Abstract
Purpose of Review
Atherosclerotic cardiovascular disease confers significant morbidity and mortality in patients with systemic lupus erythematosus (SLE) and cannot be fully explained by traditional cardiovascular risk factors. Recent immunologic discoveries have outlined putative pathways in SLE that may also accelerate the development of atherosclerosis.
Recent findings
Aberrant innate and adaptive immune responses implicated in lupus pathogenesis may also contribute to the development of accelerated atherosclerosis in these patients. Defective apoptosis, abnormal lipoprotein function, autoantibodies, aberrant neutrophil responses and a dysregulated type I interferon pathway likely contribute to endothelial dysfunction. SLE macrophages have an inflammatory phenotype that may drive progression of plaque.
Summary
Recent discoveries have placed increase emphasis on the immunology of atherosclerotic cardiovascular disease. Understanding the factors that drive the increase risk for CVD in SLE patients may provide selective therapeutic targets for reducing inflammation and improving outcomes in atherosclerosis.
Keywords: Systemic lupus erythematosus, atherosclerosis, cardiovascular disease, neutrophil extracellular traps, interferons
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune syndrome that primarily affects women of childbearing age. Although life expectancy in SLE has improved, estimated mortality rates remain approximately three times that of the general population.(1) Importantly, while deaths due to lupus manifestations have decreased, those due to atherosclerotic cardiovascular disease (CVD) in SLE have not. Indeed, CVD accounts for more than one-third of all deaths in SLE patients.(2, 3) Outcomes of percutaneous coronary intervention are worse in SLE subjects than in matched controls, with regards to the need for repeat vascularization and major adverse cardiac events.(4) Overall, SLE patients have higher incidence of CVD, present with this complication earlier than age- and gender-matched controls,(5) and have an accelerated rate of plaque progression.(6) The risk for myocardial infarction (MI) in SLE patients is 9–50 fold that of the general population, depending on the series.(7) While the Framingham risk equation (8) contributes to vascular risk in SLE, it cannot fully explain it.(9, 10) Indeed, while the absolute risk for CVD increases with age, the highest increment in relative risk for acute MI occurs in young females with SLE.(11) Furthermore, atherosclerosis in SLE is atypical not only because it affects premenopausal women but because it is not associated with the “classical” inflammatory burden characteristic of “idiopathic” atherosclerosis, as such as elevated C-reactive protein (CRP) and elevated levels of plasma low density lipoprotein (LDL).
In SLE, disease duration, higher damage index score and less aggressive immunosuppression are associated with increased CVD burden (12) (13*, 14*), suggesting that the immune dysregulation characteristic of lupus is a crucial player in plaque progression and vascular complications. Similar molecular events that drive autoimmunity have recently been proposed to influence plaque development and progression in SLE. Both the innate and adaptive immune systems contribute to the inflammatory state of lupus, and evidence suggests that similar factors may be implicated in development and progression of CVD.(15)* Furthermore, murine models suggest a synergism between genes that promote atherogenesis with those that promote autoimmunity, although the relationship between enhanced plaque and more profound systemic immune dysregulation requires further elucidation.(16)*
Endothelial Dysfunction and aberrant metabolic pathways in SLE
SLE patients demonstrate endothelial dysfunction, a phenomenon considered to correlate with poor CV outcomes in other populations. A very significant proportion of SLE patients display impaired endothelium-dependent vasorelaxation (17) and enhanced arterial stiffness.(18) The subset of patients affected by lupus nephritis appears to be at a particularly increased risk for arterial stiffness.(19) Cardiac positron-emission tomography (PET) has revealed impaired microvascular blood flow and reduced coronary flow reserve in SLE patients.(20)**
Atherosclerosis occurs in the arterial subendothelial space and is initiated by the interplay between subendothelial lipoprotein retention and endothelial dysfunction that leads to a non-resolving inflammatory response that generates endothelial damage and atherothrombosis.(21) While many atherosclerotic lesions undergo a partial fibrotic protective resolution,(22) some individuals develop “vulnerable plaques” that are more prone to breakdown and subsequent development of acute coronary syndrome. These vulnerable areas are characterized by intimal necrosis, enhanced inflammation and fibrous cap thinning. It has been proposed that plaque necrosis results from a combination of defective clearance of apoptotic cells, and primary necrosis of these cells, quite similar to defects on the immune system observed in other organs from lupus patients.(23–25) However, whether this represents a common mechanism that explains enhanced atherogenesis in SLE and whether lupus patients have increased prevalence of vulnerable plaque, remains to be determined.(26)
Thirty-five percent of adult SLE patients(27) and 60% of pediatric SLE patients have abnormal lipoprotein profiles at diagnosis(28), and prevalence increases to 60% of adults in a 3-year follow up in the SLICC cohort.(27) In SLE, a described pattern of dyslipidemia is characterized by increased VLDL and triglycerides, and decreased HDL.(29) This pattern is often seen at time of diagnosis and correlates to SLE activity.(30) Oxidized LDL/β2glycoprotein 1 immune complexes have also been described,(31) which bind oxidized LDL (oxLDL) and are phagocytosed by macrophages, promoting foam cell formation.
In addition to the effects of LDL, SLE HDL is dysfunctional and proatherogenic. Indeed, a main antiatherogenic property of HDL is the cholesterol efflux capacity, and this is impaired in SLE with no association to plasma level of the lipoprotein.(32) This proinflammatory nature of lupus HDL appears to be driven by enhanced oxidative modifications triggered, at least in part, by neutrophil extracellular traps (NETs).(33) Apolipoprotein levels and ratios are also disturbed in SLE, possibly due to anti-apolipoprotein antibodies.(34) However, outside of enhanced HDL oxidation and dysfunction, none of the dyslipidemias or apolipoproteins have been found to predict subclinical atherosclerosis or cardioprotection in SLE.(35)
Several studies have described enhanced insulin resistance and metabolic syndrome in SLE, which may be associated with insulin receptor antibody formation, inflammatory cascades or medication use.(36–39) Insulin resistance and metabolic syndrome in SLE patients are associated with increased arterial stiffness and organ damage.(40*, 41, 42*) Whether steroid use leads to insulin resistance in SLE patients is still under debate.(36, 41)
PATHOGENESIS OF PREMATURE ATHEROSCLEROSIS IN SLE
Type I IFNs and atherosclerosis in SLE
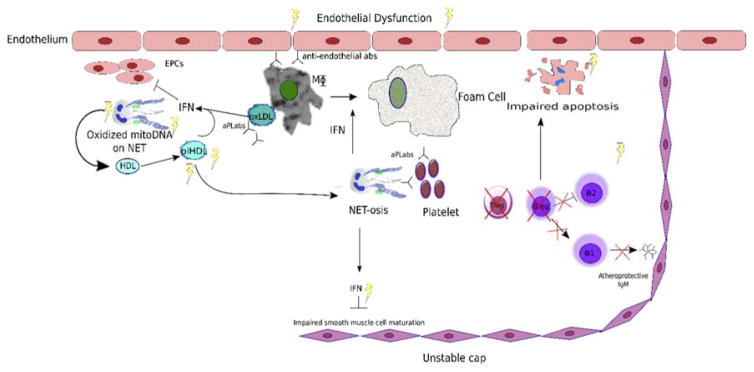
Several studies suggest that, in addition to type I IFNs role in lupus pathogenesis, they may be important contributors to premature atherosclerosis in this disease.(43, 44) Type I IFNs promote an imbalance between endothelial damage and vascular repair. Cells that are critical for vasculogenesis, such as endothelial progenitor cells (EPCs), are decreased in number and impaired in function in both adults and children with SLE (Figure 1).(43, 45*) EPC dysfunction is mediated by type I IFNs that promote an antiangiogenic signature and activation of the inflammasome machinery leading to vascular rarefaction through enhanced synthesis of IL-18.(43, 44, 46) Type I IFNs also promote foam cell formation, while blockade of these inflammatory pathways mitigates endothelial dysfunction and atherogenesis in murine systems (Figure 1).(47, 48) Type I IFNs promote platelet activation and enhance a prothrombotic phenotype.(49) Metabolic influences may also promote and enhance the antiangiogenic effects of type I IFNs, as SLE patients with metabolic syndrome have a decreased percentage of circulating EPCs and increased arterial stiffness compared to those without.(42)* Furthermore, recent evidence implicates type I IFNs in impairing maturation of smooth muscle cells that could hypothetically promote plaque rupture.(50)** How the genetic polymorphisms that influence type I IFN pathways impact atherosclerosis risk remains to be clarified and appear to be complex. For example, while IRF5 appears to be implicated in lupus pathogenesis, it appears to have a protective role in lupus-associated atherosclerosis through effects on both immune and nonimmune cells.(16)*
Figure 1.
