Abstract
Sex-specific differences have been described for a variety of infectious and autoimmune diseases. In HIV-1 infection women present with significantly lower viral loads during early infection, but during chronic infection women progress faster to AIDS for the same amount of viral replication. Recent studies have shown that sex differences during HIV-1 infection might also include the size of the latent viral reservoir, which represents a major obstacle towards a cure for HIV-1. Here we review different immunological and virological aspects that can be influenced by sex hormones and sex-specific genetic factors and their contribution to viral replication, as well as the creation and maintenance of the HIV-1 reservoir.
Keywords: HIV-1, HIV-1 reservoir, sex differences, sex hormones
Introduction
A clear dimorphism exists in the immune response of women and men towards pathogens and the incidence of autoimmune diseases. In general, men are more susceptible to infectious diseases, whereas women present with higher prevalences of autoimmune diseases [1]. The differences in the immune response between women and men can be attributed to a number of different factors. Several receptors that are of importance for the regulation of an immune response, including Toll-like receptor (TLR)7, TLR8, FoxP3, CD40L and IL2Rγ are encoded by the X chromosome [2]. It is therefore possible that incomplete inactivation of the second X chromosome [3] can lead to the higher expression of these genes in women. Sex hormones, especially testosterone and oestrogen, also have a direct influence on the immune system, as the majority of the immune cells express receptors for sex hormones and can be influenced by the different levels of oestrogen and testosterone in women and men (reviewed in [1,2,4,5]). Here we will review the implication of sex-specific differences on the manifestations of HIV-1 infection.
HIV-1 viral load, creation and maintenance of the HIV-1 reservoir
After infection with HIV-1, the majority of the infected cells will die because of direct viral cytopathic effects, recognition and destruction of infected cells by host immunity, or bystander activation leading to apoptosis [6,7]. However, a small portion of cells that have HIV-1 DNA integrated in their genome will return to a resting state, and are referred to as latently HIV-1-infected cells [6]. Those cells make up the HIV-1 DNA reservoir that cannot be targeted through antiretroviral therapy (ART) and represent at this moment the biggest obstacle towards a cure for HIV-1. The diverse factors contributing to the size of the HIV-1 reservoir remain incompletely understood [6].
Recently, it was observed in a cross-sectional study by Cuzin et al. that women had a higher chance of reaching a lower HIV-1 reservoir size in comparison with men during long-term ART [8]. There are several non-exclusive explanations for this observation of sex-specific differences in the size of the HIV-1 reservoir, including a sex-specific maintenance and regulation of the HIV-1 reservoir itself, and the establishment of a smaller HIV-1 reservoir in women due to lower viral loads during early HIV-1 infection in females compared with males [9]. Studies presented by Karn and colleagues at IAS 2015 in Vancouver, Canada, support an intriguing model of direct sex-specific regulation of the HIV-1 reservoir by demonstrating that manipulation of the oestrogen receptor α (ER-α) has a direct influence on reactivation of HIV-1 in latently infected cells in vitro and also ex vivo [10]. The authors showed that the level of oestrogen present at the peak of the menstrual cycle in women likely acts as an inhibitor of HIV-1 reactivation in latently infected cells, thus influencing the size of the viral reservoir. However, the mechanisms by which inhibition of viral reactivation in latently infected cells through oestrogen could lead to a smaller HIV-1 reservoir in women are not understood.
