Abstract
Quantitative assessment of epidermal nerve fibers (ENF) has become a widely used clinical tool for the diagnosis of small fiber neuropathies such as diabetic neuropathy and human immunodeficiency virus associated sensory neuropathy (HIV-SN). To model and investigate the pathogenesis of HIV-SN using simian immunodeficiency virus (SIV)-infected Asian macaques, we adapted the skin biopsy and immunostaining techniques currently employed in human patients, and then developed two unbiased image analysis techniques for quantifying ENF in macaque footpad skin. This report provides detailed descriptions of these tools and techniques for ENF assessment in macaques and outlines important experimental considerations that we have identified in the course of our long-term studies. Although initially developed for studies of HIV-SN in the SIV-infected macaque model, these methods could be readily translated to a range of studies involving peripheral nerve degeneration and neurotoxicity in non-human primates, as well as preclinical investigations of agents aimed at neuroprotection and regeneration.
Keywords: peripheral neuropathy, epidermal nerve fibers, image analysis, stereology, PGP 9.5, nervous system, nonhuman primate
Introduction
Transmission of afferent sensory information from the periphery to the central nervous system is accomplished by a variety of sensory nerve types, ranging from the large caliber, thickly-myelinated fibers that convey the senses of innocuous touch and limb position to the small-diameter thinly-myelinated Aδ fibers and unmyelinated C-fibers that transmit the sensations of temperature and pain (McGlone and Reilly, 2010). Small fiber neuropathies (SFN) are defined as conditions that selectively or predominantly affect the Aδ or C-fibers (Hoeijmakers et al., 2012). The clinical manifestations of SFN typically involve a spectrum of positive and negative sensory abnormalities that may include neuropathic pain, paresthesias (spontaneous tingling or burning sensations), hyperalgesia, and numbness (Hoeijmakers et al., 2012, McArthur, 2012, Gibbons, 2014). The etiologies associated with SFN are numerous and include common metabolic disorders such as diabetes mellitus (Russell and Zilliox, 2014) and hyperlipidemia (Morkavuk and Leventoglu, 2013), infectious diseases such as HIV (Kaku and Simpson, 2014) and herpesviral infections (Steiner, 2012), as well as rare hereditary (Brouwer et al., 2014) and autoimmune conditions (Oomatia et al., 2014). Relevant to neurotoxicology, several pharmaceutical compounds, including chemotherapeutic agents (Cavaletti et al., 2010) and antiretroviral drugs (Cherry et al., 2003, Ellis et al., 2008, Dorsey et al., 2015), have frequently been associated with peripheral neurotoxicity leading to SFN. For some patients, these detrimental side effects can be dose-limiting and negatively impact their quality of life (Hong et al., 2014). Thus, it is imperative to assess the peripheral neurotoxicity of new drugs during preclinical testing (Authier et al., 2009).
The diagnosis and study of SFN has historically been challenging (McArthur, 2012). Standard nerve conduction studies are relatively insensitive to alterations in small diameter nerve fibers and are often normal in patients with SFN (Herrmann et al., 1999, Gonzales-Duarte, et al., 2008, Hoeijmakers et al., 2012). Similarly, morphologic examination of peripheral nerve tissue, such as the sural or sciatic nerves, may not reflect early changes to small fibers that may begin in distal regions (Herrmann et al., 1999). In recent years, visualization and assessment of epidermal nerve fibers (ENF) in skin biopsies has become a standard clinical tool to diagnose SFN caused by a wide variety of conditions (Ebenezer et al., 2007, Lauria et al., 2010). Unlike quantitative sensory testing that relies on patient responses and is subject to bias (Hlubocky et al., 2009), ENF analysis is objective and can be applied to animal models (Lauria et al., 2005). Skin biopsy is also a relatively quick technique that is applicable to longitudinal sampling in contrast to performing sural or sciatic nerve biopsies (David, 2008). In several conditions, including HIV infection, loss of ENF density has been shown to correlate strongly with severity of neuropathic symptoms (Zhou et al., 2007, Obermann et al., 2008).
In this report, we describe the development and implementation of skin biopsy as a tool to study sensory nerve alterations in SIV-infected macaques. Previous studies by our group have shown that SIV-infected pigtailed macaques (Macaca nemestrina) develop morphologic and functional lesions of the peripheral nervous system that closely parallel those seen in HIV patients with neuropathy (Laast et al., 2011). These include inflammation, glial activation, and viral replication in the lumbar spinal cord (Mangus et al., 2014) and dorsal root ganglia, as well as decreased conduction velocity in isolated C-fibers from the sural nerve (Laast et al., 2011) Because skin biopsy and ENF quantification is currently the gold standard to diagnose and monitor SFN in HIV patients, we sought to adapt this technique for use in macaques. In addition to pigtailed macaques, we have also evaluated ENF of uninfected and SIV-infected rhesus macaques (Macaca mullata), allowing for comparison of normative and experimental data between macaque species. Through these studies, we have learned valuable lessons regarding skin sample collection and processing, quantitative image analysis of ENF, and selection of study animals. These lessons are highly applicable to any study evaluating the peripheral nervous system of non-human primates, including preclinical testing of potentially neurotoxic compounds or novel drugs aimed at the treatment of SFN.