Potential pathways promoting atherosclerosis in SLE
Endothelial dysfunction (ED) may be induced by an imbalance of vascular damage (triggered by innate and adaptive immune stimuli) and impaired vascular repair induced by a dysfunction of endothelial progenitor cells (EPCs) induced by type I IFNs and metabolic dysfunction. Various proinflammatory pathways in the plaque may induce neutrophils to undergo NETosis and promote further inflammatory cell recruitment, induce endothelial cell death, enhance local type I IFN synthesis, oxidize lipoproteins and promote thrombosis. Type I IFNs and other stimuli may promote enhanced foam cell formation. Decrease in B Regulatory cells (BRegs) may promote loss of natural IgM, impaired apoptosis and increased IFNs at the level of the plaque. Platelet activation may lead to acute coronary syndromes.
Myeloid cells and atherosclerosis in SLE
Neutrophils
A role for neutrophils in atherogenesis and plaque destabilization has recently been proposed. High-fat diet exposure can induce neutrophil recruitment into arterial walls and complement activation.(51)** Low density granulocytes (LDGs) are a distinct subset of proinflammatory neutrophils present in SLE patients(52) that may play important roles in vascular damage and atherogenesis in this disease through multiple mechanisms. For example, LDGs have an enhanced capacity to synthesize type I IFNs, propagating a vicious cycle of vascular damage, abnormal endothelial repair and atherothrombosis.(53) LDGs can activate a cell death program in endothelial cells through their enhanced capacity to form NETs.(52) NETs have been identified in atherosclerotic plaques in humans and mice(54) and matrix metalloproteinase-9 (MMP-9), found on NET surfaces,(55)** damages endothelial cells (Figure 1). Recently, it has been shown that oxidized mitochondrial DNA and mitochondrial ROS synthesis is increased in NETs, and these molecules drive enhanced IFN synthesis in target myeloid cells, thereby exacerbating potential for inflammatory responses systemically and in the vascular plaque.(56)**
NET formation induced by cholesterol crystals in atherosclerotic plaques primes macrophages for IL-1β production, leading to an IL-1β/IL-17 loop that promotes inflammation.(57)* OxLDL can stimulate NETosis (58), while NETs have a pronounced effect on HDL oxidation and may impair the anti-atherogenic roles of this lipoprotein.(33) Thrombus NET burden correlates positively with infarct size and negatively with ST-segment resolution.(59)** NETs are also prothrombotic and may play important roles in the development of acute coronary syndromes(60**, 61) Therefore, NETs seems to play a role in many crucial aspects of vascular health, and may contribute to the risk left unexplained by traditional factors.
Macrophages
Macrophages are central to the pathogenesis of atherosclerosis(62), and many SLE related factors influence macrophage behavior. SLE monocytes are more prone to oxidative damage (63)* and there is evidence for increases in macrophage proinflammatory subsets M1 and M2b, and decreases in the anti-inflammatory M2a and M2c populations in SLE, which may contribute to inflammation.(64) IFNs drive macrophages to engulf lipids,(48) and enhanced monocyte migration to plaque site increases foam cell production. FcRyIIB is an inhibitory member of the family of Fc receptors (FcR). FcRyIIB knockout mice were found to have lower lipid levels yet had larger atherosclerotic plaques.(15)* Lack of FcRyIIB promotes both autoantibody production in SLE and promotes atherosclerosis in mice.(65, 66)
Adaptive immunity
B cells and Autoantibodies
Subpopulations of B cells may alter risk for CV events, as B1 cells have been described as protective in CVD, whereas B2 cells appear to be pathogenic. B1 cells produce natural antibodies to oxidized LDLs, thus conferring potential cardioprotection (Figure 1).(67) Patients with SLE have decreased levels of protective IgM antibodies against the apoB-100 antigens p45 and p210, and these are further reduced in SLE patients with CVD.(68) Recently, a dysfunctional feedback loop between regulatory B cells and pDCs has been implicated in the pathogenesis of SLE.(69)* Interestingly, B cell depletion restores normal regulatory feedback in SLE and also reduces atherosclerosis.(70)
Immune complexes are identified in atherosclerotic plaques, but evidence remains conflicting over whether they drive plaque formation or vascular damage. Recent evidence suggests that they may amplify endothelial damage through cytolysis and interruption of phagocytosis and autophagy, causing inflammation and increase in plaque.(15)* Antiphospholipid (aPL) antibodies (found in 30–40% of SLE patients) increase risk for thrombosis, may enhance expression of adhesion molecules on endothelial cells and increase monocyte adhesion, increasing mortality in SLE patients. In SLE patients, anticardiolipin titers predict atherosclerosis and thrombus.(71*–73) Anti-β2GP1 antibodies in patient sera predisposes to an increase in NETs and thrombin production.(74)* These prothrombotic factors may add to SLE CV risk.
T cells
Activated T cells are expanded in SLE patients (33), while Treg cells are decreased in number and function; this may contribute to autoimmunity and inflammation.(75) Tregs may protect against atherosclerosis, as they improve endothelial function, inhibit B cell activation and the production of inflammatory cytokines. Proatherogenic ApoE−/− mice have significantly lower number of Tregs compared with wild type,(76) and Treg deficient mice show increased atherosclerotic lesions and plaque vulnerability.(77) Whether this Treg imbalance is implicated in atherogenesis in SLE remains to be determined.
