Abstract
Background
Magnetic resonance spectroscopy allows for the noninvasive study of brain metabolism and therefore may provide useful information about brain injuries. We examined the associations of brain metabolite ratios in very preterm infants with white matter lesions and overall health status at birth.
Methods
Spectroscopy data were obtained from 99 very preterm infants (born ≤32wk gestation) imaged shortly after birth and from 67 of these infants at term-equivalent age. These data were processed using LC Model. Multiple regression was used to examine the association of metabolite ratios with focal non cystic white matter lesions visible on conventional magnetic resonance imaging (MRI) and with at-birth illness severity scores.
Results
Within 2wk of birth, the ratio of N-acetylaspartate + N-acetylaspartylglutamate to creatine + phosphocreatine was significantly lower in those infants showing white matter abnormalities on conventional MRI. Increased lactate to creatine + phosphocreatine and lactate to glycerophosphocholine + phosphocholine ratios were significantly associated with increasing severity of Clinical Risk Index for Babies II and Apgar scores taken at 1 and 5min after birth.
Conclusion
Both overall health status at birth and white matter injury in preterm neonates are reflected in metabolite ratios measured shortly after birth. Long-term follow-up will provide additional insight into the prognostic value of these measures.
Preterm birth is an increasingly common condition with serious implications for long-term morbidity. Motor deficits will occur in 5–15% of infants born preterm, and an additional 25–50% will experience behavioral and cognitive deficits (1–4). Unfortunately, predicting long-term outcome in this population remains difficult.
Several recent studies have demonstrated the value of conventional magnetic resonance imaging (MRI) as a predictor of long-term outcome (2,3,5,6). In particular, brain abnormalities observable within the first weeks of life, such as intraventricular hemorrhage, ventricular dilation, and white matter injury, are all associated with poor neurodevelopmental outcome. Advances in understanding preterm brain injury have been made, with an increasing focus on the role of noncystic white matter injury, but there is still much to be learned about the causes and consequences of these types of insults (1,7,8).
Proton magnetic resonance spectroscopy (MRS) is one of several advanced MRI techniques that provides additional data beyond what can be seen on conventional anatomical scans. It is a safe and noninvasive way to measure the concentrations of certain brain metabolites, with the most commonly reported metabolite combinations being creatine + phosphocreatine (CR), glycerophosphocholine + phosphocholine (CHO), N-acetylaspartate + N-acetylaspartylglutamate (NAA), myo-inositol (INS), and lactate (LAC). Each of these metabolites is sensitive to multiple aspects of brain metabolism and health. CR is necessary for the regulation of energy supply in cells (9,10). CHO is involved in membrane synthesis and typically decreases in parallel with myelination; elevated levels could indicate the breakdown of myelin (9–12). The function of NAA is not well understood, but it is found in neurons, immature oligodendrocytes, and their progenitors, and decreasing levels of NAA are used as a marker of neuronal loss or depression (8–14). INS is located primarily in glial cells, is involved in membrane structure and cell growth, and is one of the major osmolytes in the central nervous system (10,11,13–15). LAC is a product of anaerobic glycolysis and reflects any disturbance in cerebral oxidative metabolism (10,11,15).
These metabolites can be used to monitor the dramatic changes in the brain that occur during the preterm period (10,13,16–18). In general, INS and CHO have relatively high concentrations in the preterm brain and decrease rapidly within the first months of life. By contrast, NAA and CR have relatively low concentrations in the preterm brain and increase over time.
Many studies of asphyxiated term neonates have demonstrated that high levels of LAC and low levels of NAA in the deep gray matter shortly after birth are predictive of poor neurodevelopmental outcome in this population (19–24). A handful of studies (13,14,25–28) have attempted to determine whether a similar relation holds in infants born very preterm, but the results have so far been inconclusive.
The current study investigated the relations of conventional at-birth illness severity scores and evidence of white matter injury on conventional MRI with brain metabolite ratios, measured both within 2 wk of birth and at term-equivalent age in a large cohort of very preterm infants.
