302 (95%) completed the vaccine series and follow-up testing according to the protocol and were therefore included in the final analysis. The time between the last vaccine dose and follow-up quantitative anti-HBs testing ranged from 14 to 245 days. The most common reason for non-completion of the vaccine protocol was that patients were no longer retained in care at the OPC.
Baseline characteristics of study subjects are shown in Table 1. Mean age was 36 years (range 16–75 years). Most patients, 183 (60.6%), were male, and 95 (31.5%) had a reported history of injection drug use. Testing for anti-HBc was available for 161 patients and 98 (60.9%) tested positive. HCV Ab was positive in 120 (39.7%) patients. The majority of patients, 249 (82.5%), were on ART at the time of the first vaccine dose with a median duration of ART use before vaccination of 327 days (range 1 to 2898 days). The mean CD4 T cell count prior to the first and third vaccine dose was 269.7 cells/μL and 336.3 cells/μL, respectively. Of those who had a plasma HIV viral load test prior to the first vaccine dose, 94/217 (43.3%) had a plasma HIV RNA <48 copies/mL. Plasma HIV RNA was <1000 copies/mL in 131 (60.4%), between 1000 and 100,000 copies/mL in 39 (18%), and >100,000 copies/mL in 47 (21.7%).
Table 1.
Baseline characteristics of 302 HIV-infected patients who received hepatitis B vaccination and follow-up quantitative anti-HBs testing
| Characteristic | Number of patients (%) |
|---|---|
| Age, mean years±SD | 36.0±8.2 |
| Gender | |
| Male | 183(60.6) |
| Female | 119(39.4) |
| Risk factor for HIV | |
| Injection drug use | 48(15.9) |
| Sexual transmission | 180(59.6) |
| Injection drug use & sexual transmission | 47(15.6) |
| Other | 27(8.9) |
| WHO clinical stage | |
| Stage 1 and 2 | 274(90.7) |
| Stage 3 and 4 | 28(9.3) |
| Hepatitis B core antibody status(n=161) | |
| Positive | 98(60.9) |
| Negative | 63(39.1) |
| Hepatitis C antibody status | |
| Positive | 120(39.7) |
| Negative | 182(60.3) |
| ALT, IU/mL, mean±SD(n=293) | 57.1±208.4 |
| Receipt of ART prior to vaccination | |
| No | 53(17.6) |
| Yes | 249(82.5) |
| Duration of ART prior to vaccination(median days [IQR]) | 327(1–2898) |
| Nadir CD4 T cell count(mean cells/μL±SD)(n=299) | 167.5±156.0 |
| CD4 T cell count prior to vaccination(mean cells/μL±SD)(n=301) | 269.7±184.1 |
| Plasma HIV RNA closest to first vaccine dose, copies/mL(n=217) | |
| <48 | 94(43.3) |
| ≥48 | 123(56.7) |
Overall, 189 (62.6%) patients had a positive Ab response to vaccination after one or three doses. Female patients had a higher response rate compared to male patients (71.4% vs 56.8%, P=0.01). Among responders, mean CD4 T cell count prior to vaccination was 309 cells/μL compared to 204 cells/μL in non-responders (P<0.0001). By univariate analysis (Table 2), female gender, ART use, clinical stage, nadir and current CD4 T cell count were significantly associated with response to vaccine. History of injection drug use, HCV Ab status, and plasma HIV RNA <48 copies/mL were not associated with vaccine response. Overall, 65 of the 98 anti-HBc-positive subjects (66.3%) responded to the vaccine series, with 40.5% (36/89) responding after the first vaccine dose (nine anti-HBc patients did not have quantitative anti-HBs testing after the first immunisation).
Table 2.