Screening
Risk factors
As traditional risk factors contribute to CV risk in SLE, it is imperative that smoking cessation, healthy weight, and exercise are encouraged. Recently published adult guidelines recommend the following: initial risk assessment of lipid profile, blood pressure and fasting glucose assessment, tobacco exposure, and plasma homocysteine evaluation; hsCRP and SLE monitoring labs at each visit, and yearly assessment of aPL antibodies.(78)** CIMT monitoring is recommended for patients with > 1 risk factor or renal disease but their utility needs to be further validated. In childhood-onset SLE, no imaging has yet proven robust for surrogate of atherosclerosis and screening guidelines remain to be developed.(13) It is unclear if these modifications will mitigate CV risk in subsets of SLE patients. Other non-invasive markers of reversible endothelial dysfunction, such as flow mediated vasodilation (FMD), and reactive hyperemia- peripheral arterial tonometry (RH-PAT) have been reported to be increased in SLE patients, but require further validation in large studies.(79, 80)
Biomarkers are needed to serve as reliable surrogates for atherosclerotic disease in SLE. Novel methods able to determine which SLE patients are at increased risk for CVD are also of interest. Recently, the PREDICTS model that uses a combination of inflammatory and metabolic markers (inflammatory HDL, leptin, TWEAK, and homocysteine, age, and diabetes status) demonstrated good predictive capacity for development of atherosclerosis in female SLE patients in short term follow up.(81) As neutrophils may play important roles involved in both SLE and atherosclerosis pathogenesis, the proteins S100A8, A9, A8/9, and A12 (secreted by activated neutrophils and associated to inflammation and atherosclerosis)(82)** have been proposed as putative biomarkers but remain to be validated. Indeed, in the general population, elevation of these proteins predicts future CV events,(83) and use of these alarmins as CVD biomarkers warrants investigation in SLE. Carotid ultrasound and CT for coronary calcification assessment have been used to predict subclinical atherosclerosis in SLE in research studies, but may only identify a small subset of individuals with damage and at risk for CV events. It is not only the quantity of plaque, but its quality, that needs to be further examined in these individuals. Assessments of vascular inflammation, unstable plaque and microvascular disease should be studied for risk stratification in SLE.18F-flouride PET/CT or MRI may identify patients with vulnerable plaque;(84)* further studies are needed to determine if it can identify SLE patients at risk for CV event (Table 1).
Table I.
Potential Imaging and Vascular Function Screening tools for CVD in SLE
| Tool | Measures | Abnormal in SLE |
|---|---|---|
| US for CIMT and carotid plaque (78) (85) | Carotid artery intima media thickness, subclinical atherosclerosis; association with coronary atherosclerosis | x |
| Flow mediated vasodilation (FMD) (79, 80) | Non-invasive method to evaluate endothelial dysfunction in conduit vessels | x |
| Reactive hyperemia- peripheral arterial tonometry (RH-PAT) (79, 80) | Non-invasive method to evaluate endothelial dysfunction in microvessels. Associates with coronary involvement | ? |
| (18)FDG PET CT or MRI(84) | Quantifies uptake by inflammatory cells in the aortic tree. | ? |
| Pulse wave velocity (PWV)(42, 86) | Non invasive measure of arterial stiffness, but depends on blood pressure; associates with CV risk. | x |
| Cardio-ankle vascular index (CAVI)(87) | Non-invasive measure of arterial stiffness, independent of blood pressure; associates with coronary abnormalities. | ? |
Therapeutic targets
Statins
Statins are a cornerstone of treatment in atherosclerosis through pleiotropic metabolic and anti-inflammatory effects.(88–90) Previous studies on the use of statins in SLE have been overall small in size and not shown improvement in outcomes, despite improved lipoprotein profiles.(91) More recently, specific statins were shown to modulate distinct inflammatory pathways.(92) Simvastatin lowers small LDL-IgG-immune complex levels more effectively than levels of LDL-cholesterol and LDL-apoB levels in individuals with atherosclerosis.(93)* Atorvastatin decreases IL-6 and IL-10 and this could have implications for SLE.(86) Fluvastatin had anti-inflammatory effects on SLE macrophages, lowering ROS production.(63)* A recent study suggested that statin therapy in Taiwanese SLE patients with hyperlipidemia may reduce the risk of mortality, CVD and end-stage renal disease.(94)** As we learn more about the shared pathogenesis of these inflammatory conditions, perhaps specific statin and patient selection will yield greater improvement in CV risk for SLE patients.
Antimalarials
Antimalarials are standard of care in SLE and they improve lipid profiles and may have vasculoprotecive effects.(95) Hydroxychloroquine has been shown to inhibit IFN production by pDCS, which may account for the reduced risk.(96) In murine models of SLE, hydroxychloroquine decreased reactive oxygen species production via NADPH, improving nitric oxide availability and preventing endothelial dysfunction. (97)** Antimalarials also inhibit NET formation in vitro and this could have a putative vasculoprotective role.(98)
Insulin resistance
Insulin resistance in SLE is associated with traditional CV risk factors as well as higher levels of oxLDL and end organ damage. (41, 99) Therapy aimed at improving insulin resistance therefore holds promise for ameliorating some of the excess CV risk of SLE. Metformin downregulates NET formation and IFN generation, and was shown to decrease SLE flares in a recent pilot study.(100) However, whether metformin will be efficacious is reducing CV risk in SLE requires further investigation. PPAR-y is a transcription factor involved in lipid metabolism, inflammation, insulin resistance, and vascular health, by inhibiting leukocyte and endothelial cell interaction in endothelial cells.(101)** PPAR-y-treated mice showed significant decreases in immune complex deposition, renal inflammation, improvement in insulin resistance, adipokine, and lipid profile and reduced SLE associated atherosclerosis,(102–104) making this an interesting prospect of therapy for CVD in SLE.(105) Indeed, a recent pilot study performed in subjects with rheumatoid arthritis who received the PPAR agonist pioglitazone indicated a significant modulatory effect on vascular function and inflammation, suggesting that this class of drugs could be investigated in SLE.
Vitamin D
Vitamin D deficiency is common in patients with SLE, and has been associated with CVD in the general population.(106) SLE patients with low vitamin D are at higher risk for CV events,(107, 108) and have increased aortic stiffness.(109, 107**) Treatment with Vitamin D improved myeloid angiogenic cell numbers and function, possibly through downregulation of IP10(110) and regulation of nitric oxide in endothelial cells.(111) A small study suggests Vitamin D repletion may benefit endothelial function in SLE,(112) but large prospective studies are needed to determine the role of Vitamin D supplementation in cardioprotection for SLE.
Biologics
Other anti-inflammatory therapies, such as anti-IL6(113) and TNF inhibitors(114) are currently in clinical trials to decrease CV risk in patients with rheumatoid arthritis. In SLE, targeting B cells through anti-BLys(67) or anti-CD20(67) may have the potential to reduce endothelial dysfunction but this remains to be further investigated. Given recent positive results with anti-type I IFN receptor trials for SLE disease activity(115) and the putative role that these cytokines play in atherogenesis in SLE, it will be important to determine their role in CV health in this disease.
SUMMARY AND CONCLUSION
CV disease is not only an important comorbidity of SLE, but similar proinflammatory and dysregulated immune pathways may lead to shared pathogenesis of these two conditions. Prospective studies are needed to determine ideal monitoring for subclinical disease (Table I), prevention of atherosclerosis, and aggressive treatment of ongoing CVD to reduce the excessive risk for SLE patients.
