Abstract
Background
The Systolic Blood Pressure Intervention Trial (SPRINT) is a multicenter, randomized clinical trial of 9,361 participants with hypertension who are ≥ 50 years old. The trial is designed to evaluate the effect of intensive systolic blood pressure control (systolic blood pressure goal <120 mm Hg) compared to standard control (systolic blood pressure goal <140 mm Hg) on cardiovascular events using commonly prescribed antihypertensive medications and lifestyle modification.
Objective
To describe the recruitment strategies and lessons learned during recruitment of the SPRINT cohort and five targeted participant subgroups: pre-existing cardiovascular disease, pre-existing chronic kidney disease, age ≥ 75 years, women, and minorities.
Methods
In collaboration with the National Institutes of Health Project Office and SPRINT Coordinating Center, five Clinical Center Networks oversaw clinical site selection, recruitment, and trial activities. Recruitment began November 8, 2010 and ended March 15, 2013 (about 28 months). Various recruitment strategies were used, including mass mailing, brochures, referrals from healthcare providers or friends, posters, newspaper ads, radio ads, and electronic medical record searches.
Results
Recruitment was scheduled to last 24 months to enroll a target of 9,250 participants; in just over 28 months, the trial enrolled 9,361 participants. The trial screened 14,692 volunteers, with 33% of initial screens originating from the use of mass mailing lists. Screening results show that participants also responded to recruitment efforts through referral by SPRINT staff, healthcare providers, or friends (45%); brochures or posters placed in clinic waiting areas (15%); and television, radio, newspaper, internet ads, or toll-free numbers (8%). The overall recruitment yield (number randomized /number screened) was 64% (9,361 randomized /14,692 screened), 77% for those with cardiovascular disease, 79% for those with chronic kidney disease, 70% for those age ≥ 75 years, 55% for women, and 61% for minorities. As recruitment was observed to lag behind expectations, additional clinics were included and inclusion criteria were broadened, keeping event rates and trial power in mind. As overall recruitment improved, a greater focus on subgroup recruitment was implemented.
Conclusion
SPRINT met its overall projected recruitment goal by using diverse, locally adapted enrollment strategies to specifically target persons with cardiovascular disease, chronic kidney disease, ≥ 75 year old, women, and minority subgroups. The trial exceeded its recruitment goal for minorities but found it a challenge to meet the competing demands of the targeted goals for recruiting into the remaining four subgroups. Important lessons include the imperative to monitor the recruitment process carefully, decide early to add new clinics or modify inclusion and exclusion criteria if recruitment lags, and consider limiting enrollment to subgroups only. We found benefit in using multiple recruitment sources simultaneously; mass mailing produced the largest number of participants, but referrals resulted in the greater randomization yield.
Keywords: SPRINT, Clinical Center Networks (CCN), Hypertension, Recruitment
Introduction
Recruitment of participants is paramount to the success of clinical research. A recent Cochrane review estimated that less than 50% of studies achieve their enrollment goal or reach the target without having to extend study duration.1 Recruitment shortfalls or prolonged enrollment time without an extension to observe the intervention’s effects often means that studies lack statistical power to address their hypotheses. In cardiovascular trials with hard clinical endpoints such as death or hospitalization for stroke or myocardial infarction, the length of time for following participants is vital to having enough statistical power to accurately answer the research question. 2,3
The Systolic Blood Pressure Intervention Trial (SPRINT) is an open design, 2-arm multicenter randomized trial to test whether lowering systolic blood pressure to <120 mm Hg versus <140 mm Hg reduces cardiovascular disease risk and other outcomes. The SPRINT recruitment plan was to enroll 9,250 participants with a systolic blood pressure ≥130 mm Hg and at least one additional cardiovascular disease risk factor over a two-year period and to follow them for four to six years.4
Eligibility for SPRINT required the presence of clinical or subclinical cardiovascular disease (excluding stroke); chronic kidney disease, defined as estimated glomerular filtration rate 20 – 59 ml/min/1.73m2; age ≥ 75; or a Framingham Risk Score for 10-year cardiovascular disease risk ≥ 15%, but excluding diabetes mellitus. The trial design and detailed inclusion/exclusion criteria have been described previously.5
In addition to the overall recruitment target of 9,250, there were five separate subgroup goals, including three clinical and two demographic subgroups, although some participants are counted within more than one subgroup: 1) Cardiovascular disease history (40% or 3,700), 2) Chronic kidney disease (46% or 4,300), 3) Age ≥ 75 (35% or 3,250), 4) Women (50% or 4,625), and 5) Racial/ethnic minorities (40% or 3,700).
In collaboration with the National Institutes of Health Project Office and the SPRINT Coordinating Center, five Clinical Center Networks managed the recruitment of 9,361 (101% of goal) SPRINT participants at 102 clinical sites across the United States and in Puerto Rico.
Prior large hypertension trials – Systolic Hypertension in the Elderly Program,3,6 Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack,7,8 and Action to Control Cardiovascular Risk in Diabetes9 – demonstrated that multiple recruitment strategies are required for successful enrollment. These trials all took longer to recruit than originally anticipated, ranging from three months, approximately 10 months, to 1.5 years longer than planned. SPRINT effectively recruited for overall numbers in just over four months longer than planned, but also concentrated efforts on meeting the specific subgroup goals as described in this paper. As noted in the Treweek Cochrane review, the effect of various types of enrollment techniques is unclear, but an open trial design is one cited as more effective at increasing recruitment.1 SPRINT was at an advantage in this regard because the target blood pressure intervention necessitated that both participants and investigators were unmasked to the participant’s assigned treatment goal.