RESULTS
Developmental Trends
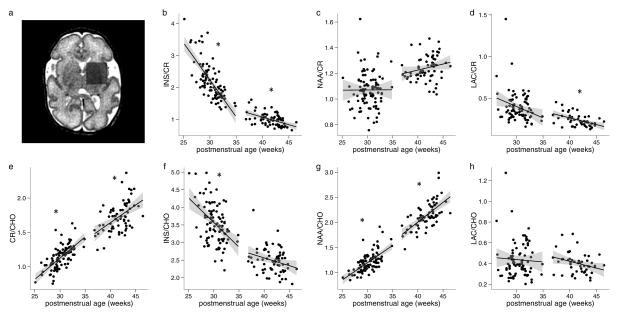
A representative example of voxel placement is shown in Figure 1a. Figure 1b–h represents the correlations between metabolite ratios and postmenstrual age at scan. Sample long and short echo time spectra from a single subject are shown in Figure 2. The average line width (full width at half maximum) across all spectra was 0.049 ± 0.017 ppm. During the pre-term time period, there were dramatic increases in CR/CHO (P < 0.001) and NAA/CHO (P < 0.001) ratios and decreases in INS/CR (P < 0.001) and INS/CHO (P < 0.001) ratios. There was also a trend toward a decreasing LAC/CR ratio, but this was not significant after correcting for multiple comparisons (P = 0.067). At term-equivalent ages, these same trends continued, with increasing CR/CHO (P < 0.001) and NAA/CHO (P < 0.001) ratios and more gradually decreasing INS/CR (P < 0.001) and LAC/CR (P = 0.002) ratios. There were also non-significant trends for increasing NAA/CR and decreasing INS/ CHO and LAC/CHO ratios around term-equivalent age. (All P values were corrected for multiple comparisons).
Figure 1.
Developmental trends in MRS data. (a) Voxel placement for MRS sequences. (b–h) Correlations between metabolite ratios and postmenstrual age at scan during the preterm and term periods, plotted with regression lines and 95% confidence bands (gray shaded area). *P < 0.05 after correction for multiple comparisons. CHO, glycerophosphocholine+ phosphocholine; CR, creatine+phosphocreatine; INS, myo-inositol; LAC, lactate; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate+N-acetylaspartylglutamate.
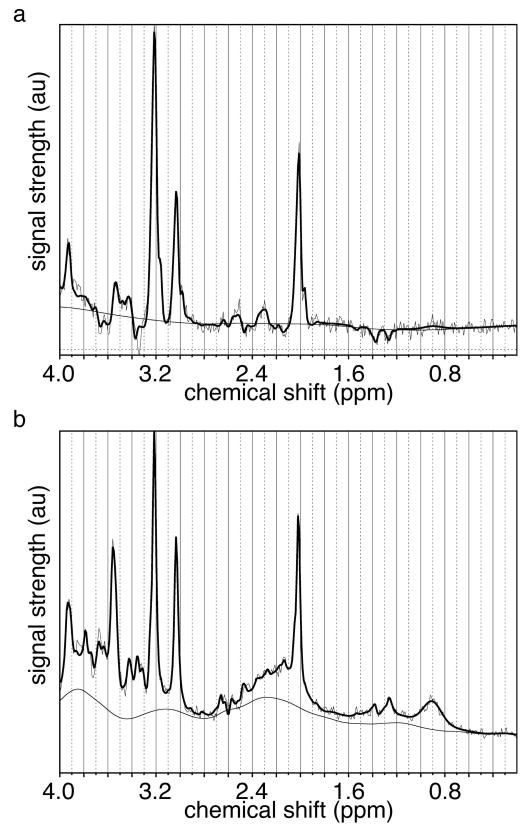
Figure 2.
