Abstract
Retinal degeneration due to chronic ambient light exposure is a common spontaneous age-related finding in albino rats, but it can also be related to exposures associated with environmental chemicals and drugs. Typically, light induced retinal degeneration has a central/hemispherical localization where as chemical induced retinal degeneration has a diffuse localization. This study was conducted to identify National Toxicology Program (NTP) rodent bioassays with treatment-related retinal degeneration, and to characterize the retinal lesions related to these retinal toxicities. A total of 3 chronic bioassays in F344/N rats were identified that had treatment-related increases in retinal degeneration (kava kava extract, acrylamide, and leucomalachite green). A retrospective light-microscopic evaluation of the retinas from rats in these three studies showed a dose-related increase in the frequencies of retinal degeneration, beginning with the loss of photoreceptor cells, followed by the inner nuclear layer cells. These dose-related increased frequencies of degenerative retinal lesions localized within the central/hemispherical region are suggestive of exacerbation of light-induced retinal degeneration.
Keywords: F344/N rat, ocular phototoxicity, retinal degeneration, light-induced exacerbation, carcinogenicity bioassay, NTP database survey, Retinal atrophy
Introduction
Ocular side effects of chemicals and drugs are a rare safety issue in nonclinical toxicity evaluations; however ocular toxicity in nonclinical studies can be a significant liability. Depending on the nature of the findings, these side effects may result in termination of the compound in the drug development process or regulation of the chemical/supplement use to avoid exposure to human. In nonclinical in vivo toxicity studies, the eye is routinely investigated by clinical examination and by histopathology in rodent and nonrodent species to evaluate the potential for ocular toxicity. A careful microscopic examination of the eye can reveal the presence of treatment-related lesions and provide information on dose responses and the type of lesion. However, spontaneous ocular lesions, especially retinal degeneration, occur relatively commonly in long term toxicity (≥ 6 months) or carcinogenicity studies in albino rats and mice.
Retinal degeneration in rats and mice can occur as an aging change or secondary to various insults such as physical trauma, detachment, inflammation, infectious agents such as viruses (Del et al. 1984), vascular derangements such as infarction (Nyska et al. 1999), increased intraocular pressure (Sun et al. 2011), nutritional deficiencies (Lee et al. 1990), or heritable factors (DiLoreto et al. 1994; Frame et al; Serfilippi et al. 1991). Retinal degeneration can also be a direct toxic effect of systemically or topically administered chemical agents (Breider et al. 1998; Illanes et al. 2006). Spontaneously occurring retinal degeneration of uncertain etiology has also been reported in laboratory rats and mice (DiLoreto et al. 1994). However, spontaneous retinal degeneration seen in the vehicle control group in long-term studies (≥ 6 months) in albino rodents is considered to be light-related, although an age effect cannot be excluded (Rao 1991; Perez and Perentes 1994; De Vera Mudry et al. 2013).
Light-induced retinal degeneration can occur when rats and mice, especially albino strains, are exposed to overly intense ambient light (Noell et al. 1996; Lai et al. 1978; Rao et al. 1991). In addition to light intensity, the development of light-induced retinal degeneration in albino rodents is influenced by wavelength, duration of exposure, length of time for dark-adaptation, age at initial exposure, maturity of the retina, gender, environmental temperature and diet including a deficiency of nutrients such as vitamins, zinc and taurine (Wenzel et al. 2005; Robison et al. 1980; Grahn et al. 2001; Organisciak et al. 1995; Rapp et al. 1988).
It is important to differentiate test article-associated retinal damage from retinal phototoxicity, or to identify the possible additive or synergistic interaction between the test article and ambient light that results in retinal degeneration. A critical component of any study that aims to investigate the cause of retinal degeneration includes the morphologic topography of the retinal damage (De Vera Mudry et al. 2013). In general, direct retinal damage by compounds tends to cause diffuse rather than more locally extensive retinal damage in the eye. On the other hand, retinal damage induced by light in rodents does not occur uniformly across the retina, at least in the early stages, but has a very specific topographic localization. Along the vertical meridian of the eye, the superior retina is more severely damaged than the inferior retina, and the central retina is more severely damaged than peripheral (Figure 1, LaVail et al. 1987; Rapp and Smith, 1992; Reme et al. 1994; Organisciak et al. 1999; Ranchon et al. 2003; Tanito et al. 2008). Additionally, rats and mice can also exhibit an incidental aging change known as peripheral retinal degeneration (DiLoreto et al. 1994). Therefore, to evaluate the mechanism of retinal degeneration in long-term rodent studies, it is very important to identify the localization of the change. Regarding the localization of damage across the retina, toxic compounds affecting the retina can be subdivided into those primarily affecting the photoreceptors or ganglion cells and those affecting the pigment epithelium (Heywood, 1982; Heywood and Gopinath, 1990). In the case of light-induced retinal degeneration, outer segments of the photoreceptor cells degenerate first, and the photoreceptor cells are involved later (Kuwabara and Gorn, 1968; Grignolo et al. 1969).
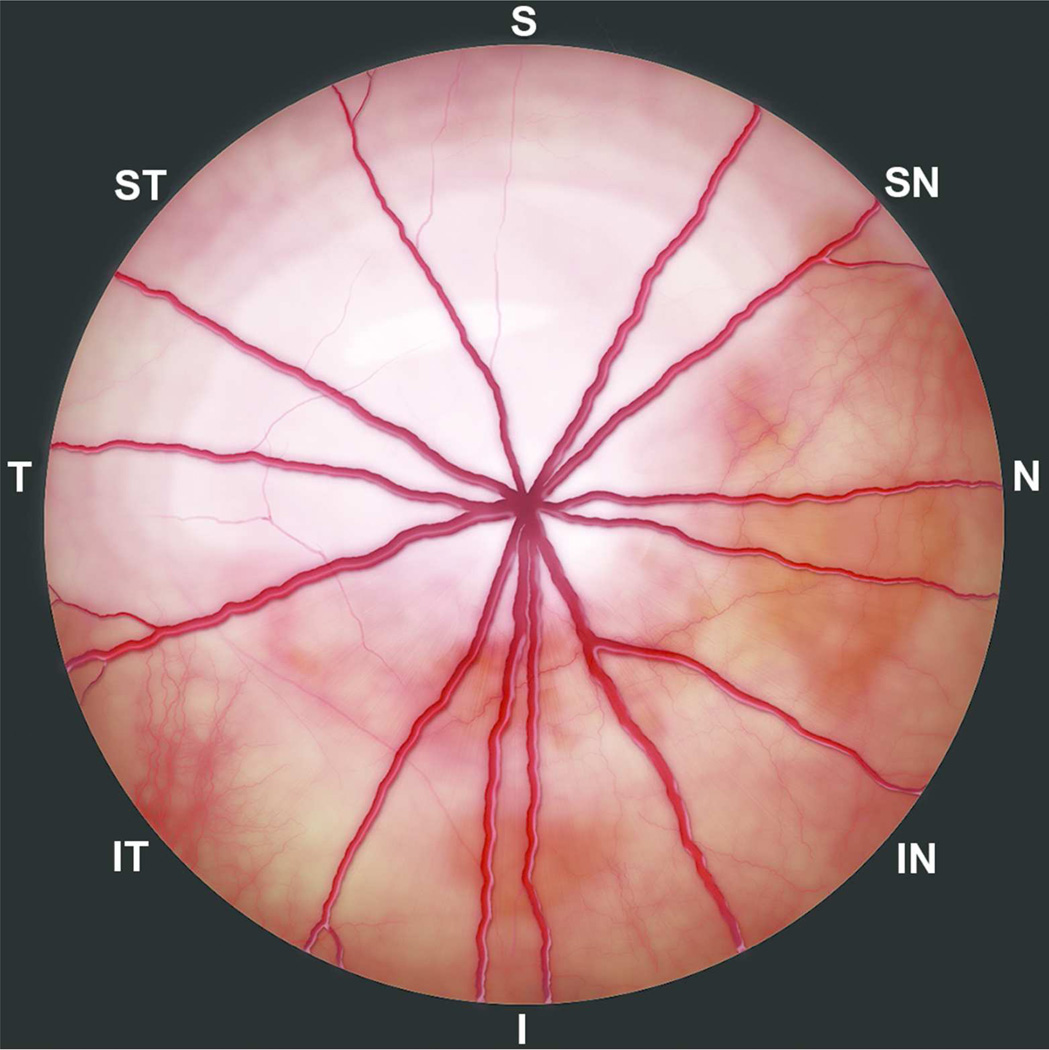
Figure 1.