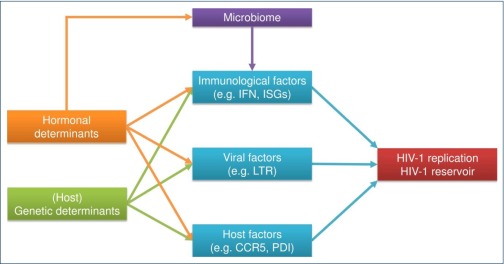
Several large cross-sectional and longitudinal cohort studies of HIV-1-infected individuals have shown that women presented with significantly lower levels of plasma HIV-1 RNA than men during the early phases of the infection [9,11,12]. Plasma viral load levels are a strong predictor of the size of the HIV-1 DNA reservoir [13,14], suggesting that sex differences in the HIV-1 reservoir after initiation of ART might be a consequence of differences in viral replication during the initial seeding of the reservoir. The reasons for lower viral load levels in women during the initial phase of HIV-1 infection, and thus the potential creation of a smaller HIV-1 reservoir, are multifactorial, as outlined in Figure 1. Genetic and sex hormonal mechanisms can influence immunological, viral and host factors, which in turn can impact viral replication as well as creation and maintenance of the HIV-1 reservoir. The sex hormonal factors possibly affecting viral replication are reflected by fluctuations of plasma viral load levels during the menstrual cycle. However, there is some controversy, as observations differed between different studies [15–18]. While two studies did not observe any changes of plasma viral RNA levels in relationship to the menstrual cycle [15,16], Greenblatt et al. found a significant fluctuation of HIV-1 RNA plasma levels between early follicular and mid-luteal phases of the menstrual cycle after excluding non-ovulating women [17]. Furthermore Benki and colleagues observed a marginally significant correlation between progesterone levels and HIV-1 viraemia [18]. In the following sections, the current state of research about the multifactorial ramifications of sex-specific factors leading to differences in HIV-1 viral load levels and the size of the HIV-1 reservoirs will be discussed.
Figure 1.
Summary of the multifactorial ramifications of sex-specific differences for HIV-1 infection. Immunological, viral and host factors regulate HIV-1 replication, as well as the creation and maintenance of the HIV-1 reservoir. These three factors are in turn regulated by genetic and hormonal determinants. The microbiome, furthermore, plays an important role in orchestrating sex differences of immune cells, as has been reviewed elsewhere [4,19,20]. IFN: interferon; ISG: interferon-stimulated genes; LTR: long terminal repeat; PDI: protein disulfide isomerase
Immunological factors impacting sex dimorphism in HIV-1 replication
Multiple studies have demonstrated that plasmacytoid dendritic cells (pDCs) from women and men respond differently to TLR7 stimulation [21], including stimulation with HIV-1-derived TLR7 ligands [22], thus contributing to sex-specific differences in HIV-1 pathogenesis. pDCs are cells of the innate immune system that produce high levels of type I interferons after stimulation via TLR7 with viral ssRNA (e.g. HIV-1) [23]. Type I interferons activate the expression of interferon-stimulated genes (ISG) that can suppress viral replication [24], but also might contribute to higher and chronic immune activation of CD4 and CD8 T cells during persistent HIV-1 infection in females [22], which is reflected by increased levels of ISGs ex vivo [25]. pDCs are the strongest producers of interferon-alpha (IFN-α) in humans, able to produce as much as 3–10 pg of IFN-α/cell [23], which is 100–1000 times more IFN-α than other immune cells are capable of producing [26]. Meier et al. have demonstrated that the number of IFN-α-producing pDCs upon HIV-1-mediated TLR7 stimulation is significantly higher in females than in males [22].