Sample (a) long and (b) short echo time spectra from a participant scanned at 34 wk gestation, with signal strength plotted in arbitrary units. The bold line represents the LCModel fit; two lighter lines represent the original spectrum and the smooth baseline calculated by LCModel.
Using the relaxed inclusion criteria (as described in the Methods section), useable LAC/CHO and LAC/CR values were obtained for 84 of 99 infants from the preterm scan and 50 of 67 infants from the term scan. On the basis of visual inspection, a lactate doublet was clearly present in all of these cases at 1.3 ppm. As can be seen in Figure 1d, h, some infants had LAC/ CHO and LAC/CR values that could be considered outliers. It is worth noting, however, that the three largest LAC values had low uncertainty estimates from LCModel, within the bounds of conventional inclusion criteria (<20%). Furthermore, all three of these infants had large and well-formed LAC doublets in their MRS spectra. One was reported as having a bilateral grade II intraventricular hemorrhage, with increased T2 in the caudate nuclei and the anterior aspect of both putamina, without diffusion restriction. The second showed a mild increase in T1 signal in both putamina without diffusion restriction. The third had one small focal white matter lesion but otherwise appeared normal. All three were also scanned at term-equivalent age and all had normal LAC/CR and LAC/CHO ratios at term (within two SDs of the mean).
Associations With Injury
After accounting for age at scan, NAA/CR ratio was significantly lower in the group of infants showing white matter injury on conventional MRI during the preterm period (corrected P= 0.006). There was no significant interaction between age and group. NAA/CHO ratio showed a similar trend toward being lower in the white matter injury group during the pre-term period (uncorrected P = 0.013) but was not significant after correcting for multiple comparisons. There were no significant differences between groups for these metabolite ratios at term-equivalent age. No other metabolite ratios showed any significant differences between groups or interactions between age and group at either time point.
Illness Severity Scores
During the preterm period, both LAC/CR and LAC/CHO ratios were significantly associated with at-birth illness severity scores, controlling for postmenstrual age at scan and correcting for multiple comparisons (corrected P < 0.05). LAC concentration increased (relative to CR and CHO) with increasing severity of illness as measured by Apgar score at 1 and 5 min and Clinical Risk Index for Babies II (CRIBII). No other metabolite ratios showed any significant interaction or association with these at-birth illness severity scores. To ensure that the possible outliers in the LAC data (as described above) were not responsible for these findings, this analysis was repeated after rejecting both one and two outliers. When the two largest outliers were rejected, all associations remained significant. When a single outlier was rejected, the associations with both CRIB II and Apgar scores at 5 min remained significant, but not the association with Apgar score at 1 min. Therefore, the effects were robust and not excessively influenced by a few data points.
DISCUSSION
Several studies have described the association between brain abnormalities observable on conventional MRI and long-term neurodevelopmental outcome in infants born very preterm. Only a few, however, have incorporated MRS as a possible source of predictive data; this is one of the primary goals of this ongoing, longitudinal study. Furthermore, most of those studies that acquired MRS data only did so at term-equivalent age. As such, this is one of the first studies to report robust MRS findings from a large cohort of very preterm infants studied shortly after birth.
There is now strong evidence that white matter injury in preterm infants is predictive of neurodevelopmental outcome. For example, in a study of 89 preterm infants scanned as soon as they could be safely transported to the MRI (3), the authors found that the common MRI abnormalities (moderate-to-severe white matter injury, ventriculomegaly, and intraventricular hemorrhage) were more strongly associated with neurodevelopmental outcome at 12–18 mo than gestational age at birth. Three other studies of preterm-born infants acquired MRI data only at term-equivalent age and found that white matter lesions were significantly associated with mental/psychomotor developmental delay and cerebral palsy at 2 y of age (6); motor impairment at 5 y of age (5); and cognitive delay, motor delay, cerebral palsy, and neurosensory impairment at 2 y of age (2). However, none of these studies included an MRS component.
