Abstract
Purpose of Review
Cutaneous lupus erythematosus (CLE) is a common manifestation among systemic lupus patients. There are no FDA approved therapies for CLE, and these lesions are frequently disfiguring and refractory to treatment. This will review will cover the recent inroads made into understanding the mechanisms behind CLE lesions and discuss promising therapeutic developments.
Recent Findings
The definition of cutaneous lupus is being refined to facilitate diagnostic and research protocols. Research into the pathogenesis of CLE is accelerating, and discoveries are now identifying genetic and epigenetic changes which may predispose to particular disease manifestations. Further, unique features of disease subtypes are being defined. Murine work supports a connection between cutaneous inflammation and systemic lupus disease activity. Importantly, human trials of type I interferon blockade hold promise for improving our treatment armamentarium for refractory CLE lesions.
Summary
Continued research to understand the mechanisms driving CLE will provide new methods for prevention and treatment of cutaneous lesions. These improvements may also have important effects on systemic disease activity, and thus, efforts to understand this link should be supported.
Keywords: cutaneous lupus, interferon, discoid, subacute, photosensitivity
Introduction
Cutaneous lupus erythematosus (CLE) is a frequent finding in systemic lupus erythematosus (SLE) patients and can also exist as a single entity without associated systemic autoimmunity. Despite ongoing research into the etiology of CLE, it remains unclear how CLE relates to SLE pathogenesis. This review will summarize the recent advances in the pathogenesis of CLE, its relation to SLE, and the evolving therapeutic approaches based on these findings.
What is CLE?
The frequency of cutaneous manifestations in SLE is as high as 70% [1], and the overall prevalence of CLE is reported as greater than 0.07% [2] and may be equivalent to SLE in some populations[3]. Subtypes of CLE are currently grouped on the basis of histology, lesion duration, clinical findings, and laboratory abnormalities [4, 5] and are summarized in Table 1. [6–9]
Table 1.
Types of cutaneous lupus erythematosus (CLE) and their manifestations.
| CLE subtype | Manifestations | Photosensitivity [6] | Systemic Disease Association |
|---|---|---|---|
| ACLE |
|
+++ | Frequent |
| SCLE |
|
+++ | Reported at 48.7% [7, 8] |
| CCLE | |||
| DLE |
|
++ | Reported at 40% [7, 8] |
| LEP |
|
−/? | Unknown |
| CHLE |
|
− | unknown |
| LET |
|
++++ | Understudied, possibly 10% [9] |
ACLE=acute CLE, SCLE=subacute CLE, CCLE=chronic CLE Included in CCLE are discoid LE (DLE), LE profundus (LEP), chilblain LE (CHLE), and LE tumidus (LET).
In 2013, the 3rd International Meeting on Cutaneous Lupus Erythematosus was held with a goal of developing a uniform definition for CLE, as well as consensus on diagnostic and classification criteria. A more formal process is currently underway, employing the Delphi consensus method with an initial goal of better characterizing DLE [10].One current diagnostic challenge is the definition of what constitutes SLE with cutaneous features vs. CLE as an independent disease. Previous studies have suggested that sCLE has a higher incidence of systemic disease [7], but most patients with SCLE who formally meet criteria for SLE do so based on mucocutaneous and laboratory criteria [11]. Furthermore, Neither the American College of Rheumatology (ACR) nor the Systemic Lupus International Collaborating Clinics (SLICC) criteria for diagnosis of SLE are able to sufficiently distinguish patients with SCLE and major internal disease from those without significant systemic manifestations [11]. This proposes a challenge for epidemiologic and mechanistic studies that try to characterize CLE only from SLE-associated skin lesions and further work in this arena is warranted.