Artistic rendering of funduscopic image of the right eye of a rat representing light-induced retinal degeneration (Modified from the color-coded outer nuclear layer thickness map (Fig. 3B) in Tanito et al. 2008). The superior temporal and central region show the greatest sensitivity to light damage and are thinner compared to the surrounding unaffected retina. Since thinner retina appears hyperreflective and white on funduscopy, the affected retinal areas are depicted in increasing degree of lighter shades. S, superior; I, inferior; N, nasal; T, temporal; SN, superionasal; ST, superiotemporal; IN, inferionasal; and IT, inferiotemporal.
In this study, we queried the National Toxicology Program (NTP) bioassay database for treatment related retinal degeneration in rats and mice. In addition, we have conducted a retrospective microscopic evaluation of the retinas in order to determine whether the retinal degeneration is due to direct effect of the chemical, or if it is an exacerbation of light induced retinal degeneration by the chemical.
Materials and Methods
Study Selection
The CEBS (Chemical Effects in Biological Systems, http://tools.niehs.nih.gov/cebs3/ui/) database, which includes over 2500 short (2 week or 13 week) or long-term (≥ 6 months) NTP bioassays, was searched (on June 5th, 2013) for studies with a statistically significant increase in retinal degeneration associated with increasing dose of chemical in rats and mice. Prior to 1995, the eyes were not a protocol-required tissue in NTP studies; only those animals with macroscopic ocular lesions have had histologic evaluation performed on the eyes. Histopathology of both eyes has been conducted on a routine basis in all animals in NTP studies since 1995. Of the 2580 short and long-term bioassays in the CEBS database, the numbers of studies where eye was a protocol-required tissue were 54 in rat 2-year study, 56 in mouse 2-year study, 27 each in rat and mouse 3-month studies, and 4 each in rat and mouse 2-week studies. The NTP technical reports and/or study reports of these studies were examined to identify studies that had a treatment-related increase in the incidence of retinal degeneration/atrophy.
Pathology
Hematoxylin and eosin (H&E)-stained sections of eyes from control and treated animals (kava kava extract and acrylamide - Davidson’s fixative, leucomalachite green - 10% neutral buffered formalin) were retrieved from the NTP archives. Sections of both eyes from each animal were examined microscopically for retinal degeneration. Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimens due to post mortem autolysis or due to early deaths. The severity of retinal lesions was graded based on the degree of degeneration using a three-point scale (Figure 2): Eyes were considered normal (Grade 0) when all of the different retinal layers were distinct, the inner and outer segments of the photoreceptor layer elongated, and the outer nuclear layer intact. Lesions were considered grade 1 (Mild) when the different layers of the retina remained distinct, but the photoreceptor layer and outer nuclear layer (approximately 1 to 3 rows of nuclei) were reduced in thickness. Grade 2 (Moderate) lesions were characterized by loss of the photoreceptors and most of the outer nuclear layer and outer plexiform layers. Grade 3 (Marked) lesions had severe disruption of the normal retinal architecture with loss of normal retinal organization, with or without the presence of remnants of the inner nuclear layer and ganglion cells.
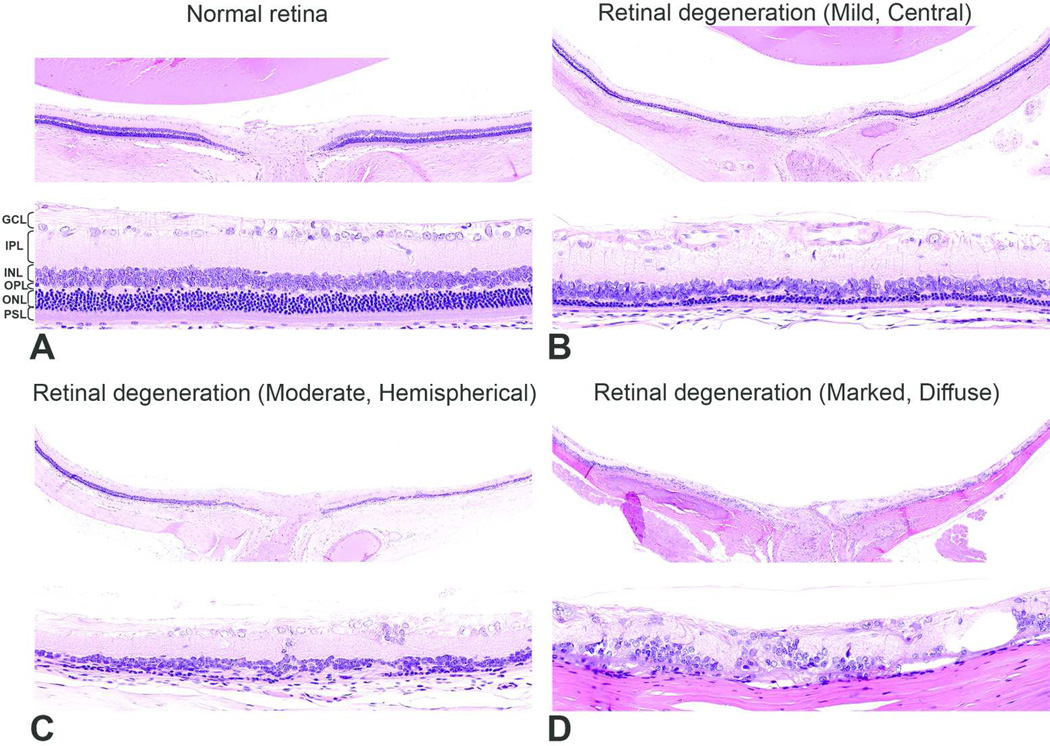
Figure 2.
Representative pictures depicting the severity of retinal degeneration. A) Grade 0 (Normal) - different retinal layers are distinct, the inner and outer segments of photoreceptor layer are elongated, outer nuclear layer is intact. B) Grade 1 (Mild) - major retinal layers remain distinct, but the photoreceptor layer and outer nuclear layer (approximately 1 to 3 rows of nuclei) are reduced in thickness. In this case, the lesion was located in the central retina. C) Grade 2 (Moderate) - photoreceptors and most of the outer nuclear layer and outer plexiform layers are lost. In this case, the lesion was located in the hemispherical retina. D) Grade 3 (Marked) - marked disruption of the normal retinal architecture with loss of normal retinal organization but with remnants of inner nuclear and ganglion cells. In this case, the lesion was diffuse. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PSL, photoreceptor segment layer. H&E.
