Abstract
Rapid estrogen actions are widely diverse across many cell types. We conducted a series of electrophysiological studies on single rat hypothalamic neurons and found that estradiol (E2) could rapidly and independently potentiate neuronal excitation/depolarizations induced by histamine (HA) and N-Methyl-D-Aspartate (NMDA). Now, the present whole-cell patch study was designed to determine whether E2 potentiates HA and NMDA depolarizations - mediated by distinctly different types of receptors, - by the same or by different mechanisms. For this, the actions of HA, NMDA, as well as E2, were investigated first using various ion channel blockers and then by analyzing and comparing their channel activating characteristics. Results indicate that: first, both HA and NMDA depolarize neurons by inhibiting K+ currents. Second, E2 potentiates both HA and NMDA depolarizations by enhancing the inhibition of K+ currents, an inhibition caused by the two transmitters. Third, E2 employs the very same mechanism, the enhancement of K+ current inhibition, thus to rapidly potentiate HA and NMDA depolarizations. These data are of behavioral importance, since the rapid E2 potentiation of depolarization synergizes with nuclear genomic actions of E2 to facilitate lordosis behavior, the primary female-typical reproductive behavior.
Keywords: Estradiol, histamine, N-Methyl-D-aspartate (NMDA), patch clamp, neuronal activity, ion channel, synergism
1. INTRODUCTION
It is now well established that estrogens have at least two kinds of actions, classically defined genomic actions and membrane-initiated rapid actions. The latter are less understood. Rapid estrogen actions can affect a wide variety of cell and tissue types [1, 2], ranging from blood cells [3, 4], bone cells [5–10], muscle cells [11–15], cancer cells [16–19], cell lines [20–22], to neurons either mature [23, 24], embryonic [25] or primary cultured [26]. In addition, estrogens are known to modulate a variety of different signaling systems [27–30].
To examine some of estrogens’ actions on neurons of importance for reproductive behavior, we investigated how they affect the responses of female rat hypothalamic ventromedial nucleus (VMN) neurons to histamine (HA) and N-Methyl-D-Aspartate (NMDA). These two neurotransmitter agents were chosen because they induce depolarizing responses via distinctly different receptor types. HA induces depolarization/excitation via a Gq-coupled receptor, and hyperpolarization/inhibition through Gi/o-coupled receptor [31, 32], while NMDA causes depolarization/excitation via a ligand-gated ion channel that does not need the activation of a G-protein [33]. Our initial study identified 3 different effects of rapid estradiol (E2) action in VMN neurons: Potentiation of HA excitatory effects, attenuation of HA inhibition, and potentiation of NMDA effects [34]. These findings raised the possibility that E2 employed three different potential mechanisms to exert these rapid actions on VMN neurons.
In the follow-up neuropharmacological analyses using specific HA agonists and antagonists [35], we confirmed that HA depolarization (which would lead to excitation) is mediated by HA receptor subtype H1 (H1R) while HA hyperpolarization (which would lead to inhibition) was mediated by H2R or H3R. As expected from our initial study, the “pure” depolarization evoked with a specific H1R agonist was potentiated rapidly by E2. But surprisingly, pure hyperpolarization evoked with a H2R or a H3R agonist was neither attenuated nor potentiated by E2. This allows us to eliminate one of the three potential rapid E2 action mechanisms in VMN neurons.
In the present study, whole-cell patch recording was used to examine and compare the rapid E2 actions on the depolarizations induced by HA and by NMDA, and found that there are several close similarities.
2. METHODS
2.1. Materials
HA and NMDA were dissolved first in saline as stock solution (50 or 100 mM) and then dissolved in bathing solutions to concentrations to be used. Ion channel blockers and their concentrations used were described at their mention. E2 and testosterone (used as a control for structurally similar E2) were dissolved initially in 100% ethanol and then diluted in distilled water to 1 mM as stock solution and finally in artificial cerebrospinal fluid (ACSF) to the concentrations to be used before experiments. The vehicle for the steroids has been tested in our previous studies and has no effect by itself on neuronal activity. Chemicals and their concentration in bathing solutions were described at their mentioning. All chemicals and test agents for electrophysiology were purchased from Sigma.
2.2. Animals
Intact 12- to 30-day old Sprague-Dawley female rats were used. The age range was chosen to guarantee successful patch clamp recordings from hypothalamic ventromedial nucleus (VMN) neurons. Also, females at these ages have little or no circulating estrogens (absence of estrogen inducible progesterone receptors in the hypothalamus) at least in the brain, but were capable of responding to estrogen, as monitored behaviorally and histochemically [36]. All procedures in handling and treating the animals were approved by The Rockefeller University’s Animal Care and Use Committee in accordance with the Animal Welfare Act and the Department of Health and Human Services’ “Guide for the Care and Use of Laboratory Animals”.
2.3. Brain slices preparation
Hypothalamic slices containing VMN were prepared as described previously [34, 37]. Briefly, rats were deeply anesthetized by urethane (160 mg/100 g B.W.) and decapitated to remove the brain. After removing the pia mater from the ventral surface, the hypothalamus was blocked out and placed on the cutting stage of a Vibratome (model 1000 Plus, The Vibratome Company, St Louis, MO). Thin (200–300 µm) coronal slices containing the VMN were cut and collected based on anatomical landmarks. For the blocking and slicing, we used ice-cold sucrose ACSF, in which all the NaCl was replaced by sucrose so as to prevent damage of neurons from over excitation. Slices were then stored in ACSF at room temperature for at least 1 hour before recording. The ACSF is composed of (mM): NaCl, 126; KCl, 5; NaH2PO4, 1.25; CaCl2, 2; MgCl2, 2; NaHCO3, 26; dextrose, 10; and oxygenated with 95% O2 / 5% CO2.
2.4. Whole-cell patch recording
One slice at a time was placed on the bottom of the recording chamber fixed to the stage of an upright microscope (Olympus BX50WI) and superfused with ACSF at 1.5–2 ml/min at room temperature. The slice was examined with infrared differential interference contrast (IR-DIC) optics. All the neurons recorded in the present study were from the ventrolateral portion of the VMN, which is rich in neurons with estrogen receptor and is critically important for estrogen induction of lordosis (Pfaff, 1980). To accomplish this, ventrolateral VMN was located under low magnification (40x) before switching to higher magnification (400x). At the end of an experiment the location of the recorded neuron was re-affirmed with low magnification. Under high magnification, cells were visually selected and patched conventionally with electrodes (2–5 MΩ) pulled from Borosilicate glass pipettes (G8515OT-4, Warner Instruments, Hamden, CT) with a Narishige PP-830 puller and filled with an internal solution, which was composed of (mM): K-Gluconate, 140; EGTA, 5; MgCl2, 2; NaHCO3, 0.6; HEPES, 10; Mg-ATP, 2; Na2-ATP, 2; CaCl2,1; Na-GTP, 0.3; and sucrose, 8.3. Whole-cell current or membrane potential was amplified with Axoclamp 200A initially and Multiclamp 700B later in this study and recorded and analyzed with Clampex and Clampfit, respectively (Axon Instruments). The extracellular solution was ACSF, unless otherwise indicated. Once a patch was obtained, membrane test was performed to check following criteria: Access resistance <15 MΩ, leak current <30 pA and membrane potential (Vm) to be equal or more negative than −45 mV. Once a criterion was violated the recording was terminated. All experiments were performed under current clamp.
2.5. General experimental procedure
The Vm was then observed for 5 min or longer and only neurons with stable Vm were studied. To ensure that the patched cell is a neuron, it was injected briefly with a depolarizing current to evoke action potentials (APs). In experiments using tetrodotoxin (TTX), action potentials were maintained with continuous current injection, if necessary, while TTX was administered to assure that the Na+ channel blocker was effective. In those without TTX, the holding membrane voltage was set to −65mV or more hyperpolarized if necessary, in attempts to avoid the spontaneous occurrence of AP.
A test agent, HA, NMDA or E2 and related agents was then applied repetitively either through the bath or by picospritzer. With bath application, the recording chamber perfusion was switched from ACSF to a modified ACSF containing a test agent. With this method, a response is usually small and slow (see Fig. 1).
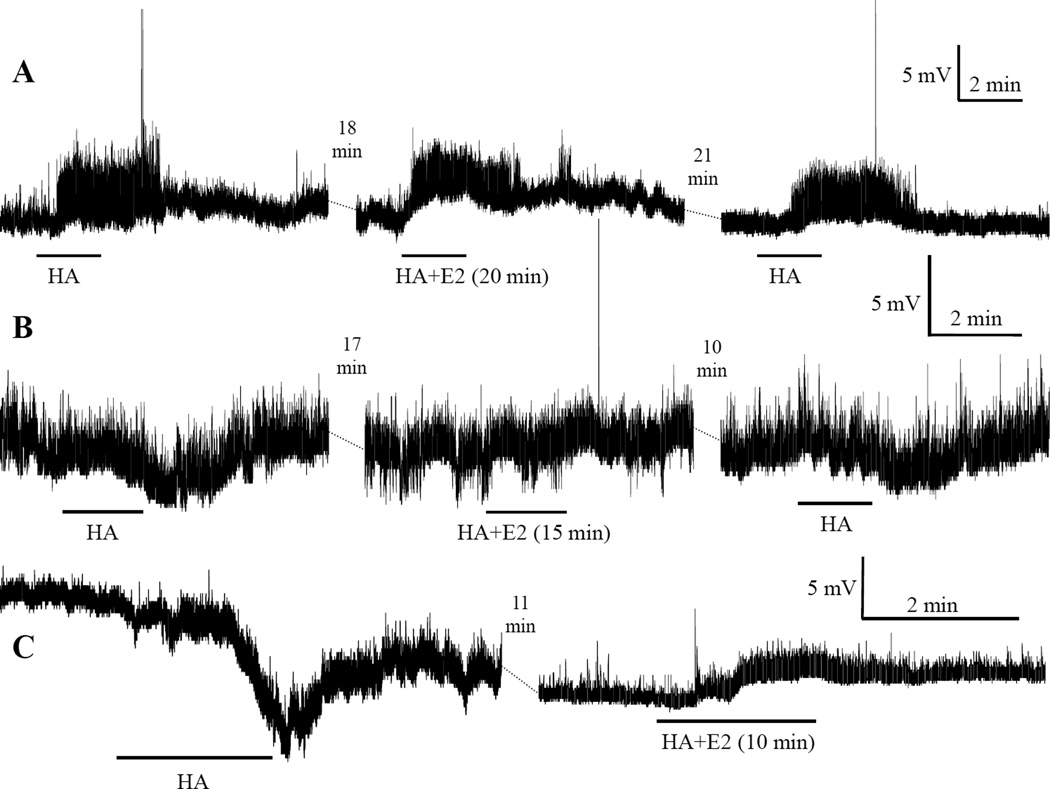
Fig. 1.
Examples of actions of bath applied histamine (HA) and the rapid E2 effects on them. Each series of traces connected by dashed lines are segments of a continuous recording from a single neuron. Tetrodotoxin (TTX) (0.5 µM) was included in the bathing solution. Horizontal bars under each trace indicate the duration of HA application. The minutes in parentheses following HA+E2 indicate the duration of E2 application at the time of HA administration. The time above the dashed lines between traces indicates the gap duration. Acutely applied E2 potentiated HA-induced depolarization in series A, abolished hyperpolarization in series B, and converted hyperpolarization into depolarization in series C.
