Abstract
Recent evidence suggests that the renin-angiotensin system (RAS) plays a vital role in adipocyte biology and the pathophysiology of metabolic syndrome. Obesity is the main culprit of metabolic syndrome and mesenchymal stem cells (MSCs) have been forwarded as a major source of adipocyte generation. Previously we reported that MSCs have a local RAS and that pharmacological blockade of angiotensin II type 2 receptor (AT2R) promotes adipogenesis in human MSCs. However, the definitive roles of AT2R and how AT2R functions in adipogenesis remains unknown. To this end, we employed AT2R-null murine MSCs to characterize how AT2R affects the differentiation of MSCs to adipocytes. Murine MSCs were isolated from AT2R-null mice and wild-type littermates, grown to confluency, and then differentiated into adipocytes. Adipogenesis was quantitated by assessing lipid droplet accumulation. Using the lipophilic fluorescent dye, the AT2R-null cells showed significantly increased total fluorescence (261.6 ± 49.6 % vs. littermate) on day 7. Oil red O staining followed by extraction of the absorbed dye and measurement of the absorbance on day 14 also exhibited significantly increased lipid droplet accumulation in the AT2R-null cells (202.7 ± 14.1 % vs. littermate). We also examined the expression of adipogenic marker genes by quantitative RT-PCR. The AT2R-null group exhibited significantly increased expression of PPAR-gamma, fatty acid synthase, and adiponectin (vs. littermate). We further examined the role of Wnt10b/beta-catenin signaling, which reportedly plays an important inhibitory role in adipogenesis. The AT2R-null group exhibited significantly decreased Wnt10b expression accompanied by decreased beta-catenin (vs. littermate). Our results thus revealed that the AT2R inhibits adipogenic differentiation in murine MSCs. Moreover, this inhibitory effect is associated with Wnt10b/beta-catenin signaling. These results provide important insights into the pathophysiology of obesity and obesity-related consequences such as metabolic syndrome, hinting at possible future therapies.
Keywords: adipocyte, differentiation, mesenchymal stem cells, metabolic syndrome, obesity, renin-angiotensin system
Metabolic syndrome is associated with an increased risk of cardiovascular disease1-3. Obesity is a major risk factor of metabolic syndrome and is associated with a combined increase in fat cell size and fat cell number4-6. Irrespective of age, new fat cells arise from a pre-existing pool of adipose stem cells7-9, which are similar to bone marrow derived mesenchymal stem cells (MSCs) 9-11. In addition, Liechty et al.12 demonstrated that human MSCs transplanted into fetal sheep marrow successfully differentiated and incorporated into normal adult adipose tissue. Developmentally, MSCs equate to the step between undifferentiated mutipotent embryonic stem cells and adipose tissue-derived preadipocytes, and MSCs are a major cell source for adipogenesis13-15. MSCs are suggested to play an important role in maintaining the mass and function of adult adipose tissue. Accordingly, in vitro differentiation of MSCs towards the adipogenic lineage provides a useful means for studying the earliest regulation of adipose cell development.
The renin-angiotensin system (RAS) is one of the most important regulators of cardiovascular homeostasis16-18. Clinical and experimental evidence suggest that RAS also plays a role in metabolic syndrome, a cluster of conditions increasing the risk of cardiovascular disease19-21. Importantly, it was reported that the adipocyte contains a complete RAS and that angiotensin influences preadipocyte maturation and differentiation, although the studies presenting such evidence are limited and conflicting22-26. Regarding the relationship between RAS and MSC-adipogenesis, we previously reported that human MSCs express all the RAS components and produce endogenous (local) angiotensin II, and that pharmacological blockade of angiotensin II (Ang II) type 2 receptor (AT2R) by PD123319 promotes adipogenesis in human MSC 27. Many pro-adipogenic stimuli or molecules have been reported to inhibit the osteogenesis of MSCs 28-30. Recently, we further reported that pharmacological blockade of AT2R inhibits osteogenesis in human MSC31. Together, these results suggest that AT2R has anti-adipogenic and pro-osteogenic effects on MSCs. However, the pharmacological blockade of AT2R in turn stimulates Ang II type 1 receptor. Thus, definitive examination of the AT2R roles in adipogenesis requires studies conducted on AT2R-null MSCs. Accordingly, we used AT2R-null murine MSCs to characterize how AT2R affects the differentiation of murine MSCs to adipocytes in this study.