Chronic light exposure is a common etiological factor in retinal degeneration and usually has a distinct topographic localization in which the superior temporal retina including the region around the optic disc is preferentially affected (Tanito et al. 2008, Figure 1). Proper orientation of the globe during dissection and processing is important to assess the topography of retinal damage. In routine rodent toxicity or carcinogenicity studies, the globes have been dissected and processed without considering the orientation and hence accurate topographic evaluation of the retinal lesions with regards to temporal/nasal and superior/inferior location was not possible. However, valuable information regarding the local/diffuse localization of retinal lesions could still be generated. To assess the contribution of light in retinal degeneration, the topography of the lesion was evaluated as follows (Figure 2 and 3): A diffuse localization grade was given to lesions in which the severity grade of the lesion was the same throughout the retina; a hemispherical localization was recorded when there was a distinct difference in the grade of the lesion on either side of the optic disc; a central localization was given when a distinct difference in the grade of the lesion was observed immediately adjacent to the optic disc. Additionally, the peripheral retina was evaluated separately because spontaneous peripheral retinal degeneration is frequently observed in albino rats, especially the F344 rat, by 24 months of age (DiLoreto et al. 1994). The severity of peripheral retinal degeneration was evaluated using the same three-point grading scale as described above (Figure 4). Due to the significant overlap of the criteria for the minimal retinal degeneration described by De Vera Mudry et al., (2013), with the normal retinas in the 2 year old albino rats, we chose not to use the minimal grade in this study.
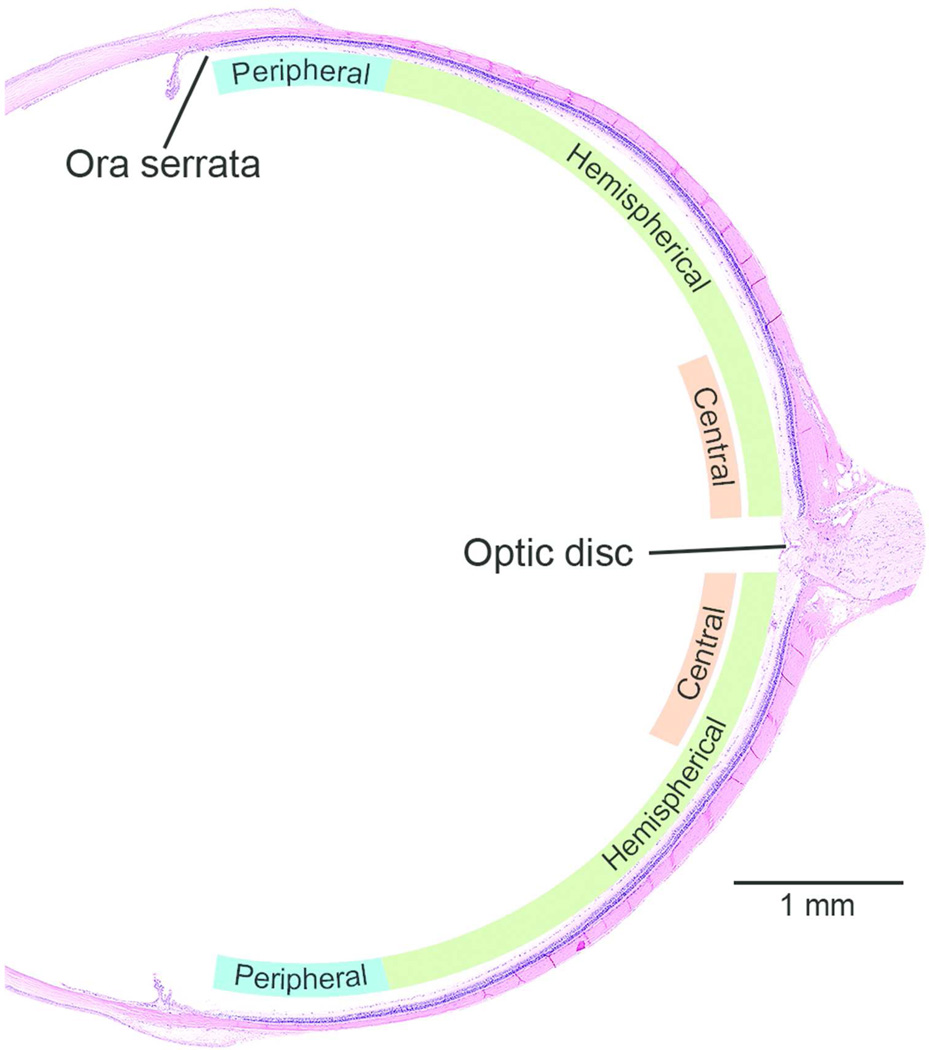
Figure 3.
Topographic localization of retinal lesions in a sagittal section. To assess whether the retinal degeneration was light-induced or not, the topography of the lesion was evaluated as follows: Diffuse - grade of the lesion is same the in the entire retina; Hemispherical - there is a distinct difference in the grade of the lesion on either side of the optic disk; Central - there is a distinct difference in the grade of the lesion just adjacent to the optic disc; and Peripheral. In general, diffuse retinal lesions are usually associated with direct chemical effect and a localized retinal lesion (at least during the early stages) may be light-induced change. Approximately 1 mm of the retina from the ora serrata was evaluated as the peripheral retina.
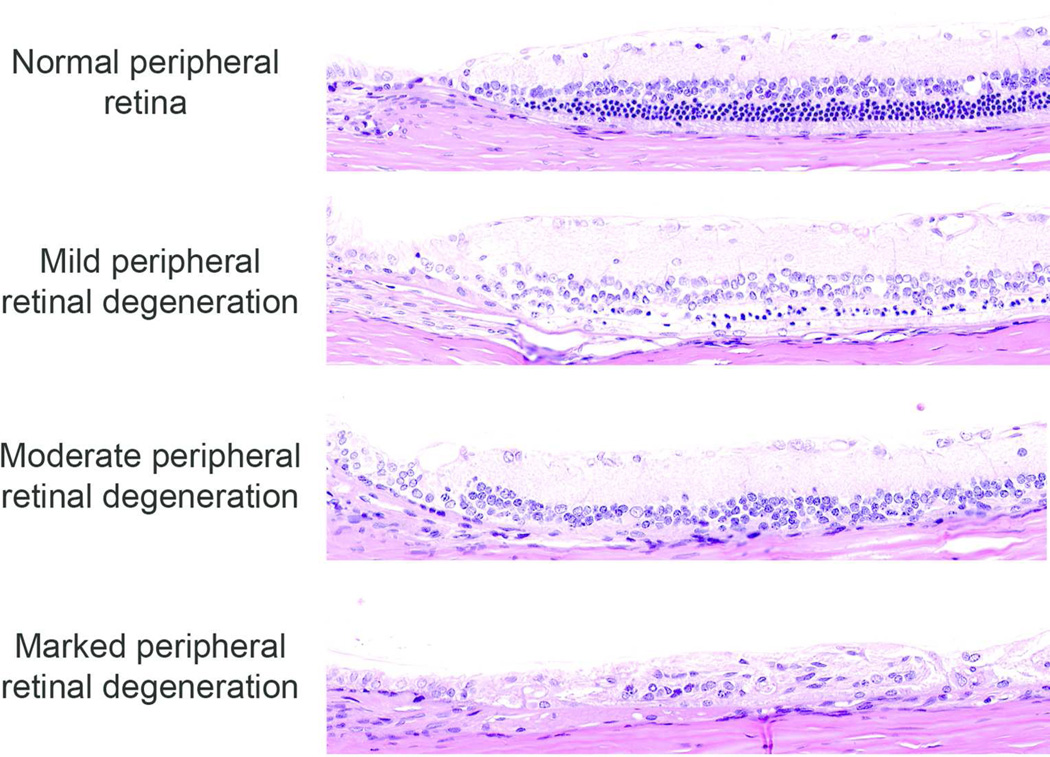
Figure 4.
