Abstract
5,6-Methylenedioxy-2-aminoindane (MDAI) has become a common substitute for (±)-3,4-methylenedioxymethamphetamine (MDMA) in Ecstasy. MDAI is known to produce MDMA-like discriminative stimulus effects, but it is not known whether MDAI has psychostimulant or hallucinogen-like effects. MDAI was tested for locomotor stimulant effects in mice and subsequently for discriminative stimulus effects in rats trained to discriminate cocaine (10 mg/kg, i.p.), methamphetamine (1 mg/kg, i.p.), ±MDMA (1.5 mg/kg, i.p.), or (−)-2,5-dimethoxy-4-methylamphetamine hydrochloride (DOM) (0.5 mg/kg, i.p.) from saline. The ability of MDAI to produce conditioned place preference was also tested in mice. MDAI (3 to 30 mg/kg) depressed locomotor activity from 10 to 60 min. A rebound stimulant effect was observed at 1 to 3.5 hr following 30 mg/kg. Lethality occurred in 8/8 mice following 100 mg/kg MDAI. Similarly, MDMA depressed locomotor activity immediately following administration of 0.25 mg/kg and stimulant effects were observed 50–70 min following administration of 0.5 and 1 mg/kg. MDAI fully substituted for the discriminative stimulus effects of MDMA (2.5 mg/kg), DOM (5 mg/kg) and cocaine (7.5 mg/kg), but produced only 73% methamphetamine-appropriate responding at a dose that suppressed responding (7.5 mg/kg). MDAI produced tremors at 10 mg/kg in one methamphetamine-trained rat. MDAI produced conditioned place preference from 0.3 to 10 mg/kg. The effects of MDAI on locomotor activity and drug discrimination were similar to those produced by MDMA, having both psychostimulant- and hallucinogen-like effects, so MDAI may have similar abuse potential as MDMA.
Keywords: MDAI, abuse potential, locomotor activity, drug discrimination, place conditioning, rat, mouse
Introduction
5,6-Methylenedioxy-2-aminoindane (MDAI), which has a similar structure to 3,4-methylenedioxymethamphetamine (MDMA), has become a popular club drug (Corkery et al., 2013; Sainsbury et al., 2011). Typically, clubs drugs such as MDMA and MDAI are taken for their social-enhancing effects rather than for any psychostimulant effects, and are reported by users to evoke feelings of empathy, closeness to others, relaxation and happiness (Cohen, 1995). Other compounds are reported to produce similar effects, and these drugs have been labeled entactogens based on their distinctive subjective effects. MDMA is a common component of the club drugs called Ecstasy, Mandy or Molly, which are used largely during “raves” (i.e., large dance parties), to increase energy and social interaction (Kalant, 2001). Use of club drugs including MDMA has been steadily increasing (Parrot & Lasky, 1998; Scholeya et al., 2004; Winstock et al., 2012). However, whereas MDMA is a controlled substance in the USA and elsewhere, MDAI is not controlled (Casale and Hayes, 2011). Because of its similarity to both stimulants and hallucinogens, MDMA produces a unique profile of abuse liability wherein compulsive use is limited relative to classical psychostimulants such as cocaine and methamphetamine, but dependence and withdrawal have been self-reported in a small subset of users (Cottler et al., 2009; Degenhardt et al., 2010).
MDMA produces a wide range of effects on several neurotransmitter systems, including blockade of dopamine, serotonin and norepinephrine transporters, release of monoamines, and acts as an agonist at 5-HT1A and 5-HT-2A receptors (see reviews by Cole and Sumnall, 2003; Schenk, 2011). Similarly, MDAI causes the release of 5-HT (Johnson et al., 1991b), but also inhibits uptake of dopamine, norepinephrine, and serotonin to a lesser extent (Johnson et al., 1991a).
Because the discriminative stimulus effects of MDMA are both serotonin- and dopamine-mediated, it is not surprising that MDMA produces effects similar to both hallucinogens and psychostimulants. MDMA produces some psychostimulant-like effects and some hallucinogen-like effects. It fully cross-substitutes with methamphetamine (i.e., MDMA fully substitutes in methamphetamine-trained rats and methamphetamine fully substitutes in MDMA-trained rats), but shows inconsistent cross-substitution with cocaine and amphetamine (see review in Gatch et al., 2009). In contrast, MDMA fully substituted in rats trained to discriminate DOM and DMT, and partially substituted in LSD-trained rats, whereas LSD produced full substitution in MDMA-trained rats, and DOM and DMT only partially substituted for MDMA (Gatch et al., 2009).
MDAI seems to have similar behavioral effects to those of MDMA, as it substituted for the discriminative stimulus effects of MDMA but not of LSD (Nichols et al., 1990). However, it is not known whether MDAI produces psychostimulant-like effects similar to MDMA. This is of more than academic interest, as there is evidence that MDAI has been advertised as a legal alternative to the controlled cathinone mephedrone, and that capsules and tablets sold as MDAI often contain cathinone compounds (Gallagher et al., 2012). Correspondingly, MDAI has been found in samples of “bath salts” or in blood samples of users increasingly over the past 4 years (Elliot and Evans 2014; Marinetti & Antonides, 2013; Uralets et al., 2014). However, there is little evidence whether MDAI also produces psychostimulant-like effects similar to other compounds regularly included in “bath salts.” MDAI failed to substitute for the discriminative stimulus effects of amphetamine, producing maximal effects of 50% drug-appropriate responding with substantial suppression or responding (Johnson et al., 1991b), but MDMA also produces inconsistent effects in amphetamine-trained subjects as previously mentioned.