Pathogenesis
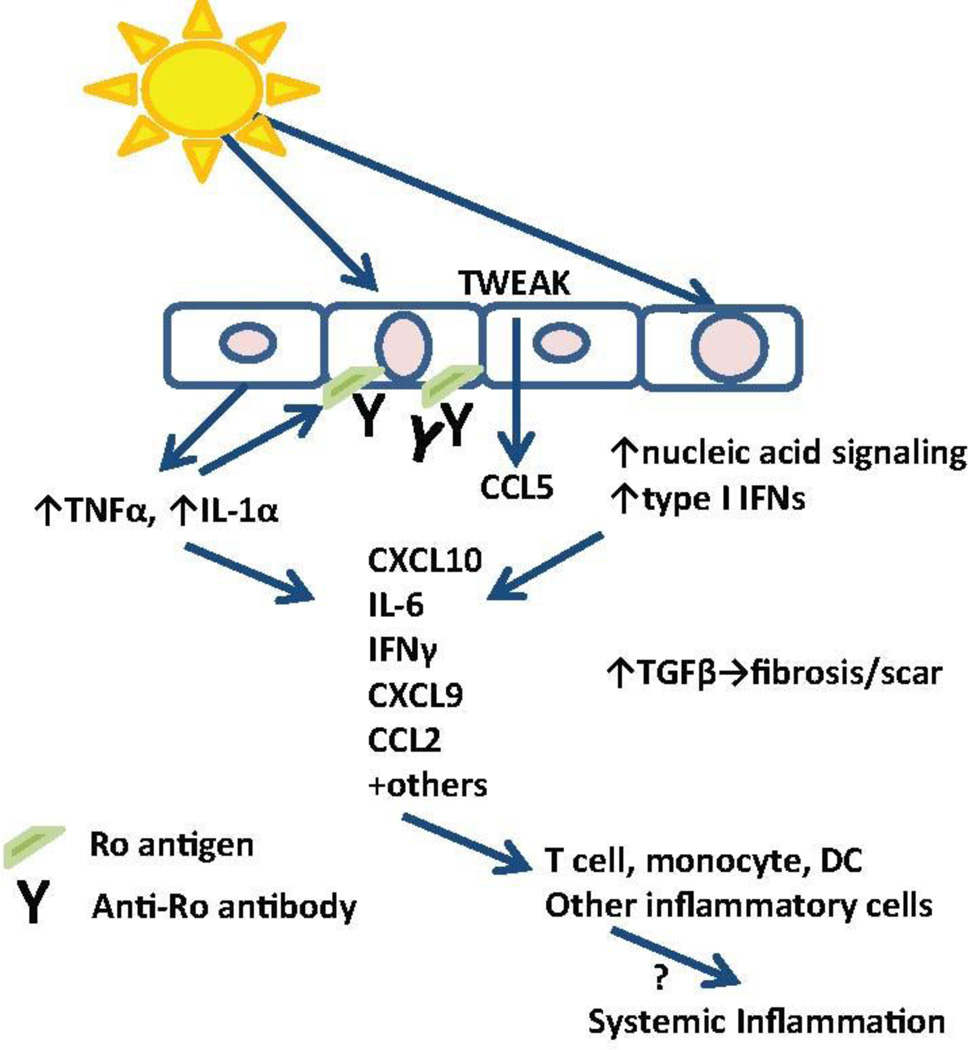
The pathogenesis of CLE is multifactorial and involves genetic predisposition, environmental triggers, and abnormalities in the innate and adaptive immune response. Current dogma points to UV irradiation as a mechanism for cellular damage and apoptosis, in addition to dendritic cell activation, T cell dysregulation, cytokine imbalances, B cell defects and autoantibody production (Figure 1). Recent advances are summarized below.
Figure 1. Summary of CLE pathogenesis.
Triggers for skin inflammation, including UV light, stimulate innate cytokine production from keratinocytes and trigger cell death which can activate nucleic acid signaling pathways. Increased autoantigen exposure on the cell surface encourages immune complex deposition, which can lead to antibody dependent cell-mediated cytotoxicity. Cytokine and chemokine production promotes inflammatory infiltrates which damage tissues, perpetuate the inflammatory cycle and lead to chronic TGFβ signaling which promotes damage and scar. The links between skin inflammation and systemic disease require further study. DC=dendritic cell
Genetics/Epigenetics/Transcriptomics
The list of genes involved in regulation of CLE disease risk is growing. Human leukocyte antigen (HLA) type may predict CLE variant risk [12, 13]. TNFα and complement promoter variants have also been linked with CLE [14]. Single nucleotide polymorphisms in tyrosine kinase 2 (TYK2), interferon regulatory factor 5 (IRF5), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) may also increase risk for CLE. Recently, a large GWAS of 183 CLE cases and 1288 controls was completed and most genes reinforced the linkage of SNPs in various HLA genes. Novel associations for CLE risk in the GWAS included polymorphisms in casein kinase 2, a gene with links to lupus nephritis, and RPP21, a subunit of RNAse P, which is involved in RNA processing pathways [15]. Other mutations in genes participating in cytosolic nucleic acid sensor signaling have also recently been identified as contributing to cutaneous lupus lesions, especially chilblains [16]. RNASEH2 variants have been identified in SLE patients that increase the risk of DNA damage by ultraviolet light [17] and may consequentially increase photosensitive responses. Interestingly, photosensitivity may decline with age of presentation, which also supports a genetic link for this SLE manifestation [18].
