Abstract
Objective
Neurotensin is a peptide whose receptor (SORT1) is linked to cardiovascular disease (CVD) development. We hypothesized concentrations of pro-neurotensin (pro-NT; stable pro-fragment of neurotensin) would predict incident CV events in community-based subjects.
Approach and Results
Blood samples from 3439 participants in the Framingham Heart Study (FHS) Offspring cohort (mean age 59.2 years, 47.1% male) were tested for pro-NT. Primary outcome of interest was incident hard CVD (myocardial infarction, stroke, and CV death); interaction between pro-NT concentration with sex, low density lipoprotein (LDL) concentrations or SORT1 single-nucleotide polymorphisms (SNP) was sought. At baseline, those in the highest log-pro-NT quartile were younger and heavier (P<0.001); across pro-NT quartiles, more prevalent hard CVD (from 3% to 7%; P<0.001) and diabetes mellitus (from 6% to 14; P<0.001) was present. In age and sex-adjusted models, log-pro-NT concentrations predicted incident hard CVD (hazard ratio [HR] = 1.24 per standard deviation [SD] change in log-pro-NT; 95% confidence intervals [CI] = 1.11–1.39; P<0.001), a finding that remained upon adjustment for standard CVD risk factors (HR 1.13; 95% CI = 1.01–1.27; P = 0.03). Elevated log-pro-NT concentrations were associated with shorter time to first event (P=0.02). We found no effect modification by sex, LDL concentration, or SORT1 SNPs. Concentrations of pro-NT were modestly associated with left ventricular mass and coronary artery calcium in these subjects.
Conclusions
Higher concentrations of pro-NT are associated with a greater risk of incident CV events in the community. This association did not vary according to sex, baseline LDL, or SORT1 genotype.
Keywords: acute myocardial infarction, stroke, risk factors
Subject codes: Vascular biology, cardiovascular disease, coronary artery disease, cerebrovascular disease/stroke
Efforts at reducing cardiovascular (CV) events rely on the accurate identification of individuals at risk. Unfortunately, patients that suffer CV events often have a paucity of traditional factors predictive of CV disease (CVD) to facilitate recognition of risk. In this regard, measurement of circulating biomarkers has been examined as an option for assessing risk for CV events beyond standard risk factors. Though potentially useful to predict and/or reclassify risk in some cohorts, studies of such testing for predicting CV events in low to intermediate risk populations have returned mixed results1, 2, suggesting further efforts are needed to better understand the role of testing of circulating substances for risk prediction in such patients. Beyond potential clinical application, biomarker measurement has also been leveraged as a tool to understand mechanism of CV disease onset. More studies of novel and established circulating markers of disease are thus needed, both to advance understanding of optimal means for risk stratification and supplement knowledge regarding mechanism of disease.
Neurotensin is a 13-amino acid peptide originally isolated from bovine hypothalamic3 and later from intestinal tissue4. Neurotensin has a wide range of biologic roles in the body5, notably including a broad range of effects on the cardiovascular system; these include regulation of heart rate, myocardial contractility, as well as vascular tone6. Effects of neurotensin are transduced primarily through three receptors: the G-protein coupled NTS1 and NTS2 receptors and the non-G protein coupled NTS3, otherwise known as SORT1, a member of the Vps10p-domain receptor family. SORT1 (also known as sortilin) is involved in the binding of a number of unrelated ligands, and plays an important role in hepatic secretion of very low density lipoprotein (VLDL) cholesterol and regulation of circulating LDL cholesterol concentrations. Additionally, genetic variation in the 1p13 locus containing the SORT1 gene is also linked to coronary artery disease development7. While SORT1 appears linked to lipid metabolism and CVD risk, it remains unclear whether neurotensin plays a role in this association.
Measurement of neurotensin in blood is challenging, due to its instability and rapid clearance from the circulation5. To overcome this issue immunoassays have been developed for the detection of the pro-peptide fragment of the peptide, which is released in equimolar amounts to mature neurotensin. Recent data from the Malmö Diet and Cancer Study suggested concentrations of pro-neurotensin (pro-NT) were independently predictive of diabetes mellitus and CVD, particularly in women8. However, beyond these preliminary findings, no other data exist regarding association of circulating pro-NT concentrations and incidence of CVD events, nor are mechanistic analyses available. Accordingly, we sought to examine links between pro-NT and CVD in a cohort of patients from the Framingham Heart Study (FHS) Offspring study. Our hypothesis was that PNT concentrations would independently and positively predict CVD events, and would do so in a manner mediated via either blood LDL cholesterol values or genetic variation in the SORT1 receptor.
MATERIALS AND METHODS
Materials and methods are available in the online-only Data Supplement.