Wnt/beta-catenin signaling is a major regulator of MSC fate 32, 33. In particular, Wnt10b was reported to be the most important endogenous regulator of adipogenesis 34, and transgenic mice overexpressing Wnt10b from the adipocyte-specific FABP4 promotor show reduced adiposity35. It was also reported that expression of Wnt10b in adipose tissue reduced adiposity in the ob/ob obesity murine model36. Thus Wnt10b/beta-catenin signaling is considered to act as a brake for adipogenic differentiation.
In the present study, we test our hypothesis that AT2R plays an important role in adipogenic differentiation of MSCs using a genetic model. Our data demonstrate that the deletion of AT2R accelerates adipogenesis in murine MSCs. Furthermore, this inhibitory effect of AT2R on adipogenesis is associated with Wnt10b/beta-catenin signaling.
Materials and Methods
Murine mesenchymal stem cell isolation and culture
All animal procedures were approved by the Duke University Institutional Animal Care and Use Committees. Eight-week-old male AT2R-null (AT2RKO) mice and wild-type male littermates were used in the present study. Because the AT2R gene is located on the X chromosome, heterozygous females were mated with wild-type males to obtain hemizygous males. Animals were genotyped as previously described37-39, and the murine MSCs were isolated by their adherence to plastic as previously described40-47. Bone marrow was collected from 8-week-old male AT2RKO mice and wild-type male littermates by flushing femurs and tibias with the murine MSC growth medium, which comprised Minimum Essential Medium alpha (MEM alpha) with GlutaMAX (Invitrogen, Carlsbad, CA), 20 % fetal bovine serum (FBS), and penicillin/streptomycin (100 U/ml and 100 μg/ml, respectively). The bone marrow cells were then filtered through a 40-μm nylon mesh filter. Mononuclear cells were separated by gradient-density centrifugation using Ficoll-Paque Plus (Amersham Bioscience, Uppsala, Sweden). Cells were then washed twice with phosphate-buffered saline and plated in plastic dishes. After 3 days, non-adherent cells were removed by two washes with phosphate-buffered saline and adherent cells were further cultured in the murine MSC growth medium. Cells were then propagated in culture. Medium was changed every 3 days.
Confluent cells were incubated in adipogenic medium (Alpha-MEM-GlutaMax medium supplemented with 10 μg/ml insulin, 0.1 μmol/l dexamethasone, 50 μmol/l 3-isobutyl-1-methyl-xanthine, 20 μmol/l indomethacin, 20 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) for 7 to 14 days. Medium was changed every 3 days.
Quantification of lipid droplet accumulation by AdipoRed assay
To quantitate adipocyte differentiation on day 7, AdipoRed Assay Reagent (Cambrex, Walkersville, MD) was employed following the manufacturer's protocol. AdipoRed is a fluorescent dye that binds to lipid droplets. Total fluorescent signal was quantitated using a fluorimeter with an excitation wavelength of 485 nm and emission wavelength of 590 nm.
Oil Red O staining
For Oil Red O staining and quantification of adipogenesis on day 14, the Adipogenesis Assay Kit (Chemicon International, Inc., Temecula, CA) was used following the manufacturer's protocol. Day-14 cells were washed with phosphate-buffered saline twice and then stained with 0.36% Oil Red O solution (Adipogenesis Assay Kit) for 15 minutes at room temperature. The staining solution was then removed and cells were washed three times with wash solution (Adipogenesis Assay Kit). The Dye Extraction Solution (Adipogenesis Assay Kit) was then added, and the plates were set on an orbital shaker for 15 minutes. The absorbance of the extracted dye was measured at 520 nm.
Quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated using Tri Reagent (Sigma, St Louis, MO), further purified on RNeasy columns (Qiagen, Valencia, CA), and quantified by concentration using spectrophotometry. First-strand cDNA was synthesized from the total RNA using a High Capacity cDNA Archive Kit (Applied Biosystem, Foster City, CA) following the manufacturer's protocol. The taqman probe primer system (Applied Biosystem) was used for quantitative RT-PCR. The primer and probe sets for murine peroxisome proliferator-activated receptor-gamma (PPAR-gamma), fatty acid synthase (FAS), adiponectin, Wnt10b, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. TaqMan PCR was performed using the ABI Prism 7700 Sequence Detection System, according to the manufacturer's instructions (Applied Biosystems). Target-gene mRNA expression was normalized to GAPDH mRNA expression and the relative amounts of all mRNAs were calculated using the comparative CT method 48.