Methods
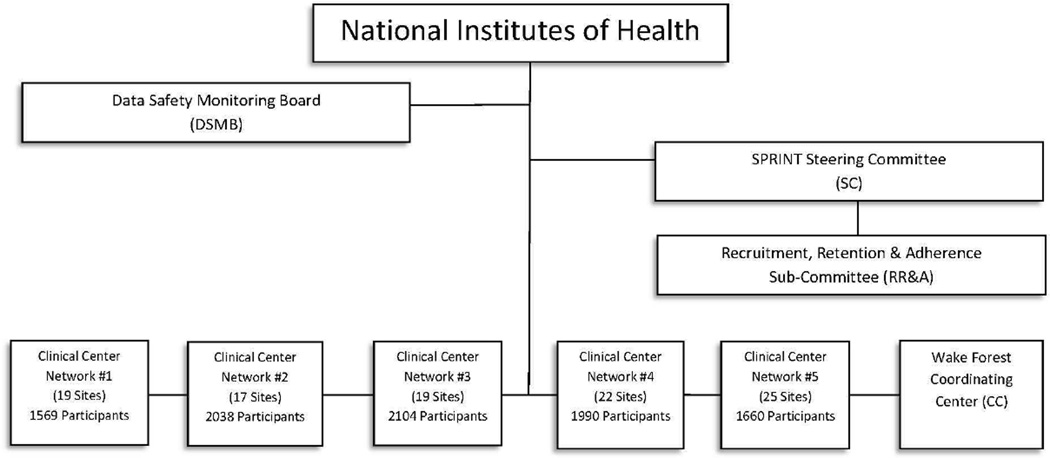
With support and coordination from the National Institutes of Health Project Office and the SPRINT Coordinating Center, the responsibilities for recruitment oversight and activities were managed by five Clinical Center Networks. SPRINT convened a Recruitment, Retention, and Adherence Subcommittee, which reports to the SPRINT Steering Committee, to oversee trial-wide recruitment strategies and progress. The Recruitment, Retention, and Adherence Subcommittee is comprised of representatives from the Coordinating Center , the Project Office, and all five of the Clinical Center Networks, including the network coordinators who oversaw, managed, and tracked the recruitment activities of all sites in their network (Figure 1).
Figure 1.
SPRINT-Organization and Reporting Chart
The plan was to recruit 9,250 participants into SPRINT over a period of 24 months and to follow participants for 4–6 years to test whether the lower systolic blood pressure goal (< 120 mm Hg) would result in a 20% reduction in cardiovascular disease events (cardiovascular disease mortality; non-fatal myocardial infarction, acute coronary syndrome, stroke, and heart failure). The sample size estimation and power calculation assumed a two-year uniform recruitment period. The recruitment efficiency (R-factor) is the ratio of the number of person years actually accrued, divided by the number of person years expected during the planned recruitment period.9–11
Recruitment strategies used in SPRINT
Recognizing that recruiting such a large number of participants is a challenge for any trial and also has been challenging in past hypertension clinical trials, the SPRINT investigators adopted a wide variety of recruitment strategies that were used simultaneously by all Clinical Center Networks, including use of mass mailing, media, posters or brochures, electronic medical record searches, and referrals by clinic staff.6–9 Most recruitment materials were developed by the SPRINT Coordinating Center (in English and Spanish) and were provided to each Clinical Center Network, with the flexibility to adapt materials prior to distribution to their target populations. In addition, the SPRINT website (www.SPRINTTRIAL.org) incorporated a public view which provided a description of the trial, basic inclusion/exclusion criteria, answers to potential participant questions, and contact information for all participating clinics across the United States and in Puerto Rico.12 Potential participants could enter their zip codes into the map section of the website to determine whether a SPRINT clinic was within a 50-mile radius of their zip code.
As the Recruitment, Retention, and Adherence Subcommittee monitored SPRINT recruitment over time, they found that using specifically targeted mass mailings were one of the most cost-effective and fruitful resources, particularly for locating potential participants based on age, gender, and zip code.
Mass mailings
To help meet the recruitment goals of the study, direct mass mailings were initially employed by four of the five Clinical Center Networks. Mailing lists were obtained from third-party vendors, and information sources for these mailing lists may have included voter registration lists, data from the United States Census Bureau, state departments of motor vehicles data, public records, phone directories, consumer surveys, self-reported data, and purchase transactions. Commercial lists, purchased from specialty marketing sources, generally included a full name and address for use on the mailing brochure. Commercial lists also included addresses with zip code information, useful for targeting mailings to those living near the clinical site, or by gender, race, or age, to meet target enrollment in these categories.
One Clinical Center Network utilized their internal Computerized Participant Records Systems, which is an integrated electronic medical record system that includes the patient problem list, pharmacy, laboratory results, consults, progress notes and an order list.13 Essentially, this Clinical Center Network used Computerized Participant Records Systems as a database for an internal mass mail campaign.
After Institutional Review Board approval, each participating SPRINT Clinical Center Network provided the mail vendor with personalized recruitment material templates. Mass mailings generally targeted potential participants within a reasonable commute of a specified SPRINT clinic. Mail volume for each clinical site varied throughout the recruitment period. Usually between 5,000 and 15,000 pieces per clinical site were customized at the printer, mail merged with the existing already enrolled participant lists to prevent duplication, and mailed at the third class bulk mail rate. Mailings were set to occur every two to four weeks, depending on recruitment goals, but were spaced to prevent clinic staff from being overwhelmed by screening phone calls, as well as to minimize the time from first participant contact to screening appointment.
The Clinical Center Networks varied on their use of targeted information to achieve subgroup goals, and use of direct mail allowed the Clinical Center Networks to rapidly change their target focus and thus adapt to the enrollment needs of the trial. For example, in an effort to fill study subgroup goals, often the Clinical Center Networks directed recruitment efforts toward people age ≥ 70 years old as a means of increasing subgroup numbers due to the increased likelihood of these individuals having other comorbid conditions such as cardiovascular disease or chronic kidney disease.
Brochures and posters
Quite a few SPRINT sites were primary care clinics, and thus participants were recruited from the pool of patients who were seen in the clinic and given brochures about the trial. The brochures were tri-fold color, on card stock paper, with photos of racially and ethnically diverse older citizens featured prominently. The goal of the brochure was to provide information to the public of the purpose of the trial, the general inclusions (age, sex, and race) as well as general exclusions (diabetes mellitus and previous stroke). Their design provided for a customizable space, which featured the local clinical site’s name and address, coordinator contact name, phone number, toll-free number, and business email address. Posters were 14”x18” and similar in design features and content, including displaying the same general inclusion/exclusion criteria as the brochure.