The purpose of the present study was to test the potential abuse liability of MDAI and to assess the degree of similarity between the behavioral effects of MDAI and MDMA. The two questions are not entirely independent, as possessing highly similar pharmacological mechanisms and behavioral effects can imply similar patterns of recreational use. Behavioral studies of the abuse liability of potential psychostimulants include locomotor activity, discriminative stimulus effects similar to controlled substances such as cocaine or methamphetamine, conditioned place preference, and finally, the ability to maintain drug-seeking behavior in a self-administration test. In the present study, the ability of MDAI to alter locomotor activity in mice was tested. Because MDMA produces both psychostimulant and hallucinogen-like discriminative stimulus effects, the ability of MDAI to substitute in rats trained to discriminate MDMA, methamphetamine, cocaine and DOM was also tested. Finally, to test for reward effects, the ability of MDAI to produce conditioned place preference was tested.
Methods
Subjects
Male Swiss–Webster mice were obtained from Harlan (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed in cages on a 12:12-h light/dark cycle and were allowed free access to food and water. Male Sprague-Dawley rats were obtained from Harlan. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 07:00 h). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) housed in sets of two within sound-attenuating chambers. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination and fans provided an 80-dB ambient noise level within the chamber.
Separate groups of 8 mice were injected with either vehicle (0.9% saline), MDMA (0.1, 0.25, 0.5, 1 mg/kg), or MDAI (1, 3, 10 or 30 mg/kg), immediately prior to locomotor activity testing. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, beginning at 0800 h (1 h after lights on).
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, a pool of rats previously trained to discriminate either MDMA (1.5 mg/kg), DOM (0.5 mg/kg), methamphetamine (1 mg/kg), or cocaine (10 mg/kg) from saline, as previously described (Gatch et al., 2009), were tested. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. The pretreatment time was 10 min for cocaine and methamphetamine, 15 min for MDMA, and 30 min for DOM. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained, or for a maximum of 20 min. Each compound was tested in groups of six rats. For dose-effect experiments, i.p. injections (1 ml/kg) of saline or MDAI (0.5, 1, 2.5, 5 and 7.5 mg/kg) were administered 60 min prior to the start of the session. For the time course experiments, the lowest dose that fully substituted without significant rate effects in the dose-effect studies was selected. The rats were injected with MDAI and placed in the test chambers 5, 15, 30, 60, 120 and 240 min after administration. A repeated-measures design was used, such that each rat was tested at all doses or all time points of a given drug.
Conditioned Place Preference
The place preference apparatus consisted of 16 acrylic test chambers (12 × 6 × 12-in, lwh) (Model 71-CFCPP, Omnitech Electronics, Inc., Columbus, OH) with interchangeable grid (bar) and hole (perforated) floors (full, 12 × 6-in, and split, 6 × 6-in). The position of the mouse within the apparatus was recorded using a photocell-based system (Model 71-CPPX, Omnitech). The acrylic chambers were housed separately in sound-attenuating chambers (Model 71-ECC, Omnitech). Ambient noise within the chambers was 64 dB and testing took place under dim illumination (31.8 ± 1.5lux).
Place conditioning, using a biased-assignment model, consisted of three phases: a pre-test for initial floor bias, four place conditioning sessions, and a final preference test. The pre-test was conducted on day 1, during which initial floor bias was examined by injecting mice intraperitoneally with 0.9% saline then allowing them free access to both floor types for 30 min. The amount of time spent on either floor was measured and the floor on which less time was spent was designated the drug-paired floor. Positioning of the floors alternated between chambers. On days 2 and 3, place conditioning occurred, wherein mice received one vehicle and one drug conditioning session on both days. In the mornings, mice were injected with saline or MDAI (0.1, 0.3, 1, 3 or 10 mg/kg) and placed in the chamber with the drug-paired floor for 30 min, then returned to their home cage. After 4 h, mice were injected with saline and immediately placed in the chambers with the non-drug-paired floors for 30 min. The final preference test, occurring on day 4, was identical to the pre-test. All subjects were administered 0.9% saline and the time spent on the drug-paired floor was measured. Sixteen mice were tested at each dose.
Drugs
(−)-Cocaine hydrochloride, (+)-methamphetamine hydrochloride, (±)-3,4-methylenedioxymethamphetamine hydrochloride (MDMA), (−)-2,5-dimethoxy-4-methylamphetamine hydrochloride (DOM), and 5,6-methylenedioxy-2-aminoindane hydrochloride (MDAI) were provided by the National Institute on Drug Abuse Drug Supply Program. All drugs were dissolved in 0.9% saline and were administered i.p. in a volume of 1 ml/kg in rats and 10 ml/kg in mice.
Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data. A two-way repeated-measures analysis of variance was conducted on horizontal activity counts/10 min interval. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as >80% drug-appropriate responding and not statistically different from the training drug.