Immunoblotting
Cells on day 14 were lysed at 4 °C with RIPA Lysis Buffer (Upstate, Lake Placid, NY). Equal amounts of proteins (20 μg per lane) were separated by NuPAGE 4–12 % Bis-Tris Gel electrophoresis (Invitrogen). Protein fractions were then electrophoretically transferred onto polyvinylidene difluoride membrane. The membrane was blocked with Blocker/Diluent solution (Western Blot Kit, Invitrogen), and then incubated with rabbit polyclonal antibody to human beta-catenin (Cell Signaling Technology, Inc., Danvers, MA) for 1 hour at room temperature. After washing in wash buffer (Western Blot Kit, Invitrogen), the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Western Blot Kit, Invitrogen) for 1 hour at room temperature. The antigen antibody-peroxidase complex was visualized using the ECL chemiluminescence solution (Western Blot Kit, Invitrogen). The blot was subsequently stripped using the Re-Blot Plus Western Blot Recycling Kit (Chemicon International, Inc.) and rehybridized with an anti-GAPDH antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) as a control for protein loading. Then, the blot was restripped and rehybridized with rabbit polyclonal antibody to PPAR-gamma (Abcam, Cambridge, MA). Densitometric quantitation was performed using ImageJ software (NIH, Bethesda, MD), and the band intensities of beta-catenin and PPAR-gamma were standardized to those of GAPDH.
Statistical analysis
All statistical procedures were performed using the Statgraphics Plus version 5.0 software (StatPoint, Inc., Herndon, VA). Comparisons between groups were made using Student's t test and the differences were considered to be significant at P < 0.05.
Results
Deletion of AT2R accelerates differentiation of murine MSCs to adipocytes
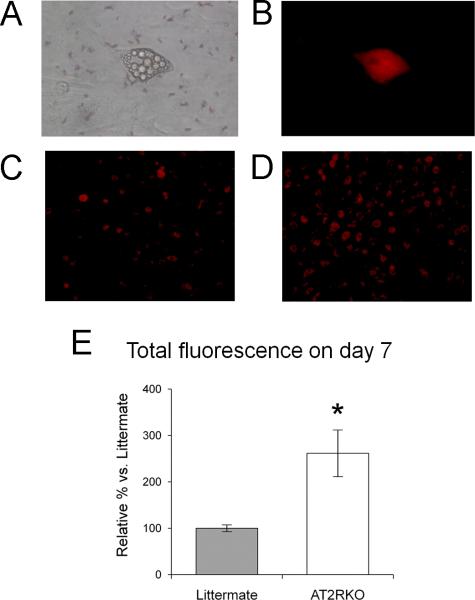
Murine MSCs were isolated from the bone marrow of male AT2RKO mice and wild-type male littermates, and then incubated in adipogenic medium for 7 days. The differentiated adipocytes exhibited lipid droplets in the cytoplasm by phase contrast microscopy (Fig. 1A) and this was verified using the AdipoRed fluorescent dye for lipids (Cambrex, Walkersville, MD) (Fig. 1B). Quantitation of the AdipoRed staining (Fig. 1C–E) revealed significantly greater lipid droplet accumulation in the differentiated adipocytes from the AT2RKO MSCs than in those from the littermate group (261.6 ± 49.6 %; n = 12; P < 0.05), suggesting an anti-adipogenic effect of AT2R on murine MSCs.
Figure 1. Deletion of AT2R accelerates adipocyte differentiation of murine MSCs.
(A) and (B) are representative micrographs of differentiated adipocytes (day 7) from wild-type littermate MSCs (A: phase contrast, B: AdipoRed fluorescence. Original magnification, x 400). (C) and (D) are representative AdipoRed fluorescence micrographs of differentiated adipocytes (day 7) from wild-type littermate MSCs (C) and AT2R-null (AT2RKO) MSCs (D). Original magnification, x 200. (E) Quantification of fluorescent lipid droplet accumulation. Relative % vs. Littermate group. Data are mean ± S.D. (n = 12). *P <0.05 vs. Littermate group.