Characteristics of hepatitis B vaccine responders and non-responders
| Characteristics | Results of testing for anti-HBs | P-value | |
|---|---|---|---|
| Negative | Positive | ||
| (n=113) | (n=189) | ||
| n (%) | n (%) | ||
| Age, mean years±SD | 36.5±6.9 | 35.8±8.8 | 0.418 |
| Female gender | 34(30.9) | 85(44.9) | 0.010 |
| History of intravenous drug use | 41(39.4) | 54(30.9) | 0.144 |
| Receipt of ART | 100(88.5) | 149(78.8) | 0.033 |
| WHO clinical stage 1 or 2 | 95(84.1) | 179(94.7) | <0.001 |
| Hepatitis B core antibody positive | 33(58.9) | 65(61.9) | 0.712 |
| Hepatitis C antibody positive | 52(46.0) | 68(36.0) | 0.084 |
| ALT ≥100 IU/mL | 7(6.4) | 24(13.0) | 0.081 |
| Nadir CD4 T cell count <100 cells/μL | 61(54.0) | 75(40.3) | 0.021 |
| CD4 T cell count prior to vaccination <200 cells/μL | 67(59.3) | 52(27.7) | <0.0001 |
| Plasma HIV RNA closest to first vaccine dose <48 copies/mL | 36(45.0) | 58(42.3) | 0.702 |
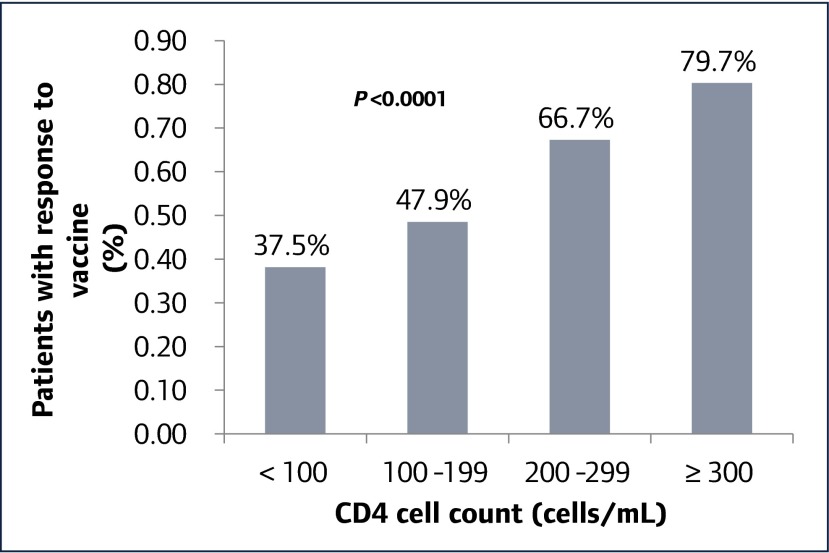
In multivariable analysis (Table 3), CD4 T cell count prior to vaccination was the only factor independently associated with a positive Ab response. Compared to CD4 T cell count <100 cells/μL, those with a CD4 T cell count between 100 and 200 cells/μL had a relative risk (RR) of vaccine response of 1.20 (95% confidence internal 0.77–1.87), those with a CD4 T cell count between 200 and 300 cells/μL had an RR of 1.61 (1.05–2.45), and those with a CD4 T cell >300 cells/μL had an RR of 1.89 (1.26–2.83). Figure 1 shows the percentage of vaccine responders stratified by CD4 T cell count prior to vaccination.
Table 3.
Multivariable analysis of factors associated with response to hepatitis B vaccination
| Risk factor | Relative risk [95% CI] | P-value |
|---|---|---|
| Female gender | 1.34 [0.78–2.32] | 0.213 |
| Receipt of ART | 1.00 [0.83–1.21] | 0.990 |
| Clinical stage 1 or 2 | 1.23 [0.71–2.15] | 0.461 |
| CD4 cell count prior to vaccination | ||
| <100 cells/μL | Referent | |
| 100–199 cells/μL | 1.20 [0.77–1.87] | 0.413 |
| 200–299 cells/μL | 1.61 [1.05–2.45] | 0.028 |
| ≥300 cells/μL | 1.89 [1.26–2.83] | 0.002 |
Figure 1.
Protective antibody response to hepatitis B vaccination by CD4 cell count prior to vaccination (n=302)
When limiting the analysis to include only patients with follow-up anti-HBs testing at least 4 weeks after the final vaccine dose (n=280), the vaccine response rate was 62.5% and nearly identical to that of the larger sample.
Discussion
We have found that nearly 63% of patients in our cohort achieved an anti-HBs titre >10 IU/L following standard dose HBV vaccination. Our study is the first to assess HBV vaccination among HIV-infected adults in Vietnam and the largest one performed in Southeast Asia. Two small studies among Thai adults have reported response rates of 46% (n=65) and 71.4% (n=28) [12,18]. The vaccine response rate in our study is higher than in a number of studies performed among HIV-infected adults in the United States [10,13,20,22,25] and similar to that found in a more recent study of HIV-infected Kenyan adults [23].
Pre-vaccination CD4 T cell count was independently associated with vaccine responsiveness in our study. This is consistent with multiple studies demonstrating an association between the CD4 T cell count at the time of vaccination and response to vaccine. For example, in one of the above referenced Thai studies, vaccine responders had a mean CD4 T cell count of 397 cells/μL compared to 301 cells/μL among non-responders, and patients who had a higher CD4 T cell count at vaccination developed higher levels of anti-HBs antibodies after vaccination [12]. By contrast, some studies have found that nadir CD4 T cell count and/or HIV viral load are more important predictors of vaccine responsiveness. In a study of 97 HIV-infected adults in the United States, nadir CD4 T cell count <200 cells/μL, rather than the current CD4 T cell count, was independently associated with a lower vaccine response rate [13].