The potencies (and 95%-confidence intervals) of MDAI in MDMA-, DOM-, cocaine-, and methamphetamine-trained rats were calculated by fitting straight lines to the linear portion of the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way repeated-measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts.
Conditioned place preference data were expressed the mean time in seconds spent on the drug-paired floor over 30 minutes. Preference scores were calculated by subtracting the pre-test time on the drug-paired floor from the post-time on the drug-paired floor. The data were analyzed using a one-way analysis of variance of preference scores over dose. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The criterion for significance was set a priori at P < 0.05.
Results
Locomotor activity
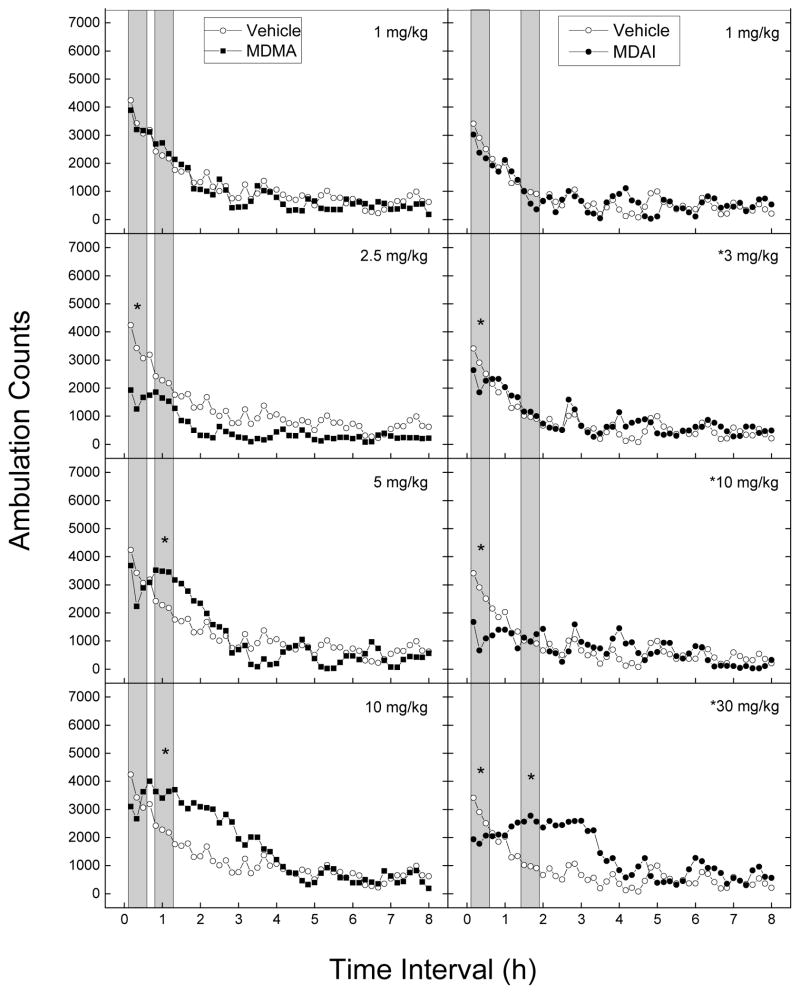
Figure 1 shows average horizontal activity counts/10 min as a function of time (0–8 h) and dose of MDMA and MDAI. Treatment with MDMA resulted in both depression and stimulation of locomotor activity. A two-way analysis of variance conducted on horizontal activity counts/10 min indicated a significant effect of Treatment [F(7,56)=4.67, p<.001], 10-Min Periods [F(47,2632)=67.40, p<.001], and the interaction of Periods and Treatment [F(329,2632)=2.08, p<.001]. Depressant effects of 2.5 mg/kg occurred within 10 min following injection [F(7,56)=4.67, p<.001] and lasted 240 min. Stimulant effects of 5 and 10 mg/kg occurred within 40–50 min [F(7,56)=4.92, p<.001] following injection and lasted 80–180 min.
Fig 1.
Time course of locomotor activity in mice. Data are represented as mean number of ambulation counts for each 10-minute period over 8 hours for each dose of MDMA (left) and MDAI (right). Data are from independent groups of 8 mice per dose. Vehicle (0.9% saline) data were obtained from one group of mice and displayed in each panel to indicate dose-dependent differences of drug-induced motor activity from vehicle-treated mice. The gray bars show the time ranges of maximal depressant and stimulant effects. * indicates doses significantly different from vehicle for the period of 0–30 minutes after injection, as determined by a two-way analysis of variance (p < 0.05).
Treatment with MDAI resulted in both stimulation and depression of locomotor activity. The two-way analysis of variance yielded a significant main effect of Treatment [F(5,42)=2.44, p<.05], 10-Minute Periods [F(47,1974)=24.06, p<.001], and the interaction of Periods and Treatment [F(235,1974)=2.34, p<.001]. Depressant effects of 3 to 30 mg/kg occurred within 10 minutes following injection and lasted 20 to 60 minutes [F(5,42)=10.26, p<.001]. Stimulant effects of 30 mg/kg MDAI occurred within 70 minutes following injection and lasted 160 minutes [F(5,42)=3.14, p<.02]. Because the depressant effects seemed to be decreasing at 30 mg/kg and because of the appearance of stimulant effects, 100 mg/kg was also tested. Data are not shown because lethality occurred in 8 of 8 mice following 100 mg/kg MDAI.