Results
Characteristics of the study sample as a function of pro-NT quartiles are shown in Table 1. The mean age of the study sample was 59.19 ± 10 years, and 53% of participants were women. Compared to subjects in log-pro-NT quartiles 1 through 3, those in the highest quartile were more likely to be younger (p=0.006), heavier (p<0.001), and more likely to smoke (p<0.001). There was no difference in LDL cholesterol concentrations across log-pro-NT quartiles; similarly, across quartiles of LDL cholesterol, there was no difference in log-pro-NT concentrations (p=0.71).
Table 1.
Baseline demographics by log-transformed pro-NT quartiles.
| Characteristic | All (N=3439) |
Quartile 1 (N=860) |
Quartile 2 (N=860) |
Quartile 3 (N=859) |
Quartile 4 (N=860) |
p-value |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean±SD (N) | 59.19±9.76 (3439) | 59.20±10.10 (860) | 59.13±9.74 (860) | 60.04±9.49 (859) | 58.39±9.65 (860) | 0.006 |
| Median (Q1,Q3) | 58.70 (51.95,66.71) | 58.70 (51.39,67.23) | 58.72 (51.83,66.27) | 59.86 (52.95,67.51) | 57.45 (51.59,65.62) | |
| Range (min,max) | (29.67,86.75) | (32.05,86.75) | (33.51,85.47) | (34.66,85.55) | (29.67,82.63) | |
| Male | 47.1% (1620/3439) | 45.3% (390/860) | 47.3% (407/860) | 47.3% (406/859) | 48.5% (417/860) | 0.626 |
| BMI | ||||||
| Mean±SD (N) | 27.91±5.14 (3430) | 27.30±4.74 (856) | 28.11±5.22 (859) | 27.92±5.33 (858) | 28.30±5.19 (857) | <.001 |
| Median (Q1,Q3) | 27.18 (24.40,30.54) | 26.76 (23.84,29.88) | 27.36 (24.67,30.69) | 26.82 (24.36,30.60) | 27.65 (24.80,30.72) | |
| Range (min,max) | (16.59,54.33) | (17.41,47.25) | (17.57,53.01) | (16.59,54.33) | (17.21,53.42) | |
| Waist Girth | ||||||
| Mean±SD (N) | 38.42±5.35 (3407) | 37.82±5.15 (854) | 38.61±5.42 (852) | 38.33±5.43 (849) | 38.94±5.32 (852) | <.001 |
| Median (Q1,Q3) | 38.25 (35.00,41.50) | 37.50 (34.50,41.00) | 38.50 (35.00,41.75) | 38.00 (34.50,41.50) | 38.88 (35.50,42.00) | |
| Range (min,max) | (23.75,60.00) | (24.25,56.75) | (25.00,60.00) | (23.75,58.25) | (25.75,59.50) | |
| Total cholesterol/HDL Ratio | ||||||
| Mean±SD (N) | 4.41±1.75 (3431) | 4.35±1.60 (859) | 4.40±1.46 (859) | 4.38±1.59 (856) | 4.51±2.26 (857) | 0.253 |
| Median (Q1,Q3) | 4.17 (3.30,5.20) | 4.09 (3.26,5.15) | 4.22 (3.33,5.28) | 4.13 (3.30,5.16) | 4.23 (3.33,5.24) | |
| Range (min,max) | (1.50,52.06) | (1.67,18.62) | (1.83,13.85) | (1.50,15.90) | (1.65,52.06) | |
| Calculated LDL | ||||||
| Mean±SD (N) | 127.14±33.63 (3373) | 126.44±33.40 (843) | 128.95±32.97 (847) | 126.17±32.52 (841) | 126.99±35.55 (842) | 0.316 |
| Median (Q1,Q3) | 125.20 (104.20,147.80) | 124.00 (103.40,146.60) | 128.00 (106.80,149.60) | 124.80 (104.40,147.00) | 124.40 (102.80,148.00) | |
| Range (min,max) | (30.00,355.00) | (30.00,261.60) | (31.40,301.00) | (31.60,258.80) | (39.20,355.00) | |
| Smoker | 15.3% (526/3439) | 11.6% (100/860) | 14.1% (121/860) | 15.9% (137/859) | 19.5% (168/860) | <.001 |
| Number of drinks per week | ||||||
| Mean±SD (N) | 5.09±7.72 (3436) | 5.56±7.91 (858) | 5.32±8.09 (860) | 4.63±7.22 (859) | 4.85±7.63 (859) | 0.048 |
| Median (Q1,Q3) | 2.00 (0.00,7.00) | 2.00 (0.00,8.00) | 2.00 (0.00,7.00) | 2.00 (0.00,7.00) | 2.00 (0.00,7.00) | |
| Range (min,max) | (0.00,85.00) | (0.00,57.00) | (0.00,85.00) | (0.00,54.00) | (0.00,70.00) | |