Several studies have demonstrated a positive association between HBV vaccine response rates and female gender. In a retrospective study of more than 600 patients in the United States, female gender was significantly associated with HBV vaccine response rate (Odds ratio 1.99, 95% CI 1.15–3.45) [14]. In our study, we found a trend towards higher vaccine response rates among women compared to men but this did not reach statistical significance on multivariate analysis.
We did not find an independent association between ART use or HIV viral load and vaccine responsiveness. This is in contrast to a number of studies in which ART use and/or viral load suppression predicted response [13,14,19,22]. Additionally, although our study population was notable for high rates of HCV Ab positivity and prior injection drug use, we did not find a significant correlation between either of these factors and response to vaccination. There was, however, a non-significant trend toward decreased responsiveness among patients with a positive anti-HCV Ab test.
The optimal timing to provide HBV vaccination in a patient who presents with low CD4 T cell counts is still an area of controversy. The United States guidelines on the prevention and treatment of opportunistic infections in HIV-infected adults recommend vaccinating HIV-infected patients before their CD4 cell counts decline to <350 cells/μL. However, for patients presenting with low CD4 T cell counts, vaccination should not be deferred until the CD4 T cell count rises above 350 cells/μL because some HIV-infected patients with CD4 T cell counts below 200 cells/μL do respond to vaccination. Our findings support these recommendations. The highest response rates (nearly 80%) were seen in patients with CD4 T cell counts above 300 cells/μL at the time of vaccination. However, more than 40% of patients with CD4 T cell counts below 200 cells/μL achieved a protective Ab response. Delaying vaccination to await immune reconstitution could put patients at risk for never being immunised [19] and acquiring HBV infection.
Whether HIV-infected patients with positive anti-HBc but negative anti-HBs (isolated anti-HBc) require vaccination is not certain [17]. Isolated anti-HBc, in the absence of acute infection, may signify one of three possible scenarios: (1) HBV exposure in the distant past with loss of anti-HBs; (2) occult chronic HBV infection with undetectable HBsAg; or (3) a false positive test result. In a highly endemic area such as Vietnam, patients with isolated anti-HBc most likely have had prior exposure to HBV and therefore may not require vaccination [26]. However, in our study, testing positive for anti-HBc was not associated with increased response to vaccine. This finding supports the recommendation to vaccinate HIV-infected patients with isolated anti-HBc [16].
We have found a high rate of patient compliance with the vaccine schedule, with 95% of patients completing vaccination and follow-up anti-HBs testing. This is particularly notable because of the high rates of injection drug use in the study population, as injection drug users have been shown to have lower adherence to vaccines [27]. HIV-infected patients in Vietnam on ART pick up their ARV drugs each month at the OPCs. Since the majority of patients in our study were taking ART at the time of vaccination, the monthly ART pick-up most likely facilitated the administration of HBV vaccine leading to high completion rates.
Our study has certain limitations. Due to the retrospective nature of the study, we had to focus our analysis on laboratory results routinely collected at HIV OPCs in Vietnam. Since testing for HBV DNA was not available for most patients in our cohort, we could not exclude occult HBV infection (presence of low levels of HBV DNA with a negative HBsAg) as a potential cause of vaccine unresponsiveness. Likewise, HCV RNA was not available so we could not determine how many of the 120 patients with positive HCV Abs had active chronic HCV infection. However, the majority of HIV-infected patients with positive HCV Abs will be RNA positive. Routine HIV viral load testing is not yet standard of care in Vietnam and so levels were not available for more than 25% of the cohort. It is possible that differences among patients with and without viral load testing could have affected our ability to show an association between an undetectable HIV viral load and vaccine responsiveness.
In summary, we found that the CD4 T cell count at the time of vaccination to be the sole predictor of response to HBV vaccination among HIV-infected Vietnamese adults. These results highlight the importance of vaccinating HIV-infected adults prior to the onset of advanced immunosuppression. In the context of Vietnam, where the majority of HIV-infected adults still present to care with advanced disease, vaccination should be offered regardless of the CD4 T cell count with post-vaccination anti-HBs testing to confirm response.
Acknowledgements
We thank the staff at Bach Mai Hospital outpatient clinic for their support in this study and dedication to improving the health of HIV-infected patients in Vietnam. We thank Sheryl Lysis and Ho Van Anh (CDC-Vietnam) and Siobhan O’Connor (CDC Division of Viral Hepatitis) for providing valuable comments on the study protocol and manuscript. This research was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the United States Centers for Disease Control and Prevention under the terms of 5U2GPS001177-05. Presented in part at the 7th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention in Kuala Lumpur, Malaysia, July 1, 2013 (Abstract WEPE478).