Representative photomicrographs of peripheral retinal degeneration. The severity of peripheral retinal degeneration was evaluated using the same three-point grading scale as in Figure 2.
Statistical Analysis
For the frequency data, a dose-related increase in lesion frequency was evaluated using a bootstrap trend test available in the software package ORIOGEN (Peddada et al., 2003, Peddada et al., 2005) using 10,000 bootstrap samples. For the localization data, a trend test was used to evaluate the statistical significance of the dose-related increase in central/hemispherical or diffuse localization in animals with retinal degeneration. If the trend was significant then pairwise comparisons were performed to test for the differences between each dose group and the respective control group using a Dunnett type test available in ORIOGEN using 10,000 bootstrap samples. Bootstrap methodology is a useful tool that enables the researcher to not make any distributional assumptions about the test procedure. Thus it is non-parametric and provides a better control of the false positive rate (Efron and Tibshirani (1993). For the severity data, the linear mixed models analysis was used. The unit of analysis was the individual animal. Thus any severity correlation between the eyes of an animal is preserved. If the overall F-test was significant then post-hoc pairwise analysis was performed using the Dunnett’s test in the mixed effects models setting. All dose groups were compared to the control group. The differences were considered significant at p < 0.05. All severity analyses were performed using the software package SAS version 9.3.
Results
The historical control range of retinal degeneration in the NTP studies where eye was a protocol-required tissue were 0 to 79% (male), 0 to 56% (female) in 2-year studies in F344/N rat, 0 to 4% (male), 0 to 2% (female) in 2-year studies in B6C3F1 mouse, 0 to 10% (male), 0% (female) in 3-month studies in F344/N rat, and 0% (male and female) in 3-month studies in B6C3F1 mouse. Among NTP studies where eye was a protocol-required tissue, three 2-year carcinogenicity studies had a dose related increased frequency of retinal degeneration. All three studies were in F344/N rats, and included the kava kava extract oral gavage study (Dose: 0, 0.1, 0.3 and 1 g/kg, NTP 2012b), acrylamide drinking water study (Dose: 0, 0.0875, 0.175, 0.35 and 0.70 mM, NTP 2012a), and leucomalachite green feed study (Dose: 0, 91, 272 and 543 ppm, NTP 2005). In these studies, the cage position had been rotated once every two weeks in order to minimize a confounding effect of chronic light exposure to the eye. Statistical analysis of the retrospective histologic evaluation of the eyes from these three studies showed that the treatment-related increased frequencies of retinal degeneration were statistically significant by the trend test. Detailed results of each of these three chemicals will be presented individually below.
Kava Kava Extract
The frequency of retinal degeneration was significantly increased in a dose-dependent manner in the 0.3 g/kg and 1.0 g/kg groups in males, and in the 1.0 g/kg group in females, compared to the control groups (Table 1). In males and females, there were no significant differences in the average severity grade of retinal degeneration (Table 1) and the localization of retinal degeneration (Figure 5). Proportionally, central/hemispherical relative to diffuse retinal degeneration increased in females with all doses (Figure 5). The proportion of bilateral change was significantly increased in the 1.0 g/kg group compared to the control group in both males and females. In the evaluation of peripheral retinal degeneration, the average severity grade was significantly increased in a dose-dependent manner in the 0.3 g/kg and 1.0 g/kg groups in males, and in the 1.0 g/kg group in females, compared to the control groups (Table 2). There were no significant differences in the frequencies of peripheral retinal degeneration between males and females, or between treated and control animals.
Table 1.
Incidence, severity and bilaterality of retinal degeneration in F344/N male and female rats treated with kava kava extract by gavage for two years.
| 2-year bioassay of kava kava extract |
Kava kava extract (g/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.3 | 1 | ||
| Males | |||||
| Retinal degeneration | 6/45d (13%)a*** | 7/46 (15%) | 16/41 (39%)** | 23/41 (56%)*** | |
| Average severityb | 1.6 | 1.3 | 1.6 | 1.4 | |
| Bilateral changec | 17%*** | 29% | 19% | 78%*** | |
| Females | |||||
| Retinal degeneration | 6/44 (14%)* | 8/46 (17%) | 8/41 (20%) | 14/44 (32%)* | |
| Average severity | 1.8 | 2.0 | 1.6 | 1.5 | |
| Bilateral change | 0%* | 25% | 38% | 29%* | |
: Frequency of retinal degeneration within all areas of the retina (central/hemispherica l/peripheral)
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
: Rate of bilateral retinal degeneration in affected rats
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
Significant at *p < 0.05 **p < 0.01 and ***p < 0.001.
Trend test under control column; pairwise test under dose group columns.
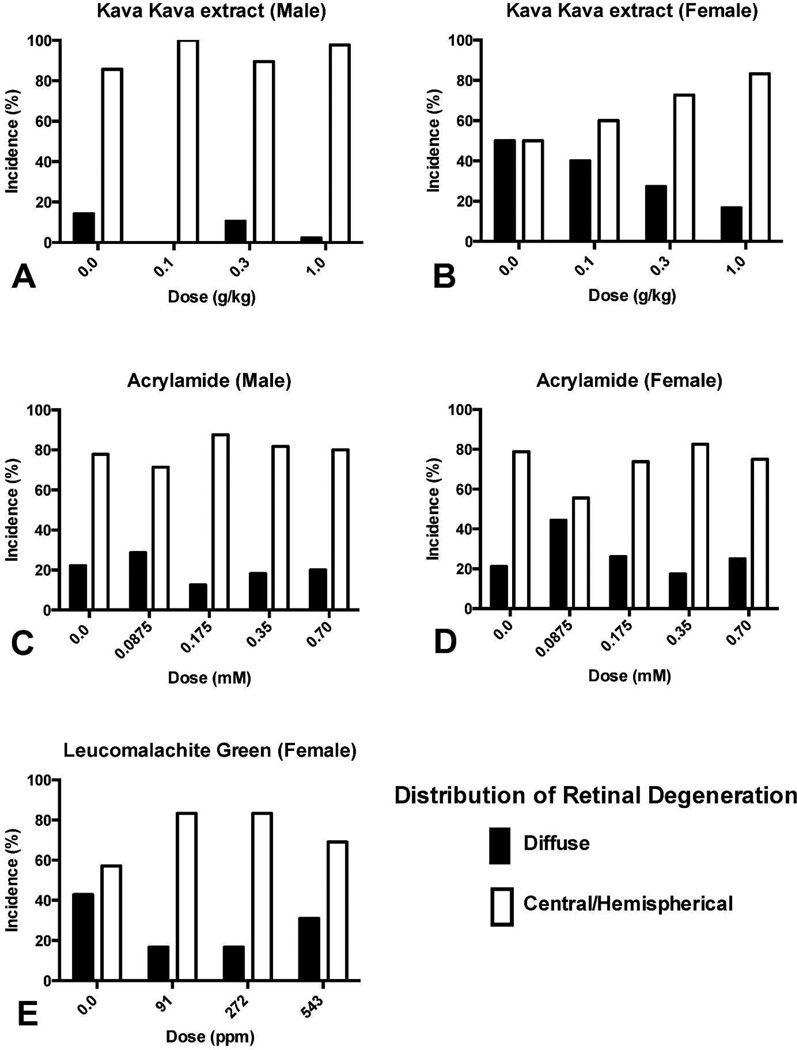
Figure 5.