| Systolic Blood Pressure | ||||||
| Mean±SD (N) | 128.44±18.80 (3439) | 127.91±19.22 (860) | 128.14±18.13 (860) | 128.33±18.24 (859) | 129.37±19.56 (860) | 0.386 |
| Median (Q1,Q3) | 126.00 (115.00,139.00) | 125.00 (114.00,138.00) | 126.00 (115.00,138.00) | 126.00 (116.00,140.00) | 127.00 (115.00,140.00) | |
| Range (min,max) | (77.00,236.00) | (77.00,236.00) | (87.00,203.00) | (91.00,212.00) | (85.00,217.00) | |
| Antihypertensive Medication Use | 28.1% (963/3427) | 25.2% (216/858) | 28.6% (245/858) | 29.2% (249/853) | 29.5% (253/858) | 0.169 |
| Diabetes | 9.9% (340/3435) | 5.5% (47/860) | 9.7% (83/859) | 11.1% (95/857) | 13.4% (115/859) | <.001 |
| Prevalent Cancer | 6.1% (210/3439) | 6.4% (55/860) | 6.2% (53/860) | 5.4% (46/859) | 6.5% (56/860) | 0.748 |
| Prevalent Breast Cancer | 1.4% (49/3439) | 1.6% (14/860) | 1.2% (10/860) | 1.3% (11/859) | 1.6% (14/860) | 0.789 |
| Characteristic |
All (N=3439) |
Quartile 1 (N=860) |
Quartile 2 (N=860) |
Quartile 3 (N=859) |
Quartile 4 (N=860) |
p-value |
| Age | ||||||
| Mean±SD (N) | 59.19±9.76 (3439) | 59.20±10.10 (860) | 59.13±9.74 (860) | 60.04±9.49 (859) | 58.39±9.65 (860) | 0.006 |
| Median (Q1,Q3) | 58.70 (51.95,66.71) | 58.70 (51.39,67.23) | 58.72 (51.83,66.27) | 59.86 (52.95,67.51) | 57.45 (51.59,65.62) | |
| Range (min,max) | (29.67,86.75) | (32.05,86.75) | (33.51,85.47) | (34.66,85.55) | (29.67,82.63) | |
| Male | 47.1% (1620/3439) | 45.3% (390/860) | 47.3% (407/860) | 47.3% (406/859) | 48.5% (417/860) | 0.63 |
| BMI | ||||||
| Mean±SD (N) | 27.91±5.14 (3430) | 27.30±4.74 (856) | 28.11±5.22 (859) | 27.92±5.33 (858) | 28.30±5.19 (857) | <0.001 |
| Median (Q1,Q3) | 27.18 (24.40,30.54) | 26.76 (23.84,29.88) | 27.36 (24.67,30.69) | 26.82 (24.36,30.60) | 27.65 (24.80,30.72) | |
| Range (min,max) | (16.59,54.33) | (17.41,47.25) | (17.57,53.01) | (16.59,54.33) | (17.21,53.42) | |
| Waist Girth | ||||||
| Mean±SD (N) | 38.42±5.35 (3407) | 37.82±5.15 (854) | 38.61±5.42 (852) | 38.33±5.43 (849) | 38.94±5.32 (852) | <.001 |
| Median (Q1,Q3) | 38.25 (35.00,41.50) | 37.50 (34.50,41.00) | 38.50 (35.00,41.75) | 38.00 (34.50,41.50) | 38.88 (35.50,42.00) | |
| Range (min,max) | (23.75,60.00) | (24.25,56.75) | (25.00,60.00) | (23.75,58.25) | (25.75,59.50) | |
| Total cholesterol/HDL Ratio | ||||||
| Mean±SD (N) | 4.41±1.75 (3431) | 4.35±1.60 (859) | 4.40±1.46 (859) | 4.38±1.59 (856) | 4.51±2.26 (857) | 0.25 |
| Median (Q1,Q3) | 4.17 (3.30,5.20) | 4.09 (3.26,5.15) | 4.22 (3.33,5.28) | 4.13 (3.30,5.16) | 4.23 (3.33,5.24) | |
| Range (min,max) | (1.50,52.06) | (1.67,18.62) | (1.83,13.85) | (1.50,15.90) | (1.65,52.06) | |
| Calculated LDL | ||||||
| Mean±SD (N) | 127.14±33.63 (3373) | 126.44±33.40 (843) | 128.95±32.97 (847) | 126.17±32.52 (841) | 126.99±35.55 (842) | 0.32 |
| Median (Q1,Q3) | 125.20 (104.20,147.80) | 124.00 (103.40,146.60) | 128.00 (106.80,149.60) | 124.80 (104.40,147.00) | 124.40 (102.80,148.00) | |
| Range (min,max) | (30.00,355.00) | (30.00,261.60) | (31.40,301.00) | (31.60,258.80) | (39.20,355.00) | |
| Smoker | 15.3% (526/3439) | 11.6% (100/860) | 14.1% (121/860) | 15.9% (137/859) | 19.5% (168/860) | <0.001 |
| Number of drinks per week | ||||||
| Mean±SD (N) | 5.09±7.72 (3436) | 5.56±7.91 (858) | 5.32±8.09 (860) | 4.63±7.22 (859) | 4.85±7.63 (859) | 0.05 |