Referrals: Hospital, clinic chart review, health care provider, and friends
All Clinical Center Networks also identified potential eligible participants for SPRINT by conducting comprehensive chart reviews. Patient databases were typically components of medical records that have been digitized to electronic medical records. These integrated applications are often used in the daily operations of healthcare and include hospital coding/billing systems, and/or data warehouse repositories. Prior to conducting large medical record searches, each clinic sought approval from its local regulatory institutional review board, including approval for preparatory research waivers or authorization for research to use and disclose private health information. After approval was granted, computerized searches were conducted from patient databases to identify participants meeting the SPRINT inclusion/exclusion criteria.
Databases were initially searched by age and International Classification of Diseases, ninth revision codes for hypertension (401.9) and excluded anyone with diabetes (250.0). Data were further filtered for other exclusionary diagnoses and laboratory values. Because of the need to increase enrollment of pre-specified subgroups (history of cardiovascular disease or chronic kidney disease; participants age ≥ 75), database search criteria were rapidly modified to direct searches toward subgroup enrollment.
For those hospitals and clinics with electronic medical records, electronic billing databases were used to generate lists of potentially eligible participants. Clinic staff manually reviewed the generated lists and contacted potentially eligible persons by mail. The electronic medical records search would exclude participants with diagnosis codes of diabetes mellitus, prior stroke, polycystic kidney disease, dementia, solid organ transplant, or those with a eGFR < 20 mL/min/1.73 m2.
Essential recruitment documents (a letter of recruitment describing the study, SPRINT brochure or frequently asked questions sheet, and any other necessary SPRINT recruitment materials) were mailed at the 3rd class bulk mail rate on behalf of the clinical site principal investigator. For potentially eligible persons identified on generated participant lists, which were external to the principal investigator clinical site practice, study staff contacted the physicians of those potentially eligible participants by letters, emails, or phone for permission to approach their patients. Once granted permission, the investigator and/or study staff contacted the potential participant to ask about his/her interest in the study. If there was interest, then pertinent study information was mailed or an in-clinic screening was scheduled. A participant was considered “screened” if any eligibility data was collected; prescreening activities were not collected centrally and are not included in this paper. Recruitment source (mass mail, media, referral, or brochure/poster) was collected directly from potential participants, and more than one recruitment source could be identified.
Use of mass media
For clinics with very challenging recruitment targets, mass media campaigns were used in conjunction with mass mail, including television, radio, and newspaper advertisements. Internet advertising was also done through the study website, with a “Find a SPRINT Clinic Near You” link on a map showing study site locations. Some networks also added advertisements to clinic on-hold messages. Advertisements included an 800 number for potential participants to find out more information about the study.
Recruitment of subgroups
In order to address specific scientific questions of interest, SPRINT recruitment focused on three trial-specific clinical subgroups: a cardiovascular disease cohort of 3,700 participants (40%), a chronic kidney disease cohort of 4,300 participants (46%), and a cohort of 3,250 participants ≥ 75 years old (35%). In addition, to ensure the generalizability of the trial results and in support of the 1994 National Institutes of Health policy on the inclusion of women and minorities in clinical research, SPRINT had demographic subgroup goals to recruit 50% (4,625) women and 40% (3,700) individuals from racial/ethnic minority backgrounds.14 A participant could be in more than one of these five subgroups. In addition, participants ≤ age 75 without cardiovascular disease or chronic kidney disease could be enrolled in the trial if they had a screening Framingham Risk Score of ≥ 15% for a 10-year risk of cardiovascular events. These participants who were enrolled solely based on their Framingham Risk Score were not included in counts of the three trial-specific clinical subgroups.
High-risk participants with cardiovascular disease other than stroke
Participants in the cardiovascular group included those with clinical cardiovascular disease (other than stroke) and subclinical cardiovascular disease. The planned subgroup recruitment was 3,700 participants (40%). The clinical cardiovascular disease definition included a history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, and acute coronary syndrome. Detailed inclusion criteria for clinical and subclinical cardiovascular disease are described in the SPRINT design paper.3 Clinics searched their medical records using these criteria to increase the number of participants with existing cardiovascular disease. The study also endeavored to include cardiology clinics as SPRINT sites, and wherever possible, participants were sought from nearby heart centers and cardiology clinics.
Chronic kidney disease
Participants with a screening eGFR between 20 - 59 mL/min/1.73m2 were sought for the trial. The overall goal was to recruit 4,300 (46%) participants with chronic kidney disease. In an effort to increase chronic kidney disease subgroup numbers, nephrology clinics referred their patients for screening. Additionally, some SPRINT sites were chosen by virtue of the fact that they were already seeing a high number of participants with existing kidney disease.
Participants age 75 and older
Regardless of cardiovascular disease history or risk, any participant who was age 75 or older at the time of screening was eligible to enroll in SPRINT if they did not have an exclusionary condition. The goal of age ≥ 75 was chosen to assess effects of blood pressure control on cognition and other outcomes in older people. Methods used to increase recruitment of this subgroup were the inclusion of sites focused on treating older individuals, recruitment at senior centers, and mailing lists targeting those ≥ 75 years old. In total, the goal was to include 3,250 (35%) participants age 75 or older.
Women
According to both 2000 and 2010 United States Census data, the ratio of women to men was 51% to 49%. Consequently, an important study goal was to enroll 4,625 (50%) female participants. Consistent with efforts seen for other subgroups, there was specific recruitment from women’s centers and clinics to bolster enrollment into this subgroup.
Minority participants
Of the total SPRINT population, it was expected that 40% of trial participants would be of minority backgrounds (African-Americans, Hispanics, Native Americans, and Asians). Recruitment strategies to maximize enrollment of persons from minority groups were used and included targeted mass mail campaigns to minority persons, use of culturally sensitive messages to encourage participation of minority groups, and translation of the recruitment materials into Spanish to maximize enrollment of Hispanic populations. Moreover, many SPRINT recruiting sites were found in dense urban locales where minority representation was higher.
Results
The first randomization occurred on November 8, 2010, and the last on March 15, 2013 (about 28 months). All clinical sites were funded within the same period; however, several clinics were later added to bolster recruitment. One Clinical Center Network was deferred from beginning screening for five months due to unforeseen delays in the Central Institutional Review Board approval process, thereby limiting recruitment in their network to 23 months.