Conflict of interests
The authors declare no conflict of interest related to this work.
References
- 1. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ.. HIV-HBV coinfection–a global challenge. N Engl J Med 2012; 366: 1749– 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization Hepatitis B fact sheet. Available at: www.who.int/mediacentre/factsheets/fs204/en/ ( accessed February 2016).
- 3. Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 2009; 49: S138– 145. [DOI] [PubMed] [Google Scholar]
- 4. Thio CL, Seaberg EC, Skolasky R Jr. et al. . HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360: 1921– 1926. [DOI] [PubMed] [Google Scholar]
- 5. Joshi D, O’Grady J, Dieterich D et al. . Increasing burden of liver disease in patients with HIV infection. Lancet 2011; 377: 1198– 1209. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen VT. Hepatitis B infection in Vietnam: current issues and future challenges. Asia Pac J Public Health 2012; 24: 361– 373. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen DB, Do NT, Shiraishi RW et al. . Outcomes of antiretroviral therapy in Vietnam: results from a national evaluation. PloS one 2013; 8: e55750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dienstag JL, Werner BG, Polk BF et al. . Hepatitis B vaccine in health care personnel: safety, immunogenicity, and indicators of efficacy. Ann Intern Med 1984; 101: 34– 40. [DOI] [PubMed] [Google Scholar]
- 9. Kim HN, Harrington RD, Crane HM et al. . Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS 2009; 20: 595– 600. [DOI] [PubMed] [Google Scholar]
- 10. Pettit NN, DePestel DD, Malani PN, Riddell Jt. Factors associated with seroconversion after standard dose hepatitis B vaccination and high-dose revaccination among HIV-infected patients. HIV Clin Trials 2010; 11: 332– 339. [DOI] [PubMed] [Google Scholar]
- 11. Launay O, Vliet D, Rosenberg AR et al. . Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011; 305: 1432– 1440. [DOI] [PubMed] [Google Scholar]
- 12. Ungulkraiwit P, Jongjirawisan Y, Atamasirikul K, Sungkanuparph S.. Factors for predicting successful immune response to hepatitis B vaccination in HIV-1 infected patients. Southeast Asian J Trop Med Public Health 2007; 38: 680– 685. [PubMed] [Google Scholar]
- 13. Kim HN, Harrington RD, Van Rompaey SE, Kitahata MM.. Independent clinical predictors of impaired response to hepatitis B vaccination in HIV-infected persons. Int J STD AIDS 2008; 19: 600– 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landrum ML, Huppler Hullsiek K, Ganesan A et al. . Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine 2009; 27: 4731– 4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vries-Sluijs TE, Hansen BE, Doornum GJ et al. . A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J Infect Dis 2008; 197: 292– 294. [DOI] [PubMed] [Google Scholar]
- 16. Masur H, Brooks JT, Benson CA et al. . Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58: 1308– 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gandhi RT, Wurcel A, Lee H et al. . Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005; 191: 1435– 1441. [DOI] [PubMed] [Google Scholar]
- 18. Paitoonpong L, Suankratay C.. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis 2008; 40: 54– 58. [DOI] [PubMed] [Google Scholar]
- 19. Tedaldi EM, Baker RK, Moorman AC et al. . Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis 2004; 38: 1478– 1484. [DOI] [PubMed] [Google Scholar]
- 20. Bailey CL, Smith V, Sands M.. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis 2008; 12: e77– 83. [DOI] [PubMed] [Google Scholar]
- 21. Fonseca MO, Pang LW, Paula Cavalheiro N et al. . Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005; 23: 2902– 2908. [DOI] [PubMed] [Google Scholar]
- 22. Overton ET, Sungkanuparph S, Powderly WG et al. . Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005; 41: 1045– 1048. [DOI] [PubMed] [Google Scholar]
- 23. Irungu E, Mugo N, Ngure K et al. . Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J Infect Dis 2013; 207: 402– 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jack AD, Hall AJ, Maine N et al. . What level of hepatitis B antibody is protective? J Infect Dis 1999; 179: 489– 492. [DOI] [PubMed] [Google Scholar]
- 25. Rey D, Krantz V, Partisani M et al. . Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000; 18: 1161– 1165. [DOI] [PubMed] [Google Scholar]
- 26. Lok AS, McMahon BJ.. Chronic hepatitis B: update 2009. Hepatology 2009; 50: 661– 662. [DOI] [PubMed] [Google Scholar]
- 27. Quaglio G, Talamini G, Lugoboni F et al. . Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction 2002; 97: 985– 992. [DOI] [PubMed] [Google Scholar]