The picospritzer (Spritzer-8, Cornerstone) was used only for administering HA or NMDA. The responses induced by spritz are usually much greater and much rapid than those by bath application (compare Fig. 1 with Fig. 2, note the difference in time scales). HA or NMDA (1–50 mM, pH 7.4 – 6.05, in ejecting pipette) was ejected with a picospritzer (2.5 × 105 Pa for 0.1–20 sec) near the patched neuron. To control for pH, 7 neurons were picospritzed with ACSFs with pH ranging from 7.05 to 6.05, equivalent to HA concentrations 5–50 mM. None of the 7 neurons was affected, neither depolarized nor hyperpolarized, by any of the ACSFs. In a previous study [35], we found the use of picospritzer was safe: mechanical disturbance, even if obviously observable, did not evoke any response and the response could be adjusted to a suitable amplitude for observing modulation by adjusting the position of the ejecting pipet. Also, picospritzer ejection has following advantages over bath application: i) because its application duration is very brief (fractions of a sec to 2 sec versus 2–3 min with bath application) and it is applied only locally near the recorded neurons, the ejected agent can be washed away quickly. This results in brief response durations (few sec versus several min by bath application) and allows for more frequent applications; to avoid desensitization or sensitization, the interval for repeated test agent applications was generally ≥10 min with bath application and 100 sec with picospritzer ejection. ii) less desensitization effect, obviously as the consequence of (i); and iii) more importantly, it is more effective and capable of evoking more and wider variety of responses. However, the concentration of the test agent that is reaching the cell is unknown when using this method.
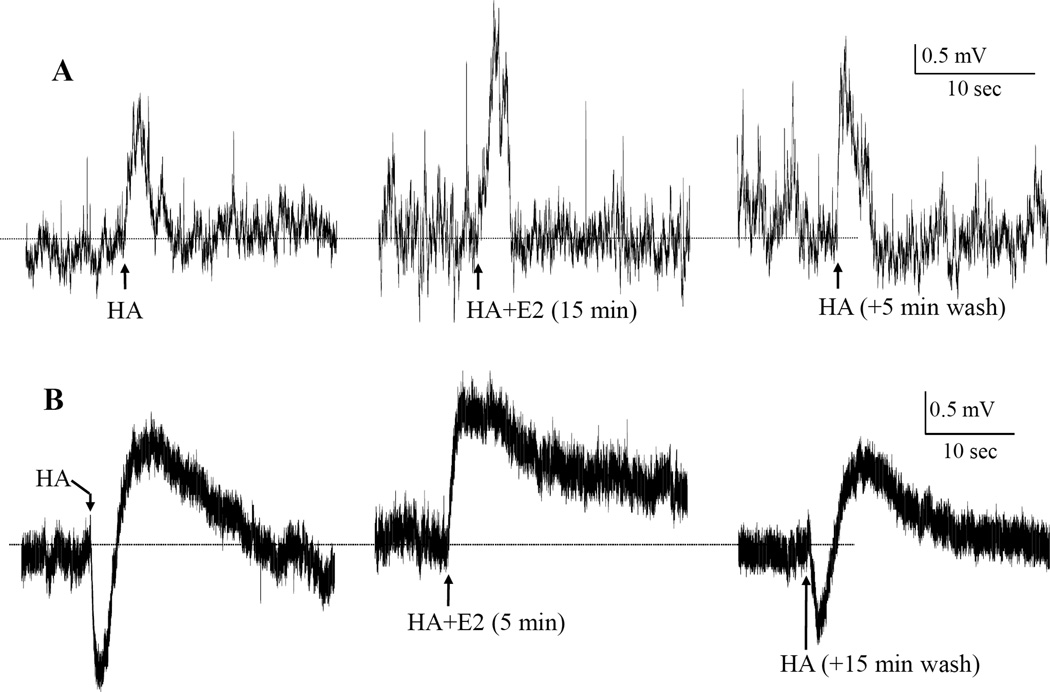
Fig. 2.
Examples of actions of picospritzed HA and the rapid E2 effects under “K+-only” condition. The figure shows two sets of 3 segments of continuous whole-cell recordings of membrane potential from two VMN neurons, A and B. The recordings were made in K+-only situation (A, in TTX plus Ca++-free; and B, Na+- and Ca++-free ACSFs; and K+-only internal solution). HA (10 mM in ejecting pipets containing respective K+-only ACSF) was picospritzed (for 2–3 sec) repetitively before, during and after acute bath application of E2 (50 nM). To facilitate comparisons, the horizontal dotted lines were drown aligning the Vm (A, −69.6 mV; B, −58.2 mV) at the point of each HA ejection. In A, HA depolarization was potentiated by E2 to 141%, from 2.44 to 3.45 mV. Neuron B responded biphasically to HA. The biphasic response was probably, as we have shown in a previous study [35], a manifestation of the colocalization of HA receptor subtypes H2/H3 and H1, which mediate hyper- and de-polarization, respectively. E2 rapidly potentiated the depolarization from 1.17 mV to 1.55 mV (or 132%) and abolished the hyperpolarization. Note, in B, the HA depolarization at HA+E2 sustained longer than those before and after E2 application. Also note that the membrane potential of each neuron remained stable despite the application of E2.
Throughout an experiment a test agent was applied several times before (Pre), 1–2 times during a conditional treatment (see below) (Dur) and several times after the treatment (Post or Recovery). The response (changes in Vm) of a neuron to repetitive applications in the Pre series were examined, and the experiment proceeded only when the responses were stable and consistent. Responses were observed continuously post-treatment until the responses stabilized (approximately same amplitude). Neurons that did not show recovery from a treatment effect were excluded from analyses. To define an “effect”, several neurons were challenged repetitively with HA, either by bath or spritzer and the random spontaneous variations in responses were recorded. In all neurons studied, this spontaneous variation in Vm was within ± 20% (Suppl. Fig. S1). Therefore, when an agent caused a Vm change that exceeded this range, it was regarded having an effect.
2.6. Specific Experimental procedures
2.6.1. Exploration of ionic mechanism(s) of HA depolarization
The mechanism was explored with the use of following ion channel blockers (concentration in ACSF): TTX (0.5 – 1.0 µM) for Na+; Cd++ (200 µM) or La+++ (100 µM) for Ca++; and tetraethylammomnium (TEA, 40 mM) or Cs+ (2 mM) or 4-Aminopyridine (4-AP, 5 mM) for K+ channels. In “K+-only” experiments three kinds of modified ACSF were employed: 1) Na+- and Ca++-free ACSF; 2) TTX plus Ca++-free ACSF; and 3) TTX-Cd-ACSF. The last one consisted of regular ACSF with TTX (0.5 – 1.0 µM) and Cd++ (200 µM) to block Na+ and Ca++ channels, respectively. The composition of the first solution was (in mM): Choline-Cl 126, choline-HCO3 26, KCl 5, KH2PO4 1.2, MgSO4 2, MgCl2 2, D-glucose 10. For the second, TTX was added to a modified ACSF, in which CaCl2 was replaced by MgSO4. The internal (pipet) solution for these experiments consisted of (in mM): K-gluconate 120, KCl 1, KHCO3 0.6, K2-ATP 2, MgCl2 2, Mg-ATP 2, Tris-GTP 0.3, HEPES-K 10, and EGTA 5. In experiments using Ca++-free ACSF, BAPTA (1,2-bis (2-aminophenoxy) ethane-N , N , N ‘, N ‘-tetraacetic acid, 11 mM) was added to the internal pipette solution to quickly chelate Ca++ released from internal stores. When adding channel blockers or other test agents to ACSF, an equal-molar amount of NaCl was replaced to maintain the osmolality.
To study the rapid action of estradiol (E2), ACSF containing E2 (10 or 50 nM), ERα agonist, PPT (4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol, 100 nM), and ERβ agonist, DPN [2,3-Bis-(4-hydroxy-phenyl)-propionitrile, 100 nM], or testosterone for control, was bath applied for up to 20 min after the base line response to repetitive applications of a test agent was established. Afterward, responses were observed for up to 30 min or longer to assess recovery from the treatment. With bath application, in which test agent responses were slow to occur and recover, the interval of test agent application was longer, 10 min, to avoid desensitization, and was applied either at 5 and 15 min or at 10 and 20 min after a hormone application. When using a picospritzer, HA or NMDA responses occurred and recovered much faster, thus HA was usually applied at an interval of 100 sec throughout the recording.
2.6.2. Current injection experiments
For studying the effects of HA and/or E2 on depolarization, a threshold current that could depolarize the neuron to evoke a minimum number (usually 1 or 2) AP was first determined and then used to inject the neuron repeatedly, initially at 15 sec interval for 2–3 min. If the responses were stable, this current was used throughout the experiment. The neuron was then giving HA or NMDA by picospritzer or less often through the bath and the repeated current injections continued at every 15 sec for 4 min or until the test agent’s effect, if ever, disappeared. If E2 was also to be studied, it was bath applied and the current injected every min for 10 min. This segment of testing is defined as “E2”. Immediately following E2 segment’s last current injection and with continued E2 application, the test agent was applied, again, and current injected at every 15 sec for 4 min thereafter. Finally, in the “Post”, both E2 and a test agent were washed away and the current was injected every minute until a recovery was observed.
2.7. Presentation of results and statistics
The average of the amplitudes of last two responses to a test agent before a treatment was regarded as the Pre value, and the largest change from Pre during a treatment was the During (Dur) value. Since the extent of recovery varied widely among neurons, it was observed but not included in the analyses. To determine whether a treatment is effective or to compare results across neurons, the Dur value was normalized as the percentage of the Pre value. Results are presented as Mean±SEM (n), where SEM is standard error of the mean and n is the number of the cases.
In the present study, each neuron served as its own control. Therefore, matched pair t test was used in most cases. In experiments involving both the slow bath application and the fast spritzing of test agents, the range of data is wide and skewed, and the variation large. In these cases the non-parametric Wilcoxon Matched-pairs Signed-ranks Test was used. A p-value <0.05, two-tailed, was regarded as significant.
3. RESULTS
3.1. Ionic mechanism of histamine depolarization
Parallel to our previous extracellular electrophysiological recording study’s finding of HA capable of evoking excitation, inhibition and biphasic responses [34], we found that HA could induce depolarization (Figs. 1A and 2A), hyperpolarization (Figs. 1B and 1C) and biphasic de- and hyper-polarization (Fig. 2B). Another previous neuropharmacological study showed that the HA depolarization is mediated by HA receptor subtype H1 (H1R), hyperpolarization by H2R and/or H3R, and biphasic response by the colocalization of H1R and H2R/H3R in the same neuron [35]. In that study, we also found that only the component of responses mediated by H1R was affected (potentiated) by acutely applied E2. Therefore, in the current study, we focused on the H1R-mediated HA depolarization.