Notably, epigenetic differences conferring susceptibility to CLE have been identified. Coit et. al performed genome-wide DNA methylation analyses of CD4+ T cells in patients with SLE and history of malar or discoid rash. The study identified 36 and 37 unique differentially methylated regions associated with malar rash and discoid rash, respectively [19]. Hypomethylation of MIR886 and TRIM69 and hypermethylation of RNF39 were identified in patients with malar rash; these genes help mediate cell proliferation and apoptosis. Discoid rash-specific hypomethylated DMRs were found in TAP1 and PSMB8, genes involved in antigen processing and presentation.
New research has also identified transcriptional changes in CLE. When compared with psoriasis, DLE has a strong Th1 signature and an absence of IL-17 signaling [20]. Others have confirmed this finding and have identified progressive TGFβ production in DLE which may contribute to scar and fibrosis of lesions over time. Further, there is substantial overlap noted between dysregulated pathways of the skin of patients with DLE and the transcriptional profiles from the blood of DLE patients- most notably in type I interferon (IFN) signaling [21]. These data support a strong role for T cells in DLE and continue to support a role for type I IFN in CLE lesions.
Triggers of CLE lesions
UV exposure is a common trigger for CLE, with photosensitivity rates reported at 81% [22]. UV induces keratinocyte apoptosis, inflammatory cytokine production, and autoantigen exposure [23]. CLE lesions highly express Fas (CD95), which activates the extrinsic apoptotic pathway [24]. It is unclear whether UV drives enhanced apoptosis in lupus vs. control skin as two studies addressing this question have disparate findings[25, 26]. However, recent studies have identified other mechanisms by which UV may influence skin disease. UV-induced apoptotic binding of the nucleolus by C1q may serve as a protective mechanism in SLE and further explain the role of C1q deficiency in SLE development [27]. Prediction of photosensitivity via global peptide profiling has identified beta-2 microglobulin as a potential predictor of photosensitive responses [28]. Further work into the role of UV activation of CLE will likely identify additional targets for treatment.
The relationship of active skin disease to systemic disease activity is another area of exploration in CLE. UV irradiation is able to trigger activation of systemic lupus disease in male BXSB mice [29]. In line with this data, photosensitive patients with robust cutaneous infiltrates have more systemic symptoms, such as fatigue and arthralgias, than patients without skin inflammation after UV exposure [22]. Other murine models of skin inflammation also suggest a link with systemic disease. Epidermal injury via tape stripping can induce chronic rash and rapid induction of nephritis in lupus-prone NZM2328 mice[30]. Further, epicutaneous stimulation with TLR7 agonists also induces a lupus-like disease in wild type mice [31]. These data suggest that cutaneous inflammation promotes systemic disease activity and that identification of the specific mediators responsible will identify novel targets to prevent disease flare. Further research into this area is needed.
Cytokines/chemokines
Various cytokines and chemokines have been identified as contributing to CLE pathogenesis. Tumor necrosis factor-α (TNF-α) is upregulated after UVB exposure at least partially through IL-1α signaling pathways [32]. Furthermore, TNFα induces surface expression of the autoantigen Ro52 in primary keratinocytes following TNF-α stimulation [33], which is interesting given known associations between Ro positivity and cutaneous lupus lesions. Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) stimulation of keratinocytes upregulates CCL5/RANTES, and skin disease in MRL/lpr mice is dependent on activation of the receptor for TWEAK [34]. Chemerin, a chemokine for plasmacytoid dendritic cells, has been identified as upregulated in CLE and by UVB exposure and consequently may participate in recruitment of this cell population in CLE [35].
CLE patients demonstrate increased expression of the IL-18 receptor on keratinocytes, and CLE keratinocytes fail to express IL-12, which is protective against apoptosis, in the presence of IL-18. [36]. Serum levels of IL-18 are higher in patients with anti-Ro antibodies [37]. Interestingly, polymorphisms in the IL-18 promoter have been identified in in some lupus patients [38].
Based on recent trial data (see below), interest in type I interferons (IFNs) as primary contributors to cutaneous lesions is strong. Increased expression of interferon-regulated genes are seen in both the dermis and epidermis of CLE lesions [39]. Type I IFN production in lupus lesions promotes a Th-1-biased inflammatory infiltrate [39]. Type I IFNs also promote upregulation of PSMB9, an immunoproteasome subunit, in the epidermis of cutaneous lupus which may lead to enhanced extracellular matrix deposition in CLE [40]. Interferonopathies, a recently identified class of genetic diseases which result in hyperactivation of type I IFN genes (reviewed in [41]) have an abundance of CLE-like lesions, emphasizing the role of type I IFNs in this process.
