Abstract
Objective
To study cardiovascular disease risk score utility we 1) compared the association between Framingham Risk Score (FRS)/pooled cohort equation (PCE) categories and coronary artery plaque presence by HIV serostatus and 2) evaluated whether D:A:D risk category more accurately identifies plaque in HIV-infected men.
Design
Cross-sectional analysis within a substudy of the Multicenter AIDS Cohort Study.
Methods
Cardiac CT was performed to assess coronary plaque. We evaluated the association of plaque with increasing CVD risk score category, stratified by HIV serostatus, using logistic regression. ROC curves compared the discrimination of the scores for plaque by HIV serostatus. The sensitivity and specificity of the risk scores were compared in HIV-infected men.
Results
The risk score category—plaque associations were stronger among HIV-uninfected men than HIV-infected men, except for non-calcified plaque. For example, the odds of coronary artery calcium (CAC)>0 were 7.03 (95% CI 4.21, 11.76) times greater among men in the PCE high risk versus low risk category among HIV-uninfected men, compared to just 3.13 (95% CI 2.13, 4.61) times greater among men in the high risk versus low risk category among HIV-infected men. Among HIV-infected men, high risk category by PCE identified the greatest percent of men with plaque/stenosis, but with lower specificity than D:A:D and FRS. The prevalence of CAC>0 among men in the PCE low risk category was 26.5% (HIV-uninfected men) and 36.0% (HIV-infected men).
Conclusions
FRS and PCE categories associate with plaque burden better in HIV-uninfected men. No risk score delivered both high sensitivity and specificity among HIV-infected men.
Keywords: cardiovascular disease, risk scores, HIV, cardiac CT, subclinical atherosclerosis
Introduction
Among HIV-infected individuals, CVD-related mortality is an important cause of death [1, 2] and epidemiologic studies have shown a greater prevalence of subclinical coronary atherosclerosis among HIV-infected compared to HIV-uninfected individuals [3–5]. Accurate CVD risk prediction, therefore, is essential for optimal long-term management of HIV-infected persons. CVD risk scores, including the Framingham Risk Score (FRS) and the American College of Cardiology/American Heart Association (ACC/AHA) PCE [6, 7], predict risk for CVD events and are recommended for use in clinical settings to determine need for preventive therapies, such as lipid-lowering medications. HIV-infected patients have higher FRS than age- and sex- matched HIV-uninfected controls due to more smoking and low HDL cholesterol levels [8]. However, FRS has been shown to underestimate cardiac events among HIV-infected persons as their duration of antiretroviral therapy (ART) increased [9]. A low level of agreement between FRS and subclinical atherosclerosis, as measured by carotid intima media thickness, has been demonstrated among HIV-infected persons, with a large proportion (56.4%) of persons with subclinical atherosclerosis classified as low risk by FRS [10]. This may reflect HIV-related immune activation despite suppressed HIV viremia [11].
Investigators from the Data Collection on Adverse Effects of Anti-HIV Drugs (D:A:D) group developed an enhanced risk equation that includes HIV-specific factors for use among HIV-infected individuals [12]. The D:A:D score performed better than the FRS in predicting subclinical atherosclerosis measured by intimal-media thickness (IMT) among HIV-infected men; however, about 17% of HIV-infected patients characterized as low risk by both equations had evidence of atherosclerosis as assessed by IMT[13]. Compared with carotid IMT, presence of CAC has been shown to improve prediction of incident CVD in the general population [14]. Currently, there is limited data on the associations between cardiovascular disease risk score and subclinical atherosclerosis (including CAC) among individuals with HIV. One study reported a higher FRS among HIV-infected individuals with versus without CAC (mean FRS of 0.19 and 0.12, respectively) [15].
Our primary objective was to examine the associations of two general population CVD risk scores (the FRS and the ACC/AHA PCE) with the presence of coronary artery plaque among well-characterized HIV-infected and similar at-risk HIV-uninfected men, and whether these associations differ by HIV serostatus.
Methods
Study Population
The MACS was initiated in 1984 as a study of men who have sex with men conducted at four study sites in Baltimore/Washington, DC, Chicago, Los Angeles, and Pittsburgh. A total of 6,972 men have been enrolled over three time periods (1984–85, 1987–90, and 2001–03) and enrollment is currently open. Details of the study design and methods have been published [16].
Selection Criteria
This analysis included data from a nested CVD CT scan study, which enrolled 1001 MACS participants who were 40–70 years of age, weighed less than 300 pounds, and had no history of coronary artery revascularization [4]. Only 2% of the sample were missing data on at least one variable required to generate the three risk scores; these participants were excluded from the analytic sample. We also excluded 10 men with adjudicated cardiovascular events (myocardial infarction, stroke, transient ischemic attack, peripheral arterial disease, or nonfatal cardiac arrest) prior to their plaque measurement. The protocol was approved by Institutional Review Boards at each site and each study participant signed informed consents.