SPRINT screened 14,692 potential participants and randomized 9,361. The R-factor for the SPRINT trial was 77%, indicating that because we enrolled for 28 months we accrued 23% less person follow-up time than planned during the 24-month recruitment period.
Despite the decreased per-person follow-up time, the SPRINT blood pressure intervention was stopped about a year earlier than anticipated, in September 2015, after a median follow-up of 3.26 years, because the lower blood pressure intervention showed a 25% benefit in the primary composite outcome, as well as a 27% reduction in all-cause mortality.15
Table 1 provides a comparison of screened participants by randomization status (non-randomized versus randomized) and key baseline subgroup. Recruitment yield is also shown within each subgroup. In accordance with the intent of the inclusion and exclusion criteria to identify a high-risk study population, randomized participants tended to have more risk factors when compared with non-randomized participants. Randomized participants were older, less likely to be female, had higher average systolic blood pressure and diastolic blood pressure but not heart rate, more clinical or sub-clinical cardiovascular disease, and a higher Framingham Risk Score.
Table 1.
Comparison of Non-Randomized to Randomized Participants
| Randomization Status* | Randomization Yield (%)** | |||
|---|---|---|---|---|
| Category | Screened (N=14692) |
Non-Randomized (N=5331) |
Randomized (N=9361) |
|
| Sex | ||||
| Female | 6060 (41.4%) | 2728 (51.6%) | 3332 (35.6%) | 55.0 |
| Male | 8591 (58.6%) | 2562 (48.4%) | 6029 (64.4%) | 70.2 |
| Race/Ethnicity | . | |||
| African American | 4801 (32.9%) | 1999 (38.2%) | 2802 (29.9%) | 58.4 |
| Hispanic | 1224 (8.4%) | 240 (4.6%) | 984 (10.5%) | 80.4 |
| Other | 331 (2.3%) | 155 (3.0%) | 176 (1.9%) | 53.2 |
| White | 8241 (56.5%) | 2842 (54.3%) | 5399 (57.7%) | 65.5 |
| CKD Status | . | |||
| eGFR ≥ 60 | 8998 (72.9%) | 2283 (76.6%) | 6715 (71.7%) | 74.6 |
| eGFR < 60 (CKD subgroup) | 3344 (27.1%) | 698 (23.4%) | 2646 (28.3%) | 79.1 |
| Age | . | |||
| < 75 Years Old | 10911 (74.4%) | 4186 (78.9%) | 6725 (71.8%) | 61.6 |
| ≥ 75 Years Old | 3756 (25.6%) | 1120 (21.1%) | 2636 (28.2%) | 70.2 |
| CVD History | . | |||
| None | 10692 (81.4%) | 3208 (85.1%) | 7484 (79.9%) | 70.0 |
| Clinical or Subclinical CVD | 2439 (18.6%) | 562 (14.9%) | 1877 (20.1%) | 77.0 |
| Framingham Risk Score - % mean | 16.0 ± 3.6 | 13.4 ± 4.0 | 17.4 ± 2.5 | |
Table 2 is an expansion of Table 1, breaking participants into the top four most used recruitment sources. While mass mail was used the most often, referrals by SPRINT staff, healthcare providers, and friends had the highest yield. It should also be noted that many participants listed more than one recruitment source; therefore, the counts in Tables 1 and 2 add to more than the total number screened (14,692) or randomized (9,361).
Table 2.
a. Recruitment Source: Mass Mailings
| Randomization Status* | Randomization Yield (%) |
|||
|---|---|---|---|---|
| Category | Screened (N=4829) |
Not Randomized (N=1827) |
Randomized (N=3002) |
|
| Sex | ||||
| Female | 1701 (35.3%) | 854 (46.8%) | 847 (28.2%) | 49.8 |
| Male | 3124 (64.7%) | 969 (53.2%) | 2155 (71.8%) | 69 |
| Race/Ethnicity | . | |||
| African American |
1491 (30.9%) | 654 (35.8%) | 837 (27.9%) | 56.1 |
| Hispanic | 169 (3.5%) |
61 (3.3%) | 108 (3.6%) | 63.9 |
| Other | 120 (2.5%) |
60 (3.3%) | 60 (2.0%) | 50 |
| White | 3048 (63.1%) | 1051 (57.6%) | 1997 (66.5%) | 65.5 |
| CKD Status | . | |||
| eGFR ≥ 60 | 2889 (73.7%) | 710 (77.3%) | 2179 (72.6%) | 75.4 |
| eGFR < 60 (CKD subgroup) |
1031 (26.3%) | 208 (22.7%) | 823 (27.4%) | 79.8 |
| Age | . | |||
| < 75 Years Old | 3098 (64.2%) | 1290 (70.8%) | 1808 (60.2%) | 58.4 |
| ≥ 75 Years Old | 1726 (35.8%) | 532 (29.2%) | 1194 (39.8%) | 69.2 |
| CVD History | . | |||
| None | 3465 (81.5%) | 1068 (85.3%) | 2397 (79.8%) | 69.2 |
| Clinical or Subclinical CVD |
789 (18.5%) | 184 (14.7%) | 605 (20.2%) | 76.7 |
| Framingham Risk Score - % mean |
16.2 ± 3.7 | 13.4 ± 4.0 | 17.8 ± 2.4 | |
| b. Recruitment Source: Media: television, radio, newspaper, internet, toll-free number | ||||
|---|---|---|---|---|
| Randomization Status* | Randomization Yield (%) |
|||
| Category | Screened (N=1244) |
Not Randomized (N=544) |
Randomized (N=700) |
|
| Sex | ||||
| Female | 635 (51.1%) | 319 (58.9%) | 316 (45.1%) | 49.8 |
| Male | 607 (48.9%) | 223 (41.1%) | 384 (54.9%) | 63.3 |
| Race/Ethnicity | . | |||