Key Points.
Cardiovascular disease confers significant risk for SLE patients, which cannot be accounted for by traditional risk factors.
Dysregulation of the innate and adaptive immune system in SLE likely contributes to premature CVD.
Novel imaging methods and biomarkers for identifying subclinical vascular inflammation and patients at high risk for poor CV outcomes are needed
Decreasing oxidative damage caused by neutrophils and interrupting the proinflammatory loop perpetuated by type I IFNs may improve CV outcomes for SLE patients.
Acknowledgments
Financial Support and Sponsorship: This work was supported by the Intramural Research Program, NIAMS/NIH, and the Lupus Research Institute.
Footnotes
Conflicts of Interest: There are no conflicts of interest.
References
- 1.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. The Journal of rheumatology. 2008 Nov;35(11):2152–8. doi: 10.3899/jrheum.080214. Epub 2008/09/17. eng. [DOI] [PubMed] [Google Scholar]
- 2.Lerang K, Gilboe IM, Steinar Thelle D, Gran JT. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus. 2014 Dec;23(14):1546–52. doi: 10.1177/0961203314551083. Epub 2014/09/12. eng. [DOI] [PubMed] [Google Scholar]
- 3.Björnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. The Journal of rheumatology. 2004;31(4):713–9. [PubMed] [Google Scholar]
- 4.Lai CH, Lai WW, Chiou MJ, Lin WC, Yang YJ, Li CY, et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Annals of the rheumatic diseases. 2015 Aug 18; doi: 10.1136/annrheumdis-2015-207719. Epub 2015/08/20. Eng. [DOI] [PubMed] [Google Scholar]
- 5.Doria A, Shoenfeld Y, Wu R, Gambari PF, Puato M, Ghirardello A, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2003 Nov;62(11):1071–7. doi: 10.1136/ard.62.11.1071. Epub 2003/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. The American journal of medicine. 2008 Oct;121(10 Suppl 1):S3–8. doi: 10.1016/j.amjmed.2008.06.010. Epub 2008/10/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. American journal of epidemiology. 1997 Mar 1;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM. Cardiovascular Risk Prediction: Basic Concepts, Current Status, and Future Directions. Circulation. 2010 Apr 20;121(15):1768–77. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 9.Gatto FBM, Larosa M, Iaccarino L, Punzi L, Doria A. Cardiovascular risk factors, burden of disease and preventive strategies in patients with systemic lupus erythematosus: a literature review. Expert Opinion on Drug Safety. 2015 Sep;14(9):1373–85. doi: 10.1517/14740338.2015.1073259. [DOI] [PubMed] [Google Scholar]
- 10.Urowitz MB, Gladman D, Ibañez D, Bae SC, Sanchez-Guerrero J, Gordon C, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care & Research. 2010;62(6):881–7. doi: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis & Rheumatism. 1999;42(2):338–46. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Roman MJ, Shanker B-A, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and Correlates of Accelerated Atherosclerosis in Systemic Lupus Erythematosus. New England Journal of Medicine. 2003;349(25):2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- *13.Barsalou J, Bradley TJ, Tyrrell PN, Slorach C, Ng LW, Levy DM, et al. Impact of Disease Duration on Vascular Surrogates of Early Atherosclerosis in Childhood-Onset Systemic Lupus Erythematosus. Arthritis & rheumatology (Hoboken, NJ) 2016 Jan;68(1):237–46. doi: 10.1002/art.39423. Epub 2015/09/12. eng Pediatric SLE patients did not have an increase in endothelial dysfunction as measured by carotid intima media thickness (CIMT), flow mediated (FMD), or pulse wave velocity (PWV). Increasing SLE disease duration increases risk of abnormal FMD in pediatric patients. [DOI] [PubMed] [Google Scholar]
- *14.Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmunity reviews. 2016 Jan;15(1):22–37. doi: 10.1016/j.autrev.2015.10.002. Epub 2015/10/13. eng A meta-analysis of 80 studies of atherosclerosis in SLE patients and controls finds that SLE patients have increased prevalence of subclinical atherosclerosis as measured by carotid plaque and higher carotid intima media thickness. Age, high density lipoprotein, triglyceride, disease duration, ESR, SLE disease activity index (SLEDAI) and steroids influence increased cardiovascular disease burden among SLE patients. [DOI] [PubMed] [Google Scholar]
- *15.Merched AJ, Daret D, Li L, Franzl N, Sauvage-Merched M. Specific autoantigens in experimental autoimmunity-associated atherosclerosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016 Feb 18; doi: 10.1096/fj.201500131. Epub 2016/02/20. Eng Autoimmunity associated mouse model of atherosclerosis generated by transplanting FCγRIIB knockout mouse bone marrow into LDL receptor knockout mice. Macrophages demonstrate reduced phagocytosis. Histology of atherosclerosis demonstrates antibodies to endothelial cells and necrotic cores and more aggressive lesions. [DOI] [PubMed] [Google Scholar]
- *16.Watkins AA, Yasuda K, Wilson GE, Aprahamian T, Xie Y, Maganto-Garcia E, et al. IRF5 deficiency ameliorates lupus but promotes atherosclerosis and metabolic dysfunction in a mouse model of lupus-associated atherosclerosis. Journal of immunology (Baltimore, Md: 1950) 2015 Feb 15;194(4):1467–79. doi: 10.4049/jimmunol.1402807. Epub 2015/01/18. eng Interferon Regulatory factory (IRF)5 increases SLE risk, and knockouts ameliorate disease. IRF5 deficient mice develop increased atherosclerosis and metabolic dysregulation and had reduced IL-10 levels in aortic tissue. IRF-5 may play contrasting roles in SLE and atherosclerosis, but IL-10 level regulation may be a shared mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004 May 15;103(10):3677–83. doi: 10.1182/blood-2003-09-3198. Epub 2004/01/17. eng. [DOI] [PubMed] [Google Scholar]
- 18.Bruce IN, Burns RJ, Gladman DD, Urowitz MB. Single photon emission computed tomography dual isotope myocardial perfusion imaging in women with systemic lupus erythematosus. I. Prevalence and distribution of abnormalities. J Rheumatol. 2000 Oct;27(10):2372–7. Epub 2000/10/19. eng. [PubMed] [Google Scholar]
- 19.Sharma SK, Rathi M, Sahoo S, Prakash M, Dhir V, Singh S. Assessment of premature atherosclerosis in systemic lupus erythematosus patients with and without nephritis. Lupus. 2015 Dec 16; doi: 10.1177/0961203315622822. Epub 2015/12/19. Eng. [DOI] [PubMed] [Google Scholar]
- **20.Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. European Heart Journal. 2016 doi: 10.1093/eurheartj/ehw018. Imaging captures how chronic inflammation in SLE can affect coronary microvascular function and the regulation of blood flow to the myocardium due to endothelial dysfunction. ROS, T cells, and monocyte dysregulation contribute to microvascular dysfunction. [DOI] [PubMed] [Google Scholar]
- 21.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. The Journal of cell biology. 2015 Apr 13;209(1):13–22. doi: 10.1083/jcb.201412052. Epub 2015/04/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013 Mar;34(10):719–28. doi: 10.1093/eurheartj/ehs411. Epub 2012/12/18. eng. [DOI] [PubMed] [Google Scholar]