A recent study (29) on the association between chorioamnionitis and focal white matter injury, and the effects of chorioamnionitis on brain development, in a large cohort of very preterm infants included both MRS and diffusion tensor imaging. Although chorioamnionitis was not associated with white matter injury or any of the MRS or diffusion tensor imaging measurements, the authors did find that NAA/CHO ratio was significantly lower in those infants suffering from white matter injury, based on MRS data acquired in the basal ganglia and superior white matter. LAC/CHO ratios were also investigated, but no significant results were found. Ratios relative to CR were not reported. These findings are consistent with the significantly lower NAA/CR ratio that we observed in the basal ganglia of those infants with white matter injury in our population, as well as the trend toward lower NAA/CHO ratio, which lost significance due to a correction for multiple comparisons.
Four other recent preterm MRI studies also acquired MRS data but differed in terms of timing, brain regions, and data processing. The largest study (30) did not report long-term outcomes but found that those infants suffering from infection showed significantly reduced NAA/CHO ratios in various brain locations, suggesting a possible causal link between these findings. Another large study (27) acquired MRS data in the cerebellum. The authors found that NAA/CHO ratio measured in the cerebellum, as well as cerebellar volume, was significantly associated with cognitive scores at 2 y corrected age, highlighting the importance of considering multiple locations with MRS. However, the infants were imaged only at term-equivalent age, and the authors note that the cohort was relatively healthy overall as compared with other preterm study cohorts.
A third study (28) involved 20 preterm infants and included an MRS sequence in the periventricular white matter at term-equivalent age. The authors found that NAA/CHO ratio was positively correlated with white matter, gray matter, and cerebellar volumes, but not with motor scores, at 6 mo of age. Finally, a study conducted in 2008 (25) included 36 infants born at ≤32 wk gestation and/or having birth weight ≤1,500 g who were scanned between 35 and 43 wk postmenstrual age. Although the expected developmental trends were found for various metabolites, the authors were unable to find any correlation between metabolite ratios in the basal ganglia and neurodevelopmental outcome at 18 or 24 mo. Furthermore, they were unable to distinguish the LAC peaks from noise, and thus LAC levels were not reported. A concern with these studies was the delay between birth and the time of MRI; our study suggests that MRS investigations closer to the time of birth may yield information more sensitive to possible brain insults related to preterm birth.
Two metabolites that can be measured by MRS stand out as particularly relevant, both in the literature and in this article—NAA and LAC. As summarized in the introduction, elevated LAC and decreased NAA are found in the basal ganglia of asphyxiated term neonates. Elevated LAC found in preterm infant brains is hypothesized to be the result of low levels of mitochondria in the immature brain, which therefore has a greater dependence on glycolytic energy production. Therefore, the presence of LAC in preterm infants is not in itself thought to be indicative of brain injury (11,15,17,31). The current study, however, suggests that there is a relation between the amount of LAC in the basal ganglia of very preterm infants and overall illness severity at birth, even when the total amount of LAC is small. For most infants in this study, although lactate was clearly present in the spectrum based on visual inspection, the concentration of LAC would be difficult for a human observer to accurately quantify, because of the small size of the peaks. Automated fitting software, such as LCModel, however, has the ability to reliably quantify even small peaks, along with an uncertainty estimate, given data of sufficient quality. Although we have included LAC data in our study with higher uncertainty than would typically be included, the strong correlations described above between LAC/CR and LAC/CHO ratios during the preterm period and the measures of illness severity at birth are highly suggestive of clinical relevance. The original CRIB score, upon which the CRIB II is based, has also been investigated as a possible predictor of neurodisability, with inconclusive results (32), but the same has not yet been done with the CRIB II. Ultimately, it is only through follow-up data that we will know if these LAC measurements have long-term predictive value.