Mechanism(s) potentially underlying HA (10 µM) depolarization were investigated using ion channel blockers. Application of Na+ channel blocker, TTX, at concentrations (0.5 – 1.0 µM) effective for suppressing inward currents had practically no effect on HA depolarization (Fig. 3A). Initially, calcium channels were blocked with cadmium (Cd++, 100 mM) and were found to severely attenuate, but not usually to abolish, HA depolarization (Fig. 3B). Since this result was not consistent with our findings in mice [38], lanthanum (La+++, 100 µM) was also employed with the same results (Fig. 3B). The involvement of K+ channels in HA-caused depolarization was first investigated with the wide-spectrum blocker, TEA (40 mM). Treatment with TEA attenuated (by 64.2%) but did not abolish HA depolarization. To also block TEA-resistant K+ channels, another wide-spectrum blocking approach, Cs+ (2 mM), and 4-AP (5 mM) that block fast acting channels, such as the A-current, were added to TEA. TTX (0.5 µM) was also added. With this combination, HA depolarization was essentially abolished (Fig. 3C). This result shows that K+ currents are essential for mediating HA depolarization. However, 4-AP combined with TTX had only a very small (changed from 100% to 96±19% (n=10) and inconsistent (6 inhibitions and 4 potentiations) effect. Hence, the fast-acting K+ currents may be excluded from further consideration for this study.
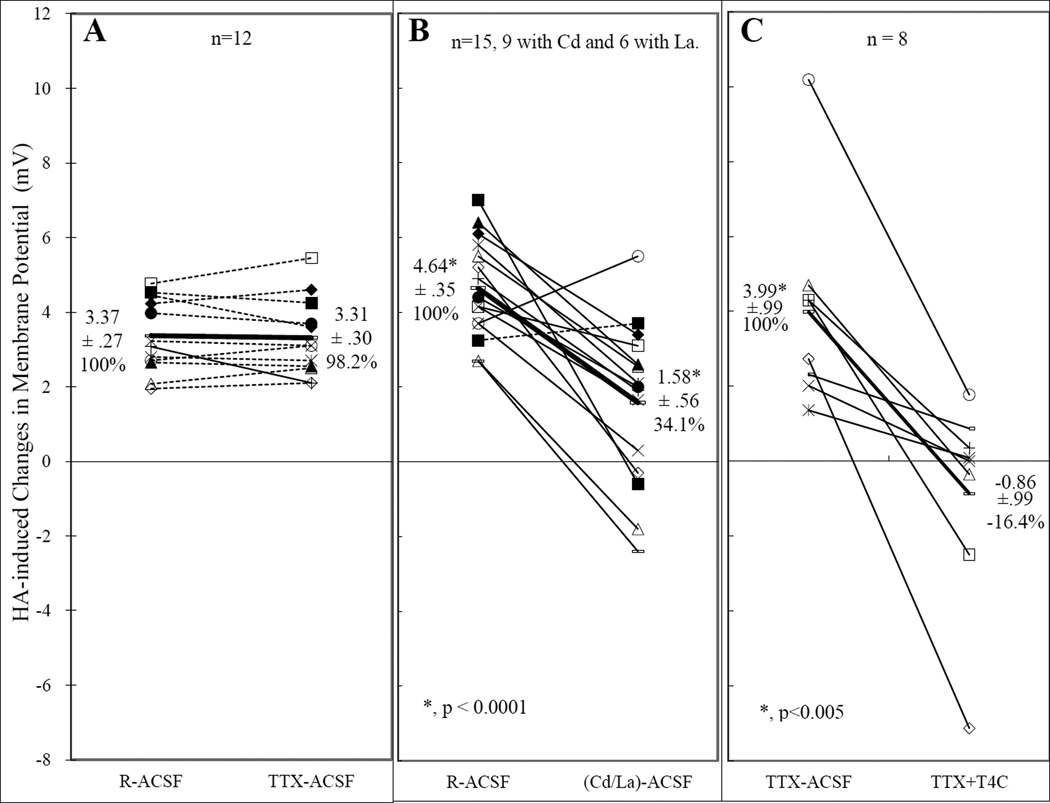
Fig. 3.
Effects of Na+ (A), Ca++ (B) and K+ (C) channel blockers on responses of VMN neurons to histamine (HA). Each line connecting a pair of identical symbols denotes the change in HA induced responses (changes in membrane potential, Vm) measured from a single neuron in control solution and in solution containing test channel blockers. Solid lines indicate that the test blockers caused a change beyond ±20% of the control (R-ACSF) and is regarded as having an effect. Dotted lines indicate that the changes were within ±20% of the control. The thick lines are the averages, with the values (mean ±SEM and % over the control) listed next to them. In A, all lines, except the thick one, are dotted, indicating that TTX had no effect on HA depolarization. In B, Ca++ channel blockers reduced HA depolarization in 13 neurons and converted 4 of them from depolarization into hyperpolarization. However, one neuron was not affected and another was even enhanced. Neurons in C were initially bath in TTX-ACSF (ACSF containing 0.5 µM of TTX) and then switched to TTX+T4C, ACSF containing TTX and a combination of K+ channel blockers, including tetraethylammonium (TEA, 40 mM), 4-aminopyridine (4-AP, 5 mM) and Cs+ (2 mM). This combination reduced HA depolarization in all of the neurons tested and virtually abolished the depolarization of this group of neurons.
The result of the three K+ channel blockers combined with TTX also indicate that the remaining Ca++ currents are not sufficient to mediate HA depolarization. Their involvement in the depolarization is most likely an indirect one, such as inactivation of Ca++-dependent K+ channels.
To determine whether K+ currents are also sufficient to support HA depolarization, 12 neurons were tested in a “K+-only” environment, where K+ was the only permeable cation in both internal and external solutions (see Methods). HA depolarized 9 neurons and induced 3 biphasic responses in 3 VMN neurons (Table 1, where, for analyses, each biphasic neuron was considered as two with opposite responses). Examples for HA actions under this condition are illustrated in Fig. 2. Thus, K+ currents are both necessary and sufficient for HA depolarization.
Table 1.
Effects of E2 (estradiol), PPT (ERa agonist), DPN (ERβ agonist), testosterone (Ttt) or vehicle on responses of VMN neurons to HA (histamine) or NMDA (N-Methyl-D-aspartate) administered by bath application (Bath) or by spritzer.
| E2 or related agents |
Transmitter Agents | n | HA or NMDA induced changes in Vm (mV) at: | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Agent | Applied by |
Responsea | Before hormone |
+ Hormone | |||||
| Effectb | % over Pre |
p valuec |
|||||||
| E2 (10 nM) |
HA | Bath | DP | 10 | 1.91 ± .27 | 3.32 ± .43 | ↑ | 181 ± 13 | < .001 |
| 9 | 4.2 ± .84 | 4.43 ± .99 | ↔ | 106 ± 2.8 |
ns | ||||
| 1 | 3.1 | 1.95 | ↓ | 41 | n/a | ||||
| HP | 3 | −4.8 ± 2.58 | 2.55 ± .74 | ← | 189 ± 43 | <0.05d | |||
| 4 | −3.99 ± .27 | −2.03 ± .38 | ↓ | 61 ± 9 | <0.05 | ||||
| 4 | −0.7 ± .32 | −0.7 ± .37 | ↔ | 104 ± 4 | ns | ||||
| NR | 5 | ∼0 | ∼0 | ↔ | ∼100 | n/a | |||
| E2 (10–50 nM) |
Spritzer | DP | 10 | 38.8 ± 28.1 | 53.5 ± 34.1 | ↑ | 197 ± 21 | <0.05 | |
| DP, HP | 15 | 34.6 ± 12.4 | 35.0 ± 12.7 | ↔ | 103 ± 2 | ns | |||
| HP | 2 | −2.2 ± 0.5 | −1.4 ± 0.5 | ↓ | 60 ± 7 | <0.05 | |||
| Spritzer K+-only | DP | 8 | 1.25±0.20 | 1.98±0.31 | ↑ | 170 ±18 | <0.005 | ||
| DP | 2 | 2.32±1.08 | 2.12±0.75 | ↔ | 98 ±13 | ns | |||
| DP | 2 | 0.49±0.06 | 0.24±0.00 | ↓ | 49 ±6 | ns | |||
| HP | 3 | −1.30±0.05 | −0.23±0.23 | ↓ | 17 ±17 | <0.03 | |||
| PPT (100 nM) |
Spritzer | DP | 8 | 4.46 ± 0.69 | 6.2 ± 0.66 | ↑ | 159 ± 17 | <0.05e | |
| DP | 1 | 5.11 | 6.29 | ↔ | 113 | n/a | |||
| DPN (100 nM) |
Spritzer | DP | 3 | 8.7 ± 1.34 | 13.7 ± 2.61 | ↑ | 158 ± 35 | ns | |
| DP | 7 | 6.45 ± 1.32 | 7.48 ± 1.94 | ↔ | 107 ± 1 | ns | |||
| DP | 2 | 5.5 ± 0.03 | 3.3 ± 0.16 | ↓ | 74 ± 4 | ns | |||
| Ttt (10nM) |
Bath | DP, HP | 6 | 0.5 ± .31 | 0.57 ± .37 | ↔ | 102 ± 4 | ns | |
| E2 (10–50 nM) |
NMDA | Bath | DP | 21 | 7.15 ± 0.83 | 10.8 ± 1.2 | ↑ | 138 ± 11 | <0.001 |
| DP | 19 | 13.8 ± 1.6 | 12.7 ± 1.7 | ↔ | 96 ± 4 | ns | |||
| Spritzer | DP | 2 | 6.15 ± 2.45 | 8.40 ± 3.80 | ↑ | 133 ± 9 | n/a | ||
| DP | 5 | 12.1 ± 2.0 | 12.2 ± 2.3 | ↔ | 99 ± 4 | ns | |||
| Vehicle | Bath | DP | 7 | 13.4 ± 2.5 | 13.1 ± 2.6 | ↔ | 99 ± 5 | ns | |
DP stands for depolarization; HP for hyperpolarization; and NR for no response.
↑, potentiation; ↔, no effect; ↓, attenuation; and ←, reversal.
matched t test; 2-tailed unless otherwise indicated.
one-tailed
ANOVA
3.2. HA actions on VMN neurons
A logical extension of the section above is that HA-caused depolarization is due, mainly, to the inhibition of K+ currents. This inference can also be tested by examining the effect of HA on depolarization induced by current injection. A neuron was injected repeatedly (see Methods, Current injection experiments) with a predetermined threshold current (10 to 50 pA) just sufficient to depolarize the neuron to evoke a minimum number (1 or 2, usually) of AP (Fig. 4, Pre). In 7 of the 9 neurons examined, HA application, either through the bath (n=2) or by picospritzer (n=5), induced changes in response to current injection. These changes are illustrated in Fig. 4. Most obvious are the increases in the number of APs and shortening of the latency (the time from the point of current injection to the peak of the first AP). AP numbers increased from one before, to 3 soon after, and then back to one, again, much later after HA application (Fig. 4, traces Pre, HA and Rcv, respectively). The occurrence of the first AP was advanced by HA and then fell back after recovery (Fig. 4). This is more clearly illustrated by expanding the initial segments and superimposing them - - aligned by the point of current injection - -as shown in Fig. 5, in which the HA effects are defined.