Deletion of AT2R increases the expression of adipogenic marker genes
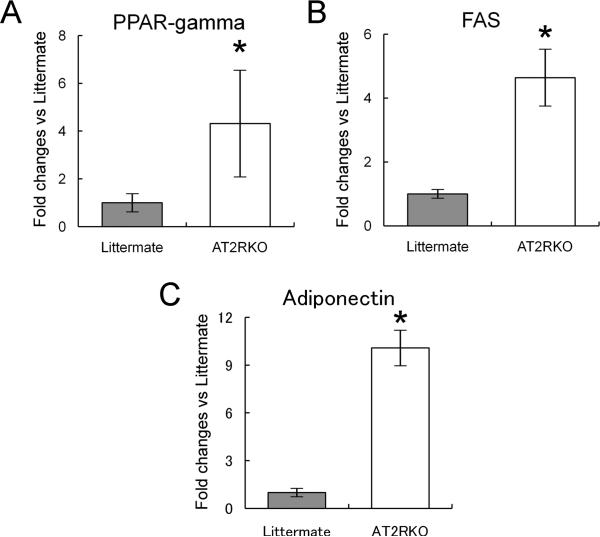
We also examined the expression of adipogenic marker genes by quantitative RT-PCR. Murine MSCs from AT2RKO mice and wild-type littermates were incubated in adipogenic medium for 7 days, then subjected to total RNA extraction and analyzed by real-time RT-PCR for mRNA expression of PPAR-gamma, FAS, and adiponectin. Consistent with the analysis of lipid droplet accumulation, PPAR-gamma, FAS, and adiponectin were upregulated in the AT2RKO-derived cells compared with the littermate controls (4.32 ± 2.21-, 4.64 ± 0.87-, and 10.08 ± 1.08-fold increases, respectively; n = 4; P < 0.05; Fig. 2A-C).
Figure 2. Expression of adipogenic marker genes.
Murine MSCs from wild-type littermate (Littermate) and AT2R-null (AT2RKO) mice were incubated in adipogenic medium for 7 days. Total RNA was analyzed by quantitative RT-PCR for mRNA expression of PPAR-gamma (A), fatty acid synthase (FAS) (B), and adiponectin (C) on day 7. Fold changes vs. Littermate group. Data are mean ± S.D. (n = 4). *P <0.05 vs. Littermate group.
Deletion of AT2R prolongs the acceleration of adipogenesis
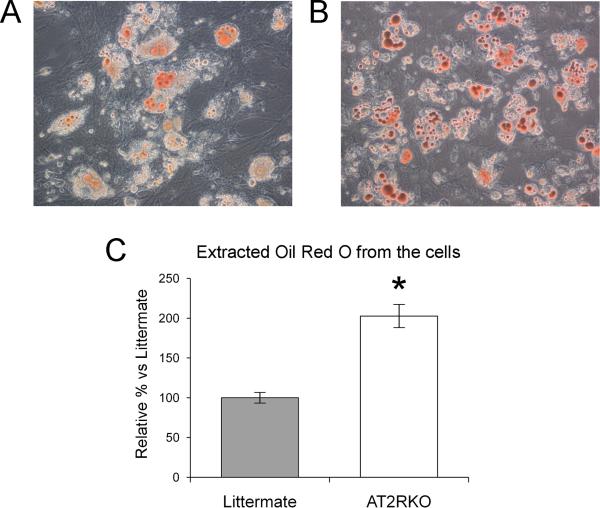
We further examined the effect of deleting of AT2R on adipogenesis over a prolonged period. Murine MSCs from AT2RKO mice and wild-type littermates were incubated in adipogenic medium for 14 days and then stained using Oil Red O to quantify the lipid droplet accumulation. Oil Red O staining, followed by extraction of the absorbed dye and measurement of the absorbance on day 14, exhibited that the AT2RKO group showed significantly increased lipid droplet accumulation (202.7 ± 14.1 % vs. littermate), confirming the anti-adipogenic effect of AT2R on murine MSCs (Fig. 3A-C).
Figure 3. Deletion of AT2R prolongs acceleration of adipogenesis.
(A) and (B) are representative micrographs of Oil red O staining of differentiated adipocytes (day 14) from wild-type littermate MSCs (A) and AT2R-null (AT2RKO) MSCs (B). Original magnification, x 200. (C) Quantification of extracted Oil red O from the cells. Relative % vs. Littermate group. Data are mean ± S.D. (n = 4). *P <0.05 vs. Littermate group.
Deletion of AT2R decreases Wnt10b expression
Since Wnt10b/beta-catenin signaling is reported to inhibit adipogenesis, we examined the expression of Wnt10b by quantitative RT-PCR. Murine MSCs from AT2RKO mice and wild-type littermates were incubated in adipogenic medium for 7 days, and then analyzed for the mRNA expression of Wnt10b. As shown in Figure 4, the AT2RKO group showed a remarkably decreased expression of Wnt10b compared with the littermate group (0.0041 ± 0.0015-fold changes vs. the littermate group, respectively; n = 4; P < 0.05).
Figure 4. Deletion of AT2R remarkably decreases Wnt10b expression.