| African American | 460 (37.0%) | 253 (46.5%) | 207 (29.6%) | 45 |
| Hispanic | 146 (11.7%) | 23 (4.2%) | 123 (17.6%) | 84.2 |
| Other | 25 (2.0%) | 11 (2.0%) | 14 (2.0%) | 56 |
| White | 613 (49.3%) | 257 (47.2%) | 356 (50.9%) | 58.1 |
| CKD Status | . | |||
| eGFR ≥ 60 | 829 (84.8%) | 252 (90.6%) | 577 (82.4%) | 69.6 |
| eGFR < 60 (CKD subgroup) |
149 (15.2%) | 26 (9.4%) | 123 (17.6%) | 82.6 |
| Age | . | |||
| < 75 Years Old | 1041 (83.7%) | 457 (84.0%) | 584 (83.4%) | 56.1 |
| ≥ 75 Years Old | 203 (16.3%) | 87 (16.0%) | 116 (16.6%) | 57.1 |
| CVD History | . | |||
| None | 962 (91.1%) | 337 (94.7%) | 625 (89.3%) | 65 |
| Clinical or Subclinical CVD |
94 (8.9%) | 19 (5.3%) | 75 (10.7%) | 79.8 |
| Framingham Risk Score - % mean |
15.3 ± 3.5 | 12.9 ± 3.7 | 16.9 ± 2.3 | |
| c. Recruitment Source: Brochure or Poster | ||||
|---|---|---|---|---|
| Randomization Status* | Randomization Yield (%) |
|||
| Category | Screened (N=2195) |
Not Randomized (N=879) |
Randomized (N=1316) |
|
| Sex | ||||
| Female | 923 (42.1%) | 452 (51.5%) | 471 (35.8%) | 51 |
| Male | 1271 (57.9%) | 426 (48.5%) | 845 (64.2%) | 66.5 |
| Race/Ethnicity | . | |||
| African American |
728 (33.2%) | 325 (37.1%) | 403 (30.6%) | 55.4 |
| Hispanic | 131 (6.0%) | 26 (3.0%) | 105 (8.0%) | 80.2 |
| Other | 63 (2.9%) | 31 (3.5%) | 32 (2.4%) | 50.8 |
| White | 1271 (58.0%) | 495 (56.4%) | 776 (59.0%) | 61.1 |
| CKD Status | . | |||
| eGFR ≥ 60 | 1415 (79.5%) | 374 (80.8%) | 1041 (79.1%) | 73.6 |
| eGFR < 60 (CKD subgroup) |
364 (20.5%) | 89 (19.2%) | 275 (20.9%) | 75.5 |
| Age | . | |||
| < 75 Years Old | 1584 (72.3%) | 663 (75.7%) | 921 (70.0%) | 58.1 |
| ≥ 75 Years Old | 608 (27.7%) | 213 (24.3%) | 395 (30.0%) | 65 |
| CVD History | . | |||
| None | 1672 (85.7%) | 562 (88.6%) | 1110 (84.3%) | 66.4 |
| Clinical or Subclinical CVD |
278 (14.3%) | 72 (11.4%) | 206 (15.7%) | 74.1 |
| Framingham Risk Score - % mean |
15.7 ± 3.7 | 13.1 ± 3.9 | 17.3 ± 2.4 | |
| d. Recruitment Source: Referral from SPRINT Staff, Healthcare Provider, Friends | ||||
|---|---|---|---|---|
| Randomization Status* | Randomization Yield (%) |
|||
| Category | Screened (N=6555) |
Not Randomized (N=2069) |
Randomized (N=4486) |
|
| Sex | ||||
| Female | 2820 (43.0%) | 1076 (52.0%) | 1744 (38.9%) |
61.8 |
| Male | 3734 (57.0%) | 992 (48.0%) | 2742 (61.1%) |
73.4 |
| Race/Ethnicity | . | |||
| African American | 2302 (35.3%) | 851 (41.7%) | 1451 (32.3%) |
63 |
| Hispanic | 807 (12.4%) | 131 (6.4%) | 676 (15.1%) | 83.8 |
| Other | 122 (1.9%) | 58 (2.8%) | 64 (1.4%) | 52.5 |
| White | 3298 (50.5%) | 1003 (49.1%) | 2295 (51.2%) |
69.6 |
| CKD Status | . | |||
| eGFR ≥ 60 | 3968 (68.2%) | 952 (71.4%) | 3016 (67.2%) |
76 |
| eGFR < 60 (CKD subgroup) |
1851 (31.8%) | 381 (28.6%) | 1470 (32.8%) |
79.4 |
| Age | . | |||
| < 75 Years Old | 5145 (78.6%) | 1695 (82.2%) | 3450 (76.9%) |
67.1 |
| ≥ 75 Years Old | 1402 (21.4%) | 366 (17.8%) | 1036 (23.1%) |
73.9 |
| CVD History | . | |||
| None | 4699 (78.0%) | 1248 (81.0%) | 3451 (76.9%) |
73.4 |
| Clinical or Subclinical CVD |
1327 (22.0%) | 292 (19.0%) | 1035 (23.1%) |
78 |
| Framingham Risk Score - % mean |
16.2 ± 3.5 | 13.7 ± 4.1 | 17.3 ± 2.6 | |
p-value for difference in randomization status between groups ≤ 0.001 for all categories except CKD (p-value=0.0004)
p-value for difference in randomization status between groups ≤ 0.001 for all categories except age (p=0.78)
p-value for difference in randomization status between groups ≤ 0.001 for all categories except age (p=0.004) and CVD (p=0.011)
p-value for difference in randomization status between groups ≤ 0.001 for all categories except CKD (p=0.004)
Targeted recruitment results
With the exception of racial/ethnic minorities, the trial fell short in reaching subgroup targets. The cardiovascular disease subgroup accrual rate was half of the trial goal (20%), with only one Clinical Center Network exceeding recruiting in this subgroup (28%), which may be attributed to the proportionally higher prevalence of cardiovascular disease in men versus women. Among participants qualifying for this subgroup, 47% had coronary revascularization, 35% had prior myocardial infarction, 23% had acute coronary syndrome with or without electrocardiogram changes (or electrocardiogram changes on graded test, or positive cardiac imaging), 16% had carotid endarterectomy or carotid stenting, 7% had peripheral artery disease with revascularization, and 41% had other clinical or subclinical cardiovascular disease within the past two years.