| Median (Q1,Q3) | 2.00 (0.00,7.00) | 2.00 (0.00,8.00) | 2.00 (0.00,7.00) | 2.00 (0.00,7.00) | 2.00 (0.00,7.00) | |
| Range (min,max) | (0.00,85.00) | (0.00,57.00) | (0.00,85.00) | (0.00,54.00) | (0.00,70.00) | |
| Systolic Blood Pressure | ||||||
| Mean±SD (N) | 128.44±18.80 (3439) | 127.91±19.22 (860) | 128.14±18.13 (860) | 128.33±18.24 (859) | 129.37±19.56 (860) | 0.39 |
| Median (Q1,Q3) | 126.00 (115.00,139.00) | 125.00 (114.00,138.00) | 126.00 (115.00,138.00) | 126.00 (116.00,140.00) | 127.00 (115.00,140.00) | |
| Range (min,max) | (77.00,236.00) | (77.00,236.00) | (87.00,203.00) | (91.00,212.00) | (85.00,217.00) | |
| Antihypertensive Medication Use | 28.1% (963/3427) | 25.2% (216/858) | 28.6% (245/858) | 29.2% (249/853) | 29.5% (253/858) | 0.17 |
| Diabetes | 9.9% (340/3435) | 5.5% (47/860) | 9.7% (83/859) | 11.1% (95/857) | 13.4% (115/859) | <0.001 |
| Prevalent Cancer | 6.1% (210/3439) | 6.4% (55/860) | 6.2% (53/860) | 5.4% (46/859) | 6.5% (56/860) | 0.75 |
| Prevalent Breast Cancer | 1.4% (49/3439) | 1.6% (14/860) | 1.2% (10/860) | 1.3% (11/859) | 1.6% (14/860) | 0.79 |
SD denotes: standard deviation; BMI denotes: body-mass index; HDL denotes: high density lipoprotein cholesterol; LDL denotes: low density lipoprotein cholesterol; mg/dL denotes: milligrams per deciliter; Q denotes: quartile; mm Hg denotes: millimeter of mercury.
In multivariable linear regression analyses, variables independently correlated with log-pro-NT concentrations included waist girth (β=0.0044; p=0.005), smoking (β=0.0818; p<0.001), and prevalent diabetes mellitus (β=0.1438; p<0.001); concentrations of LDL cholesterol were not predictive of log-pro-NT concentrations (β=-0.0002; p=0.39). Furthermore, neither age (β=-0.0011; p=0.10) or male sex (β=-0.0007; p=0.96) significantly correlated/predicted concentrations of log-pro-NT.
As detailed in Table 2, at baseline, across pro-NT quartiles study participants with higher concentrations were more likely to have prevalent diabetes mellitus (from 6% to 14%; p<0.001), prevalent hard CVD (from 3% to 7%; p<0.001) or prevalent hard CHD (from 3% to 5%; p=0.06). No association between log-pro-NT and prevalent cancer (including breast cancer) was observed.
Table 2.
Prevalent medical history as a function of log-transformed pro-NT quartiles.
| Characteristic | Quartile 1 (N=860) |
Quartile 2 (N=860) |
Quartile 3 (N=859) |
Quartile 4 (N=860) |
p-value |
|---|---|---|---|---|---|
| Diabetes | 6.3% (54/860) | 10.7% (92/860) | 12.1% (104/859) | 14.3% (123/860) | <0.001 |
| Hard CVD | 3.0% (26/860) | 4.5% (39/860) | 6.8% (58/859) | 6.6% (57/860) | <0.001 |
| Hard CHD | 2.8% (24/860) | 3.6% (31/860) | 5.0% (43/859) | 4.9% (42/860) | 0.06 |
| Cancer | 6.4% (55/860) | 6.2% (53/860) | 5.4% (46/859) | 6.5% (56/860) | 0.75 |
| Breast Cancer | 1.6% (14/860) | 1.2% (10/860) | 1.3% (11/859) | 1.6% (14/860) | 0.79 |
CVD denotes: cardiovascular disease; CHD denotes: coronary heart disease.
Biomarker concentrations and outcomes
During a mean follow up of 14.0 years, 342 (10.5%) individuals suffered a hard CVD event, with 166 myocardial infarctions, 148 strokes, 27 CHD deaths, and 1 stroke death. During similar follow up time 209 (6.3%) suffered a hard CHD event.