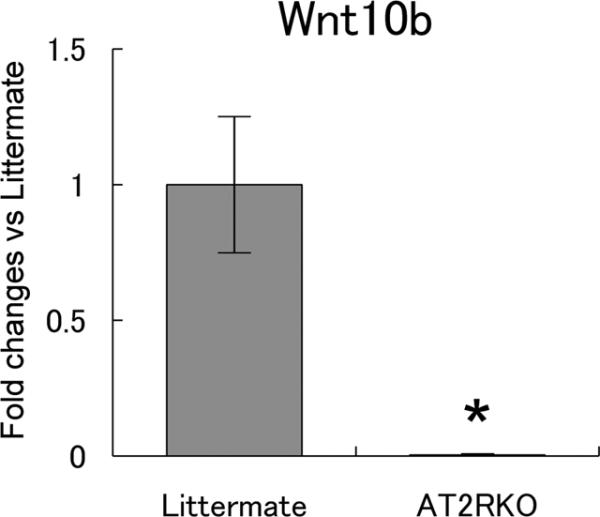
Murine MSCs from wild-type littermate (Littermate) and AT2R-null (AT2RKO) mice were incubated in adipogenic medium for 7 days. Total RNA was analyzed by quantitative RT-PCR for mRNA expression of Wnt10b on day 7. Fold changes vs. Littermate group. Data are mean ± S.D. (n = 4). *P <0.05 vs. Littermate group.
Deletion of AT2R decreases cellular beta-catenin
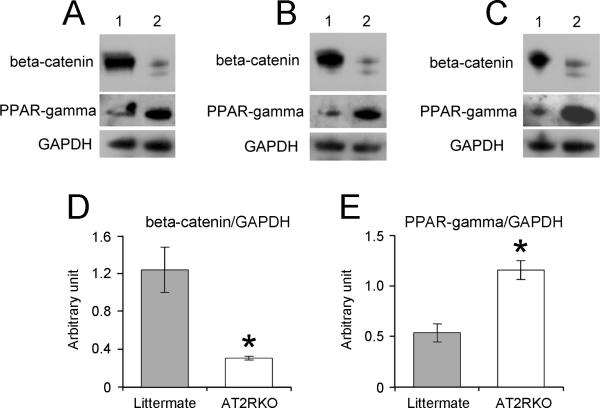
To confirm the suppression of Wnt10b/beta-catenin signaling in the AT2RKO group, we further analyzed cellular beta-catenin protein expression by immunoblotting. Murine MSCs from AT2RKO mice and wild-type littermates were incubated in adipogenic medium for 14 days. Cell lysates were then isolated and subjected to immunoblotting analysis using antibodies for beta-catenin, PPAR-gamma, and GAPDH. Cellular beta-catenin protein expression was decreased in the AT2RKO group compared with the littermate group (Fig. 5A-C), and densitometric quantification from three independent experiments revealed this decrease in beta-catenin expression to be significant (Fig. 5D). Consistent with the results of mRNA expression, PPAR-gamma protein expression was increased in the AT2RKO cells compared with those from littermates (Fig. 5E). These results suggest that the inhibitory effect of AT2R on the adipogenesis of murine MSCs is associated with Wnt10b/beta-catenin signaling.
Figure 5. Deletion of AT2R decreases cellular beta-catenin.
Murine MSCs from wild-type littermate (Littermate) and AT2R-null (AT2RKO) mice were incubated in adipogenic medium for 14 days. Cell lysates were isolated and subjected to immunoblotting analysis using antibodies for beta-catenin, PPAR-gamma, and GAPDH. (A)-(C) The blots from three independent experiments are shown. Lane 1, Littermate; Lane 2, AT2RKO. (D) and (E): Densitometric quantitation for beta-catenin/GAPDH (D) and PPAR-gamma/GAPDH (E) from three independent experiments. Data are mean ± S.E.M.; *P <0.05 vs. Littermate group.
Discussion
This study demonstrates that the deletion of AT2R accelerates adipogenesis of MSCs, suggesting that AT2R has an inhibitory effect on adipogenesis. Furthermore, the inhibitory effect of AT2R on adipogenesis was associated experimentally with Wnt10b/beta-catenin signaling, which plays a vital role in inhibiting adipocyte differentiation. Indeed, our data suggest that the increase in AT2R mRNA levels during adipogenesis27 contributes to the modulation of adipogenesis and obesity.
Obesity predisposes individuals to both type 2 diabetes and metabolic syndrome. Severe obesity is associated with an increase in adipocyte size combined with increased adipocyte number4-6. New adipocytes arise from a pre-existing pool of adipose stem cells regardless of age7-9, and recent studies likened adipose stem cells to bone marrow stromal MSCs9-11. In addition to resident stem cells, Crossno et al.49 reported that adipocyte progenitor cells originating from bone marrow also contribute to development of new adipocytes in adult animals. Taken together, MSCs are suggested to play an important role in the maintenance of the mass and function of adult adipose tissue. Thus, the in vitro differentiation of MSCs towards the adipogenic lineage is the major research target for studying the regulation of adipogenesis and obesity27, 50.