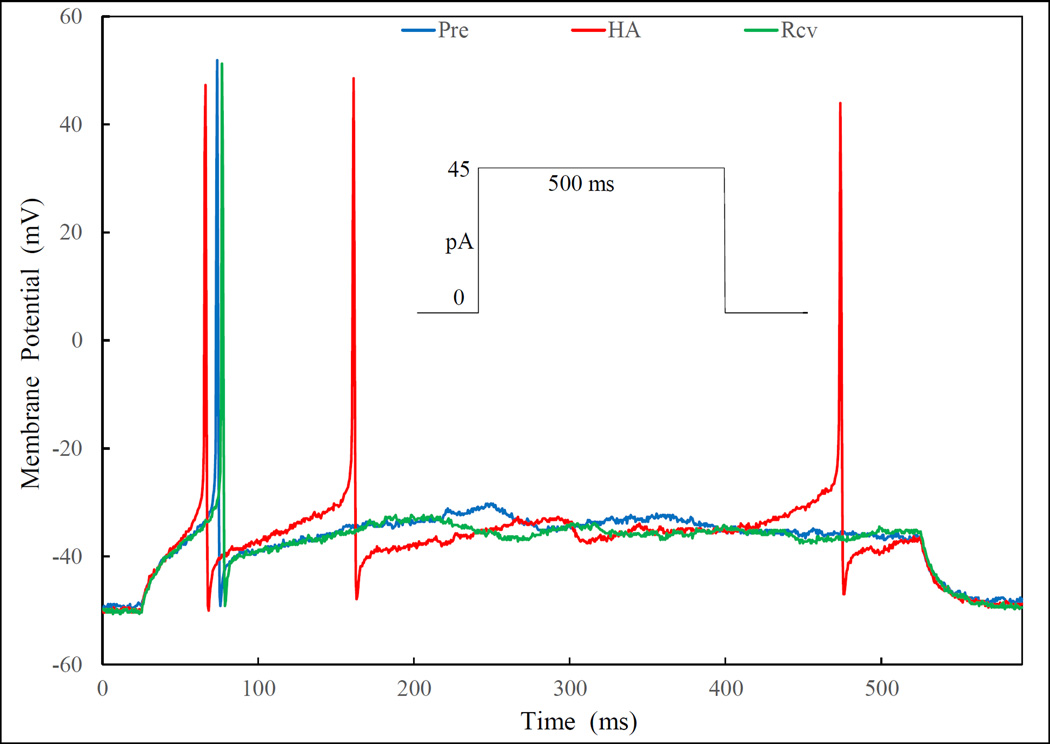
Fig. 4.
Histamine (HA) enhances the depolarization induced by current injection. Superimposed traces are depolarizations of the neuron injected with a “threshold” current of 45 pA (see protocol in the inset) before (Pre, with one AP), peak response after HA application (HA, with three APs) and recovery following wash out (Rcv, with one AP). In addition to increasing number of AP, HA also shortened the latency to the first APs and increase the rate of depolarization. Effects on “relative depolarization” and AP threshold are illustrated in Fig. 5.
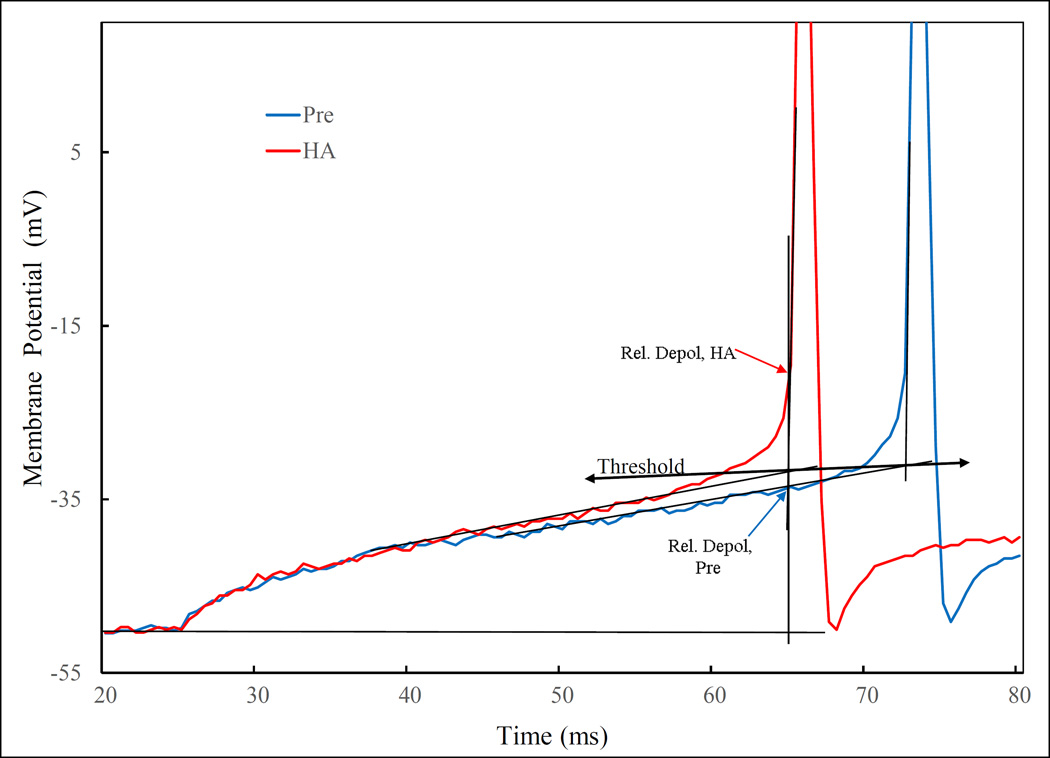
Fig. 5.
Definition of some of effects of HA on current injection induced depolarization. Superimposition of the initial segment of Pre and HA traces from Fig. 4 aligned by the current injection point. The relative depolarization is defined with the thin vertical line through the point of the upswing of the first AP. The net voltage difference between the holding potential (Vh, denoted by the thin horizontal line) and intersection between the vertical line and Pre or HA trace are relative depolarization for the respective traces. For this neuron, the relative depolarization is approximately doubled by HA. Threshold for AP is defined by the intersection lines drown along the depolarization slope and the rising slope of the AP. There is a slight, non-significant lowering of the threshold from Pre to HA in this neuron.
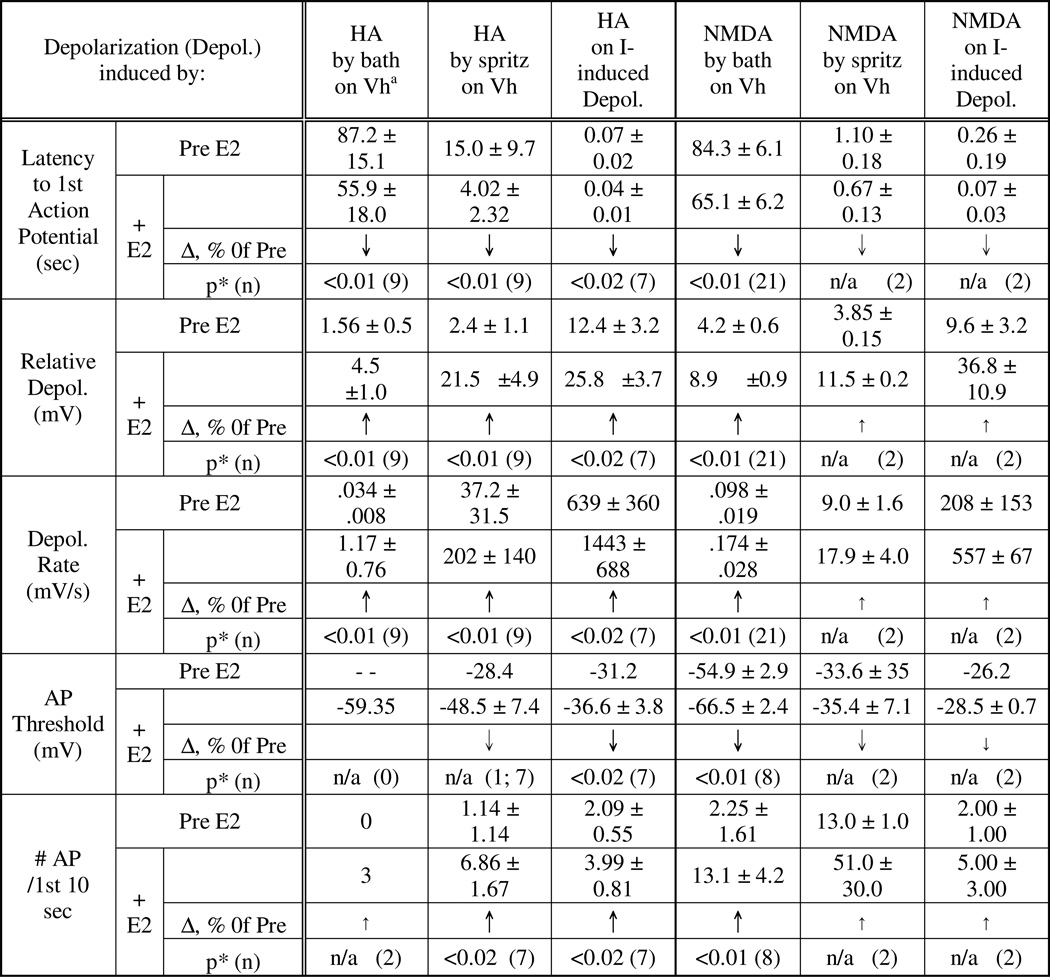
Comparison of “Pre” with “HA” revealed the following HA effects (Fig. 5): (i.) increased ‘relative depolarization’, in which ; (ii.) accelerated depolarization rate, a measure of the slope in Figure 5 which also contributes to measure (i.); (iii.) increase in the number of APs; (iv.) shortened latency to the first AP; and (v.) a lowering of AP threshold. These are summarized in Table 2. As will be discussed below, all of these HA effects can be explained by an enhancement of the inhibition of K+ currents.
Table 2.
Effects of HA (histamine) and NMDA (N-Methyl-D-Aspartate) on current injection-induced depolarization
| Test Agent | HA | NMDA | ||
|---|---|---|---|---|
| Latency to 1st AP or Peak (ms) |
Pre Agent | 174 ± 77 | 102 ± 26 | |
| + Agent |
102 ± 66 | 50 ± 9 | ||
| Δ, % of Pre | ↓ | ↓ | ||
| p* (n) | <0.02a (7) |
<0.05 (10) | ||
| Relative Depol. (mV) |
Pre Agent | 11.3 ± 4.2 |
20.9 ± 2.6 | |
| + Agent |
18.5 ± 5.9 | 33.7 ± 4.2 | ||
| Δ, % of Pre | ↑ | ↑ | ||
| p* (n) | <0.02 (7) | <0.001 (10) |
||
| Depolarization Rate (mV/s) |
Pre Agent | 417 ± 189 |
400 ± 125 | |
| + Agent |
823 ± 294 |
942 ± 182 | ||
| Δ, % of Pre | ↑ | ↑ | ||
| p* (n) | <0.05 (7) | <0.001 (10) |
||
| AP Threshold (mV) |
Pre Agent | −28.5 ± 3.9 |
−31.7 ± 2.4 |
|
| + Agent |
−30.6 ± 3.5 |
−29.0 ± 7.2 |
||
| Δ, % of Pre | ↓ | ↓ | ||
| pa (n) | <0.02a (7) |
<0.01 (10) | ||
| # of A P /10 sec after 1st AP |
Pre Agent | 2.43 ± 0.61 |
8.9 ± 3.6 | |
| + Agent |
3.86 ± 0.70 |
13.8 ± 3.9 | ||
| Δ, % of Pre | ↑ | ↑ | ||
| p* (n) | <0.02 (7) | <0.02 (10) | ||
Matched-pair t test, 2-tailed.