Although the recruitment goal for chronic kidney disease participants was not met, two Clinical Center Networks had relatively greater success. The key reason for the higher rates of chronic kidney disease participants in one Clinical Center Network is that this network was comprised mainly of nephrologists. The other Clinical Center Networks also included a high number of nephrologists and the ability to target specific chronic kidney disease groups by using their large electronic medical records nationwide database.
The age distribution of randomized participants is as follows: 609 (6.5%) age < 55; 3,221 (34.4%) age 55–64; 2,895 (30.9%) age 65–74; 2,250 (24.0%) age 75–84; and 386 (4.1%) age 85 and older. Although not achieving the overall study goal of 35% of participants ≥ 75 years old, two Clinical Center Networks (both 39%), exceeded the study goal due to extensive recruitment efforts in ≥ age 75 populations. Recruitment of this number of older participants proved to be challenging, and actual enrollment was only 28% (N=2,636) despite targeted efforts to increase the participation of this subgroup.
None of the Clinical Center Networks achieved their goal (50%) for women, in part reflecting the lower risk status of women in comparison to men. Although 6,061 (41%) of total recruitment screened were women, only 3,332 (36%) were randomized. However, four of the five Clinical Center Networks recruited over 40% women. It was expected that one Clinical Center Network would not be able to contribute significantly to the recruitment of women (2%) due to their predominantly male population.
Minority recruitment exceeded the study goal through heavy recruitment in the African-American communities in two of the Clinical Center Networks (46% and 47% respectively), as well as high numbers of participants in the Hispanic communities (52%) through the eight sites affiliated with the Clinical Center Networks with responsibilities in Puerto Rico and New York City.
Successful strategies to overcome recruitment challenges
Intervention 1: Additional sites
In the five Clinical Center Networks, there were projected to be 90 sites at the onset of the study. Three months into the recruiting process accrual numbers were less than anticipated, so the decision was made to expand the number of SPRINT sites; thus, 12 sites were subsequently added as a strategy to bolster overall recruitment as well as enrollment into the specified subgroups. Since the trial opened for enrollment in November of 2010, 6 clinical sites have been closed, with the participant population transferred to another clinical site when possible, leaving 96 total clinics in the SPRINT trial.
Intervention 2: Protocol changes to age-based recruitment and lower estimated glomerular filtration rate limit
The initial design of SPRINT included recruitment of participants age 55 and older, and individuals with chronic kidney disease with an estimated glomerular filtration rate of 25 - 59 mL/min/1.73m2 While recruiting participants greater than 55 years was feasible, SPRINT investigators recognized that a significant proportion of high-risk patients (cardiovascular disease and chronic kidney disease) could be recruited if the eligibility age was reduced to 50 years old or older. In addition, investigators judged that lowering the estimated glomerular filtration rate from 25 to 20 mL/min/1.73m2 would expand the number of potential participants with chronic kidney disease eligible for the trial, thus enhancing recruitment and generalizability. Therefore, in May of 2011, the SPRINT investigators asked the trial’s independent data and safety monitoring board for a recommendation in favor of modifying the trial’s inclusion criteria. This recommendation was approved, and the SPRINT protocol was subsequently changed to allow this wider inclusion criteria. As indicated above, lowering the age threshold resulted in a substantial number of additional participants (n=609) recruited. Lowering the estimated glomerular filtration threshold resulted in 57 additional chronic kidney disease participants.
Intervention 3: Close of non-clinical-subgroup recruitment
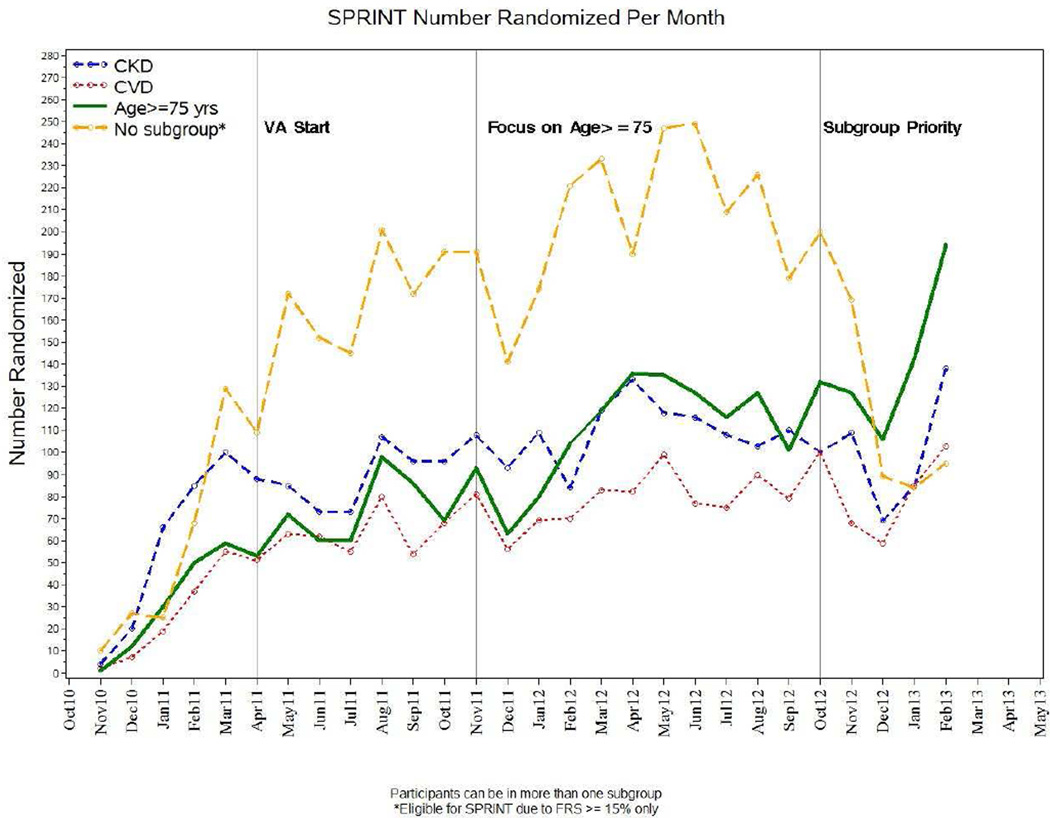
One strategy for recruiting participants with cardiovascular disease and chronic kidney disease was to target recruitment of participants who were ≥ 75 years old. While somewhat effective, clinical subgroup recruitment still lagged. Figures 2 and 3 portray the recruitment experience overall and by subgroup over the duration of the enrollment period. While variable, overall clinical subgroup recruitment did increase with time. However, a key decision was whether to focus all efforts on recruiting participants in the three trial-specific clinical subgroups (cardiovascular disease, chronic kidney disease, ≥ 75 year olds). Study leadership was concerned about the overall recruitment rate and thus was apprehensive about whether closing off all but clinical subgroup enrollment would further reduce the accrual rate. While this strategy of subgroup-only recruitment caused some debate because of the possibility that it would greatly slow total study enrollment and increase the time to reach the overall recruitment goal, it proved to be an effective strategy for increasing subgroup enrollment while at the same time not negatively impacting overall accrual numbers substantially. About 6 months prior to the end of the trial’s recruitment period, the National Institutes of Health Project Office made the decision to limit enrollment into SPRINT to the three major clinical subgroups of cardiovascular disease, chronic kidney disease, or ≥ 75-year-old participants.