The renin-angiotensin system (RAS) is central to the regulation of cardiovascular homeostasis16-18. Previously, we reported that human MSCs contain a local RAS and produce endogenous angiotensin II27. We also showed that AT2R mRNA is increased during adipogenesis of human MSC27. Using a genetic model in the present study, we further built on our previous findings on the role of AT2R in adipogenesis27 to show that deletion of AT2R accelerates adipogenesis of murine MSCs. Together, these studies strongly indicate that AT2R could regulate adipogenesis in MSCs and, potentially, modulate obesity.
In general, failure to produce new adipocytes is considered to result in the increase in large insulin-resistant adipocytes and the predisposition to developing diabetes and metabolic syndrome 51. Thus, promoting the production of small, insulin-sensitive adipocytes is thought to yield medical benefits. Yvan-Charvet et al.52 reported that AT2RKO mice fed a low-fat diet had an increased number of small adipocyte cells as compared with wild-type mice. Based on our results, we speculate that such small adipocytes might be recruited from MSCs.
Since obesity is considered the main culprit of metabolic syndrome, studies into obesity control are of note. For example, based on the theory that obesity can be prevented through exercise, Rubin et al.53 showed that 15 weeks of brief, daily exposure to high-frequency mechanical signals, induced at a magnitude well below that encountered during walking, inhibited adipogenesis in mice. These authors further demonstrated that irradiated mice receiving bone marrow transplants from heterozygous green fluorescent protein+ mice showed a reduced commitment of MSC differentiation into adipocytes following 6 weeks of these low-magnitude mechanical signals, suggesting that such signals would suppress adiposity by suppressing the differentiation of MSCs into adipocytes, rather than by metabolizing existing adipose tissue53. In the clinical setting, the most effective treatment for severe obesity is bariatric surgery54-57. Importantly, such surgery to treat obesity was also reported to resolve hypertension, diabetes, and dyslipidemia, resulting in an overall reduction in cardiovascular risk54-57. To investigate the mechanisms of this surgical treatment of obesity, Chen et al.58 examined RAS-related gene expressions in human adipose-derived MSCs and differentiated adipocytes from three groups: non-obese control subjects, obese subjects undergoing bariatric surgery, and subjects 1 year or more after bariatric surgery. These authors demonstrated upregulation of the RAS-related gene expressions in MSCs and differentiated adipocytes of obese individuals and that such upregulation resolved in post-bariatric surgery subjects58. These data together suggest that the treatment of obesity could ameliorate metabolic syndrome, possibly via the modulation of MSC adipogenesis. However, simple inhibition of adipogenesis alone is an inappropriate approach to managing obesity and metabolic syndrome, and obesity derived from an imbalance between energy intake and output must also be considered59. Simply inhibiting the requisite adipogenesis would require an alternative site for the excess calories and ectopic fat deposition is associated with insulin resistance, type 2 diabetes, and adverse metabolic phenotypes60-64. An appropriate balance between promoting requisite adipogenesis and inhibiting excess adipogenesis seems important in maintaining a caloric equilibrium. Thus, although the roles of MSC adipogenesis in obesity and metabolic syndrome hold significant promise for new treatment strategies, further studies are needed to support successful clinical applications of controlling MSC adipogenesis.
We also studied the mechanism by which AT2R could inhibit adipogenesis of murine MSCs. Wnt/beta-catenin signaling is an important regulator of MSC fate 32, 33 and also plays a vital role in the adipogenic differentiation of preadipocytes 34, 65. In particular, Wnt10b is the most important endogenous regulator of adipogenesis34, 66. Wnt10b is considered to act as a brake on adipogenic differentiation by activating the canonical Wnt signaling pathway that leads to stabilization of cytosolic beta-catenin34, and myoblasts from Wnt10b-null mice showed accelerated adipogenic potential67. In addition, Longo et al.35 created transgenic mice overexpressing Wnt10b from the adipocyte-specific FABP4 promotor (FABP4-Wnt10b mice) that showed reduced adiposity and were resistant to diet-induced obesity, while Wright et al.36 reported that expression of Wnt10b in adipose tissue reduces adiposity in the ob/ob obesity model and that Wnt10b protected against genetic obesity in mice due to ectopic expression of agouti (Ay). In the present study, deletion of AT2R significantly decreased Wnt10b and beta-catenin expression, confirming that Wnt10b/beta-catenin signaling is suppressed in the AT2R-null cells. These findings suggest that the inhibitory effect of AT2R on MSC adipogenesis is associated with Wnt10b/beta-catenin signaling; however, it should be noted that this may not be causal and may instead reflect Wnt-dependent and/or Wnt-independent changes. In addition, although Wnt10b/beta-catenin signaling has been suggested as one of the most important pathways, there are a number of molecules that regulate adipogenesis. Therefore, further studies are needed into the anti-adipogenic effect of AT2R, particularly in light of the potential therapeutic applications of such findings.