Drug discrimination
Dose effect
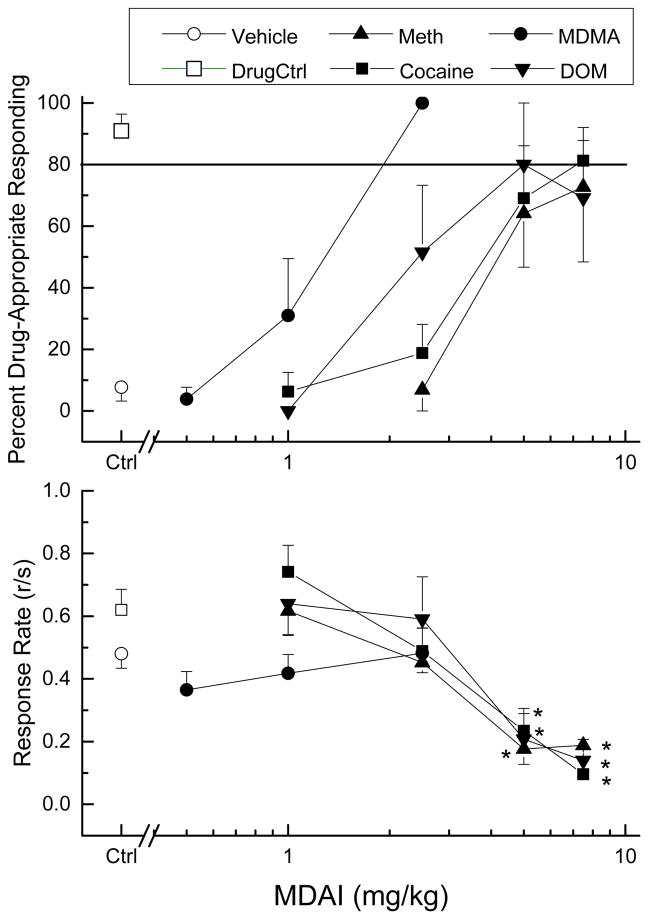
MDAI fully substituted for the discriminative stimulus effects of MDMA (ED50= 1.07 mg/kg, 95% confidence interval 0.27 – 4.28 mg/kg), with peak effects (100%) following 2.5 mg/kg MDAI (Fig. 2). MDAI fully substituted in cocaine-trained rats (ED50=3.66 mg/kg, 0.86 – 15.26 mg/kg) with a peak of 81±11% following 7.5 mg/kg. MDAI also fully substituted in DOM-trained rats (ED50=2.62 mg/kg, 0.49 – 14.09 mg/kg) with a peak of 80±20% at 5 mg/kg; however, when tested at 7.5 mg/kg, drug-appropriate responding dropped to 69±19%. MDAI produced a peak of only 73±24% drug-appropriate responding in methamphetamine-trained rats following 7.5 mg/kg (Figure 2). A methamphetamine-trained rat tested following administration of 10 mg/kg evidenced tremors, so 7.5 mg/kg was used as the maximum dose in all subsequent testing. MDAI (5 and 7.5 mg/kg) decreased rate in cocaine-trained rats [F(4,20)=46.67, p<.001], methamphetamine-trained rats [F(4,25)=12.07, p<.001], and DOM-trained rats [F(4,20)=6.98, p<.001]. MDAI (tested at doses from 0.3 to 3 mg/kg) did not alter response rate in MDMA-trained rats [F(4,20)=1.31, p=0.302].
Fig. 2.
Substitution of MDAI for the discriminative stimulus effects of MDMA, cocaine, methamphetamine, or DOM in rats. The upper panel shows percentage of total responses on the drug-appropriate lever as a function of log dose of MDAI. The lower panel shows rate of responding in responses per second (r/s). Data are from groups of 6 rats. Ctrl indicates average of responding to vehicle (0.9% saline) and drug controls. * indicates response rate different from vehicle control (p < 0.05).