Autoantibodies
Lupus is characterized by production of multiple autoantibodies. In CLE, autoantibodies frequently deposit at the dermal-epidermal junction and may facilitate antibody-dependent cell-mediated cytotoxicity. However, their specific role in the pathogenesis of cutaneous lupus remains unclear. Recent work has focused on identifying correlations between autoantibody production and CLE subtypes and clinical presentations. In a study by Biazar et. al, anti-Ro/SSA antibodies were found in 47.4% of patients with ACLE, 72.1% of patients with SCLE, and 22% of patients with DLE. Anti-LA/SSB antibodies were detected in 27.5% of patients with ACLE, 36.2% of patients with SCLE, and 7% of patients with DLE [42].
Additional studies have looked at the utility of autoantibodies as prognostic indicators. One analysis in a primarily Caucasian population identified an association between anti-Smith (Sm) antibodies and discoid rash and photosensitivity; an association between anti-Ro/SSA antibodies and malar rash, oral ulcers, and presence of rheumatoid factor; and an association between anti-U1RNP antibodies and Raynaud’s and malar rash [43]. Another study performed in a primarily Chinese SLE population demonstrated that photosensivity and discoid rash are associated with anti-SSA and SSB antibodies, whereas malar rash, mucositis, serositis, and arthritis are associated with anti-Sm, anti-ribonuclear protein (anti-RNP), and antiphospholipid (anti-PL) antibodies. Anti-double stranded DNA (dsDNA) antibodies were associated only with renal involvement in this study [44]. The discordant findings may be due to differences in ethnic backgrounds, but additional studies are needed to better clarify the relationship between autoantibody presence and disease manifestations.
Treatment
The treatment of cutaneous lupus remains a challenge. This is partly due to varying and often unpredictable response to therapy among different subtypes of cutaneous lupus, and even among different patients within subtypes. Additionally, there has been a paucity of studies dedicated to the treatment of cutaneous lupus, and no agent specifically for cutaneous lupus has been approved to date. The authors of a 2009 Cochrane Database review were only able to conclude that fluocinonide cream may be more effective than hydrocortisone cream in DLE, and that acitretin is likely equally effective compared with hydroxychloroquine, but carries with it more frequent and severe adverse effects [45]. A study published this year by Reich et. al evaluated current practices in the management of cutaneous lupus and highlighted a significant amount of variability between countries and even among individual physicians [46]. However, there have been several studies in recent years introducing novel and potentially effective treatment options.
Standard Therapies
Prevention is a cornerstone in the management of CLE, as UV irradiation is known to induce lesions and trigger flares of disease [47]. Consistent protection with sunscreen and avoidance of sun and UV exposure have been associated with better clinical outcomes in SLE [48] and these precautions should be a part of any treatment plan.
Topical corticosteroids remain the established first-line treatment of localized CLE [45, 49]. Topical tacrolimus has additionally demonstrated efficacy in treatment of localized lesions [50]. Intralesional steroids can be beneficial for DLE [49]. For wide-spread and recalcitrant disease, however, corticosteroid use is clearly limited by side effects. Several immunosuppressive and immunomodulatory drugs have therefore been tried as steroid-sparing agents. Among these, antimalarials are the most established treatment approach. Currently, chloroquine and hydroxychloroquine are first-line systemic treatment, according to dermatological guidelines [49, 51]. Mycophenolate and methotrexate have been used as part of combination therapy for cutaneous disease partially responsive or unresponsive to antimalarial therapies, with varying effect ([52–55]. There is limited data available for the efficacy of azathioprine, with approximately 10 patients described in the literature ([56, 57].
Evolving Therapeutic Approaches
A restrospective analysis published this year by Klebes et. al evaluated 34 patients treated with Dapsone, either as monotherapy or combined with antimalarials [58]. The study demonstrated that dapsone with/without antimalarials was effective in over 50% of patients. Four patients discontinued the drug due to side effects, including drug eruptions, peripheral neuropathy, and hemolytic anemia. Overall, the study suggests that Dapsone may be a good second line therapy in CLE.
Another recent study evaluated the efficacy of increased hydroxychloroquine dosing in patients with refractory CLE [59]. Thirty four patients with hydroxychloroquine blood levels less than or equal to 750 ng/ml were included in this open-label study. The daily dose of hydroxychloroquine was increased to reach blood concentrations of greater than 750 ng/mL. The primary endpoint in the study was defined as a 20% improvement in the CLE Disease Area and Severity Index (CLASI) score. Eighty one percent of patients in the study reached the primary endpoint and hydroxychloroquine doses were able to be decreased without subsequent flare in 15/26 responders. The potential side effects of increased hydroxychloroquine dosing (eg. retinal toxicity) need to be considered, but in patients able to reduce dose without subsequent flare, this approach may be effective and could avoid risks associated with more immunosuppressive therapies.