In conclusion, the present study demonstrates that AT2R inhibits adipocyte differentiation of murine MSCs with Wnt10b/beta-catenin signaling, which might be physiologically important in maintaining the mass and function of adult adipose tissue. Our results thus support a key role for AT2R in adipose tissue that could be important in future research of obesity. Further characterization of this AT2R function in adipocyte biology could potentially reveal therapeutic implications for obesity and obesity-related consequences, such as metabolic syndrome.
Supplementary Material
Acknowledgments
The authors thank Hui Mu for her technical assistance. This work was supported, in whole or in part, by grants from the National Heart, Lung, and Blood Institute (RO1 HL35610, HL58516, HL72010, and HL73219 to Victor. J. Dzau), the Edna Mandel Foundation (to Victor. J. Dzau), the Leducq Foundation (to Victor. J. Dzau), the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI 21790745 to Kenichi Matsushita), the Japan Society for the Promotion of Science (KAKENHI 26461086 to Kenichi Matsushita), and the Uehara Memorial Foundation (to Kenichi Matsushita).
Abbreviations
- AT2R
angiotensin II type 2 receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MSCs
mesenchymal stem cells
- PPAR-gamma
peroxisome proliferator-activated receptor-gamma
- RAS
renin-angiotensin system
- RT-PCR
reverse transcription–polymerase chain reaction
Footnotes
Conflict of interest
None declared.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Brook CG, Lloyd JK, Wolf OH. Relation between age of onset of obesity and size and number of adipose cells. Br Med J. 1972;2(5804):25–27. doi: 10.1136/bmj.2.5804.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faust IM, Johnson PR, Stern JS, et al. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235(3):E279–286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5(2):299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 7.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40(4):229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 8.Phillips BW, Vernochet C, Dani C. Differentiation of embryonic stem cells for pharmacological studies on adipose cells. Pharmacol Res. 2003;47(4):263–268. doi: 10.1016/s1043-6618(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 11.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4-6):311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 12.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 13.Bost F, Aouadi M, Caron L, et al. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Hausman DB, DiGirolamo M, Bartness TJ, et al. The biology of white adipocyte proliferation. Obes Rev. 2001;2(4):239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 15.Scavo LM, Karas M, Murray M, et al. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab. 2004;89(7):3543–3553. doi: 10.1210/jc.2003-031682. [DOI] [PubMed] [Google Scholar]
- 16.Dzau V. The cardiovascular continuum and renin-angiotensin-aldosterone system blockade. J Hypertens Suppl. 2005;23(1):S9–17. [PubMed] [Google Scholar]
- 17.Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77(6 Pt 2):I4–13. [PubMed] [Google Scholar]
- 18.Dzau VJ, Hirsch AT. Emerging role of the tissue renin-angiotensin systems in congestive heart failure. Eur Heart J. 1990;11(Suppl B):65–71. doi: 10.1093/eurheartj/11.suppl_b.65. [DOI] [PubMed] [Google Scholar]
- 19.de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100(5):525–534. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad A, Quyyumi AA. Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation. 2004;110(11):1507–1512. doi: 10.1161/01.CIR.0000141736.76561.78. [DOI] [PubMed] [Google Scholar]
- 21.Putnam K, Shoemaker R, Yiannikouris F, et al. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302(6):H1219–1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darimont C, Vassaux G, Ailhaud G, et al. Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Endocrinology. 1994;135(5):2030–2036. doi: 10.1210/endo.135.5.7956925. [DOI] [PubMed] [Google Scholar]
- 23.Janke J, Engeli S, Gorzelniak K, et al. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51(6):1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 24.Palominos MM, Dunner NH, Wabitsch M, et al. Angiotensin II directly impairs adipogenic differentiation of human preadipose cells. Mol Cell Biochem. 2015;408(1-2):115–122. doi: 10.1007/s11010-015-2487-y. [DOI] [PubMed] [Google Scholar]
- 25.Schling P. Expression of angiotensin II receptors type 1 and type 2 in human preadipose cells during differentiation. Horm Metab Res. 2002;34(11-12):709–715. doi: 10.1055/s-2002-38240. [DOI] [PubMed] [Google Scholar]
- 26.Schling P, Loffler G. Effects of angiotensin II on adipose conversion and expression of genes of the renin-angiotensin system in human preadipocytes. Horm Metab Res. 2001;33(4):189–195. doi: 10.1055/s-2001-14951. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita K, Wu Y, Okamoto Y, et al. Local renin angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension. 2006;48(6):1095–1102. doi: 10.1161/01.HYP.0000248211.82232.a7. [DOI] [PubMed] [Google Scholar]