Plaque Outcomes
The details of the CT scanning have been published previously [4, 17]. Briefly, non-contrast cardiac CT scans for coronary artery calcium (CAC) and coronary CT angiography (CTA) for other plaque outcomes were performed between January 2010 and August 2013. Men with atrial fibrillation, chronic kidney disease [estimated glomerular filtration rate (GFR) <60 ml/min/m2 during a prior MACS study visit] or a history of IV contrast allergy were excluded from CTA studies, and GFR>60 ml/min/m2 was verified within one month for those men undergoing CTA. Images from the scans were evaluated at a core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA) by trained, experienced readers who were blinded to participant characteristics including HIV serostatus. CAC scores were calculated using the Agatston method. For the CTA studies, each segment was analyzed using the modified 15-segment model of the American Heart Association [18].
Each coronary segment was classified as normal or containing non-calcified plaque, mixed plaque (<50% of plaque area occupied by calcium) or calcified plaque (definitions provided in previous manuscript) and/or stenosis ≥ 50% [4, 17]. The outcomes for this analysis included the presence of plaque (CAC>0 on non-contrast CT, and any coronary plaque, non-calcified plaque or mixed plaque on CTA), and coronary artery stenosis ≥ 50%.
Risk Score Calculation
For each participant, we calculated the following cardiovascular disease risk scores: the Framingham hard coronary heart disease (CHD) Risk Score using code based on the FRS score sheets in the Adult Treatment Panel III (http://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf) [6] the ACC/AHA PCE [7], and for HIV-infected participants, the D:A:D Score [12]. The participant characteristics that are included in the calculation of each risk score, the risk time horizon, and the outcome(s) predicted by each score are listed (see Table, Supplemental Digital Content 1, which shows each risk score). Score calculation is described in detail (see Text file, Supplemental Digital Content 2, describing the variables that are included in the risk score).
For the FRS, individuals with >20% estimated risk of MI and coronary death within 10 years are categorized as high risk; persons with 10–20% risk are moderate risk, and persons with <10% risk are low risk. All diabetic participants were classified as FRS high risk a priori, regardless of FRS score. The PCE classifies as high risk, individuals with ≥ 7.5% risk of coronary death or nonfatal MI, or fatal or nonfatal stroke within 10 years; moderate risk for those with 5–7.5% risk, and low risk for those with <5% risk. The D:A:D score classifies individuals with >10% risk of nonfatal and fatal MI within 5 years as very high risk, 5–10% as high risk, 1–5% as moderate risk, and <1% as low risk. We combined the D:A:D very high and high risk categories. Our aim was to compare the extent to which the risk categories used clinically as specified by the various risk algorithms are associated with contemporaneous subclinical coronary disease, therefore, risk categories, rather than risk scores, were our primary predictors.
Statistical Analysis
Distributions of demographic, behavioral, and clinical characteristics of HIV-infected and uninfected men were compared using the chi-square or Wilcoxon rank sum tests for categorical and continuous variables, respectively.
For each plaque outcome, we fit a logistic regression model using predictors of risk score category and HIV serostatus. Interaction terms were included to evaluate whether the association between risk score category and plaque differed by HIV serostatus. Separate models were run for each general risk score equation (PCE and FRS). Discrimination, the ability of the risk score to classify participants with and without plaque, was assessed separately among HIV-infected and HIV-uninfected men based on the receiver operating characteristic (ROC) curve. The area under the ROC curve was calculated using the trapezoidal rule and was compared by HIV status using a test for two independent samples.
We evaluated the discrimination of the D:A:D score compared to the FRS and PCE among HIV-infected men, using sensitivity and specificity of the risk category cutpoints in addition to the global area under the ROC curve metric. We evaluated the sensitivity and specificity at two thresholds: 1) greater than or equal to the high risk category, and alternatively 2) greater than or equal to the moderate risk categories, for each of the three scores. Sensitivity was the probability of being classified as ≥ high risk (alternatively ≥ moderate risk) risk among men with atherosclerosis. Specificity was the probability of being classified as < high risk (alternatively < moderate risk) risk among men without atherosclerosis. We compared the sensitivity and specificity of the algorithms using McNemar’s test for dependent samples. The area under the curve was calculated for the thresholds using the trapezoidal rule. Because age is a strong determinant of risk score, we stratified the analysis by age greater than 50 to evaluate whether the discrimination metrics differed by age category.
Statistical significance was defined as a p-value <0.05. All analyses used Stata 13.1 (StataCorp, College Station, TX).
Results
Demographic and clinical characteristics
Table 1 shows the distribution of relevant demographic and clinical characteristics by HIV serostatus. The HIV-infected men (n= 599, 61% of sample) were younger and more likely to report being Hispanic/Other or African-American race. The HIV-infected men had higher fasting glucose and were more likely to have diabetes, lower total, HDL- and LDL- cholesterol, higher triglycerides, and lower body mass index (BMI). The HIV-infected men were more likely to be current smokers and to have hepatitis C virus (HCV) co-infection.
Table 1.