Addition of quinacrine to low dose hydroxychloroquine has also been suggested for management of CLE [60]. This approach has been widely used in previous years and has been reported in a recent prospective, longitudinal study to be effective when hydroxychloroquine monotherapy fails [61]. Further studies are needed to evaluate risk associated with this additive approach.
Novel Therapeutic Targets
Type I IFNs (IFN-α/IFN-β) have been a focus in the development of new drugs for the treatment of systemic lupus with generally disappointing results. Sifalimumab, an anti-IFN-α antibody, demonstrated modest improvements on skin disease activity [62]. Another anti-IFN-α antibody, rontazilumab, was ineffective in a phase II study [63]. More recently, anifrolumab, a monoclonal antibody targeting the type I IFN receptor (IFNAR), the common receptor for all type I IFNs, has been developed. The drug is currently being tested in patients with SLE with preliminarily positive results. A phase II randomized, double-blinded, placebo-controlled trial demonstrated significant reduction in arthritis and improvement in cutaneous disease in 305 patients with moderate to severe lupus [64], making this agent a potentially important treatment option for CLE. These studies suggest that other type I IFNs, besides IFNα, may have synergistic effects in CLE pathogenesis.
Tocilizumab, a humanized monoclonal anti-IL-6 antibody, has additionally been studied in the treatment of systemic lupus in recent years. There is limited evidence for the efficacy of tocilizumab in cutaneous disease, but a case report describing marked improvement in severe tumid lupus lesions suggests that the drug could be a promising treatment for CLE [65]. Belimumab, a monoclonal antibody targeting B lymphocyte stimulator, has not been studied in CLE, but case reports also support consideration of this for future use [66].
Conclusion
CLE encompasses several cutaneous diseases with common and unique pathogenic factors. Further research will identify and refine the mechanisms that lead to disease and facilitate development of specific therapies which go beyond general immunosuppressive approaches, especially for recalcitrant disease.
Key Points.
Definitions of cutaneous lupus or cutaneous lupus associated with systemic lupus are being refined.
Many genetic risk factors for cutaneous lupus involve HLA or interferon-related pathways.
More work is needed to identify the pathogenic mechanisms behind cutaneous lupus manifestations.
Type I interferon receptor blockade may be a promising therapy for cutaneous lupus.
Acknowledgments
None
Financial support and sponsorship:
JNS was supported by the US Department of Veterans Affairs. JMK was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under Award Number K08AR063668.
Footnotes
Conflict of interest:
None
References Cited
- 1.Mikita N, et al. Recent advances in cytokines in cutaneous and systemic lupus erythematosus. J Dermatol. 2011;38(9):839–849. doi: 10.1111/j.1346-8138.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen LK, Davis MD. Prevalence of Skin and Skin-Related Diseases in the Rochester Epidemiology Project and a Comparison with Other Published Prevalence Studies. Dermatology. 2016 doi: 10.1159/000444580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarukitsopa S, et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken) 2015;67(6):817–828. doi: 10.1002/acr.22502. * This is a retrospective cohort study of predominantly white SLE and CLE patients, which demonstrates that CLE is more common than SLE in men and older adults, but the overall incidences of CLE and SLE are similar.
- 4.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4(4):471–475. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 5.Hejazi EZ, Werth VP. Cutaneous Lupus Erythematosus: An Update on Pathogenesis, Diagnosis and Treatment. Am J Clin Dermatol. 2016 doi: 10.1007/s40257-016-0173-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim A, Chong BF. Photosensitivity in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed. 2013;29(1):4–11. doi: 10.1111/phpp.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronhagen CM, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–1341. doi: 10.1111/j.1365-2133.2011.10272.x. [DOI] [PubMed] [Google Scholar]
- 8.Wieczorek IT, et al. Systemic symptoms in the progression of cutaneous to systemic lupus erythematosus. JAMA Dermatol. 2014;150(3):291–296. doi: 10.1001/jamadermatol.2013.9026. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn A, et al. Lupus erythematosus tumidus--a neglected subset of cutaneous Lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136(8):1033–1041. doi: 10.1001/archderm.136.8.1033. [DOI] [PubMed] [Google Scholar]