Frequency of retinal degeneration in F344/N rats treated with kava kava extract (A, B), acrylamide (C, D) and leucomalachite green (E) for two years. In control animals, 50–86% of retinal degeneration occurred in the central or hemispherical retina, the typical site for light-induced retinal degeneration. Diffuse retinal degeneration, more likely due to a direct chemical effect, was proportionally less frequent and the preponderance of central/hemispherical retinal degeneration suggested that these findings were treatment-related exacerbation of light-induced retinal degeneration. The total number of F344/N rats with retinal degeneration in each group are indicated in tables 1, 3, and 5 for kava kava extract, acrylamide and leucomalachite green, respectively.
Table 2.
Incidence and severity of peripheral retinal degeneration in F344/N male and female rats treated with kava kava extract by gavage for two years.
| 2-year bioassay of kava kava extract |
Kava kava extract (g/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.3 | 1 | ||
| Males | |||||
| Retinal degeneration, peripheral | 44/45c (98%)a | 45/46 (98%) | 46/46 (100%) | 44/47 (94%) | |
| Average severityb | 1.6** | 1.8 | 1.8* | 1.9** | |
| Females | |||||
| Retinal degeneration, peripheral | 46/48 (96%) | 47/49 (96%) | 43/46 (93%) | 42/46 (91%) | |
| Average severity | 1.6** | 1.8 | 1.7 | 2.0*** | |
: Frequency
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
Significant at *p < 0.05 **p < 0.01 and ***p < 0.001.
Trend test under control column; pairwise test under dose group columns.
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
Acrylamide
In males, the frequency of retinal degeneration was significantly increased in the 0.70 mM group compared to the control group. There were no significant differences in the average severity grade and the proportion of bilateral change of retinal degeneration (Table 3), and the localization of retinal degeneration (Figure 5). In the evaluation of peripheral retinal degeneration, there were no changes in the frequency or the average severity grade in any of the groups (Table 4).
Table 3.
Incidence, severity and bilaterality of retinal degeneration in F344/N male and female rats treated with acrylamide in drinking water for two years.
| 2-year bioassay of Acrylamide |
Acrylamide (mM) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Males | ||||||
| Retinal degeneration | 7/42d (17%)a* | 6/46 (13%) | 13/45 (29%) |
10/45 (22%) |
16/44 (36%)* |
|
| Average severityb | 1.4 | 1.7 | 1.1 | 1.4 | 1.5 | |
| Bilateral changec | 14% | 17% | 23% | 0% | 25% | |
| Females | ||||||
| Retinal degeneration | 16/44 (36%)* | 12/46 (26%) | 17/46 (37%) |
17/45 (38%) |
24/46 (52%) |
|
| Average severity | 1.3 | 1.8 | 1.9 | 1.6 | 1.8 | |
| Bilateral change | 19%* | 50% | 35% | 35% | 50%* | |
: Frequency of retinal degeneration within all areas of the retina (central /hemispherical/peripheral)
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
: Rate of bilateral retinal degeneration in affected rats
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
Significant at *p < 0.05.
Trend test under control column; pairwise test under dose group columns.
Table 4.
Incidence and severity of peripheral retinal degeneration in F344/N male and female rats treated with acrylamide in drinking water for two years.
| 2-year bioassay of acrylamide |
Acrylamide (mM) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Males | ||||||
| Retinal degeneration, peripheral | 40/42c (95%)a | 40/47 (85%) | 38/46 (83%) |
45/46 (98%) |
41/44 (93%) |
|
| Average severityb | 1.8 | 1.8 | 1.9 | 1.8 | 1.9 | |
| Females | ||||||
| Retinal degeneration, peripheral | 43/45 (96%) | 46/46 (100%) | 45/46 (98%) |
40/44 (91%) |
41/46 (89%) |
|
| Average severity | 1.9 | 1.9 | 1.9 | 2.0 | 1.9 | |
: Frequency
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
In females, there were no changes in the frequency of retinal degeneration in any of the groups when compared to the control group, although the trend was significant (Table 3). The proportion of bilateral change was significantly increased in the 0.70 mM group compared to the control group (Table 3). There were no significant differences in the average severity grade of retinal degeneration (Table 3) and the localization of retinal degeneration (Figure 5). In the evaluation of peripheral retinal degeneration, there were no changes in the frequency or the average severity grade in any of the groups (Table 4).
Leucomalachite Green
In females, the frequency of retinal degeneration was significantly increased in the 543 ppm group compared to the control group, but there were no significant differences in the average severity grade of retinal degeneration (Table 5) and the localization of retinal degeneration (Figure 5). Proportionally, central/hemispherical relative to diffuse retinal degeneration increased in all doses (Figure 5). In the evaluation of peripheral retinal degeneration, the average severity grade was significantly increased in the 543 ppm group compared to the control group, but there were no significant changes in the frequency of peripheral retinal degeneration in any of the groups (Table 6).
Table 5.
Incidence, severity and bilaterality of retinal degeneration in F344/N female rats treated with Leucomalachite green in feed for two years.
| 2-year bioassay of Leucomalachite green |
Leucomalachite Green (ppm) | ||||
|---|---|---|---|---|---|
| 0 | 91 | 272 | 543 | ||
| Females | |||||
| Retinal degeneration | 4/33d (12%)a*** | 8/37 (22%) | 8/33 (24%) | 22/35 (63%)*** |
|
| Average severityb | 1.7 | 1.7 | 1.3 | 1.8 | |
| Bilateral changec | 50% | 25% | 38% | 27% | |
: Frequency of retinal degeneration within all areas of the retina (central/hemispherical/peripheral)
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
: Rate of bilateral retinal degeneration in affected rats
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
Significant at ***p < 0.001.
Trend test under control column; pairwise test under dose group columns.
Table 6.
Incidence and severity of peripheral retinal degeneration in F344/N female rats treated with Leucomalachite green in feed for two years.
| 2-year bioassay of Leucomalachite green |
Leucomalachite green (ppm) | ||||
|---|---|---|---|---|---|
| 0 | 91 | 272 | 543 | ||
| Females | |||||
| Retinal degeneration, peripheral | 41/42c (98%)a | 40/42 (95%) | 40/41 (98%) | 37/37 (100%) | |
| Average severityb | 1.6** | 1.7 | 1.7 | 2.0*** | |
: Frequency
: Average severity grade of lesions in affected eyes: 1 = mild, 2 = moderate, 3 = marked
: Fifty animals per group were initially assigned to each study however some animals were excluded from the assessment because of improper specimen handling, post mortem autolysis or due to early deaths.
Significant at **p < 0.01 and ***p < 0.001.
Trend test under control column; pairwise test under dose group columns.
Discussion
Among NTP studies where eye was a protocol-required tissue, only three 2-year carcinogenicity bioassays could be identified that showed an increase in the frequencies of retinal degeneration in F344/N rats. Retrospective microscopic evaluation of retinas from these three studies was conducted in order to determine whether the retinal degeneration was due to a direct chemical effect, or chemical exacerbation of light-induced retinal degeneration.