Alteration of NAA has been associated with abnormalities in the development of oligodendroglia and their precursors, which impair normal myelination, as in the case of Canavan’s disease (9). Nevertheless, in predominantly gray matter areas, such as the basal ganglia, decreased NAA, relative to the overall preterm population, may be considered a marker of neuronal loss (15). In one of the first MRS studies of preterm infants (26), the authors hypothesized that those infants with white matter lesions would show similarities on MRS to asphyxiated term neonates, expecting to find a reduced NAA/CR ratio in the injured group. By contrast, they found significantly higher LAC/CR and INS/CR ratios in the periventricular white matter of the white matter injury group, with no significant difference in NAA/CR ratio. However, their study included only 30 infants, and the MRI scans were done at ~10 wk after birth. Our study suggests that their original hypothesis might have been confirmed if they had imaged their participants closer to delivery. We found a reduction in NAA/CR ratio in the basal ganglia of those infants with noncystic white matter lesions, as compared with those without, but only in the preterm period. There was no difference between groups when the neonates were studied again at term-equivalent age, suggesting that NAA levels normalize after several weeks of life, perhaps reflecting restored, but potentially abnormal, connectivity. Although we did not find the differences in LAC/CR or INS/ CR ratios observed by the above-mentioned authors, there were several differences in methodology that could explain this discrepancy, including their use of line-shape fitting, as opposed to LCModel to quantify metabolite concentrations, and their placement of the MRS voxel in the periventricular white matter, as opposed to the basal ganglia.
In a review article (13), Vigneron similarly reported lower NAA and higher LAC in preterm infants with evidence of brain injury as compared with those without. He also noted that elevated LAC is seen in preterm infants who go on to have abnormal neurodevelopmental outcome at 1 y of age, as compared with those with normal development. Follow-up papers from the same group (16,33) have reported on only those infants who went on to have normal neurodevelopmental outcome, in an effort to establish normative metabolite concentrations for preterm infants. Nevertheless, the findings of the current study are in line with Vigneron’s original observations. Although we have examined white matter abnormality specifically, rather than brain injury in general, the reductions in NAA/CR and NAA/CHO ratios that we observed in the group with white matter lesions agree with Vigneron’s findings. Our results, in combination with the literature described above, firmly establish this as a reliable pattern. Although we did not find the increased LAC reported by Vigneron, we did find a correlation between elevated LAC and higher at-birth illness severity; the discrepancy may be a result of the type of brain injuries considered. We anticipate that the results of our follow-up data at 2 and 4 y of age will determine the significance of these findings with respect to long-term outcome.
There were several limitations to this study. In general, single-voxel MRS studies are limited in their spatial coverage and resolution. In light of the need for adequate signal-to-noise ratio, localizing the MRS voxel to a specific anatomical location without any spectral contamination from adjacent tissue is hardly achievable, especially in the very small preterm brains. An additional limitation was the reduction in numbers from the initial to the term MRI scan. The majority were due to families being unwilling to return from significant distances at this early age. Finally, because of the relatively low concentrations of LAC in this population, we have included estimates with higher uncertainty than would normally be included. More advanced sequences designed specifically to provide a better quantification of LAC are available, and this research suggests that there would be considerable value in using them in preterm populations in the future.
Conclusions
Along with other recent preterm and neonatal MRS studies, our results strongly indicate a consistent pattern of reduced NAA with focal white matter lesions in the brains of very preterm infants scanned shortly after birth. Elevated LAC, by contrast, is associated with increased severity of illness at birth. These results, in combination with other recent research, demonstrate the power and importance of MRS in assessing the health of very preterm infant brains.
METHODS
Participants
One hundred and five infants born at ≤32 wk gestation were enrolled in this study from the neonatal intensive care unit at the Hospital for Sick Children in Toronto and scanned within 2 wk of birth. Seventy of these were also scanned at term-equivalent age. Infants with known chromosomal defects or major congenital abnormalities were excluded. This study was approved by the hospital’s research ethics board, and informed, written consent was obtained from the parents of all of the infants.