- 10.Schultz HY, et al. From pathogenesis, epidemiology, and genetics to definitions, diagnosis, and treatments of cutaneous lupus erythematosus and dermatomyositis: a report from the 3rd International Conference on Cutaneous Lupus Erythematosus (ICCLE) 2013. J Invest Dermatol. 2015;135(1):7–12. doi: 10.1038/jid.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tiao J, et al. Using the American College of Rheumatology (ACR) and Systemic Lupus International Collaborating Clinics (SLICC) criteria to determine the diagnosis of systemic lupus erythematosus (SLE) in patients with subacute cutaneous lupus erythematosus (SCLE) J Am Acad Dermatol. 2016 doi: 10.1016/j.jaad.2015.12.029. ** This study recognizes challenges in differentiating cutaneous lupus from systemic lupus using current classification criteria. The study points out that neither ACR nor SLICC criteria are useful in distinguishing SCLE patients with systemic involvement from SCLE patients with only cutaneous disease
- 12.Osmola A, et al. Genetic background of cutaneous forms of lupus erythematosus: update on current evidence. J Appl Genet. 2004;45(1):77–86. [PubMed] [Google Scholar]
- 13.Fischer GF, et al. Association between chronic cutaneous lupus erythematosus and HLA class II alleles. Hum Immunol. 1994;41(4):280–284. doi: 10.1016/0198-8859(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 14.Werth VP, et al. Association of a promoter polymorphism of tumor necrosis factor-alpha with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol. 2000;115(4):726–730. doi: 10.1046/j.1523-1747.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 15. Kunz M, et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Exp Dermatol. 2015;24(7):510–555. doi: 10.1111/exd.12708. ** This is the largest GWAS study for cutaneous lupus erythematosus to date and identifies multiple genes involved in antigen presentation, apoptosis regulation, and interferon response, which may play key roles in the pathogenesis of CLE.
- 16.Crow YJ, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A(2):296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gunther C, et al. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest. 2015;125(1):413–424. doi: 10.1172/JCI78001. ** This study determined that heterozygous parents of Aicardi-Goutières syndrome (AGS) patients demonstrate impaired RNase H2 function, leading to chronic, low-level DNA damage and an intermediate autoimmune phenotype. This highlights the importance of DNA damage-associated pathways in the development of autoimmunity
- 18. Medlin JL, et al. A systematic review and meta-analysis of cutaneous manifestations in late- versus early-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2016 doi: 10.1016/j.semarthrit.2016.01.004. * A meta-analysis of over 12,000 SLE patients demonstrating that, aside from sicca symptoms, cutaneous manifestations are less common in late-onset SLE patients
- 19. Renauer P, et al. DNA methylation patterns in naive CD4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus Sci Med. 2015;2(1):e000101. doi: 10.1136/lupus-2015-000101. * This study identifies multiple new methylation sites unique to both SCLE and DLE, which primarily localize to genes involved in cell proliferation and apoptosis
- 20.Jabbari A, et al. Dominant Th1 and minimal Th17 skewing in discoid lupus revealed by transcriptomic comparison with psoriasis. J Invest Dermatol. 2014;134(1):87–95. doi: 10.1038/jid.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dey-Rao R, Sinha AA. Genome-wide transcriptional profiling of chronic cutaneous lupus erythematosus (CCLE) peripheral blood identifies systemic alterations relevant to the skin manifestation. Genomics. 2015;105(2):90–100. doi: 10.1016/j.ygeno.2014.11.004. * This study identifies important transcriptional abnormalities in the blood of DLE patients, including alterations in pathways for type I interferon (IFN) signaling.
- 22.Foering K, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69(2):205–213. doi: 10.1016/j.jaad.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa F, et al. Keratinocytes from patients with lupus erythematosus show enhanced cytotoxicity to ultraviolet radiation and to antibody-mediated cytotoxicity. Clin Exp Immunol. 1999;118(1):164–170. doi: 10.1046/j.1365-2249.1999.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toberer F, et al. Apoptotic signal molecules in skin biopsies of cutaneous lupus erythematosus: analysis using tissue microarray. Exp Dermatol. 2013;22(10):656–659. doi: 10.1111/exd.12216. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn A, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54(3):939–950. doi: 10.1002/art.21658. [DOI] [PubMed] [Google Scholar]
- 26.Reefman E, et al. Is disturbed clearance of apoptotic keratinocytes responsible for UVB-induced inflammatory skin lesions in systemic lupus erythematosus? Arthritis Res Ther. 2006;8(6):R156. doi: 10.1186/ar2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Y, et al. C1q protein binds to the apoptotic nucleolus and causes C1 protease degradation of nucleolar proteins. J Biol Chem. 2015;290(37):22570–22580. doi: 10.1074/jbc.M115.670661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calderon C, et al. A multicenter photoprovocation study to identify potential biomarkers by global peptide profiling in cutaneous lupus erythematosus. Lupus. 2015;24(13):1406–1420. doi: 10.1177/0961203315596077. * This study identifies a unique plasma peptide signature among CLE subjects with rash development following photoprovocation and thus indicates there are systemic changes which reflect photosensitivity.