Several studies have started to assess why pDCs from females produce more IFN-α following TLR7 stimulation than pDCs of males [21,22,27]. One potential mechanism could be due to the location of the tlr7 gene on the X chromosome. Incomplete inactivation occurs in up to 25% of X-linked genes [3] and the location Xp22.2 of the tlr7 gene [5] lies close to a locus where escape of inactivation has been demonstrated [28], meaning that the TLR7-encoding region of the second X chromosome in women could lead to higher TLR7 mRNA and protein expression. However, several studies have demonstrated that pDCs derived from females do not have a higher expression of TLR7 mRNA compared to pDCs derived from males (in humans [29,30] and in mice [31]). A current study by Seillet and colleagues has shown that 17β-oestradiol treatment of postmenopausal women increases IFN-α production of pDCs following TLR7 stimulation. Moreover, using NOD/SCID/β2m-/- mice of both sexes and transplanting them with human CD34 female progenitor cells, the authors showed that after in vitro TLR7 stimulation there were significantly more IFN-α-producing pDCs in female mice compared to male mice, providing further evidence for a hormonal and not a genetic regulation of TLR7-stimulated IFN-α production [21]. These sex differences in TLR7 responsiveness should be taken into account in the design of future studies using TLR7 agonists as latency reversal agents. Recently, a study by our group has demonstrated a higher basal level of interferon regulatory factor (IRF)5 in pDCs from women compared to men that correlated with the percentage of IFN-α-producing pDCs following TLR7 stimulation. Furthermore, knocking-out of the ER-α reduced IRF5 protein levels and the amount of IFN-α-secreting pDCs, suggesting that oestrogen can regulate IFN-α production by pDCs through a modulation of IRF5 expression [27]. Taken together, these studies suggest that sex hormones regulate IFN-α production by pDCs. Higher production of IFN-α by pDCs from women can have beneficial effects on viral control during early infection, leading to lower HIV-1 replication. In contrast, during chronic persistent HIV-1 infection the continuous IFN-α production contributes to chronic immune activation and HIV-1 disease progression (reviewed in [32]).
Viral factors contributing to sex differences in HIV-1 replication
In addition to immunological factors, viral factors might also contribute to the observed sex-specific differences in viral load levels and viral reservoirs [9]. Only a few studies have investigated the possible direct influence of sex hormones such as oestrogen and progesterone on the transcription and replication of HIV-1 to date [33–35]. Szotek et al. showed that high concentrations of 17β-oestradiol (equivalent to the oestrogen peak during the follicular phase of the menstrual cycle [36,37]) inhibited the production of HIV-1 in human PBMCs in vitro by roughly 40% without inhibition of HIV-1 reverse transcription or integration [33]. Through further experiments the authors concluded that 17β-oestradiol can inhibit HIV-1 replication at the transcriptional level through enabling the association of the transcriptional co-regulator β-catenin and the transcription factor TCF-4 with ER-α [33]. On the contrary, in a different study, HEK293 cells were transfected with different plasmids in order to become sensitive to oestrogen treatment and to express HIV-1 LTR luciferase constructs. Using this model, the experiments suggested an oestrogen-based increase of DNA-binding activity of the transcription factor Sp1, causing augmented transcriptional activity of the HIV-1 LTR [34]. The discrepancies in the results might be due to the usage of different in vitro experimental setting (primary PBMCs [33] vs HEK293 cell line [34]) and different oestrogen concentrations used (1.5 nM [33] vs 10 nM [34]). In an additional study, Ragupathy et al. suggested that the hormonal effects on HIV-1 replication are subtype and donor specific [38], further complicating any conclusions. Asin et al. isolated PBMCs from HIV-1-seronegative donors, infected them in vitro with HIV-1, and then analysed viral replication under oestrogen and progesterone concentrations simulating mid-follicular phase (mid-proliferative [39]) or mid-luteal phase (mid-secretory [39]) conditions of the menstrual cycle [35]. In these studies, the authors observed that mid-follicular hormone concentrations (low progesterone and low oestrogen) increased replication of HIV-1 through an enhanced activity of the LTR. A decreased HIV-1 replication was observed for mid-luteal phase conditions (high progesterone, high oestrogen). This would indicate a dual effect of both sex hormones; however, using low oestrogen concentrations as representation of the follicular phase, when oestrogen levels normally peak [36,37], is certainly debatable. Overall, the current research regarding direct effects of sex hormones on the transcription of HIV-1 is contradictory, which is likely to be due to different experimental conditions. More research will be needed to draw definite conclusions about the direct influence of sex hormones on HIV-1 replication.
Sex-specific regulation of host factors influencing HIV-1 replication
Apart from their influence on immune function and viral factors, sex hormones have also been suggested to directly regulate host proteins that are influencing HIV-1 replication.