Wilcoxon Match-pairs Signed-ranks Test, 2-tailed.
3.3. Effects of acutely applied E2 on responses of VMN neurons to HA
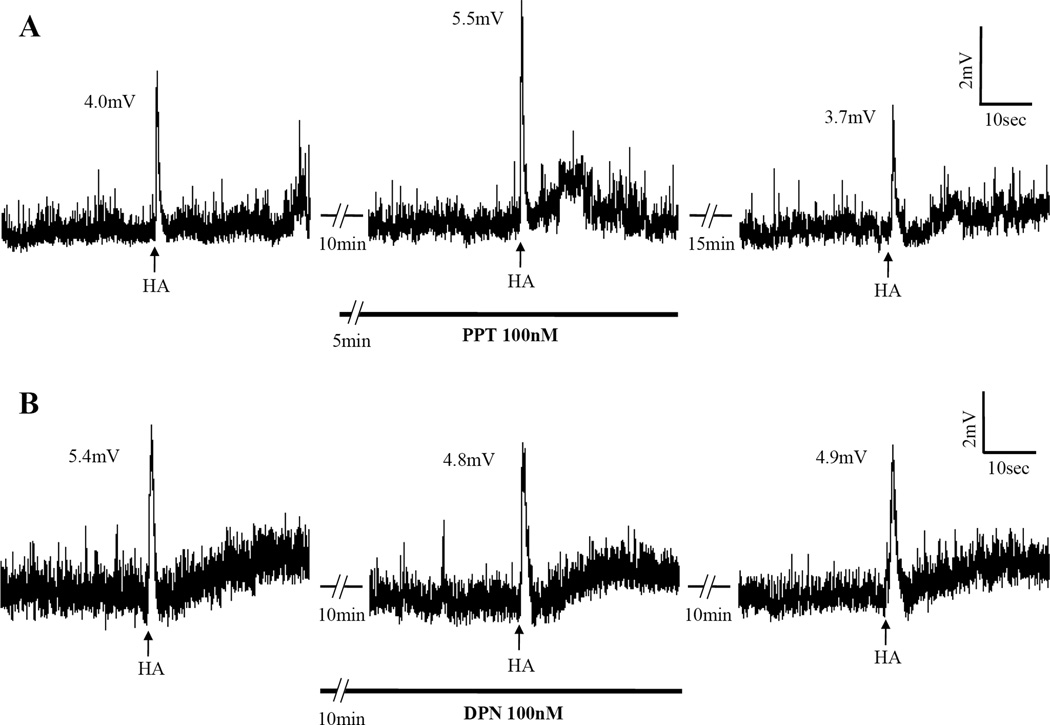
Acutely applied E2 can rapidly potentiate depolarization (Fig. 1A and 2A) or abolish (Fig. 1B and 2B) or even reverse the hyperpolarization (Fig. 1C) induced by HA, applied either through the bath or by picospritz (Table 1). These rapid E2 actions are estrogen-specific in that neither testosterone (Ttt), another hormonal steroid, nor vehicle in experiments with NMDA had any effect (Table 1). This specificity is further confirmed by the experiments using estrogen agonists PPT for estrogen receptor subtype α (ERα); and DPN for ERβ. As summarized in Table 1, PPT acted like E2 to potentiate depolarization (Fig. 6A) in 8 out of 9 neurons, whereas DPN had no significant effect (Fig. 6B). We infer that the rapid potentiating action of E2 is mediated via ERα.
Fig. 6.
Examples of depolarization induced by picospritzed HA and the effect of ERα agonist, PPT, and the lack of an effect of ERβ agonist, DPN. A, PPT potentiated the depolarization induced by HA. B, DPN had no effect on a separate neuron. Each series of traces are segments of a continuous recording from a neuron with gaps denoted by dashed lines. Arrows below the traces indicate the time points when HA were picospritzed. The number (in mV) near the peak of responses indicates the extent of depolarization induced by HA. PPT and DPN were applied through the bath.
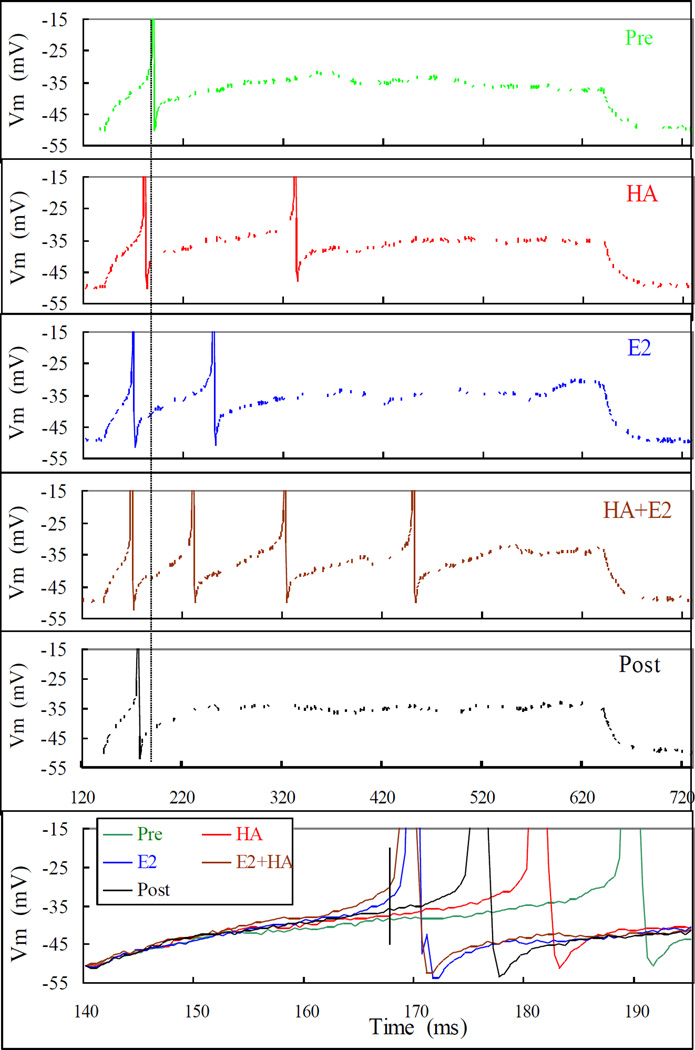
An interesting aspect of this rapid E2 action is its similarity to HA’s depolarizing action. First, as for HA-induced depolarization, E2 can also achieve its rapid potentiation of the HA depolarization in “K+-only” environment (Fig. 2 and Table 1), indicating that both HA depolarization and its potentiation by E2 need only the modulation of K+ currents. Second, as exemplified in Fig. 7 and summarized in Table 3, we found that, like HA, E2-enhancements of both current injection-induced as well as HA induced depolarization also have the same 5 characteristics (see above). To compare the depolarizing actions of HA and E2, their effects on the depolarization induced by the repeated injections of a threshold depolarizing current were observed. Before any application, the neuron usually depolarized to produce approximately 1 or 2 AP (Fig.7, Pre). Application of HA, as expected from experiments above, produced a transient peak HA response (Fig. 7, HA). After the neuron recovered from HA action, E2 (10–50 nM) was applied through the bath. E2 alone also enhanced depolarization and that was invariably greater than that by HA (Fig. 7, E2). Once an E2 effect was detected HA was administered again. After the peak of HA action was observed (Fig. 7, HA+E2), E2 and HA were washed out to observe the Recovery (Fig. 7, Post). In Fig. 7, the successive increases in AP number and advances of the latency (see vertical line) by HA, E2 and HA+E2, and subsequent recovery upon wash out were clearly discernable in Fig. 7 (bottom panel, which illustrates the superimposed and expanded initial segment of each trace aligned by the point of current injection). The lowering of AP threshold that is not obvious in Fig. 7, is illustrated with another example in Fig. 8. In this example, the neuron was depolarized by repeated application of HA by picospritzer without firing an AP. Administration of E2 quickly enhanced the HA-induced depolarization to evoke an AP and then a train of APs. In Fig. 8’s insets, one can see that, during E2 exposure, the neuron fired an AP at a membrane potential well below the peak depolarizations induced by the same HA applications before E2 administration. The increases in depolarization rate and AP number are also obvious.
Fig. 7.
Rapid actions of E2 and HA on depolarization induced by current injection. The neuron was depolarized by injecting 50 pA for 500 ms repetitively under different treatments. The Pre trace shows the effect of the threshold (for action potential evocation) current injection alone. The HA trace was recorded within one minute of an HA picospritz (50 mM in ejecting pipette, for 1 sec). Trace E2 was recorded 9 min after HA picospritz and 4 min after bath application of E2 (50 nM). HA+E2 was recorded after more than 7 min of continuous E2 application and less than 2 min of a second HA picospritz. Post was observed 6 and 12 min after washing out of E2 and HA, respectively. Note, although both HA and E2 evoked 2 action potentials (APs), under E2 they appeared sooner and the inter-spike interval shorter. The long vertical line across the top 5 panels is positioned to highlight the shortening of the latency, while the short vertical line in the bottom panel facilitates the comparison of relative depolarization defined in Fig. 5. The differences in latency and relative depolarization are also better illustrated in the bottom panel, where the initial portions recorded under all conditions were expanded, aligned and superimposed. Note, the relative depolarization is highest for E2+HA followed by E2, Post, HA and lastly, Pre. Note also that E2 effect was far greater (higher relative depolarization and shorter latency) than that of HA. Likewise, Post is also greater than Pre, indicating that the recovery was incomplete at this time point. The threshold for action potential under each treatment was measured as defined in Fig. 5, and was lowered (in mV) successively from −27.1 to −28.1, −32.3, −31.4 and back to −29.6 at Pre, HA, E2, E2+HAand Post, respectively.
Table 3.
Similarities in the characteristics of rapid estradiol (E2) actions on the depolarizations evoked by HA (histamine) and by NMDA (N-Methyl-D-Aspartate)
Wilcoxon Matched-pairs Signed-ranks Test, 2-tailed. Significant changes are indicated by bold faced arrows (↑, ↓). Thinner arrows (↑, ↓) indicate non-significant trends.
Vh = holding potential.