Materials and Methods
Animals
In total, 117 pigtailed macaques (Macaca nemestrina) and 22 rhesus macaques (Macaca mulatta) were evaluated in this study. Pigtailed macaques had a mean age of 4 years (range 1 to 9 years) and were male; rhesus macaques had a mean age of 12 (range 7 to 15 years). The rhesus macaque group included three males and 19 females. For SIV studies, macaques were inoculated intravenously with a combination of the neurovirulent clone SIV/17E–Fr and the immunosuppressive viral swarm SIV/DeltaB670 as previously described (Clements et al., 2011). Age-matched uninfected macaques served as procedural controls (i.e. control animals). At study endpoints, animals were euthanized and whole-body perfused with room temperature sterile saline. This removes blood from vasculature, thus allowing for quantification of virus and other factors, such as cytokines, in tissues with minimal blood contamination. For the pigtailed macaques, which demonstrate an accelerated, predictable SIV disease progression (Mankowski et al., 2002), specific end points were predetermined according to stage of disease [acute phase at 7 and 10 days post infection (dpi), asymptomatic phase at 21, 35, 42, and 56 dpi, and terminal AIDS at 84 dpi]. Because the course of disease progression is more variable in rhesus macaques, these animals were euthanized when two or more AIDS-defining criteria were met (mean length of infection 213 days)(Beck et al., 2015).
Macaques were pair-housed in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAS), and all procedures were performed according to principles set forth by the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC) and the National Research Council’s Guide for the Care and Use of Laboratory Animals. Macaques were fed a complete commercial diet (Harlan Laboratories, Cambridgeshire, UK) and provided with daily enrichment including fresh fruits and vegetables.
Skin biopsy collection and fixation
Skin biopsies for ENF analysis in human patients are typically obtained from an area of the distal leg above the lateral malleolus (Hoeijmakers et al., 2012). However, the high density of hair follicles and adnexa present in haired macaque skin results in variation among sections and obscures visualization of ENF. We therefore obtained skin samples from the glabrous, (non-haired), skin of the metatarsal footpad between the third and fourth digits. To obtain pre-infection biopsies, which were utilized for longitudinal ENF studies, animals were anesthetized with ketamine hydrochloride (15–20 mg/kg) and 3mm punch biopsies were collected from the left metatarsal footpad. Biopsy sites were then covered with antibiotic ointment, followed by a sterile non-adherent dressing and elastic bandaging, which animals typically removed themselves within 24 hours. No systemic antibiotics or analgesics were needed postoperatively and the procedure was generally well tolerated. Post-infection biopsies were obtained from the right metatarsal footpad at necropsy. The biopsy sample was handled carefully using fine-tipped forceps to grasp only the deep dermal fat and avoid any crush artifact to the epidermis.
Skin biopsies were immersed in Zamboni’s fixative (Newcomer Supply, Middleton, WI) and fixed for 12 to 24 hours at 4°C. Compared to other standard fixatives, such as formalin and paraformaldehyde, we have found that Zamboni’s better preserves antigenicity in the ENF, and it is stable for long periods at room temperature. After fixation, sections were rinsed with 0.08M Sorensen’s phosphate buffer (prepared from 0.4M Sorenson’s buffer: 7.176g Na3PO4 monobasic monohydrate and 49.4g Na3PO4 dibasic anhydrous dissolved in 1L distilled water, pH 7.6). Fixed sections were transferred to a cryprotective buffer (20% glycerol in 0.08M Sorensen’s phosphate buffer) and stored at 4°C until processing (typically within one week).