Descriptive Characteristics of the MACS Participants, by HIV Status, 2010–2013
| HIV+ n=599 |
HIV− n=376 |
P-value | |
|---|---|---|---|
| Age (years) | 52 (47, 58) | 54 (50, 61) | <0.001 |
| Race | <0.001 | ||
| Caucasian | 315 (52.6%) | 253 (67.3%) | |
| Hispanic/Other | 80 (13.4%) | 32 (8.5%) | |
| African-American | 204 (34.1%) | 91 (24.2%) | |
| Systolic Blood Pressure (mmHg) | 126 (115, 137) | 128 (118, 137) | 0.24 |
| On Hypertensive Medications | 212 (35.4%) | 116 (30.9%) | 0.14 |
| Fasting Glucose (mg/dL) | 98 (90, 108) | 96 (89, 105) | 0.02 |
| Diabetes (1) | 109 (18.2%) | 49 (13.0%) | 0.03 |
| Total Cholesterol (mg/dL) | 185 (157, 212) | 190 (167, 216) | 0.03 |
| HDL-Cholesterol (mg/dL) | 46 (38, 55) | 52 (42, 60) | <0.001 |
| LDL-Cholesterol (mg/dL) | 104 (82, 129) | 112 (91, 137) | <0.001 |
| Triglycerides (mg/dL) | 132 (95, 201) | 104 (74, 147) | <0.001 |
| On Lipid Lowering Medications | 202 (34.6%) | 111 (30.0%) | 0.14 |
| Body Mass Index (kg/m2) | 26 (23, 28) | 27 (24, 30) | <0.001 |
| Smoking Status | <0.01 | ||
| Current | 183 (30.6%) | 82 (21.8%) | |
| Former | 265 (44.2%) | 201 (53.5%) | |
| Never | 151 (25.2%) | 93 (24.7%) | |
| Hepatitis C Infection | 66 (11.0%) | 17 (4.5%) | <0.001 |
| HIV-specific factors | |||
| Undetectable Viral Load (< 50 copies) | 491 (82.0%) | ||
| HIV RNA (copies/mL)(2) | 661 (139, 19500) | ||
| On HAART | 530 (88.5%) | ||
| Time on HAART (years) | 9.4 (6.3, 12.4) | ||
| Current Indinavir use | 6 (1.0%) | ||
| Indinavir exposure (years) | 0.0 (0.0, 0.8) | ||
| Current Lopinavir use | 50 (8.3%) | ||
| Lopinavir exposure (years) | 0.0 (0.0, 0.8) | ||
| Current Abacavir | 26 (4.3%) | ||
| CD4+ T-cell count (cells/mm3) | 594 (419, 765) | ||
| CD4+ T-cell nadir (cells/mm3) | 284 (172, 399) | ||
| History of AIDS | 89 (14.9%) | ||
HDL= high-density lipoprotein, LDL = low-density lipoprotein, HAART = highly active antiretroviral therapy. Prevalence (%) or median and interquartile range. P-values determined using the Wilcoxon rank sum or chi-square test as appropriate. (1) Diabetes was defined as having a fasting glucose >=126 or being on diabetes medication, either at the current visit, or at two or more previous visits (2) HIV viral load among the 109 HIV+ men with detectable current HIV RNA (>50 copies/mL) levels.
Most HIV-infected men (89%) were receiving highly active antiretroviral therapy (HAART), median duration of therapy was 9.4 years, and most participants (82%) had undetectable HIV RNA levels. Few were currently taking a regimen that included indinavir, lopinavir, or abacavir (1.0%, 8.3% and 4.3%, respectively), antiretroviral drugs in the D:A:D risk score.
Of the 975 participants included in our study sample, 975 underwent a non-contrast CT (974 non-contrast CT results available; 1 scan was technically limited) for CAC score. Coronary CT angiography was performed in 749 out of 975 (77% of the total).
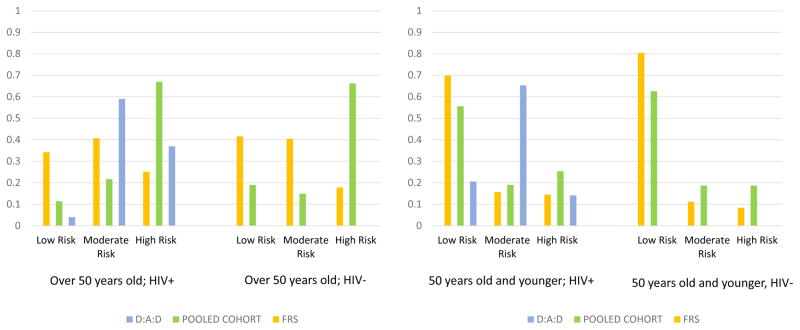
The distribution of the risk categories for study participants by HIV serostatus is shown in Figure 1 for each CVD risk score algorithm, stratified by age. As expected, men older than 50 years were more likely to be classified as high risk. More HIV-infected compared to uninfected men were classified as high risk in both age groups and across risk algorithms, except for the PCE among men older than 50 years, which categorized two-thirds of evaluated men as high risk, regardless of HIV serostatus. Among younger men, the D:A:D risk score classified fewer HIV-infected men as low risk and more as moderate risk compared to the two general population risk categories; it classified fewer as high risk compared to the PCE but more than the FRS for men older than 50 years. Regardless of HIV serostatus, more men were classified as high risk by the PCE.
Figure 1.
Distribution of the Categories of 10 Year CVD Risk* by HIV Status for the three Risk Scores among MACS Participants, 2010–2013, stratified by age
Association of PCE Category with Coronary Plaque by HIV Serostatus
We expected that plaque presence would be higher in moderate/high risk men compared to the low risk men, which was generally true (Table 2). For example, odds of CAC>0 among high-risk HIV-infected men was three times higher than low-risk HIV-infected men (OR 3.13, p<0.001). Odds of CAC>0 was even higher among high risk HIV-uninfected men compared with low risk HIV-uninfected men (OR of 7.03 (p<0.001)). The risk group-HIV interaction was statistically significant (p value =0.01).