Table 3 details predictive value of log-pro-NT for hard CVD events. In age and sex-adjusted Cox proportional hazards models (Table 3A), log-pro-NT concentrations were positively associated with incident hard CVD (HR = 1.242 per one SD change in log-pro-NT; 95% CI = 1.11–1.39; P <0.001). In models adjusted for standard risk factors (including BMI), log-pro-NT remained significantly associated with incident hard CVD (HR = 1.13 per one SD change in log-pro-NT; 95% CI = 1.01–1.27; P = 0.03). The HR for log-pro-NT remained significant in models forcing concentrations of LDL (HR = 1.121 per one SD change in log-pro-NT; 95% CI = 1.002–1.254; P =0.05), the interaction factor of male*LDL (HR = 1.121 per one SD change in log-pro-NT; 95% CI = 1.002–1.254; P =0.05), or an LDL cholesterol above the median for the group (HR = 1.122 per one SD change in log-pro-NT; 95% CI = 1.003–1.215; P =0.05).
Table 3.
Cox proportional hazards analysis for predictors of hard CVD using stepwise selection.
| Parameter | Hazard Ratio |
95% CI | p-value |
|---|---|---|---|
| Proneurotensin (Standardized log transform) | 1.136 | 1.017, 1.270 | 0.03 |
| Age | 1.063 | 1.049, 1.078 | <0.001 |
| Male | 1.636 | 1.313, 2.040 | <0.001 |
| Total cholesterol/HDL Ratio | 1.052 | 1.018, 1.086 | 0.002 |
| Smoker | 2.390 | 1.825, 3.130 | <0.001 |
| Systolic Blood Pressure | 1.008 | 1.002, 1.014 | 0.007 |
| Antihypertensive Medication Use | 1.536 | 1.213, 1.945 | 0.004 |
| Diabetes | 1.759 | 1.319, 2.345 | 0.001 |
HDL denotes high density lipoprotein; CI denotes: confidence intervals.
Examining risk across log-pro-NT quartiles in age and sex adjusted models (Supplemental Table 1), greatest risk for incident hard CVD was observed in log-pro-NT quartile 4 (HR = 1.53 vs quartile 1; p=0.005). Using stepwise selection for prediction of hard CVD, comparable results were found, with higher concentrations of log-pro-NT predicting risk (Table 3).
Considering hard CHD, similar results were found, with higher concentrations of log-pro-NT predicting hard CHD in adjusted models that also contained prevalent CVD (Supplemental Tables 1 and 2); in fully adjusted models for hard CHD, we found a HR of 1.156 per one SD change in log-pro-NT for predicting hard CHD (95% CI 1.005–1.330; P =0.04).
Addition of further biomarker results for highly sensitive troponin I, growth differentiation factor-15, or soluble ST2 (previously reported to be predictive of CV events in this cohort9) in a stepwise model resulted in retention of growth differentiation factor-15 as a predictor of hard CVD (HR = 1.25 per one SD change in log-transform; 95% CI = 1.11–1.40; P <0.001), while log-pro-NT became marginally non-significant (HR = 1.11 per one SD change in log-pro-NT; 95% CI = 0.99–1.24; P = 0.07).
In interaction testing with fully adjusted models, we did not observe a pro-NT*LDL interaction (p=0.97) for prediction of hard CVD events. Similarly, we found no pro-NT*SORT1 SNP interactions for prognostication of hard CVD (pro-NT*rs629301, p =0.76; pro-NT*rs646776, p =0.56; pro-NT*rs12740374, p=0.65). Similarly negative results were found in pro-NT*LDL or pro-NT*SNP interaction analyses for hard CHD.
In contrast to prior data8, we found no interaction term with respect to sex and pro-NT-based prognostication (Table 4); although the HR for log-pro-NT predicting hard CVD was numerically higher in women (HR = 1.175 [95% CI 0.993–1.390]) compared to men (HR 1.118 [95% CI = 0.962–1.300]), these differences did not approach statistical significance.
Table 4.
Multivariable adjusted Cox proportional hazards model for pro-NT prediction of hard CVD events in males and females.
| Males | |||
|---|---|---|---|
| Parameter | Hazard Ratio per SD |
95% CI | p-value |
| Proneurotensin (Standardized log transform) | 1.118 | 0.962, 1.300 | 0.15 |
| Females | |||
| Proneurotensin (Standardized log transform) | 1.175 | 0.993, 1.390 | 0.06 |
Model adjusted for age, sex, waist girth, total cholesterol/HDL ratio, valve disease, smoking, number of alcoholic beverages per week, systolic blood pressure, antihypertensive medication use, diabetes mellitus, and prevalent cancer. SD denotes: standard deviation; CI denotes: confidence intervals.
Notably, log-transformed concentrations of pro-NT were not related to incident change in body-mass index or waist girth. Log-pro-NT did not predict incident diabetes mellitus, through during follow up, there were only 32 incident cases. Over a mean follow up of 8.7 years, the HR for log-pro-NT to predict incident diabetes mellitus was 0.942 (95% CI 0.662–1.340; p=0.74).