Immunohistochemistry
Protein gene product 9.5 (PGP9.5) is widely expressed in neuronal cell bodies and axons, including epidermal nerve fibers (McCarthy et al., 1995). Although PGP9.5 antibodies can be used in paraffin embedded tissues, cryosectioning reduces tissue shrinkage and allows for the production of consistent, thick sections, which are necessary when implementing stereological methods. For these studies, cryoprotected skin samples were cut perpendicular to the epidermis at a thickness of 50µm using a freezing-sliding microtome (Microm HM 440E, GMI, Ramsey, MN). Each biopsy yielded approximately 48–50 sections, from which 4 sections were chosen for immunostaining. Sections were selected at roughly fixed intervals (eg.10th,20th,30th,40th sections) to be representative of the whole biopsy. Individual sections were transferred to 96 well plates and immunostained for the pan-axonal marker PGP9.5 using a free floating section method as previously described (McCarthy et al., 1995). All staining procedures were conducted at room temperature (RT) and all incubations were performed with gentle agitation on an orbital shaker (Mini Orbital Shaker, VWR, Radnor, PA). Sections were first bleached with 0.25% potassium permanganate for 5 minutes followed by 5.0% oxalic acid for 2 minutes to reduce melanin pigmentation. The sections were blocked for 10 minutes with a solution of 1.0% Triton X-100 (Sigma, St. Louis, MO) with 0.5% powdered milk (Bio Rad, Hercules, CA) and 5% normal goat serum (Vector, Burlingame, CA) in Tris-buffered saline (TBS: 250mM Tris base, 250mM NaCl, pH 7.4), then incubated with the primary anti-PGP9.5 antibody overnight (rabbit polyclonal, 1:10,000, product #7863-1004, ABD Serotec, Oxford, UK). The following day, sections were incubated with a biotinylated secondary antibody (goat anti-rabbit, 1:100, product #BA-1000, Vector) for 1 hour, and treated with 1% hydrogen peroxide in 30% methanol/PBS for 30 minutes to quench endogenous peroxidase activity. Sections were then incubated for 1 hour in avidin/biotin complex solution (ABC, product #PK-6100, Vector) and developed for 5–8 minutes using the Vector SG peroxidase substrate kit (product #SK-4700), which produces dark blue-gray chromogenic staining. The stained sections were mounted on chrome alum-gelatin subbed slides, air-dried for 30 minutes at RT, and rinsed in RT tap water for 1 minute to remove dried salt residues. Finally, slides were lightly counterstained with dilute eosin (1%) for 1 minute and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). This procedure results in final section thickness ranging from 35 to 40µm.
Quantitative epidermal nerve fiber assessment
Measurement of ENF density in human skin biopsies is typically achieved by manually counting all PGP9.5-stained epidermal nerve fibers that cross the dermal-epidermal junction and dividing this number by the linear length of the skin section in millimeters (McCarthy et al., 1995). Although this method is highly sensitive and reproducible in human skin samples (Smith et al., 2004a), and has also been utilized for peripheral neuropathy studies in feline-immunodeficiency virus (FIV)-infected cats (Kennedy et al., 2004), the remarkably rich sensory innervation of the macaque footpad makes manual counting of individual fibers cumbersome and poorly reproducible. Thus, we developed two quantitative image analysis techniques, one based on ENF density and another on ENF length, to facilitate ENF assessment in our macaque skin samples. For both techniques, slides were blinded prior to analysis to reduce operator bias.
Measurement of epidermal nerve fiber density
The method used to determine ENF density in macaque footpad samples was adapted from the confocal ‘optical sectioning’ technique described by Kennedy and colleagues (Kennedy, 2005). It combines the thick-sections required to visualize linear objects in tissues with standard, two-dimensional image anaylsis tools to determine the percentage of epidermal area occupied by PGP9.5-stained nerve fibers. Using a brightfield microscope equipped with a Z-axis motor (Carl Zeiss, Oberkochen, Germany) and IP Lab software (Version 3.9, Scanalytics, Campbell, CA), Z-stack images were obtained from adjacent, non-overlapping, 400X fields for each immunostained skin section. To reduce operator bias during image acquisition, the stage was moved in a systematic grid-like manner to collect non-overlapping images encompassing the entire epidermal region on the section (typically 10–15 fields). Section thickness (Z-distance) was measured at each imaging field by the operator focusing on the top and bottom of the tissue. Serial Z-stack images for each field were collected at 0.5µm intervals throughout the Z-axis, and then collapsed into a single image. For each collapsed image, the epidermal region of interest (ROI) was defined by tracing along the dermal-epidermal junction and the stratum corneum using the paint function. Using iVision software (BioVision Technologies, Exton, PA, Version 4.0.14), collapsed images were then binarized by setting the threshold at the right-most inflection point in the image intensity histogram, and PGP9.5 immunoreactivity was measured as percent of the ROI (%ROI) area occupied by positively stained pixels. To adjust for minor variations in skin section thickness, results were normalized to the thickness of each sample by dividing the %ROI value by the average Z-distance of the section.