- 23.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European Journal of Immunology. 2011;41(9):2515–8. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouts YM, Wolthuis DFGJ, Dirkx MFM, Pieterse E, Simons EMF, Van Boekel AM, et al. Apoptosis and NET formation in the pathogenesis of SLE. Autoimmunity. 2012 Dec 01;45(8):597–601. doi: 10.3109/08916934.2012.719953. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson J, Hansson G. The changing face of atherosclerotic plaque inflammation. Journal of internal medicine. 2015;278(5):430–2. doi: 10.1111/joim.12403. [DOI] [PubMed] [Google Scholar]
- 26.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: basic mechanisms oxidation, inflammation, and genetics. Circulation. 1995;91(9):2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 27.Urowitz MB, Gladman D, Ibañez D, Fortin P, Sanchez-Guerrero J, Bae S, et al. Accumulation of coronary artery disease risk factors over three years: Data from an international inception cohort. Arthritis Care & Research. 2008;59(2):176–80. doi: 10.1002/art.23353. [DOI] [PubMed] [Google Scholar]
- 28.Ardoin SP, Schanberg LE, Sandborg C, Yow E, Barnhart HX, Mieszkalski K, et al. Laboratory markers of cardiovascular risk in pediatric SLE: the APPLE baseline cohort. Lupus. 2010 Oct;19(11):1315–25. doi: 10.1177/0961203310373937. Epub 2010/09/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tselios K, Koumaras C, Gladman DD, Urowitz MB. Dyslipidemia in systemic lupus erythematosus: just another comorbidity? Seminars in Arthritis and Rheumatism. doi: 10.1016/j.semarthrit.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Borba E, Bonfa E. Dyslipoproteinemias in systemic lupus erythematosus: influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6(6):533–9. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 31.Vaarala O, Aho K, Palosuo T, Alfthan G, Jauhiainen M, Leirisalo-Repo M. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. The Lancet. 1993;341(8850):923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 32.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Annals of the rheumatic diseases. 2014 Mar;73(3):609–15. doi: 10.1136/annrheumdis-2012-202914. Epub 2013/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 33.Smith CK, Vivekanandan-Giri A, Tang CR, Knight JS, Mathew A, Padilla RL, et al. Neutrophil Extracellular Trap-Derived Enzymes Oxidize High-Density Lipoprotein An Additional Proatherogenic Mechanism in Systemic Lupus Erythematosus. Arthritis & Rheumatology. 2014 Sep;66(9):2532–44. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croca S, Davari M, Isenberg D, Rahman A. 323. Serum Anti-Apolipoprotein 1 Antibodies are Present in a Quarter of Patients with Systemic Lupus Erythematosus at the Time of Diagnosis and are Associated with Earlier Mortality. Rheumatology. 2014 Apr 1;53(suppl 1):i180–i1. [Google Scholar]
- 35.Kiani AN, Fang H, Akhter E, Quiroga C, Simpson N, Alaupovic P, et al. Apolipoprotein-Containing Lipoprotein Subclasses and Subclinical Atherosclerosis in Systemic Lupus Erythematosus. Arthritis Care & Research. 2015;67(3):442–6. doi: 10.1002/acr.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posadas-Romero C, Torres-Tamayo M, Zamora-González J, Aguilar-Herrera BE, Posadas-Sánchez R, Cardoso-Saldaña G, et al. High insulin levels and increased low-density lipoprotein oxidizability in pediatric patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2004;50(1):160–5. doi: 10.1002/art.11472. [DOI] [PubMed] [Google Scholar]
- 37.Parker B, Urowitz MB, Gladman DD, Lunt M, Bae S-C, Sanchez-Guerrero J, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Annals of the rheumatic diseases. 2013;72(8):1308–14. doi: 10.1136/annrheumdis-2012-202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Annals of the rheumatic diseases. 2007 Feb 1;66(2):208–14. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lidar M, Braf A, Givol N, Langevitz P, Pauzner R, Many A, et al. Anti-insulin antibodies and the natural autoimmune response in systemic lupus erythematosus. Lupus. 2001;10(2):81–6. doi: 10.1191/096120301669081314. [DOI] [PubMed] [Google Scholar]
- *40.Sabio JM, Vargas-Hitos JA, Martinez-Bordonado J, Navarrete-Navarrete N, Diaz-Chamorro A, Olvera-Porcel C, et al. Association between low 25-hydroxyvitamin D, insulin resistance and arterial stiffness in nondiabetic women with systemic lupus erythematosus. Lupus. 2015 Feb;24(2):155–63. doi: 10.1177/0961203314551811. In females with SLE, low vitamin D was associated with increased insulin resistance independent of their BMI. Pulse wave velocity, a marker of arterial stiffness, was significantly higher in the SLE patients with the lowest vitamin D levels. [DOI] [PubMed] [Google Scholar]
- 41.Tso TK, Huang WN. Elevation of fasting insulin and its association with cardiovascular disease risk in women with systemic lupus erythematosus. Rheumatology international. 2009 May;29(7):735–42. doi: 10.1007/s00296-008-0781-7. Epub 2008/11/28. eng. [DOI] [PubMed] [Google Scholar]
- *42.Castejon R, Jimenez-Ortiz C, Rosado S, Tutor-Ureta P, Mellor-Pita S, Yebra-Bango M. Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus. 2015 Sep 9; doi: 10.1177/0961203315603138. Metabolic syndrome in SLE patients was associated with increased organ damage, decreased number of endothelial progenitor cells, and increase in arterial stiffness compared to SLE patients without metabolic syndrome. This study suggests it is prudent to monitor SLE patients with metabolic syndrome closely for atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 43.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007 Oct 15;110(8):2907–15. doi: 10.1182/blood-2007-05-089086. Epub 2007/07/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thacker SG, Zhao W, Smith CK, Luo W, Wang H, Vivekanandan-Giri A, et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis & Rheumatism. 2012;64(9):2975–85. doi: 10.1002/art.34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Mohan S, Barsalou J, Bradley TJ, Slorach C, Reynolds JA, Hasni S, et al. Endothelial progenitor cell phenotype and function are impaired in childhood-onset systemic lupus erythematosus. Arthritis & rheumatology (Hoboken, NJ) 2015 May;67(8):2257–62. doi: 10.1002/art.39149. Epub 2015/04/22. eng. This is the first study to observe decreased number and function of circulating endothelial progenitor cells in childhood-onset SLE, which is likely due to high levels of type I IFNs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. Journal of immunology (Baltimore, Md: 1950) 2011 Dec 1;187(11):6143–56. doi: 10.4049/jimmunol.1101284. Epub 2011/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goossens P, Gijbels MJJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, et al. Myeloid Type I Interferon Signaling Promotes Atherosclerosis by Stimulating Macrophage Recruitment to Lesions. Cell Metabolism. 2010 Aug 4;12(2):142–53. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: A novel link between interferon-α and atherosclerosis in lupus. Arthritis & Rheumatism. 2011;63(2):492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
- 49.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010 Sep 16;116(11):1951–7. doi: 10.1182/blood-2010-03-274605. Epub 2010/06/12. eng. [DOI] [PubMed] [Google Scholar]