The cytokine receptor CCR5 plays an important role in the susceptibility towards HIV-1 infection, since it is a necessary co-receptor for viral entry [40]. An in vitro study by Vassiliadou et al. assessing the effect of progesterone on CCR5 expression showed a significant inhibition of CCR5 upregulation on activated peripheral blood-derived CD4 T cells following progesterone pre-treatment [41]. It has to be noted, however, that these effects were dose-dependent, and only at concentrations of 1 μM and higher were significant effects observed, while at a progesterone concentration of 100 nM no effect was displayed. The upper physiological ranges of the progesterone peak during menstrual cycle range between 60 nM [36] and 92 nM [37], thus representing concentrations at which no effects were observed in vitro. However, the results of Vassiliadou et al. are supported by the observation that postmenopausal women, who have low progesterone levels, demonstrate a significant increase of CCR5 receptor expression on cervical CD4 T cells [42].
An influence of sex hormones on the susceptibility of cells to infection with HIV-1 has also been suggested for oestrogen. A recent study concluded that in vitro treatment of CD4 T cells and macrophages with 17β-oestradiol prior to HIV-1 challenge renders the cells less susceptible to HIV-1 infection, and this effect was independent of CCR5 downregulation [43]. Again, the hormone concentrations used in vitro (up to 50 nM) were multiple times higher than the oestrogen peaks observed during menstrual cycle, which range up to 2 nM [36,37]. Of interest, an in vivo study of male-to-female transsexual individuals treated with oestrogens and antiandrogens showed a significant increase in CCR5 expression on T cells over a 4-month period [44]. These observations are in line with a study by Mo et al. in mice, demonstrating significant higher expression of CCR5 in female mice. In addition, gonadectomised female mice supplemented with oestrogen had a higher expression of CCR5 than gonadectomised female mice treated with a placebo. However, CCR5 expression remained lower in hormone-supplemented female mice than in wildtype female mice, indicating that the factors involved in regulating CCR5 expression are more complex [45]. In conclusion, the multifactorial influences on CCR5 expression need to be validated under more physiological conditions, as sex differences in CCR5 expression levels might contribute to differences in the size of the HIV-1 reservoir.
Antimicrobial peptides such as secretory leukocyte protease inhibitor, α- and β-defensins can also be regulated by sex hormones, and might influence HIV-1 transmission through their presence in the female genital tract (reviewed in [39]). The enzyme protein disulfide isomerase (PDI) is capable of catalysing disulfide isomerisation, reduction, oxidation and chaperone activity [46]. It has been shown that the enzymatic activity of PDI as a reductase is necessary for HIV-1 infection and that inhibition of PDI effectively prevents entry of HIV-1 into target cells (reviewed in [47]). In vitro studies have furthermore shown that oestrogens are able to bind to PDI [48]. However, there remains some controversy on whether oestrogen binding leads to a decline of PDI reductase activity, with one study showing partial PDI inhibition at (unphysiologically) high (up to 1 μM) oestrogen concentrations [49]. In contrast, Klett and colleagues showed a dose-dependent inhibition of PDI at a more physiological concentration (10 nM) of oestrogen [50]. In summary, little is known about the direct impact of sex hormones on the regulation of host factors that can modulate HIV-1 replication.
Conclusion
Current research clearly demonstrates how sex-specific differences in the manifestations of HIV-1 infection are shaped on multiple levels, and this complexity has to be taken into account in the interpretation of studies investigating the precise underlying mechanisms. The elucidation of the effects of X-chromosomal genetic factors and of sex hormones in modulating HIV-1 acquisition, HIV-1 replication/reservoir formation, and HIV-1 pathogenesis will be critical in order to design new and personalised strategies against HIV-1 that take sex differences into account.
Acknowledgements
We thank Susanne Ziegler for helpful comments and critical reading of the manuscript.