Time course
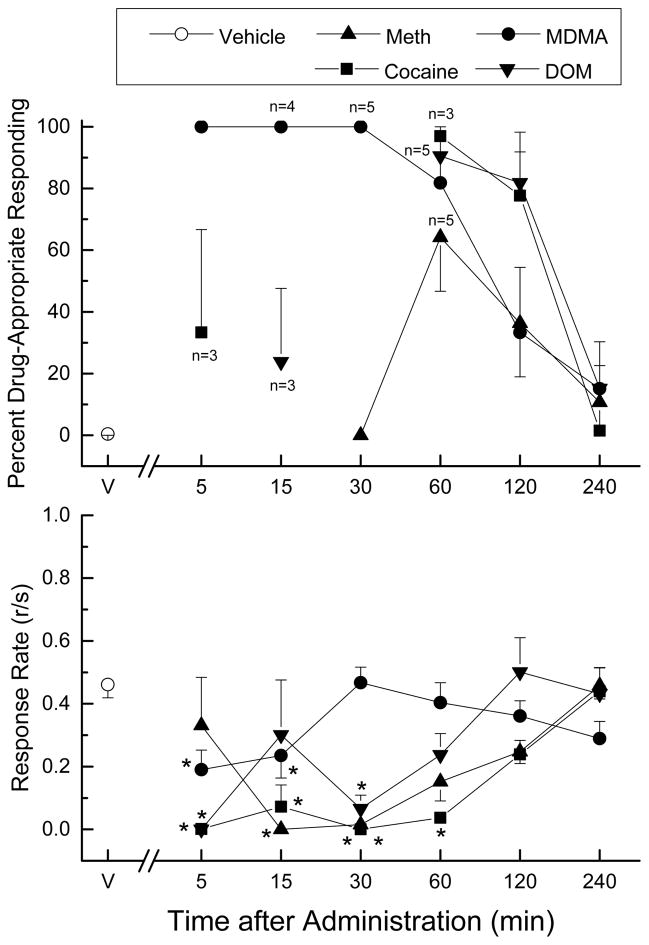
Doses of MDAI that produced peak effects without substantial rate effects were selected for use in the time course studies (Fig. 3). MDAI (2.5 mg/kg) produced 100% MDMA-appropriate responding within 5 min that lasted 30 min. MDMA-appropriate responding was still above 80% at 60 min, and then dropped to 49±22% at 120 min, and then to 15% at 240 min. Response rate was suppressed at 5 and 15 min following administration [F(6,30)=3.49, p<.01]. In DOM-trained rats, MDAI (5 mg/kg) suppressed responding at 5 and 30 min. At 15 min, 24±24% DOM-appropriate responding was observed. Responding peaked at the 60 min time point (90.5±9.5%), and then decreased to 82±16% at 120 min, and 15±15% at 240 min after administration of MDAI. Response rate was suppressed from 5 to 30 min following administration [F(6,30)=4.94, p<.001]. None of the 6 rats responded at 5 min following administration, 3 of 6 rats responded at 15 min, and 2 of 6 rats responded at 30 min.
Fig. 3.
Time course of the discriminative stimulus effects of MDAI. Percentage of total responses made on the drug-appropriate lever as a function of time (shown in log increments). The dose of MDAI was the dose that produced peak effects in the dose-effect study without suppressing response rate to the point that rats failed to respond (2.5 mg/kg in MDMA-trained rats, and 5 mg/kg in cocaine-, methamphetamine- and DOM-trained rats. The upper panel shows percentage of total responses on the drug-appropriate lever. The lower panel shows rate of responding in responses per second (r/s). n=6 except where shown. V indicates vehicle values. * indicates rate different (p < 0.05) from vehicle control.
In cocaine trained rats, MDAI (5 mg/kg) suppressed responding until the 60 min time point in cocaine-trained rats. Peak cocaine-appropriate responding (97±3%) occurred at 60 min, dropped to 78±14% at 120 min, and then to 2±2% at 240 min after administration of MDAI. Response rate was suppressed from 5 to 60 min following administration of MDAI [F(6,30)=20.02, p<.001]. A time course of 5 mg/kg MDAI in methamphetamine-trained rats showed maximal effects of 64% methamphetamine-appropriate responding at 60 min following administration (Figure 3). Low levels of methamphetamine-appropriate responding (31–32%) were observed at 5 and 120 min and the effects of MDAI had diminished to 11% at 240 min after administration. Responding was suppressed at 15 and 30 min [F(6,30)=6.59, p<0.001].
Conditioned Place Preference
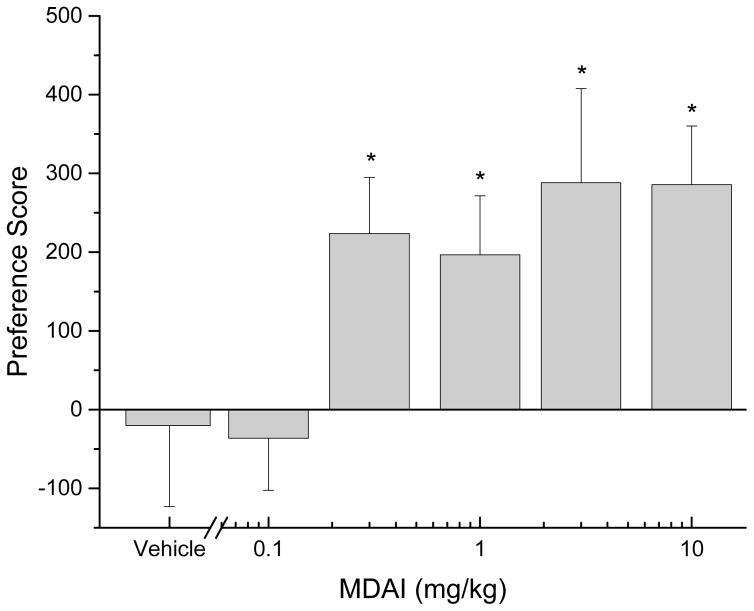
MDAI (0.1 to 10 mg/kg) was tested for its ability to produce a conditioned place preference (Fig 4). There was a significant overall effect of dose [F(5,90)=2.86, p<.02]. MDAI produced significant levels of CPP following administration of 0.3 to 10 mg/kg, whereas vehicle (saline) and 0.1 mg/kg MDAI produced effects no different from baseline. Higher doses were not tested due to the apparent plateau of effect and because of adverse effects observed in mice in the locomotor activity study.