- 29.Ansel JC, et al. Effects of UV radiation on autoimmune strains of mice: increased mortality and accelerated autoimmunity in BXSB male mice. J Invest Dermatol. 1985;85(3):181–186. doi: 10.1111/1523-1747.ep12276652. [DOI] [PubMed] [Google Scholar]
- 30. Clark KL, et al. Epidermal injury promotes nephritis flare in lupus-prone mice. J Autoimmun. 2015;65:38–48. doi: 10.1016/j.jaut.2015.08.005. ** This study describes a novel model of lupus flare induction via epicutaneous stimulation in NZM2328 mice, identifying an important link between epidermal injury and development of systemic disease.
- 31.Yokogawa M, et al. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol. 2014;66(3):694–706. doi: 10.1002/art.38298. [DOI] [PubMed] [Google Scholar]
- 32.Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol. 2009;129(4):994–1001. doi: 10.1038/jid.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerl V, et al. The intracellular 52-kd Ro/SSA autoantigen in keratinocytes is up-regulated by tumor necrosis factor alpha via tumor necrosis factor receptor I. Arthritis Rheum. 2005;52(2):531–538. doi: 10.1002/art.20851. [DOI] [PubMed] [Google Scholar]
- 34. Doerner JL, et al. TWEAK/Fn14 Signaling Involvement in the Pathogenesis of Cutaneous Disease in the MRL/lpr Model of Spontaneous Lupus. J Invest Dermatol. 2015;135(8):1986–1995. doi: 10.1038/jid.2015.124. ** This article is the first to demonstrate a role for TWEAK signaling in cutaneous lupus and gives a mechanistic explanation for the role of this protein through its upregulation of CCL5.
- 35.Yin Q, et al. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity. 2014;47(3):185–192. doi: 10.3109/08916934.2013.866105. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, et al. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 2008;58(10):3205–3215. doi: 10.1002/art.23868. [DOI] [PubMed] [Google Scholar]
- 37.Kahlenberg JM, et al. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187(11):6143–6156. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YJ, et al. Disease association of the interleukin-18 promoter polymorphisms in Taiwan Chinese systemic lupus erythematosus patients. Genes Immun. 2007;8(4):302–307. doi: 10.1038/sj.gene.6364387. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel J, et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205(4):435–442. doi: 10.1002/path.1721. [DOI] [PubMed] [Google Scholar]
- 40. Nakamura K, et al. The role of PSMB9 up-regulated by interferon signature in the pathophysiology of cutaneous lesions of dermatomyositis and lupus erythematosus. Br J Dermatol. 2015 doi: 10.1111/bjd.14385. * This study identifies IFN-driven upregulation of PSMB9, an immunoproteasome subunit, in the epidermis of cutaneous lupus. This protein may play a role in increased extracellular matrix deposition in CLE lesions.
- 41. Crow YJ, Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15(7):429–440. doi: 10.1038/nri3850. * A thorough review of the type I interferonopathies. This paper recognizes frequent CLE-like lesions in these diseases, emphasizing the role of type I IFNs in the pathogenesis of CLE.
- 42.Biazar C, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE) Autoimmun Rev. 2013;12(3):444–454. doi: 10.1016/j.autrev.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 43. Fredi M, et al. Rare autoantibodies to cellular antigens in systemic lupus erythematosus. Lupus. 2014;23(7):672–677. doi: 10.1177/0961203314524850. * This is a cohort study identifying associations between autoantibodies and different manifestations of systemic lupus in 540 Italian patients.