Measurement of epidermal nerve fiber length
An advantage of quantifying length, rather than density of linear structures in tissue sections is the ability to employ non-biased, software-guided stereologic image analysis techniques. These methods are based on counting random intersections of the object of interest, such as an axon or blood vessel, with a geometric probe that is superimposed over the image of the tissue (Mouton et al., 2002). Additionally, stereologic algorithms provide an estimate of the total length of objects in a specimen by systematic random sampling (SRS), which saves time compared to analyzing an entire specimen. To measure length of epidermal nerve fibers in macaque footpad samples, we used the Stereo Investigator space ball probe (MBF Biosciences, Williston, VT, Version 9), similar to the method described by Ebenezer and colleagues (2011) and (2012). Three, 50µm thick, PGP9.5 immunostained skin sections were evaluated per animal. For each section, a region of interest (ROI) in the epidermis, extending from the dermal-epidermal junction to the stratum corneum, was traced under a Nikon 4x/0.2 Plan Apo objective on a Nikon Eclipse E600 light microscope equipped with a motorized stage (see Fig 1, upper panel for illustration). The Stereo Investigator software then created a SRS grid of rectangular counting frames encompassing the entire ROI and moved the slide to random sampling sites via the motorized stage. At each sampling site, section thickness was measured by focusing on the top and bottom of the tissue using a Nikon 60x/1.4 oil Plan Apo objective. Total ENF length was estimated by counting intersections of nerve fibers with a hemispheric probe that had a 30µm radius and 2µm guard zones (to account for tissue irregularity at the edges of the sections). Only nerve fibers that were clearly within the epidermis (single, finely-beaded fibers visualized within the epidermal ROI contour) were counted (Fig 1, lower panel). All stereology measurements were obtained using Stereo Investigator DAT files and results were expressed as the estimated total length of PGP9.5-stained epidermal nerve fibers.
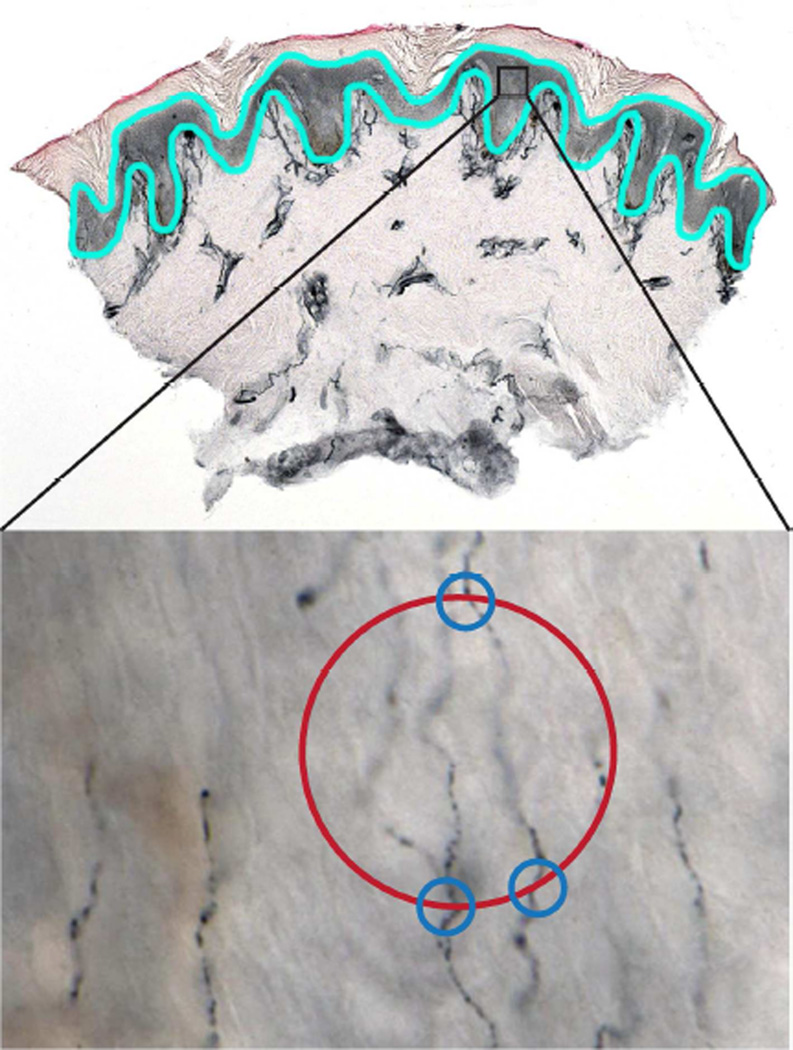
Figure 1. Illustration of the stereologic technique for measuring ENF length in a footpad skin biopsy.
As depicted in the upper panel, the epidermis is traced (light blue outline) in a PGP9.5-stained skin section under low magnification. This serves as the region of interest (ROI). The Stereo Investigator software then systematically selects a subset of fields within the ROI for measuring ENF (represented by the black rectangle). After switching to high magnification, as shown in the lower panel (60X oil objective), the probe is moved through the Z-axis of the tissue by a motorized stage and points of intersection between ENF and a hemispheric probe (represented by red circle) are marked by the user (blue circles). Intersections are only counted when the nerve fiber is in sharp focus at the point of crossing.
Statistics
All statistical analyses were performed using Prism software (GraphPad, San Diego, CA Version 5.0d) and nonparametric methods. The Mann-Whitney U test was used for two-group comparisons and the Spearman correlation coefficient was used to analyze relationships between continuous variables. Statistical inferences were considered significant when p < 0.05.