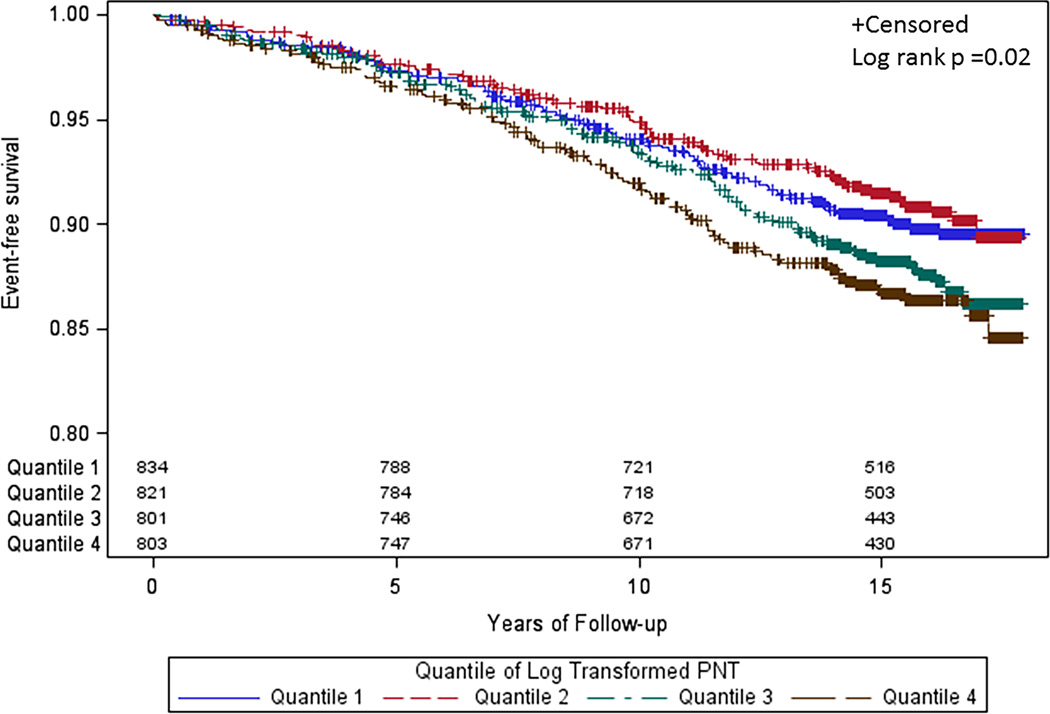
Lastly, in Kaplan Meier analyses, shorter time to first event was seen in higher log-pro-NT values (Figure 1; log rank P = 0.02). Similar results were found for hard CHD.
Figure 1.
Kaplan-Meier survival curves for hard CVD events in the Framingham Heart Study Offspring Study as a function of log-pro-NT quartiles. Those with higher pro-NT values had shorter time to first hard CVD event.
Cardiac structure and function
In age, sex, and height-adjusted regression, pro-NT concentrations were associated with LVM (parameter estimate 0.016 per standard deviation [SD] of log-transformed pro-NT; p=0.0002) and presence of LVSD (p=0.05). Across pro-NT quartiles (Q), significantly higher mean LVM (p=0.001) was observed; LVSD was least likely in pro-NT Q1 vs Q4 (p=0.05). In multivariable-adjusted models these findings were attenuated (p = 0.10 for LVM; p=0.22 for LVSD). Pro-NT concentrations were associated with extent of CAC in age and sex-adjusted regression analyses (parameter estimate 0.145 per SD of log-transformed pro-NT; p=0.02), however in multivariable-adjusted analyses, this finding was no longer significant (p=0.10). Findings were similar when excluding subjects with prevalent CVD.
Discussion
The principal findings of our analysis were that concentrations of PNT were associated cross-sectionally with a more deleterious cardiometabolic phenotype, and were prospectively associated with incident CV events in the population, independently of LDL concentrations or SORT1 genotypes relevant to development of atherosclerosis. The association of PNT with hard CVD and hard CHD remained robust after adjustment for traditional cardiovascular risk factors, and provided modest risk reclassification for predicting events. Concentrations of pro-NT were modestly associated with cardiac structure and function in echocardiographic and CAC imaging, but in rigorously adjusted models, these associations were less obvious. These results support a possible role for the neurotensin system in the development of clinical cardiovascular disease but further evaluation is needed.
Neurotensin is believed to work as a local hormone on peripheral organs, including the heart; it has a broad range of cardiovascular effects, regulating heart rate, myocardial contractility, and blood pressure6. Though measurement of neurotensin had been challenging due to analytical instability, recent development of a pro-peptide assay to quantify neurotensin has overcome this challenge. We therefore measured pro-NT in the FHS Offspring Study in an effort to examine the prognostic role of the neurotensin system for CV disease incidence.