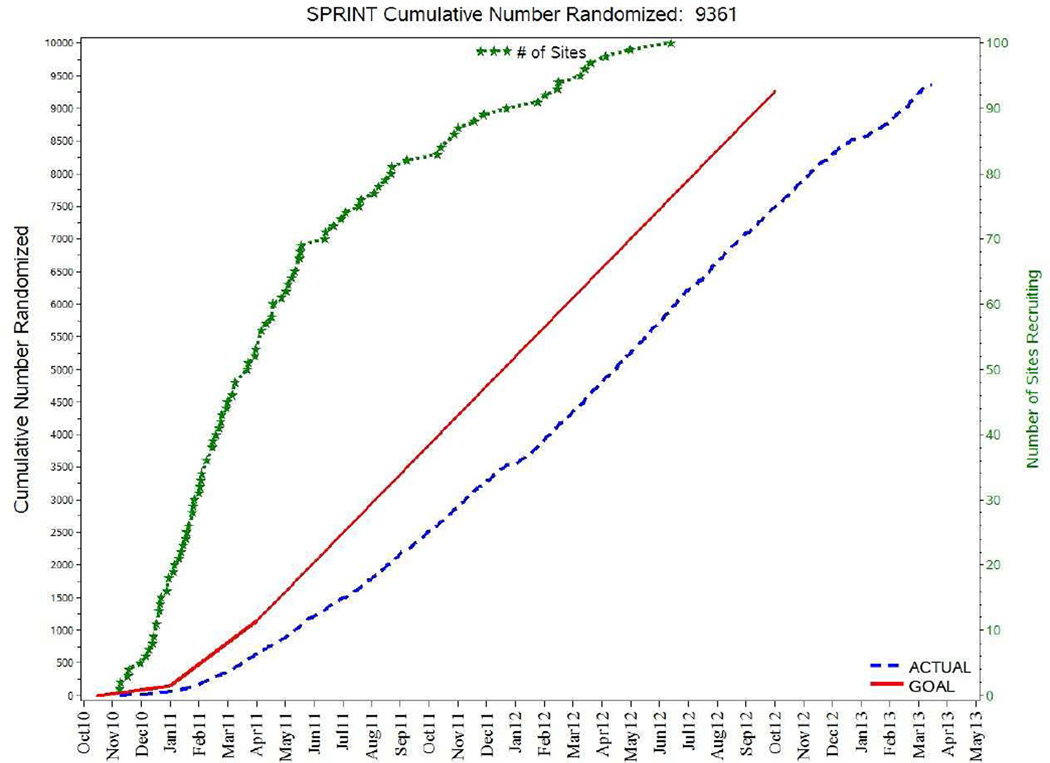
Figure 2.
Cumulative Recruitment Compared to Site Start Dates
* Cumulative IRB approvals are shown on the green line indexed to the right (green) axis
Figure 3.
SPRINT-Specific Subgroup Recruitment by Month with Intervention Changes
A proviso to this decision was that clinics could request exemption from subgroup-only recruitment if doing so would affect clinic viability. Approximately 20% of clinical sites asked for exemption to continue to enroll non-clinical subgroup participants, and these decisions were made on a “case-by-case” basis by each Clinical Center Network in collaboration with the National Institutes of Health Project Office and the SPRINT Coordinating Center. Often the decision to continue non-subgroup enrollment was allowed due to concern that the site’s ability to continue enrolling and thus its overall viability would be adversely affected (i.e., a site needed to enroll a minimum number of participants to support the staff required to carry out the remainder of the trial).
Discussion
Large multicenter randomized trials such as SPRINT offer multiple unique challenges faced in recruitment at each level of the organization from the National Institutes of Health Project Office to the clinical sites. First, as in the Systolic Hypertension in the Elderly Program and the Action to Control Cardiovascular Risk in Diabetes trials, our findings indicate that mass mailing is one of the most important strategies in recruitment for a large interventional trial.3,16 In addition, the Systolic Hypertension in the Elderly Program team noted that the involvement of experienced recruitment staff is important, and that although the total time spent by staff on recruitment may also improve results, it matters less than the recruiting staff’s level of experience.3,6,16 SPRINT was fortunate because many of the staff had experience in previous large clinical trials, including hypertension trials such as Systolic Hypertension in the Elderly Program , Action to Control Cardiovascular Risk in Diabetes , and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial.7,8
Second, because of a priori specified high-risk clinical subgroups, the SPRINT trial required flexibility in recruitment methods that fit the needs and schedule of the investigative staff in order to enrich the participant population with the pre-specified clinical subgroups. This was achieved in multiple ways, including targeted direct mail campaigns and electronic medical records searches, as well as the use of clinics specializing in nephrology, hypertension, and geriatrics.