Funding
SH is supported by the Leibniz Center Infection. MA receives funding from the National Health Institute (NIH), the Deutsche Forschungsgemeinschaft (DFG), the German Center for Infection Research (DZIF), the Ragon Institute of MGH, MIT and Harvard, and the Leibniz Gemeinschaft.
References
- 1. Klein SL. Immune cells have sex and so should journal articles. Endocrinology 2012; 153: 2544– 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8: 737– 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrel L, Willard HF.. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005; 434( 7031): 400– 404. [DOI] [PubMed] [Google Scholar]
- 4. Markle JG, Fish EN.. SeXX matters in immunity. Trends Immunol 2014; 35( 3): 97– 104. [DOI] [PubMed] [Google Scholar]
- 5. Libert C, Dejager L, Pinheiro I.. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010; 10( 8): 594– 604. [DOI] [PubMed] [Google Scholar]
- 6. Deeks SG, Autran B, Berkhout B et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 2012; 12( 8): 607– 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silvestri G, Feinberg MB.. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest 2003; 112: 821– 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuzin L, Pugliese P, Saune K et al. Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS 2015; 29: 1665– 1671. [DOI] [PubMed] [Google Scholar]
- 9. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC.. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344: 720– 725. [DOI] [PubMed] [Google Scholar]
- 10. Karn J, Das B, Dobrowolski C et al. Estrogen blocks HIV re-emergence from latency and points to gender-specific differences in HIV reservoirs. 2015 Towards an HIV Cure Symposium. July 2015. Vancouver, Canada. Abstract OA1-4 LB.
- 11. Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM.. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35: 313– 322. [DOI] [PubMed] [Google Scholar]
- 12. Meditz AL, MaWhinney S, Allshouse A et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203: 442– 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosn J, Deveau C, Chaix ML et al. Despite being highly diverse, immunovirological status strongly correlates with clinical symptoms during primary HIV-1 infection: A cross-sectional study based on 674 patients enrolled in the ANRS CO 06 PRIMO cohort. J Antimicrob Chemother 2010; 65: 741– 748. [DOI] [PubMed] [Google Scholar]
- 14. Ngo-Giang-Huong N, Deveau C, Da Silva I et al. Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS 2001; 15: 665– 673. [DOI] [PubMed] [Google Scholar]
- 15. Money DM, Arikan YY, Remple V et al. Genital tract and plasma human immunodeficiency virus viral load throughout the menstrual cycle in women who are infected with ovulatory human immunodeficiency virus. Am J Obstet Gynecol. 2003; 188: 122– 128. [DOI] [PubMed] [Google Scholar]
- 16. Reichelderfer PS, Coombs RW, Wright DJ et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS 2000; 14: 2101– 2107. [DOI] [PubMed] [Google Scholar]
- 17. Greenblatt RM, Ameli N, Grant RM, Bacchetti P, Taylor RN.. Impact of the ovulatory cycle on virologic and immunologic markers in HIV-infected women. J Infect Dis 2000; 181: 82– 90. [DOI] [PubMed] [Google Scholar]
- 18. Benki S, Mostad SB, Richardson BA, Mandaliya K, Kreiss JK, Overbaugh J.. Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J Infect Dis 2004; 189: 2192– 2201. [DOI] [PubMed] [Google Scholar]
- 19. Klein SL, Marriott I, Fish EN.. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015; 109: 9– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziegler S, Altfeld M.. Sex differences in HIV-1-mediated immunopathology. Curr Opin HIV AIDS 2016; 11: 209– 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seillet C, Laffont S, Trémollières F et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012; 119: 454– 464. [DOI] [PubMed] [Google Scholar]
- 22. Meier A, Chang JJ, Chan ES et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature Medicine 2009; 15: 955– 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitzgerald-Bocarsly P, Dai J, Singh S.. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 2008; 19: 3– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katze MG, He Y, Gale M Jr.. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002; 2: 675– 687. [DOI] [PubMed] [Google Scholar]