Most of the spontaneous retinal degeneration observed in long-term (≥ 6 months) toxicity studies in albino rodents is attributed to chronic light exposure (Rao 1991; Perez and Perentes 1994). Indeed, 50 to 86% of retinal degeneration in control groups in this retrospective study was observed in the central or hemispherical retina (Figure 5), which is a primary feature of light-induced retinal degeneration during early stages of disease (Figure 1 and 2). Therefore, in long-term albino rat and mouse studies, it is important to differentiate test article-associated direct retinal damage from light-induced retinal degeneration, or to identify a possible interaction between test article and light that could result in retinal degeneration.
To determine whether the retinal degeneration is light-related or not, it is essential to evaluate the topography of the retinal lesions (De Vera Mudry et al. 2013). At least in the early stages of light-related retinal degeneration, the superior temporal retina, including the region around the optic disc, is more severely damaged along the vertical median of the eye (Tanito et al. 2008, Figure 1). On the other hand, direct retinal damage by systemic chemical exposure is thought to cause bilateral and diffuse involvement rather than more locally extensive lesions. Taking into consideration the factors mentioned above, in addition to the frequency of retinal degeneration, laterality (unilateral/bilateral) and topographic localization of the retinal degeneration was evaluated in this retrospective study. In general, the globes in these and other rodent toxicity studies are dissected and processed without consideration to the orientation. It would be preferable to fix the globe orientation while preparing eye sections to allow for more accurate topographic evaluation, especially in the long-term (≥ 6 months) rodent toxicity studies in which light-induced retinal degeneration may be observed spontaneously.
As a result of the retrospective microscopic evaluation, treatment-related increases in the frequency of retinal degeneration were found in all three chemicals (Table 1, 2 and 3) and they were in good agreement with those previously reported by the NTP (NTP 2012a; NTP 2012b; NTP 2005). Diffuse retinal degeneration, a change which might indicate direct chemical damage to the retina, did not increase relative to central/hemispherical retinal degeneration (Figure 5). Therefore, these data suggest that all three chemicals exacerbated the light-induced retinal degeneration that is spontaneously observed in long-term toxicity studies in albino rats.
At least three different mechanisms of exacerbation of light induced retinal degeneration in long-term rat toxicity studies have been reported; (1) pupillary dilation, (2) taurine deficiency and (3) reduction of disc shedding. Clonidine, an α2 adrenergic agonist, induced a dose-dependent increase in the frequency and severity of retinal degeneration in albino rats (Weisse et al. 1971). Clonidine causes pupillary dilation, and a causal association was confirmed between light intensity and retinal damage (Weisse et al. 1971).
Taurine is critical for photoreceptor development, and acts as a cytoprotectant against stress-related neuronal damage (Ripps and Shen 2012). Deficiency of taurine is a well known cause of retinal phototoxicity (Rapp et al. 1988; Cocker and Lake 1989; Leon et al. 1995; Neuringer and Sturman 1987; Imaki et al. 1987). Also, chemicals that alter taurine metabolism or cause taurine deficiency induce retinal degeneration (Jammoul et al. 2009). Vigabatrin, a GABA transaminase inhibitor, exacerbates light-induced retinal degeneration in albino rats, which can be partially prevented by taurine supplementation (Izumi et al. 2004; European Medicines Agency [EMEA] 1999; Jammoul et al. 2009). The vigabatrin-elicited increase in GABA could explain this observation because GABA is a known competitive inhibitor of the taurine transporter (Lee and Kang 2004).
Disc shedding, which involves phagocytosis of the outer segments of photoreceptors by retinal pigment epithelium, is essential to photoreceptor homeostasis (Young and Droz 1968; Kevany and Palczewski 2010). Perturbations in disc shedding lead to excessive accumulation of photo-oxidized products, which are believed to be an underlying cause of degenerative retinal diseases (Kevany and Palczewski 2010). Pramipexole dihydrochloride, a dopamine agonist, induced retinal degeneration in albino rats in a 2- year carcinogenicity study, which was enhanced by light intensity (U.S. Food and Drug Administration [FDA] 2013). Investigative studies demonstrated that pramipexole reduced the rate of disc shedding, which was associated with enhanced sensitivity to damaging effects of light in albino rats (FDA 1997). In addition to these three mechanisms, chemicals or drugs that cause phototoxicity can exacerbate light-induced retinal degeneration (Shimoda et al. 1993).
Kava kava extract is an herbal product used extensively as an alternative to anti-anxiety drugs (NTP 2012b). Facilitation of GABAergic transmission and changes in dopamine concentrations in the brain may contribute to the anti-anxiety and sedative properties of Kava kava extract (Singh et al. 2002). Pupil dilation has also been reported in humans consuming Kava kava extract (Garner et al. 1985) but similar changes have not been documented in the rat. Therefore, some of the mechanisms which have been reported to cause exacerbation of light induced retinal degeneration may also contribute to the retinal degeneration observed in the Kava kava extract 2-year NTP rat study.
Acrylamide is used in industry to produce polyacrylamides, but it is also produced in small amounts during baking and frying of starch-rich foods (NTP 2012a). Acrylamide is a neurotoxic agent that causes central and peripheral axonopathy, characterized by distal retrograde degeneration of long and large diameter axons (Burek et al. 1980; Fullerton and Barnes 1966; Spencer and Schaumburg 1977). Experimentally induced acrylamide intoxication has been shown to cause abnormal retinal function and microscopic changes in retinal ganglion cells and retinogeniculate axons in rats and nonhuman primates (Wild and Kulikowski 1984; Eskin et al. 1985; Eskin and Merigan 1986; Merigan and Eskin 1986). In the 2-year NTP rat study, although axonal degeneration of the sciatic nerve was found in a dose-related manner in both sexes, there were no histologic changes observed in the ganglion cell layer or optic nerve in the light microscopic evaluation of H&E stained slides. Glycidamide, a metabolite of acrylamide, has also been evaluated in NTP chronic studies and did not induce the retinal degeneration observed in the acrylamide study (NTP 2014).
Leucomalachite green is formed by the reduction of malachite green chloride, a dye used in the fish industry as an antifungal agent, and persists in the tissues of exposed fish. Malachite green chloride was also evaluated by the NTP, and showed a slight increase in retinal degeneration in female rats, although this frequency was not statistically significant (NTP 2005). Similar to Malachite green chloride, an increased frequency of retinal degeneration was found only in females in the 2-year study of leucomalachite green. The cause of this sex difference is unknown; however, female rats are known to be more sensitive than male rats to the toxic effects of malachite green chloride (NTP 2005). Although there is no literature demonstrating a definitive link between these chemicals and retinal toxicity, in general dyes affect light absorption with the potential to exacerbate light-induced retinal degeneration in albino rats. For example, indocyanine green and halogen or xenon light sources induce histologic and functional damage to the retina in human eyes (Haritoglou et al. 2005). A photosensitizing effect might be induced by an overlap of the emission spectrum of the light source and the absorption band of the indocyanine green, resulting in morphologic damage to the retina.
In the 2-year pigmented B6C3F1 mouse studies, Kava kava extract, acrylamide, and leucomalachite green did not induce retinal degeneration. It is possible that the exacerbation of light induced retinal degeneration in these studies is a characteristic of the albino rat, since similar changes have not been reported in humans. On the other hand, vigabatrin is known to induce visual field defects in humans, although the exacerbation of light induced retinal degeneration with vigabatrin has only been observed in albino rats in pre-clinical toxicity studies (EMEA 1999). Therefore, it is uncertain whether or not the exacerbation of light-induced retinal degeneration in albino rats is relevant to humans. Further studies are needed in order to better understand the mechanisms of this change, and to further evaluate the risk of these three chemicals to humans.