Although SPRINT had experienced clinicians and staff at all organizational levels in the trial and a plan for targeting the patient populations relevant to the trial, the SPRINT leadership found that additional measures were required to meet the overall recruitment goal as well as to increase the subgroup enrollment numbers. The National Institutes of Health Project Office encouraged the addition of sites just three months after recruitment had begun, thus increasing the number of SPRINT sites relatively quickly as a way to prevent further enrollment shortfalls. Those selected sites were noted to have a participant population that was highly reflective of the subgroups being sought in SPRINT. This targeted approach for the addition of clinical sites was found to be a useful strategy. While adding clinics increases costs, this additional expense must be considered in relation to the potential cost of extending the recruitment period and trial duration.
The changes in eligibility criteria increased the feasibility of recruitment. When considering a change in inclusion criteria, it is important to balance recruitment efficiency alongside consideration of event rates and intervention effects.
The strategy of closing off recruitment to focus on SPRINT subgroup populations was judged risky in terms of overall recruitment yield; however, it ended up being an effective strategy for increasing subgroup enrollment and did not result in substantially lower accrual numbers during the last six months of recruitment.
Regarding barriers to subgroup recruitment for cardiovascular disease, the desire of usual care physicians to maintain control of the use of blood pressure lowering medications may have served as a barrier to recruiting participants with cardiovascular. Three issues that may have reduced recruitment of chronic kidney disease participants were the exclusion of participants with diabetes, severity of illness (i.e., too low estimated glomerular filtration rate in some chronic kidney disease participants), and the desire of usual care physicians to maintain control of the use of blood pressure lowering medications. Potential barriers for recruitment of those ≥ 75 years old included protocol-specified exclusion criteria that restricted comorbid conditions such as diabetes and previous stroke. Finally, we believe that the lower rate of enrollment of women in SPRINT was partly due to the limited number of women in one of the Clinical Center Networks and because the women screened proved not to have the higher risk required for inclusion in the study.
SPRINT took four months longer than expected to finish randomization, but enrolled 111 more participants than planned. The R factor was 77%, indicating that we lost some follow-up time during the recruitment period. However, this likely had little effect on study power in a study of this duration (4–6 years of follow-up time).
The primary limitation of this paper is that we did not collect data on pre-screening activity. This decreased our ability to provide true recruitment yield starting with all potential participants reached. We did not collect any data on the cost of our recruitment sources and can make no comment on recruitment cost-effectiveness. Recruitment source was collected directly from participants. No additional confirmation was done. We recommend that future studies track recruitment source, possibly by attaching a code to each advertisement or mailing that the participant would use when contacting the clinical site.
Recruitment in SPRINT was challenging in and of itself because the overall goal of 9,250 is a large number of participants to recruit. However, it was even more challenging to balance the competing demands of the three trial-specific clinical subgroups of cardiovascular disease, chronic kidney disease, and ≥ 75-year-old participants, as well as goals for recruiting women and minorities. Thus, one of the key lessons learned in SPRINT is that it is difficult to simultaneously meet multiple subgroup goals. Careful monitoring of recruitment performance, combined with early decision-making on altered recruitment strategies such as adding clinics or modifying inclusion criteria, are critical to success. Closing recruitment to all but important subgroups of participants could possibly be considered relatively early in the recruitment phase of the trial, but should be weighed against the overall accrual status of the trial.
As previous large blood pressure trials have found, we conclude that as many recruitment sources as possible are necessary for successful enrollment. Future trials may benefit from our finding that mass mailing brought in the largest number of participants but that the largest randomization yield was from referrals.
Acknowledgments
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It is also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: ClinicalTrials.gov Identifier: NCT01206062.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 &UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433.
Glossary
Definition of Terms
- SPRINT
Systolic Blood Pressure Intervention Trial
References
- 1.Treweek S, Mitchell E, Pitkethly M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;4 doi: 10.1002/14651858.MR000013.pub5. [DOI] [PubMed] [Google Scholar]
- 2.Lovato LC, Hill K, Hertert S, et al. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–352. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing staff experience in a total recruitment program for a clinical trial: the SHEP experience. Systolic Hypertension in the Elderly Program Cooperative Research Group. Control Clin Trials. 1999;20:133–148. doi: 10.1016/s0197-2456(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SPRINT Protocol. SPRINT Protocol and MOP. 2015 [Google Scholar]
- 6.Petrovitch H, Byington R, Bailey G, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 2: Screening and recruitment. Hypertension. 1991;17:II16–II23. doi: 10.1161/01.hyp.17.3_suppl.ii16. [DOI] [PubMed] [Google Scholar]
- 7.Wright JT, Cushman WC, Davis BR, Jr, et al. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT): clinical center recruitment experience. Control Clin Trials. 2001;22:659–673. doi: 10.1016/s0197-2456(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 8.Pressel S, Davis BR, Louis GT, et al. Participant recruitment in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Control Clin Trials. 2001;22:674–686. doi: 10.1016/s0197-2456(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 9.Kingry C, Bastien A, Booth G, et al. Recruitment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:68i–79i. doi: 10.1016/j.amjcard.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Probstfield JL, Wittes JT, Hunninghake DB. Recruitment in NHLBI population-based studies and randomized clinical trials: data analysis and survey results. Control Clin Trials. 1987;8:141s–149s. doi: 10.1016/0197-2456(87)90017-1. [DOI] [PubMed] [Google Scholar]
- 11.Carew BD, Ahn SA, Boichot HD, et al. Recruitment strategies in the studies of left ventricular dysfunction (SOLVD): strategies for screening enrollment in two concurrent but separate trials The SOLVD Investigators. Control Clin Trials. 1992;13:325–338. doi: 10.1016/0197-2456(92)90035-x. [DOI] [PubMed] [Google Scholar]
- 12.SPRINT. [Accessed 29 December 2015];Sprint Trial Website. 2015 www.SPRINTTRIAL.org.
- 13.Veteran’s Health Administration Health Information Management and Health Records. Veteran’s Health Administation Health and Records Management. 2014 [Google Scholar]
- 14.National Institutes of Health. National Institutes of Health. [Retrieved March 25, 2015];NIH guidelines for the inclusion of women and minorities as subjects in clinical research. 1994 [Google Scholar]
- 15.Wright JT, Williamson JD, Whelton PK, Jr, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: Design and methods. Am J Cardiol. 2007;99:21I–33I. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]