- 28.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4(3):290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Takada I, Kouzmenko AP, Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets. 2009;13(5):593–603. doi: 10.1517/14728220902915310. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Wu Y, Pratt RE, et al. Blockade of angiotensin II type 2 receptor by PD123319 inhibits osteogenic differentiation of human mesenchymal stem cells via inhibition of extracellular signal-regulated kinase signaling. J Am Soc Hypertens. 2015;9(7):517–525. doi: 10.1016/j.jash.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Davis LA, Zur Nieden NI. Mesodermal fate decisions of a stem cell: the Wnt switch. Cell Mol Life Sci. 2008;65(17):2658–2674. doi: 10.1007/s00018-008-8042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem cells and development. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 34.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 35.Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 36.Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56(2):295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 37.Akishita M, Ito M, Lehtonen JY, et al. Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J Clin Invest. 1999;103(1):63–71. doi: 10.1172/JCI5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akishita M, Iwai M, Wu L, et al. Inhibitory effect of angiotensin II type 2 receptor on coronary arterial remodeling after aortic banding in mice. Circulation. 2000;102(14):1684–1689. doi: 10.1161/01.cir.102.14.1684. [DOI] [PubMed] [Google Scholar]
- 39.Hein L, Barsh GS, Pratt RE, et al. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377(6551):744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 40.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 41.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18(8):2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 44.Matsushita K, Morello F, Wu Y, et al. Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha. J Biol Chem. 2010;285(16):11974–11982. doi: 10.1074/jbc.M109.099671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira RF, Halford KW, O'Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92(11):4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Crossno JT, Jr., Majka SM, Grazia T, et al. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116(12):3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mogi M, Iwai M, Horiuchi M. Emerging concept of adipogenesis regulation by the renin-angiotensin system. Hypertension. 2006;48(6):1020–1022. doi: 10.1161/01.HYP.0000248196.14826.31. [DOI] [PubMed] [Google Scholar]
- 51.Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101(6):1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yvan-Charvet L, Even P, Bloch-Faure M, et al. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54(4):991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]
- 53.Rubin CT, Capilla E, Luu YK, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batsis JA, Sarr MG, Collazo-Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102(7):930–937. doi: 10.1016/j.amjcard.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heneghan HM, Meron-Eldar S, Brethauer SA, et al. Effect of bariatric surgery on cardiovascular risk profile. Am J Cardiol. 2011;108(10):1499–1507. doi: 10.1016/j.amjcard.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 56.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 57.Torquati A, Wright K, Melvin W, et al. Effect of gastric bypass operation on Framingham and actual risk of cardiovascular events in class II to III obesity. J Am Coll Surg. 2007;204(5):776–782. doi: 10.1016/j.jamcollsurg.2006.12.038. discussion 782-773. [DOI] [PubMed] [Google Scholar]
- 58.Chen JG, Spagnoli A, Torquati A. Adipogenic differentiation of adipose tissue-derived human mesenchymal stem cells: effect of gastric bypass surgery. Surg Endosc. 2012;26(12):3449–3456. doi: 10.1007/s00464-012-2353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosen ED, Walkey CJ, Puigserver P, et al. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):1293–1307. [PubMed] [Google Scholar]
- 60.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Current diabetes reports. 2010;10(4):306–315. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- 61.Hocking S, Samocha-Bonet D, Milner KL, et al. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463–500. doi: 10.1210/er.2012-1041. [DOI] [PubMed] [Google Scholar]
- 62.Lim S. Ectopic fat assessment focusing on cardiometabolic and renal risk. Endocrinology and metabolism (Seoul, Korea) 2014;29(1):1–4. doi: 10.3803/EnM.2014.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma X, Lee P, Chisholm DJ, et al. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol (Lausanne) 2015;6:1. doi: 10.3389/fendo.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sattar N, Gill JM. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277(34):30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 66.Wend P, Wend K, Krum SA, et al. The role of WNT10B in physiology and disease. Acta physiologica (Oxford, England) 2012;204(1):34–51. doi: 10.1111/j.1748-1716.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- 67.Vertino AM, Taylor-Jones JM, Longo KA, et al. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16(4):2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.