Fig. 4.
Conditioned place preference in mice. Preference scores (time spent on drug-paired floor during pre-test subtracted from time spent on drug-paired floor during post-test) as a function of log dose of MDAI. Data are from independent groups of n=16 mice per dose. * indicates doses significantly different from baseline (p < 0.05).
Discussion
Recreational use of MDAI as a substitute for MDMA or for cathinone stimulants has been reported (Elliot and Evans 2014; Marinetti & Antonides, 2013; Uralets et al., 2014). However, MDAI is not currently scheduled as a controlled substance (Casale and Hayes, 2011) and there is limited research on its behavioral effects in laboratory models of substance abuse. In the present study, MDAI produced locomotor and discriminative stimulus effects similar to those produced by MDMA, and produced conditioned place preference, as does MDMA (Bilsky et al., 1990; Daza-Losada et al., 2009; Roger-Sánchez et al., 2013). MDAI produced lethality in all mice tested at 100 mg/kg. The LD50 of MDMA is 97 mg/kg (i.p.) in mice (Hardman et al., 1973), so MDMA may be somewhat less potent at producing lethal effects than MDA. Taken together, these findings suggest that MDAI may have abuse liability comparable to that of MDMA.
MDAI depressed locomotor activity within the first 30 min of administration, although a stimulant effect was observed 1 to 3 h following administration of the highest dose (30 mg/kg). These effects may seem curious, as MDMA (which is structurally and pharmacologically similar to MDAI) produces a stimulation of locomotor activity (Callaway et al., 1990; Gold et al., 1989). However, a broader assessment of the time course and dose-effect of MDMA uncovered effects very similar to those of MDAI. In the present study, both compounds produced rapid onset locomotor depression at lower doses, and slower onset, long-lasting locomotor stimulation at higher doses. In addition, the cathinone flephedrone (Gatch et al., 2013), the sympathomimetic dimethylamylamine (Dolan & Gatch, 2015), and the phenethylamine hallucinogens 2C-D and DOC (Eshleman et al., 2014) produced a profile similar to that of MDAI and MDMA: depressant effects followed by rebound stimulant effects. In contrast, a wide range of psychostimulants produce locomotor stimulation, often in an-inverted U-shaped function, such that increasing doses produce increases in locomotor activity, with the highest doses producing marked decreases in locomotor activity (Carroll et al., 2009; Gatch et al., 2013; 2015a; 2015b; Katz, 2001). When tested in the same manner, most hallucinogens produce dose-dependent depression of locomotor activity (Eshleman et al., 2014; Gatch et al., 2011). It is not clear why a pharmacologically diverse group of compounds would all produce this unusual biphasic effect.
MDAI fully substituted for the discriminative-stimulus effects of MDMA (100%). MDAI also fully substituted for cocaine and DOM in both the dose-effect and time-course studies, but at doses higher than those required to substitute for MDMA and which produced substantial suppression of response rate. However, MDAI produced less than 80% drug-appropriate responding in methamphetamine-trained rats in both the dose-effect and time-course studies, also at doses that considerably suppressed rate of responding. Despite the differences in doses at which MDAI substituted, there were no statistically significant differences in ED50 values, as evidenced by completely overlapping 95% confidence intervals. However, a qualitative difference in the effects of MDAI between the training compounds was apparent in the time-course study. Full substitution with little rate suppression was observed in the MDMA-trained rats during the time interval of 5 to 30 min, whereas substantial rate suppression was observed in the DOM-, methamphetamine-, and cocaine-trained rats, with only modest drug-appropriate responding during the same time period. Full substitution took 60 min to appear in the DOM- and cocaine-trained rats. The differences in suppression of response rate can be attributed to the different doses tested (2.5 mg/kg in MDMA-trained rats, and 5 mg/kg in the cocaine-, methamphetamine-, and DOM-trained rats. However, the difference in onset of the discriminative stimulus effects is striking. Why MDAI should substitute for MDMA at earlier time points than for cocaine, methamphetamine and DOM is not clear.
These findings agree with earlier work that MDAI substituted for the discriminative stimulus effects of MDMA (Nichols et al., 1990) and partially substituted (50%) for the discriminative stimulus effects of amphetamine at a dose that produced substantial rate suppression (Johnson et al., 1991b). However, the finding in the present study that MDAI substituted for the discriminative stimulus effects of DOM does not appear to agree with the finding that MDMA did not substitute in rats trained to discriminate LSD (Nichols et al., 1990). A reason for the apparent discrepancy is that in the earlier study, MDAI was tested 30 min following administration in the LSD-trained rats. In present study, MDAI produced no DOM-appropriate responding 30 min after administration in agreement with the earlier study, and full substitution was not observed until the 60 min time point. This suggests that MDAI does produce hallucinogen-like effects, but at times later than those tested in the earlier study.