Table 2.
Association between ACC/AHA Pooled Cohort Equation Risk Categories and Coronary Plaque by HIV serostatus, among men in the MACS, 2010–2013
| HIV-infected (n=445 CTA/n=599 CT) | HIV-uninfected (n=304 CTA/n=375 CT) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | OR and 95% CI | N | % | OR and 95% CI | Interaction p-value | |
| CAC>0 | |||||||
|
| |||||||
| Low Risk | 178 | 36.0% | 1 (ref) | 117 | 26.5% | 1 (ref) | |
| Moderate Risk | 123 | 49.6% | 1.75* [1.10,2.80] | 60 | 36.7% | 1.61 [0.82,3.13] | 0.83 |
| High Risk | 298 | 63.8% | 3.13*** [2.13,4.61] | 198 | 71.7% | 7.03*** [4.21,11.76] | 0.01 |
|
| |||||||
| Any Plaque on CCTA | |||||||
|
| |||||||
| Low Risk | 146 | 63.0% | 1 (ref) | 93 | 52.7% | 1 (ref) | |
| Moderate Risk | 91 | 76.9% | 1.96* [1.08,3.54] | 52 | 69.2% | 2.02 [0.99,4.13] | 0.95 |
| High Risk | 208 | 88.0% | 4.30*** [2.51,7.35] | 159 | 89.3% | 7.50*** [3.93,14.33] | 0.19 |
|
| |||||||
| Non Calcified Plaque Presence | |||||||
|
| |||||||
| Low Risk | 146 | 47.3% | 1 (ref) | 93 | 40.9% | 1 (ref) | |
| Moderate Risk | 91 | 61.5% | 1.79* [1.05,3.04] | 52 | 55.8% | 1.82 [0.92,3.62] | 0.96 |
| High Risk | 208 | 74.5% | 3.26*** [2.08,5.12] | 159 | 59.1% | 2.09** [1.24,3.52] | 0.21 |
|
| |||||||
| Mixed Plaque Presence | |||||||
|
| |||||||
| Low Risk | 146 | 24.7% | 1 (ref) | 93 | 15.1% | 1 (ref) | |
| Moderate Risk | 91 | 36.3% | 1.74 [0.98,3.07] | 52 | 21.2% | 1.51 [0.63,3.63] | 0.80 |
| High Risk | 208 | 39.4% | 1.99** [1.25,3.18] | 159 | 45.3% | 4.67*** [2.44,8.93] | 0.04 |
|
| |||||||
| Coronary Stenosis >=50% | |||||||
|
| |||||||
| Low Risk | 146 | 11.6% | 1 (ref) | 93 | 5.4% | 1 (ref) | |
| Moderate Risk | 91 | 17.6% | 1.62 [0.77,3.39] | 52 | 7.7% | 1.47 [0.38,5.72] | 0.90 |
| High Risk | 208 | 20.2% | 1.92* [1.04,3.53] | 159 | 22.6% | 5.15*** [1.94,13.65] | 0.09 |
Exponentiated coefficients; 95% confidence intervals in brackets
p < 0.05,
p < 0.01,
p < 0.001
Similarly, for the presence of any plaque on coronary CT angiography, mixed plaque, and coronary stenosis ≥ 50%, we observed higher odds of these outcomes in moderate risk men compared to low risk men and in high risk men compared to low risk men. Among those outcomes, the high risk category was more strongly associated with plaque presence among HIV-uninfected compared to HIV-infected men (only statistically significant for mixed plaque (p=0.04)).
In contrast to other plaque outcomes, the high risk category was more strongly associated with non-calcified plaque in HIV-infected men compared to uninfected men (OR 3.26, 95% CI 2.08, 5.12, v. 2.09, 95% CI 1.24, 3.52; interaction not significant).
For each plaque outcome, plaque prevalence in the low risk group was higher among HIV-infected than HIV-uninfected men, though the differences were not statistically significant. For example, 36.0% of low risk HIV-infected participants had CAC>0 compared to 26.5% of low risk HIV-uninfected men (p-value = 0.09) (see Table, Supplemental Digital Content 3, comparing odds of plaque by HIV serostatus among men categorized as low risk).
Association of Framingham Risk Score with plaque by HIV status
The corollary results using the FRS categories are shown (see Table, Supplemental Digital Content 4, showing the FRS category—coronary plaque by HIV serostatus). Again, for each plaque outcome, the plaque prevalence in the low risk group was higher in the HIV-infected compared to the HIV-uninfected group. For example, 41.6% of low risk HIV-infected men had CAC>0 compared to 36.0% of low risk HIV-uninfected men. More participants characterized as low risk with the FRS had plaque, compared with men characterized as low risk with the PCE, the result of more men being categorized as low risk with the FRS compared with the pooled cohort.
As described in the prior section, the odds of plaque generally increased with increasing PCE risk category, with the odds ratio for the men in the high risk category greater than the odds ratio for the men in the moderate risk category. However, the increase in odds with increasing FRS risk category was not as consistent. This is the result of few men being categorized as high risk with the FRS. When moderate/high risk category men are considered in a combined fashion for PCE and FRS, odds of plaque are consistently higher compared with low risk category men.