RESULTS
Two ENF quantification methods yield strongly correlative results
In our SIV/macaque studies, we have found that epidermal nerve fibers can be effectively visualized in footpad biopsies by immunostaining thick cryosections with the pan-neuronal marker PGP9.5. Using these immunostained sections, two different image analysis techniques, one assessing nerve fiber density and the other measuring fiber length, were developed and found to be effective means of objectively quantifying ENF. Furthermore, when both quantification methods were applied to the same skin samples, we found strong, direct correlation between the results (Fig 2), showing that either technique could serve as a valid tool in other macaque ENF studies. Once optimized, the time required to perform these techniques is similar (roughly 60 to 90 minutes per animal), and choice of methods would be likely depend on available imaging equipment and software.
Figure 2. Two ENF quantification methods yield strongly correlative results.
Two different image analysis techniques, one measuring nerve fiber density and the other measuring fiber length, were developed. When both quantification methods were applied to the same skin samples from uninfected control (open squares) and SIV-infected pigtailed macaques (solid circles), there was strong direct correlation between the techniques.
Species differences in ENF loss during SIV infection
The species of macaque used in our group’s SIV work is based on the desired phenotypic outcome for each study. Since most of our studies have aimed to model the neurologic consequences of HIV infection, the vast majority of our study animals are pigtailed macaques, which more quickly and consistently develop SIV-associated CNS and PNS disease (Mankowski et al., 2002). Smaller numbers of rhesus macaques have been used in studies involving HIV-associated cardiac dysfunction, as this species survives longer following SIV infection and more readily develops functional cardiac abnormalities (Kelly et al., 2012). We have collected normative ENF data on uninfected control groups of both pigtailed (Fig 3A) and rhesus (Fig 3B) macaques. While the median ENF density among control rhesus was lower than that of pigtailed macaques, this difference did not reach statistical significance (p = 0.081, data not shown). However, in the context of SIV infection, only infected pigtailed macaques exhibited a significant decline in ENF density compared to control animals (p = 0.0033, Fig 3A). There was no significant difference in ENF density between rhesus macaques euthanized at terminal time points and control animals (p = 0.26, Fig 3B). This finding demonstrates the potential for considerable variation in host responses between closely related primate species, and underscores the need for careful species selection in studies evaluating the peripheral nervous system.
Figure 3. Species differences in ENF loss during SIV infection.
Pigtailed macaques exhibited significant decline in ENF density at 84 days post SIV infection (A; p = 0.0033), but there was no significant difference in ENF density between rhesus macaques euthanized at terminal SIV time points versus control animals (B; p = 0.26, Mann-Whitney U test). Photomicrographs of skin biopsies from control (C) and SIV-infected (D) pigtailed macaques demonstrate a marked decrease in the number of thin, finely-beaded PGP9.5-stained nerve fibers in the epidermis. The light blue line delineates the region of interest (ROI) for assessing epidermal nerve fibers. Bundles of thicker PGP9.5-stained dermal nerve fibers can be seen below the dermoepidermal junction.
Pigtailed macaques of US and Indonesian origin have different baseline ENF length
In addition to considering potential difference in ENF response between species, we also examined whether pigtailed macaques from different originating countries had comparable ENF status prior to entering SIV studies. Interestingly, we found that pigtailed macaques imported from Indonesia had significantly lower baseline ENF length than macaques born in the United States (p = 0.0048, Fig 4). Whether this observation results from genetic or environmental differences between Indonesian and US pigtailed macaque populations is currently unclear. However, this finding suggests that uniformity of animal origin may be a critical factor when designing studies evaluating ENF status of primates.
Figure 4. Differences in baseline ENF length based on country of origin.
When comparing baseline ENF length of pigtailed macaques originating from different countries, animals from the United States had significantly higher baseline ENF than animals born in Indonesia (p = 0.0048, Mann-Whitney U test).
Measuring change from baseline allows longitudinal assessment of ENF loss
Skin biopsies are a minimally-invasive and repeatable tool for assessing the status of peripheral sensory nerves in humans and animal models, and therefore can be used for longitudinal assessment of individual subjects. In our more recent macaque studies, we collected pre-infection footpad biopsies and quantified baseline ENF lengths that were then compared to values obtained from necropsy skin samples. Among pigtailed macaques, we found that SIV-infected animals euthanized during terminal disease (84 dpi) had a median decrease in ENF length of nearly 5×104 µm from their baseline value and that this change was significantly different from that observed among uninfected controls that were also euthanized at 84 days post mock infection (p = 0.0024, Fig 5). We believe that this longitudinal manner of ENF assessment produces more reliable data than cross-sectional sampling and measurement, and plan to utilize this method in future studies of SIV neuropathy.
Figure 5. Measuring change from baseline allows longitudinal assessment of ENF loss.