Like MDAI, MDMA also fully substituted for the discriminative-stimulus effects of DOM (Schechter 1998; Gatch et al., 2009). However, unlike MDAI, MDMA fully substituted in methamphetamine-trained rats (Gatch et al., 2009), but did not substitute in cocaine- or amphetamine-trained rats (Khorana et al. 2004; Oberlender and Nichols 1988), although one study reported partial substitution in cocaine-trained rats (Kueh and Baker 2007). The drug discrimination data presented here are in general accordance with the mechanistic data previously presented for MDAI (Simmler et al., 2014b; Johnson et al., 1991a). Of the training drugs utilized, the pharmacological profile of MDAI is most similar to that of MDMA, demonstrating relative affinities of NET > SERT > DAT, with MDAI producing affinities of 0.65, 8.3, and 31 micromolar (respectively) and MDMA producing affinities of 0.45, 1.36, and 17 micromolar for the three transporters (Simmler et al., 2014a; 2014b)
This explains the potent substitution by MDAI for the discriminative stimulus effects of MDMA and, to a lesser extent, DOM, a 5-HT2 partial agonist (Sanders-Bush et al., 1988). Furthermore, these data suggest a complex discriminative stimulus profile for MDAI, similar to that of MDMA, which is mediated by both serotonin and dopamine (Goodwin & Baker, 2000; Goodwin et al., 2003). It has previously been demonstrated that MDMA adopts a more stimulant-like discriminative stimulus profile at higher doses that is mediated by dopaminergic signaling (Gatch et al., 2009; Harper et al., 2014). The data from the current study demonstrate similar effects with higher doses of MDAI (5–7.5 mg/kg) in cocaine- and methamphetamine-trained rats, the effects of which are primarily mediated by dopamine (Callahan et al., 1990; Kleven et al., 1990; Tidey & Bergman, 1998).
MDAI produced conditioned place preference at doses from 0.3 to 10 mg/kg, which provides evidence that MDAI produces reward-like effects and may maintain self-administration. These findings are comparable with earlier reports that MDMA produces conditioned place preference (Bilsky et al., 1990; Daza-Losada et al., 2009; Roger-Sánchez et al., 2013), and are not surprising given that MDAI is being used on the street as a substitute for MDMA (Corkery et al., 2013; Sainsbury et al., 2011) or mephedrone (Gallagher et al., 2012).
In conclusion, MDAI produced locomotor, discriminative stimulus, rewarding, and lethal effects similar to those of MDMA. MDAI, like MDMA, produced locomotor, discriminative stimulus and rewarding effects not only like those of psychostimulants, but also produced locomotor and discriminative stimulus effects like those of hallucinogens. These data suggest that MDAI has an abuse liability similar to that of MDMA, and is agreement with reports that MDAI is increasingly being used as a substitute for MDMA on the street.
Acknowledgments
Support: Supported by NIH N01DA-13-8908.
References
- Bilsky EJ, Hui YZ, Hubbell CL, Reid LD. Methylenedioxymethamphetamine’s capacity to establish place preferences and modify intake of an alcoholic beverage. Pharmacol Biochem Behav. 1990;37:633–8. doi: 10.1016/0091-3057(90)90538-s. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Appel JB, Cunningham KA. Dopamine D1 and D2 mediation of the discriminative stimulus properties of d-amphetamine and cocaine. Psychopharmacology (Berl) 1990;103:50–55. doi: 10.1007/BF02244073. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–64. [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Abraham P, Mills AC, Holleman JA, Wolckenhauer SA, Decker AM, Landavazo A, McElroy KT, Navarro HA, Gatch MB, Forster MJ. Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for cocaine addiction. J Med Chem. 2009;52:6768–81. doi: 10.1021/jm901189z. [DOI] [PubMed] [Google Scholar]
- Casale JF, Hays PA. Characterization of the “Methylenedioxy-2-aminoindans”. Microgram J. 2011;8:43–52. [Google Scholar]
- Cohen RS. Subjective reports on the effects of the MDMA (‘ecstasy’) experience in humans. Prog Neuropsychopharmacol Biol Psychiatr. 1995;19:1137–1145. doi: 10.1016/0278-5846(95)00231-6. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Corkery JM, Elliott S, Schifano F, Corazza O, Ghodse AH. MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; ‘sparkle’; ‘mindy’) toxicity: a brief overview and update. Hum Psychopharmacol. 2013;28:345–55. doi: 10.1002/hup.2298. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Leung KS, Abdallah AB. Test-re-test reliability of DSM-IV adopted criteria for 3,4-methylenedioxymethamphetamine (MDMA) abuse and dependence: A cross-national study. Addiction. 2009;104:1679–1690. doi: 10.1111/j.1360-0443.2009.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daza-Losada M, Rodriguez-Arias M, Aguilar MA, Minarro J. Acquisition and reinstatement of MDMA-induced conditioned place preference in mice pre-treated with MDMA or cocaine during adolescence. Addiction Biol. 2009;14:447–456. doi: 10.1111/j.1369-1600.2009.00173.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bruno R, Topp L. Is ecstasy a drug of dependence? Drug Alcohol Depend. 2010;107:1–10. doi: 10.1016/j.drugalcdep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Dolan SB, Gatch MB. Abuse liability of the dietary supplement dimethylamylamine. Drug Alcohol Depend. 2015;146:97–102. doi: 10.1016/j.drugalcdep.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243C:55–60. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl) 2014;231:875–888. doi: 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CT, Assi S, Stair JL, Fergus S, Corazza O, Corkery JM, Schifano F. 5,6- Methylenedioxy-2-aminoindane: from laboratory curiosity to ‘legal high’. Hum Psychopharmacol. 