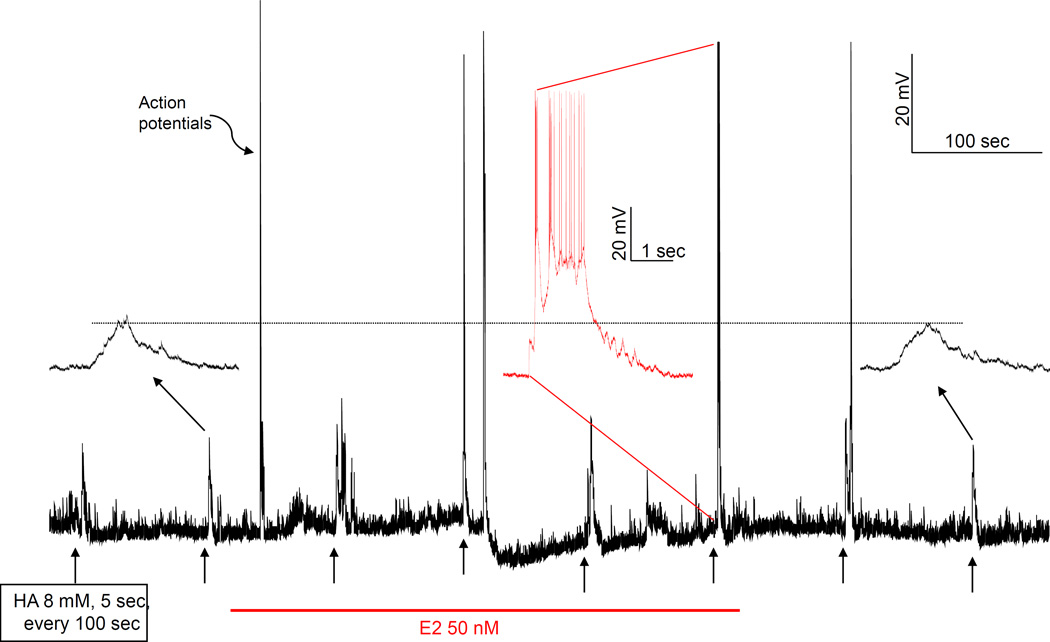
Fig. 8.
Examples of rapid E2 potentiation of depolarization induced by picospritzed HA. The trace is a continuous monitoring of the membrane potential of a VMN neuron. HA (8 mM in the ejecting pipet) was picospritzed for 5 sec every 100 sec, as indicated by short arrows below the trace. E2 was administered soon after the second HA application. In the first HA response after E2 administration, there was more activity but no action potential. By the second stimulation after E2, HA triggered action potentials and even a depolarization block that affected the next response. The second, sixth and eighth responses (indicated by longer arrows) to HA were expanded and their baseline aligned. It is clear from these insets that E2 accelerated the rate of depolarization by HA. The dashed line shows that the depolarizations induced by second and eighth HA applications were actually more depolarized than the threshold that triggered action potential in the sixth response, indicating that acute E2 lowered the threshold. Note, the neuron recovered from E2 influence gradually soon after the termination of E2 administration. Note also that E2 had no significant effect on baseline membrane potential, except for the depolarization blockade.
These illustrations are supported by the results summarized in Table 3. In addition to neurons with depolarization induced by current injection (as the one illustrated in Fig. 7), Table 3 also includes neurons depolarized directly by bath-applied or picospritzed HA (as that in Fig. 8).
3.4. Characteristics of NMDA depolarization – similar to those of HA
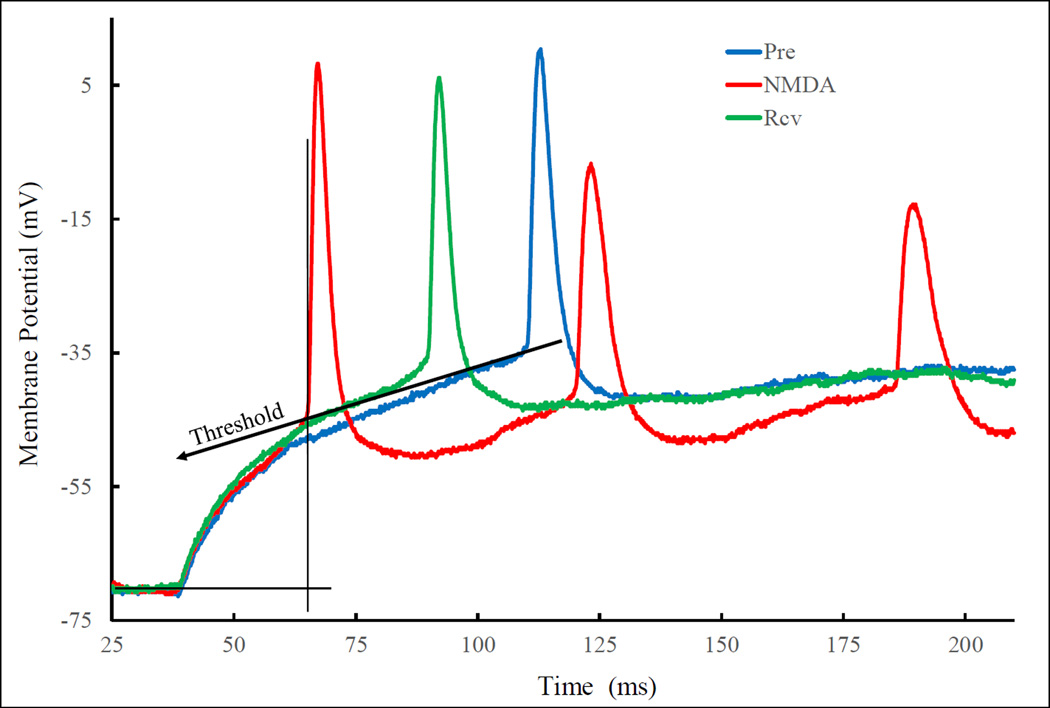
As the most common excitatory neurotransmitter in the brain, it is expected that NMDA would depolarize VMN neurons. Indeed, NMDA whenever effective, evoked only depolarizations in this study. But like HA, NMDA’s evocation of depolarization also had five characteristics. These characteristics are illustrated with Fig. 9, where one can see NMDA: (i) reduced the latency to AP; (ii) increased AP number; (iii) increased relative depolarization; (iv.) increased depolarization rate; and (v.) lowered AP threshold. Results observed from ten neurons are summarized in Table 2. These characteristics are qualitatively the same as those for HA.
Fig. 9.
Characteristics of the depolarizing effects of NMDA on current injection induced depolarization. Superimposition of traces from before (Pre), after (NMDA) and recovery (Rcv) from NMDA application. Similar protocol for that shown in Fig. 4 was employed. For illustration clarity, only parts of the traces are shown. On this neuron, NMDA clearly lowered AP threshold, as defined in Fig. 5, and increased relative depolarization slightly.
3.5. Acutely administered E2 can also potentiate NMDA depolarization
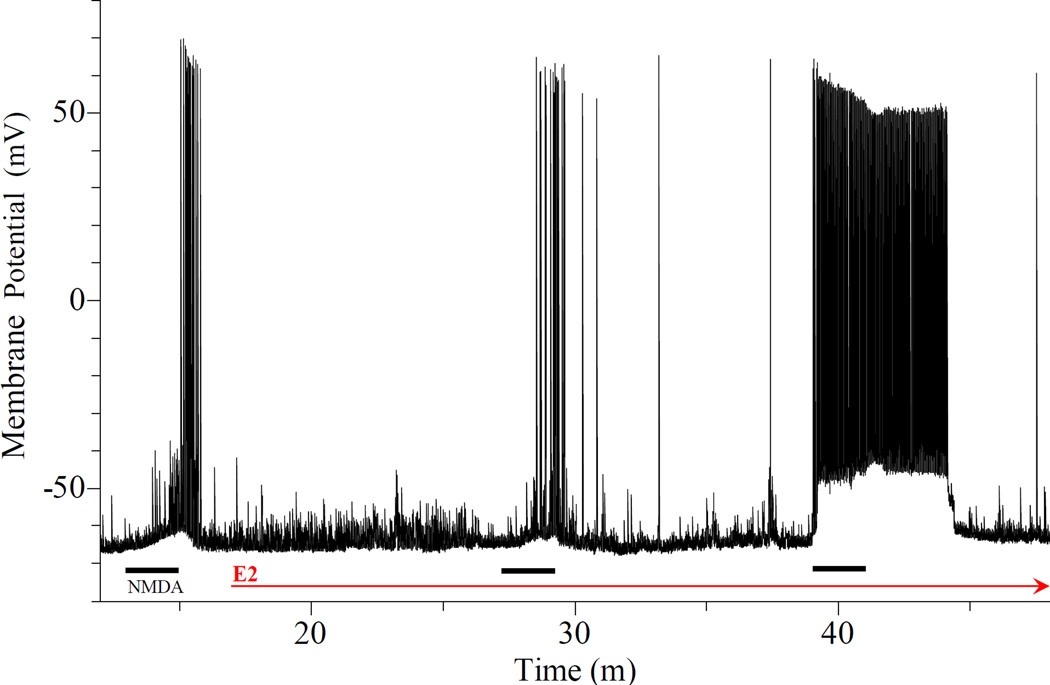
Estradiol can rapidly potentiate the depolarization induced directly by bath-applied NMDA (Fig. 10), over that induced by current injection (Fig. 11) and by picospritzed NMDA applied at a holding potential Vh. The results from all the neurons observed are summarized in Table 1. NMDA-caused depolarizations are not affected by vehicle (Table 1). Table 1 also shows that the proportions of neurons affected by the rapid E2 action are similar whether the neurons were stimulated by HA or NMDA.
Fig. 10.
Rapid E2 potentiation of the depolarization induced by bath applied NMDA. Three bath applied NMDA (15 µM for 2 min) are indicated by thick bars below the trace. The thin arrow indicate the time of E2 administration. Rapid E2 action increased with time. It was not obvious, except for prolonged spiking, 10 min after E2. But at 20 min, NMDA depolarization was greatly potentiated. The decrease in latency (from the beginning of NMDA application to the occurrence of first AP) and increases in AP firing, relative depolarization and depolarization rate are obvious. Upon expansion (not shown), the lowering of AP threshold is also apparent. Also note that the baseline membrane potential was stable over approximately 30 min of E2 exposure while responses to NMDA were greatly potentiated.
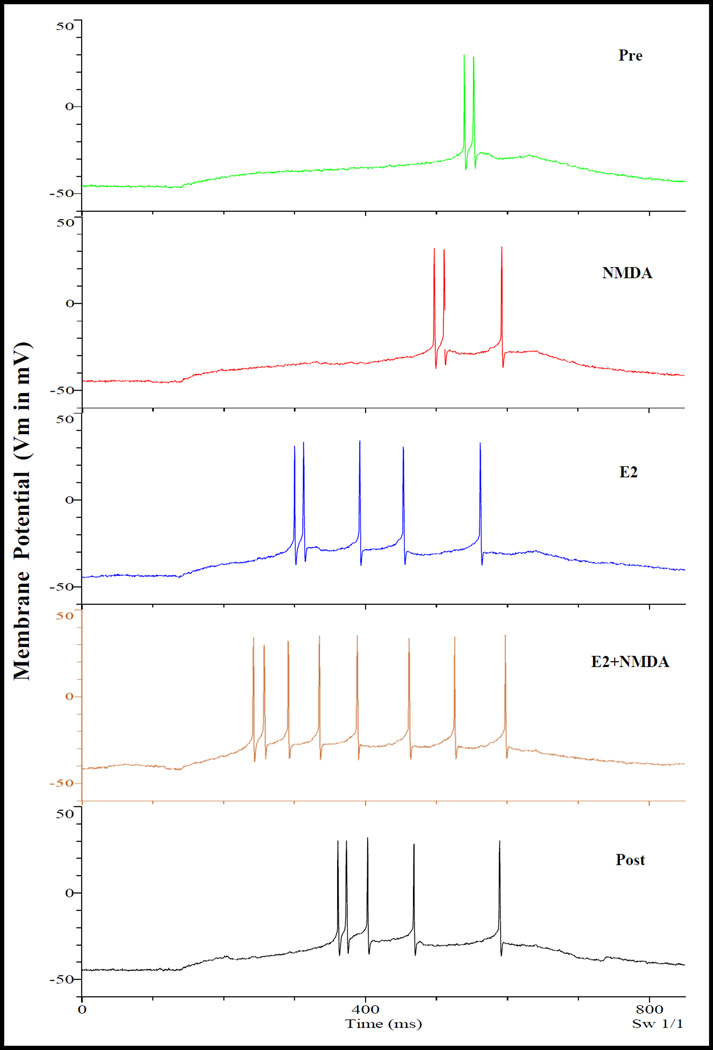
Fig. 11.