Footpad skin biopsies were collected from pigtailed macaques at the time of inoculation (SIV or mock) and at necropsy, 84 dpi. ENF length measurements obtained from each animal’s necropsy skin samples were compared to measurement from their pre-infection samples, and results were plotted as “change from baseline.” SIV-infected pigtailed macaques had a median decrease of approximately 5×104 µm, which was significantly different from the median change observed in uninfected animals, which was close to zero (p = 0.0028, Mann-Whitney U test)
DISCUSSION
When studying an animal model of human disease or toxicity, a priority is to employ experimental outcome measures similar to those currently used in clinical settings. Over the past decade, assessment of sensory nerve fibers in skin biopsies has emerged as a sensitive indicator of early damage to distal unmeylinated nerve fibers, and has supplanted older methods, such as sural nerve morphometry and electrodiagnostic studies, in the clinical diagnosis of HIV-associated sensory neuropathy and other small fiber neuropathies. In line with this advancement, our group has worked to develop and implement techniques to measure progressive ENF loss in SIV-infected macaques. These techniques have proven to be key assets in our investigations of the pathogenesis of SIV-SN (Laast et al., 2011, Mangus et al., 2014, Dorsey et al., 2014). While distal ENF loss has been widely reported rodent models of metabolic and toxic neuropathies, such as diabetic and chemotherapy-induced peripheral neuropathy (Lauria et al., 2005, Siau et al., 2006, Drel et al., 2006, Hoke and Ray, 2014), published data on the ENF responses of non-human primates outside of SIV research is scarce. We propose that broader application of the techniques described herein for quantitative ENF analysis in Asian macaques could greatly advance preclinical studies of peripheral neurodegeneration and neurotoxicity in non-human primates.
Our work with both pigtailed and rhesus macaques has also revealed important experimental variables that need to be considered when planning ENF studies in primates. First, we found that there was no significant difference in footpad ENF density between uninfected control macaques of the two species despite a substantial age difference between the groups. However, only pigtailed macaques exhibited significant ENF loss during the course of SIV infection. ENF density in SIV-infected rhesus macaques with terminal disease was not significantly different from controls. In contrast, Lakritz and colleagues (2015) recently reported significant ENF decline in SIV-infected rhesus macaques that were also depleted of CD8+ cells including lymphocytes and NK cells. This discrepancy in experimental outcome between closely-related macaque species emphasizes the importance of host immune responses in animal studies of peripheral neuropathy. Rodent studies have also revealed marked differences in the susceptibility of various mouse strains to peripheral neuropathy (Höke, 2012). For example, in a study investigating the sensitivity of different inbred mouse strains to paclitaxel-induced neuropathy, Smith and colleagues (2004b) showed that DBA/2J mice exhibited especially robust mechanical hypersensitivity after exposure to paclitaxel, while the C57BL/6J mice were relatively resistant to this change. The influence of genetic background has also been shown to be a critical variable in mouse models of diabetic neuropathy (Sullivan et al., 2008).
Second, when evaluating pigtailed macaques originating from different countries, we found that animals from Indonesia had significantly lower baseline ENF length than animals born in the United States. Although a relatively low number of Indonesian animals were included in this analysis, the robust difference was nonetheless evident in our dataset. Whether this observation is related to genetic or environmental factors is not clear. Significant differences in ENF density have also been reported in people of distinct geographic origins (Shikuma et al., 2013). A recent study investigating ENF density as a marker of neuropathy risk in Thai HIV patients showed that very few subjects in that cohort developed neuropathic signs or symptoms despite use of stavudine, a known neurotoxic antiretroviral drug that is still often used in developing countries (Shikuma et al., 2015). While several factors likely contributed to this finding, the authors note that HIV seronegative Thai subjects have higher ENF density compared to published US control values, and that this higher baseline ENF density may provide Thai subjects with greater “ENF reserve.” Together, our findings suggest that both species and geographic origin are important variables to consider when planning studies of peripheral neuropathy in Asian macaques. While these variables are potential confounding factors in preclinical studies of peripheral neuropathy in primates, they could ultimately be informative in future research aimed at identifying ‘neuroprotective’ host genes and environmental factors. Furthermore, these findings should encourage investigators to exercise caution when comparing macaque ENF values among different laboratories, especially regarding control data, as animal source and environment may affect the results.
Finally, we have shown that by obtaining footpad skin biopsies prior to SIV infection, each animal’s ENF measurement at necropsy can then be compared to its own pre-infection ENF measurement. With this longitudinal sampling method, we were able to demonstrate that SIV-infected pigtailed macaques experienced a significantly greater loss of distal ENF length compared to control animals at 84 dpi or post mock-infection. By using ‘change-from-baseline’ data rather than single, cross-sectional ENF measurements, each macaque serves as its own control, which helps account for individual variation in baseline ENF values. Longitudinal sampling could be of great benefit for ENF studies facilitating use of smaller groups of animals or when uniformity of animals (age, origin, etc.) is not feasible. In addition, longitudinal ENF studies can be used to monitor epidermal nerve fiber regeneration, as demonstrated by Ebenezer and colleagues in SIV-infected pigtailed macaques (Ebenezer et al., 2009).