Neonatal Data Collection
The illness severity scores that were collected were CRIB II and Apgar scores at 1 and 5 min. CRIB II is a composite measure specifically designed for premature and low–birth-weight infants to determine the severity of illness and risk of morbidity and mortality. The variables in the score are gender, birth weight, gestational age, temperature at admission, and maximum base excess over a 12-h period (34).
Radiological Evaluation
Clinical scans were evaluated by two pediatric neuroradiologists with more than 10 y of experience in neonatal imaging, and findings were reported to the neonatal intensive care unit. Preterm scans were evaluated for the presence of germinal matrix hemorrhage graded according to Volpe (8), periventricular hemorrhagic infarction, noncystic white matter lesions (identified as abnormal foci of T1 hyperintensities without concomitant T2 hypointensity), cerebellar lesions, and extra-axial hemorrhage. MRS scans were obtained from 99 infants within 2wk of birth and from 67 of these infants at term-equivalent age. In the remaining cases, scanning was aborted before MRS sequences could be completed. The cohort of 99 comprised 52 males and 47 females and included 17 infants from 9 sets of twins. Three infants died while still in the hospital, and two passed away after being discharged. Forty infants showed no brain abnormalities on the preterm morphological MRI. The basic clinical information for this cohort is summarized in Table 1. The radiological findings from clinical scans collected at birth are summarized in Table 2.
Table 1.
Basic demographic and clinical data
| Demographic | Median (interquartile range) or number (%) |
|---|---|
| GA at birth (wk) | 28.9 (27.5–30) |
| Cesarean-section delivery | 55 (56%) |
| Age at birth scan (d) | 10 (7–13) |
| Postmenstrual age at birth scan (wk) | 30.1 (29.1–31.6) |
| Postmenstrual age at term scan (wk) | 42.1 (40.2–43.1) |
| Weight at birth (kg) | 1.12 (0.96–1.39) |
| Apgar score at 1 min | 6 (4–8) |
| Apgar score at 5 min | 8 (6–9) |
| CRIB II | 7 (5–9) |
| Mechanical ventilation (d) | 5 (2–15) |
| Resuscitation required (CPR) | 11 (11%) |
| Respiratory distress syndrome | 93 (96%) |
| Chronic lung disease | 18 (18%) |
| Patent ductus arteriosis | 26 (27%) |
| Sepsis (cultures positive) | 33 (34%) |
| Meningitis (cultures positive) | 2 (4%) |
| Necrotizing enterocolitis | 11 (12%) |
CPR, cardiopulmonary resuscitation; CRIBII, Clinical Risk Index for Babies II; GA, gestational age.
Table 2.
Radiological findings on conventional MRI from preterm scan
| Incidence (%) among all infants in cohort (n=99) | Incidence (%) at birth, among infants scanned at term (n= 67) | |
|---|---|---|
| Diffusion restriction in deep gray matter | 2 (2) | 1 (1) |
| Germinal matrix hemorrhage | 39 (39) | 27 (40) |
| Intraventricular hemorrhage | 29 (29) | 21 (31) |
| Ventricular dilation | 20 (20) | 14 (21) |
| Periventricular hemorrhagic infarction | 8 (8) | 4 (6) |
| Noncystic white matter lesions | 38 (38) | 23 (34) |
| Cerebellar lesions | 9 (9) | 6 (9) |
| Extra-axial hemorrhage | 19 (19) | 14 (21) |
MRI, magnetic resonance imaging.
MRI Sequences and Processing
All scans were performed on a 1.5T MRI scanner (Signa Excite; GE, Milwaukee, WI), using an MR-compatible incubator and neonatal head coil (AIR, Cleveland, OH) for the preterm scans. At both time points, the battery of clinical scans included T1- and T2-weighted anatomical, gradient echo and diffusion-weighted sequences. Spectroscopy data were collected using two single-voxel point-resolved spectroscopy sequences (TE=35 and 144ms, TR =2,000 ms, 64 averages). Both MRS sequences used the same voxel, which was placed so as to maximize coverage of the basal ganglia while attempting to minimize the presence of white matter and cerebral spinal fluid. The average voxel size was 5.16 cm3, with an SD of 1.44 cm3. This location was chosen because it has been shown to be a sensitive location in term infants and for comparison with previous MRS studies of pre-term infants (13,14,19,20,22,24,25).