Rapid E2 potentiation of depolarizations induced by current injection and by NMDA. Same experimental procedure as that shown in Fig. 7, except that NMDA was used in place of HA. The results are qualitatively identical to those illustrated in Fig. 7. That is, NMDA, E2 alone and E2 together with NMDA caused successively greater potentiation of current injection induced depolarization that include: 1) shortening of latency; 2) increase in number of AP; 3) increase in relative depolarization; and 4) increase in depolarization rate. Measurements also revealed a lowering of AP threshold.
3.6. Characteristics of rapid E2 potentiation of NMDA depolarization
As illustrated in Fig. 11 and summarized in Table 3, E2 affects NMDA depolarization with the same characteristics as it affects HA depolarization. That is, E2 can: 1) shorten the AP latency; 2) increase the number of AP; 3) increase relative depolarization; 4) increase depolarization rate; and 5) lower AP threshold (Fig. 11 and Table 2).
4. DISCUSSION
Using whole-cell patch recording under current clamp, we obtained results indicating that both HA and NMDA depolarize VMN neurons by inhibiting K+ currents, and that enhancement of this inhibition appeared to be a common mechanism by which E2 rapidly potentiates depolarizations induced by both HA and NMDA.
4.1. Ionic mechanism of HA and NMDA-induced depolarizations
Pharmacological analyses using ion channel blockers show that blockade of practically all K+ channels with a combination of TEA, Cs and 4-AP virtually abolished HA (evoked) depolarization (Fig. 3C). Furthermore, HA could still depolarize VMN neurons under the “K+-only” condition (Fig. 2). These results clearly indicate that K+ currents are both necessary and sufficient for HA depolarization.
In contrast, TTX-sensitive Na+ currents are not involved in HA depolarization (Fig. 3A), and while Ca++ currents may be involved indirectly (Fig. 3B), they are not capable of mediating HA depolarization (Fig. 3C). With both Na+ and Ca++ currents excluded, HA depolarization appears to rely solely on change in K+ currents. With rare exceptions, the change in K+ current that would lead to depolarization is a reduction of the current. The rare exceptions are those K+ currents, such as HCN or H currents [39], whose activation induces depolarization, rather than inhibition. However, these currents are activated by hyperpolarization, rather than depolarization [39]. Since HA depolarization mediated via H1R is a pure depolarization [35], such hyperpolarization-activated channels can be excluded. Therefore, it is safe to conclude from our analyses that HA depolarization is due to inhibition of K+ currents alone.
The above conclusion can be derived from our findings that HA increased the relative depolarization and accelerated the depolarization induced by current injection (Fig. 4 and Fig. 5, and Table 2). It is understood that the cell membrane is not an electrically linear substrate, and Ohm’s law cannot be applied strictly, that is, it cannot be used for precise numerical calculation. However, the relationship between membrane currents, resistance and potential should still apply. Since the HA effects were observed with repeated injections of a constant current, the increase in depolarization must be, according to the principle of Ohm’s law, due to an increase in membrane resistance, which in turn, is due to closing of certain ion channels. The most likely targets are K+ channels, because closing of these channels would cause depolarization, and because K+ currents are both essential and sufficient for HA action. It appears, therefore, HA induces depolarization by inhibiting K+ channels, i.e., reducing their numbers or average conductance.
Our conclusion is consistent with reports by others that HA induces depolarization by inhibiting Kca channels [40–47], a leak current [38, 48–51], M-current [52], voltage-gated [53], inward rectified [54] and some unspecified K+ currents [55, 56].
No further pharmacological analysis was carried out on NMDA actions. However, NMDA evokes only depolarization, and its depolarizing actions are identical to those caused by HA (Figs. 4 and 5 vs 9; Table 2). Therefore, the same arguments used in the case of HA also apply to NMDA. Hence, it is reasonable to conclude that, like HA, NMDA induces depolarization by inhibiting K+ currents.
4.2. Mechanism of rapid E2 potentiation of HA and NMDA depolarization
In a previous extracellular recording study we found that both HA and NMDA could evoke excitations and both types of excitation could be independently potentiated by E2 [34]. Following on from this study, we now find that both HA (Figs. 1A, 2A, and 4–8) and NMDA (Figs. 9, 10 and 11) could also evoke depolarization in VMN neurons, and both HA (Fig. 1A, 2A, 7 and 8; Table 1) and NMDA depolarization (Figs. 10 and 11; Table 1) could also be potentiated by E2.
Since E2 potentiates HA and NMDA depolarizations, and since these transmitter agents depolarize VMN neurons by inhibiting K+ currents, E2 may simply enhance the agents’ inhibition of K+ currents. If this were true, one would expect that the characteristics of the rapid E2 potentiation of HA and NMDA depolarization would be similar to those of HA and NMDA, alone, in depolarizing VMN neurons. Indeed, these are revealed for HA by comparing the illustrations in Figs. 4 and 5 to that in Fig. 7, and for NMDA comparing Figs. 9 to 11. These comparisons and the more thorough comparison of results in Table 2 and Table 3 show that rapid E2, like HA and NMDA, also: (i.) shortens the latency; (ii.) increases relative depolarization; (iii.) accelerates depolarization rate; (iv.) lowers AP threshold; and (v.) increases the numbers of AP evoked. The similarities between E2 characteristics and those of E2’s targets, HA and NMDA, suggest that one mechanism – inhibition of K+ currents – was employed. As well, we have earlier found that E2 by itself can rapidly inhibit voltage ramp induced depolarization [37].
The above suggestion is supported by two other findings. First, by simply enhancing the inhibition of K+ currents, as suggested above, E2 potentiation of HA and NMDA depolarization requires K+ currents but not Na+ or Ca++ currents. Indeed, acute E2 could still potentiate HA depolarization under the “K+-only” condition, where K+ is the only permeable cation inside and outside the neuron (Fig. 2 and Table 1). Second, E2 by itself does not inhibit K+ currents. If E2 by itself could inhibit K+ currents then the resting or the holding firing rate or membrane potential would be increased or depolarized, respectively. This has never been observed. In fact, application of E2 had no effect on base line firing rate seen in the previous extracellular study [34] or the resting or holding membrane potential in the present study, at least to the point when E2 potentiation was observed or even recovered. For example, see the Vh indicated by the dotted lines in Fig. 2, the relatively stable Vh in Figs. 8 and 10. Thus, the rapid potentiation of HA and NMDA depolarization by E2 is not a convergent effect of a separate E2 mechanism with the inhibition of K+ currents by the transmitter agents, or the summation two types of K+ current inhibitions. E2 simply enhances the agents’ induced inhibition of K+ currents.
4.3. Enhancement of inhibition of K+ currents can be a common mechanism for rapid E2 potentiation of HA and NMDA depolarizations
In a previous study [34], acutely applied E2 was capable of three modulatory actions on VMN neurons: potentiation of HA (evoked) excitation, abbreviation of HA inhibition and, independently, potentiation of NMDA excitation. Since HA excitation (depolarization) and inhibition (hyperpolarization) are mediated by different HA receptor subtypes coupling to G-proteins with opposite functions [35], and since NMDA action is mediated by ligand gated ion channel [33], E2 actions appeared to utilize three separate mechanisms. However, in a pharmacological study we found that HA hyperpolarization, which would lead to inhibition, was mediated by HA receptor subtypes H2 and/or H3 (H2R and/or H3R, respectively), and furthermore, HA responses mediated by these receptors were insensitive to E2 [35]. The abbreviation of HA inhibition we found with extracellular recording [34] was most likely due to the neutralization by the excitation potentiated by E2, because HA excitation/depolarization potentiated by E2 is mediated by H1R that can co-localize with H2R/H3R in individual VMN neurons [35]. Therefore, one of the three mechanisms for the rapid E2 action can be eliminated.
As discussed above, using whole-cell patch clamp we now find that E2 rapidly potentiates both HA and NMDA depolarizations by enhancing the inhibition of K+ currents induced by the transmitter agents themselves. What’s surprising is that the characteristics of the rapid E2 actions on HA and NMDA depolarizations are virtually identical (see Table 3). This is true whether the agents were applied through the bath, by picospritzer or on current induced depolarization (Table 3), but is most obvious in the last case, as can be seen by comparing Figs. 7 and 11. This similarity strongly indicates that the enhancement of the inhibition of K+ currents is a common mechanism by which E2 rapidly potentiates depolarizations induced by HA and NMDA.
What’s even more striking is the fact that HA and NMDA depolarizations are mediated by distinctively differently types of receptors, G-protein coupled receptor and ligand-gated ion channel, respectively. This brings up the interesting and important question of how E2 potentiates differently derived depolarizations with a common mechanism. For this question it is relevant to point out our findings of the close similarities, this time, between the characteristics of E2 actions with those of HA’s and NMDA’s (compare results in Tables 2 and 3). E2’s dynamic characteristics overlap with the latter perfectly. This overlap and our observation of the lack of a rapid E2 effect on the baseline activity or membrane potential of the neurons suggest that E2 simply amplifies whatever the neurotransmitter actions are.
4.4. Behavioral implications
In a series of studies we have found that E2’s rapid actions could work synergistically with its genomic actions in the induction of luciferase in neuroblastoma cells [57] and the breast cancer cell line MCF-7 cells [58], and even in the induction of the female sexual behavior, lordosis [59]. However, in the case of lordosis induction, at least, it was not known exactly how rapid actions could facilitate the genomic action of estrogen, which is required for inducing lordosis.
Roy et al. [60] published an interesting report about the conditions for successful estrogen priming to induce lordosis. They found that an estrogen priming effective on ovariectomized rats failed to induce lordosis when applied while the rats were anesthetized with one of three anesthetics, including pentobarbital. However, priming after rats woke up from anesthesia was effective. So, the failure was not due to anesthetization per se but due to the presumed suppression of neuronal activity in the brain during estrogen priming.
To verify this presumption, the activity and responses to HA of VMN neurons were recorded. They were then challenged with pentobarbital, and with doses comparable to that used by Roy et al., [60] the excitatory response to HA and resting neuronal activity were suppressed [34]. These data, together with results from Roy et al., [60] indicate that sufficiently high neuronal activity in VMN is required for estrogenic priming to induce lordosis. It is possible to infer that E2’s potentiation of electrical activity in VMN neurons, demonstrated here, plays a permissive role in estrogen’s genomic induction of lordosis, thus lending support to our discovery of a synergistic relationship between the two types of estrogen actions (59) in supporting lordosis behavior.