2012;7:106–12. doi: 10.1002/hup.1255. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Carbonaro T, Forster MJ. Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology (Berl) 2009;204:715–724. doi: 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “Bath Salt” cathinones. Behav Pharmacol. 2013;24:437–47. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharmacol Exp Ther. 2015a;354:103–10. doi: 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 2015b;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Hubner CB, Koob GF. A role for the mesolimbic dopamine system in the psychostimulant actions of MDMA. Psychopharmacology (Berl) 1989;99:40–7. doi: 10.1007/BF00634450. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Baker LE. A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (±)-MDMA in rats. Exp Clin Psychopharmacol. 2000;8:415–423. doi: 10.1037//1064-1297.8.3.415. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE. Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a three-choice discrimination. Pharmacol Biochem Behav. 2003;74:987–995. doi: 10.1016/s0091-3057(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Hardman HF, Haavik CO, Seevers MH. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicol Appl Pharmacol. 1973;25:299–309. doi: 10.1016/s0041-008x(73)80016-x. [DOI] [PubMed] [Google Scholar]
- Harper DN, Langen A-L, Schenk S. A 3-lever discrimination procedure reveals differences in the subjective effects of low and high doses of MDMA. Pharmacol Biochem Behav. 2014;116:9–15. doi: 10.1016/j.pbb.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang X-P, Roth BL. Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol. 2013;700:147–51. doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Conarty PF, Nichols DE. [3H]Monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur J Pharmacol. 1991a;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Frescas SP, Oberlender R, Nichols DE. Synthesis and pharmacological examination of 1(-3-methoxy-4-methylphenyl)-2-amino propane and 5-methoxy-6-methyl-2-aminosindan: Similarities to 3,4-(methylenedioxy)methamphetamine (MDMA) J Med Chem. 1991b;34:1662–1668. doi: 10.1021/jm00109a020. [DOI] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Assoc J. 2001;165:917–928. [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, Newman AH. Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 2001;154:362–374. doi: 10.1007/s002130000667. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Khorana N, Pullogurla MR, Young R, Glennon RA. Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: asymmetric generalization. Drug Alcohol Depend. 2004;74:281–287. doi: 10.1016/j.drugalcdep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kueh D, Baker LE. Reinforcement schedule effects in rats trained to discriminate 3,4-methylenedioxymethamphetamine (MDMA) or cocaine. Psychopharmacology (Berl) 2007;189:447–457. doi: 10.1007/s00213-006-0523-z. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Brewster WK, Johnson MP, Oberlender R, Riggs RM. Nonneurotoxic tetralin and Indan analogues of 3,4-(methylenedioxy)amphetamine. J Med Chem. 1990;33:703–710. 703. doi: 10.1021/jm00164a037. [DOI] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J Anal Toxicol. 2013;37:135–46. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology (Berl) 1988;95:71–6. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Parrot AC, Lasky J. Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance. Psychopharmacology (Berl) 1998;139:261–268. doi: 10.1007/s002130050714. [DOI] [PubMed] [Google Scholar]
- Roger-Sánchez C, Rodríguez-Arias M, Miñarro J, Aguilar MA. Involvement of 5-hydroxytryptamine 5-HT3 serotonergic receptors in the acquisition and reinstatement of the conditioned place preference induced by MDMA. Eur J Pharmacol. 2013;714:132–41. doi: 10.1016/j.ejphar.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Sainsbury PD, Kicman AT, Archer RP, King LA, Braithwaite RA. Aminoindanes--the next wave of ‘legal highs’? Drug Test Anal. 2011;3:479–82. doi: 10.1002/dta.318. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamine and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- Schechter MD. MDMA-like stimulus effects of hallucinogens in male Fawn-Hooded rats. Pharmacol Biochem Behav. 1998;59:265–270. doi: 10.1016/s0091-3057(97)00415-2. [DOI] [PubMed] [Google Scholar]
- Schenk S. MDMA (“Ecstasy”) abuse as an example of dopamine neuroplasticity. Neurosci Biobehav Rev. 2011;35:1203–18. doi: 10.1016/j.neubiorev.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Scholeya AB, Parrott AC, Buchanan T, Heffernan TM, Ling J, Rodgers J. Increased intensity of Ecstasy and polydrug usage in the more experienced recreational Ecstasy/MDMA users: A WWW study. Addict Behav. 2004;29:743–752. doi: 10.1016/j.addbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacol. 2014a;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Schramm Y, Hoener MC, Leichti ME. Pharmacological profiles of aminoindanes, piperazines, and pipradol derivatives. Biochem Pharmacol. 2014b;88:237–244. doi: 10.1016/j.bcp.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–1174. [PubMed] [Google Scholar]
- Uralets V, Rana S, Morgan S, Ross W. Testing for designer stimulants: metabolic profiles of 16 synthetic cathinones excreted free in human urine. J Anal Toxicol. 2014;38:233–41. doi: 10.1093/jat/bku021. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mictheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2012;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]