- 25. Chang JJ, Woods M, Lindsay RJ et al. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis 2013; 208: 830– 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siegal FP, Kadowaki N, Shodell M et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999; 284( 5421): 1835– 1837. [DOI] [PubMed] [Google Scholar]
- 27. Griesbeck M, Ziegler S, Laffont S et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J Immunol 2015; 195: 5327– 5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrel L, Cottle AA, Goglin KC, Willard HF.. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci U S A 1999; 96: 14440– 14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H.. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 2006; 177: 2088– 2096. [DOI] [PubMed] [Google Scholar]
- 30. Laffont S, Rouquié N, Azar P et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol 2014; 193: 5444– 5452. [DOI] [PubMed] [Google Scholar]
- 31. Karnam G, Rygiel TP, Raaben M et al. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathog 2012; 8: e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Addo MM, Altfeld M.. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209( Suppl 3): S86– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szotek EL, Narasipura SD, Al-Harthi L.. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology 2013; 443: 375– 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katagiri D, Hayashi H, Victoriano AF, Okamoto T, Onozaki K.. Estrogen stimulates transcription of human immunodeficiency virus type 1 (HIV-1). Int Immunopharmacol 2006; 6: 170– 181. [DOI] [PubMed] [Google Scholar]
- 35. Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL.. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses 2008; 24: 701– 716. [DOI] [PubMed] [Google Scholar]
- 36. Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R.. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med 2006; 44: 883– 887. [DOI] [PubMed] [Google Scholar]
- 37. Harlow SD, Ephross SA.. Epidemiology of menstruation and its relevance to women's health. Epidemiol Rev 1995; 17: 265– 286. [DOI] [PubMed] [Google Scholar]
- 38. Ragupathy V, Devadas K, Tang S et al. Effect of sex steroid hormones on replication and transmission of major HIV subtypes. J Steroid Biochem Mol Biol 2013; 138: 63– 71. [DOI] [PubMed] [Google Scholar]
- 39. Cole AM, Cole AL.. Antimicrobial polypeptides are key anti-hiv-1 effector molecules of cervicovaginal host defense. Am J Reprod Immunol 2008; 59: 27– 34. [DOI] [PubMed] [Google Scholar]
- 40. Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR.. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med 1997; 185: 621– 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vassiliadou N, Tucker L, Anderson DJ.. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol 1999; 162: 7510– 7518. [PubMed] [Google Scholar]
- 42. Meditz AL, Moreau KL, MaWhinney S et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr 2012; 59: 221– 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodriguez-Garcia M, Biswas N, Patel MV et al. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One 2013; 8: e62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giltay EJ, Fonk JC, Blomberg BM, Drexhage HA, Schalkwijk C, Gooren LJ.. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metab 2000; 85: 1648– 1657. [DOI] [PubMed] [Google Scholar]
- 45. Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL.. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol 2005; 174: 6023– 6029. [DOI] [PubMed] [Google Scholar]
- 46. Wilkinson B, Gilbert HF.. Protein disulfide isomerase. Biochim Biophys Acta 2004; 1699: 35– 44. [DOI] [PubMed] [Google Scholar]
- 47. Ryser HJ, Flückiger R.. Progress in targeting HIV-1 entry. Drug Discov Today 2005; 10: 1085– 1094. [DOI] [PubMed] [Google Scholar]
- 48. Fu XM, Wang P, Zhu BT.. Characterization of the estradiol-binding site structure of human protein disulfide isomerase (PDI). PLoS One 2011; 6: e27185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsibris JC, Hunt LT, Ballejo G, Barker WC, Toney LJ, Spellacy WN.. Selective inhibition of protein disulfide isomerase by estrogens. J Biol Chem 1989; 264: 13967– 13970. [PubMed] [Google Scholar]
- 50. Klett D, Cahoreau C, Villeret M, Combarnous Y.. Effect of pharmaceutical potential endocrine disruptor compounds on protein disulfide isomerase reductase activity using di-eosin-oxidized-glutathione. PLoS One 2010; 5: e9507. [DOI] [PMC free article] [PubMed] [Google Scholar]