- **50.Diao Y, Mohandas R, Lee P, Liu Z, Sautina L, Mu W, et al. Effects of Long-Term Type I Interferon on the Arterial Wall and Smooth Muscle Progenitor Cells Differentiation. Arteriosclerosis, thrombosis, and vascular biology. 2016 Feb;36(2):266–73. doi: 10.1161/ATVBAHA.115.306767. Epub 2015/12/05. eng.Smooth muscle progenitor cells, which have been implicated in development of atherosclerosis, remain in an immature state when exposed to Type I IFNs. This may lead to fewer smooth muscle cells in the atherosclerotic plaque, and thus a plaque which is more likely to rupture. [DOI] [PubMed] [Google Scholar]
- **51.Osaka M, Ito S, Honda M, Inomata Y, Egashira K, Yoshida M. Critical role of the C5a-activated neutrophils in high-fat diet-induced vascular inflammation. Scientific Reports. 2016 Feb 19;6:21391. doi: 10.1038/srep21391. 12/09/received; 01/22/accepted. Live in vitro microscopy of vascular inflammation demonstrates feeding mice a high fat diet upregulates production of C5a, which is able attract and activate neutrophils. C5a also induces production of inflammatory cytokine MCP-1, which directs lesional neutrophil infiltration. These findings show a link between high fat diet and neutrophils in atherosclerosis pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Seminars in Immunopathology. 2013;35(4):455–63. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. Journal of immunology (Baltimore, Md: 1950) 2010 Mar 15;184(6):3284–97. doi: 10.4049/jimmunol.0902199. Epub 2010/02/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Megens RT, Vijayan S, Lievens D, Doring Y, van Zandvoort MA, Grommes J, et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thrombosis and haemostasis. 2012 Mar;107(3):597–8. doi: 10.1160/TH11-09-0650. Epub 2012/02/10. eng. [DOI] [PubMed] [Google Scholar]
- **55.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Annals of the rheumatic diseases. 2015 Jul;74(7):1417–24. doi: 10.1136/annrheumdis-2013-204837. Epub 2014/02/27. eng. In SLE, neutrophil NETs externalize matrix metalloproteinase-9 (MMP-9) which activates MMP-2 in endothelial cells causing imparied relaxation and endothelial cell death. Immune complexes containing MMP-9 enhance NET-osis in LDGs, resulting in a proinflammmatory loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nature medicine. 2016 Feb;22(2):146–53. doi: 10.1038/nm.4027. Epub 2016/01/19. eng. In SLE, mitochondrial reactive oxygen species (ROS) play an important role in autoimmunity. Mitochondrial ROS on neutrophil NETs induce an increase in Type I IFN synthesis and drive NET-osis in low density granulocytes. Inhibiting mitochondrial ROS attenuates autoimmune phenotype, suggesting a therapeutic role for mitochondrial ROS inhibitors in SLE and atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science (New York, NY) 2015 Jul 17;349(6245):316–20. doi: 10.1126/science.aaa8064. Epub 2015/07/18. eng. This study describes a positive inflammatory loop between cholesterol, NETs, and macrophages. Cholesterol crytals stimulate neutrophils to release NETs. NETs prime macrophages to release inflammatory cytokines, which increase immune cell recruitment to atherosclerotic plaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awasthi D, Nagarkoti S, Kumar A, Dubey M, Singh AK, Pathak P, et al. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radical Biology and Medicine. 2016 Apr;93:190–203. doi: 10.1016/j.freeradbiomed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- **59.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circulation research. 2015 Mar 27;116(7):1182–92. doi: 10.1161/CIRCRESAHA.116.304944. Epub 2014/12/31. eng. Studying coronary lesions in ST-elevation acute coronary syndromes reveals highly active neutrophils which readily undergo NET-osis and form aggregates with platelets. NETs were part of the thrombus lattice and larger NET burden correlated with increasing infarct size and decreasing likelihood of ST segment resolution. [DOI] [PubMed] [Google Scholar]
- **60.Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. European Heart Journal. 2015;36(22):1405–14. doi: 10.1093/eurheartj/ehv007. 2015-06-07 00:00:00. Authors propose a novel mechanism for the prothrombotic role of neutrophils. Platelet-NET interactions at plaque rupture site in ST-elevation MI activate neutrophils. NETs allow neutrophils to display functional tissue factor, inducing thrombin formation, further activating platelets, propogating and stablizing the thrombus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Annals of the rheumatic diseases. 2014 Oct 1;73(10):1854–63. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 62.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015 Jan;12(1):10–7. doi: 10.1038/nrcardio.2014.173. //print. [DOI] [PubMed] [Google Scholar]
- *63.Ruiz-Limon P, Barbarroja N, Perez-Sanchez C, Aguirre MA, Bertolaccini ML, Khamashta MA, et al. Atherosclerosis and cardiovascular disease in systemic lupus erythematosus: effects of in vivo statin treatment. Annals of the rheumatic diseases. 2015 Jul 1;74(7):1450–8. doi: 10.1136/annrheumdis-2013-204351. Here, the antiinflammatory and anti-oxidative effectiveness of fluvastatin is demonstrated in vitro in SLE monocytes and in vivo, reducing SLEDAI, anti-dsDNA, IL-6 and MCP-1 in SLE patients after 1 month of therapy. Fluvastatin increased monocyte mitochondrial biosynthesis and lowered ROS levels. [DOI] [PubMed] [Google Scholar]
- 64.Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discovery medicine. 2012;13(69):151–8. [PubMed] [Google Scholar]
- 65.Takai T. Fc Receptors and Their Role in Immune Regulation and Autoimmunity. Journal of Clinical Immunology. 25(1):1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 66.Mendez-Fernandez YV, Stevenson BG, Diehl CJ, Braun NA, Wade NS, Covarrubias R, et al. The inhibitory FcγRIIb modulates the inflammatory response and influences atherosclerosis in male apoE−/− mice. Atherosclerosis. 2011 Jan;214(1):73–80. doi: 10.1016/j.atherosclerosis.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsiantoulas D, Sage AP, Mallat Z, Binder CJ. Targeting B Cells in Atherosclerosis Closing the Gap From Bench to Bedside. Arteriosclerosis Thrombosis and Vascular Biology. 2015 Feb;35(2):296–302. doi: 10.1161/ATVBAHA.114.303569. [DOI] [PubMed] [Google Scholar]
- 68.Svenungsson E, Engelbertsen D, Wigren M, Gustafsson JT, Gunnarsson I, Elvin K, et al. Decreased levels of autoantibodies against apolipoprotein B-100 antigens are associated with cardiovascular disease in systemic lupus erythematosus. Clinical and experimental immunology. 2015 Sep;181(3):417–26. doi: 10.1111/cei.12651. Epub 2015/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *69.Menon M, Blair Paul A, Isenberg David A, Mauri C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity. 2016 Mar 15;44(3):683–97. doi: 10.1016/j.immuni.2016.02.012. PDCs titrate IFN-α to control differentiation of B cells and Bregs, and Bregs reciprocally regulate IFN production. This interaction is dysregulated in pDCs from SLE patients, and potentially may be modified with rituximab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. The Journal of Immunology. 2010;185(7):4410–9. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- *71.Perez-Sanchez C, Barbarroja N, Messineo S, Ruiz-Limon P, Rodriguez-Ariza A, Jimenez-Gomez Y, et al. Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Annals of the rheumatic diseases. 2015 Jul;74(7):1441–9. doi: 10.1136/annrheumdis-2013-204600. Epub 2014/03/13. eng. Gene expression patterns from monocytes of patients with SLE and aPL antibody syndrome reveal increased in markers of early atherosclerosis and thrombotic events, adding to evidence that aPL antibodies may be an additive risk for thrombosis in SLE. [DOI] [PubMed] [Google Scholar]