Again, the associations with non-calcified plaque did not follow the pattern observed with other outcomes. The odds of non-calcified plaque among high-risk HIV-infected men was over three times higher than low-risk HIV-infected men (OR 3.19, p<0.001). However, the odds of non-calcified plaque among HIV-uninfected men in the high risk category compared to the low risk category was 1.39 (p=0.08) (risk score-HIV interaction not significant).
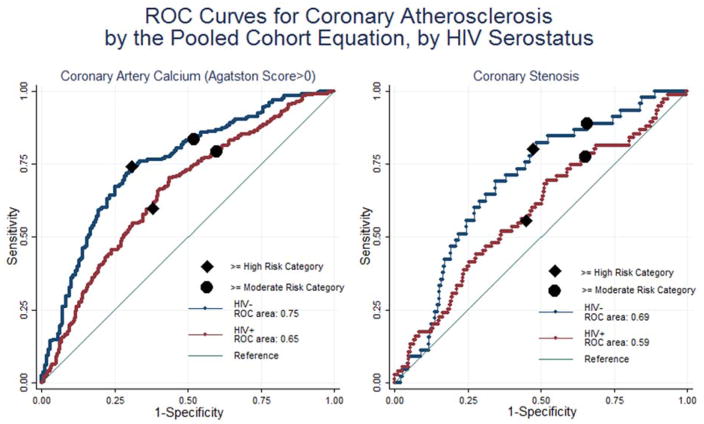
We also evaluated the discrimination of the continuous risk scores for the presence of coronary plaque. The ROC curves plot the ability of the scores to separate those with and without coronary plaque (CAC>0 and stenosis>=50%) using each observed PCE risk score value as a threshold associated with a sensitivity (y-axis) and 1-specificity (x-axis for CAC>0) (Figure 2). The area under the ROC curve is significantly higher for the HIV-uninfected compared to HIV-infected men for CAC>0 (0.75 vs 0.65, p<0.01) and marginally higher for coronary stenosis (0.69 vs 0.59, p=0.06). Across the range of PCE risk scores for HIV-infected men, a cutpoint with a given level of sensitivity will have worse specificity in the HIV-infected compared to HIV-uninfected men. The ROC curves are similar for the FRS (data not shown). Despite the relative higher performance of the scores among the HIV-uninfected men, the areas under the ROC curve are less than 0.8, a commonly cited threshold for sufficient discrimination, regardless of serostatus.
Figure 2.
D:A:D compared to general population CVD risk score categories
Our secondary objective was to evaluate whether the D:A:D score better classified plaque presence than the general population score categories in the HIV-infected men. Table 3 shows that the high risk category by PCE identified the most men with plaque and stenosis ≥ 50%, though its specificity was lower than the D:A:D and Framingham. For example, among men > 50 years, the high risk category included 71.4% of men with CAC>0 using the PCE, compared to 40.5% with CAC>0 using the D:A:D and just 24.7% with CAC>0 using the FRS (p<0.001 comparing each to pooled cohort). For men ≤ 50 years, the high risk category captured fewer of those with atherosclerosis for each respective risk equation (e.g., for CAC>0, sensitivity was 31.8% with the PCE, 17% with FRS, and 15.9% with D:A:D). Conversely, the specificity of the PCE high risk category was lower compared with D:A:D and FRS (e.g., among men > 50 years old for CAC>0, 41.1% for pooled cohort, 69.4% for D:A:D, and 74.2% for FRS). The gains in sensitivity when combining the moderate/high risk categories are sizeable, particularly for the D:A:D, which classifies the largest proportion of men with plaque as moderate risk, as shown in Figure 1. When considering moderate/high as a combined group, the D:A:D has significantly greater sensitivity than the PCE and FRS. None of the risk score categories had both high sensitivity and specificity, and the area under the curves indicated inadequate discrimination (<70%) of all three scores among HIV-infected men.
Table 3.