All spectroscopy data were processed using LCModel (35) to obtain metabolite concentrations and uncertainty estimates from each MRS sequence. Using the estimates from LCModel, the following metabolite ratios were calculated: CR/CHO, NAA/CHO, NAA/CR, INS/ CHO, INS/CR, LAC/CHO, and LAC/CR. All ratios were calculated from the long echo time sequence, which tends to have a flatter baseline, except for those involving INS, which can be more accurately measured at a short echo time. The use of ratios controls for changes in brain water content, T1 and T2 relaxation times of water, and possible cerebral spinal fluid contamination of the voxel. All uncertainty estimates from LCModel were<20%, except in the case of LAC. Because of overall low LAC levels in the preterm brain, only 22 infants at birth and 11 infants at term-equivalent age had LAC uncertainty estimates <20%, which is commonly used as a standard inclusion criteria (36). To obtain a more representative data sample, this was relaxed to <40% in the case of LAC. All spectra meeting this criterion were also visually evaluated to ensure that a lactate doublet was evident at 1.3 ppm. Although this relaxed inclusion criteria introduced additional uncertainty into the LAC concentrations, it provided estimates of LAC concentration for most of the subjects in this cohort.
Statistical Analyses
Statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria). Three analyses were performed. First, all seven metabolite ratios were correlated with age at scan using univariate regression (age in all analyses refers to postmenstrual age). Data from preterm and term scans were considered separately. Second, the cohort was divided into two groups, those with noncystic white matter lesions identified on the clinical MRI scans shortly after birth and those without. There were no significant differences between these groups in terms of mean gestational age at birth, birth weight, delivery type, Apgar or CRIB II scores, days of mechanical ventilation, or age at scan. Metabolite ratios from both time points were then analyzed using multiple regression with a linear mixed-effects model using age, group, and the age–group interaction as factors. In cases in which the interaction was not significant, the regression was computed again without the interaction term to evaluate the effect of white matter injury on metabolite ratios, controlling for age. Third, this procedure was repeated to evaluate the relations between metabolite ratios from the preterm scan and the three illness severity scores (CRIB II and Apgar scores at 1 and 5min), controlling for age. In all three analyses, the Holm–Bonferroni method was used to correct for multiple comparisons (with n values = 14, 14, and 21, respectively), and all corrected P values <0.05 were considered to be statistically significant.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT
This research was funded by the Canadian Institutes of Health Research (grant MOP-84399).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 3.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112(1 Pt 1):176–80. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 5.Spittle AJ, Cheong J, Doyle LW, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol. 2011;53:1000–6. doi: 10.1111/j.1469-8749.2011.04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants : a 2-year follow-up study. Radiology. 2011;261:899–906. doi: 10.1148/radiol.11110797. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpe JJ. Neurology of the Newborn. 5. Philadelphia, PA: Saunder Elsevier; 2008. pp. 415–543. [Google Scholar]
- 9.Dezortova M, Hajek M. (1) H MR spectroscopy in pediatrics. Eur J Radiol. 2008;67:240–9. doi: 10.1016/j.ejrad.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Cecil KM, Jones BV. Magnetic resonance spectroscopy of the pediatric brain. Top Magn Reson Imaging. 2001;12:435–52. doi: 10.1097/00002142-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Panigrahy A, Borzage M, Blüml S. Basic principles and concepts underlying recent advances in magnetic resonance imaging of the developing brain. Semin Perinatol. 2010;34:3–19. doi: 10.1053/j.semperi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi CG, Ulug AM, Deck MD, Zimmerman RD, Heier LA. Developmental delay in children: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2002;23:882–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Vigneron DB. Magnetic resonance spectroscopic imaging of human brain development. Neuroimaging Clin N Am. 2006;16:75–85. viii. doi: 10.1016/j.nic.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Roelants-van Rijn AM, van der Grond J, Stigter RH, de Vries LS, Groenendaal F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr Res. 2004;56:285–90. doi: 10.1203/01.PDR.0000132751.09067.3F. [DOI] [PubMed] [Google Scholar]