- 72.Ballocca F, D’Ascenzo F, Moretti C, Omedè P, Cerrato E, Barbero U, et al. Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. European journal of preventive cardiology. 2014 doi: 10.1177/2047487314546826. 2047487314546826. [DOI] [PubMed] [Google Scholar]
- 73.Gustafsson JT, Simard JF, Gunnarsson I, Elvin K, Lundberg IE, Hansson L-O, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis research & therapy. 2012;14(2):1–11. doi: 10.1186/ar3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated With Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis & Rheumatology. 2015;67(11):2990–3003. doi: 10.1002/art.39247. Neutrophils from patients with APS are predisposed to NET release, and levels of circulating NETs correlated with levels of circulating aPL antibodies. This study supports role of aPLs in promoting NET release, which may contribute to thrombotic events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonelli M, Smolen JS, Scheinecker C. Treg and lupus. Annals of the rheumatic diseases. 2010 Jan 1;69(Suppl 1):i65–i6. doi: 10.1136/ard.2009.117135. [DOI] [PubMed] [Google Scholar]
- 76.Pastrana JL, Sha X, Virtue A, Mai J, Cueto R, Lee IA, et al. Regulatory T cells and Atherosclerosis. Journal of clinical & experimental cardiology. 2012;2012(Suppl 12):002. doi: 10.4172/2155-9880.S12-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nature medicine. 2006;12(2):178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- **78.Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal Monitoring For Coronary Heart Disease Risk in Patients with Systemic Lupus Erythematosus: A Systematic Review. The Journal of rheumatology. 2016;43(1):54–65. doi: 10.3899/jrheum.150460. Guildelines for evidence based risk stratification and monitoring for CVD risk in adult SLE patients are proposed. [DOI] [PubMed] [Google Scholar]
- 79.Matsuzawa Y, Guddeti RR, Kwon T-G, Lerman LO, Lerman A. Treating Coronary Disease and the Impact of Endothelial Dysfunction. Progress in Cardiovascular Diseases. 2015 Mar;57(5):431–42. doi: 10.1016/j.pcad.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos MJ, Carmona-Fernandes D, Canhao H, Canas da Silva J, Fonseca JE, Gil V. Early vascular alterations in SLE and RA patients--a step towards understanding the associated cardiovascular risk. PloS one. 2012;7(9):e44668. doi: 10.1371/journal.pone.0044668. Epub 2012/09/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMahon M, Skaggs BJ, Grossman JM, Sahakian L, FitzGerald J, Wong WK, et al. A Panel of Biomarkers Is Associated With Increased Risk of the Presence and Progression of Atherosclerosis in Women With Systemic Lupus Erythematosus. Arthritis & Rheumatology. 2014;66(1):130–9. doi: 10.1002/art.38204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **82.Oesterle A, Hofmann Bowman MA. S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets. Arteriosclerosis, thrombosis, and vascular biology. 2015 Dec 1;35(12):2496–507. doi: 10.1161/ATVBAHA.115.302072. S100A12, which has been associated both with atherosclerosis in patients with CVD and also in SLE disease activity, is identified as a biomarker for human atherosclerosis, and may be a marker of plaque instability. Inhibiting S100A12 mediated activation of RAGE decreases atherosclerosis in hyperlipidemic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. EN-RAGE A Novel Inflammatory Marker for Incident Coronary Heart Disease. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(12):2695–9. doi: 10.1161/ATVBAHA.114.304306. [DOI] [PubMed] [Google Scholar]
- *84.Adamson PD, Vesey AT, Joshi NV, Newby DE, Dweck MR. Salt in the wound: (18)F-fluoride positron emission tomography for identification of vulnerable coronary plaques. Cardiovascular Diagnosis and Therapy. 2015;5(2):150–5. doi: 10.3978/j.issn.2223-3652.2015.03.01. 01/19/received02/05/accepted. (18) F fluoride binds to expose, early active regions of microcalcification, thereby localizing to coronary plaques most at risk for rupture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeevarethinam A, Venuraju S, Weymouth M, Atwal S, Lahiri A. Carotid Intimal Thickness and Plaque Predict Prevalence and Severity of Coronary Atherosclerosis A Pilot Study. Angiology. 2015;66(1):65–9. doi: 10.1177/0003319714522849. [DOI] [PubMed] [Google Scholar]
- 86.Meyer ML, Tanaka H, Palta P, Patel MD, Camplain R, Couper D, et al. Repeatability of central and peripheral pulse wave velocity measures: the atherosclerosis risk in communities (ARIC) study. American journal of hypertension. 2015:hpv127. doi: 10.1093/ajh/hpv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kusunose K, Sato M, Yamada H, Saijo Y, Bando M, Hirata Y, et al. Prognostic Implications of Non-Invasive Vascular Function Tests in High-Risk Atherosclerosis Patients. Circulation Journal. 2016:advpub. doi: 10.1253/circj.CJ-15-1356. [DOI] [PubMed] [Google Scholar]
- 88.Forrester JS, Libby P. The Inflammation Hypothesis and Its Potential Relevance to Statin Therapy. The American Journal of Cardiology. 2007 Mar 1;99(5):732–8. doi: 10.1016/j.amjcard.2006.09.125. [DOI] [PubMed] [Google Scholar]
- 89.Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin Restores Lck Expression and Lipid Raft-Associated Signaling in T Cells from Patients with Systemic Lupus Erythematosus. The Journal of Immunology. 2006 Nov 15;177(10):7416–22. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 90.Meschiari CA, Pinheiro LC, Guimaraes DA, Gerlach RF, Tanus-Santos JE. Sodium nitrite attenuates MMP-9 production by endothelial cells and may explain similar effects of atorvastatin. Naunyn-Schmiedeberg’s archives of pharmacology. 2016 Feb;389(2):223–31. doi: 10.1007/s00210-015-1192-4. Epub 2015/11/29. eng. [DOI] [PubMed] [Google Scholar]
- 91.Ye Y, Zhao X, Xie H, Tian Z, Zhang S. Efficacy and safety of statins in the prevention of atherosclerosis in patients with systemic lupus erythematosus — A meta-analysis of randomized controlled trials. International Journal of Cardiology. 2013 Jul 15;167(1):301–3. doi: 10.1016/j.ijcard.2012.09.190. [DOI] [PubMed] [Google Scholar]