Discrimination metrics of the D:A:D, Framingham Hard CVD Risk Categories, and pooled cohort equation for coronary plaque by age strata, among HIV-infected men in the MACS, 2010–2013
| Sensitivity 95% CI |
Specificity 95% CI |
Area under the ROC curve |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N with plaque |
D:A:D | FRS | PCE | N without plaque |
D:A:D | FRS | PCE | D:A:D | FRS | PCE | ||
| ≤ 50 years of age (n=201 CTA/n=248 CT) | ||||||||||||
| CAC>0 | 88 | ≥ Moderate Risk Category | 87.5% (78.7,93.6) | 34.1% (24.3,45.0) *** | 48.9% (38.1,59.8) *** | 160 | 25.0% (18.5,32.4) | 71.9% (64.2,78.7) *** | 58.1% (50.1,65.9) *** | 0.56 | 0.53 | 0.55 |
| ≥ High Risk Category | 15.9% (9.0,25.2) | 17.0% (9.9,26.6) | 31.8% (22.3,42.6) *** | 86.9% (80.6,91.7) | 86.9% (80.6,91.7) | 78.1% (70.9,84.3) * | ||||||
| Any plaque on CTA | 129 | ≥ Moderate Risk Category | 85.3% (78.0,90.9) | 33.3% (25.3,42.2) *** | 49.6% (40.7,58.5) *** | 72 | 37.5% (26.4,49.7) | 79.2% (68.0,87.8) *** | 66.7% (54.6,77.3) *** | 0.64 | 0.57 | 0.59 |
| ≥ High Risk Category | 17.1% (11.0,24.7) | 16.3% (10.4,23.8) | 30.2% (22.5,38.9) *** | 94.4% (86.4, 98.5) | 94.4% (86.4, 98.5) | 84.7% (74.3,92.1) * | ||||||
| Non-calcified plaque | 97 | ≥ Moderate Risk Category | 84.5% (75.8,91.1) | 38.1% (28.5,48.6) *** | 53.6% (43.2,63.8) *** | 104 | 29.8% (21.2,39.6) | 79.8% (70.8,87.0) *** | 65.4% (55.4,74.4) *** | 0.60 | 0.60 | 0.61 |
| ≥ High Risk Category | 18.6% (11.4,27.7) | 20.6% (13.1,30.0) | 33.0% (23.8,43.3) ** | 92.3% (85.4,96.6) | 95.2% (89.1, 98.4) | 82.7% (74.0,89.4) ** | ||||||
| Stenosis > 50% | 18 | ≥ Moderate Risk Category | 77.8% (52.4,93.6) | 44.4% (21.5,69.2) * | 44.4% (21.5,69.2) * | 183 | 23.0% (17.1,29.7) | 72.7% (65.6, 79.0) *** | 56.3% (48.8,63.6) *** | 0.54 | 0.61 | 0.52 |
| ≥ High Risk Category | 22.2% (6.4,47.6) | 33.3% (13.3,59.0) | 33.3% (13.3,59.0) | 88.0% (82.4,92.3) | 89.6% (84.3,93.6) | 76.0% 69.1,82.0) *** | ||||||
|
| ||||||||||||
| >50 years of age (n=244 CTA/n=351 CT) | ||||||||||||
| CAC>0 | 227 | ≥ Moderate Risk Category | 96.9% (93.7,98.8) | 71.8% (65.5, 77.6) *** | 91.6% (87.2,94.9) ** | 124 | 5.6% (2.3,11.3) | 45.2% (36.2,54.3) *** | 16.9% (10.8,24.7) ** | 0.56 | 0.56 | 0.57 |
| ≥ High Risk Category | 40.5% (34.1,47.2) | 24.7% (19.2,30.8) *** | 71.4% (65.0,77.2) *** | 69.4% (60.4,77.3) | 74.2% (65.6,81.6) | 41.1% (32.4,50.3) *** | ||||||
| Any plaque on CTA | 216 | ≥ Moderate Risk Category | 95.4% (91.7,97.8) | 62.5% (55.7,69.0) *** | 87.5% (82.3,91.6) *** | 28 | 3.6% (0.1,18.3) | 46.4% (27.5,66.1) *** | 21.4% (8.3,41.0) | 0.56 | 0.50 | 0.59 |
| ≥ High Risk Category | 33.8% (27.5,40.5) | 19.9% (14.8,25.9) *** | 66.7% (60.0,72.9) *** | 78.6% (59.0,91.7) | 67.9% (47.6,84.1) | 50.0% (30.6,69.4) * | ||||||
| Non-calcified plaque | 183 | ≥ Moderate Risk Category | 95.6% (91.6,98.1) | 62.3% (54.8,69.3) *** | 86.9% (81.1,91.4) *** | 61 | 4.9% (1.0,13.7) | 41.0% (28.6,54.3) *** | 14.8% (7.0,26.2) * | 0.52 | 0.53 | 0.55 |
| ≥ High Risk Category | 33.3% (26.6,40.7) | 22.4% (16.6,29.1) ** | 67.2% (59.9,74.0) *** | 70.5% (57.4,81.5) | 82.0% (70.0,90.6) | 42.6% (30.0,55.9) ** | ||||||
| Stenosis > 50% | 57 | ≥ Moderate Risk Category | 96.2% (90.6,99.0) | 64.9% (51.1,77.1) *** | 87.7% (76.3,94.9) * | 187 | 5.1% (2.1,10.2) | 39.6% (32.5,47.0) *** | 13.9% (9.3,19.7) *** | 0.56 | 0.52 | 0.49 |
| ≥ High Risk Category | 40.4% (27.6,54.2) | 22.8% (12.7, 35.8) ** | 63.2% (49.3,75.6) ** | 70.1% (62.9, 76.5) | 79.1% (72.6,84.7) * | 34.8% (28.0,42.1) *** | ||||||
Difference in the sensitivity and specificity of the FRS and pooled cohort equation, each compared to the D:A:D was assessed using McNemar’s test;
p < 0.05,
p < 0.01,
p < 0.001
The prevalence of any plaque was high among the older HIV-infected men classified as low risk using each of the three scores: 86% (81/94) by FRS, 82% (27/33) by pooled cohort, and 91% (10/11) by D:A:D (see Figure, Supplemental Digital Content 5). Despite the similar prevalence across algorithms, the absolute number of men with plaque who are classified as having low risk of cardiovascular outcomes (false negatives), was minimized with the D:A:D, which classifies fewer HIV-infected men as low risk. Among the men aged 50 and younger, the prevalence of any plaque among the men in the low risk category was significantly lower in the D:A:D (41%, 19/46) versus the FRS (60%, 86/143) and marginally lower versus the pooled cohort algorithm (58%, 65/113).