Supplementary Material
Highlights.
-
➢
In VMN neurons, greater inhibition of K+ channels can be a common mechanism for estradiol (E2) to potentiate depolarization mediated by distinctly different types of membrane receptors.
-
➢
This common mechanism emphasizes estrogen actions initiated through membrane receptors.
-
➢
Through the membrane receptor-initiated actions, estrogens can enhance a wide variety of downstream signaling systems to induce a wide variety of rapid actions.
-
➢
This work has behavioral import: E2’s rapid potentiation of excitatory responses sets the conditions for E2’s genomic induction of lordosis behavior.
Acknowledgments
The authors want to acknowledge the support by a grant HD 05751 from NIH for this work.
Abbreviations
- 4-AP
4-Aminopyridine
- ACSF
artificial cerebrospinal fluid
- AP
action potential
- DPN
[2,3-Bis-(4-hydroxy-phenyl)-propionitrile
- E2
17β-estradiol
- ER
estrogen receptor
- ERα
estrogen receptor subtype α
- ERβ
estrogen receptor subtype β
- H1R
H1R and H1R, histamine receptor subtypes H1, H2 and H3, respectively
- HA
histamine
- NMDA
N-Methyl-D-aspartate
- mER
membrane estrogen receptor
- NMDAR
NMDA receptor
- PPT
(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol
- TEA
tetraethylammonium
- TTX
tetrodotoxin
- Vh
holding potential
- Vm
membrane potential
- VMN
hypothalamic ventromedial nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol. 1995;52(2):113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- 2.Watson CS, et al. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol Cell Endocrinol. 2007;274(1–2):1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benten WP, et al. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422(3):349–353. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 4.Stefano GB, et al. Estradiol coupling to human monocyte nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J Immunol. 1999;163(7):3758–3763. [PubMed] [Google Scholar]
- 5.Endoh H, et al. Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235(1):99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- 6.Grosse B, et al. Membrane signalling and progesterone in female and male osteoblasts. I. Involvement of intracellular Ca2+, inositol trisphosphate, and diacylglycerol, but not cAMP. J Cell Biochem. 2000;79(2):334–345. [PubMed] [Google Scholar]
- 7.Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed] [Google Scholar]
- 8.Okabe K, et al. Estrogen directly acts on osteoclasts via inhibition of inward rectifier K+ channels. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(6):610–620. doi: 10.1007/s002100000243. [DOI] [PubMed] [Google Scholar]
- 9.Sylvia VL, et al. 17β-estradiol regulation of protein kinase C activity in chondrocytes is sex-dependent and involves nongenomic mechanisms. J Cell Physiol. 1998;176(2):435–444. doi: 10.1002/(SICI)1097-4652(199808)176:2<435::AID-JCP22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Sylvia VL, et al. 17β-estradiol-BSA conjugates and 17β-estradiol regulate growth plate chondrocytes by common membrane assocaited mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem. 2001;81(3):413–429. doi: 10.1002/1097-4644(20010601)81:3<413::aid-jcb1055>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.de Beer EL, Keizer HA. Direct action of estradiol-17β on the atrial action potential. Steroids. 1982;40(2):223–231. doi: 10.1016/0039-128x(82)90035-6. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, Okabe K, Soeda H. Augmentation and suppression of action potentials by estradiol in the myometrium of pregnant rat. Can J Physiol Pharmacol. 1999;77(6):447–453. [PubMed] [Google Scholar]
- 13.Moller RA, Datta S, Strichartz GR. β-estradiol acutely potentiates the depression of cardiac excitability by lidocaine and bupivacaine. J Cardiovasc Pharmacol. 1999;34(5):718–727. doi: 10.1097/00005344-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Shenfeld OZ, et al. Rapid effects of estrogen and progesterone on tone and spontaneous rhythmic contractions of the rabbit bladder. Urol Res. 1999;27(5):386–392. doi: 10.1007/s002400050168. [DOI] [PubMed] [Google Scholar]
- 15.Stefano GB, et al. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;101(13):1594–1597. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- 16.Audy MC, Vacher P, Dufy B. 17β-estradiol stimulates a rapid Ca2+ influx in LNCaP human prostate cancer cells. Eur J Endocrinol. 1996;135(3):367–373. doi: 10.1530/eje.0.1350367. [DOI] [PubMed] [Google Scholar]
- 17.Filardo EJ, et al. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 18.Improta-Brears T, et al. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci U S A. 1999;96(8):4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliaccio A, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15(6):1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 20.Watters JJ, et al. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol. Endocri. 2000;14(11):1872–1881. doi: 10.1210/mend.14.11.0551. [DOI] [PubMed] [Google Scholar]
- 21.Morales A, et al. Estradiol modulates acetylcholine-induced Ca2+ signals in LHRH-releasing GT1–7 cells through a membrane binding site. Eur J Neurosci. 2003;18(9):2505–2514. doi: 10.1046/j.1460-9568.2003.02997.x. [DOI] [PubMed] [Google Scholar]
- 22.Watters JJ, et al. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138(9):4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 23.Minami T, et al. 17 β-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519(1–2):301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 24.Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17β-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543(1):148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- 25.Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur J Neurosci. 1998;10(1):255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 26.Cordey M, et al. Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem. 2003;84(6):1340–138. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 27.Hammes SR, Levin ER. Minireview: Recent Advances in Extranuclear Steroid Receptor Actions. Endocrinology. 2011;152(12):4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207(3):594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- 29.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147(12):5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 30.Norman AW, Mizwicki MT, Norman DPG. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3(1):27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 31.Haas HL, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 32.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 33.Dingledine R, et al. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 34.Kow L-M, Easton A, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2005;1043(1–2):124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 35.Dupré C, et al. Histaminergic responses by hypothalamic neurons that regulate lordosis and their modulation by estradiol. Proc Natl Acad Sci U S A. 2010;107(27):12311–12316. doi: 10.1073/pnas.1006049107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kow L-M, et al. Hormonal induction of lordosis and ear wiggling in rat pups: gender and age differences. Endocrine. 2007;32(3):287–296. doi: 10.1007/s12020-008-9046-1. [DOI] [PubMed] [Google Scholar]
- 37.Kow L-M, et al. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006;1116(1):1–11. doi: 10.1016/j.brainres.2006.07.104. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, et al. Histamine-Induced excitatory responses in mouse ventromedial hypothalamic neurons: Ionic mechanisms and estrogenic regulation. J Neurophysiol. 2007;98:3143–3152. doi: 10.1152/jn.00337.2007. [DOI] [PubMed] [Google Scholar]
- 39.Kuisle M, Lüthi A. In: Hyperpolarization-activated cation channels, in Ion Channels: From Structure to Function. Davies JKaC., editor. Oxford: Oxford University Press; 2010. pp. 183–203. [Google Scholar]
- 40.Christian EP, Undem BJ, Weinreich D. Endogenous histamine excites neurones in the guinea-pig superior cervical ganglion in vitro. J. Physiol. 1989;409(1):297–312. doi: 10.1113/jphysiol.1989.sp017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christian EP, Weinreich D. Long-duration spike afterhyperpolarizations in neurons from the guinea pig superior cervical ganglion. Neuroscience Letters. 1988;84(2):191–196. doi: 10.1016/0304-3940(88)90406-5. [DOI] [PubMed] [Google Scholar]
- 42.Greene RW, Haas HL. Effects of histamine on dentate granule cells in vitro. Neuroscience. 1990;34(2):299–303. doi: 10.1016/0306-4522(90)90140-y. [DOI] [PubMed] [Google Scholar]
- 43.Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- 44.Haas HL. Histamine potentiates neuronal excitation by blocking a calcium-dependent potassium conductance. Agents Actions. 1984;14(3–4):534–537. doi: 10.1007/BF01973865. [DOI] [PubMed] [Google Scholar]
- 45.Haas HL, Greene RW. Effects of histamine on hippocampal pyramidal cells of the rat in vitro. Exp Brain Res. 1986;62(1):123–130. doi: 10.1007/BF00237408. [DOI] [PubMed] [Google Scholar]
- 46.Jacklet J, Grizzaffi J, Tieman D. Serotonin, nitric oxide and histamine enhance the excitability of neuron MCC by diverse mechanisms. Acta Biol Hung. 2004;55(1–4):201–210. doi: 10.1556/ABiol.55.2004.1-4.25. [DOI] [PubMed] [Google Scholar]
- 47.Pellmar TC. Histamine decreases calcium-mediated potassium current in guinea pig hippocampal CA1 pyramidal cells. J Neurophysiol. 1986;55(4):727–738. doi: 10.1152/jn.1986.55.4.727. [DOI] [PubMed] [Google Scholar]
- 48.Jacklet JW, Tieman DG. Nitric oxide and histamine induce neuronal excitability by blocking background currents in neuron MCC of aplysia. J Neurophysiol. 2004;91(2):656–665. doi: 10.1152/jn.00409.2003. [DOI] [PubMed] [Google Scholar]
- 49.Jafri MS, et al. Histamine H1 receptor activation blocks two classes of potassium current, IK(rest) and IAHP, to excite ferret vagal afferents. J. Physiol. 1997;503(Pt 3):533–546. doi: 10.1111/j.1469-7793.1997.533bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Hatton G. Histamine-induced prolonged depolarization in rat supraoptic neurons: G-protein-mediated, Ca2+-independent suppression of K+ leakage conductance. Neuroscience. 1996;70(1):145–158. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- 51.Reiner PB, Kamondi A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59(3):579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 52.Liu B, et al. Phosphatidylinositol 4,5-bisphosphate hydrolysis mediates histamine-induced KCNQ/M current inhibition. Am J Physiol Cell Physiol. 2008;295(1):C81–C91. doi: 10.1152/ajpcell.00028.2008. [DOI] [PubMed] [Google Scholar]
- 53.Atzori M, et al. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci. 2000;3(8):791–798. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- 54.Gorelova N, Reiner PB. Histamine depolarizes cholinergic septal neurons. J Neurophysiol. 1996;75(2):707–714. doi: 10.1152/jn.1996.75.2.707. [DOI] [PubMed] [Google Scholar]
- 55.Munakata M, Akaike N. Regulation of K+ conductance by histamine H1 and H2 receptors in neurones dissociated from rat neostriatum. J. Physiol. 1994;480(Pt 2):233–245. doi: 10.1113/jphysiol.1994.sp020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whyment AD, et al. Histamine excites neonatal rat sympathetic preganglionic neurons in vitro via activation of H1 receptors. J Neurophysiol. 2006;95(4):2492–2500. doi: 10.1152/jn.01135.2004. [DOI] [PubMed] [Google Scholar]
- 57.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc. Natl. Acad. Sci. USA. 2001;98(21):12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devidze N, Pfaff DW, Kow L-M. Potentiation of genomic actions of estrogen by membrane actions in MCF-7 cells and the involvement of PKC activation. Endocrine. 2005;27(3):253–258. doi: 10.1385/ENDO:27:3:253. [DOI] [PubMed] [Google Scholar]
- 59.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101(33):12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy EJ, Lynn DM, Clark AS. Inhibition of sexual receptivity by anesthesia during estrogen priming. Brain Res. 1985;337(1):163–166. doi: 10.1016/0006-8993(85)91624-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.