The chemical induced dose-related exacerbation of light-induced retinal degeneration occurred only in the albino F344/N rats but not in the B6C3F1 mice. Pigmented B6C3F1 mice are used in the NTP studies and it is well known that ocular pigmentation protects against damage primarily by lowering the retinal irradiance (Rapp and Williams, 1980). Therefore, it is not surprising that the exacerbation of light-induced retinal degeneration was not found in the B6C3F1 mice in NTP studies.
The cause of regional variations in light-induced retinal damage is still unclear. Differences in light exposure among retinal regions may explain the regional variation. Meanwhile, various factors, such as rhodopsin content, fatty acid composition and expression of cytoprotective molecules, could affect the susceptibility to retinal damage (Rapp and Williams, 1977; Anderson and Penn, 2004; Faktorovich et al., 1992; LaVail et al., 1992). Therefore, differences in those factors among retinal regions may also explain the regional variations of retinal damage, but the mechanism still remains to be clarified.
Peripheral retinal degeneration is known to occur as an aging change in the F344 rat after 12 to 18 months of age and this change is observed in almost all F344 rats at 24 months of age (DiLoreto et al. 1994). Hence, the frequency and the grade of peripheral retinal degeneration were evaluated separately from the other areas of the retina. Although there were no effects on the frequency, the severity of peripheral degeneration increased in a dose-related manner in the kava kava extract and leucomalachite green studies. These changes in the peripheral retina could be related to the exacerbation of light-induced change since this change is known to be exacerbated by ambient light (Lai et al. 1978). In acrylamide, the peripheral retina of treated animals did not show any significant differences compared to the control groups in either males or females. However, since the severity grade in the control group of the acrylamide study was relatively high compared to that in the kava kava extract and leucomalachite green studies, a possible effect could have been masked. Evaluation of peripheral retina may give additional evidence on the exacerbation of light-induced retinal degeneration.
In summary, only three chemicals studied in the F344/N rats but not in B6C3F1 mice induced treatment-related increase in retinal degeneration in the NTP studies. Based on the localization of the lesions, it is hypothesized that these three chemicals might have exacerbated the light-induced retinal degeneration. Light microscopic evaluation with slides prepared by proper orientation of the eye is an important starting point to differentiate test article-associated retinal damage from retinal phototoxicity or to identify the interaction between the test article and ambient light that results in retinal degeneration. In cases in which retinal degeneration is found in a dose-dependent manner in a long-term albino rodent toxicity study, evaluation of the morphology and topography of the retinal lesions is warranted to distinguish exacerbation of the light-induced retinal degeneration or direct phototoxicity of the test article. If the retinal lesions are characteristic of light-induced changes, additional careful evaluation in other species including pigmented animals as well as additional mechanistic studies may help to better understand the underlying mechanisms and to assess human risk.
Acknowledgments
We would like to thank Mr. David Sabio, Experimental Pathology Laboratories Inc, for the artistic rendering of the funduscopic image based on figure 3B from Tanito et al., 2008 al. We appreciate the assistance provided by the NTP archives, as well as at the National Center for Toxicologic Research (NCTR). We acknowledge Drs. Gordon Flake, Peter Little and David Malarkey for helpful comments on this manuscript and Ms. Beth Mahler for preparing the images for publication. We would also like to thank Mr. Marcus Jackson and Ms. Carolyn Favaro for generating the F344/N rat and B6C3F1 mouse historical control data on retinal degeneration/atrophy in NTP studies.
References
- Anderson RE, Penn JS. Environmental light and heredity are associated with adaptive changes in retinal DHA levels that affect retinal function. Lipids. 2004;39:1121–1124. doi: 10.1007/s11745-004-1338-8. [DOI] [PubMed] [Google Scholar]
- Breider MA, Pilcher GD, Graziano MJ, Gough AW. Retinal degeneration in rats induced by CI-1010, a 2-nitroimidazole radiosensitizer. Toxicol Pathol. 1998;26:234–239. doi: 10.1177/019262339802600207. [DOI] [PubMed] [Google Scholar]
- Burek JD, Albee RR, Beyer JE, Bell TJ, Carreon RM, Morden DC, Wade CE, Hermann EA, Gorzinski SJ. Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J Environ Pathol Toxicol. 1980;4:157–182. [PubMed] [Google Scholar]
- Cocker SE, Lake N. Effects of dark maintenance on retinal biochemistry and function during taurine depletion in the adult rat. Vis Neurosci. 1989;3:33–38. doi: 10.1017/s0952523800012487. [DOI] [PubMed] [Google Scholar]
- Del Cerro M, Grover D, Monjan A, Pfau C, Dematte J. Congenital retinitis in the rat following maternal exposure to lymphocytic choriomeningitis virus. Exp Eye Res. 1984;38:313–324. doi: 10.1016/0014-4835(84)90169-6. [DOI] [PubMed] [Google Scholar]
- De Vera Mudry MC, Kronenberg S, Komatsu S, Aguirre GD. Blinded by the light: retinal phototoxicity in the context of safety studies. Toxicol Pathol. 2013;41:813–825. doi: 10.1177/0192623312469308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLoreto D, Jr, Cox C, Grover DA, Lazar E, del Cerro C, del Cerro M. The influences of age, retinal topography, and gender on retinal degeneration in the Fischer 344 rat. Brain Res. 1994;647:181–191. doi: 10.1016/0006-8993(94)91316-1. [DOI] [PubMed] [Google Scholar]
- DiLoreto DA, Jr, Del Cerro C, Cox C, Del and Cerro M. Changes in visually guided behavior of Royal College of Surgeons rats as a function of age: a histologic, morphometric, and functional study. Invest Ophthalmol Vis Sci. 1998;39:1058–1063. [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall/CRC Monographs on Statistics & Applied Probability; 1993. [Google Scholar]
- EMEA. Committee for proprietary medicinal products opinion following an article 12 referral. Vigabatrin. 1999 CPMP/1357/99. [Google Scholar]
- Eskin TA, Lapham LW, Maurissen JP, Merigan WH. Acrylamide effects on the macaque visual system. II. Retinogeniculate morphology. Invest Ophthalmol Vis Sci. 1985;26:317–329. [PubMed] [Google Scholar]
- Eskin TA, Merigan WH. Selective acrylamide-induced degeneration of color opponent ganglion cells in macaques. Brain Res. 1986;378:379–384. doi: 10.1016/0006-8993(86)90941-8. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Mirapex (pramipexole dihydrochloride) Pharmacology review. 1997 [Google Scholar]
- FDA. Mirapex (pramipexole dihydrochloride) Prescribing information. 2013 [Google Scholar]
- Fullerton PM, Barnes JM. Peripheral neuropathy in rats produced by acrylamide. Br J Ind Med. 1966;23:210–221. doi: 10.1136/oem.23.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame SR, Carlton WW. Toxic retinopathy, rat, mouse, and hamster. In: Jones TC, Mohr U, Hunt RD, editors. International Life Sciences Institute Monographs on Pathology of Laboratory Animals, Eye and Ear. Berlin: Springer-Verlag; 1991. pp. 116–124. [Google Scholar]