Discussion
In this large, well-characterized group of HIV-infected and uninfected men, moderate and high risk general population score categories (FRS and PCE) were more strongly associated with plaque presence among HIV-uninfected men than among HIV-infected men. Among HIV-infected men, the PCE high risk category was more sensitive for plaque presence than both the D:A:D and the FRS, however, the specificity was lower. Current ACC/AHA CVD risk reduction guidelines recommend statin therapy for patients in the PCE high risk category, and our results show that for HIV-infected men aged 50 and younger, this will miss most men with plaque and most men with stenosis ≥ 50%. In both age groups, stenosis ≥ 50% represents a potentially high risk lesion that could lead to ischemia. CAC>0 is predictive of CVD events compared to CAC=0 [19], with the significance of a particular lesion depending on both the degree of CAC and patient’s age.
In previous work, FRS and PCE have both been shown to underestimate risk for cardiac events [9, 20, 21] and subclinical atherosclerosis assessed by carotid IMT [10, 13] in HIV-infected persons. The D:A:D score has performed better than general population scores in some studies in predicting myocardial infarction [9] and carotid IMT [13] among HIV-infected people. However, an analysis of HIV Outpatient Study (HOPS) data revealed more observed CVD events than expected by the D:A:D score, with a ratio of expected events to observed events of 0.75. The ratio of expected events to observed events was higher using the FRS (0.85) and the PCE (0.83) [21]. Our study did not show any advantage of the D:A:D score over the general population risk scores for the intermediate outcome of coronary plaque. D:A:D high risk category was not very sensitive for plaque in either age group examined, and when the moderate/high risk groups were combined (versus the low risk group), although the sensitivity increased to include almost all of the men with plaque, specificity dropped sharply.
Chronic effects of HIV infection itself (inflammation, hypercoagulability, endothelial dysfunction), effects of certain ART drugs (hyperlipidemia, insulin resistance), lifestyle risk factors, particularly smoking, and other chronic medical comorbidities (hypertension, diabetes, and hyperlipidemia) [22–25] all contribute to CVD risk among HIV-infected persons. Some factors that contribute to increased risk of CVD among HIV-infected patients (e.g. chronic inflammation) are not incorporated into risk prediction scores [11, 26]. The D:A:D score accounts for certain ART agents (abacavir, indinavir, and lopinavir) that contribute to cardiovascular risk independent of the metabolic derangement expected with any ART [27–30]. However, this adjustment was of limited importance in our study population with low levels of use of these ART agents. The D:A:D uses an algorithm that weights the effects of traditional risk factors such as age and hypertension based on their associations with cardiovascular outcomes in an HIV-infected rather than the general population. Even so, we did not see an enhanced performance of the D:A:D compared to the general population risk scores in our analysis. Because the upper threshold for the low risk category (<1% 5-year risk) is so low, few people are classified in the low risk group, contributing to the score’s low specificity.
The strengths of our study include a large sample size with standardized collection of CV risk factors from which to calculate risk scores and the presence of an HIV-uninfected comparison group. We classified subclinical coronary atherosclerosis using coronary CT angiography, which is a very specific and sensitive measure. Limitations of our study include the cross sectional analysis and the limited number of hard coronary outcomes, preventing us from looking at associations between risk scores and hard coronary endpoints over time. CVD risk scores were developed to predict hard coronary outcomes, not subclinical CVD, and therefore we might not expect very high discrimination metrics for our intermediate outcomes. However, both CAC and plaque have been associated with subsequent coronary events [19, 31–33]. In a large study of outpatients undergoing coronary CTA, cumulative probability of a major coronary endpoint increased with the degree of CAC from 2.1% among individuals with a CAC of 0 to 16.3% among individuals with a CAC > 400. No plaque was associated with cumulative probability of major adverse cardiac events of 0.8% and cumulative probability of event increased as the number of involved vessels increased. Calcified plaque had the lowest risk of subsequent event (5.5%), while noncalcified and mixed plaque had the highest (22.7% and 37.7%, respectively) [19]. In another study examining plaque characteristics among individuals with acute coronary syndrome, noncalcified plaque presence was two-fold higher comparing ACS with non-ACS group members (43% vs. 22%, p < 0.01) [33]. There was variation in the prevalence of our plaque outcomes, with mixed plaque and coronary stenosis >50% being the least prevalent. Even with the lower prevalence, we observed a stronger risk score-plaque association for these outcomes among HIV-uninfected men when general population risk scores were used.
In summary, risk score-plaque associations for the general population risk scores were weaker among HIV-infected men compared with HIV-uninfected men, except for non-calcified plaque. The high risk category by PCE identified the greatest percent of men with plaque, regardless of serostatus, however, with very low specificity. No risk score delivered both high sensitivity and specificity among HIV-infected men. Clinicians must balance the limitations of each of the scores, and the risk of side effects with statin therapy, when making individual treatment decisions for their patients.