Given its extensive distribution and anatomic complexity, systematic sampling and assessment of the PNS can be challenging, especially in large animal models. Skin biopsy and subsequent ENF analysis provide relatively simple methods for obtaining information about the status of peripheral sensory neurons, and can be performed objectively with little specialized training. Moreover, loss of ENF density is a more sensitive indicator of early damage to small unmeylinated nerves than traditional histologic or morphometric nerve analyses. Our group’s efforts to evaluate ENF in the context of SIV-associated sensory neuropathy has led to the development of reliable tools and unbiased, quantitative techniques that could be applied to a wide range of peripheral neuropathy and neurotoxicity studies in primate species. Additionally, skin biopsies are relatively non-traumatic and well tolerated by humans and animals. Thus they are repeatable and can be used for longitudinal assessment of individuals, both in the clinic and in the context of drug safety studies.
References
- Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, Coudore F, Bourinet E, Eschalier A. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, Mankowski JL. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. Journal of neurovirology. 2015;21:148–158. doi: 10.1007/s13365-015-0313-7. [DOI] [PubMed] [Google Scholar]
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. Journal of the peripheral nervous system : JPNS. 2014;19:53–65. doi: 10.1111/jns5.12071. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P, Cortinovis D, Bidoli P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. European journal of cancer (Oxford, England : 1990) 2010;46:479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2003;26:195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Clements J, Gama L, Graham D, Mankowski J, Zink M. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Current opinion in HIV and AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NH. Noninvasive and minimally invasive detection and monitoring of peripheral neuropathies. Expert review of neurotherapeutics. 2008;8:1807–1816. doi: 10.1586/14737175.8.12.1807. [DOI] [PubMed] [Google Scholar]
- Dorsey JL, Mangus LM, Hauer P, Ebenezer GJ, Queen SE, Laast VA, Adams RJ, Mankowski JL. Persistent Peripheral Nervous System Damage in Simian Immunodeficiency Virus-Infected Macaques Receiving Antiretroviral Therapy. Journal of neuropathology and experimental neurology. 2015;74:1053–1060. doi: 10.1097/NEN.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey JL, Mangus LM, Oakley JD, Beck SE, Kelly KM, Queen SE, Metcalf Pate KA, Adams RJ, Marfurt CF, Mankowski JL. Loss of corneal sensory nerve fibers in SIV-infected macaques: an alternate approach to investigate HIV-induced PNS damage. The American journal of pathology. 2014;184:1652–1659. doi: 10.1016/j.ajpath.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. Journal of neuropathology and experimental neurology. 2007;66:1059–1073. doi: 10.1097/nen.0b013e31815c8989. [DOI] [PubMed] [Google Scholar]
- Ebenezer GJ, Laast VA, Dearman B, Hauer P, Tarwater PM, Adams RJ, Zink MC, McArthur JC, Mankowski JL. Altered cutaneous nerve regeneration in a simian immunodeficiency virus / macaque intracutaneous axotomy model. The Journal of comparative neurology. 2009;514:272–283. doi: 10.1002/cne.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer GJ, McArthur JC, Polydefkis M, Dorsey JL, O’Donnell R, Hauer P, Adams RJ, Mankowski JL. SIV-induced impairment of neurovascular repair: a potential role for VEGF. Journal of neurovirology. 2012;18:222–230. doi: 10.1007/s13365-012-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer GJ, O’Donnell R, Hauer P, Cimino NP, McArthur JC, Polydefkis M. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain : a journal of neurology. 2011;134:1853–1863. doi: 10.1093/brain/awr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, Simpson DM, Ake C, Collier AC, Gelman BB, McCutchan JA, Morgello S, Grant I, Group C. Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Annals of neurology. 2008;64:566–572. doi: 10.1002/ana.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH. Small fiber neuropathies. Continuum (Minneapolis, Minn.) 2014;20:1398–1412. doi: 10.1212/01.CON.0000455874.68556.02. [DOI] [PubMed] [Google Scholar]
- Gonzales-Duarte A, Ronbinson-Papp J, Simpson DM. Diagnosis and management of HIV-associated neuropathy. Neurologic clinics. 2008;26:821–831. doi: 10.1016/j.ncl.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- Hlubocky A, Wellik K, Ross MA, Smith BE, Hoffman-Snyder C, Demaerschalk BM, Wingerchuk DM. Skin biopsy for diagnosis of small fiber neuropathy: a critically appraised topic. The neurologist. 2009;16:61–63. doi: 10.1097/NRL.0b013e3181c9c303. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG. Small-fibre neuropathies--advances in diagnosis, pathophysiology and management. Nature reviews. Neurology. 2012;8:369–379. doi: 10.1038/nrneurol.2012.97. [DOI] [PubMed] [Google Scholar]
- Höke A. Animal models of peripheral neuropathies. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:262–269. doi: 10.1007/s13311-012-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A, Ray M. Rodent models of chemotherapy-induced peripheral neuropathy. ILAR J. 2014;54:273–281. doi: 10.1093/ilar/ilt053. [DOI] [PubMed] [Google Scholar]
- Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- Hong JS, Tian J, Wu LH. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Current oncology (Toronto, Ont.) 2014;21:174–180. doi: 10.3747/co.21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M, Simpson DM. HIV neuropathy. Current opinion in HIV and AIDS. 2014;9:521–526. doi: 10.1097/COH.0000000000000103. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Tarwater PM, Karper JM, Bedja D, Queen SE, Tunin RS, Adams RJ, Kass DA, Mankowski JL. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS (London, England) 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Hoke A, Zhu Y, Johnston JB, van Marle G, Silva C, Zochodne DW, Power C. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS. 2004;18:1241–1250. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur JC. Pathology and Quantification of Cutaneous Innervation. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Philadelphia: Saunders; 2005. pp. 869–896. [Google Scholar]
- Laast V, Shim B, Johanek L, Dorsey J, Hauer P, Tarwater P, Adams R, Pardo C, McArthur J, Ringkamp M, Mankowski J. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. The American journal of pathology. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakritz JR, Bodair A, Shah N, O’Donnell R, Polydefkis MJ, Miller AD, Burdo TH. Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy. The American journal of pathology. 2015 doi: 10.1016/j.ajpath.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Hsieh S-T, Johansson O, Kennedy W, Leger J-M, Mellgren S, Nolano M, Merkies I, Polydefkis M, Smith AG, Sommer C, Valls-Sole J. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Journal of the peripheral nervous system : JPNS. 2010;15:79–92. doi: 10.1111/j.1529-8027.2010.00269.x. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. Journal of the peripheral nervous system : JPNS. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- Mangus LM, Dorsey JL, Laast VA, Hauer P, Queen SE, Adams RJ, McArthur JC, Mankowski JL. Neuroinflammation and virus replication in the spinal cord of simian immunodeficiency virus-infected macaques. Journal of neuropathology and experimental neurology. 2014;74:38–47. doi: 10.1097/NEN.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski J, Clements J, Zink M. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. The Journal of infectious diseases. 2002;(186 Suppl 2):208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- McArthur JC. Painful small fiber neuropathies. Continuum (Minneapolis, Minn.) 2012;18:106–125. doi: 10.1212/01.CON.0000411570.79827.25. [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- McGlone F, Reilly D. The cutaneous sensory system. Neuroscience and biobehavioral reviews. 2010;34:148–159. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Morkavuk G, Leventoglu A. Small fiber neuropathy associated with hyperlipidemia: utility of cutaneous silent periods and autonomic tests. ISRN neurology. 2013;2014 doi: 10.1155/2014/579242. Article ID: 579242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. Journal of microscopy. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Obermann M, Katsarava Z, Esser S, Sommer C, He L, Selter L, Yoon M-S, Kaube H, Diener H-C, Maschke M. Correlation of epidermal nerve fiber density with pain-related evoked potentials in HIV neuropathy. Pain. 2008;138:79–86. doi: 10.1016/j.pain.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Oomatia A, Fang H, Petri M, Birnbaum J. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five-year study period. Arthritis & rheumatology (Hoboken, N.J.) 2014;66:1000–1009. doi: 10.1002/art.38302. [DOI] [PubMed] [Google Scholar]
- Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneapolis, Minn.) 2014;20:1226–1240. doi: 10.1212/01.CON.0000455884.29545.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma CM, Bennett K, Ananworanich J, Gerschenson M, Teeratakulpisarn N, Jadwattanakul T, DeGruttola V, McArthur JC, Ebenezer G, Chomchey N, Praihirunkit P, Hongchookiat P, Mathajittiphun P, Nakamoto B, Hauer P, Phanuphak P, Phanuphak N, team Sp. Distal leg epidermal nerve fiber density as a surrogate marker of HIV-associated sensory neuropathy risk: risk factors and change following initial antiretroviral therapy. Journal of neurovirology. 2015;21:525–534. doi: 10.1007/s13365-015-0352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma CM, McArthur JC, Ebenezer GJ, Ananworanich J, Teeratakulpisarn N, Jadwattanakul T, Valcour VG, Bennett K, Phanuphak N, Team S. Ethnic differences in epidermal nerve fiber density. Muscle & nerve. 2013;48:462–464. doi: 10.1002/mus.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Experimental neurology. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, James RH, Randi K, Pam R, Peter H, Singleton JR, Justin M. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. Journal of the neurological sciences. 2004a;228:65–69. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life sciences. 2004b;74:2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Steiner I. Herpes virus infection of the peripheral nervous system. Handbook of clinical neurology. 2012;115:543–558. doi: 10.1016/B978-0-444-52902-2.00031-X. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Lentz SI, Roberts JL, Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Current drug targets. 2008;9:3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebenezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, McArthur JC Narc and Group, A.A.S. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]