- 44.Li PH, et al. Relationship between autoantibody clustering and clinical subsets in SLE: cluster and association analyses in Hong Kong Chinese. Rheumatology (Oxford) 2013;52(2):337–345. doi: 10.1093/rheumatology/kes261. [DOI] [PubMed] [Google Scholar]
- 45.Jessop S, Whitelaw DA, Delamere FM. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2009;4:CD002954. doi: 10.1002/14651858.CD002954.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Reich A, et al. Treatment of cutaneous lupus erythematosus: current practice variations. Lupus. 2016 doi: 10.1177/0961203316628997. [DOI] [PubMed] [Google Scholar]
- 47.Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of Cutaneous Lupus Erythematosus: Review and Assessment of Treatment Benefits Based on Oxford Centre for Evidence-based Medicine Criteria. J Clin Aesthet Dermatol. 2013;6(1):27–38. [PMC free article] [PubMed] [Google Scholar]
- 48.Vila LM, et al. Association of sunlight exposure and photoprotection measures with clinical outcome in systemic lupus erythematosus. P R Health Sci J. 1999;18(2):89–94. [PubMed] [Google Scholar]
- 49.Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65(6):e179–e193. doi: 10.1016/j.jaad.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Tzung TY, Liu YS, Chang HW. Tacrolimus vs. clobetasol propionate in the treatment of facial cutaneous lupus erythematosus: a randomized, double-blind, bilateral comparison study. Br J Dermatol. 2007;156(1):191–192. doi: 10.1111/j.1365-2133.2006.07595.x. [DOI] [PubMed] [Google Scholar]
- 51.Sigges J, et al. Therapeutic strategies evaluated by the European Society of Cutaneous Lupus Erythematosus (EUSCLE) Core Set Questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun Rev. 2013;12(7):694–702. doi: 10.1016/j.autrev.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Wenzel J, et al. Efficacy and safety of methotrexate in recalcitrant cutaneous lupus erythematosus: results of a retrospective study in 43 patients. Br J Dermatol. 2005;153(1):157–162. doi: 10.1111/j.1365-2133.2005.06552.x. [DOI] [PubMed] [Google Scholar]
- 53.Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18(2):59–62. doi: 10.1007/s002960050058. [DOI] [PubMed] [Google Scholar]
- 54.Kreuter A, et al. Mycophenolate sodium for subacute cutaneous lupus erythematosus resistant to standard therapy. Br J Dermatol. 2007;156(6):1321–1327. doi: 10.1111/j.1365-2133.2007.07826.x. [DOI] [PubMed] [Google Scholar]
- 55.Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;65(4):717–721. doi: 10.1016/j.jaad.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Tsokos GC, Caughman SW, Klippel JH. Successful treatment of generalized discoid skin lesions with azathioprine. Its use in a patient with systemic lupus erythematosus. Arch Dermatol. 1985;121(10):1323–1325. [PubMed] [Google Scholar]
- 57.Shehade S. Successful treatment of generalized discoid skin lesions with azathioprine. Arch Dermatol. 1986;122(4):376–377. [PubMed] [Google Scholar]
- 58. Klebes M, Wutte N, Aberer E. Dapsone as Second-Line Treatment for Cutaneous Lupus Erythematosus? A Retrospective Analysis of 34 Patients and a Review of the Literature. Dermatology. 2016;232(1):91–96. doi: 10.1159/000441054. *This study further clarifies a potential role for Dapsone in the treatment of CLE.
- 59. Chasset F, et al. The effect of increasing the dose of hydroxychloroquine (HCQ) in patients with refractory cutaneous lupus erythematosus (CLE): An open-label prospective pilot study. J Am Acad Dermatol. 2016;74(4):693–699. e3. doi: 10.1016/j.jaad.2015.09.064. * This is the first study suggesting that temporary increase in hydroxychloroquine dosing could lead to lasting improvement in CLE.
- 60.McCune WJ, Gonzalez-Rivera T. Should very low doses of hydroxychloroquine and quinacrine be employed in combination for long-term maintenance of remission in systemic lupus to reduce the risk of ocular toxicity? Curr Opin Rheumatol. 2015;27(3):213–215. doi: 10.1097/BOR.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 61.Chang AY, et al. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Arch Dermatol. 2011;147(11):1261–1267. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khamashta M, et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208562. * This randomised, controlled trial of Sifalimumab in the treatment of SLE demonstrates modest improvements in disease activity, including cutaneous manifestations of disease.
- 63.Kalunian KC, et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE) Ann Rheum Dis. 2016;75(1):196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 64. Richard Furie JM, Werth VP, Khamashta M, Kalunian K, Brohawn P, Illei G, Drappa J, Wang L, Yoo S. American College of Rheumatology Annual Meeting. San Franscisco, CA: 2015. Anifrolumab, an Anti-Interferon Alpha Receptor Monoclonal Antibody, in Moderate to Severe Systemic Lupus Erythematosus (SLE) * This abstract reported the results of a phase II study of Anifrolumab, demonstrating promising preliminary results in the treatment of systemic lupus.
- 65.Makol A, Gibson LE, Michet CJ. Successful use of interleukin 6 antagonist tocilizumab in a patient with refractory cutaneous lupus and urticarial vasculitis. J Clin Rheumatol. 2012;18(2):92–95. doi: 10.1097/RHU.0b013e31823ecd73. [DOI] [PubMed] [Google Scholar]
- 66.Husein-ElAhmed H, et al. Refractory subacute cutaneous lupus erythematous responding to a single course of belimumab: a new anti-BLyS human monoclonal antibody. Indian J Dermatol Venereol Leprol. 2014;80(5):477–478. doi: 10.4103/0378-6323.140335. [DOI] [PubMed] [Google Scholar]