- 15.Cady EB, Penrice J, Amess PN, et al. Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med. 1996;36:878–86. doi: 10.1002/mrm.1910360610. [DOI] [PubMed] [Google Scholar]
- 16.Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Magn Reson Imaging. 2011;33:306–11. doi: 10.1002/jmri.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZJ, Vigneron DB, Miller SP, et al. Brain metabolite levels assessed by lactate-edited MR spectroscopy in premature neonates with and without pentobarbital sedation. AJNR Am J Neuroradiol. 2008;29:798–801. doi: 10.3174/ajnr.A0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Hüppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48:949–58. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 19.Cheong JL, Cady EB, Penrice J, Wyatt JS, Cox IJ, Robertson NJ. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR Am J Neuroradiol. 2006;27:1546–54. [PMC free article] [PubMed] [Google Scholar]
- 20.Khong PL, Tse C, Wong IY, et al. Diffusion-weighted imaging and proton magnetic resonance spectroscopy in perinatal hypoxic-ischemic encephalopathy: association with neuromotor outcome at 18 months of age. J Child Neurol. 2004;19:872–81. doi: 10.1177/08830738040190110501. [DOI] [PubMed] [Google Scholar]
- 21.Kadri M, Shu S, Holshouser B, et al. Proton magnetic resonance spectroscopy improves outcome prediction in perinatal CNS insults. J Perinatol. 2003;23:181–5. doi: 10.1038/sj.jp.7210913. [DOI] [PubMed] [Google Scholar]
- 22.Zarifi MK, Astrakas LG, Poussaint TY, Plessis AdAd, Zurakowski D, Tzika AA. Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology. 2002;225:859–70. doi: 10.1148/radiol.2253011797. [DOI] [PubMed] [Google Scholar]
- 23.Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol. 2001;22:1786–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Amess PN, Penrice J, Wylezinska M, et al. Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol. 1999;41:436–45. [PubMed] [Google Scholar]
- 25.Augustine EM, Spielman DM, Barnes PD, et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol. 2008;28:611–8. doi: 10.1038/jp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson NJ, Kuint J, Counsell TJ, et al. Characterization of cerebral white matter damage in preterm infants using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2000;20:1446–56. doi: 10.1097/00004647-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Van Kooij BJM, Benders MJNL, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54:260–6. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 28.Gadin E, Lobo M, Paul DA, et al. Volumetric MRI and MRS and early motor development of infants born preterm. Pediatr Phys Ther. 2012;24:38–44. doi: 10.1097/PEP.0b013e31823e069d. [DOI] [PubMed] [Google Scholar]
- 29.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–64. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 30.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71:274–9. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leth H, Toft PB, Pryds O, Peitersen B, Lou HC, Henriksen O. Brain lactate in preterm and growth-retarded neonates. Acta Paediatr. 1995;84:495–9. doi: 10.1111/j.1651-2227.1995.tb13681.x. [DOI] [PubMed] [Google Scholar]
- 32.Dorling JS, Field DJ, Manktelow B. Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed. 2005;90:F11–6. doi: 10.1136/adc.2003.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu D, Vigneron D. Magnetic resonance spectroscopy imaging of the newborn brain–a technical review. Semin Perinatol. 2010;34:20–7. doi: 10.1053/j.semperi.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry G, Tucker J, Tarnow-Mordi W UK Neonatal Staffing Study Collaborative Group. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 35.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 36.Provencher SW. [Accessed 1 February 2013];LCModel & LCMgui User’s Manual. ( http://s-provencher.com/pub/LCModel/manual/manual.pdf)