Our results are notable since little information exists presently about neurotensin and risk for heart disease. Data from Melander and colleagues from the Malmö Diet and Cancer Study suggest that concentrations of pro-NT predicted adverse cardiometabolic outcome, including CVD and incident diabetes mellitus8. An intriguing interaction between pro-NT-based prognostication and sex was found by Melander, et al, suggesting pro-NT provided unique prognostic information particularly in women, correctly re-classifying as much as 40% of women for risk of cardiovascular death. Additionally, in Malmö, pro-NT predicted incident breast cancer. Our results are reasonably comparable to those from Malmö with respect to the ability of pro-NT to predict hard CVD and hard CHD, though such ability to predict is much more modest in our more rigorously adjusted analyses. Additionally, we could not confirm a sex-specific value of pro-NT. Further, while pro-NT was associated with more deleterious cardiometabolic state at enrollment (with higher values of pro-NT associated with more prevalent obesity and diabetes mellitus), we could not confirm pro-NT predicted incident obesity or diabetes mellitus. Lastly, no predictive ability to prognosticate incident cancers was seen in our analysis. The differences in results may be explained on the basis of the fact our study sample was smaller, and participants differ considerably in terms of baseline cardiometabolic risk compared to those in the Malmö analysis. Additionally, our statistical models were more rigorously adjusted. Despite more modest results, our findings are important as they clarify those from Malmö.
We initially hypothesized the deleterious effect(s) of neurotensin might be explainable on the basis of binding to SORT1. This receptor is intracellular and non-G protein coupled and plays a role in endocytosis and trafficking of several molecules (including various cholesterol particles); SORT1 has been implicated in LDL cholesterol metabolism as well as VLDL and proprotein convertase subtililisin/kexin type 9 (PCSK9) secretion10. Genetic variation of SORT1 is pivotally linked to coronary artery disease development in humans, in part through its effects on lipoprotein metabolism7. In theory, therefore, higher values of pro-NT could influence cardiac risk through interactions at the level of the SORT1 receptor via interference with normal lipid processing. In our analysis, however, we found no association between pro-NT concentrations and LDL cholesterol values; additionally, in evaluating various SORT1 SNPs important to CVD development, we could not detect any pro-NT*SORT1 interaction for prognosis; this lack of significant interaction is not due to solely lack of power as the outcome hazard ratio per 1 SD increase of log-PNT for participants above and below median rs629301 were 1.23 and 1.21, respectively, indicating consistent log-PNT effect across the values of this SNP. As another example, a similar trend was seen for rs1274074 (hazard ratio was 1.20 for participants above the median and 1.24 for participants below it). Indeed, with the current sample size/event rates, we have approximately 90% power to detect an interaction with SNP if HR for participants below median SNP was approximately twice that for participants above median SNP, or vice-versa. Lastly, though we did not perform interaction testing between pro-NT and other variables influenced by the SORT1 receptor (such as PCSK9), our results suggest the deleterious link(s) between pro-NT and CV disease may be mediated via effects of neurotensin on receptors other than SORT1.
While neurotensin binds both NTS1 and NTS2, substantial differences exist between the two receptors. While both are G-protein coupled, NTS1 has considerably higher affinity for neurotensin. Further, while both receptors are found in cardiovascular tissue, NTS1 is currently suspected to be more involved in regulation of cardiovascular effects of neurotensin. Manipulation of NTS1 receptor function with SR48692 (a selective non-peptide NTS1 inhibitor) resulted in dose-dependent effects on blood pressure, heart rate, myocardial contractility, vascular tone and permeability and endothelial cell survival11–14. Hypothetically, therefore, higher concentrations of neurotensin may result in cardiac stimulation, increased vascular tone, and accelerated atherogenesis as a consequence of binding to the NTS1 receptor. In contrast, NTS2—a low affinity receptor for neurotensin—is not currently believed to play a role in cardiovascular responses. Of importance, it remains unclear if circulating pro-NT concentrations reflect tissue-based concentrations of neurotensin. More studies are needed to better understand the role of both NTS1 as well as tissue-based neurotensin as participants in development of CV disease.
Limitations of our analysis include the fact our sample size is relatively small, and our event rates are modest. Nonetheless, our results support the primary hypothesis that pro-NT is predictive of CV events in the community, though in a manner somewhat more modest than that demonstrated by Melander and colleagues8; our results provide balance to the literature. While the more modest prognostic implication of pro-NT in our study may be due to limited event rates, for both hard CVD and hard CHD outcomes we had over 85% power to detect a difference between each of the upper and the reference quartiles of log-pro-NT. The highly skewed nature of pro-NT makes association between graded variables (such as age or BMI) somewhat challenging. We have log transformed pro-NT in order to best address this fact. While binding of neurotensin to NTS1 remains a potential mechanistic explanation for the increased CV risk associated with higher values of pro-NT, we cannot directly inform mechanism of how the neurotensin system predicts CVD or CHD. Lastly, unfortunately, we lack SNP data regarding NTS1 polymorphisms.