- Garner LF, Klinger JD. Some visual effects caused by the beverage kava. J Ethnopharmacol. 1985;13:307–311. doi: 10.1016/0378-8741(85)90076-5. [DOI] [PubMed] [Google Scholar]
- Grahn BH, Paterson PG, Gottschall-Pass KT, Zhang Z. Zinc and the eye. J Am Coll Nutr. 2001;20(suppl 2):106–118. doi: 10.1080/07315724.2001.10719022. [DOI] [PubMed] [Google Scholar]
- Grignolo A, Orzalesi N, Castellazzo R, Vittone P. Retinal damage by visible light in albino rats. An electron microscope study. Ophthalmologica. 1969;157:43–59. doi: 10.1159/000305619. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Priglinger S, Gandorfer A, Welge-Lussen U, Kampik A. Histology of the vitreoretinal interface after indocyanine green staining of the ILM, with illumination using a halogen and xenon light source. Invest Ophthalmol Vis Sci. 2005;46:1468–1472. doi: 10.1167/iovs.04-0838. [DOI] [PubMed] [Google Scholar]
- Illanes O, Anderson S, Niesman M, Zwick L, Jessen BA. Retinal and peripheral nerve toxicity induced by the administration of a pan-cyclin dependent kinase (cdk) inhibitor in mice. Toxicol Pathol. 2006;34:243–248. doi: 10.1080/01926230600713186. [DOI] [PubMed] [Google Scholar]
- Imaki H, Moretz R, Wisniewski H, Neuringer M, Sturman J. Retinal degeneration in 3-month-old rhesus monkey infants fed a taurine-free human infant formula. J Neurosci Res. 1987;18:602–614. doi: 10.1002/jnr.490180414. [DOI] [PubMed] [Google Scholar]
- Jammoul F, Wang Q, Nabbout R, Coriat C, Duboc A, Simonutti M, Dubus E, Craft CM, Ye W, Collins SD, Dulac O, Chiron C, Sahel JA, Picaud S. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann Neurol. 2009;65:98–107. doi: 10.1002/ana.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Gorn RA. Retinal damage by visible light. An electron microscopic study. Arch Ophthalmol. 1968;79:69–78. doi: 10.1001/archopht.1968.03850040071019. [DOI] [PubMed] [Google Scholar]
- Lai YL, Jacoby RL, Jonas AM. Age-related and light-associated retinal changes in Fischer rats. Invest Ophthamol Vis Sci. 1978;17:634–638. [PubMed] [Google Scholar]
- LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EW, Render JA, Garner CD, Brady AN, Li LC. Unilateral degeneration of retina and optic nerve in Fischer-344 rats. Vet Pathol. 1990;27:439–444. doi: 10.1177/030098589902700609. [DOI] [PubMed] [Google Scholar]
- Lee NY, Kang YS. The brain-to-blood efflux transport of taurine and changes in the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain Res. 2004;1023:141–147. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Leon A, Levick WR, Sarossy MG. Lesion topography and new histological features in feline taurine deficiency retinopathy. Exp Eye Res. 1995;61:731–741. doi: 10.1016/s0014-4835(05)80024-7. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Eskin TA. Spatio-temporal vision of macaques with severe loss of P beta retinal ganglion cells. Vision Res. 1986;26:1751–1761. doi: 10.1016/0042-6989(86)90125-2. [DOI] [PubMed] [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of malachite green chloride and leucomalachite green. (CAS NOS. 569-64-2 and 129-73-7) in F344/N rats and B6C3F1 mice (feed studies) Natl Toxicol Program Tech Rep Ser. 2005:1–312. [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of acrylamide (CASRN 79-06-1) in F344/N rats and B6C3F1 mice (feed and drinking water studies) Natl Toxicol Program Tech Rep Ser. 2012a:1–234. [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 2012b:1–186. [PubMed] [Google Scholar]
- NTP. NTP TR 588. Research Triangle Park, NC: NIEHS; 2014. Toxicology and Carcinogenesis Studies of Glycidamide (CAS No. 5694-00-8) in F344/N Nctr Rats and B6C3F1/Nctr Mice (Drinking Water Studies) [Google Scholar]
- Neuringer M, Sturman J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. J Neurosci Res. 1987;18:597–601. doi: 10.1002/jnr.490180413. [DOI] [PubMed] [Google Scholar]
- Nyska A, Maronpot RR, Ghanayem BI. Ocular thrombosis and retinal degeneration induced in female F344 rats by 2-butoxyethanol. Hum Exp Toxicology. 1999;18:577–582. doi: 10.1191/096032799678845070. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Noell WK, Blanks JC. Hyperthermia accelerates retinal light damage in rats. Invest Ophthalmol Vis Sci. 1995;36:997–1008. [PubMed] [Google Scholar]
- Peddada SD, Harris S, Zajd J, Harvey E. ORIOGEN: Order restricted inference for ordered gene expression data. Bioinformatics. 2005;21:3933–3934. doi: 10.1093/bioinformatics/bti637. [DOI] [PubMed] [Google Scholar]
- Perez J, Perentes E. Light-induced retinopathy in the albino rat in long-term studies. An immunohistochemical and quantitative approach. Exp Toxicol Pathol. 1994;46:229–235. doi: 10.1016/S0940-2993(11)80088-6. [DOI] [PubMed] [Google Scholar]
- Rao GN. Light intensity-associated eye lesions of Fischer 344 rats in long-term studies. Toxicol Pathol. 1991;19:148–155. doi: 10.1177/019262339101900209. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Thum LA, Anderson RE. Synergism between environmental lighting and taurine depletion in causing photoreceptor cell degeneration. Exp Eye Res. 1988;46:229–238. doi: 10.1016/s0014-4835(88)80080-0. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Williams TP. Rhodopsin content and electroretinographic sensitivity in light-damaged rat retina. Nature. 1977;267:835–836. doi: 10.1038/267835a0. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Williams TP. The role of ocular pigmentation in protecting against retinal light damage. Vision Res. 1980;20:1127–1131. doi: 10.1016/0042-6989(80)90050-4. [DOI] [PubMed] [Google Scholar]
- Ripps H, Shen W. Review: taurine: a "very essential" amino acid. Mol Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Robison WG, Jr, Kuwabara T, Bieri JG. Deficiencies of vitamins E and A in the rat. Retinal damage and lipofuscin accumulation. Invest Ophthalmol Vis Sci. 1980;19:1030–1037. [PubMed] [Google Scholar]
- Serfilippi LM, Pallman DR, Gruebbel MM, Kern TJ, Spainhour CB. Assessment of retinal degeneration in outbred albino mice. Comp Med. 2004;54:69–76. [PubMed] [Google Scholar]
- Singh YN, Singh NN. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs. 2002;16:731–743. doi: 10.2165/00023210-200216110-00002. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process. IV. Differential vulnerability of PNS and CNS fibers in experimental central553 peripheral distal axonopathies. J Neuropathol Exp Neurol. 1977;36:300–320. doi: 10.1097/00005072-197703000-00006. [DOI] [PubMed] [Google Scholar]
- Sun H, Wang Y, Pang IH, Shen J, Tang X, Li Y, Liu C, Li B. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Mol Vis. 2011;17:864–875. [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Kaidzu S, Ohira A, Anderson RE. Topography of retinal damage in light-exposed albino rats. Exp Eye Res. 2008;87:292–295. doi: 10.1016/j.exer.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wild HM, Kulikowski JJ. Neurotoxic effects of acrylamide on rat retinogeniculate fibres. Behav Brain Res. 1984;13:201–207. doi: 10.1016/0166-4328(84)90162-1. [DOI] [PubMed] [Google Scholar]
- Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]