Supplementary Material
Acknowledgments
Grant Support: This study was funded by the National Heart, Lung, and Blood Institute (grant R01 HL095129 to Dr. Post). Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). The research described was additionally supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124. MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Footnotes
Conflicts of Interest:
F.J.P. is on the speaker’s bureaus for Gilead Sciences, Janssen Pharmaceuticals, Merck, and Bristol Myers Squibb. T.T.B. is a consultant for Gilead Sciences, Bristol Myers Squibb, Merck, Abbvie, EMD-Serono, and ViiV Healthcare. M.D.W. is a consultant for Gilead Sciences.
Contributions of authors: A.K.M. – study design, interpretation of results, and manuscript writing; S.A.H. - study design, data analysis, interpretation of results, and manuscript writing; W.S.P. – study design, interpretation of results, funding, administrative support, and manuscript writing F.J.P. – funding, administrative support, and manuscript writing; L.A.K – funding, administrative support, and manuscript writing M.D.W. – interpretation of results, administrative support, and manuscript writing M.B. – Cardiac CT scans, funding, administrative support, manuscript writing L.P.J. – study design, interpretation of results, funding, administrative support, and manuscript writing – T.T.B - study design, interpretation of results, and manuscript writing
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. JAIDS J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kingsley L, Cuervo-Rojas J, Muñoz A, Palella F, Post W, Witt M, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22:1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergersen B, Sandvik L, Bruun J, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23:625–630. doi: 10.1007/s10096-004-1177-6. [DOI] [PubMed] [Google Scholar]
- 9.Law M, Friis-Møller N, El-Sadr W, Weber R, Reiss P, Monforte AD, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 10.Parra S, Coll B, Aragones G, Marsillach J, Beltrán R, Rull A, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 11.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2014;211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Villar S, Estrada V, Gómez-Garre D, Ávila M, Fuentes-Ferrer M, San RJ, et al. Diagnosis of subclinical atherosclerosis in HIV-infected patients: higher accuracy of the D:A:D risk equation over Framingham and SCORE algorithms. Eur J Prev Cardiol. 2014;21:739–748. doi: 10.1177/2047487312452964. [DOI] [PubMed] [Google Scholar]
- 14.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow D, Young R, Valcour N, Kronmal RA, Lum CJ, Parikh NI, et al. HIV and coronary artery calcium score: comparison of the Hawaii Aging with HIV Cardiovascular Study and Multi-Ethnic Study of Atherosclerosis (MESA) cohorts. HIV Clin Trials. 2015;16:130–138. doi: 10.1179/1528433614Z.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 17.Hacıoğlu Y, Gupta M, Choi T-Y, George RT, Deible CR, Jacobson LP, et al. Use of cardiac CT angiography imaging in epidemiology study--the methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu Kardiyol Derg. 2013;13:207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austen W, Edwards J, Frye R, Gensini G, Gotts V, Griffith L, et al. A reporting system on patients evaluated for coronary artery disease: Report of the Ad Hoc Committee for Grading Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(Suppl 4):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 19.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Regan S, Meigs JB, Massaro J, Triant V. Evaluation of the ACC/AHA CVD risk prediction algorithm among HIV-infected patients. Conference on Retroviruses and Opportunistic Infections; 2015. Available from: http://www.croiconference.org/sessions/evaluation-accaha-cvd-risk-prediction-algorithm-among-hiv-infected-patients. [Google Scholar]
- 21.Thompson-Paul A, Lichtenstein KA, Armon C, Buchacz K, Hart R, Chmiel JS, et al. Cardiovascular disease risk prediction in the HIV Outpatient Study (HOPS). Conference onRetroviruses and Opportunistic Infections; 2015. Available from: http://www.croiconference.org/sessions/cardiovascular-disease-risk-prediction-hiv-outpatient-study-hops. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 23.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and Risk Factors for New-Onset Diabetes in HIV-Infected Patients. Diabetes care. 2008;31:1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26:2126–2133. doi: 10.1097/HJH.0b013e32830ef5fb. [DOI] [PubMed] [Google Scholar]
- 25.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 26.El-Sadr W, Lundgren J, Neaton J, Gordin F, Abrams D, Arduino R, et al. CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 27.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study) Circulation. 2009;119:805–811. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalska J, Kirk O, Mocroft A, Høj L, Friis-Møller N, Reiss P, et al. Implementing the number needed to harm in clinical practice: risk of myocardial infarction in HIV-1-infected patients treated with abacavir. HIV Med. 2010;11:200–208. doi: 10.1111/j.1468-1293.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 29.Sabin CA, Worm SW. Conventional cardiovascular risk factors in HIV infection: how conventional are they? Curr Opin HIV AIDS. 2008;3:214–219. doi: 10.1097/COH.0b013e3282f6a613. [DOI] [PubMed] [Google Scholar]
- 30.Sabin CPRL, Ryom S, de Wit O, Kirk R, Weber C, Pradier F, Dabis AN, Phillips JD. Lundgren, for the D:A:D study group Is there continued evidence for an association between abacavir and myocardial infarction risk?. 21st CROI; 3–6 March 2014; Boston. Poster abstract 747 LB. [Google Scholar]
- 31.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:689–691. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: A systematic review and meta-analysis. JACC. 2011;57:1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–160. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.