In summary, concentrations of pro-NT predicted onset of hard CVD and hard CHD events in a community based cohort. The ability of pro-NT to prognosticate such events appears to be independent of LDL cholesterol values or SORT1 genotypes, suggesting deleterious cardiovascular effects of neurotensin are mediated via another mechanism, such as binding to the NTS1 receptor. Further data are needed regarding the role(s) played by the neurotensin system in cardiovascular risk.
Supplementary Material
Highlights.
Neurotensin is a neuropeptide with a broad range of effects in the body, including feeding behavior. In the heart neurotensin regulates heart rate, myocardial contractility, and blood pressure.
There are three receptors for neurotensin including the sortilin receptor, which is also involved in lipid trafficking.
Measurement of neurotensin is challenging due to instability; we measured concentrations of pro-neurotensin, which is a stable pro-fragment equivalent of neurotensin.
Higher concentrations of pro-neurotensin were found to be cross-sectionally associated with a greater risk of incident cardiovascular events in the community. This association did not vary according to sex, baseline cholesterol values, or sortilin genotype.
Concentrations of pro-neurotensin were modestly associated with cardiac structure and function as well as coronary calcium.
More data are needed to better understand the link(s) between neurotensin and cardiovascular disease in the community.
Acknowledgments
The authors wish to thank SphingoTec for pro-NT analysis.
Funding sources: Dr. Januzzi is supported in part by the Hutter Family Professorship in the Field of Cardiology, as well as the DeSanctis Endowed Clinical Scholar in Medicine Fund. Dr. Gaggin is supported in part by the Clark Fund for Cardiac Research Innovation. Dr. Vasan is supported in part by National Heart Lung and Blood Institute contracts N01-HC-25195 and HHSN268201500001I.
Dr. Januzzi has received grant support from Siemens, Singulex and Prevencio, consulting income from Roche Diagnostics, Critical Diagnostics, Sphingotec, Phillips, and Novartis, and participates in clinical endpoint committees for Novartis, Amgen, Janssen, and Boehringer Ingelheim. Dr. Gaggin has received consulting income from Roche Diagnostics, American Regent, EchoSense, Boston Heart Diagnostics and Critical Diagnostics. Dr. Maisel has received grant support from Abbott, Roche Diagnostics, Alere, and has received consulting income from Critical Diagnostics and Sphingotec. Dr. Wang reports research support from Diasorin and consulting income from Takeda.
Abbreviations
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CHD
Coronary heart disease
- SORT1
Sortilin receptor 1
- VLDL
Very low density lipoprotein
- LDL
Low density lipoprotein
- Pro-NT
Proneurotensin
- FHS
Framingham Heart Study
- MI
Myocardial infarction
- SNP
Single nucleotide polymorphism
- NRI
Net reclassification improvement
- PCSK9
Proprotein convertase subtililisin/kexin type 9
Footnotes
Disclosures: The other authors have no disclosures to report.
References
- 1.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 2.de Lemos JA, Lloyd-Jones DM. Multiple biomarker panels for cardiovascular risk assessment. N Engl J Med. 2008;358:2172–2174. doi: 10.1056/NEJMe0801721. [DOI] [PubMed] [Google Scholar]
- 3.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- 4.Kitabgi P, Carraway R, Leeman SE. Isolation of a tridecapeptide from bovine intestinal tissue and its partial characterization as neurotensin. J Biol Chem. 1976;251:7053–7058. [PubMed] [Google Scholar]
- 5.Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- 6.Osadchii OE. Emerging role of neurotensin in regulation of the cardiovascular system. Eur J Pharmacol. 2015;762:184–192. doi: 10.1016/j.ejphar.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, Engstrom G, Kilger U, Nilsson P, Bergmann A, Orho-Melander M. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308:1469–1475. doi: 10.1001/jama.2012.12998. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsen C, Kjolby M, Nyegaard M, Mattheisen M, Lundhede J, Buttenschon H, Mors O, Bentzon JF, Madsen P, Nykjaer A, Glerup S. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19:310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci U S A. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard JC, Poncelet M, Gueudet C, Heaulme M, Leyris R, Brouard A, et al. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci U S A. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osadchii O, Norton G, Deftereos D, Badenhorst D, Woodiwiss A. Impact and mechanisms of action of neurotensin on cardiac contractility in the rat left ventricle. Eur J Pharmacol. 2005;520:108–117. doi: 10.1016/j.ejphar.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer P, Laplace MC, Savi P, Pflieger AM, Gully D, Herbert JM. Human umbilical vein endothelial cells express high affinity neurotensin receptors coupled to intracellular calcium release. J Biol Chem. 1995;270:3409–3413. doi: 10.1074/jbc.270.7.3409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.