- 92.Ardoin SP, Schanberg LE, Sandborg CI, Barnhart HX, Evans GW, Yow E, et al. Secondary analysis of APPLE study suggests atorvastatin may reduce atherosclerosis progression in pubertal lupus patients with higher C reactive protein. Annals of the rheumatic diseases. 2014 Mar 1;73(3):557–66. doi: 10.1136/annrheumdis-2012-202315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *93.Horl G, Froehlich H, Ferstl U, Ledinski G, Binder J, Cvirn G, et al. Simvastatin Efficiently Lowers Small LDL-IgG Immune Complex Levels: A Therapeutic Quality beyond the Lipid-Lowering Effect. PloS one. 2016;11(2):e0148210. doi: 10.1371/journal.pone.0148210. Epub 2016/02/04. eng. Statins have effects which extend beyond lipid reduction. Simvastatin was highly effective in lowering small LDL-IgG complexes, which are potentially atherogenic and contribute to foam cell formation. Simvastatin reduced small LDL-IgG complexes more than LDL cholesterol and LDL-apo-B levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **94.Yu HH, Chen PC, Yang YH, Wang LC, Lee JH, Lin YT, et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: A nationwide population-based cohort study. Atherosclerosis. 2015 Nov;243(1):11–8. doi: 10.1016/j.atherosclerosis.2015.08.030. Epub 2015/09/08. eng. A nationwide population based cohort study in Taiwan demonstrated statin use in SLE patients with hyperlipidemia resulted in primary prevention for CVD. Statins reduced both morbidity and mortality in this large cohort. Higher dose of statins correlate with more significant reduction in risk. [DOI] [PubMed] [Google Scholar]
- 95.Durcan L, Winegar DA, Connelly MA, Otvos JD, Magder LS, Petri M. Longitudinal Evaluation of Lipoprotein Variables in Systemic Lupus Erythematosus Reveals Adverse Changes with Disease Activity and Prednisone and More Favorable Profiles with Hydroxychloroquine Therapy. The Journal of rheumatology. 2016 Feb 1;2016 doi: 10.3899/jrheum.150437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Research & Therapy. 2012;14(3):1–10. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **97.Virdis A, Tani C, Duranti E, Vagnani S, Carli L, Kühl AA, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Research & Therapy. 2015;17(1):1–9. doi: 10.1186/s13075-015-0790-3. In a murine SLE model, endothelial dysfunction is driven by NADPH ROS. Administration of HCQ normalized endothelium dependent relaxation in 8 week old and 24 week old mice, indicating endothelial dysfunction begins early in SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis & rheumatology (Hoboken, NJ) 2014 Sep;66(9):2532–44. doi: 10.1002/art.38703. Epub 2014/05/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El Magadmi M, Ahmad Y, Turkie W, Yates AP, Sheikh N, Bernstein RM, et al. Hyperinsulinemia, insulin resistance, and circulating oxidized low density lipoprotein in women with systemic lupus erythematosus. J Rheumatol. 2006 Jan;33(1):50–6. Epub 2006/01/06. eng. [PubMed] [Google Scholar]
- 100.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin. Arthritis & Rheumatology. 2015;67(12):3190–200. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- **101.Jin H, Gebska MA, Blokhin IO, Wilson KM, Ketsawatsomkron P, Chauhan AK, et al. Endothelial PPAR-γ Protects Against Vascular Thrombosis by Downregulating P-Selectin Expression. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(4):838–44. doi: 10.1161/ATVBAHA.115.305378. A murine model expressing mutant PPAR-γ demonstrated accelerated occlusive thrombosis via upregulation of P selectin. This atheroprotective effect of PPAR-γ may be through suppressing P-selectin mediated adhesion of leukocytess on endothelium. PPAR-y agonists may warrant study as cardioprotective agents in SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mok CC. Prognostic factors in lupus nephritis. Lupus. 2005;14(1):39–44. doi: 10.1191/0961203305lu2057oa. Epub 2005/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 103.Zhao W, Thacker SG, Hodgin JB, Zhang H, Wang JH, Park JL, et al. The peroxisome proliferator-activated receptor gamma agonist pioglitazone improves cardiometabolic risk and renal inflammation in murine lupus. Journal of immunology (Baltimore, Md: 1950) 2009 Aug 15;183(4):2729–40. doi: 10.4049/jimmunol.0804341. Epub 2009/07/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang L-K, Sato K, et al. The Peroxisome Proliferator-Activated Receptor γ Agonist Rosiglitazone Ameliorates Murine Lupus by Induction of Adiponectin. The Journal of Immunology. 2009 Jan 1;182(1):340–6. doi: 10.4049/jimmunol.182.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marder W, Khalatbari S, Myles JD, Hench R, Lustig S, Yalavarthi S, et al. The Peroxisome Proliferator Activated Receptor-γ Pioglitazone Improves Vascular Function and Decreases Disease Activity in Patients With Rheumatoid Arthritis. Journal of the American Heart Association. 2013 Dec 19;2(6) doi: 10.1161/JAHA.113.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205(1):255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 107.Lertratanakul A, Wu P, Dyer A, Urowitz M, Gladman D, Fortin P, et al. 25-hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res (Hoboken) 2014 Aug;66(8):1167–76. doi: 10.1002/acr.22291. Epub 2014/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. Journal of the American Society of Nephrology. 2009;20(8):1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reynolds JA, Haque S, Berry JL, Pemberton P, Teh L-S, Ho P, et al. 25-Hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology. 2011:ker352. doi: 10.1093/rheumatology/ker352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **110.Reynolds JA, Haque S, Williamson K, Ray DW, Alexander MY, Bruce IN. Vitamin D improves endothelial dysfunction and restores myeloid angiogenic cell function via reduced CXCL-10 expression in systemic lupus erythematosus. Scientific Reports. 6:22341. doi: 10.1038/srep22341. 201603/01; 09/18/received 02/09/accepted. SLE myeloid angiogenic cells are dysfunctional and inflammatory. Treatment with vitamin D reduced IL-6 production, augmented angiogenic capacity via downregulation of CXCL-10 (IP-10), and modulated eNOS expression in myeloid angiogenic cells in vitro. In vivo, vitamin D supplementation improves endothelial function in SLE patients, independent of disease activity, suggesting this may reduce CVD risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, et al. 1α, 25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cellular Physiology and Biochemistry. 2011;27(6):661–8. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 112.Kamen DL, Oates JC. A Pilot Study to Determine if Vitamin D Repletion Improves Endothelial Function in Lupus Patients. The American journal of the medical sciences. 2015 Oct;350(4):302–7. doi: 10.1097/MAJ.0000000000000556. Epub 2015/09/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JS, Chapman MJ, Piraino P, Lamerz J, Schindler T, Cutler P, et al. Remodeling of plasma lipoproteins in patients with rheumatoid arthritis: Interleukin-6 receptor-alpha inhibition with tocilizumab. PROTEOMICS – Clinical Applications. 2016;10(2):183–94. doi: 10.1002/prca.201500036. [DOI] [PubMed] [Google Scholar]
- 114.Cheung T, Tsoi M, Cheung B. SAT0099 Effect of TNF Inhibitors on Subclinical Atherosclerosis in Patients with Rheumatoid Arthritis: A Meta-Analysis. Annals of the rheumatic diseases. 2015;74(Suppl 2):685. [Google Scholar]
- 115.Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Annals of the rheumatic diseases. 2016 doi: 10.1136/annrheumdis-2015-208562. annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]